Abstract
An enzyme-linked immunoelectrotransfer blot for the diagnosis of human hydatid disease was performed, and the different antibody responses were analyzed by a discriminant analysis. This multivariate technique gave us, first, a selection of the most important responses against Echinococcus granulosus infection and, second, a procedure for the classification of patients into two groups: patients with hydatid disease and patients without a history of hydatid disease. This method was applied to 67 patients, 25 with active hydatid cysts (24 hepatic and 1 pulmonary) and 42 without a history of hydatid disease and was compared with the results obtained by conventional serology: indirect hemagglutination, latex particle agglutination, and basophil degranulation. An immunoelectrotransfer blot coupled to a discriminant analysis was more sensitive than conventional serological diagnosis and detected 100% of patients with an active hepatic hydatid cyst with a specificity of 100%. This method, however, failed to detect an uncomplicated hyaline pulmonary hydatid cyst.
Hydatidosis is the parasitization of tissue by the larval stage of different cestodes of the Echinococcus genus and represents a public health problem with important economic implications (15).
The diagnosis of hydatidosis is mainly based on two phenomena: analysis by morphologic methods (radiology, echography, and nuclear magnetic resonance imaging) and analysis by immunologic methods (detection of antibodies, antigens, circulating immune complexes, and delayed hypersensitivity and lymphoproliferative assays). The morphologic methods as a whole have better sensitivity than immunologic ones, but they require adequate equipment and the uncomplicated hydatid cysts are poorly differentiated from idiopathic cysts. Immunologic methods are more available as screening tests because they are technologically simpler, but they lack sufficient sensitivity for the detection of extrahepatic cysts (2, 10, 13).
The enzyme-linked immunoelectrotransfer blot (EITB) is a test which combines the high sensitivity of the immunoenzymatic tests with the high resolution of sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE). The detection of antibodies against certain proteins by EIBT is considered highly specific for detection of the Echinococcus genus, and it has a sensitivity of about 90%, with a specificity of 100% for the diagnosis of hepatic hydatidosis, but it is less sensitive for detection of uncomplicated cysts located in the lung and brain (14).
The main subject of this paper is use of the serological pattern obtained by EITB in order to improve the sensitivity of this procedure for the diagnosis of hydatid disease, but without the loss of specificity. The problem is to combine the bands obtained by EITB in the classification task. This question has been studied by means of a usual statistical procedure: discriminant analysis.
MATERIALS AND METHODS
Patients studied.
Sixty-seven patients were included in the study and were separated into the groups outlined below.
(i) Group 1.
Twenty-five patients with active hydatid cysts comprised group 1. Twenty-two of them had fertile hepatic cysts. One patient had an infertile giant hydatid cyst of the liver; another patient had a fertile cyst of the lung. The last one was a patient who was previously treated for hepatic hydatidosis but who actually had a fertile meningeal relapse. The activities and fertilities of the cysts were confirmed by postsurgical parasitological examinations.
(ii) Group 2.
Group 2 consisted of 42 patients with no history of hydatidosis. Twenty patients presenting with chronic hepatopathies (hepatocarcinoma, cirrhosis, or chronic hepatitis) were selected. Ten patients were children from 6 months to 2 years of age who had no evidence of parasitic diseases. Eight patients were selected from among the adult patients without hydatid disease; they had suspected parasitosis, and hydatid serology was required by the physician. Another four patients were children with transient eosinophilia, and for these patients hydatid disease had been ruled out. All the patients except the children younger than age 2 years were selected from different clinical conditions classically linked to false-positive results by hydatid serology.
For all patients a chest X-ray and abdominal ultrasonography were performed, as were conventional tests for the serological diagnosis of hydatidosis: the indirect hemagglutination (IHA) and latex agglutination (LA) tests. The basophil degranulation (BD) test was performed for 22 of the 24 patients in group 1 but for only 1 of the patients in group 2.
None of the patients received antihelmintic drugs, and those whose cysts had calcified walls were excluded from the study.
Serum samples from all the patients described above were stored at −40°C. Anticoagulated complete blood samples were drawn from 23 of the patients for immediate performance of BD tests.
IHA test.
The commercial Cellognost Echinococcosis (Behring) test was used. Results equal to or greater than 1:64 were considered positive when hemagglutinins against type O erythrocytes were absent.
LA test.
The commercial Agglutinotest echinococcosis (Ismunit) test was performed according to the manufacturer’s recommendations, and those sera whose titers were equal to or greater than 1:2 were considered positive.
BD test.
