Abstract
A series of novel aloe-emodin–coumarin hybrids were designed and synthesized. The antitumor activity of these derivatives was evaluated against five human tumor cell lines (A549, SGC-7901, HepG2, MCF-7 and HCT-8). Some of the synthesized compounds exhibited moderate to good activity against one or more cell lines. Particularly, compound 5d exhibited more potent antiproliferative activity than the reference drug etoposide against all tested tumor cell lines, indicating that it had a broad spectrum of antitumor activity and that it may provide a promising lead compound for further development as an antitumor agent by structural modification. Furthermore, the structure–activity relationship study of the synthesized compounds was also performed.
Keywords: aloe-emodin, coumarin, hybrid, structural modification, antitumor activity
1. Introduction
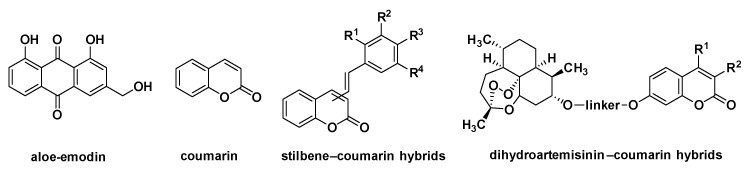
Aloe-emodin (1,8-dihydroxy-3-hydroxymethyl-anthraquinone), a representative natural anthraquinone compound, is isolated from some traditional medicinal plants such as Rheum palmatum L. and Aloe vera L (Figure 1). Modern pharmacological studies have shown that aloe-emodin exhibits a broad range of bioactivity profiles, which include antitumor [1,2,3,4,5], anti-inflammatory [6,7], antiviral [8], antimicrobial [9] and antifibrosis effects [10]. It is noteworthy that its antitumor activity has attracted special attention. Some studies have shown that aloe-emodin exerts the anti-tumor effect through inhibiting proliferation and inducing the apoptosis of certain cancer cells [11,12,13,14,15]. However, aloe-emodin itself is not sufficient to apply as an anti-tumor drug, and the development of its derivatives to improve its therapeutic efficacies is necessary.
Figure 1.
Structures of aloe-emodin, coumarin and the hybrid derivatives of coumarin.
Coumarin represents an important class of heterocyclic skeletons, which is widely distributed in diverse natural products. Coumarin-based natural and synthetic derivatives exhibit a wide range of pharmacological properties [16,17,18,19], and antitumor activity is one of the most important pharmacological activities among them [20,21,22]. Biological investigations have revealed that coumarin derivatives can exert antitumor efficiency through binding to various biological targets such as sulfatase [23], aromatase [24] and protein kinase [25]. Owing to its privileged structure and impressive antitumor activity, coumarin is often introduced into many natural and synthetic compounds in the design of antitumor drugs [26,27]. For example, Belluti et al. adopted a molecular hybridization strategy to produce stilbene-coumarin hybrids, and the hybrid compounds showed higher antitumor activities than the reference compound resveratrol [28]. Guo and co-workers synthesized a series of dihydroartemisinin-coumarin hybrids as potential antitumor agents, and the evaluation of their antitumor activity also proved that the synthetic compounds had great antitumor activity against the MDA-MB-231 and HT-29 cell lines [29]. The above research indicates that the hybridization of coumarin with other antitumor compounds is an effective strategy to search for new antitumor agents, and the resulting hybrid compounds often have more potent antitumor activity than the parent compound. These stimulated us to design and synthesize the hybrids of aloe-emodin and coumarin as antitumor agents by a molecular hybridization strategy to improve the affinity and efficacy of aloe-emodin.
Herein, a series of aloe-emodin-coumarin hybrids were synthesized, and their antiproliferative activity was evaluated against a panel of human tumor cell lines using etoposide as a reference. The structure-activity relationship of the derivatives is also discussed.
2. Results and Discussion
2.1. Chemistry
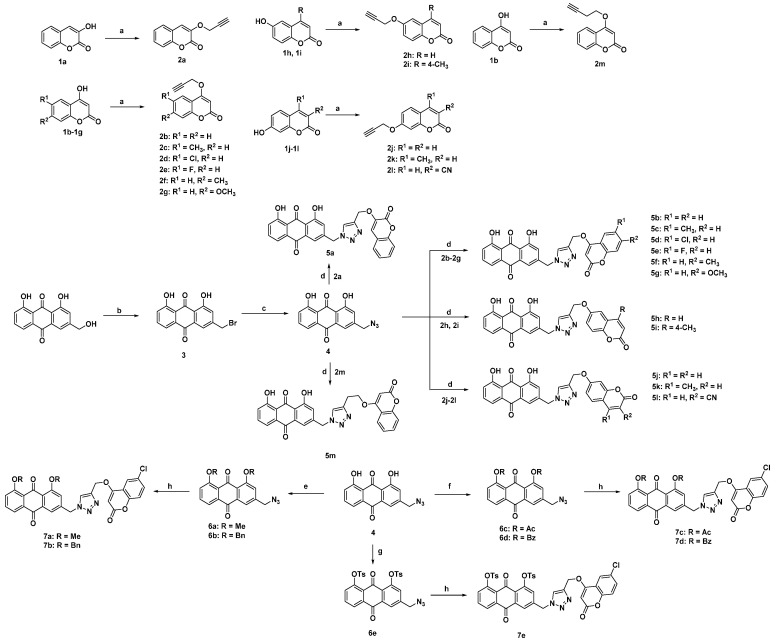
The synthetic strategy employed for the synthesis of aloe-emodin–coumarin derivatives is depicted in Scheme 1. O-propargyl and -butynyl coumarin derivatives 2a–m were obtained in a single step by the reaction of corresponding hydroxycoumarin with propargyl bromide or 4-bromobut-1-yne in the presence of K2CO3 in DMF. The treatment of commercially available aloe-emodin with CBr4 and PPh3 in tetrahydrofran afforded bromide 3 in 94% yields [30]. Then, the intermediate bromide 3 was reacted with NaN3 through nucleophilic substitution to provide azido derivative 4, which was finally subjected to the click reaction with O-propargyl or -butynyl coumarin derivatives (2a–m) in the presence of copper(I) thiophene-2-carboxylate in dichloromethane to afford the target products (5a–m) [31]. To further investigate the structure-activity relationship of 1- and 8-disubstitution on the anthraquinone core of compound 5d (the most active compound among derivatives 5a–m), a series of 5d analogs 7a–e were synthesized. The methylation or benzylation of 4 with methyl iodide or benzyl bromide in the presence of K2CO3 was performed to give dimethylated or dibenzylated intermediates 6a and 6b, respectively. Diacetylated intermediate 6c and dibenzoylated intermediate 6d were obtained by the acylation of 4. The sulfonylation of 4 with p-toluenesulfonyl chloride gave disulfonylated intermediate 6e. Finally, 6a–e reacted with 2d under the catalysis of copper(I) thiophene-2-carboxylate to give 7a–e. The structures of the target compounds are shown in Table 1 and were identified by HRMS, 1H-NMR and 13C-NMR spectral analysis (1H-NMR and 13C-NMR spectra of the synthesized compounds are available in the Supplementary Materials).
Scheme 1.
Reagents and conditions: (a) K2CO3, 3-bromoprop-1-yne (for 2a–l) or 4-bromobut-1-yne (for 2m), DMF, 60 °C, 2 h; (b) PPh3, CBr4, THF, room temperature, 6 h; (c) NaN3, CH3CN, 60 °C, 3 h; (d) 2a–m, copper(I) thiophene-2-carboxylate, CH2Cl2, room temperature, 6 h; (e) K2CO3, methyl iodide (for 6a) or benzyl bromide (for 6b), DMF, 60 °C, 4 h; (f) acetyl chloride (for 6c) or benzoyl chloride (for 6d), DMAP, DIPEA, CH2Cl2, room temperature, 4 h; (g) K2CO3, TsCl, CH3COCH3, 60 °C, 5 h; (h) 2d, copper(I) thiophene-2-carboxylate, CH2Cl2, room temperature, 6 h.
Table 1.
The structures, yields and melting points of target compounds 5a–m and 7a–e.
| Comp. | Structure | Yield | M.p. | Comp. | Structure | Yield | M.p. |
|---|---|---|---|---|---|---|---|
| 5a |
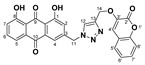
|
57% | 252.3–254.1 °C | 5j |
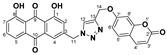
|
94% | 265.2–266.9 °C |
| 5b |
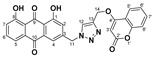
|
46% | 272.5–273.8 °C | 5k |
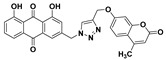
|
88% | 233.7–235.6 °C |
| 5c |
|
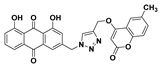
49% | 274.9–276.9 °C | 5l |
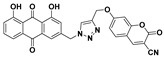
|
54% | 258.4–260.2 °C |
| 5d |
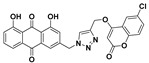
|
82% | 286.3–288.1 °C | 5m |
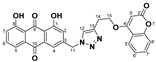
|
82% | 250.4–251.8 °C |
| 5e |
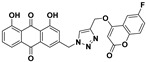
|
58% | 245.9–247.8 °C | 7a |
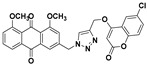
|
82% | 251.8–252.6 °C |
| 5f |
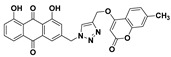
|
85% | 283.3–284.8 °C | 7b |
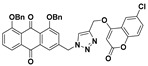
|
40% | 258.3–259.8 °C |
| 5g |
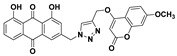
|
48% | 281.1–283.3 °C | 7c |
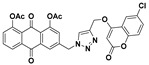
|
55% | 245.6–247.2 °C |
| 5h |
|
80% | 248.3–250.2 °C | 7d |
|
83% | 231.1–232.6 °C |
| 5i |
|
79% | 249.7–251.5 °C | 7e |

|
95% | 195.6–197.1 °C |
2.2. Biological Results and Discussion
All of the target compounds were evaluated for their antiproliferative activities against five cancer cell lines (A549, SGC-7901, HepG2, MCF-7 and HCT-8) with etoposide as a positive reference using the MTT assay. To determine the selectivity, the cytotoxic activities of several compounds with good antitumor activities were also evaluated against the Hk-2 normal cell line (Human Kidney-2). The results are summarized in Table 2.
