Abstract
Background: Dental caries commonly occurs during orthodontic treatment because fixed appliances can impede effective oral hygiene practices. This study investigated the effects of fixed orthodontic treatment on dental biofilm maturity and virulence gene (gtfB, ldh, brpA, spaP, luxS, and gbpB) expression. Methods: Dental biofilms and virulence gene expression were determined in 24 orthodontic patients before and after treatment of ≥6 months. A three-tone disclosing gel was used to stain dental biofilm and assess its maturity by its color change—pink (new dental biofilm), purple (mature dental biofilm), and light blue (cariogenic dental biofilm). Gene expression levels were determined using real-time PCR. Results: After fixed orthodontic appliance insertion, the percentage of new dental biofilm decreased, whereas that of cariogenic dental biofilm significantly increased (p < 0.05). There was no significant difference in the percentage of mature dental biofilm (p > 0.05). Fixed orthodontic appliances increased gtfB, ldh, brpA, and gbpB gene expression above 1.5-fold in dental biofilm. In contrast, there was no change in spaP or luxS gene expression after treatment. Conclusions: Fixed orthodontic appliance insertion induced ecological changes and cariogenic virulence gene expression in dental biofilm.
Keywords: brackets, cariogenicity, dental biofilm, dental caries, fixed orthodontic appliances, oral health, virulence genes
1. Introduction
Dental caries is one of the most common public health problems and is induced by multiple risk factors involving dental biofilm (also known as dental plaque), diet habits, oral hygiene, socioeconomic status, and related genetic disorders such as molar/incisor hypomineralization [1]. Dental caries occurs due to a substrate disruption of the bacterial community, as described in the “ecological plaque hypothesis”. The ecological plaque hypothesis proposes that any major changes in local environmental conditions, e.g., high sucrose consumption, induces the colonization of cariogenic bacteria in the dental biofilm and results in cariogenic dental biofilm formation [2]. In addition to focusing on specific bacteria, the bacterial virulence/function and dental biofilm ecology should be considered when assessing the cariogenicity of dental biofilm [3,4].
Dental caries is commonly observed in patients undergoing orthodontic treatment [5]. Fixed orthodontic appliances, including brackets, springs, and arch wires, impede access to the tooth surface, making it difficult to remove dental biofilm by mechanical cleaning [6,7]. A systematic review revealed that the presence of fixed appliances influences the quantity and quality of the oral microbiota. These appliances create an ecological environment favorable to qualitative and quantitative changes in dental biofilm microorganisms [8]. The number of cariogenic bacteria, including Streptococcus mutans and lactobacilli, increases in the dental biofilm on teeth with fixed orthodontic appliances [9].
S. mutans is an oral bacterium belonging to the mutans streptococci (MS) group. It is an important bacterium that participates in dental biofilm formation and dental caries. Virulence genes in S. mutans can be classified into genes involved in bacterial adhesion, extracellular polysaccharide formation, dental biofilm formation, sugar uptake and metabolism, acid tolerance, and biofilm regulation mechanisms [10]. The genes involved in bacterial adhesion are gbps (gbpA, gbpB, and gbpC) and spaP. The gbp genes encode glucan-binding proteins (GBP) [11]. The spaP gene encodes the cell surface antigen (SpaP), which is the adhesion protein mediating early bacterial attachment in dental biofilm [12]. S. mutans contains gtfB, gtfC, and gtfD genes, each of which encodes glucosyltransferases (GTFs). GTFs convert sucrose to glucans. Glucans mediate sucrose-dependent adherence of S. mutans to the tooth surface, and act as a component of the dental biofilm matrix [13]. In S. mutans, ldh encodes lactate dehydrogenase (LDH), which is involved in lactic acid production [14]. The brpA gene regulates S. mutans cell envelope integrity [15], biofilm formation, and acid tolerance [16,17]. The luxS gene regulates quorum sensing within dental biofilm and regulates cellular metabolism, DNA repair, and stress tolerance in S. mutans [18,19].
Various methods have been used to assess caries risk, including dental biofilm staining, bacterial culture, and molecular biology techniques, such as polymerase chain reaction (PCR). Among these methods, PCR has been preferentially used to detect cariogenic bacteria and/or putative MS virulence genes because it is quick, sensitive, and simple [10,20].
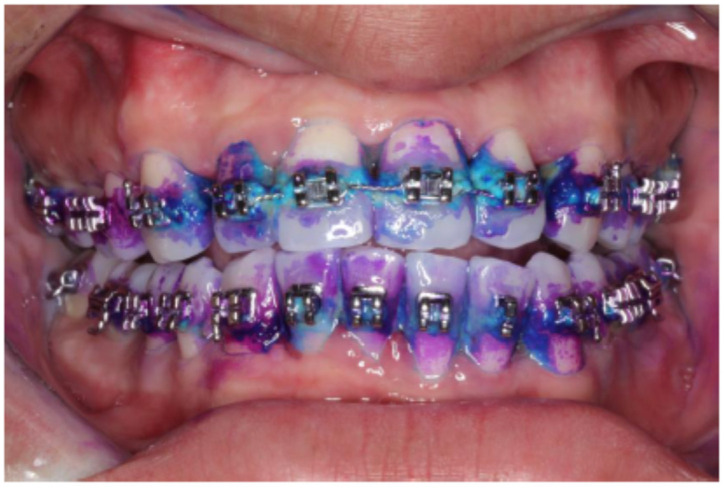
Assessing dental biofilm is difficult for patients and dentists because the tooth and dental plaque often appear similar, especially if dental biofilm is present in small amounts. The dental biofilm is typically detected by clinicians, either by directly using an explorer or with a disclosing solution, and is quantified using indices based on the area of the tooth covered or its thickness [21,22]. Furthermore, most orthodontic trials have used plaque indices that scored the extent and thickness of dental biofilm as ordinal scales [23,24]. Therefore, in previous studies, the usefulness of a dental biofilm index for patients with fixed orthodontic appliances depended on the subjective evaluation of the extent of stained dental biofilm by the dentist. Furthermore, the disclosing agents previously used to stain mature and newly formed dental biofilm lack specificity. These assessment methods have a limitation of being subjective; therefore, the results may vary between clinicians, especially when little dental biofilm is present. Thus, there is a need to more precisely evaluate the dental biofilm in orthodontic patients [23,25]. A three-tone plaque disclosing gel (GC Tri Plaque ID GelTM) was developed to identify the caries risk in individuals [26]. This gel contains red and blue pigments, and sucrose. The new dental biofilm is stained pink/red because its structure is sparse, and the blue pigment easily washes off. In contrast, the mature dental biofilm (>48 h) structure is dense; thus, the blue and red pigments are trapped, resulting in blue/purple staining. In the acid-producing mature dental biofilm, the sucrose in the disclosing gel is metabolized by acidogenic bacteria in the dental biofilm. When the dental biofilm pH drops to <pH 4.5, the dental biofilm becomes light blue. In addition, the light blue-stained dental biofilm has been found to have high levels of S. mutans [27]. Therefore, the light blue dental biofilm is cariogenic dental biofilm, which is the most virulent, followed by the blue/purple, and pink dental biofilm.