The BD test was performed by the method of Mir et al. (16).
Immunoelectrotransfer performance.
Hydatid cyst fluid from a human fertile hepatic cyst was obtained by sterile puncture during surgery. The fluid was centrifuged at 900 × g for 15 min, and the supernatant was sterilized by filtration through a Millipore filter (pore diameter, 0.45 μm). Thereafter, it was dialyzed in phosphate-buffered saline (PBS) at 4°C for 24 h and was kept at −100°C in aliquots for no longer than a month.
Two tests were standardized for the detection of immunoglobulin G (IgG) antibodies against the different antigens present in hydatid cyst fluid by previously described methods (5, 27). The first one was performed with unreduced antigens of hydatid fluid. The other one was performed with antigens previously treated with 2-mercaptoethanol in order to reduce disulfide bonds.
For SDS-PAGE, gels of 1 mm in thickness and 17 cm in width with continuous gradients of 5 to 20% acrylamide were used. The gels contained SDS at 0.1% and were buffered at pH 8.8 with 375 mM Tris-HCl. Ammonium persulfate (0.00052% [wt/vol]) and N,N,N′,N′-tetramethylethylenediamine (0.052% [vol/vol]; Bio-Rad) were used as polymerization catalyzers (5, 27).
The stacking gel contained 4% acrylamide in 125 mM Tris-HCl buffer at pH 6.8 with SDS at 0.1%.
EITB was performed as described previously (4–6, 14, 23, 27), but with some modifications. Briefly, samples consisting of 1.5 ml of hydatid fluid prepared as described above were mixed with 0.75 ml of sample buffer (62 mM Tris-HCl [pH 6.8], 2% [wt/vol] SDS, 10% [vol/vol] glycerol, 0.00125% [wt/vol] bromophenol blue). The final protein concentration was between 60 and 100 μg/ml. This antigenic mixture was denatured in a boiling bath for 5 min. For the test with reduced antigens, 5% (vol/vol) 2-mercaptoethanol was added to the sample buffer.
Electrophoresis was performed in Tris-glycine buffer (pH 8.3) with 0.1% SDS at a constant intensity of 7 mA per gel (approximately 12 h).
The separated proteins were electrotransferred to nitrocellulose sheets with a constant voltage of 80 V for 8 h in a Tris-glycine buffer without SDS and with 20% (vol/vol) methanol. The sheets were coated with 3% (vol/vol) bovine serum albumin in PBS for 15 min and were stored in a dry atmosphere at 4°C until their use. In order to calculate the molecular weights of the different protein bands, we used the method of Plikaytis et al. (20) with high- and low-molecular-mass markers which were separated in the same gel with a hydatid fluid sample, electrotransferred to the same sheet, and dyed with India ink (7).
The sheets were cut into 3-mm-wide strips, and a standard procedure for the immunoenzymatic test was followed (5, 22, 27). The patients’ sera were diluted 1:100, and a peroxidase-conjugated goat antibody to human IgG was used as a second antibody. The specific antigenic bands were visualized by the addition of 3,3′-diaminobenzidine which was diluted 1/1,000 in 0.05 M citrate buffer (pH 5.6) containing 0.03% (vol/vol) oxygenated water (110 volumes).
In all EITBs (up to 15), a positive serum sample belonging to a patient with multiple peritoneal hydatidosis with an IHA level of greater than 1:4,096 and a negative serum sample from an 11-month-old child without a clinical history of hydatidosis were included. Only the bands which constantly appeared with the positive control serum in all tests were used for statistical analysis of the patients’ reactive bands. The band with an approximate molecular mass of 170 kDa which appeared in all strips with unreduced antigens and another one with an approximate molecular mass of 70 kDa which appeared in all strips with reduced antigens and which corresponded to the presence of human IgG in the hydatid fluid were discarded for the statistical analysis described below.
Statistical analysis.
In order to classify each patient as having active hydatid disease or not, the results of conventional serology and bands obtained by EITB were used. The results of conventional serology were considered in a qualitative manner; that is, sera were coded a 1 if they were considered positive for hydatid disease by each conventional test and were coded a 0 if they were negative. Similarly, the presence of a specific band in a patient’s serum was coded 1 and the absence of the band was coded 0. As pointed out above, only the bands that constantly appeared with the positive control serum in all tests were considered. A previous and descriptive point is the evaluation of the sensitivity and specificity of each band and conventional serology, which were calculated by analyzing the relative frequencies. High values correspond to potentially important variables for the classification of a patient.