Table 2.
In vitro antitumor activities of target compounds 5a–m and 7a–e.
| Compound | IC50 (μmol/L) a, b | |||||
|---|---|---|---|---|---|---|
| A549 | SGC-7901 | HepG2 | MCF-7 | HCT-8 | Hk-2 | |
| 5a | 2.57 ± 0.48 | 3.28 ± 0.67 | 7.06 ± 0.27 | 2.34 ± 0.48 | 1.56 ± 0.45 | <1.0 |
| 5b | >40 | >40 | 21.86 ± 0.75 | 25.27 ± 6.072 | >40 | - |
| 5c | >40 | 19.30 ± 1.28 | 14.23 ± 2.38 | 31.34 ± 0.62 | 34.07 ± 1.38 | - |
| 5d | 1.12 ± 0.09 | 0.90 ± 0.12 | 0.99 ± 0.12 | 1.59 ± 0.58 | 0.48 ± 0.06 | <1.0 |
| 5e | >40 | >40 | >40 | >40 | >40 | |
| 5f | 3.82 ± 0.31 | 2.17 ± 0.24 | 1.02 ± 0.44 | 1.68 ± 0.21 | 4.49 ± 0.24 | <10 |
| 5g | 4.05 ± 1.99 | 9.04 ± 1.17 | 5.01 ± 1.27 | 3.60 ± 0.92 | 5.79 ± 1.24 | <10 |
| 5h | >40 | >40 | 14.95 ± 1.52 | >40 | >40 | - |
| 5i | >40 | 16.92 ± 0.12 | 6.96 ± 1.02 | >40 | >40 | - |
| 5j | 15.34 ± 4.87 | 10.01 ± 1.21 | 4.68 ± 0.66 | 11.16 ± 3.75 | 24.23 ± 1.07 | - |
| 5k | >40 | 13.45 ± 0.93 | 2.04 ± 0.71 | 9.26 ± 1.00 | >40 | - |
| 5l | >40 | >40 | >40 | 21.87 ± 2.16 | >40 | - |
| 5m | >40 | 10.67 ± 0.40 | 3.56 ± 1.03 | 2.35 ± 0.65 | 2.84 ± 1.88 | - |
| 7a | >40 | 13.95 ± 2.79 | 3.14 ± 1.33 | 3.12 ± 1.31 | >40 | - |
| 7b | >40 | >40 | >40 | >40 | >40 | - |
| 7c | >40 | 1.29 ± 0.32 | 1.94 ± 0.74 | 1.56 ± 1.00 | 3.53 ± 0.91 | <10 |
| 7d | >40 | 32.09 ± 1.23 | >40 | >40 | >40 | - |
| 7e | >40 | >40 | >40 | >40 | >40 | - |
| aloe-emodin | 16.38 ± 2.82 | 10.78 ± 3.54 | 3.61 ± 0.81 | 10.8 ± 0.41 | 16.33 ± 0.67 | - |
| etoposide | 3.18 ± 1.36 | 8.30 ± 1.09 | 5.77 ± 0.99 | 5.45 ± 1.14 | 3.40 ± 0.42 | - |
a IC50 values are shown as mean ± SD from the average of three replicates; b IC50: concentration that causes a 50% reduction in cell growth; “-”: not detected.
Compared to the parent compound aloe-emodin, some compounds displayed promising antiproliferative activity against one or more cell lines. Among them, compound 5d exhibited the most potent antiproliferative activity against all the tested cell lines. It showed 3–9-fold increased activity compared to the positive control, etoposide. In addition, 5f also exhibited strong potency against all five cell lines, which was comparable or superior to that of etoposide. It is worth noting that five synthesized compounds (5a, 5d, 5f, 5g and 5j) exhibited moderate to good activities against all the tested cell lines, which indicated that these derivatives possessed a broad spectrum of antitumor activity. The results of the cytotoxic activities of 5a, 5d, 5f, 5g and 7c against the Hk-2 cell line showed that the synthetic compounds also displayed similar cytotoxic activity compared with the tested tumor cell lines, which did not show obvious selectivity.
The preliminary structure–activity relationship (SAR) study indicated that the linking position of coumarin moiety had an effect on their antiproliferative activity. The derivatives linked at the 3-, 4- and 7-position of coumarin were more active than others linked at the 6-position. In the same series of derivatives (containing the same linking position of coumarin), both the position and electron donor–acceptor properties of the substituents on coumarin significantly affected the activity. For the derivatives linked at the 4-position of coumarin, the chloro group in the 5-position of coumarin (5d) showed better activity than others, and the derivatives bearing a methyl (5f) or methoxy (5g) group in the 6-position of coumarin showed slightly weaker activity than 5d. It was noted that the replacement of the chloro with the fluoro group (5e) in the 5-position of coumarin resulted in the loss of activity. Regarding the derivatives linked at the 6-position of coumarin, the substituent at the 3-position of coumarin (5k) was better than that at the 4-position (5l). Moreover, to investigate the influence of the linker length to activity, 5m with an extended linker length was then synthesized, and the activity of it showed equal to or slightly less than that of 5a against four tumor cell lines (SGC-7901, HepG2, MCF-7 and HCT-8). The results indicated that extending the linker length had no significant effect on activity.
The effect of the substituent at the 1- and 8-position on an anthraquinone core was also investigated. The results suggested that the substituent size and steric properties had a remarkable effect on their antiproliferative activity. 1, 8-diacetyl-substituted derivative 7c displayed similar or slightly decreased potency to 1, 8-unsubstituted derivative 5d against four tumor cell lines (SGC-7901, HepG2, MCF-7 and HCT-8). However, after replacement of the acetyl group with the bulky benzoyl and benzenesulfonyl moieties, compounds 7d and 7e exhibited weak or no activity. A similar phenomenon could also be found by the replacement of the methyl (7a) group with the benzyl (7b). The results suggested that the introduction of the bulky substituents at the 1- and 8-position on anthraquinone was detrimental for activity.
3. Materials and Methods
3.1. General Information
NMR spectra were recorded on a Bruker AVANCE III 600 or a Bruker AVANCE III 500 NMR spectrometer instrument (Bruker, Rheinstetten, Germany). Solvent signals (DMSO-d6: δH = 2.50 ppm/δC = 39.52 ppm) were used as references. High-resolution mass spectra (HRMS) were recorded on a Waters SYNAPT G2 HDMS (Waters, Milford, MA, USA). The reactions were monitored by thin-layer chromatography on plates (GF254) supplied by Yantai Chemicals (Yantai, China). Silica gel column chromatography was performed using 200–300 mesh silica gel supplied by Tsingtao Haiyang Chemicals (Tsingtao, China). Unless otherwise noted, all common reagents and solvents were obtained from commercial suppliers (Beijing InnoChem Science & Technology Co., Ltd., Beijing, China) without further purification.
3.2. Chemistry
3.2.1. General Procedures for O-Propargyl and O-Butynyl Coumarin Derivatives (2a–m)
3-bromoprop-1-yne or 4-bromobut-1-yne (2.0 mmol) and anhydrous K2CO3 (1.5 mmol) were added to a solution of the corresponding hydroxycoumarin (1.0 mmol) in DMF (5 mL). The reaction was heated to 60 °C for 2 h. Upon completion, the reaction was cooled down to room temperature, and then the reaction mixture was diluted with water and extracted three times with ethyl acetate. The organic layers were dried with dry Na2SO4, they were filtered, and the filtrate was removed under reduced pressure. The residue was purified by column chromatography to give compounds 2a–m (Yield: 40~96%).
3.2.2. General Procedure for the Synthesis of 3-(Bromomethyl)-1,8-Dihydroxyanthracene-9,10-Dione (3)
CBr4 (15.34 g, 46.25 mmol) was added portion-wise to a solution of PPh3 (12.13 g, 46.25 mmol) in THF (50 mL) at room temperature. The mixture was left stirring for 10 min, and then aloe-emodin (5.0 g, 18.5 mmol) was added. After being stirred at room temperature for 6 h, the reaction mixture was filtered, and the filtrate was evaporated to give a residue that was subjected to column chromatography (petroleum ether:CH2Cl2 = 1:1) to obtain compound 3 (5.83 g, 94%). 1H-NMR (600 MHz, DMSO-d6) δ: 11.92 (s, 1H, OH-8), 11.91 (s, 1H, OH-1), 7.82 (dd, J = 8.3 Hz, 7.5 Hz, 1H, H-6), 7.78 (d, J = 1.7 Hz, 1H, H-4), 7.72 (dd, J = 7.5 Hz, 1.1 Hz, 1H, H-5), 7.47 (d, J = 1.7 Hz, 1H, H-2), 7.40 (dd, J = 8.3 Hz, 1.1 Hz, 1H, H-7), 4.82 (s, 2H, H-11).
