Abstract
Cytotoxic T-lymphocyte (CTL) responses to E6 and E7 were previously shown to be more commonly detectable in human papillomavirus type 16 (HPV-16)-positive women without squamous intraepithelial neoplasia (SIL) than in HPV-16-positive women with SIL (M. Nakagawa, D. P. Stites, S. Farhat, J. R. Sisler, B. Moss, F. Kong, A. B. Moscicki, and J. M. Palefsky, J. Infect. Dis. 175:927–931, 1997). The objective of this study was to characterize the phenotype(s) of the effector cell population responsible for HPV-16 E6- and E7-specific cytotoxic responses. Peripheral blood mononuclear cells were stimulated with HPV-16 E6 or E7 fusion protein. Cells from an autologous B-lymphoblastoid cell line, infected with vaccinia virus expressing E6 or E7, served as target cells. The effector cells were characterized by using natural-killer-cell removal, antibody blocking, and T-cell subset separation. Our results suggest that both CD4 and CD8 T lymphocytes contribute to HPV-16 E6- and E7-specific CTL responses although their relative contributions vary from individual to individual. On the other hand, natural killer cells in the effector cell population contribute to background activities but not to HPV-specific responses in this assay system.
The link between human papillomavirus (HPV) and the development of squamous intraepithelial lesions (SIL) as well as cervical cancer is well known. Although immune responses to HPV are thought to be important in the prevention of neoplastic development, they have not been well characterized. In a previous study (13), we showed that cytotoxic T-lymphocyte (CTL) responses to HPV type 16 (HPV-16) E6 and E7 proteins appear to be important in the prevention of SIL in that responses to both E6 and E7 proteins were more commonly found in HPV-16-positive women without SIL than in HPV-16-positive women with SIL.
Traditionally, CD8-positive CTLs are known to be the primary effector cells involved in protection from intracellular pathogens. However, several investigators have shown that CD4-positive cells may behave as CTLs, contributing to the observed antigen-specific cytotoxicity (1, 4, 9, 10, 11, 14). In our HPV-CTL assay, recombinant fusion protein containing HPV-16 E6 or E7 was used for in vitro stimulation of effector cells. Exogenously introduced proteins, such as those used in our assays, may be taken up in endocytic compartments where they are degraded and processed for antigen presentation by major histocompatibility complex class II molecules. Therefore, CD4 cells could be preferentially stimulated and thus could have contributed solely to the observed CTL responses. The objective of this study was to determine whether the HPV-specific effectors responsible for the CTL response were primarily CD4 T lymphocytes and whether natural killer (NK) cells contributed to the observed killing.
MATERIALS AND METHODS
Subjects.
Twenty-one subjects who had recent HPV-16 infections of the cervix, detected by PCR analysis (17), but who had not developed SIL were chosen from participants in an ongoing prospective study (12) of cervical HPV infection. This study was approved by the institution’s Committee on Human Research, and informed consent was obtained from all subjects.
HPV-CTL assay.
Sixty to 80 ml of heparinized whole blood was collected from each subject and an HPV-CTL assay was set up as previously described (13). The peripheral blood mononuclear cells (106 cells/well in 2 ml of medium), isolated by a Ficoll-Hypaque density gradient (Pharmacia, Piscataway, N.J.), were stimulated for 7 days at 37°C in a 5% CO2 atmosphere with E6-–glutathione S-transferase (GST) or E7-GST (1 μg/ml) in RPMI 1640 with 15% pooled human serum (Norml Cera-Plus; NABI, Miami, Fla.), penicillin G (100 U/ml), streptomycin (100 μg/ml), gentamicin (500 μg/ml), and 20 U of recombinant interleukin-2 (Chiron Corporation, Emeryville, Calif.) per ml. At the same time, a mixed lymphocyte culture was set as a positive cytotoxicity control with an irradiated (40 Gy) allogeneic Epstein-Barr virus (EBV)-transformed B-lymphoblastoid cell line (LCL) (106 cells/well) for stimulation.
