Abstract
The basis for many febrile nonhemolytic transfusion reactions associated with platelet transfusion therapy is cytokine elaboration and accumulation in the storage bag, which correlate with the leukocyte content and the length of platelet storage. We propose that a possible additional variable in the elaboration and accumulation of cytokines is the differential adhesion of mononuclear cells to the plastic substrate of the platelet storage bag. We hypothesize that mononuclear cell adhesion-induced cytokine release is greater in random-donor platelet bags composed of the polyolefin polymer compared to the single-donor apheresis platelet bags composed of the polyvinyl chloride polymer with the tri-(2-ethylhexyl) trimellitate (TEHTM) plasticizer. For four blood donors, we demonstrate preferential mononuclear cell adhesion, in vitro, to discs of polyolefin polymer versus discs of polyvinyl chloride polymer with the TEHTM plasticizer. Scanning electron microscopy corroborates this. In addition, proinflammatory cytokine (interleukin 1β [IL-1β] and tumor necrosis factor alpha [TNF-α]) levels are greater in culture wells containing discs of polyolefin polymer than in those containing discs of polyvinyl chloride polymer with the TEHTM plasticizer, and even more so in storage bags containing polyolefin polymer versus polyvinyl chloride polymer with the TEHTM plasticizer (IL-1β, TNF-α, IL-6, and IL-8). This study suggests, for the first time, that differential plastic substrate mononuclear cell adhesion may contribute to cytokine release during platelet storage. This may represent an additional variable in the pathophysiology of febrile nonhemolytic transfusion reactions in patients receiving stored platelet units.
Platelet transfusions are frequently accompanied by febrile nonhemolytic transfusion reactions (FNHTRs) (11, 16). It is believed that during room temperature platelet storage cytokine release by contaminating mononuclear leukocytes results in accumulation of proinflammatory cytokines sufficient to produce the majority of these FNHTRs (1, 2, 5, 10, 12, 13, 22, 24, 25). In support of this, the incidence of FNHTRs has been related to both the leukocyte content (1, 12, 13, 22, 25) and the transfusional age (21, 22) of the platelet unit. Indeed, the association of cytokine levels with leukocyte content has been, in part, the impetus for techniques to reduce the leukocyte content of platelet units. Today, virtually all single-donor platelet (SDP) apheresis units, and an increasing number of random-donor platelet (RDP) units, are subjected to leukocyte reduction during or shortly after manufacture. This has resulted in a diminution in the occurrence of FNHTRs.
We hypothesize that an additional stimulus leading to cytokine elaboration during in vitro platelet storage is mononuclear cell (MC) substrate adhesion. While a variety of different plastic substrates and plasticizers have been used in the manufacture of platelet storage bags, it is presumed that most SDP bags in the United States at the time of our study were manufactured with polyvinyl chloride polymers (PVC) while most RDP bags were manufactured with either PVC or polyolefin polymers (POF) (8). Thus, differences in the levels of cytokine production might also result from differential MC adhesive interactions with the particular plastic substrates used in the manufacture of different platelet storage bags. In likely support of this hypothesis is the paradoxical observation, in a well-controlled study, of a significantly higher rate of FNHTRs in recipients of pooled RDP units despite a lower number of contaminating leukocytes compared to SDP units (9).
We thus developed an in vitro model to investigate the differential adhesion of MCs to plastic discs obtained from two types of platelet storage bags—an RDP storage bag constructed of a POF (8) (PL 732; Fenwal, Deerfield, Ill.) and an SDP unit storage bag constructed of a PVC with the TEHTM plasticizer (8) (CLX; Haemonetics, Braintree, Mass.). We then correlated MC adhesion with the in vitro production and accumulation of proinflammatory cytokines. We studied the quantitative and qualitative features of MC adherence to POF and PVC with scanning electron microscopy (SEM). Finally, we assessed the surface hydrophilic properties of PVC and POF as a corollary to cell adhesion and activation. Information on the differential adhesion of MCs to plastic substrates used in the manufacture of platelet (and blood) storage bags may be important in optimizing the methods and timing of leukocyte reduction strategies.
(This work was presented in part at the 48th Annual Meeting of the American Association of Blood Banks, New Orleans, Louisiana on 14 November 1995.)
