Abstract
Background
Data are limited on whether several easily measured indices are independent predictors of type 2 diabetes mellitus (T2DM) in hypertensive patients. This study aimed to assess the association of hypertriglyceridemic-waist phenotype, triglyceride glucose (TyG) index, lipid accumulation product (LAP), and visceral adiposity index (VAI) with T2DM risk in hypertensive patients.
Methods
This cross-sectional study included 5321 hypertensive patients from the baseline survey of the Guangzhou Heart Study. Face-to-face questionnaire survey, physical examination, and fasting blood sample collection were completed for all subjects. Odds ratio (OR) with 95% confidence interval (95% CI) were calculated by using the logistic regression model. The potential nonlinear relationship was examined using restricted cubic spline regression.
Results
The prevalence of T2DM was 19.98% among hypertensive patients. After adjusting for confounders, participants with elevated triglyceride levels and enlarged waist circumference (HTGW) were associated with a 2.57-fold risk of T2DM (OR 2.57, 95% CI 2.05, 3.23). When comparing with subjects within the lowest quartile of the indices, those in the highest quartile of TyG, LAP, and VAI were associated with 5.35-fold (95% CI 4.33, 6.64), 2.65-fold (95% CI 2.11, 3.34), and 2.17-fold (95% CI 1.77, 2.67) risk of T2DM after adjusting for confounders. Every 1-unit increment of TyG, LAP, and VAI was associated with 81%, 38%, and 31% increased risk of T2DM, respectively. The nonlinear association was observed for TyG, LAP, and VAI (all P Non-linear < 0.001).
Conclusions
The results found that among hypertensive patients, HTGW and a higher level of TyG, LAP, and VAI were associated with an elevated risk of T2DM. The findings suggested that HTGW, TyG, LAP, and VAI may serve as simple and effective tools for T2DM risk assessment in the prevention and management of main chronic diseases.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12933-022-01660-z.
Keywords: Hypertriglyceridemic-waist phenotype, Triglyceride glucose index, Lipid accumulation product, Visceral adiposity index, Diabetes, Hypertension
Background
Type 2 diabetes mellitus (T2DM) has been found to be associated with an increased risk of cardiovascular diseases and reduced life expectancy, imposing a huge economic burden on societies [1–3]. The International Diabetes Federation estimated that the number of individuals with diabetes will be 700 million in 2045, and 80% will be found in low- and middle-income countries [4]. China has the largest number of diabetics in the world, with nearly 140.9 million diabetics in 2021 [5, 6]. With lifestyle changes and nutritional transitions over the past decades, diabetes has become a serious public health problem in China [5, 6].
Visceral adiposity and insulin resistance are associated with an increased risk of diabetes [7]. Imaging techniques, such as computed tomography and magnetic resonance imaging, can measure fat distribution and evaluate visceral adiposity quantitatively [8]; the hyperinsulinemic-euglycemic clamp is the gold standard test for the measurement of insulin resistance [9]. However, these techniques are time-consuming and costly for regular medical examinations and large-scale epidemiological studies. Thus, a simple and cost-effective predictor is required for detecting subjects with a high risk of T2DM.
Some anthropometric measures were usually used to evaluate the risk of cardiovascular diseases and metabolic syndrome [10–14]. By combining these anthropometric measures with biochemical measures, some novel lipid indicators can be established. The hypertriglyceridemic-waist phenotype, defined as an elevated triglyceride (TG) level and enlarged waist circumference (WC), was firstly introduced in 2000 as a useful and easy-to-measure marker of excess visceral adiposity [15]. In addition, the triglyceride glucose (TyG) index, the product of TG and fasting plasma glucose (FPG), has been considered as a surrogate measure to identify insulin resistance and define the status of metabolic health [16, 17]. Moreover, another two inexpensive and reliable lipid indices have been proposed to estimate insulin resistance from visceral adiposity dysfunction [18, 19]. One is lipid accumulation product (LAP), a model calculated from WC and TG levels [18]; the other is visceral adiposity index (VAI), which is computed based on body mass index (BMI), WC, TG, and high-density lipoprotein cholesterol (HDL-C) [19].
Prior studies have indicated that elevated triglyceride level and enlarged waist circumference (HTGW) was risk factor for developing T2DM [20–23]. Several studies have also demonstrated that higher levels of TyG, LAP, and VAI indices were associated with an increased risk of T2DM [24–26]. However, most of the previous studies were conducted among the general population. Compared with normotensive subjects, diabetes mellitus is more frequent in hypertensive patients [27, 28]. Hypertension could induce endothelial dysfunction, increase inflammatory markers, and further lead to the incidence of diabetes [29, 30]. Hence, hypertension may be a provoking factor for developing diabetes. However, whether these lipid indices have similar predictive effects on T2DM among hypertension patients is still unclear. Therefore, this population-based study was performed to evaluate the association between HTGW, TyG, LAP, and VAI indices and the risk of T2DM in subjects with hypertension, and to examine any possible effect modifiers.
Methods
Setting and subjects
The data used in this population-based cross-sectional study was derived from the baseline survey of Guangzhou Heart Study, an ongoing prospective cohort study in Guangzhou, China. Detailed information about Guangzhou Heart Study has been shown in our previous reports [31–35]. Briefly, a total of 12,013 permanent residents aged 35 years or above were recruited and accomplished the baseline survey between July 2015 and August 2017. Those who had mental or cognitive disorders, had mobility difficulties, had any history of cancer, were pregnant or lactating women, and were non-Guangzhou permanent residents were excluded when recruiting subjects. In this study, we further excluded those with missing data on the blood pressure measurement or diabetes-related information (n = 6), and subjects without hypertension (n = 6686). Finally, a total of 5321 subjects with hypertension were included for further analysis. No significant difference in terms of age and sex was observed between subjects included and not included. The flow chart of the selection of hypertensive patients were shown in Additional file 1: Fig. S1.
This study was approved by the Ethical Review Committee for Biomedical Research, School of Public Health, Sun Yat-sen University, and the Research Ethics Committee for Guangdong Provincial People’s Hospita1, Guangdong Academy of Medical Sciences. The study was performed in line with the Declaration of Helsinki and all participants provided informed consent.
Data collection and measurements
A structured questionnaire conducted with a face-to-face approach was used to collect demographic and lifestyle information, including age (years), sex (male, female), education (< high school, ≥ high school), marital status (married, others), retirement status (yes, no), smoking (yes, no), and alcohol drinking (yes: occasion and frequent, no: never drinking). Leisure-time physical activity was estimated by using the modified Global Physical Activity Questionnaire (GPAQ) with in-person interview [33, 34]. The volume of each type of leisure-time physical activity was calculated by multiplying the intensity of the physical activity by its frequency and its duration, and the total volume of leisure-time physical activity (MET-h/week) was the sum of the volume of all types of leisure-time physical activity [33, 34]. Medical history including physician-diagnosed T2DM (yes, no), hypertension (yes, no), and cardiovascular diseases (yes, no) was required to report.