Currently, there is no evidence supporting the use of three-tone plaque disclosing gel to assess the cariogenic status of dental biofilm in orthodontic patients. The present study used a three-tone disclosing agent and investigated the virulence-related gene expression by real-time PCR, to determine dental biofilm cariogenicity in fixed orthodontic patients. In this study, we tested the hypothesis that fixed orthodontic appliances affect the cariogenicity (primary outcome) and virulence of dental biofilm (secondary outcome). Therefore, the aim of the present study was to investigate the effect of orthodontic treatment on dental biofilm maturity and virulence gene expression levels, using a three-tone plaque disclosing gel and real-time PCR, respectively. The association between the virulence genes of cariogenic bacteria and the cariogenicity of dental biofilm was also evaluated.
2. Materials and Methods
2.1. Participants and Background
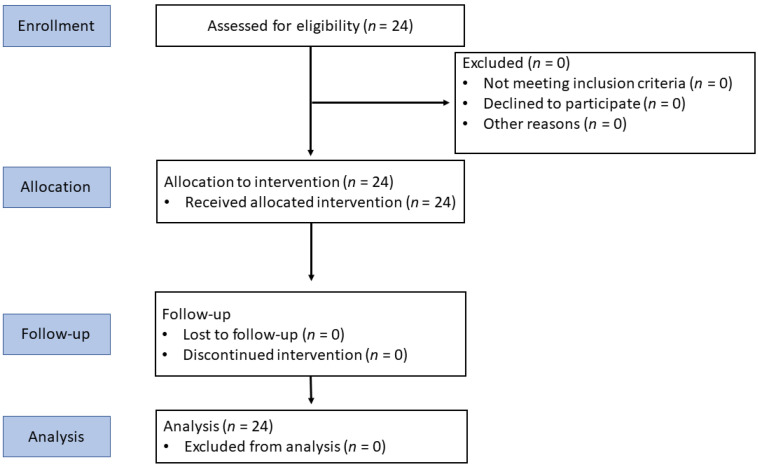
This study received ethical approval from the Human Research Ethics Committee of Walailak University, Thailand (no. WUEC-20-225-01/2), on 10 August 2020. The principles of the Declaration of Helsinki were followed in the present study [28]. This study was a nonrandomized open label trial (Figure 1). The patients were ≥ 12-year-old individuals, who were undergoing fixed orthodontic treatment at the Center for Advanced Oral Health, Walailak University. The exclusion criteria were having a systemic disease or being on a long-term/recent/current regimen of medications affecting salivary flow, receiving antibiotic therapy within the previous 6 months, and/or severe periodontal problems. Written consent was obtained from the participants or a parent of the participants under 16 years old on the first day of the study. The participants were recruited using a purposive sampling method, based on the inclusion and exclusion criteria, which resulted in 24 subjects.
Figure 1.
Flow diagram. Twenty-four patients completed the full study protocol.
2.2. Sample Size Calculation
The sample size calculation was performed to detect the effect of orthodontic treatments on the primary outcome (dental biofilm maturity) [29,30] using G*power 3.1.9.4. (Heinrich-Heine University, Dusseldorf, Germany). Because there were no exact effect size values in previous reports, an estimation of the effect size was computed using Cohen’s d to obtain the standardized effect size [31]. The paired t-test was used to detect the effects of fixed orthodontic treatment on dental biofilm maturity (power of 0.8, α error of 0.05, and Cohen’s d of 0.8). This calculation determined that a minimum of 15 patients were required. To compensate for dropouts, 24 patients were enrolled in this study.
2.3. Questionnaire and Food Diary
Interview questionnaires were used to obtain information about the patients’ demographic characteristics, orthodontic treatment duration, and oral hygiene practice. A 3-day food diary was distributed to each patient. The patients were required to record their food intake in the food diary on two weekdays and one weekend day [32]. In the present study, the patients were educated and motivated to enter everything that he/she consumed from morning until bedtime in the chart. They were also requested to include any medications, chewing gum, and cough lozenges. The chart was analyzed after orthodontic treatment for at least 6 months by the dentist–patient duo for the frequency of acid foods, including frank or occult sugar intake between meals. The average number of exposures to sugar and acid between meals over the 3 d was calculated for each patient [33]. In addition, the patients were educated on essential oral hygiene maintenance at home during orthodontic treatment. The patients were instructed to regularly brush their teeth with the modified Bass technique, using a manual or powered toothbrush with fluoride toothpaste, for at least 2 min after every meal. An interdental brush was recommended to clean the small spaces under the wires and around the bands and brackets.