One day prior to the CTL assay, autologous EBV-transformed LCLs were infected with either E6-vac, E7-vac, or WR at a multiplicity of infection of 1. Positive-control cells (the allogeneic EBV-transformed LCL used for stimulation was also used as a target for the mixed lymphocyte culture) and cells infected with E6-vac, E7-vac, and WR were labeled with 200 μCi of sodium chromate (Na251CrO4; specific activity, 5 mCi/ml) (Amersham Corp., Arlington Heights, Ill.) for 90 min at 37°C on the day of the assay. Labeled cells were washed and plated in triplicate in 96-well round-bottom plates at 5 × 103 cells/well. Effector lymphocytes were added at four different effector-to-target ratios in a final volume of 200 μl/well. Supernatants were harvested with a Skatron harvesting press after 5 h of incubation, and the 51Cr disintegrations were counted by Cobra II Auto-Gamma counter (Packard Instruments, Meriden, Conn.). The spontaneous release and maximum release of the 51Cr target cells were determined by adding 100 μl of assay medium and 100 μl of 5% sodium dodecyl sulfate, respectively. Percent specific lysis for each effector-to-target cell ratio was calculated with the following formula: percent specific lysis = (experimental release − spontaneous release)/(maximum release − spontaneous release) × 100. When experimental specific lysis was at least 11% above the specific lysis of the corresponding negative control at at least two of the four effector-to-target ratios, the response was considered positive (13).
The NK-cell depletion, antibody blocking, and T-cell subset separation experiments were performed concurrently with the same effectors. None of the samples was used for more than one of the three analyses due to the limitation of the number of cells available.
NK-cell depletion.
NK-cell depletion experiments were performed to assess the role of NK cells in the assay system. One day prior to the 51Cr release assay, magnetic beads (Dynal, Lake Success, N.Y.) coupled with CD16-specific mouse monoclonal antibody (Becton Dickinson, San Jose, Calif.) were incubated with anti-E6 or anti-E7 effector cells at 4°C for 30 min. Although the estimated bead-to-NK-cell ratio was 50 to 1, the actual ratios varied from 13 to 1 to 201 to 1 due to the variations in the frequency of NK cells in the effector cell population. The percent NK cells before and after was measured by labeling with anti-CD56 antibody (Beckton Dickinson).
Antibody blocking.
51Cr-labeled target cells were incubated with anti-HLA class I antibody (50 μl of W6/32; American Type Culture Collection, Manassas, Va.) to block CD8 T-lymphocyte activity or with a cocktail of anti-HLA class II antibodies (25 μl each of IVA12 and 9.3F10; American Type Culture Collection) to block CD4 T-lymphocyte activity. On the day of the 51Cr release assay, radiolabeled target cells were incubated in hybridoma supernatant for 30 min at 37°C. Excess antibody was removed by washing with medium prior to plating target cells. The formula used was as follows: percent inhibition = [1 − (HPV-specific lysis in the presence of antibodies/HPV-specific lysis in the absence of antibodies)] × 100.
T-cell subset separation.
A magnetic bead separation system was used to separate effector cells into CD4 or CD8 T lymphocytes (Miltenyi Biotec, Auburn, Calif.) by magnetically labeling and depleting cells of other phenotypes. For selection of CD4 T lymphocytes, a cocktail of hapten-conjugated antibodies against CD8, CD11b, CD16, CD19, CD36, and CD56 was used. A cocktail for isolating CD8 T lymphocytes contained anti-CD4 antibody in place of the anti-CD8 antibody. These selections were performed immediately prior to CTL assays. The efficiencies of CD4-, CD8-, and NK-cell removal were monitored by fluorescence-activated cell sorter analysis with two-colored combinations of antibodies: anti-CD4–anti-CD8 and anti-CD3–anti-CD16 (Caltag, Burlingame, Calif.).