3.2.3. General Procedure for the Synthesis of 3-(Azidomethyl)-1,8-Dihydroxyanthracene-9,10-Dione (4)
NaN3 (117 mg, 1.8 mmol) was added to a solution of compound 3 (500 mg, 1.5 mmol) in dry CH3CN (10 mL) at room temperature. The reaction mixture was heated to 60 °C for 3 h. Upon completion, the reaction was cooled down to room temperature, and then the reaction mixture was diluted with water and extracted three times with CH2Cl2. The organic layers were dried with dry Na2SO4, they were filtered, and the filtrate was removed under reduced pressure. The residue was purified by column chromatography (petroleum ether:CH2Cl2 = 1:1) to give compound 4 (375 mg, 85%). 1H-NMR (600 MHz, DMSO-d6) δ: 11.92 (s, 1H, OH-8), 11.91 (s, 1H, OH-1), 7.81 (dd, J = 8.4 Hz, 7.4 Hz, 1H, H-6), 7.71 (dd, J = 7.4 Hz, 1.1 Hz, 1H, H-5), 7.68 (d, J = 1.6 Hz, 1H, H-4), 7.39 (dd, J = 8.4 Hz, 1.1 Hz, 1H, H-7), 7.36 (d, J =1.6 Hz, 1H, H-2), 4.68 (s, 2H, H-11).
3.2.4. General Procedures for the Synthesis of Aloe-Emodin–Coumarin Hybrids (5)
Compound 2 (0.24 mmol) and copper(I) thiophene-2-carboxylate (0.08 mmol) were added to a solution of compound 4 (0.2 mmol) in CH2Cl2 (10 mL). After being stirred at room temperature for 6 h, the reaction mixture was concentrated, and the residue was purified by silica gel column chromatography to give compound 5.
1,8-dihydroxy-3-((4-(((2-oxo-2H-chromen-3-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)methyl)anthracene-9,10-dione (5a) yellow solid. Yield: 57%; mp: 252.3–254.1 °C; 1H-NMR (500 MHz, DMSO-d6) δ 11.91–11.89 (m, 2H, OH-1, OH-8), 8.49 (s, 1H, H-12), 7.81 (dd, J = 8.4 Hz, 7.4 Hz, 1H, H-6), 7.71 (dd, J = 7.4 Hz, 0.9 Hz, 1H, H-5), 7.62–7.59 (m, 2H, H-4, H-5′), 7.57 (s, 1H, H-4′), 7.44 (ddd, J = 8.0 Hz, 7.4 Hz, 1.5 Hz, 1H, H-7′), 7.39 (dd, J = 8.4 Hz, 0.9 Hz, 1H, H-7), 7.36 (d, J = 8.0 Hz, 1H, H-8′), 7.32 (ddd, J = 7.6 Hz, 7.4 Hz, 0.9 Hz, 1H, H-6′), 7.28 (d, J = 1.4 Hz, 1H, H-2), 5.83 (s, 2H, H-11), 5.26 (s, 2H, H-14); 13C-NMR (125 MHz, DMSO-d6) δ 191.5, 181.2, 161.4, 161.3, 156.5, 149.2, 145.6, 142.5, 142.0, 137.5, 133.9, 133.3, 128.6, 127.0, 125.8, 124.8, 124.5, 122.9, 119.6, 119.4, 118.3, 116.0, 115.8, 115.7, 115.1, 62.1, 52.1; HRMS m/z calcd for C27H18N3O7 [M + H]+: 496.1145; found: 496.1147.
1,8-dihydroxy-3-((4-(((2-oxo-2H-chromen-4-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)methyl)anthracene-9,10-dione (5b) yellow solid. Yield: 46%; mp: 272.5–273.8 °C; 1H-NMR (500 MHz, DMSO-d6) δ 11.91 (s, 1H, OH-8), 11.90 (s, 1H, OH-1), 8.58 (s, 1H, H-12), 7.82 (dd, J = 8.4 Hz, 7.5 Hz, 1H, H-6), 7.76 (dd, J = 7.9 Hz, 1.5 Hz, 1H, H-5′), 7.72 (dd, J = 7.5 Hz, 0.9 Hz, 1H, H-5), 7.66 (ddd, J = 8.1 Hz, 7.4 Hz, 1.5 Hz, 1H, H-7′), 7.59 (d, J = 1.5 Hz, 1H, H-4), 7.41 (d, J = 8.1 Hz, 1H, H-8′), 7.40 (dd, J = 8.4 Hz, 0.9 Hz, 1H, H-7), 7.34 (ddd, J = 7.9 Hz, 7.4 Hz, 0.8 Hz, 1H, H-6′), 7.29 (d, J = 1.5 Hz, 1H, H-2), 6.16 (s, 1H, H-3′), 5.85 (s, 2H, H-11), 5.46 (s, 2H, H-14); 13C-NMR (150 MHz, DMSO-d6) δ 191.6, 181.3, 164.4, 161.6, 161.4, 161.4, 152.8, 145.6, 141.6, 137.5, 133.9, 133.3, 132.9, 126.1, 124.6, 124.3, 122.9, 119.5, 118.2, 116.5, 116.1, 115.8, 115.1, 91.4, 62.8, 52.1; HRMS m/z calcd for C27H18N3O7 [M + H]+: 496.1145; found: 496.1148.
1,8-dihydroxy-3-((4-(((6-methyl-2-oxo-2H-chromen-4-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)methyl)anthracene-9,10-dione (5c) yellow solid. Yield: 49%; mp: 274.9–276.9 °C; 1H-NMR (500 MHz, DMSO-d6) δ 11.91 (s, 1H, OH-8), 11.90 (s, 1H, OH-1), 8.57 (s, 1H, H-12), 7.82 (dd, J = 8.4 Hz, 7.5 Hz, 1H, H-6), 7.71 (dd, J = 7.5 Hz, 0.8 Hz, 1H, H-5), 7.58 (d, J = 1.5 Hz, 1H, H-4), 7.51 (d, J = 2.0 Hz, 1H, H-5′), 7.46 (dd, J = 8.5 Hz, 2.0 Hz, 1H, H-7′), 7.40 (dd, J = 8.4 Hz, 0.8 Hz, 1H, H-7), 7.32–7.27 (m, 2H, H-2, H-8′), 6.13 (s, 1H, H-3′), 5.85 (s, 2H, H-11), 5.45 (s, 2H, H-14), 2.34 (s, 3H, CH3-6′); 13C-NMR (125 MHz, DMSO-d6) δ 191.5, 181.3, 164.4, 161.7, 161.4, 161.4, 151.0, 145.6, 141.5, 137.5, 133.9, 133.7, 133.6, 133.3, 126.1, 124.6, 122.9, 122.3, 119.4, 118.1, 116.3, 116.0, 115.8, 114.8, 91.3, 62.7, 52.1, 20.3; HRMS m/z calcd for C28H20N3O7 [M + H]+: 510.1301; found: 510.1299.
3-((4-(((6-chloro-2-oxo-2H-chromen-4-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)methyl)-1,8-dihydroxyanthracene-9,10-dione (5d) yellow solid. Yield: 82%; mp: 286.3–288.1 °C; 1H-NMR (500 MHz, DMSO-d6) δ 11.92–11.89 (m, 2H, OH-1, OH-8), 8.59 (s, 1H, H-12), 7.82 (dd, J = 8.4 Hz, 7.5 Hz, 1H, H-6), 7.73–7.67 (m, 3H, H-5, H-5′, H-7′), 7.58 (d, J = 1.5 Hz, 1H, H-4), 7.47 (d, J = 8.7 Hz, 1H, H-8′), 7.40 (dd, J = 8.4 Hz, 1.0 Hz, 1H, H-7), 7.29 (d, J = 1.5 Hz, 1H, H-2), 6.24 (s, 1H, H-3′), 5.85 (s, 2H, H-11), 5.47 (s, 2H, H-14); 13C-NMR (150 MHz, DMSO-d6) δ 191.6, 181.2, 163.2, 161.4, 161.4, 161.1, 151.5, 145.6, 141.4, 137.5, 133.9, 133.3, 132.6, 128.3, 126.1, 124.6, 122.9, 122.0, 119.5, 118.8, 118.1, 116.6, 116.0, 115.8, 92.3, 63.1, 52.2; HRMS m/z calcd for C27H17ClN3O7 [M + H]+: 530.0755; found: 530.0759.