2.4. Dental Biofilm Staining
At the beginning of the study, dental biofilm staining and collection were performed before orthodontic treatment. The dental biofilm was stained and collected again after treatment for at least six months. All tooth surfaces were stained, except for the occlusal surface, as previously described [34,35]. The dental biofilm maturity was assessed using GC Tri Plaque ID Gel TM (GC Corporation, Tokyo, Japan), according to the manufacturer’s protocol. Briefly, the gel was applied with a microbrush on all tooth surfaces and left undisturbed for 2 min. The tooth surfaces were then gently rinsed with water for 30 sec, and the changes in dental biofilm color were observed (Figure 2). The original dark blue changed to light blue when the pH in dental biofilm was less than 5. Immature and mature dental biofilm stained pink and purple, respectively. The most mature dental biofilm was recorded for each tooth. Based on the color changes on the tooth surfaces, the dental biofilm (plaque) maturing staining (PMS) was obtained using the formula:
| % PMS = (number of teeth with each plaque color/total number of teeth examined) × 100 |
as previously described [35].
Figure 2.
Use of the three-tone plaque disclosing gel, showing cariogenic, mature, and new dental biofilm as light blue, dark purple/blue, and pink/red stained areas, respectively.
2.5. Dental Biofilm Collection
Dental biofilm samples were collected from the tooth surfaces with a sterile dental explorer. Each sample was placed in a sterile 1.5 mL microcentrifuge tube. The sample was kept on dry ice and transported to the laboratory and stored at −80 °C until use.
2.6. Determination of Gene Expression by Real-Time PCR
Total RNA of the dental biofilm samples was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA). Complementary DNA was synthesized from total RNA (1 µg) using a PrimeScript 1st strand cDNA Synthesis Kit (Takara Bio Inc., Shiga, Japan). Real-time PCR was performed using a KAPA SYBR® FAST qPCR Kit Master Mix (Kapa Biosystems, Wilmington, MA, USA) in a QuantStudio™ 3 Real-Time PCR System (Thermo Fisher Scientific, Waltham, MA, USA). The PCR primer sequences were obtained from previous studies (Table 1). The gtfB, ldh, brpA, spaP, luxS, and gbpB primers were obtained from Wen et al. [36]. The 16S rRNA gene served as the internal control [37]. The relative gene expression levels were determined using the 2−ΔΔCT equation. In this case, ΔCT = CT (After treatment group)—CT (Before treatment group). In this study, we defined Differentially Expressed Genes (DEGs) based on the fold changes in gene expression between before and after orthodontic treatments. The DEGs were considered if their expression demonstrated at least a 1.5-fold increase [38].
Table 1.
Primer sequences for real-time PCR.
| Primers | Forward Primers (5′–3′) | Reverse Primers (5′–3′) | Size (bp) |
|---|---|---|---|
| gtfB | AGCAATGCAGCCATCTACAAAT | ACGAACTTTGCCGTTATTGTCA | 98 |
| ldh | TTGGCGACGCTCTTGATCTTAG | GTCAGCATCCGCACAGTCTTC | 92 |
| brpA | CGTGAGGTCATCAGCAAGGTC | CGCTGTACCCCAAAAGTTTAGG | 148 |
| spaP | TCCGCTTATACAGGTCAAGTTG | GAGAAGCTACTGATAGAAGGGC | 121 |
| luxS | ACTGTTCCCCTTTTGGCTGTC | AACTTGCTTTGATGACTGTGGC | 93 |
| gbpB | CGTGTTTCGGCTATTCGTGAAG | TGCTGCTTGATTTTCTTGTTGC | 108 |
| 16S rRNA | TCCACGCCGTAAACGATGA | TTGTGCGGCCCCCGT | 119 |
2.7. Statistical Analysis
The statistical analysis was performed using GraphPad Prism Version 7.0 (GraphPad software Inc., La Jolla, CA, USA) and the Statistical Package of Social Sciences (SPSS) version 25 (IBM Corp., Armonk, NY, USA). Descriptive statistics are displayed as the mean, standard deviation (SD), and median and range for quantitative variables where appropriate. Frequencies and percentages were used to describe qualitative data. Numerical data were plotted in the figures as the mean ± the standard error of the mean (SEM) and analyzed for statistical significance using the paired t-test. Multiple linear regression analysis was used to predict the association of the outcome variable (the PMS of the cariogenic dental biofilm) with the predictor variables (the expression level of virulence genes). Multicollinearity was assessed using the variance inflation factor (VIF) and tolerance values. A variance inflation factor (VIF) > 10 and/or intolerance < 0.1 indicates serious multicollinearity [39]. The significance level for all tests was defined as p-value ≤ 0.05.
3. Results
3.1. The Study Population
The patients’ demographic characteristics, treatment durations, oral hygiene practices, and dietary habits are shown in Table 2. The study comprised 24 patients, 14–41 years old (mean ± SD = 27.21 ± 6.45). There were 12 (50%) males and 12 (50%) females. Dental biofilm staining and determination of virulence gene expression were performed after orthodontic treatment for 7.74 ± 0.65 months (mean ± SD). All patients used fluoride toothpaste. The brushing frequencies of most patients were ≥2 times/day. They occasionally used dental floss, an interdental brush, and mouthwash. The patients consumed sugary and acidic food between meals 0.9 (0–3) and 0 (0–0.4) (median and range) times per day, respectively.
Table 2.