From a statistical point of view, our problem can be studied by means of a discriminant analysis because two conditions are present: a classification problem and a training sample, i.e., it is known whether each serum sample belonged to a patient with hydatid disease. First, this technique has as its main goal the design of a classification rule by using the training sample in such a manner that the rate of occurrence of misclassification errors (false positives and false negatives) is as low as possible. Essentially, a linear combination of the original variables (presence or absence of bands obtained by EITB) is used to classify a patient. This index is the discriminant score. If this discriminant score is greater than a certain value, then the patient is placed in one of the two groups (patients with hydatid disease or patients without hydatid disease), and if this score is lower than this value, the patient is placed in the other group. Note that in our case, each variable corresponded to the presence or absence of a concrete band in EITB or positive or negative conventional tests (IHA, LA, and BD tests). Second, this technique can be used to select the most important variables in such a manner that the accuracy of the method that used the smaller set of variables will be similar to the one that used all variables. A simpler and easier method is achieved when this smaller set of sampling information is used (1, 21, 24).
All subsequent analyses were done by using the discriminant procedure of SPSS for Windows, version 6.1.3 (17). Briefly, the selection of variables has been done by using the stepwise method. Then, by using the selected variables, a discriminant score (Z) is determined and the observations are classified. Note that because equal prior possibilities are used, the classification rule can be formulated in two equivalent ways: If Z1 and Z2 are the mean values of the discriminant score in groups 1 and 2 (named centroids), respectively, an observation with a score Z is assigned to one or another group depending on whether Z ≥ (Z1 + Z2)/2 or Z < (Z1 + Z2)/2.
This is equivalent to calculating them by using Bayes’ theorem and assigning an observation to the group with the highest posterior probability. For instance, when the discriminant function is applied to a particular patient, the presence or absence of the bands included in the discriminant function will allow us to calculate the particular score for the patient. The final classification for the patient would be made by calculating the posterior probabilities with the statistical package (using Bayes’ theorem), or more easily, the patient may be classified into the group whose centroid (mean of the scores for the group) is closer to the patient’s score.
The statistical significance of the classification made with the discriminant function and the real situation was calculated by the chi-square test.
RESULTS
Conventional serology.
As shown in Table 1, the agglutination of latex particles is highly specific (100% in our series), with a sensitivity of about 74%, while the IHA test had a sensitivity of 95.5% and a specificity of 90.5% even though a titer as low as 1:64 was considered significant. The BD test had great sensitivity (91.3%), but because we did not dispose of recently drawn blood samples from patients from group 2 specificity studies could not be performed.
TABLE 1.
Results obtained with conventional serology expressed as sensitivity and specificity values
| Test | Sensitivity (%) | Specificity (%) |
|---|---|---|
| IHA | 92 | 90.5 |
| BD | 91.3 | NEa |
| LA | 74 | 100 |
NE, not evaluated.
EITB.
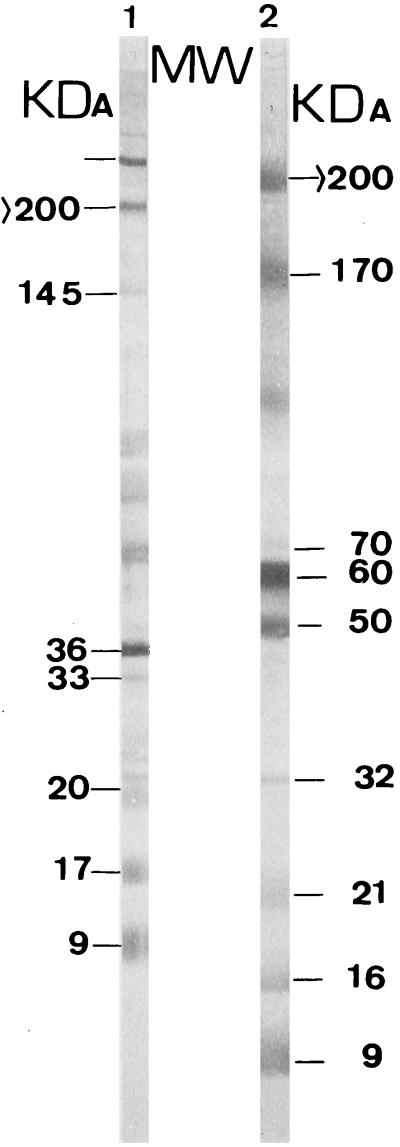
The bands obtained by EITB with or without 2-mercaptoethanol treatment during the processing of the hydatid fluid are shown in Fig. 1. To facilitate the statistical analysis of the results, the different bands were given separate designations (see Table 4).