3-((4-(((6-fluoro-2-oxo-2H-chromen-4-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)methyl)-1,8-dihydroxyanthracene-9,10-dione (5e) yellow solid. Yield: 58%; mp: 245.9–247.8 °C; 1H-NMR (500 MHz, DMSO-d6) δ 11.94–11.80 (m, 2H, OH-1, OH-8), 8.60 (s, 1H, H-12), 7.79 (dd, J = 8.4 Hz, 7.4 Hz, 1H, H-6), 7.68 (dd, J = 7.4 Hz, 0.5 Hz, 1H, H-5), 7.55 (d, J = 1.3 Hz, 1H, H-4), 7.51 (dd, J = 8.3 Hz, 2.8 Hz, 1H, H-5′), 7.48–7.44 (m, 2H, H-7′, H-8′), 7.37 (dd, J = 8.4 Hz, 0.5 Hz, 1H, H-7), 7.27 (d, J = 1.3 Hz, 1H, H-2), 6.23 (s, 1H, H-3′), 5.84 (s, 2H, H-11), 5.47 (s, 2H, H-14); 13C-NMR (150 MHz, DMSO-d6) δ 191.5, 181.2, 163.5, 163.5, 161.4, 161.4 (d, J = 2.3 Hz), 158.1 (d, J = 241.3 Hz), 149.2, 145.6, 141.5, 137.5, 133.8, 133.3, 126.1, 124.6, 122.9, 120.3 (d, J = 24.6 Hz), 119.4, 118.8 (d, J = 8.5 Hz), 118.1, 116.2 (d, J = 9.3 Hz), 116.0, 115.8, 108.5 (d, J = 25.5 Hz), 92.2, 63.1, 52.2; HRMS m/z calcd for C27H17FN3O7 [M + H]+: 514.1051; found: 514.1053.
1,8-dihydroxy-3-((4-(((7-methyl-2-oxo-2H-chromen-4-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)methyl)anthracene-9,10-dione (5f) yellow solid. Yield: 85%; mp: 283.3–284.8 °C; 1H-NMR (600 MHz, DMSO-d6) δ 11.94–11.90 (m, 2H, OH-1, OH-8), 8.58 (s, 1H, H-12), 7.82 (dd, J = 8.4 Hz, 7.5 Hz, 1H, H-6), 7.72 (dd, J = 7.5 Hz, 0.8 Hz, 1H, H-5), 7.63 (d, J = 8.1 Hz, 1H, H-5′), 7.60 (d, J = 1.2 Hz, 1H, H-4), 7.40 (dd, J = 8.4 Hz, 0.8 Hz, 1H, H-7), 7.30 (d, J = 1.2 Hz, 1H, H-2), 7.24 (d, J = 0.7 Hz, 1H, H-8′), 7.16 (dd, J = 8.1 Hz, 0.7 Hz, 1H, H-6′), 6.10 (s, 1H, H-3′), 5.85 (s, 2H, H-11), 5.44 (s, 2H, H-14), 2.40 (s, 3H, CH3-7′); 13C-NMR (150 MHz, DMSO-d6) 191.6, 181.3,164.6 161.8, 161.4, 161.4, 152.9, 145.6, 143.7, 141.6, 137.5, 133.9, 133.3, 126.1, 125.3, 124.6, 122.9, 122.7, 119.5, 118.2, 116.5, 116.1, 115.8, 112.6, 90.5, 62.7, 52.1, 21.2; HRMS m/z calcd for C28H20N3O7 [M + H]+: 510.1301; found: 510.1301.
1,8-dihydroxy-3-((4-(((7-methoxy-2-oxo-2H-chromen-4-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)methyl)anthracene-9,10-dione (5g) yellow solid. Yield: 48%; mp: 281.1–283.3 °C; 1H-NMR (500 MHz, DMSO-d6) δ 11.92 (s, 1H, OH-8), 11.91 (s, 1H, OH-1), 8.56 (s, 1H, H-12), 7.82 (dd, J = 8.4 Hz, 7.5 Hz, 1H, H-6), 7.72 (dd, J = 7.5 Hz, 1.0 Hz, 1H, H-5), 7.64 (d, J = 8.9 Hz, 1H, H-5′), 7.60 (d, J = 1.5 Hz, 1H, H-4), 7.40 (dd, J = 8.4 Hz, 1.0 Hz, 1H, H-7), 7.29 (d, J = 1.5 Hz, 1H, H-2), 6.99 (d, J = 2.4 Hz, 1H, H-8′), 6.91 (dd, J = 8.9 Hz, 2.4 Hz, 1H, H-6′), 6.00 (s, 1H, H-3′), 5.84 (s, 2H, H-11), 5.43 (s, 2H, H-14), 3.84 (s, 3H, OCH3-7′); 13C-NMR (150 MHz, DMSO-d6) δ 191.6, 181.3, 164.8, 163.0, 162.0, 161.4, 161.3, 154.7, 145.6, 141.6, 137.5, 133.9, 133.3, 126.0, 124.6, 124.0, 122.9, 119.4, 118.2, 116.0, 115.8, 112.3, 108.2, 100.6, 88.7, 62.6, 56.0, 52.1; HRMS m/z calcd for C28H20N3O8 [M + H]+: 526.1250; found: 526.1248.
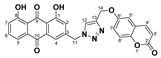
1,8-dihydroxy-3-((4-(((2-oxo-2H-chromen-6-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)methyl)anthracene-9,10-dione (5h) yellow solid. Yield: 80%; mp: 248.3–250.2 °C; 1H-NMR (500 MHz, DMSO-d6) δ 11.92–11.89 (m, 2H, OH-1, OH-8), 8.43 (s, 1H, H-12), 7.99 (d, J = 9.6 Hz, 1H, H-4′), 7.82 (dd, J = 8.4 Hz, 7.5 Hz, 1H, H-6), 7.71 (dd, J = 7.5 Hz, 1.0 Hz, 1H, H-5), 7.58 (d, J = 1.5 Hz, 1H, H-4), 7.42 (d, J = 3.0 Hz, 1H, H-5′), 7.40 (dd, J = 8.4 Hz, 1.0 Hz, 1H, H-7), 7.35 (d, J = 9.1 Hz, 1H, H-8′), 7.28 (dd, J = 9.1 Hz, 3.0 Hz, 1H, H-7′), 7.24 (d, J = 1.5 Hz, 1H, H-2), 6.49 (d, J = 9.6 Hz, 1H, H-3′), 5.81 (s, 2H, H-11), 5.24 (s, 2H, H-14); 13C-NMR (125 MHz, DMSO-d6) δ 191.5, 181.2, 161.4, 161.3, 160.1, 154.2, 148.1, 145.6, 144.0, 142.9, 137.5, 133.8, 133.3, 125.4, 124.5, 122.8, 120.1, 119.4, 119.2, 118.2, 117.4, 116.7, 116.0, 115.7, 112.1, 61.7, 52.0; HRMS m/z calcd for C27H18N3O7 [M + H]+: 496.1145; found: 496.1141.
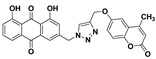
1,8-dihydroxy-3-((4-(((4-methyl-2-oxo-2H-chromen-6-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)methyl)anthracene-9,10-dione (5i) yellow solid. Yield: 79%; mp: 249.7–251.5 °C; 1H-NMR (600 MHz, DMSO-d6) δ 11.91–11.89 (m, 2H, OH-1, OH-8), 8.44 (s, 1H, H-12), 7.81 (dd, J = 8.4 Hz, 7.5 Hz, 1H, H-6), 7.70 (dd, J = 7.5 Hz, 1.0 Hz, 1H, H-5), 7.56 (d, J = 1.6 Hz, 1H, H-4), 7.39 (dd, J = 8.4 Hz, 1.0 Hz, 1H, H-7), 7.36 (d, J = 2.8 Hz, 1H, H-5′), 7.34 (d, J = 9.0 Hz, 1H, H-8′), 7.30 (dd, J = 9.0 Hz, 2.8 Hz, 1H, H-7′), 7.24 (d, J = 1.6 Hz, 1H, H-2), 6.38 (d, J = 1.2 Hz, 1H, H-3′), 5.81 (s, 2H, H-11), 5.29 (s, 2H, H-14), 2.41 (d, J = 1.2 Hz, 3H, CH3-4′); 13C-NMR (150 MHz, DMSO-d6) 191.6, 181.3, 161.4, 161.4, 159.9, 154.2, 153.1, 147.5, 145.7, 143.0, 137.5, 133.8, 133.3, 125.5, 124.6, 122.8, 120.2, 119.8, 119.5, 118.2, 117.6, 116.0, 115.8, 114.8, 109.6, 61.7, 52.1, 18.3; HRMS m/z calcd for C28H20N3O7 [M + H]+: 510.1301; found: 510.1305.