Demographic data, treatment duration, oral hygiene practices, and dietary habits of the orthodontic patients.
| Variables | Values |
|---|---|
| Demographic characteristics | |
| Age (years) Mean ± SD Min–Max |
27.21 ± 6.45 14–41 |
| Sex Male, n (%) Female, n (%) |
12 (50%) 12 (50%) |
| Orthodontic treatment duration | |
| Fixed orthodontic therapy follow-up (months) Mean ± SD Min–Max |
7.74 ± 0.63 6.46–8.70 |
| Oral hygiene practices | |
| Tooth brushing frequency, n (%) 1 time a day ≥2 times a day |
1 (4.7) 23 (95.83) |
| Use of dental floss, n (%) Daily Occasionally No |
4 (16.67) 15 (62.5) 5 (20.83) |
| Use an interdental brush, n (%) Daily Occasionally No |
9 (37.5) 15 (62.5) 0 (0) |
| Use a fluoride toothpaste, n (%) Yes No |
24 (100) 0 (0) |
| Use mouthwash, n (%) Yes No |
13 (54.17) 11 (45.83) |
| Dietary habits | |
| Sugary intake between meals/day (Median and range) |
0.90 (0–3) |
| Acidic food intake between meals/day (Median and rage) |
0 (0–0.4) |
3.2. Dental Biofilm Maturity
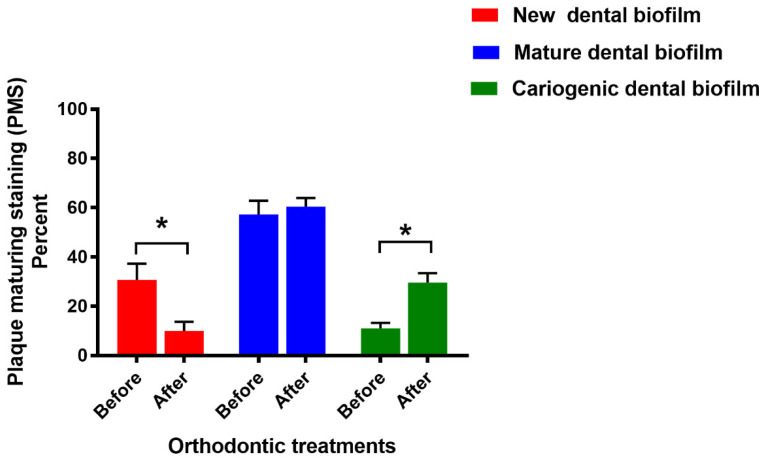
The percent PMS of new dental biofilm significantly decreased after fixed orthodontic treatment (p < 0.05) (Figure 3). In contrast, the cariogenic dental biofilm percentage significantly increased after treatment (p < 0.05). However, there was no significant difference in the PMS of mature dental biofilm before and after treatment (p > 0.05).
Figure 3.
Percent PMS in the patients before and after fixed orthodontic treatment (n = 24). Bars represent the PMS mean ± SEM. Asterisks indicate a significant difference (p < 0.05) using the paired t-test.
3.3. Cariogenic Virulence Gene Expression in Dental Biofilm
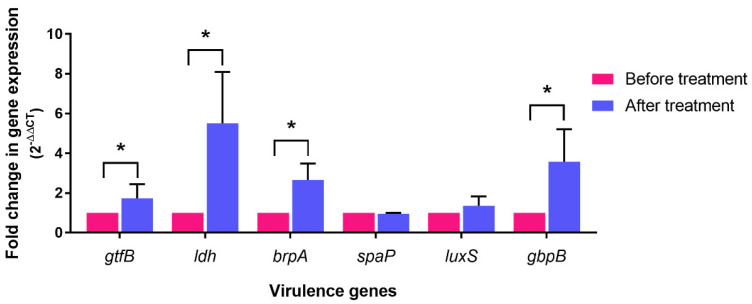
Representative cariogenic genes (gtfB, ldh, brpA, spaP, luxS, and gbpB) in S. mutans were investigated in dental biofilm using real-time PCR. After fixed orthodontic treatment, DEGs were found in gtfB, ldh, brpA, and gbpB (Figure 4). The expression of spaP or luxS following the insertion of fixed orthodontic appliances was similar to that pre-insertion.
Figure 4.
Fold change in gtfB, ldh, brpA, spaP, luxS, and gbpB gene expression in dental biofilm after orthodontic treatment (n = 24). Bars represent the mean ± SEM of gene expression level relative to that of the expression level before treatment. Asterisks indicate Differentially Expressed Genes (DEGs), which were defined as at least a 1.5-fold increase.
3.4. Association between PMS and Virulence Gene Expression Level
Multiple linear regression analyses were used to predict the association between the PMS of cariogenic dental biofilm and virulence gene expression levels. In this study, the outcome and predictor variables were the PMS and gtfB, ldh, brpA, spaP, luxS, and gbpB expression levels, respectively. The results revealed no significant correlation between cariogenicity and the expression level of virulence genes in dental biofilm after fixed orthodontic treatment (p > 0.05, R2 = 0.25) (Table 3). In addition, the predictor’s tolerance values were greater than 0.1 and the VIF values were less than 10. Therefore, there was no significance of multicollinearity among our predictor variables.
Table 3.