Physical measurements, including height, weight, waist circumference (WC), and blood pressure, were performed by trained medical workers in line with standard instruments and protocols. BMI was calculated by dividing weight by height squared (kg/m2). WC was measured with a tape at the midway between the lower edge of the costal arch and the superior border of the iliac crest while standing. Participants were instructed to rest for at least 10 min in a quiet room before blood pressure measurement. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured three times by trained medical workers, and then the mean of three measurements was calculated. Hypertension was defined as SBP ≥ 140 mmHg or DBP ≥ 90 mmHg, or having self-reported physician-diagnosed hypertension [33].
Fasting blood sample from each subject was collected, and serum concentrations of FPG, TG, HDL-C, low-density lipoprotein cholesterol (LDL-C), and total cholesterol were detected. T2DM was defined as fasting blood glucose ≥ 7.0 mmol/L, or having self-reported physician-diagnosed diabetes excluding gestational diabetes mellitus, type 1 diabetes mellitus, or other types of diabetes [36].
Definition of indicators
Central obesity was defined as a WC ≥ 85 cm in women or ≥ 90 cm in men [37]. TG level ≥ 1.7 mmol/L was defined as increased TG [38]. The hypertriglyceridemic-waist phenotypes were categorized into four groups: (1) normal triglyceride level and normal waist circumference (NTNW); (2) normal triglyceride level and enlarged waist circumference (NTGW); (3) elevated triglyceride level and normal waist circumference (HTNW); (4) elevated triglyceride level and enlarged waist circumference (HTGW). TyG, LAP and, VAI were calculated using the following formulas [39]:
Statistical analysis
The normality was examined by the Kolmogorov–Smirnov test. The continuous variables with normal distribution were displayed using mean and standard deviation (SD), and the continuous variables with non-normal distribution were shown using median and interquartile range (IQR). The distribution of categorical variables was represented as frequency and proportion (%). The distribution difference of baseline demographics, lifestyle information, and other covariables among different groups was estimated by chi-square test for a categorical variable and by t-test or Wilcoxon rank-sum test for a continuous variable. The continuous variables of TyG, LAP, and VAI were transformed into categorical variables by using quartile methods.
Logistic regression models were performed to estimate the association of T2DM risk with triglyceridemic-waist phenotypes, TyG, LAP, and VAI; odds ratios (ORs) with 95% confidence intervals (CIs) were calculated with and without adjustment for covariates. The potential nonlinear relationship was examined using restricted cubic spline regression (knots on the 5th, 25th, 75th, and 95th percentiles), with OR and 95% CI being calculated based on the logistic regression model. The restricted cubic spline regression was performed with the “rms” package.
Stratified analysis was conducted by age group (< 60 or ≥ 60 years), sex (male or female), retirement status (retirement or non-retirement), education (< High school or ≥ High school), BMI (< 24 or ≥ 24 kg/m2), smoking (yes or no), and alcohol drinking (yes or no). The multiplicative interaction of HTGW, TyG, LAP, and VAI with age group, sex, retirement status, education, BMI, smoking, and alcohol drinking was estimated respectively using the likelihood ratio test, with a comparison of the likelihood scores of the two models with and without the interaction terms. In addition, to examine the robustness of the results, we conducted the sensitivity analyses by defining WC ≥ 80 cm in women as enlarged waist circumference according to the new International Diabetes Federation; and by adding total cholesterol and LDL-C as additional covariates to control potential influence; and by defining hypertension as SBP ≥ 130 mmHg or DBP ≥ 85 mmHg, or having self-reported physician-diagnosed hypertension. All statistical analyses were performed using R 4.0.1 (R Development Core Team, Vienna, Austria); the tests were two-tailed, and the P value of less than 0.05 was considered statistically significant.
Results
A total of 5,321 subjects with hypertension were included in the present study, of which 4,258 subjects (80.02%) were classified into the non-T2DM group and 1,063 (19.98%) into the T2DM group (Table 1). Of all subjects, the mean (SD) of age, BMI, SBP, DBP and TyG index was 64.08 (10.72) years, 24.96 (3.61) kg/m2, 142.70 (16.79) mmHg, 85.71 (11.19) mmHg, and 8.82 (0.63). The median (IQR) value of the leisure-time physical activity, LAP, and VAI was 34.65 (39.90) MET-h/week, 38.92 (38.07), and 1.74 (1.85). More participants in the T2DM group than in the non-T2DM group were non-married, retirees, and non-alcohol drinker, had a history of cardiovascular diseases, were in the HTGW group, and were in the highest quartile of TyG, LAP and VAI (all P < 0.05).
Table 1.
Baseline characteristic of the subjects with hypertension
| Characteristic | Total (N = 5321) | Non-T2DM (N = 4258) | T2DM (N = 1063) | P |
|---|---|---|---|---|
| Age (years), mean (S.D.) | 64.08 (10.72) | 63.44 (10.79) | 66.66 (10.00) | < 0.001* |
| BMI (kg/m2), mean (S.D.) | 24.96 (3.61) | 24.85 (3.59) | 25.41 (3.67) | < 0.001* |