FIG. 1.
Description of the different bands detected by EITB with a positive serum sample. Only the bands which appeared in all the tests performed with the same positive control serum are marked, and they are the only ones which were used in the statistical analysis. Lane 1, strip with reduced antigens; lane 2, strip with unreduced antigens.
TABLE 4.
Results of EITB with relative frequencies of appearance of the different bands among the patients with hydatid cysts at whatever location (group 1) and patients with no history of hydatid disease (group 2)
| Protein reduction and band size (kDa [band designation]) | Relative frequency (%) of band
appearance
|
|
|---|---|---|
| Group 1 | Group 2 | |
| Reduced | ||
| >200 (X1) | 96 | 14.28 |
| 145 (X2) | 84 | 0 |
| 36 (X3) | 100 | 40.47 |
| 33 (X4) | 92 | 2.38 |
| 20 (X5) | 88 | 0 |
| 17 (X6) | 80 | 0 |
| 9 (X7) | 80 | 0 |
| Unreduced | ||
| >200 (X8) | 96 | 28.57 |
| 70 (X9) | 88 | 0 |
| 60 (X10) | 100 | 47.61 |
| 50 (X11) | 96 | 2.38 |
| 32 (X12) | 92 | 0 |
| 21 (X13) | 88 | 0 |
| 16 (X14) | 80 | 0 |
| 9 (X15) | 80 | 0 |
The results obtained by EITB and conventional tests are presented in Tables 2 and 3. In Table 4 we summarize the relative frequencies of IgG antibodies against E. granulosus proteins obtained by EITB.
TABLE 2.
Results obtained by EITB and classical tests for patients in group 1
| Patient no. | Immunoblot results with reduced
or unreduced proteins of the indicated molecular mass
(kDa)a:
|
Clinical classification | Conventional serologic test
resultb
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reduced
proteins
|
Unreduced proteins
|
LA (IT) | IHA (IT) | BD (DI) | |||||||||||||||
| >200 | 145 | 36 | 33 | 20 | 17 | 9 | >200 | 70 | 60 | 50 | 32 | 21 | 16 | 9 | |||||
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Hepatic relapse | 4 | 2,048 | 87 |
| 2 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | Hepatic relapse | 8 | 4,096 | 100 |
| 3 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | Hepatic relapse | 8 | 8,192 | 100 |
| 4 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | Hepatic relapse | 1 | 8,192 | 69 |
| 5 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Hepatic relapse | 4 | 4,094 | 83 |
| 6 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Infected hepatic relapse | NEG | 128 | 6 |
| 7 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Original hepatic cyst | 8 | 2,048 | 55 |
| 8 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Original hepatic cyst | 4 | 4,096 | 62 |
| 9 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Original hepatic cyst | 4 | 2,048 | 87 |
| 10 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Original hepatic cyst | 4 | 1,024 | 87 |
| 11 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Original hepatic cyst | 2 | 256 | 79 |
| 12 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Original hepatic cyst | 2 | 4,096 | 80 |
| 13 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | Meningeal relapse | NEG | 64 | NP |
| 14 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Original hepatic cyst | 1 | 4,096 | 71 |
| 15 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Original hepatic cyst | 4 | 4,096 | 100 |
| 16 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Original hepatic cyst | 4 | 1,024 | 77 |
| 17 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Original hepatic cyst | 2 | 1,024 | 83 |
| 18 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Original hepatic cyst | 2 | 4,096 | 98 |
| 19 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Original hepatic cyst | 4 | 2,048 | 69 |
| 20 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Original hepatic cyst | 1 | 1,024 | 65 |
| 21 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Original hepatic cyst | 1 | 1,024 | 64 |
| 22 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Original hepatic cyst | 2 | 4,096 | 84 |
| 23 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | Original hepatic cyst | 2 | 1,024 | 70 |
| 24 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Original lung cyst | NEG | NEG | NP |
| 25 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | Unfertile hepatic cyst | NEG | NEG | 2 |
0, band absence; 1, band presence.
Positive results by conventional serologic tests were as follows: LA test, ≥2; IHA test, ≥64; BD test, ≥40. IT, inverse titer (titer−1); DI, degranulation index; NP, not performed; NEG, absence of agglutination.
TABLE 3.