Analysis of the association between the PMS of cariogenic dental biofilm and the expression level of virulence genes using multiple linear regression.
| Predictor Variables | Coefficients | p-Value | Collinearity Statistics |
||
|---|---|---|---|---|---|
| Unstandardized Coefficient (B) | Coefficients Standard Error | Tolerance | VIF | ||
| gtfB | −1.15 | 1.2 | 0.35 | 0.88 | 1.14 |
| ldh | −0.19 | 0.44 | 0.67 | 0.49 | 2.06 |
| brpA | −0.71 | 0.16 | 0.65 | 0.39 | 2.56 |
| spaP | −16.37 | 23.1 | 0.49 | 0.98 | 1.02 |
| luxS | −2.45 | 1.71 | 0.17 | 0.96 | 1.04 |
| gbpB | 0.14 | 0.59 | 0.82 | 0.68 | 1.46 |
4. Discussion
Dental caries is a multifactorial dynamic disease; the presence of dental biofilm and its interaction between host factors and sugar is very important in caries development [40]. This interaction influences the dental biofilm environment, as explained by the ecological plaque hypothesis, and acidogenic bacteria play an important role in dental biofilm acidogenicity [40,41]. A previous study determined the association between acidogenic bacterial counts and dental biofilm maturity using three-tone plaque disclosing gels [42]. A high amount of acidogenic bacteria was found in light blue dental biofilm, followed by purple and pink. The present study found that the PMS of cariogenic dental biofilm was significantly (18.54%) higher in the orthodontic patients after fixed appliance insertion. Therefore, our results confirm those of previous reports.
The presence of fixed orthodontic appliances makes oral hygiene more challenging for orthodontic patients. Fixed appliances impede access to the tooth surface and increase the complexity of the mechanical cleaning that the patient must perform during orthodontic treatment. Poor oral hygiene practices, such as ineffective tooth brushing, and dental biofilm accumulation on orthodontic appliances, can drive ecological changes in dental biofilm around the appliances. Multicolor plaque disclosing dyes allow clinicians to rapidly identify where patients are struggling with mechanical cleaning. This can be very informative for identifying high caries risk areas around brackets that can guide dental professionals to efficiently provide targeted oral hygiene education [26,27,34].
In this study, gtfB, gbpB, ldh, and brpA expression levels and dental biofilm cariogenicity in orthodontic patients increased after fixed orthodontic treatment. These findings are partially in line with our previous study in asthmatic patients [35]. However, in addition to gtfB, gbpB, ldh, and brpA, spaP and luxS gene expression was significantly increased in asthmatic patients. It is possible that a relatively high caries risk status is common in asthmatic patients. A meta-analysis found that the caries risk level was double in asthmatic patients [43]. The most asthmatic patients have been found to have a significant decrease in salivary flow rates, pH, and buffer capacity, which are known to predispose individuals to dental caries.
The ldh gene in S. mutans has been reported to be constitutively expressed [36]. During the sugar fermentation process, LDH catalyzes the transformation of pyruvate (plus NADH) to lactic acid plus NAD+ at the final step. This reaction is exergonic, thermodynamically preferred, but reversible, and lactate can be oxidized to pyruvate and NADH, if needed. Most oral acidogenic bacteria produce LDH by activating ldh gene expression, which directly correlates with their acid production and cariogenicity [44]. We therefore hypothesize that ldh expression could be a suitable biomarker to correlate the relative metabolic activity with the acid production within cariogenic dental biofilm. Indeed, 5.5-fold increases in ldh expression and cariogenic dental biofilm were observed following orthodontic treatment.
Similar results were also observed in the expression of gtfB, gbpB, and brpA. GTFs and GBP of S. mutans are known to be differentially expressed in response to environmental conditions, such as carbohydrate source and availability, pH, and growth of the bacteria on surfaces [10]. GTFs and GBP facilitate bacterial adherence and biofilm accumulation [11,13,45]. In the present study, gtfB and gbpB demonstrated increased expression after orthodontic treatment, whereas there was no change in spaP expression in the cariogenic dental biofilm of our patients. The spaP gene is considered the primary factor mediating the early attachment of S. mutans to tooth enamel in the absence of sucrose [46]. In acidic dental biofilm, which had the highest %PMS in our patients, spaP might not be predominantly expressed compared with gtfB and gbpB. The expression of brpA in S. mutans has been proposed to be associated with acid tolerance and biofilm development [15,17]. The increased brpA expression found in the present study might play an important role in dental biofilm cariogenicity after fixed orthodontic appliance insertion.
Although luxS expression in S. mutans is associated with biofilm formation and biofilm structure maintenance, its roles in regulating the factors critical to biofilm formation are unresolved [36]. However, it has been found that the LuxS quorum sensing system is considered the main system that most of oral bacteria use to communicate within biofilm [47]. The most common and widespread quorum sensing signal among bacteria is autoinducer-2, which is a signaling molecule induced by LuxS that regulates the expression of numerous genes, including gtfB/C genes [48]. Our results suggest that luxS gene expression might upregulate gtfB. In addition, luxS might not be involved in the cariogenic environment induced in dental biofilm after fixed orthodontic treatment. These results imply that the expression level of virulence genes and their related factors may be altered in dental biofilm, when the patients have fixed orthodontic appliances inserted.
We also determined the influence of virulence genes on the cariogenicity of dental biofilm. Multiple linear regression analysis indicated that there was no significant correlation between cariogenicity and virulence gene expression in dental biofilm after fixed orthodontic treatment. Our results suggest that the high cariogenicity of dental biofilm, formed in the presence of fixed orthodontic appliances, may be explained by the overexpression of the virulence genes involved in bacterial adherence and biofilm formation. However, the cariogenicity of dental biofilm cannot be totally explained by virulence gene activation. Other components/conditions in dental biofilm, such as a high concentration of insoluble glucans in its matrix, the low inorganic concentration, and its protein composition may contribute to dental biofilm cariogenicity [49,50].
Surprisingly, high sugary and acidic food consumption were not observed in our patients. A high frequency of sugary (>1 time/day) and acidic food (>2 times/day) intake between meals is considered high caries risk behavior [51]. Most of our patients were exposed to sugary and acidic food between meals at a frequency of less than 1 time/day. Therefore, sugary and acidic food consumption in our patients might not be factors involved in the increase in cariogenic dental biofilm after fixed orthodontic appliance insertion. These dietary history results did not agree with our previous findings in asthmatic patients, who were high caries risk individuals [35]. The average frequency of sugary food intake between meals in asthmatics was three times/day, which was higher than in the control subjects. The disparate results in orthodontic patients might be due to the impact of dietary counseling during orthodontic treatment.