1,8-dihydroxy-3-((4-(((2-oxo-2H-chromen-7-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)methyl)anthracene-9,10-dione (5j) yellow solid. Yield: 94%; mp: 265.2–266.9 °C; 1H-NMR (500 MHz, DMSO-d6) δ 11.91–11.88 (m, 2H, OH-1, OH-8), 8.46 (s, 1H, H-12), 7.98 (d, J = 9.5 Hz, 1H, H-4′), 7.81 (dd, J = 8.4 Hz, 7.5 Hz, 1H, H-6), 7.70 (dd, J = 7.5 Hz, 0.9 Hz, 1H, H-5), 7.63 (d, J = 8.7 Hz, 1H, H-5′), 7.58 (d, J = 1.4 Hz, 1H, H-4), 7.39 (dd, J = 8.4 Hz, 0.9 Hz, 1H, H-7), 7.25 (d, J = 1.4 Hz, 1H, H-2), 7.15 (d, J = 2.4 Hz, 1H, H-8′), 7.02 (dd, J = 8.7 Hz, 2.4 Hz, 1H, H-6′), 6.29 (d, J = 9.5 Hz, 1H, H-3′), 5.81 (s, 2H, H-11), 5.31 (s, 2H, H-14); 13C-NMR (125 MHz, DMSO-d6) δ 191.5, 181.2, 161.4, 161.3, 161.0, 160.2, 155.3, 145.6, 144.3, 142.5, 137.5, 133.8, 133.2, 129.5, 125.6, 124.5, 122.8, 119.4, 118.2, 116.0, 115.7, 112.9, 112.7, 112.6, 101.6, 61.6, 52.1; HRMS m/z calcd for C27H18N3O7 [M + H]+: 496.1145; found: 496.1146.
1,8-dihydroxy-3-((4-(((4-methyl-2-oxo-2H-chromen-7-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)methyl)anthracene-9,10-dione (5k) yellow solid. Yield: 88%; mp: 233.7–235.6 °C; 1H-NMR (600 MHz, DMSO-d6) δ 11.92–11.89 (m, 2H, OH-1, OH-8), 8.47 (s, 1H, H-12), 7.81 (dd, J = 8.4 Hz, 7.5 Hz, 1H, H-6), 7.70 (dd, J =7.5 Hz, 0.9 Hz, 1H, H-5), 7.68 (d, J = 8.8 Hz, 1H, H-5′), 7.58 (d, J = 1.3 Hz, 1H, H-4), 7.39 (dd, J = 8.4 Hz, 0.9 Hz, 1H, H-7), 7.26 (d, J = 1.3 Hz, 1H, H-2), 7.15 (d, J = 2.5 Hz, 1H, H-8′), 7.03 (dd, J = 8.8 Hz, 2.5 Hz, 1H, H-6′), 6.21 (d, J = 1.0 Hz, 1H, H-3′), 5.81 (s, 2H, H-11), 5.31 (s, 2H, H-14), 2.39 (d, J = 1.0 Hz, 3H, CH3-4′); 13C-NMR (150 MHz, DMSO-d6) δ 191.6, 181.3, 161.4, 161.4, 161.0, 160.2, 154.7, 153.5, 145.6, 142.5, 137.5, 133.9, 133.3, 126.6, 125.7, 124.6, 122.9, 119.5, 118.2, 116.0, 115.8, 113.4, 112.7, 111.4, 101.6, 61.6, 52.1, 18.2; HRMS m/z calcd for C28H20N3O7 [M + H]+: 510.1301; found: 510.1303.
1,8-dihydroxy-3-((4-(((3-cyano-2-oxo-2H-chromen-7-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)methyl)anthracene-9,10-dione (5l) yellow solid. Yield: 54%; mp: 258.4–260.2 °C; 1H-NMR (600 MHz, DMSO-d6) δ 11.91–11.88 (m, 2H, OH-1, OH-8), 8.85 (s, 1H, H-4′), 8.49 (s, 1H, H-12), 7.80 (dd, J = 8.4 Hz, 7.4 Hz, 1H, H-6), 7.73 (d, J = 8.8 Hz, 1H, H-5′), 7.69 (d, J = 7.4 Hz, 1H, H-5), 7.55 (d, J = 1.3 Hz, 1H, H-4), 7.39 (d, J = 8.4 Hz, 1H, H-7), 7.29 (d, J = 2.3 Hz, 1H, H-8′), 7.25 (d, J = 1.3 Hz, 1H, H-2), 7.13 (dd, J = 8.8 Hz, 2.3 Hz, 1H, H-6′), 5.82 (s, 2H, H-11), 5.38 (s, 2H, H-14); 13C-NMR (150 MHz, DMSO-d6) δ 191.5, 181.2, 163.9, 161.4, 161.4, 157.4, 156.5, 153.2, 145.6, 142.1, 137.5, 133.8, 133.2, 131.4, 125.9, 124.6, 122.9, 119.4, 118.1, 116.0, 115.7, 115.1, 114.5, 111.6, 101.9, 97.8, 62.0, 52.1; HRMS m/z calcd for C28H17N4O7 [M + H]+: 521.1097; found: 521.1090.
1,8-dihydroxy-3-((4-(2-((2-oxo-2H-chromen-4-yl)oxy)ethyl)-1H-1,2,3-triazol-1-yl)methyl)anthracene-9,10-dione (5m) yellow solid. Yield: 82%; mp: 250.4–251.8 °C; 1H-NMR (600 MHz, DMSO-d6) δ 11.90 (s, 1H, OH-8), 11.88 (s, 1H, OH-1), 8.22 (s, 1H, H-12), 7.81 (dd, J = 8.4 Hz, 7.7 Hz, 1H, H-6), 7.69–7.66 (m, 2H, H-5, H-5′), 7.56 (ddd, J = 8.4 Hz, 7.4 Hz, 1.6 Hz, 1H, H-7′), 7.48 (d, J = 1.6 Hz, 1H, H-4), 7.39 (dd, J = 8.4 Hz, 0.9 Hz, 1H, H-7), 7.31 (dd, J = 8.4 Hz, 0.9 Hz, 1H, H-8′), 7.25 (ddd, J = 7.8 Hz, 7.4 Hz, 0.9 Hz, 1H, H-6′), 7.21 (d, J = 1.6 Hz, 1H, H-2), 5.94 (s, 1H, H-3′), 5.76 (s, 2H, H-11), 4.47 (t, J = 6.2 Hz, 2H, H-15), 3.25 (t, J = 6.2 Hz, 2H, H-14); 13C-NMR (150 MHz, DMSO-d6) 191.5, 181.2, 164.8, 161.7, 161.4, 161.3, 152.7, 146.0, 143.8, 137.5, 133.7, 133.2, 132.6, 124.6, 124.1, 123.8, 122.8, 122.7, 119.4, 118.0, 116.4, 116.0, 115.6, 115.2, 90.8, 68.4, 51.9, 24.9; HRMS m/z calcd for C28H20N3O7 [M + H]+: 510.1301; found: 510.1298.
3.2.5. Procedure for the Synthesis of Compound 6a and 6b
Iodomethane (2.0 mmol) or benzyl bromide (1.0 mmol) and anhydrous K2CO3 (2.0 mmol) were added to a solution of 4 (0.2 mmol) in DMF (3 mL). The reaction was heated to 60 °C for 4 h. Upon completion, the reaction was cooled down to room temperature, and then the reaction mixture was diluted with water and extracted three times with CH2Cl2. The organic layers were dried with dry Na2SO4, they were filtered, and the filtrate was removed under reduced pressure. The residue was purified by column chromatography to give compound 6a or 6b.
3-(azidomethyl)-1,8-dimethoxyanthracene-9,10-dione (6a) yellow solid. Yield: 87%; mp: 133.6–135.0 °C; 1H-NMR (600 MHz, CDCl3) δ 7.83 (dd, J = 7.7 Hz, 0.8 Hz, 1H, H-5), 7.75 (d, J = 1.3 Hz, 1H, H-4), 7.64 (dd, J = 8.1 Hz, 7.7 Hz, 1H, H-6), 7.31 (d, J = 8.1 Hz, 1H, H-7), 7.25 (d, J = 1.3 Hz, 1H, H-2), 4.50 (s, 2H, H-11), 4.03 (s, 3H, OCH3-8), 4.01 (s, 3H, OCH3-1); 13C-NMR (150 MHz, CDCl3) 183.9, 182.6, 160.2, 159.7, 142.0, 135.2, 134.7, 134.2, 124.0, 123.7, 119.1, 118.3, 118.0, 116.9, 56.8, 56.7, 54.3.
3-(azidomethyl)-1,8-bis(benzyloxy)anthracene-9,10-dione (6b) yellow solid. Yield: 67%; mp: 133.9–135.5 °C; 1H-NMR (600 MHz, CDCl3) δ 7.85 (dd, J = 7.7 Hz, 0.7 Hz, 1H, H-5), 7.77 (d, J = 0.9 Hz, 1H, H-4), 7.66–7.62 (m, 4H, CH2C6H5-1, CH2C6H5-8), 7.60 (dd, J = 8.1 Hz, 7.7 Hz, 1H, H-6), 7.42–7.38 (m, 4H, CH2C6H5-1, CH2C6H5-8), 7.36–7.33 (m, 3H, H-7, CH2C6H5-1, CH2C6H5-8), 7.30 (d, J = 0.9 Hz, 1H, H-2), 5.34 (s, 2H, CH2C6H5-8), 5.33 (s, 2H, CH2C6H5-1), 4.46 (s, 2H, H-11); 13C-NMR (150 MHz, CDCl3) 183.8, 181.9, 158.9, 158.4, 141.9, 136.6, 136.4, 135.3, 134.9, 134.0, 128.8, 128.7, 128.0, 127.9, 126.9, 126.9, 124.8, 124.5, 120.4, 119.6, 118.9, 118.5, 71.3, 71.2, 54.2.