Results obtained by EITB and classical serologic tests for patients to group 2
| Patient no. | Immunoblot results with reduced
or unreduced proteins of the indicated molecular
massa:
|
Clinical classification | Conventional serologic test
resultb
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reduced
proteins
|
Unreduced proteins
|
LA (IT) | IHA (IT) | BD (DI) | |||||||||||||||
| >200 | 145 | 36 | 33 | 20 | 17 | 9 | >200 | 70 | 60 | 50 | 32 | 21 | 16 | 9 | |||||
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | Adult (SP) | NEG | 64 | 0 |
| 2 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | Adult (SP) | NEG | NEG | 0 |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Adult (SP) | NEG | 64 | 0 |
| 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Adult (SP) | NEG | 32 | 0 |
| 5 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | Adult (SP) | NEG | 64 | 0 |
| 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Adult (SP) | NEG | 32 | 0 |
| 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Adult (SP) | NEG | NEG | 0 |
| 8 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | Adult (SP) | NEG | NEG | 21 |
| 9 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | Child with eosinophilia | NEG | NEG | 0 |
| 10 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | Child with eosinophilia | NEG | NEG | NP |
| 11 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | Child with eosinophilia | NEG | NEG | NP |
| 12 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | Child with eosinophilia | NEG | NEG | NP |
| 13 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Pediatric patient (WP) | NEG | NEG | NP |
| 14 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | Pediatric patient (WP) | NEG | NEG | NP |
| 15 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | Pediatric patient (WP) | NEG | NEG | NP |
| 16 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | Pediatric patient (WP) | NEG | NEG | NP |
| 17 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | Pediatric patient (WP) | NEG | NEG | NP |
| 18 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | Pediatric patient (WP) | NEG | NEG | NP |
| 19 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Pediatric patient (WP) | NEG | NEG | NP |
| 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Pediatric patient (WP) | NEG | NEG | NP |
| 21 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | Pediatric patient (WP) | NEG | NEG | NP |
| 22 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Pediatric patient (WP) | NEG | NEG | NP |
| 23 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Hepatic carcinoma | NEG | NEG | NP |
| 24 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Hepatic carcinoma | NEG | NEG | NP |
| 25 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | Hepatic carcinoma | NEG | NEG | NP |
| 26 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Chronic hepatitis | NEG | NEG | NP |
| 27 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Chronic hepatitis | NEG | NEG | NP |
| 28 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Hepatic carcinoma | NEG | NEG | NP |
| 29 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Chronic hepatitis | NEG | NEG | NP |
| 30 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Chronic hepatitis | NEG | NEG | NP |
| 31 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Chronic hepatitis | NEG | NEG | NP |
| 32 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | Hepatic cirrhosis | NEG | NEG | NP |
| 33 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Hepatic carcinoma | NEG | NEG | NP |
| 34 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | Hepatic cirrhosis | 1 | 512 | NP |
| 35 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Hepatic cirrhosis | NEG | NEG | NP |
| 36 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Hepatic cirrhosis | NEG | NEG | NP |
| 37 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Hepatic cirrhosis | NEG | NEG | NP |
| 38 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Hepatic cirrhosis | NEG | NEG | NP |
| 39 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Chronic hepatitis | NEG | NEG | NP |
| 40 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | Chronic hepatitis | NEG | NEG | NP |
| 41 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | Hepatic cirrhosis | NEG | 16 | NP |
| 42 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | Hepatic carcinoma | NEG | NEG | NP |
0, band absence; 1, band presence.
Positive results by conventional serology were as follows: LA test, ≥2; IHA test, ≥64; BD test, ≥40. IT, inverse titer (titer−1); DI, degranulation index; NP, not performed; NEG, absence of agglutination; WP, without parasitosis; SP, suspected parasitosis.
A stepwise selection of variables (with Wilks’ lambda) has been applied. The selected variables are X3, X4, X8, X11, and X12. The discriminant score (DS) is calculated by the following expression: DS = 1.08(X3) − 8.99 (X4) − 1.03 (X8) + 8.78 (X11) + 8.88 (X12) − 3.27, where X3, X4, X8, X11, and X12 are 1 or 0, depending on whether antibodies against the corresponding proteins are found or not found, respectively.
The mean values of the discriminating score (centroids) for each group were as follows: for patients with active hydatid cyst, 5.2561 = Z1; for patients without a hydatid cyst, −3.1286 = Z2. The patients were classified in group 1 (with hydatid cyst) if Z (for this patient) was greater than or equal to half the sum of the centroids and in group 2 if Z was less than half the sum of the centroids.