| Leisure-time physical activity (MET-h), median (Interquartile) | 34.65 (39.90) | 34.65 (40.79) | 34.65 (38.91) | 0.631† |
| SBP (mmHg), mean (S.D.) | 142.70 (16.79) | 142.80 (16.67) | 142.30 (17.29) | 0.340* |
| DBP (mmHg), mean (S.D.) | 85.71 (11.19) | 86.45 (11.03) | 82.74 (11.30) | < 0.001* |
| TyG index, mean (S.D.) | 8.82 (0.63) | 8.73 (0.55) | 9.19 (0.76) | < 0.001* |
| LAP, median (interquartile) | 38.92 (38.07) | 37.27 (35.63) | 47.92 (46.61) | < 0.001† |
| VAI, median (interquartile) | 1.74 (1.85) | 1.65 (1.73) | 2.14 (2.32) | < 0.001† |
| Sex, N (%) | 0.909‡ | |||
| Male | 2078 (39.05) | 1665 (39.10) | 413 (38.85) | |
| Female | 3243 (60.95) | 2593 (60.90) | 650 (61.15) | |
| Education, N (%) | 0.540‡ | |||
| < high school | 3668 (68.93) | 2944 (69.14) | 724 (68.11) | |
| ≥ high school | 1653 (31.07) | 1314 (30.86) | 339 (31.89) | |
| Material status, N (%) | 0.029‡ | |||
| Married | 4331 (81.39) | 3491 (81.99) | 840 (79.02) | |
| Others | 990 (18.61) | 767 (18.01) | 223 (20.98) | |
| Retirement status, N (%) | < 0.001‡ | |||
| Non-retirement | 1555 (29.22) | 1339 (31.45) | 216 (20.32) | |
| Retirement | 3766 (70.78) | 2919 (68.55) | 847 (79.68) | |
| Smoking, N (%) | 0.252‡ | |||
| No | 4107 (77.18) | 3272 (76.84) | 835 (78.55) | |
| Yes | 1214 (22.82) | 986 (23.16) | 228 (21.45) | |
| Alcohol drinking, N (%) | 0.021‡ | |||
| No | 4252 (79.91) | 3375 (79.26) | 877 (82.50) | |
| Yes | 1069 (20.09) | 883 (20.74) | 186 (17.50) | |
| History of cardiovascular diseases, N (%) | < 0.001‡ | |||
| No | 4951 (93.05) | 3989 (93.68) | 962 (90.50) | |
| Yes | 370 (6.95) | 269 (6.32) | 101 (9.50) | |
| Triglyceridemic-waist phenotypes, N (%) | < 0.001‡ | |||
| NTNW | 1628 (30.60) | 1412 (33.16) | 216 (20.32) | |
| NTGW | 1652 (31.05) | 1309 (30.74) | 343 (32.27) | |
| HTNW | 774 (14.55) | 622 (14.61) | 152 (14.30) | |
| HTGW | 1267 (23.81) | 915 (21.49) | 352 (33.11) | |
| TyG (quartile), N (%) | < 0.001‡ | |||
| Quartile 1 (≤ 8.39) | 1330 (25.00) | 1185 (27.83) | 145 (13.64) | |
| Quartile 2 (8.39–8.76) | 1330 (25.00) | 1153 (27.08) | 177 (16.65) | |
| Quartile 3 (8.76–9.18) | 1331 (25.00) | 1088 (25.55) | 243 (22.86) | |
| Quartile 4 (> 9.18) | 1330 (25.00) | 832 (19.54) | 498 (46.85) | |
| LAP (quartile), N (%) | < 0.001‡ | |||
| Quartile 1 (≤ 24.31) | 1330 (25.00) | 1151 (27.00) | 179 (16.80) | |
| Quartile 2 (24.31–38.92) | 1330 (25.00) | 1097 (25.80) | 233 (21.90) | |
| Quartile 3 (38.92–62.38) | 1330 (25.00) | 1050 (24.70) | 280 (26.30) | |
| Quartile 4 (> 62.38) | 1331 (25.00) | 960 (22.50) | 371 (34.90) | |
| VAI (quartile), N (%) | < 0.001‡ | |||
| Quartile 1 (≤ 1.05) | 1330 (25.00) | 1124 (26.40) | 206 (19.40) | |
| Quartile 2 (1.05–1.74) | 1330 (25.00) | 1113 (26.10) | 217 (20.40) | |
| Quartile 3 (1.74–2.90) | 1331 (25.00) | 1052 (24.70) | 279 (26.20) | |
| Quartile 4 (> 2.90) | 1330 (25.00) | 969 (22.80) | 361 (34.00) |
BMI body mass index, HTGW elevated triglyceride level and enlarged waist circumference, HTNW elevated triglyceride level and normal waist circumference, LAP lipid accumulation product, MET-h metabolic equivalent values-hours, NTGW normal triglyceride level and enlarged waist circumference, NTNW normal triglyceride level and normal waist circumference, TyG triglyceride-glucose, VAI visceral adiposity index, SBP systolic blood pressure, DBP diastolic blood pressure
*P value from t-test
†P value from Wilcoxon rank sum test
‡P value Chi-square test
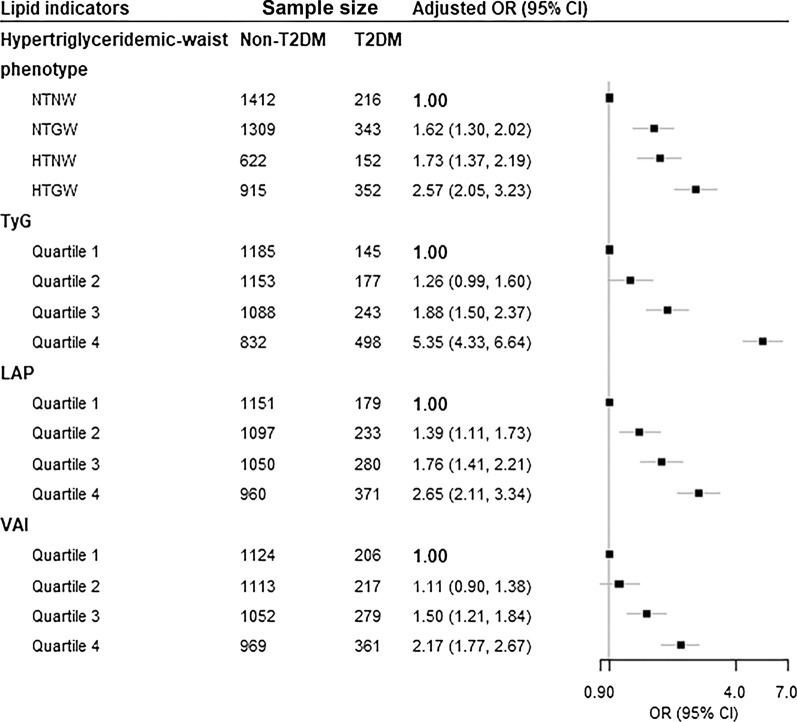
The association of different hypertriglyceridemic-waist phenotypes and T2DM risk was shown in Fig. 1 and Additional file 1: Table S1. In comparison to participants with NTNW, those with NTGW, HTNW and HTGW were associated with a 1.62-fold (OR 1.62, 95% CI 1.30, 2.02), 1.73-fold (OR 1.73, 95% CI 1.37, 2.19), and 2.57-fold (OR 2.57, 95% CI 2.05, 3.23) risk of T2DM, respectively, after adjusting for confounders.
Fig. 1.
Association between lipid indicators and the risk of T2DM. HTGW elevated triglyceride level and enlarged waist circumference, HTNW elevated triglyceride level and normal waist circumference; LAP, lipid accumulation product, NTGW normal triglyceride level and enlarged waist circumference, NTNW normal triglyceride level and normal waist circumference, TyG triglyceride-glucose, VAI visceral adiposity index. Adjustment for age, sex, education, marital status, retirement status, smoking, alcohol drinking, leisure-time physical activity, body mass index, systolic blood pressure, diastolic blood pressure, and history of cardiovascular disease
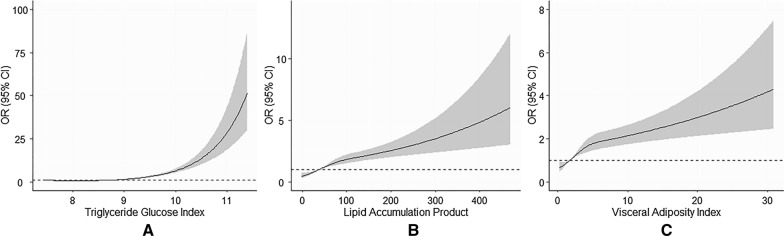
The risk of T2DM increased with the increment of TyG, LAP, and VAI (Fig. 1; Additional file 1: Table S1). After considering possible confounders, every 1-unit increment of TyG, LAP, and VAI were associated with 81% (OR 1.81, 95% CI 1.69, 1.94), 38% (OR 1.38, 95% CI 1.28, 1.48), and 31% (OR 1.31, 95% CI 1.23, 1.40) increased risk of T2DM, respectively. When comparing with the lowest quartile of the indices, the highest quartile of TyG, LAP, and VAI were associated with 5.35-fold (95% CI 4.33, 6.64, P-trend < 0.001), 2.65-fold (95% CI 2.11, 3.34, P-trend < 0.001), and 2.17-fold (95% CI 1.77, 2.67, P-trend < 0.001) risk of T2DM after adjusting for potential confounders. Figure 2 displayed the associations of T2DM risk with TyG, LAP, and VAI through the restricted cubic spline curve, and the significant non-linear associations were found (all P Non-linear < 0.001).