3.2.6. Procedure for the Synthesis of Compound 6c and 6d
Acetyl chloride (0.6 mmol) or benzoyl chloride (0.6 mmol), 4-dimethylaminopyridine (0.04 mmol) and N,N-diisopropylethylamine (0.6 mmol) were added to a solution of 4 (0.2 mmol) in CH2Cl2 (3 mL). After being stirred at room temperature for 4 h, the reaction mixture was diluted with water and extracted three times with CH2Cl2. The organic layers were dried with anhydrous Na2SO4, and the solvent was removed under reduced pressure. The residue was purified by silica gel column chromatography to give compound 6c or 6d.
3-(azidomethyl)-9,10-dioxo-9,10-dihydroanthracene-1,8-diyl diacetate (6c) yellow solid. Yield: 89%; mp: 161.2–162.8 °C; 1H-NMR (600 MHz, CDCl3) δ 8.23 (dd, J = 7.8 Hz, 1.3 Hz, 1H, H-5), 8.15 (d, J = 1.8 Hz, 1H, H-4), 7.78 (dd, J = 8.0 Hz, 7.8 Hz, 1H, H-6), 7.42 (dd, J = 8.0 Hz, 1.3 Hz, 1H, H-7), 7.39 (d, J = 1.8 Hz, 1H, H-2), 4.56 (s, 2H, H-11), 2.46 (s, 3H, COCH3-8), 2.45 (s, 3H, COCH3-1); 13C-NMR (150 MHz, CDCl3) 181.8, 180.5, 169.6, 169.5, 150.7, 150.2, 143.4, 134.9, 134.8, 134.5, 130.6, 129.0, 125.7, 125.6, 125.1, 124.3, 53.6, 21.3, 21.2.
3-(azidomethyl)-9,10-dioxo-9,10-dihydroanthracene-1,8-diyl dibenzoate (6d) yellow solid. Yield: 83%; mp: 175.6–176.8 °C; 1H-NMR (600 MHz, CDCl3) δ 8.26 (dd, J = 7.8 Hz, 1.1 Hz, 1H, H-5), 8.18 (d, J = 1.7 Hz, 1H, H-4), 8.03–8.00 (m, 4H, COC6H5-1, COC6H5-8), 7.81 (dd, J = 8.2 Hz, 7.8 Hz, 1H, H-6), 7.55 (dd, J = 8.2 Hz, 1.1 Hz, 1H, H-7), 7.53–7.48 (m, 3H, H-2, COC6H5-1, COC6H5-8), 7.29–7.25 (m, 4H, COC6H5-1, COC6H5-8), 4.58 (s, 2H, H-11); 13C-NMR (150 MHz, CDCl3) 182.0, 180.6, 165.5, 165.4, 150.7, 150.3, 143.2, 134.8, 134.7, 134.5, 133.4, 133.3, 130.6, 130.3, 130.3, 129.4, 129.3, 129.1, 128.4, 128.3, 126.1, 125.6, 125.6, 124.2, 53.7.
3.2.7. Procedure for the Synthesis of Compound 6e
p-Toluenesulfonyl chloride (82 mg, 0.43 mmol) and anhydrous K2CO3 (70 mg, 0.51 mmol) were added to a solution of 4 (50 mg, 0.17 mmol) in acetone (5 mL). The reaction was heated to 60 °C for 5 h. Upon completion, the reaction was cooled down to room temperature, and then the reaction mixture was diluted with water and extracted three times with CH2Cl2. The organic layers were dried with dry Na2SO4, they were filtered, and the filtrate was removed under reduced pressure. The residue was purified by column chromatography (petroleum ether:ethyl acetate = 5:1) to give compound 6e.
3-(azidomethyl)-9,10-dioxo-9,10-dihydroanthracene-1,8-diyl bis(4-methylbenzenesulfonate) (6e) yellow solid. Yield: 83%; mp: 131.3–133.1 °C; 1H-NMR (600 MHz, CDCl3) δ 8.19 (dd, J = 7.7 Hz, 1.3 Hz, 1H, H-5), 8.12 (d, J = 1.7 Hz, 1H, H-4), 7.95 (d, J = 8.3 Hz, 2H, SO2C6H4-8), 7.92 (d, J = 8.3 Hz, 2H, SO2C6H4-1), 7.72 (dd, J = 8.1 Hz, 7.7 Hz, 1H, H-6), 7.65 (dd, J = 8.1 Hz, 1.3 Hz, 1H, H-7), 7.62 (d, J = 1.7 Hz, 1H, H-2), 7.36–7.31 (m, 4H, SO2C6H4-1, SO2C6H4-8), 4.52 (s, 2H, H-11), 2.43 (s, 3H, C6H4CH3-8), 2.43 (s, 3H, C6H4CH3-1); 13C NMR (150 MHz, CDCl3) 181.4, 178.6, 147.8, 147.4, 146.1, 145.9, 143.0, 134.9, 134.5, 134.4, 132.3, 132.2, 130.3, 130.1, 130.0, 129.2, 129.1, 128.9, 127.7, 127.2, 126.2, 124.9, 53.5, 21.9, 21.9.
3.2.8. General Procedures for the Synthesis of Aloe-Emodin–Coumarin Hybrids (7a–e)
Compound 2d (0.18 mmol) and copper(I) thiophene-2-carboxylate (0.03 mmol) were added to a solution of compound 6a (0.15 mmol) in CH2Cl2 (5 mL). After being stirred at room temperature for 6 h, the reaction mixture was concentrated, and the residue was purified by silica gel column chromatography to give compounds 7a–e.
3-((4-(((6-chloro-2-oxo-2H-chromen-4-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)methyl)-1,8-dimethoxyanthracene-9,10-dione (7a) yellow solid. Yield: 82%; mp: 251.8–252.6 °C; 1H-NMR (600 MHz, DMSO-d6) δ 8.59 (s, 1H, H-12), 7.73 (dd, J = 8.3 Hz, 7.5 Hz, 1H, H-6), 7.69 (dd, J = 8.8 Hz, 2.6 Hz, 1H, H-7′), 7.66–7.63 (m, 2H, H-5, H-5′), 7.57 (d, J = 0.7 Hz, 1H, H-4), 7.52 (d, J = 8.3 Hz, 1H, H-7), 7.49 (d, J = 0.7 Hz, 1H, H-2), 7.45 (d, J = 8.8 Hz, 1H, H-8′), 6.24 (s, 1H, H-3′), 5.83 (s, 2H, H-11), 5.46 (s, 2H, H-14), 3.91 (s, 3H, OCH3-8), 3.90 (s, 3H, OCH3-1); 13C-NMR (150 MHz, DMSO-d6) 183.0, 180.9, 163.2, 161.1, 159.0, 158.8, 151.5, 142.3, 141.3, 134.5, 134.4, 134.0, 132.6, 128.3, 126.0, 123.3, 123.1, 122.0, 119.1, 118.8, 118.2, 118.1, 117.0, 116.6, 92.3, 63.1, 56.5, 56.4, 52.5; HRMS m/z calcd for C29H21ClN3O7 [M + H]+: 558.1068; found: 558.1070.
1,8-bis(benzyloxy)-3-((4-(((6-chloro-2-oxo-2H-chromen-4-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)methyl)anthracene-9,10-dione (7b) yellow solid. Yield: 40%; mp: 258.3–259.8 °C; 1H-NMR (600 MHz, DMSO-d6) δ 8.57 (s, 1H, H-12), 7.76 (dd, J = 8.3 Hz, 7.5 Hz, 1H, H-6), 7.72–7.66 (m, 4H, H-5, H-5′, H-4, H-7′), 7.65–7.60 (m, 5H, H-7, CH2C6H5-1, CH2C6H5-8), 7.56 (d, J = 1.0 Hz, 1H, H-2), 7.46 (d, J = 8.7 Hz, 1H, H-8′), 7.43–7.38 (m, 4H, CH2C6H5-1, CH2C6H5-8), 7.37–7.33 (m, 2H, CH2C6H5-1, CH2C6H5-8), 6.26 (s, 1H, H-3′), 5.83 (s, 2H, H-11), 5.46 (s, 2H, H-14), 5.33 (s, 2H, CH2C6H5-8), 5.32 (s, 2H, CH2C6H5-1); 13C-NMR (150 MHz, DMSO-d6) 182.9, 180.9, 163.2, 161.1, 158.0, 157.7, 151.5, 142.2, 141.3, 136.9, 136.6, 134.6, 134.4, 134.2, 132.6, 128.4, 128.3, 127.8, 127.7, 127.0, 126.9, 126.0, 123.8, 123.6, 122.0, 120.6, 119.7, 118.7, 118.7, 117.5, 116.6, 92.3, 70.3, 70.1, 63.1, 52.4; HRMS m/z calcd for C41H29ClN3O7 [M + H]+: 710.1694; found: 710.1690.