RESULTS
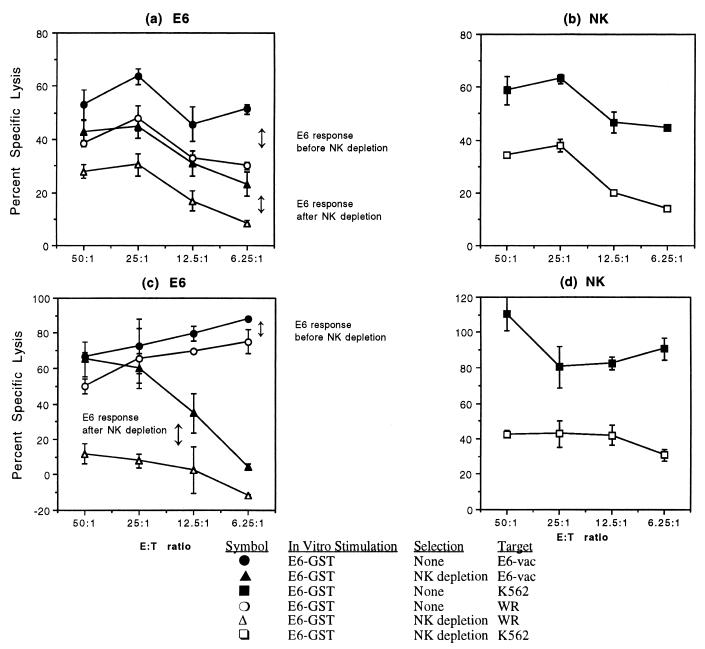
To assess whether the HPV-16 E6- and E7-specific responses demonstrated in our assay system were mediated by T lymphocytes in an antigen-specific manner, the role of NK cells was studied. In the two experiments addressing the E6-specific response, the positive HPV-16 E6-specific response, shown by the difference between the experimental percent specific lysis and the negative-control percent specific lysis, was preserved (Fig. 1a) or enhanced (Fig. 1c) after the removal of NK cells. NK-cell response, measured by killing of the 51Cr-labeled NK-cell-sensitive erythroleukemia cell line K562, was decreased in both experiments (Fig. 1b and d). In eight other experiments, no E6- or E7-specific responses were detected. The NK cells from the effector cell population were decreased from 17.0 to 3.0% in one experiment (Fig. 1a and b) and from 6.1 to 2.8% in the other experiment (Fig. 1c and d). These results show that the NK cells present in the effector cell population did not contribute to HPV-specific killing.
FIG. 1.
The HPV-16 E6-specific CTL response is preserved after removal of NK cells by using magnetic beads coupled with anti-CD16 antibody. Each point represents the mean ± standard error. (a and c) In two subjects, the HPV-16 E6-specific CTL response present prior to NK-cell depletion (the difference between the experimental lysis [filled circles] and the negative-control lysis [open circles]) was maintained or enhanced after NK-cell depletion (the difference between the experimental lysis [filled triangles] and the negative-control percent specific lysis [open triangles]). (b and d) Analysis showed reduced NK-cell activity in the NK-cell-depleted effector cells (open squares) compared to the untreated effector cells (filled squares) in both subjects. E:T ratio, effector-to-target ratio.