Fig. 2.
Restricted cubic spline of the relationship of T2DM with TyG (A), LAP (B), and VAI (C). Potential nonlinear relationships were examined using restricted cubic splines (knots on the 5th, 25th, 75th and 95th percentiles), with hazard ratios (HRs) based on logistic regression models. The dash line represents the OR equal to 1. The ORs was adjusted for age, sex, education, marital status, retirement status, smoking, alcohol drinking, leisure-time physical activity, BMI, SBP, DBP and history of cardiovascular diseases
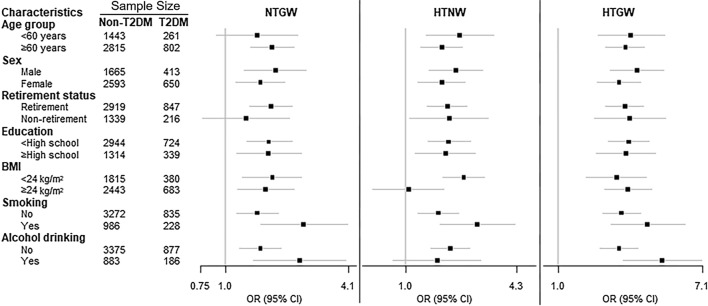
Compared with the NTNW group, the HTGW group was significantly associated with an increased risk of T2DM in all subgroups (Fig. 3; Additional file 1: Table S2). Compared with the NTNW group, participants with NTGW were significantly associated an increased risk of T2DM in all stratified groups except for non-retirees and those aged < 60 years, participants with HTNW were significant associated with an increased risk of T2DM in all stratified groups except for alcohol drinkers and those with BMI ≥ 24 kg/m2. We also found that BMI was a significant effect modifier in the association between hypertriglyceridemic-waist phenotype and T2DM risk among hypertensive patients (P-interaction = 0.008), while no interaction was found between other factors and hypertriglyceridemic-waist phenotype.
Fig. 3.
Stratified analyses on the association between triglyceridemic-waist phenotypes and T2DM risk. BMI body mass index, HTGW elevated triglyceride level and enlarged waist circumference, HTNW elevated triglyceride level and normal waist circumference, NTGW normal triglyceride level and enlarged waist circumference, NTNW normal triglyceride level and normal waist circumference. Adjustment for age, sex, education, marital status, retirement status, smoking, alcohol drinking, leisure-time physical activity, BMI, SBP, DBP and history of cardiovascular diseases except the corresponding stratification variable
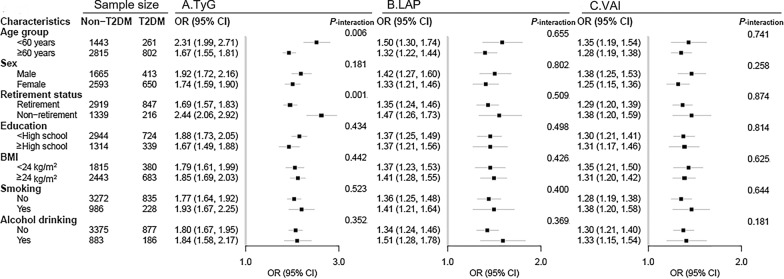
In stratified analysis, the positive relationship of TyG, LAP, and VAI with T2DM risk remained significant in all subgroups (Fig. 4; Additional file 1: Tables S3–S5). The association between TyG index and T2DM risk appeared stronger in subjects < 60 years and in non-retirees (both P-interaction < 0.05). No interaction was found for other factors with TyG, LAP, and VAI.
Fig. 4.
Stratified analyses on the association between TyG, LAP, and VAI and T2DM risk. BMI body mass index, TyG triglyceride-glucose, VAI visceral adiposity index, LAP lipid accumulation product. Adjustment for age, sex, education, marital status, retirement status, smoking, alcohol drinking, leisure-time physical activity, BMI, SBP, DBP and history of cardiovascular diseases except the corresponding stratification variable
In sensitivity analysis, similar results on the association between HTGW phenotype and T2DM risk were found when defining WC ≥ 80 cm in women as enlarged waist circumference (Additional file 1: Table S6). In addition, additional adjustment for total cholesterol and LDL-C did not alter the results remarkably (Additional file 1: Table S7); and similar results were also found by using different definition of hypertension (Additional file 1: Table S8).
Discussion
The present study demonstrated that among hypertensive patients, HTGW was associated with an increased T2DM risk, and TyG, LAP, and VAI were positively associated with the T2DM risk. Furthermore, our study indicated that HTGW was a stable risk factor for T2DM in hypertension patients regardless of any characteristics; and the positive association of TyG index with T2DM was more pronounced in participants < 60 years and in non-retirees.
The hypertriglyceridemic-waist phenotype, defined as elevated TG and enlarged WC, was a simple marker of visceral adiposity [15]. Previous studies indicated that HTGW was associated with higher risk of diabetes, hypertension, and cardiovascular diseases [20–22, 40, 41]. A meta-analysis further indicated that HTGW was closely associated with an increased risk of diabetes in the general population [20]. In the present study, we further found that HTGW was associated with a higher risk of diabetes among hypertensive patients, which is consistent with the results in the general population [20]. Our study showed that the risk effect of HTNW on T2DM was better than subjects with NTGW, which is consistent with several studies [42, 43]; however, some studies indicated that NTGW had a stronger association with T2DM than NTGW [21, 23]. The discrepancies among the studies may be due to the heterogeneity of study designs and study populations. Meanwhile, we found that HTGW was associated with a higher diabetes risk than solely-increased TG or solely-increased WC, which further supported previous studies pointing that participants’ cardiovascular disease risk depended jointly on their body size and metabolic profile [44, 45]. Furthermore, our study indicated that HTGW was a stable risk factor for diabetes in all stratified analyses, implicating that hypertriglyceridemic-waist phenotype might be a good and stable indicator of visceral adiposity in hypertensive patients.