3-((4-(((6-chloro-2-oxo-2H-chromen-4-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)methyl)-9,10-dioxo-9,10-dihydroanthracene-1,8-diyl diacetate (7c) yellow solid. Yield: 55%; mp: 245.6–247.2 °C; 1H-NMR (600 MHz, DMSO-d6) δ 8.61 (s, 1H, H-12), 8.11 (d, J = 7.6 Hz, 1H, H-5), 7.98 (s, 1H, H-4), 7.93 (dd, J = 7.9 Hz, 7.6 Hz, 1H, H-6), 7.71 (dd, J = 8.7 Hz, 2.2 Hz, 1H, H-7′), 7.69 (d, J = 2.2 Hz, 1H, H-5′), 7.63 (d, J =7.9 Hz, 1H, H-7), 7.53 (s, 1H, H-2), 7.48 (d, J = 8.7 Hz, 1H, H-8′), 6.25 (s, 1H, H-3′), 5.92 (s, 2H, H-11), 5.47 (s, 2H, H-14), 2.40–2.35 (m, 6H, COCH3-1, COCH3-8); 13C-NMR (150 MHz, DMSO-d6) 181.1, 180.3, 169.1, 169.1, 163.2, 161.1, 151.5, 149.9, 149.6, 143.6, 141.4, 135.4, 134.5, 134.1, 132.6, 130.7, 129.2, 128.3, 126.2, 125.2, 125.0, 124.8, 123.7, 122.0, 118.8, 116.6, 92.3, 63.1, 51.8, 20.9, 20.9; HRMS m/z calcd for C31H21ClN3O9 [M + H]+: 614.0966; found: 614.0961.
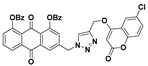
3-((4-(((6-chloro-2-oxo-2H-chromen-4-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)methyl)-9,10-dioxo-9,10-dihydroanthracene-1,8-diyl dibenzoate (7d) yellow solid. Yield: 83%; mp: 231.1–232.6 °C; 1H-NMR (600 MHz, DMSO-d6) δ 8.63 (s, 1H, H-12), 8.16 (dd, J = 7.8 Hz, 1.1 Hz, 1H, H-5), 8.04 (d, J = 1.6 Hz, 1H, H-4), 7.98 (dd, J = 8.0 Hz, 7.8 Hz, 1H, H-6), 7.94–7.91 (m, 4H, COC6H5-1, COC6H5-8), 7.78 (dd, J = 8.0 Hz, 1.1 Hz, 1H, H-7), 7.73 (d, J = 1.6 Hz, 1H, H-2), 7.70 (dd, J = 8.8 Hz, 2.6 Hz, 1H, H-7′), 7.68 (d, J = 2.6 Hz, 1H, H-5′), 7.65–7.61 (m, 2H, COC6H5-1, COC6H5-8), 7.46 (d, J = 8.8 Hz, 1H, H-8′), 7.43–7.37 (m, 4H, COC6H5-1, COC6H5-8), 6.25 (s, 1H, H-3′), 5.95 (s, 2H, H-11), 5.47 (s, 2H, H-14); 13C-NMR (150 MHz, DMSO-d6) 181.2, 180.6, 164.6, 164.5, 163.2, 161.1, 151.5, 149.5, 149.2, 143.4, 141.4, 135.3, 134.6, 134.2, 133.8, 133.7, 132.6, 130.7, 129.7, 129.7, 129.2, 128.8, 128.7, 128.7, 128.6, 128.3, 126.2, 125.8, 125.4, 125.0, 123.8, 122.0, 118.7, 116.6, 92.3, 63.1, 51.9; HRMS m/z calcd for C41H25ClN3O9 [M + H]+: 738.1279; found: 738.1281.
3-((4-(((6-chloro-2-oxo-2H-chromen-4-yl)oxy)methyl)-1H-1,2,3-triazol-1-yl)methyl)-9,10-dioxo-9,10-dihydroanthracene-1,8-diyl bis(4-methylbenzenesulfonate) (7e) yellow solid. Yield: 95%; mp: 195.6–197.1 °C; 1H-NMR (600 MHz, DMSO-d6) δ 8.56 (s, 1H, H-12), 8.10 (dd, J = 7.8 Hz, 1.1 Hz, 1H, H-5), 8.03 (d, J = 1.6 Hz, 1H, H-4), 7.89 (dd, J = 8.1 Hz, 7.8 Hz, 1H, H-6), 7.80 (d, J = 8.3 Hz, 2H, SO2C6H4-8), 7.75 (d, J = 8.3 Hz, 2H, SO2C6H4-1), 7.69–7.65 (m, 2H, H-5′, H-7′), 7.57 (dd, J = 8.1 Hz, 1.1 Hz, 1H, H-7), 7.49 (d, J = 1.6 Hz, 1H, H-2), 7.45 (d, J = 8.7 Hz, 1H, H-8′), 7.41 (d, J = 8.1 Hz, 2H, SO2C6H4-8), 7.39 (d, J = 8.1 Hz, 2H, SO2C6H4-1), 6.28 (s, 1H, H-3′), 5.92 (s, 2H, H-11), 5.49 (s, 2H, H-14), 2.37 (s, 3H, C6H4CH3-8), 2.36 (s, 3H, C6H4CH3-1); 13C-NMR (150 MHz, DMSO-d6) 180.5, 178.2, 163.2, 161.1, 151.5 146.7, 146.3, 146.1, 146.0, 143.3, 141.4, 135.1, 134.8, 134.5, 132.6, 131.6, 131.4, 130.2, 130.2, 129.5, 128.5, 128.4, 128.3, 127.7, 127.2, 126.5, 126.1, 125.8, 124.5, 122.0, 118.7, 116.6, 92.3, 63.0, 51.7, 21.2, 21.2; HRMS m/z calcd for C41H29ClN3O11S2 [M + H]+: 838.0932; found: 838.0931.
3.3. Evaluation of the Biological Activity
A549, HepG2, MCF-7 and HCT-8 were obtained from the Cell Resource Center, Peking Union Medical College (which is the headquarters of the National Infrastructure of Cell Line Resource, NSTI) (Beijing, China), SGC-7901 was purchased from Beyotime (Beijing, China), and Hk-2 was obtained from the American Type Culture Collection (ATCC) (Manassas, VA, USA). The antiproliferative activity of the synthetic compounds was evaluated by the MTT assay in vitro, with etoposide as a positive control. In brief, the cells were seeded into 96-well plates at 5 × 103 cells/well and were cultured overnight for adherence. The test compounds were prepared from 10 mM or 5 mM stock solution in DMSO according to preliminary screening results. Then, these stocks solution were serially diluted with DMEM medium with a final DMSO concentration no greater than 0.5%. The concentration was set to 10 μM, 5 μM, 2.5 μM, 1.25 μM, 0.625μM, 0.3125 μM and 0.15625 μM for potent compounds and 40 μM, 20 μM, 10 μM, 5 μM and 2.5 μM for less potent compounds. The cells were treated with different concentrations of test compounds for 48 h. After that, 20 μL MTT (5 mg/mL) was added to each well, and the plates were further incubated for 4 h. The medium was carefully removed, and the formazan crystals were dissolved in 150 μL DMSO. The absorbance at 490 nm was measured on a microplate reader (TECAN Infinite M1000, Austria). The cell viability was determined in terms of IC50 value.
4. Conclusions
In summary, a new series of aloe-emodin–coumarin hybrids were designed, synthesized and evaluated for their anticancer activity against five human cancer cell lines. Some of the synthesized compounds showed potent antiproliferative activity against one or more cell lines. Particularly, compound 5d exhibited a broad spectrum of antitumor activity against all tested cancer cell lines. Preliminary SAR analysis showed that the introduction of the chloro group in the 5-position of coumarin for the derivatives linked at the 4-position of coumarin was beneficial for the antitumor activity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27196153/s1, 1H-NMR and 13C-NMR spectra of the synthesized compounds.