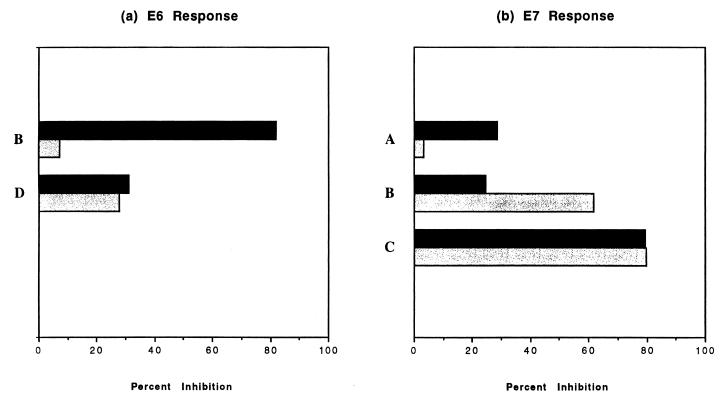
Monoclonal antibodies with specificities to HLA class I and class II molecules were utilized to determine the phenotype(s) of effector cells responsible for HPV-16 E6- and E7-specific responses (Fig. 2). Four subjects were tested for both anti-E6 and anti-E7 responses except for subject A, who was tested only for anti-E7 response. Subjects B and D had positive anti-E6 CTL responses, and the magnitude of the responses, as determined by the difference between the experimental lysis of the E6-vac-infected target and the background lysis of the WR-infected target, was 17% for both subjects. Subjects A, B, and C had positive anti-E7 CTL responses, and the magnitudes of these responses were 36, 65, and 44%, respectively. For two subjects (E7 response for subject A and E6 response for subject B), class I-restricted CD8 lymphocytes seemed to have a more dominant role in HPV-specific killing than class II-restricted CD4 lymphocytes. The reverse was true for the E7 response for subject B. In two subjects (E7 response for subject C and E6 response for subject D), CD4 and CD8 seemed to make equal contributions toward HPV-16-specific killing. These experiments show that both CD4 and CD8 T lymphocytes contribute to HPV-16 E6- and E7-specific CTL responses, although their relative contributions vary.
FIG. 2.
Antibodies with specificities to HLA class I (solid bars) or class II (shaded bars) molecules inhibit HPV-16 E6- and E7-specific responses. The results at the effector-to-target ratio of 50 to 1 are shown.
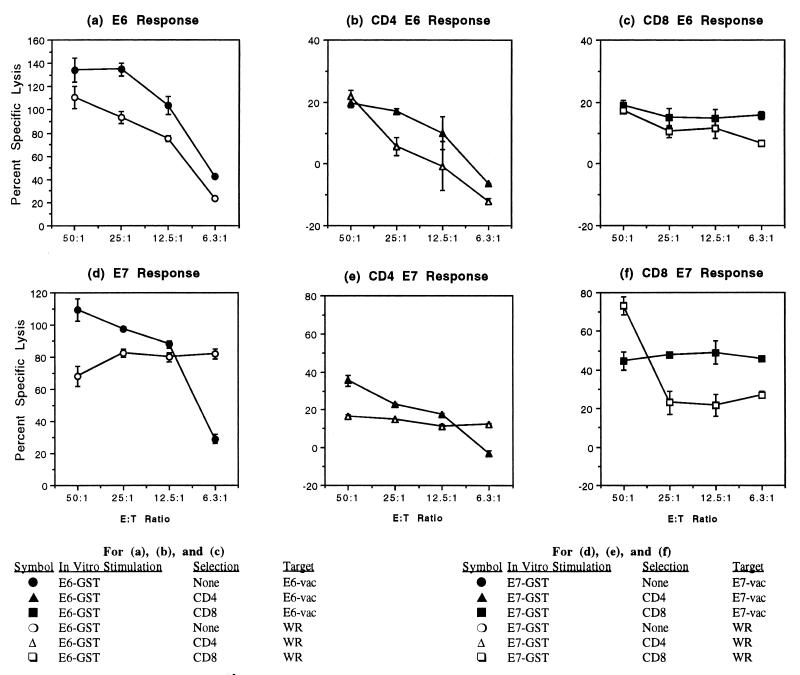
CD4 and CD8 T lymphocytes were separated by removing other cell types. In the CD4-selected E6-specific effector cell population shown in Fig. 3b, the CD8 T lymphocytes were reduced from 18.8 to 0.1% and the NK cells were reduced from 6.0 to 0.3%. In the CD8-selected E6-specific effector cell population, CD4 T lymphocytes were reduced from 43.8 to 0.5% and NK cells were reduced from 6.0 to 0.4% (Fig. 3c). Similarly, the CD8 T lymphocytes were reduced from 54.2 to 1.2% and NK cells were reduced from 11.3 to 0.1% in the CD4-selected E7-specific effector cell population (Fig. 3e), and the CD4 T lymphocytes were reduced from 21.8 to 0.1% and the NK cells were reduced from 11.3 to 0.0% in the CD8-selected E7-specific effector cell population (Fig. 3f).