The TyG index, derived from TG and FPG, has been used as a marker to identify insulin resistance in many epidemiological studies. A study conducted in the Mexican population showed that the TyG index performed high sensitivity (96.5%) and specificity (85.0%), compared with the hyperinsulinemic-euglycemic clamp test [16]; another study conducted in the Brazilian population indicated that the TyG index was better than homeostasis model assessment in identifying patients with insulin resistance [46]; a real-world study suggested that compared with homeostasis model assessment for insulin resistance, the TyG index is more strongly associated with arterial stiffness in T2DM patients [47]. A recent meta-analysis included 14 studies showed that TyG index was associated with an increased risk of T2DM in a nonlinear fashion, which is consistent with our study [24]. Li et al. also found that TyG index was positively associated with the risk of diabetes in the subjects with SBP ≥ 140 mmHg and DBP ≥ 90 mmHg after conducting subgroup analyses among Chinese [48]. In our study, we found that the positive relationship between TyG index and T2DM risk was consistent in all subgroups among hypertension patients, and seemed to be more evident in younger subjects or in non-retirees. This result was reasonable because the retirees tended to be older and with poorer physical functioning [49]. Based on the stratified analysis, our findings suggested that among hypertension patients, the TyG index may be promising for screening risk of T2DM, especially in subjects without high-risk factors, such as retirement and older age, which expanded the results of previous study conducted in apparently healthy adults [48].
The LAP was reported as a reliable visceral adiposity index combining WC and TG levels [18]. Several studies have found that LAP was a better predictor of metabolic syndrome and diabetes than the common obesity index [50–52]. A recent cohort study conducted among 15,717 Chinese participants showed that high LAP was associated with high risk of developing T2DM, suggesting that LAP may be a potential indicator for T2DM [26]. Similar results were also found in Japanese and Korean populations [53, 54]. Our study indicated that the positive relationship between LAP and T2DM risk was in a nonlinear fashion among hypertension patients, which is also consistent with prior evidence conducted in the general populations [26]. The VAI is another reliable lipid index to estimate insulin resistance from visceral adiposity dysfunction [19]. A meta-analysis included ten observational studies involving 112,603 participants found that VAI may be related to a high risk of prediabetes [55]. A recent meta-analysis included nine cohort studies showed that each 1-unit increment of VAI was associated with 42% higher risk of T2DM [25]. Yu et al. also conducted a cohort analysis and found that high-VAI level was associated with increased risk of new-onset T2DM [56]. Expanded the notions of previous studies, our study indicated that increased LAP and VAI levels were associated with increased risk of T2DM among subjects with hypertension, and these positive relationships persisted in all stratification variables, which suggested that LAP and VAI may serve as early markers of T2DM risk in hypertensive patients.
Although the exact mechanisms for the relationship of these indicators with T2DM among hypertensive patients are unclear, several hypotheses were proposed. Increased visceral adipose is linked to an increased risk of insulin resistance and β-cell dysfunction [55]. As the markers of visceral adiposity, increased LAP, VAI, and enlarged WC could promote the accumulation of various inflammatory cytokines, which could accelerate the progression of insulin resistance and glucose intolerance and cause the development of diabetes directly [57]. Additionally, excessive adipose tissue could produce excessive free fatty acids, which may deteriorate insulin sensitivity and exacerbate T2DM risk subsequently [58]. Moreover, the increased TG level in the blood could lead to the inhibited insulin activity and muscle catabolism, and overloaded TG in the pancreatic islet cells could also lead to β-cell dysfunction [59]. And elevated glucose concentrations could raise the level of reactive oxygen species and exert toxic effects on β-cells [60]. High TyG index, as the product of TG and FPG, reflected decreased β-cells and the increased IR, which contributed to the development of T2DM [61]. As a common comorbidity of diabetes, hypertension is a major risk factor for T2DM [62]. Our analyses demonstrated that HTGW, TyG, LAP, and VAI were positively associated with T2DM, even when the subjects recruited were limited to those with high risk of T2DM, which further confirmed the possibility that these indicators may serve as novel and simple non-invasive markers to evaluate the risk of T2DM. More mechanistic researches are needed to reveal the role of these indicators in the development of diabetes among hypertensive patients.
Our study has several strengths. First, the present study examined the associations of four novel indicators with the risk of T2DM among a large number of hypertensive participants, and thus, the results are specifically applicable and dependable for the participants with hypertension. Second, we have taken TyG, LAP, and VAI as both continuous variable and categorical variable when doing the analysis, and sensitivity analysis and trend examination were performed to improve the reliability of the results. Finally, we conducted subgroup analyses and interaction test to enhance the credibility of the results and identify potential interactions with other variables.
There are also some limitations. First, this is a cross-sectional study, which limited the ability of determining the direction of the association and inferring a causal association. Second, the diagnosis of T2DM was determined by self-reported or fasting blood glucose. A lack of oral glucose tolerance test and determination of glycosylated haemoglobin might underestimate the strength of association. Third, all the participants included in the present study were Chinese adults with hypertension, which made it more useful for specific patients.
In conclusion, the results found that among hypertensive patients, HTGW and higher level of TyG, LAP, and VAI were associated with the elevated risk of T2DM. The findings suggest that HTGW, TyG, LAP, and VAI may serve as simple and effective tools for T2DM risk assessment in the prevention and management of main chronic diseases.
Supplementary Information
Additional file 1: Figure S1. Flow chart of selection of hypertensive patients. Table S1. Association between lipid indicators and the risk of T2DM. Table S2. Stratified analyses on the association between triglyceridemic-waist phenotypes and the risk of T2DM. Table S3. Stratified analyses on the association between triglyceride glucose index and the risk of T2DM. Table S4. Stratified analyses on the association between lipid accumulation product and the risk of T2DM. Table S5. Stratified analyses on the association between visceral adiposity index and the risk of T2DM. Table S6. Association between triglyceridemic-waist phenotypes and the risk of T2DM by defining WC ≥ 80 cm in women as enlarged waist circumference. Table S7. Association between lipid indices and the risk of T2DM with additional adjustment for total cholesterol and LDL-C. Table S8. Association between lipid indicators and the risk of T2DM by using different definition of hypertension.
Acknowledgements
The author would like to thank epidemiologists, nurses, and doctors in Guangdong Provincial People’s Hospital, in Guangzhou Center for Disease Control and Prevention, and in community healthcare centers in data collection, and thank all study subjects for their participation.
Abbreviations
- BMI
Body mass index
- CI
Confidence interval
- DBP
Diastolic blood pressure
- FPG
Fasting plasma glucose
- HDL-C
High-density lipoprotein cholesterol
- HTGW
Elevated triglyceride level and enlarged waist circumference
- HTNW
Elevated triglyceride level and normal waist circumference
- IQR
Interquartile range
- LAP
Lipid accumulation product
- LDL-C
Low-density lipoprotein cholesterol
- NTNW
Normal triglyceride level and normal waist circumference
- NTGW
Normal triglyceride level and enlarged waist circumference
- OR
Odds ratios
- SBP
Systolic blood pressure
- SD
Standard deviation
- T2DM
Type 2 diabetes mellitus
- TG
Triglyceride
- TyG index
Triglyceride glucose index
- VAI
Visceral adiposity index
- WC
Waist circumference
Author contributions
XL conceived the study; XL, WZ and MZ supervised the study; XW, MY, MZ, HD, CW, HL, ML, XD and HZ collected the data, PH analyzed the data, PH and HD drafted the manuscript, XQL, WZ, XL, MZ reviewed and edited the manuscript. All co-authors provided comments and approved the final version. All authors read and approved the final manuscript.