Fixed orthodontic therapy may provide retentive site-associated dental biofilm accumulation [52]. Our findings imply that fixed orthodontic appliances may induce oral ecologic changes, which lead to increased dental biofilm cariogenicity. Increased expression of specific virulence genes related to dental biofilm formation, acid production, and acid tolerance contributed to greater cariogenicity. Moreover, acidogenicity and virulence gene expression in dental biofilm may be altered in response to cariogenic conditions, including orthodontic treatment and asthma. This study suggests that dental biofilm staining by a three-tone plaque disclosing gel is useful for assessing the cariogenic dental biofilm in orthodontic patients. Using a three-tone plaque disclosing gel for assessing dental biofilm in combination with the application of a biomimetic hydroxyapatite product may improve the efficiency of caries prevention in orthodontic patients, by reducing the incidence of white spot lesions along the surface of the brackets [53]. However, our results should be interpreted with caution because the level of acidogenic bacteria, particularly S. mutans in the dental biofilm, was not investigated in this study.
5. Conclusions
This study’s results indicate that staining dental biofilm with a three-tone disclosing gel allows for cariogenic dental biofilm assessment, following orthodontic treatment. Real-time PCR analysis demonstrated that the expression of virulence genes related to biofilm formation (gtfB and gbpB), acid production (ldh), and acid tolerance (brpA) were altered in response to fixed orthodontic appliance insertion. Our results suggest that cariogenicity and virulence gene expression in dental biofilm can be used in the early identification of caries risk status in orthodontic patients. A three-tone plaque disclosing dye can allow orthodontists to quickly identify the tooth surfaces where patients are struggling with mechanical cleaning. In combination with good oral hygiene maintenance, assessing cariogenicity and virulence gene expression in dental biofilm may synergistically reduce the incidence of carious lesions in orthodontic patients.
Acknowledgments
We thank Christian Estacio, a native English speaker and Academic Counselor for International Affairs, Walailak University, International College of Dentistry, for his English editing.
Author Contributions
Conceptualization, S.K. and K.U.; methodology, K.U.; validation, S.K., K.U. and W.T.; formal analysis, S.K. and K.U.; investigation, W.T.; resources, S.K. and W.T.; writing—original draft preparation, S.K. and K.U.; writing—review and editing, K.U. and W.T.; visualization, W.T. and S.K.; funding acquisition, S.K. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Human Research Ethics Committee of Walailak University, Thailand (no. WUEC-20-225-01/2).
Informed Consent Statement
Informed consent was obtained from individual patients or a parent of the patients under 16 years old who were involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors have no commercial interest in the 3-tone disclosing gel used in this study. We declare that the manufacturer has no commercial or associated interest that represents a conflict of interest in connection with the manuscript.
Funding Statement
This work was supported by Walailak University, International College of Dentistry (Grant Number: WUICD-C2020).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Butera A., Maiorani C., Morandini A., Simonini M., Morittu S., Trombini J., Scribante A. Evaluation of children caries risk factors: A narrative review of nutritional aspects, oral hygiene habits, and bacterial alterations. Children. 2022;9:262. doi: 10.3390/children9020262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marsh P.D., Head D.A., Devine D.A. Dental plaque as a biofilm and a microbial community—Implications for treatment. J. Oral Biosci. 2015;57:185–191. doi: 10.1016/j.job.2015.08.002. [DOI] [Google Scholar]
- 3.Kutsch V.K. Dental caries: An updated medical model of risk assessment. J. Prosthet. Dent. 2014;111:280–285. doi: 10.1016/j.prosdent.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 4.Nyvad B., Crielaard W., Mira A., Takahashi N., Beighton D. Dental caries from a molecular microbiological perspective. Caries Res. 2013;47:89–102. doi: 10.1159/000345367. [DOI] [PubMed] [Google Scholar]
- 5.Sundararaj D., Venkatachalapathy S., Tandon A., Pereira A. Critical evaluation of incidence and prevalence of white spot lesions during fixed orthodontic appliance treatment: A meta-analysis. J. Int. Soc. Prev. Community Dent. 2015;5:433–439. doi: 10.4103/2231-0762.167719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown M.D., Campbell P.M., Schneiderman E.D., Buschang P.H. A practice-based evaluation of the prevalence and predisposing etiology of white spot lesions. Angle Orthod. 2016;86:181–186. doi: 10.2319/041515-249.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hadler-Olsen S., Sandvik K., El-Agroudi M.A., Ogaard B. The incidence of caries and white spot lesions in orthodontically treated adolescents with a comprehensive caries prophylactic regimen—A prospective study. Eur. J. Orthod. 2012;34:633–639. doi: 10.1093/ejo/cjr068. [DOI] [PubMed] [Google Scholar]