The accuracies of the classifications are as follows. Among the 25 patients in group 1 whose clinical condition was confirmed, 24 were predicted to be in group 1 and 1 was predicted to be in group 2. All 42 patients in group 2 whose clinical condition was confirmed were correctly predicted to be in group 2. The proportion of correctly classified patients was 98.51%.
The discriminant function outlined above has a sensitivity of 96% and a specificity of 100% for the immunological diagnosis of hydatid disease (P < 0.0001).
The positive predictive value of this methodology was 100%, while the negative predictive value was 97.6%.
As indicated above, only one patient was erroneously classified. This patient had a hydatid hyaline cyst of the lung which was not detected by the test; so, for hepatic hydatid disease, the method had a sensitivity and a specificity of 100%.
DISCUSSION
Of the 25 patients with active hydatid cysts, 23 had hepatic cysts, 1 had a hyaline cyst of the lung, and another had a meningeal relapse after arterial dissemination of a giant hepatic cyst that spread into the aorta. Our results obtained by the IHA and LA tests are similar to those obtained by other researchers (8, 19).
Many studies have analyzed the predictive value of having antibodies against several antigens in hydatid cyst fluid (3, 9, 11, 12, 14, 18, 22, 23, 25, 26, 28, 29). The presence of antibodies against the unreduced 60-kDa protein or against the reduced 36-kDa protein, which are part of Capron’s antigen 5 (3), in 47.6 and 40.47% of the healthy population, respectively, is not surprising because the antigen 5 molecule contains the phosphoryl-choline epitope, a widely distributed hapten. Furthermore, it has been proved that antibodies against phosphoryl-choline could cross-react when highly sensitive tests are used (14, 25), and the 36- to 38-kDa reduced subunit of antigen 5 cross-reacts with human antibodies to other cestode, trematode, and nematode parasites and to blood group P1 antigen (29).
The presence of antibodies against the unreduced 50-kDa protein was far more specific. Antibodies against this protein were observed only in a patient in the group that did not have history of hydatidosis; but in another series that included patients with parasitosis other than hydatidosis (14), the detection of antibodies against the 50-kDa protein was less specific due to the cross-reactions.
In accordance with the majority of studies (9, 12, 14, 22, 23), the maximal specificity in the diagnosis of hydatidosis is obtained by detecting antibodies against the B-antigen complex of Oriol et al. (18), which corresponds to proteins with approximate molecular masses of 20, 16, and 10 kDa. The presence of antibodies against the 8- to 12-kDa protein, the smallest subunit of the B antigen (which is not altered by reduction with 2-mercaptoethanol), represents a specific indicator of a previous contact with Echinococcus genus cestodes (100% specificity) in our population. These antibodies, however, did appear in only 80% of the patients with active hydatid cysts (12, 14). Similarly, when antibodies against the 32-kDa unreduced protein, which perhaps corresponds to the 38-kDa protein reported by Verasategui et al. (28), are present, the diagnosis of cystic echinococcosis had a sensitivity of 92% and a specificity of 100%.
Despite the high sensitivity and specificity obtained with some single-band results (Table 4), when a discriminant analysis was applied by using the linear functions of the variables (the discriminant score) instead of analyzing the different variables separately, the sensitivity of EITB was increased, but without a notable loss of specificity.
EITB coupled with a discriminant analysis showed excellent sensitivity (100%) for the detection of cysts located in the liver, but it was unsuccessful for the diagnosis of infection in our only patient with pulmonary hydatidosis; that patient was also negative by conventional methods. Uncomplicated pulmonary cysts are most frequently serologically negative, and a method with a high level of sensitivity is necessary. We may not be able to predict the usefulness of EITBs until we study larger numbers of patients.
The results of sensitivity and specificity are based on the cyst location in the patients included in this study. The inclusion of only a single patient with pulmonary hydatidosis has the effect of raising the calculated sensitivity.
It may seem peculiar that the appearance of antibodies against the 33-kDa reduced protein, which appears to have a high degree of specificity when it is analyzed alone, may diminish the discriminant value and, apparently, also the probability of having hydatid cysts. This interpretation would be erroneous. By using the discriminant function, the combination of variables that allow a good classification are evaluated, although a single value for each variable included in the function could have a different meaning if it were considered alone.
However, when additional patients are included in such a study, the discriminant score will develop and will progressively better adjust to the population values.