Funding
This work was supported by the Science and Technology Program of Guangzhou City (No. 202102080404), and the Guangdong Basic and Applied Basic Research Foundation (No. 2022A1515010686), the Guangdong Provincial Key R&D Program (No. 2019B020230004) and the National Key R&D Program of China (No. 2018YFC1312502).
Availability of data and materials
The data used to support the findings of this study are available from the corresponding author upon request. A proposal with detailed description of study objectives and statistical analysis plan will be needed for evaluation of the reasonability of requests if someone requests data sharing.
Declarations
Ethics approval and consent to participate
This study was approved by the Ethical Review Committee for Biomedical Research, School of Public Health, Sun Yat-sen University and the Research Ethics Committee for Guangdong Provincial People’s Hospita1, Guangdong Academy of Medical Sciences. The study was performed in accordance with the Declaration of Helsinki and written informed consent was obtained from each participant before they joined in the study.
Consent for publication
All co-authors provided comments and approved the final version.
Competing interests
The authors state that they have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hai Deng, Peng Hu, Huoxing Li, and Huanning Zhou have equal contribution
Contributor Information
Murui Zheng, Email: zhengmr@163.com.
Wenjing Zhao, Email: zhaowj@sustech.edu.cn.
Xudong Liu, Email: xdliu.cn@hotmail.com.
References
- 1.Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet (London, England). 2018; 392(10159):1923–1994. [DOI] [PMC free article] [PubMed]
- 2.Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet (London, England) 2010;375(9733):2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cunningham SA, Riosmena F, Wang J, Boyle JP, Rolka DB, Geiss LS. Decreases in diabetes-free life expectancy in the U.S. and the role of obesity. Diabetes Care. 2011;34(10):2225–2230. doi: 10.2337/dc11-0462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Wang DD, Ley SH, Vasanti M, Howard AG, He Y, Hu FB. Time trends of dietary and lifestyle factors and their potential impact on diabetes burden in China. Diabetes Care. 2017;40(12):1685–1694. doi: 10.2337/dc17-0571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.IDF Diabetes Atlas [Internet]. 10th edition. Brussels: International Diabetes Federation; 2021. Available from https://www.diabetesatlas.org.
- 7.Neeland IJ, Turer AT, Ayers CR, Powell-Wiley TM, Vega GL, Farzaneh-Far R, Grundy SM, Khera A, McGuire DK, de Lemos JA. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. JAMA. 2012;308(11):1150–1159. doi: 10.1001/2012.jama.11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheung AS, de Rooy C, Hoermann R, Gianatti EJ, Hamilton EJ, Roff G, Zajac JD, Grossmann M. Correlation of visceral adipose tissue measured by Lunar Prodigy dual X-ray absorptiometry with MRI and CT in older men. Int J Obes (2005) 2016;40(8):1325–1328. doi: 10.1038/ijo.2016.50. [DOI] [PubMed] [Google Scholar]
- 9.Tam CS, Xie W, Johnson WD, Cefalu WT, Redman LM, Ravussin E. Defining insulin resistance from hyperinsulinemic-euglycemic clamps. Diabetes Care. 2012;35(7):1605–1610. doi: 10.2337/dc11-2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bichteler A, Barton JM, Lumeng JC, Gershoff ET. Patterns of childhood body mass index percentile gains as predictors of adolescent body mass index, waist circumference, and blood pressure. Acad Pediatr. 2022;22(5):769–776. doi: 10.1016/j.acap.2021.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopez-Lopez JP, Cohen DD, Ney-Salazar D, Martinez D, Otero J, Gomez-Arbelaez D, Camacho PA, Sanchez-Vallejo G, Arcos E, Narvaez C, et al. The prediction of metabolic syndrome alterations is improved by combining waist circumference and handgrip strength measurements compared to either alone. Cardiovasc Diabetol. 2021;20(1):68. doi: 10.1186/s12933-021-01256-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ke JF, Wang JW, Lu JX, Zhang ZH, Liu Y, Li LX. Waist-to-height ratio has a stronger association with cardiovascular risks than waist circumference, waist-hip ratio and body mass index in type 2 diabetes. Diabetes Res Clin Pract. 2022;183:109151. doi: 10.1016/j.diabres.2021.109151. [DOI] [PubMed] [Google Scholar]
- 13.Fu L, Zhou Y, Sun J, Zhu Z, Xing Z, Zhou S, Wang Y, Tai S. Atherogenic index of plasma is associated with major adverse cardiovascular events in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2021;20(1):201. doi: 10.1186/s12933-021-01393-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caiano LM, Costanzo S, Panzera T, Di Castelnuovo A, de Gaetano G, Donati MB, Ageno W, Iacoviello L, Moli-sani Study I Association between body mass index, waist circumference, and relative fat mass with the risk of first unprovoked venous thromboembolism. Nutr Metab Cardiovasc Dis NMCD. 2021;31(11):3122–3130. doi: 10.1016/j.numecd.2021.07.018. [DOI] [PubMed] [Google Scholar]
- 15.Lemieux I, Pascot A, Couillard C, Lamarche B, Tchernof A, Alméras N, Bergeron J, Gaudet D, Tremblay G, Prud'homme D, et al. Hypertriglyceridemic waist: a marker of the atherogenic metabolic triad (hyperinsulinemia; hyperapolipoprotein B; small, dense LDL) in men? Circulation. 2000;102(2):179–184. doi: 10.1161/01.CIR.102.2.179. [DOI] [PubMed] [Google Scholar]
- 16.Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, Martínez-Abundis E, Ramos-Zavala MG, Hernández-González SO, Jacques-Camarena O, Rodríguez-Morán M. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95(7):3347–3351. doi: 10.1210/jc.2010-0288. [DOI] [PubMed] [Google Scholar]
- 17.Lee SH, Han K, Yang HK, Kim MK, Yoon KH, Kwon HS, Park YM. Identifying subgroups of obesity using the product of triglycerides and glucose: the Korea National Health and Nutrition Examination Survey, 2008–2010. Clin Endocrinol (Oxf) 2015;82(2):213–220. doi: 10.1111/cen.12502. [DOI] [PubMed] [Google Scholar]