- 8.Freitas A.O., Marquezan M., Mda C.N., Alviano D.S., Maia L.C. The influence of orthodontic fixed appliances on the oral microbiota: A systematic review. Dent. Press J. Orthod. 2014;19:46–55. doi: 10.1590/2176-9451.19.2.046-055.oar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sawhney R., Sharma R., Sharma K. Microbial colonization on elastomeric ligatures during orthodontic therapeutics: An overview. Turk. J. Orthod. 2018;31:21–25. doi: 10.5152/TurkJOrthod.2018.17050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.You Y.-O. Virulence genes of Streptococcus mutans and dental caries. Int. J. Oral Biol. 2019;44:31–36. doi: 10.11620/IJOB.2019.44.2.31. [DOI] [Google Scholar]
- 11.Banas J.A., Vickerman M.M. Glucan-binding proteins of the oral streptococci. Crit. Rev. Oral Biol. Med. 2003;14:89–99. doi: 10.1177/154411130301400203. [DOI] [PubMed] [Google Scholar]
- 12.Yang J., Deng D., Brandt B.W., Nazmi K., Wu Y., Crielaard W., Ligtenberg A.J.M. Diversity of SpaP in genetic and salivary agglutinin mediated adherence among Streptococcus mutans strains. Sci. Rep. 2019;9:19943. doi: 10.1038/s41598-019-56486-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowen W.H., Koo H. Biology of Streptococcus mutans-derived glucosyltransferases: Role in extracellular matrix formation of cariogenic biofilms. Caries Res. 2011;45:69–86. doi: 10.1159/000324598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishimura J., Saito T., Yoneyama H., Bai L.L., Okumura K., Isogai E. Biofilm formation by Streptococcus mutans and related bacteria. Adv. Microbiol. 2012;2:208–215. doi: 10.4236/aim.2012.23025. [DOI] [Google Scholar]
- 15.Bitoun J.P., Liao S., Yao X., Ahn S.J., Isoda R., Nguyen A.H., Brady L.J., Burne R.A., Abranches J., Wen Z.T. BrpA is involved in regulation of cell envelope stress responses in Streptococcus mutans. Appl. Environ. Microbiol. 2012;78:2914–2922. doi: 10.1128/AEM.07823-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lemos J.A., Burne R.A. A model of efficiency: Stress tolerance by Streptococcus mutans. Microbiology. 2008;154:3247–3255. doi: 10.1099/mic.0.2008/023770-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wen Z.T., Baker H.V., Burne R.A. Influence of BrpA on critical virulence attributes of Streptococcus mutans. J. Bacteriol. 2006;188:2983–2992. doi: 10.1128/JB.188.8.2983-2992.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wen Z.T., Nguyen A.H., Bitoun J.P., Abranches J., Baker H.V., Burne R.A. Transcriptome analysis of LuxS-deficient Streptococcus mutans grown in biofilms. Mol. Oral Microbiol. 2011;26:2–18. doi: 10.1111/j.2041-1014.2010.00581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshida A., Ansai T., Takehara T., Kuramitsu H.K. LuxS-based signaling affects Streptococcus mutans biofilm formation. Appl. Environ. Microbiol. 2005;71:2372–2380. doi: 10.1128/AEM.71.5.2372-2380.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mira A. Oral microbiome studies: Potential diagnostic and therapeutic implications. Adv. Dent. Res. 2018;29:71–77. doi: 10.1177/0022034517737024. [DOI] [PubMed] [Google Scholar]
- 21.Pretty I.A., Edgar W.M., Smith P.W., Higham S.M. Quantification of dental plaque in the research environment. J. Dent. 2005;33:193–207. doi: 10.1016/j.jdent.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 22.Wei S.H.Y., Lang N.P. Periodontal epidemiological indices for children and aldolescents: II Evaluation of oral hygiene; III Clinical applications. Pediatr. Dent. 1982;4:64–73. [PubMed] [Google Scholar]
- 23.Al-Anezi S.A., Harradine N.W. Quantifying plaque during orthodontic treatment. Angle Orthod. 2012;82:748–753. doi: 10.2319/050111-312.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oikonomou E., Foros P., Tagkli A., Rahiotis C., Eliades T. Impact of aligners and fixed appliances on oral Health duringo rthodontic treatment: A systematic review and meta-analysis. Oral Health Prev. Dent. 2021;19:659–672. doi: 10.3290/j.ohpd.b2403661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kozak U., Lasota A., Chalas R. Changes in distribution of dental biofilm after insertion of fixed orthodontic appliances. J. Clin. Med. 2021;10:5638. doi: 10.3390/jcm10235638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walsh L.J., Healey D.L. Prevention and caries risk management in teenage and orthodontic patients. Aust. Dent. J. 2019;64((Suppl. S1)):S37–S45. doi: 10.1111/adj.12671. [DOI] [PubMed] [Google Scholar]
- 27.Jayanthi M., Shilpapriya M., Reddy V.N., Elangovan A., Sakthivel R., Vijayakumar P. Efficacy of three-tone disclosing agent as an adjunct in caries risk assessment. Contemp. Clin. Dent. 2015;6:358–363. doi: 10.4103/0976-237X.161887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schulz K.F., Altman D.G., Mother D. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. J. Pharmacol. Pharmacother. 2010;1:100–107. doi: 10.4103/0976-500X.72352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferreira J.C., Patino C.M. Types of outcomes in clinical research. J. Bras. Pneumol. 2017;43:5. doi: 10.1590/s1806-37562017000000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pannuti C.M., Sendyk D.I., GraCas Y.T.D., Takai S.L., SabOia V.P.A., Romito G.A., Mendes F.M. Clinically relevant outcomes in dental clinical trials: Challenges and proposals. Braz. Oral Res. 2020;34((Suppl. S2)):e073. doi: 10.1590/1807-3107bor-2020.vol34.0073. [DOI] [PubMed] [Google Scholar]