REFERENCES
- 1.Anderson T V. An introduction to multivariate analysis. 2nd ed. New York, N.Y: John Wiley & Sons, Inc.; 1984. Classifications of observations; pp. 195–243. [Google Scholar]
- 2.Babba H, Messedi A, Masmoudi S, Zribi M, Grillot R, Ambriose-Thomas P, Beyrouti I, Sahnoun Y. Diagnosis of human hydatidosis: comparison between imagery and six serologic techniques. Am J Trop Med Hyg. 1994;50:64–68. doi: 10.4269/ajtmh.1994.50.64. [DOI] [PubMed] [Google Scholar]
- 3.Capron A, Yarzabal L, Vernes A, Fruit J. Le diagnostic immunologique de l’equinococcose humaine. Pathol Biol. 1970;18:357–363. [PubMed] [Google Scholar]
- 4.D’Amelio R, Pontesilli O, Dayal R, de Rosa F, Barnet M, Teggi A, Brighouse G. Characterization of parasite antigens from human hydatid cyst fluid by SDS-PAGE and IEF. Med Microbiol Immunol. 1985;174:43–50. doi: 10.1007/BF02123670. [DOI] [PubMed] [Google Scholar]
- 5.Gottstein B, Eckert J, Michael S A, Thompson R C A. Echinococcus granulosus antigens: immunoelectrophoretic and Western blot analysis of hydatid cysts fluids. Parasitol Res. 1987;73:186–189. doi: 10.1007/BF00536480. [DOI] [PubMed] [Google Scholar]
- 6.Gottstein B, Tsang V V W, Schantz P M. Demonstration of species-specific and cross-reactive components of Taenia solium metacestode antigens. Am J Trop Med Hyg. 1986;35:308–313. doi: 10.4269/ajtmh.1986.35.308. [DOI] [PubMed] [Google Scholar]
- 7.Hancock K, Tsang V C W. India ink staining of proteins on nitrocellulose paper. Anal Biochem. 1988;133:157–162. doi: 10.1016/0003-2697(83)90237-3. [DOI] [PubMed] [Google Scholar]
- 8.Hira P R, Shweiki H M, Siboo R, Behbehani K. Counterimmunoelectrophoresis using an arc 5 antigen for the rapid diagnosis of hydatidosis and comparison with the indirect hemagglutination test. Am J Trop Med Hyg. 1987;36:592–597. doi: 10.4269/ajtmh.1987.36.592. [DOI] [PubMed] [Google Scholar]
- 9.Kanwar J R, Kaushick S P, Sawahaney I M S, Kamboj M S, Mehta S K, Vinayak V K. Specific antibodies in serum of patients with hydatidosis recognized by immunoblotting. J Med Microbiol. 1992;36:46–51. doi: 10.1099/00222615-36-1-46. [DOI] [PubMed] [Google Scholar]
- 10.Kharebov A, Nnahmias J, El-On J. Cellular and humoral immune responses of hydatidosis patients to Echinococcus granulosus purified antigens. Am J Trop Med Hyg. 1997;57:619–625. doi: 10.4269/ajtmh.1997.57.619. [DOI] [PubMed] [Google Scholar]
- 11.Larralde C, Montoya R M, Sciutto E, Díaz M L, Govezensky T, Coltorti E. Deciphering Western blots of tapeworm antigens (Taenia solium, Echinococcus granulosus and Taenia crassiceps) reacting with sera from neurocysticercosis and hydatid disease patients. Am J Trop Med Hyg. 1989;40:282–289. doi: 10.4269/ajtmh.1989.40.282. [DOI] [PubMed] [Google Scholar]
- 12.Leggatt G R, McManus D P. Identification and diagnostic value of a major antibody epitope on the 12 kDa antigen from Echinococcus granulosus (hydatid disease) cyst fluid. Parasite Immunol. 1994;16:87–96. doi: 10.1111/j.1365-3024.1994.tb00327.x. [DOI] [PubMed] [Google Scholar]
- 13.Macpherson C N L, Romig T, Zeyhle E, Rees P H, Were J B O. Portable ultrasound scanner versus serology in screening for hydatid cysts in a nomadic population. Lancet. 1987;ii:259–261. doi: 10.1016/s0140-6736(87)90839-7. [DOI] [PubMed] [Google Scholar]
- 14.Maddison S E, Slemenda S B, Schantz P M. A specific diagnostic antigen of Echinococcus granulosus with an apparent molecular weight of 8 kDa. Trans R Soc Trop Med Hyg. 1989;40:337–383. doi: 10.4269/ajtmh.1989.40.377. [DOI] [PubMed] [Google Scholar]