Author Contributions
Conceptualization, H.S. and Z.Z.; methodology, H.S., Y.H., J.L., Y.T. and Q.W.; formal analysis, J.L. and L.L.; investigation, H.S., Y.T. and X.L.; writing—original draft preparation, H.S.; writing—review and editing, L.L. and Z.Z.; supervision, Z.Z.; project administration, H.S.; funding acquisition, H.S. and Z.Z. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
Funding Statement
This research was funded by CAMS Innovation Fund for Medical Sciences (CIFMS, 2021-I2M-1-028) and the Drug Innovation Major Project (2018ZX09711001).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Suboj P., Babykutty S., Gopi D.R.V., Nairc R.S., Srinivasc P., Gopala S. Aloe emodin inhibits colon cancer cell migration/angiogenesis by downregulating MMP-2/9, RhoB and VEGF via reduced DNA binding activity of NF-Κb. Eur. J. Pharm. Sci. 2012;45:581–591. doi: 10.1016/j.ejps.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 2.Chen S.H., Lin K.Y., Chang C.C., Fang C.L., Lin C.P. Aloe-emodin-induced apoptosis in human gastric carcinoma cells. Food Chem. Toxicol. 2007;45:2296–2303. doi: 10.1016/j.fct.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y.Y., Chiang S.Y., Lin J.G., Yang J.S., Ma Y.S., Liao V.L., Lai T.Y., Tang N.Y., Chung J.G. Emodin, aloe-emodin and rhein induced DNA damage and inhibited DNA repair gene expression in SCC-4 human tongue cancer cells. Anticancer Res. 2010;30:945–951. [PubMed] [Google Scholar]
- 4.Tabolacci C., Cordella M., Turcano L., Rossi S., Lentini A., Mariotti S., Nisini R., Sette G., Eramo A., Piredda L., et al. Aloe-emodin exerts a potent anticancer and immunomodulatory activity on BRAF-mutated human melanoma cells. Eur. J. Pharmacol. 2015;762:283–292. doi: 10.1016/j.ejphar.2015.05.057. [DOI] [PubMed] [Google Scholar]
- 5.Shen F.G., Ge C.P., Yuan P. Aloe-emodin induces autophagy and apoptotic cell death in non-small cell lung cancer cells via Akt/mTOR and MAPK signaling. Eur. J. Pharmacol. 2020;886:173550. doi: 10.1016/j.ejphar.2020.173550. [DOI] [PubMed] [Google Scholar]
- 6.Hu B., Zhang H., Meng X., Wang F., Wang P. Aloe-emodin from rhubarb (Rheum rhabarbarum) inhibits lipopolysaccharide-induced inflammatory responses in RAW 264.7 macrophages. J. Ethnopharmacol. 2014;153:846–853. doi: 10.1016/j.jep.2014.03.059. [DOI] [PubMed] [Google Scholar]
- 7.Park M.Y., Kwon H.J., Sung M.K. Evaluation of aloin and aloe-emodin as anti-inflammatory agents in aloe by using murine macrophages. Biosci. Biotechnol. Biochem. 2009;73:828–832. doi: 10.1271/bbb.80714. [DOI] [PubMed] [Google Scholar]
- 8.Li S.W., Yang T.C., Lai C.C., Huang S.H., Liao J.M., Lei W., Line Y.J., Lina C.W. Antiviral activity of aloe-emodin against influenza A virus via galectin-3 up-regulation. Eur. J. Pharmacol. 2014;738:125–132. doi: 10.1016/j.ejphar.2014.05.028. [DOI] [PubMed] [Google Scholar]
- 9.Agarwal S.K., Singh S.S., Verma S., Kumar S. Antifungal activity of anthraquinone derivatives from Rheum emodi. J. Ethnopharmacol. 2000;72:43–46. doi: 10.1016/S0378-8741(00)00195-1. [DOI] [PubMed] [Google Scholar]
- 10.Dou F., Liu Y., Liu L., Wang J., Sun T., Mu F., Guo Q., Guo C., Jia N., Liu W., et al. Aloe-emodin ameliorates renal fibrosis via inhibiting PI3K/Akt/mTOR signaling pathway in vivo and in vitro. Rejuvenation Res. 2019;22:218–229. doi: 10.1089/rej.2018.2104. [DOI] [PubMed] [Google Scholar]
- 11.Cheng C., Dong W. Aloe-emodin induces endoplasmic reticulum stress-dependent apoptosis in colorectal cancer cells. Med. Sci. Monit. 2018;24:6331–6339. doi: 10.12659/MSM.908400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin M.L., Lu Y.C., Chung J.G., Li Y.C., Wang S.G., Ng S.H., Wu C.Y., Su H.L., Chen S.S. Aloe-emodin induces apoptosis of human nasopharyngeal carcinoma cells via caspase-8-mediated activation of the mitochondrial death pathway. Cancer Lett. 2010;291:46–58. doi: 10.1016/j.canlet.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 13.Du Y.Q., Zhang J., Tao Z.H., Wang C.C., Yan S.Y., Zhang X.W., Huang M.Z. Aloe emodin exerts potent anticancer effects in MIAPaCa-2 and PANC-1 human pancreatic adenocarcinoma cell lines through activation of both apoptotic and autophagic pathways, sub-G1 cell cycle arrest and disruption of mitochondrial membrane potential (ΛΨm) J. Buon. 2019;24:746–753. [PubMed] [Google Scholar]
- 14.Quan Y., Gong L., He J., Zhou Y., Liu M., Cao Z., Li Y., Peng C. Aloe emodin induces hepatotoxicity by activating NF-κB inflammatory pathway and P53 apoptosis pathway in zebrafish. Toxicol. Lett. 2019;306:66–79. doi: 10.1016/j.toxlet.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Dong X., Fu J., Yin X., Qu C., Yang C., He H., Ni J. Induction of apoptosis in HepaRG cell line by aloe-emodin through generation of reactive oxygen species and the mitochondrial pathway. Cell. Physiol. Biochem. 2017;42:685–696. doi: 10.1159/000477886. [DOI] [PubMed] [Google Scholar]
- 16.Sandhu S., Bansal Y., Silakari O., Bansal G. Coumarin hybrids as novel therapeutic agents. Bioorg. Med. Chem. 2014;22:3806–3814. doi: 10.1016/j.bmc.2014.05.032. [DOI] [PubMed] [Google Scholar]
- 17.Salehian F., Nadri H., Jalili-Baleh L., Youseftabar-Miri L., Abbas Bukhari S.N., Foroumadi A., Tüylü Küçükkilinç T., Sharifzadeh M., Khoobi M. A review: Biologically active 3,4-heterocycle-fused coumarins. Eur. J. Med. Chem. 2021;212:113034. doi: 10.1016/j.ejmech.2020.113034. [DOI] [PubMed] [Google Scholar]
- 18.Fylaktakidou K.C., Hadjipavlou-Litina D.J., Litinas K.E., Nicolaides D.N. Natural and synthetic coumarin derivatives with anti-inflammatory/antioxidant activities. Curr. Pharm. Des. 2004;10:3813–3833. doi: 10.2174/1381612043382710. [DOI] [PubMed] [Google Scholar]
- 19.Qin H.L., Zhang Z.W., Ravindar L., Rakesh K.P. Antibacterial activities with the structure-activity relationship of coumarin derivatives. Eur. J. Med. Chem. 2020;207:112832. doi: 10.1016/j.ejmech.2020.112832. [DOI] [PubMed] [Google Scholar]
- 20.Thakur A., Singla R., Jaitak V. Coumarins as anticancer agents: A review on synthetic strategies, mechanism of action and SAR studies. Eur. J. Med. Chem. 2015;101:476–495. doi: 10.1016/j.ejmech.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 21.Emami S., Dadashpour S. Current developments of coumarin-based anti-cancer agents in medicinal chemistry. Eur. J. Med. Chem. 2015;102:611–630. doi: 10.1016/j.ejmech.2015.08.033. [DOI] [PubMed] [Google Scholar]
- 22.Rubab L., Afroz S., Ahmad S., Hussain S., Nawaz I., Irfan A., Batool F., Kotwica-Mojzych K., Mojzych M. An Update on synthesis of coumarin sulfonamides as enzyme inhibitors and anticancer agents. Molecules. 2022;27:1604. doi: 10.3390/molecules27051604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Musa A.M., Cooperwood J.S., Khan M.O.F. A Review of coumarin derivatives in pharmacotherapy of breast Cancer. Curr. Med. Chem. 2008;15:2664–2679. doi: 10.2174/092986708786242877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen S., Cho M., Karlsberg K., Zhou D.Y., Yuan C. Biochemical and biological characterization of a novel anti-aromatase coumarin derivative. J. Biol. Chem. 2004;279:48071–48078. doi: 10.1074/jbc.M406847200. [DOI] [PubMed] [Google Scholar]
- 25.Xu J., Li H., Wang X., Huang J., Li S., Liu C., Dong R., Zhu G., Duan C., Jiang F., et al. Discovery of coumarin derivatives as potent and selective cyclin-dependent kinase 9 (CDK9) inhibitors with high antitumour activity. Eur. J. Med. Chem. 2020;200:112424. doi: 10.1016/j.ejmech.2020.112424. [DOI] [PubMed] [Google Scholar]
- 26.Bhatia R., Rawal R.K. Coumarin Hybrids: Promising scaffolds in the treatment of breast cancer. Mini Rev. Med. Chem. 2019;19:1443–1458. doi: 10.2174/1389557519666190308122509. [DOI] [PubMed] [Google Scholar]
- 27.Zhang L., Xu Z. Coumarin-containing hybrids and their anticancer activities. Eur. J. Med. Chem. 2019;181:111587. doi: 10.1016/j.ejmech.2019.111587. [DOI] [PubMed] [Google Scholar]
- 28.Belluti F., Fontana G., Dal Bo L., Carenini N., Giommarelli C., Zunino F. Design, synthesis and anticancer activities of stilbene-coumarin hybrid compounds: Identification of novel proapoptotic agents. Bioorg. Med. Chem. 2010;18:3543–3550. doi: 10.1016/j.bmc.2010.03.069. [DOI] [PubMed] [Google Scholar]
- 29.Yu H., Hou Z., Tian Y., Mou Y., Guo C. Design, synthesis, cytotoxicity and mechanism of novel dihydroartemisinin-coumarin hybrids as potential anti-cancer agents. Eur. J. Med. Chem. 2018;151:434–449. doi: 10.1016/j.ejmech.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Cui X.R., Takahashi K., Shimamura T., Koyanagi J., Komada F., Saito S. Preparation of 1,8-di-O-alkylaloe-emodins and 15-amino-, 15-thiocyano-, and 15-selenocyanochrysophanol derivatives from aloe-emodin and studying their cytotoxic effects. Chem. Pharm. Bull. 2008;56:497–503. doi: 10.1248/cpb.56.497. [DOI] [PubMed] [Google Scholar]
- 31.Raushel J., Fokin V.V. Efficient synthesis of 1-sulfonyl-1,2,3-triazoles. Org. Lett. 2010;12:4952–4955. doi: 10.1021/ol102087r. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.