- 31.Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: A practical primer for t-tests and ANOVAs. Front. Psychol. 2013;4:863. doi: 10.3389/fpsyg.2013.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Y.J., Kim M.K., Hwang S.H., Ahn Y., Shim J.E., Kim D.H. Relative validities of 3-day food records and the food frequency questionnaire. Nutr. Res. Pract. 2010;4:142–148. doi: 10.4162/nrp.2010.4.2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wongkamhaeng K., Poachanukoon O., Koontongkaew S. Dental caries, cariogenic microorganisms and salivary properties of allergic rhinitis children. Int. J. Pediatr. Otorhinolaryngol. 2014;78:860–865. doi: 10.1016/j.ijporl.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Brostek A.M., Walsh L.J. Minimal intervention dentistry in general practice. Oral Health Dent. Manag. 2014;13:285–294. [PubMed] [Google Scholar]
- 35.Widhianingsih D., Koontongkaew S. Enhancement of cariogenic virulence properties of dental plaque in asthmatics. J. Asthma. 2021;58:1051–1057. doi: 10.1080/02770903.2020.1753211. [DOI] [PubMed] [Google Scholar]
- 36.Wen Z.T., Yates D., Ahn S.-J., Burne R.A. Biofilm formation and virulence expression by Streptococcus mutans are altered when grown in dual-species model. BMC Microbiol. 2010;10:111. doi: 10.1186/1471-2180-10-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Utispan K., Chitkul B., Monthanapisut P., Meesuk L., Pugdee K., Koontongkaew S. Propolis extracted from the stingless bee Trigona sirindhornae Inhibited S. mutans activity in vitro. Oral Health Prev. Dent. 2017;15:279–284. doi: 10.3290/j.ohpd.a38528. [DOI] [PubMed] [Google Scholar]
- 38.Zhao B., Erwin A., Xue B. How many differentially expressed genes: A perspective from the comparison of genotypic and phenotypic distances. Genomics. 2018;110:67–73. doi: 10.1016/j.ygeno.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 39.Kim J.H. Multicollinearity and misleading statistical results. Korean J. Anesthesiol. 2019;72:558–569. doi: 10.4097/kja.19087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pitts N.B., Zero D.T., Marsh P.D., Ekstrand K., Weintraub J.A., Ramos-Gomez F., Tagami J., Twetman S., Tsakos G., Ismail A. Dental caries. Nat. Rev. Dis. Prim. 2017;3:17030. doi: 10.1038/nrdp.2017.30. [DOI] [PubMed] [Google Scholar]
- 41.Marsh P.D. Dental plaque as a biofilm and a microbial community—Implications for health and disease. BMC Oral Health. 2006;6((Suppl. S1)):S14. doi: 10.1186/1472-6831-6-S1-S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee J., Park H., Lee J., Seo H., Lee S. Study of bacteria associated with dental daries using a 3 tone disclosing agent. J. Korean Acad. Pediatr. Dent. 2018;45:32–40. [Google Scholar]
- 43.Alavaikko S., Jaakkola M.S., Tjaderhane L., Jaakkola J.J. Asthma and caries: A systematic review and meta-analysis. Am. J. Epidemiol. 2011;174:631–641. doi: 10.1093/aje/kwr129. [DOI] [PubMed] [Google Scholar]
- 44.Len A.C.L., Harty D.W.S., Jacques N.A. Proteome analysis of Streptococcus mutans metabolic phenotype during acid tolerance. Microbiology. 2004;150:1353–1366. doi: 10.1099/mic.0.26888-0. [DOI] [PubMed] [Google Scholar]
- 45.Bowen W.H., Burne R.A., Wu H., Koo H. Oral Biofilms: Pathogens, matrix, and polymicrobial interactions in microenvironments. Trends Microbiol. 2018;26:229–242. doi: 10.1016/j.tim.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bowen W.H., Schilling K., Giertsen E., Pearson S., Lee S.F., Bleiweis A., Beeman D. Role of a cell surface-associated protein in adherence and dental caries. Infect. Immun. 1991;59:4606–4609. doi: 10.1128/iai.59.12.4606-4609.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Niazy A.A. LuxS quorum sensing system and biofilm formation of oral microflora: A short review article. Saudi Dent. J. 2021;33:116–123. doi: 10.1016/j.sdentj.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Q., Ma Q., Wang Y., Wu H., Zou J. Molecular mechanisms of inhibiting glucosyltransferases for biofilm formation in Streptococcus mutans. Int. J. Oral Sci. 2021;13:30. doi: 10.1038/s41368-021-00137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cury J.A., Rebelo M.A., Del Bel Cury A.A., Derbyshire M.T., Tabchoury C.P. Biochemical composition and cariogenicity of dental plaque formed in the presence of sucrose or glucose and fructose. Caries Res. 2000;34:491–497. doi: 10.1159/000016629. [DOI] [PubMed] [Google Scholar]
- 50.Wolff M.P., Larson C. The cariogenic dental biofilm: Good, bad or just something to control? Braz. Oral Res. 2009;23:31–38. doi: 10.1590/S1806-83242009000500006. [DOI] [PubMed] [Google Scholar]
- 51.Ngo H.C., Wolff M.S., Hume W.R. Preservation and Restoration of Tooth Structure. 3rd ed. John Wiley & Sons; Chichester, UK: 2016. Dental caries: Activity and risk assessments as a logical and effective path to both prevention and cure; pp. 33–49. [Google Scholar]
- 52.Raju A.S., Hegde N.A., Reddy V.P., Chandrashekar B.S., Mahendra S., Harishkoushik S.R. An in vivo study on bacterial colonization with metal, ceramic and self-ligating brackets: A scanning ekectron microscopy study. J. Indian Orthod. Soc. 2013;47:88–96. doi: 10.1177/0974909820130206. [DOI] [Google Scholar]
- 53.Butera A., Pascadopoli M., Gallo S., Lelli M., Tarterini F., Giglia F., Scribante A. SEM/EDS evaluation of the mineral deposition on a polymeric composite resin of a toothpaste containing biomimetic Zn-carbonate hydroxyapatite (microRepair (R)) in oral environment: A Randomized Clinical Trial. Polymers. 2021;13:2740. doi: 10.3390/polym13162740. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.