- 18.Kahn HS. The lipid accumulation product is better than BMI for identifying diabetes: a population-based comparison. Diabetes Care. 2006;29(1):151–153. doi: 10.2337/diacare.29.01.06.dc05-1805. [DOI] [PubMed] [Google Scholar]
- 19.Amato MC, Giordano C, Galia M, Criscimanna A, Vitabile S, Midiri M, Galluzzo A. Visceral Adiposity Index: a reliable indicator of visceral fat function associated with cardiometabolic risk. Diabetes Care. 2010;33(4):920–922. doi: 10.2337/dc09-1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma CM, Liu XL, Lu N, Wang R, Lu Q, Yin FZ. Hypertriglyceridemic waist phenotype and abnormal glucose metabolism: a system review and meta-analysis. Endocrine. 2019;64(3):469–485. doi: 10.1007/s12020-019-01945-6. [DOI] [PubMed] [Google Scholar]
- 21.Ren Y, Zhang M, Zhao J, Wang C, Luo X, Zhang J, Zhu T, Li X, Yin L, Pang C, et al. Association of the hypertriglyceridemic waist phenotype and type 2 diabetes mellitus among adults in China. J Diabetes Investig. 2016;7(5):689–694. doi: 10.1111/jdi.12489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ren Y, Luo X, Wang C, Yin L, Pang C, Feng T, Wang B, Zhang L, Li L, Yang X, et al. Prevalence of hypertriglyceridemic waist and association with risk of type 2 diabetes mellitus: a meta-analysis. Diabetes Metab Res Rev. 2016;32(4):405–412. doi: 10.1002/dmrr.2725. [DOI] [PubMed] [Google Scholar]
- 23.Chen D, Liang Z, Sun H, Lu C, Chen W, Wang HHX, Guo VY. Association between hypertriglyceridemic-waist phenotype and risk of type 2 diabetes mellitus in middle-aged and older Chinese population: a longitudinal cohort study. Int J Environ Res Public Health. 2021;18(18):9618. doi: 10.3390/ijerph18189618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pranata R, Huang I, Irvan, Lim MA, Vania R The association between triglyceride-glucose index and the incidence of type 2 diabetes mellitus—a systematic review and dose-response meta-analysis of cohort studies. Endocrine. 2021; 74(2):254–262. [DOI] [PubMed]
- 25.Jayedi A, Soltani S, Motlagh SZ, Emadi A, Shahinfar H, Moosavi H, Shab-Bidar S. Anthropometric and adiposity indicators and risk of type 2 diabetes: systematic review and dose-response meta-analysis of cohort studies. BMJ (Clin Res Ed) 2022;376:e067516. doi: 10.1136/bmj-2021-067516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu M, Huang M, Qiang D, Gu J, Li Y, Pan Y, Yao X, Xu W, Tao Y, Zhou Y, et al. Hypertriglyceridemic waist phenotype and lipid accumulation product: two comprehensive obese indicators of waist circumference and triglyceride to predict type 2 diabetes mellitus in Chinese population. J Diabetes Res. 2020;2020:9157430. doi: 10.1155/2020/9157430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayashi T, Tsumura K, Suematsu C, Endo G, Fujii S, Okada K. High normal blood pressure, hypertension, and the risk of type 2 diabetes in Japanese men. The Osaka Health Survey. Diabetes Care. 1999;22(10):1683–1687. doi: 10.2337/diacare.22.10.1683. [DOI] [PubMed] [Google Scholar]
- 28.Yang X, Chen J, Pan A, Wu JHY, Zhao F, Xie Y, Wang Y, Ye Y, Pan XF, Yang CX. Association between higher blood pressure and risk of diabetes mellitus in middle-aged and elderly Chinese adults. Diabetes Metab J. 2020;44(3):436–445. doi: 10.4093/dmj.2019.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mordi I, Mordi N, Delles C, Tzemos N. Endothelial dysfunction in human essential hypertension. J Hypertens. 2016;34(8):1464–1472. doi: 10.1097/HJH.0000000000000965. [DOI] [PubMed] [Google Scholar]
- 30.Hu FB, Meigs JB, Li TY, Rifai N, Manson JE. Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes. 2004;53(3):693–700. doi: 10.2337/diabetes.53.3.693. [DOI] [PubMed] [Google Scholar]
- 31.Deng H, Guo P, Zheng M, Huang J, Xue Y, Zhan X, Wang F, Liu Y, Fang X, Liao H, et al. Epidemiological characteristics of atrial fibrillation in southern China: results from the Guangzhou Heart Study. Sci Rep. 2018;8(1):17829. doi: 10.1038/s41598-018-35928-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duan X, Huang J, Zheng M, Zhao W, Lao L, Li H, Wang Z, Lu J, Chen W, Deng H, et al. Association of healthy lifestyle with risk of obstructive sleep apnea: a cross-sectional study. BMC Pulm Med. 2022;22(1):33. doi: 10.1186/s12890-021-01818-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duan X, Zheng M, He S, Lao L, Huang J, Zhao W, Lao XQ, Deng H, Liu X. Association between physical activity and risk of obstructive sleep apnea. Sleep Breathing Schlaf Atmung. 2021;25(4):1925–1934. doi: 10.1007/s11325-021-02318-y. [DOI] [PubMed] [Google Scholar]
- 34.Hu P, Zheng M, Huang J, Zhao W, Wang HHX, Zhang X, Chen Y, Deng H, Qin P, Liu X. Association of habitual physical activity with the risk of all-cause mortality among Chinese adults: a prospective cohort study. Front Public Health. 2022;10:919306. doi: 10.3389/fpubh.2022.919306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu P, Zheng M, Huang J, Fan HY, Fan CJ, Ruan HH, Yuan YS, Zhao W, Wang HHX, Deng H, et al. Effect of healthy lifestyle index and lifestyle patterns on the risk of mortality: a community-based cohort study. Front Med. 2022;9:920760. doi: 10.3389/fmed.2022.920760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Classification of diabetes mellitus 2019. https://www.who.int/publications/i/item/classification-of-diabetes-mellitus.
- 37.Bao Y, Lu J, Wang C, Yang M, Li H, Zhang X, Zhu J, Lu H, Jia W, Xiang K. Optimal waist circumference cutoffs for abdominal obesity in Chinese. Atherosclerosis. 2008;201(2):378–384. doi: 10.1016/j.atherosclerosis.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 38.China adult dyslipidemia control guidelines revision joint committee: Guidelines for the prevention and treatment of dyslipidemia in Chinese adults (revised edition, 2016). Chin Circul J. 2016; 31(10).