FIG. 3.
CD4- and CD8-positive effector cell populations demonstrate HPV-16 E6- and E7-specific CTL responses. Each point represents the mean ± standard error. (a through c) E6-specific CTL responses of an unselected effector cell population (a), CD4-positive effector cell population (b), and CD8-positive effector cell population (c). (d through f) E7-specific CTL response of an unselected effector cell population (d), CD4-positive effector cell population (e), and CD8-positive effector cell population (f). E:T ratio, effector-to-target ratio.
In CTL assays with separated cells, two subjects demonstrated a greater contribution of CD4 T lymphocytes than CD8 T lymphocytes in the anti-E6 effector cell population (Fig. 3a to c). Of six subjects tested for E7, three were negative for E7 response. The other three subjects showed a greater contribution of CD8 T lymphocytes than CD4 T lymphocytes (Fig. 3d to f). Therefore, the results of antibody blocking were corroborated with those of T-cell subset separation experiments, which demonstrated that both CD4 and CD8 T lymphocytes contribute to HPV-16 E6- and E7-specific CTL responses.
DISCUSSION
To date, a number of protocols to measure HPV-specific CTL responses in humans have been described (2, 3, 7, 8, 13). Various methods have been used to stimulate HPV responses in vitro, including the use of HPV-16-derived peptides (2, 8), HPV-16 recombinant fusion proteins (13), HPV-16 recombinant adenovirus (3), and an HPV-16-positive cervical carcinoma cell line (7). Some of these approaches limit the scope of the analysis to HLA-A2-positive individuals (2, 7, 8). Our approach with recombinant fusion proteins has the advantage of being able to include individuals of all HLA types as well as requiring only 1 week for in vitro stimulation.
Soluble antigens have been shown to be able to stimulate specific CTL responses in vitro in spleen cells previously primed with live virus. For example, a nuclear protein of influenza A virus has been shown to induce CTL responses in vitro in a mouse model (19). Another study has shown that purified hemagglutinin is able to stimulate CTL responses in vitro in spleen cells from mice previously primed with live virus (5). However, attempts to stimulate effectors in vitro with soluble proteins more commonly fail. It is possible that an infection with live virus is a prerequisite for an organism’s ability to demonstrate specific CTL responses when in vitro stimulation with soluble protein is used, since the mice were infected with influenza A virus and the subjects in our study were infected with HPV-16.
Our present study shows that NK cells do not contribute to the observed HPV-16 E6- and E7-specific responses in the CTL assay but that both CD4 and CD8 T lymphocytes do. Although the contribution of CD8 cells was certainly expected, the mechanism associated with the stimulation of CD8 cells in our assay is not clear. In vitro stimulation of HPV antigens through an HLA class I-dependent classical pathway was supported by data from Evans et al. (7) and Ressing et al. (15). Evans et al. reported that HLA class I-specific antibody, but not HLA class II-specific antibody, inhibits cytotoxicity (7). However, these results were obtained with only one subject. Ressing et al. also showed that peptide-specific human CTL responses induced in vitro appeared to be due to HLA class I-restricted CD8 T lymphocytes (15). A representative example was shown, but the number of experiments performed was not mentioned, limiting the interpretation of their finding. On the other hand, the HPV-16-specific CD8-positive CTL observed in our system may have been stimulated via an alternative antigen presentation pathway described by Rock (16) which has been shown to process and present antigens from intact proteins, such as ovalbumin.