- 15.Matossian R M. The immunological diagnosis of human hydatid disease. Trans R Soc Trop Med Hyg. 1987;71:101–104. doi: 10.1016/0035-9203(77)90070-0. [DOI] [PubMed] [Google Scholar]
- 16.Mir A, García de Lomas J, Olmos P, Nogueira J M, Buesa F. Degranulación de Basófilos humanos en el diagnóstico de la hidatidosis. Clin Esp. 1982;167:31–34. [PubMed] [Google Scholar]
- 17.Norušis M J. SPSS professional statistics™ 6.1. Discriminant analysis. Chicago, Ill: SPSS Inc.; 1994. [Google Scholar]
- 18.Oriol R, Williams J F, Pérez-Esandi M V, Oriol C. Purification of lipoprotein antigens of Echinococcus granulosus from sheep hydatid fluid. Am J Trop Med Hyg. 1971;20:569–575. doi: 10.4269/ajtmh.1971.20.569. [DOI] [PubMed] [Google Scholar]
- 19.Peláez A, Sastre A, Morales C, Martínez M L, Basomba A. A comparative study of the EAST, indirect haemagglutination, basophil degranulation and latex tests in the diagnosis of human hydatid disease. Allergol Immunopathol. 1988;16:109–112. [PubMed] [Google Scholar]
- 20.Plikaytis B D, Carlone G H, Edmans P, Mayer L W. Robust estimation of standard curves for protein molecular weight and linear-duplex DNA base pair number after gel electrophoresis. Anal Biochem. 1986;152:346–364. doi: 10.1016/0003-2697(86)90420-3. [DOI] [PubMed] [Google Scholar]
- 21.Seber G A F. Multivariate observations. New York, N.Y: John Wiley & Sons, Inc.; 1989. pp. 279–346. [Google Scholar]
- 22.Shambesh M K, Craig P S, Gusbi A M, Echtuish E F, Wen H. Immunoblot evaluation of the 100 and 130 kDa antigens in camel hydatid cyst fluid for serodiagnosis of human cystic echinococcosis in Libya. Trans R Soc Trop Med Hyg. 1995;89:276–279. doi: 10.1016/0035-9203(95)90538-3. [DOI] [PubMed] [Google Scholar]
- 23.Shapiro S Z, Bahr G M, Hira P R. Analysis of host components in hydatid cyst fluid and immunoblot-diagnosis of human Echinococcus granulosus infection. Ann Trop Med Parasitol. 1992;86:503–509. doi: 10.1080/00034983.1992.11812699. [DOI] [PubMed] [Google Scholar]
- 24.Sharma S. Applied multivariate techniques. New York, N.Y: John Wiley & Sons, Inc.; 1996. Two group discriminant analysis; pp. 237–286. [Google Scholar]
- 25.Shepherd J C, McManus D P. Specific and cross-reactive antigens of Echinococcus granulosus hydatid cyst fluid. Mol Biochem Parasitol. 1987;25:143–154. doi: 10.1016/0166-6851(87)90003-x. [DOI] [PubMed] [Google Scholar]
- 26.Siracusano A, Ioppolo S, Notargiacomo S, Ortona E, Riganó R, Teggi A, De Rosa F, Vicari G. Detection of antibodies against Echinococcus granulosus major antigens and their subunits by immunoblotting. Trans R Soc Trop Med Hyg. 1991;85:239–243. doi: 10.1016/0035-9203(91)90039-2. [DOI] [PubMed] [Google Scholar]
- 27.Tsang V C W, Peralta J M, Simons A R. Enzyme-linked immunoelectrotransfer blot techniques (EITB) for studying the specificities of antigens and antibodies separated by gel electrophoresis. Methods Enzymol. 1983;92:377–391. doi: 10.1016/0076-6879(83)92032-3. [DOI] [PubMed] [Google Scholar]
- 28.Verasategui M, Moro P, Guevara A, Rodríguez T, Miranda E, Gilman R H. Enzyme-linked immunoelectrotransfer blot test for diagnosis of human hydatid disease. J Clin Microbiol. 1992;30:1557–1561. doi: 10.1128/jcm.30.6.1557-1561.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang L-H, Leggatt G R, McManus D P. Further characterization of the 38 KDa antigen from Echinococcus granulosus (hydatid disease) cyst fluid: evidence for antigenic heterogeneity and reactivity with anti-P1 antibodies. Parasite Immunol. 1995;17:287–296. doi: 10.1111/j.1365-3024.1995.tb00894.x. [DOI] [PubMed] [Google Scholar]