- 39.Wang B, Zhang M, Liu Y, Sun X, Zhang L, Wang C, Li L, Ren Y, Han C, Zhao Y, et al. Utility of three novel insulin resistance-related lipid indices for predicting type 2 diabetes mellitus among people with normal fasting glucose in rural China. J Diabetes. 2018;10(8):641–652. doi: 10.1111/1753-0407.12642. [DOI] [PubMed] [Google Scholar]
- 40.Xuan Y, Shen Y, Wang S, Gao P, Gu X, Tang D, Wang X, Zhu F, Lu L, Chen L. The association of hypertriglyceridemic waist phenotype with hypertension: a cross-sectional study in a Chinese middle aged-old population. J Clin Hypertens (Greenwich) 2022;24(2):191–199. doi: 10.1111/jch.14424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang A, Li Z, Zhou Y, Wang C, Luo Y, Liu X, Guo X, Wu S, Zhao X. Hypertriglyceridemic waist phenotype and risk of cardiovascular diseases in China: results from the Kailuan Study. Int J Cardiol. 2014;174(1):106–109. doi: 10.1016/j.ijcard.2014.03.177. [DOI] [PubMed] [Google Scholar]
- 42.Du T, Sun X, Huo R, Yu X. Visceral adiposity index, hypertriglyceridemic waist and risk of diabetes: the China Health and Nutrition Survey 2009. Int J Obes (2005) 2014;38(6):840–847. doi: 10.1038/ijo.2013.181. [DOI] [PubMed] [Google Scholar]
- 43.Ren YC, Liu Y, Sun XZ, Wang BY, Liu Y, Ni H, Zhao Y, Liu D, Liu X, Zhang D, et al. Prevalence and relationship of hypertriglyceridaemic-waist phenotype and type 2 diabetes mellitus among a rural adult Chinese population. Public Health Nutr. 2019;22(8):1361–1366. doi: 10.1017/S1368980019000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kip KE, Marroquin OC, Kelley DE, Johnson BD, Kelsey SF, Shaw LJ, Rogers WJ, Reis SE. Clinical importance of obesity versus the metabolic syndrome in cardiovascular risk in women: a report from the Women's Ischemia Syndrome Evaluation (WISE) study. Circulation. 2004;109(6):706–713. doi: 10.1161/01.CIR.0000115514.44135.A8. [DOI] [PubMed] [Google Scholar]
- 45.Katzmarzyk PT, Janssen I, Ross R, Church TS, Blair SN. The importance of waist circumference in the definition of metabolic syndrome: prospective analyses of mortality in men. Diabetes Care. 2006;29(2):404–409. doi: 10.2337/diacare.29.02.06.dc05-1636. [DOI] [PubMed] [Google Scholar]
- 46.Vasques AC, Novaes FS, de Oliveira MS, Souza JR, Yamanaka A, Pareja JC, Tambascia MA, Saad MJ, Geloneze B. TyG index performs better than HOMA in a Brazilian population: a hyperglycemic clamp validated study. Diabetes Res Clin Pract. 2011;93(3):e98–e100. doi: 10.1016/j.diabres.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 47.Wang S, Shi J, Peng Y, Fang Q, Mu Q, Gu W, Hong J, Zhang Y, Wang W. Stronger association of triglyceride glucose index than the HOMA-IR with arterial stiffness in patients with type 2 diabetes: a real-world single-centre study. Cardiovasc Diabetol. 2021;20(1):82. doi: 10.1186/s12933-021-01274-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li X, Li G, Cheng T, Liu J, Song G, Ma H. Association between triglyceride-glucose index and risk of incident diabetes: a secondary analysis based on a Chinese cohort study: TyG index and incident diabetes. Lipids Health Dis. 2020;19(1):236. doi: 10.1186/s12944-020-01403-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nilsen C, Agahi N, Kåreholt I. Work stressors in late midlife and physical functioning in old age. J Aging Health. 2017;29(5):893–911. doi: 10.1177/0898264316654673. [DOI] [PubMed] [Google Scholar]
- 50.Ray L, Ravichandran K, Nanda SK. Comparison of lipid accumulation product index with body mass index and waist circumference as a predictor of metabolic syndrome in Indian population. Metab Syndr Relat Disord. 2018;16(5):240–245. doi: 10.1089/met.2017.0119. [DOI] [PubMed] [Google Scholar]
- 51.Song J, Chen X, Jiang Y, Mi J, Zhang Y, Zhao Y, Wu X, Gao H. Association and interaction analysis of lipid accumulation product with impaired fasting glucose risk: a cross-sectional survey. J Diabetes Res. 2019;2019:9014698. doi: 10.1155/2019/9014698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tian T, Pei H, Chen Z, Hailili G, Wang S, Sun Y, Yao H, Jianghong D. Comparison of lipid accumulation product and body mass index as indicators of diabetes diagnosis among 215,651 Chinese adults. PeerJ. 2020;8:e8483. doi: 10.7717/peerj.8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wakabayashi I, Daimon T. A strong association between lipid accumulation product and diabetes mellitus in Japanese women and men. J Atheroscler Thromb. 2014;21(3):282–288. doi: 10.5551/jat.20628. [DOI] [PubMed] [Google Scholar]
- 54.Lee JW, Lim NK, Park HY. The product of fasting plasma glucose and triglycerides improves risk prediction of type 2 diabetes in middle-aged Koreans. BMC Endocr Disord. 2018;18(1):33. doi: 10.1186/s12902-018-0259-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang D, Fang R, Han H, Zhang J, Chen K, Fu X, He Q, Yang Y. Association between visceral adiposity index and risk of prediabetes: a meta-analysis of observational studies. J Diabetes Investig. 2022;13(3):543–551. doi: 10.1111/jdi.13685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu J, Yi Q, Chen G, Hou L, Liu Q, Xu Y, Qiu Y, Song P. The visceral adiposity index and risk of type 2 diabetes mellitus in China: a national cohort analysis. Diabetes Metab Res Rev. 2022;38(3):e3507. doi: 10.1002/dmrr.3507. [DOI] [PubMed] [Google Scholar]
- 57.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444(7121):840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 58.Papaetis GS, Papakyriakou P, Panagiotou TN. Central obesity, type 2 diabetes and insulin: exploring a pathway full of thorns. Arch Med Sci AMS. 2015;11(3):463–482. doi: 10.5114/aoms.2015.52350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Unger RH. Lipotoxicity in the pathogenesis of obesity-dependent NIDDM. Genetic and clinical implications. Diabetes. 1995;44(8):863–870. doi: 10.2337/diab.44.8.863. [DOI] [PubMed] [Google Scholar]
- 60.Robertson RP, Harmon J, Tran PO, Poitout V. Beta-cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes. 2004;53(Suppl 1):S119–124. doi: 10.2337/diabetes.53.2007.S119. [DOI] [PubMed] [Google Scholar]
- 61.Alejandro EU, Gregg B, Blandino-Rosano M, Cras-Méneur C, Bernal-Mizrachi E. Natural history of β-cell adaptation and failure in type 2 diabetes. Mol Aspects Med. 2015;42:19–41. doi: 10.1016/j.mam.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wu Y, Hu H, Cai J, Chen R, Zuo X, Cheng H, Yan D. Association of hypertension and incident diabetes in Chinese adults: a retrospective cohort study using propensity-score matching. BMC Endocr Disord. 2021;21(1):87. doi: 10.1186/s12902-021-00747-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Flow chart of selection of hypertensive patients. Table S1. Association between lipid indicators and the risk of T2DM. Table S2. Stratified analyses on the association between triglyceridemic-waist phenotypes and the risk of T2DM. Table S3. Stratified analyses on the association between triglyceride glucose index and the risk of T2DM. Table S4. Stratified analyses on the association between lipid accumulation product and the risk of T2DM. Table S5. Stratified analyses on the association between visceral adiposity index and the risk of T2DM. Table S6. Association between triglyceridemic-waist phenotypes and the risk of T2DM by defining WC ≥ 80 cm in women as enlarged waist circumference. Table S7. Association between lipid indices and the risk of T2DM with additional adjustment for total cholesterol and LDL-C. Table S8. Association between lipid indicators and the risk of T2DM by using different definition of hypertension.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request. A proposal with detailed description of study objectives and statistical analysis plan will be needed for evaluation of the reasonability of requests if someone requests data sharing.