The mixed CD4 and CD8 CTL responses specific to HPV-16 observed in our study certainly are not unique to our assay system. Many investigators have observed the contribution of CD4 cells to cytotoxity (1, 4, 9, 10, 11, 14). The contribution of CD4 T lymphocytes in our system is most likely due to the use of recombinant fusion proteins for in vitro stimulation and their processing through the endocytic major histocompatibility complex class II pathway, which results in the preferential stimulation of CD4 cells. However, we also found a similar contribution of CD4 cells in EBV-specific responses by using effectors that were stimulated in vitro with an irradiated autologous EBV-transformed LCL, which is believed to present antigen through the classical pathway in which CD8 T lymphocytes are stimulated in a HLA class I-dependent manner (6). Whether the presence of these CD4 CTLs is due to an artifact of the in vitro system or whether it has functional significance needs to be determined by adaptive transfer experiments similar to those performed by Wang et al. (18).
In summary, our results have shown that both CD4 and CD8 T lymphocytes contributed to the HPV-16 E6- and E7-specific CTL responses in our assay system, which utilized soluble proteins for in vitro stimulation. However, the NK cells present in the effector cell population did not contribute to the HPV-specific responses.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health (NCI CA51323, NCI K07 CA75974, and M01 RR01271), the University of California AIDS Clinical Research Center, the University of California Cancer Research Coordinating Committee (2-513803-36240), and the Cancer Research Institute (fellowship to M.N. and Pre-Clinical Grant-HPV).
REFERENCES
- 1.Ab B K, Kiessling R, Van Embden J D, Thole J E, Kumararatne D S, Pisa P, Wondimu A, Ottenhoff T H. Induction of antigen-specific CD4+ HLA-DR-restricted cytotoxic T lymphocytes as well as nonspecific nonrestricted killer cells by the recombinant mycobacterial 65-kDa heat-shock protein. Eur J Immunol. 1990;20:369–377. doi: 10.1002/eji.1830200221. [DOI] [PubMed] [Google Scholar]
- 2.Alexander M, Salgaller M L, Celis E, Sette A, Barnes W A, Rosenberg S A, Steller M A. Generation of tumor-specific cytolytic T lymphocytes from peripheral blood of cervical cancer patients by in vitro stimulation with a synthetic human papillomavirus type 16 E7 epitope. Am J Obstet Gynecol. 1996;175:1586–1593. doi: 10.1016/s0002-9378(96)70110-2. [DOI] [PubMed] [Google Scholar]
- 3.Borysiewicz L K, Fiander A, Nimako M, Man S, Wilkinson G W, Westmoreland D, Evans A S, Adams M, Stacey S N, Boursnell M E, Rutherford E, Hickling J K, Inglis S C. A recombinant vaccinia virus encoding human papillomavirus types 16 and 18, E6 and E7 proteins as immunotherapy for cervical cancer. Lancet. 1996;347:1523–1527. doi: 10.1016/s0140-6736(96)90674-1. [DOI] [PubMed] [Google Scholar]
- 4.Bourgault I, Gomez A, Gomard E, Picard F, Levy J P. A virus-specific CD4+ cell-mediated cytolytic activity revealed by CD8+ cell elimination regularly develops in uncloned human antiviral cell lines. J Immunol. 1989;142:252–256. . (Erratum, 142:4118.) [PubMed] [Google Scholar]
- 5.Braciale T J. Specificity of cytotoxicity T cells directed to influenza virus hemagglutinin. J Exp Med. 1979;149:856–869. doi: 10.1084/jem.149.4.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Jong, A., J. M. Palefsky, D. P. Stites, and M. Nakagawa. Submitted for publication.
- 7.Evans C, Bauer S, Grubert T, Brucker C, Baur S, Heeg K, Wagner H, Lipford G B. HLA-A2-restricted peripheral blood cytolytic T lymphocyte response to HPV type 16 proteins E6 and E7 from patients with neoplastic cervical lesions. Cancer Immunol Immunother. 1996;42:151–160. doi: 10.1007/s002620050265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans E M, Man S, Evans A S, Borysiewicz L K. Infiltration of cervical cancer tissue with human papillomavirus-specific cytotoxic T-lymphocytes. Cancer Res. 1997;57:2943–2950. [PubMed] [Google Scholar]
- 9.Hammond S A, Bollinger R C, Stanhope P E, Quinn T C, Schwartz D, Clements M L, Siliciano R F. Comparative clonal analysis of human immunodeficiency virus type 1 (HIV-1)-specific CD4+ and CD8+ cytolytic T lymphocytes isolated from seronegative humans immunized with candidate HIV-1 vaccines. J Exp Med. 1992;176:1531–1542. doi: 10.1084/jem.176.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khanna R, Burrows S R, Thomson S A, Moss D J, Cresswell P, Poulsen L M, Cooper L. Class I processing-defective Burkitt’s lymphoma cells are recognized efficiently by CD4+ EBV-specific CTLs. J Immunol. 1997;158:3619–3625. [PubMed] [Google Scholar]
- 11.Koelle D M, Posavad C M, Barnum G R, Johnson M L, Frank J M, Corey L. Clearance of HSV-2 from recurrent genital lesions correlates with infiltration of HSV-specific cytotoxic T lymphocytes. J Clin Investig. 1998;101:1500–1508. doi: 10.1172/JCI1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moscicki A B, Shiboski S, Broering J, Powell K, Clayton L, Jay N, Darragh T M, Brescia R, Kanowitz S, Miller S B, Stone J, Hanson E, Palefsky J. The natural history of human papillomavirus infection as measured by repeated DNA testing in adolescent and young women. J Pediatr. 1998;132:277–284. doi: 10.1016/s0022-3476(98)70445-7. [DOI] [PubMed] [Google Scholar]
- 13.Nakagawa M, Stites D P, Farhat S, Sisler J R, Moss B, Kong F, Moscicki A B, Palefsky J M. Cytotoxic T lymphocyte responses to E6 and E7 proteins of human papillomavirus type 16: relationship to cervical intraepithelial neoplasia. J Infect Dis. 1997;175:927–931. doi: 10.1086/513992. [DOI] [PubMed] [Google Scholar]
- 14.Renia L, Grillot D, Marussig M, Corradin G, Miltgen F, Lambert P H, Mazier D, Del Giudice G. Effector functions of circumsporozoite peptide-primed CD4+ T cell clones against Plasmodium yoelii liver stages. J Immunol. 1993;150:1471–1478. [PubMed] [Google Scholar]
- 15.Ressing M E, Sette A, Brandt R M, Ruppert J, Wentworth P A, Hartman M, Oseroff C, Grey H M, Melief C J, Kast W M. Human CTL epitopes encoded by human papillomavirus type 16 E6 and E7 identified through in vivo and in vitro immunogenicity studies of HLA-A∗0201-binding peptides. J Immunol. 1995;154:5934–5943. [PubMed] [Google Scholar]
- 16.Rock K L. A new foreign policy: MHC class I molecules monitor the outside world. Immunol Today. 1996;17:131–137. doi: 10.1016/0167-5699(96)80605-0. [DOI] [PubMed] [Google Scholar]
- 17.Ting Y, Manos M M. Detection and typing of genital human papillomaviruses. In: Innis M A, Gelfand D H, Sninsky J J, White T J, editors. PCR protocols: a guide to methods and applications—1990. San Diego, Calif: Academic Press; 1990. pp. 356–367. [Google Scholar]
- 18.Wang R, Charoenvit Y, Corradin G, De La Vega P, Franke E D, Hoffman S L. Protection against malaria by Plasmodium yoelii sporozoite surface protein 2 linear peptide induction of CD4+ T cell- and IFN-gamma-dependent elimination of infected hepatocytes. J Immunol. 1996;157:4061–4067. [PubMed] [Google Scholar]
- 19.Wraith D C, Askonas B A. Induction of influenza A virus cross-reactive cytotoxic T cells by a nucleoprotein/haemagglutinin preparation. J Gen Virol. 1985;66:1327–1331. doi: 10.1099/0022-1317-66-6-1327. [DOI] [PubMed] [Google Scholar]