MATERIALS AND METHODS
Culture wells.
Preparation of the plastic polymer discs, human MC isolation and culture, and cytokine enzyme-linked immunosorbent assay (ELISA) determinations were accomplished as described by Yun et al. (26). Following informed consent, MCs were isolated from four healthy donors and plated at a density of 1,340 cells/mm2 on four 16-mm-diameter discs of POF and PVC resting in tissue culture wells. These plate cultures were incubated in RPMI 1640 supplemented with 10% autologous human serum in a humidified 5% CO2 tissue culture incubator at 37°C. For each of the four donors, seven such plate cultures were prepared for the POF and PVC discs. On each of seven consecutive days, supernatant was harvested from the wells and stored at −80°C for cytokine ELISAs (interleukin 1β [IL-1β], IL-6, tumor necrosis factor alpha [TNF-α], IL-8; R&D Systems, Minneapolis, Minn.). Following removal of the supernatant, the polymer discs were washed with phosphate-buffered saline, fixed in 10% buffered formalin, and then stained with May-Grunwald-Giemsa stain. Adherent MCs were counted under phase microscopy (16 fields, 0.403 mm2 each, per donor were counted for each polymer on each of seven consecutive days).
Plateletpheresis.
These same donors then underwent plateletpheresis (MCS+; Haemonetics). The sensor device that helps separate the buffy coat from the platelet collection was removed in order to maximize the leukocyte spill to levels associated with cytokine elaboration (>2,000/μl) (2). The total leukocyte concentrations in the four collections were 4,300, 7,700, 7,300, and 7,600/μl in total initial volumes of 516, 428, 474, and 425 ml, respectively. Immediately following collection, one-half was maintained in the original PVC apheresis bag (two instances) or divided equally among the original and two additional identical PVC apheresis bags; the other half was divided equally among three POF bags (65 ml each). The platelets were stored in a 22°C platelet agitator for 8 days. Each day, 7 cc of supernatant was removed, utilizing a sterile connecting device (Terumo, Elkton, Md.), from one each of the PVC and POF bags for each of the four donors and stored at −80°C for later cytokine ELISAs.
SEM.
The SEM studies were accomplished at 10 KV at magnifications of ×200, ×600, and ×1,000 (JSM 840A; Jeol, Tokyo, Japan).
Contact angles.
Advancing and receding water contact angles were measured by the sessile drop method with deionized distilled water at a pH of 5.6. The advancing and receding contact angles were measured (n = 3) with a goniometer. The receding contact angles were determined after removal of one drop of water at a time from the surface.
RESULTS
Adhesion assays.
Analysis of substrate adhesion reveals that there is preferential quantitative adhesion, in the in vitro culture well model, of the MCs to the POF substrate compared to the PVC substrate throughout the 7 days of storage (P < 0.01 for each day by a two-tailed Student’s t test) (Fig. 1).
FIG. 1.
Mean MC adhesion in culture wells to discs of POF and PVC. The error bars represent ±1 standard error of the mean (n = 4 donors; P < 0.01).
SEM.
The quantitative preference for adhesion to the POF surface compared to the PVC surface was corroborated by SEM. Figure 2 shows representative scanning electron micrographs magnified at ×438 of human monocytes/macrophages adherent to the POF or PVC surfaces. Human monocytes/macrophages adhered to the POF surface (Fig. 2A) and were well spread. The central area of the uppermost surfaces showed raised membranous projections. The edges of the macrophages showed smoothness, with many fine filopodial processes and pinocytotic pits. These filopodial processes made contact with similar processes on adjacent cells. Many macrophages showed extending or stout cytoplasmic processes interconnecting cells. In contrast, macrophages adherent to the PVC surface (Fig. 2B) were often isolated from other macrophages. Furthermore, macrophages on the PVC surface did not show any raised membranous projections, and the edges of the cells showed only a few filopodial processes. Figure 3 shows the representative micrographs, magnified at ×146 and ×730, of human monocytes/macrophages adherent to the POF and PVC surfaces. In general, the POF surface (Fig. 3C and D) showed higher numbers of adherent monocytes/macrophages than the PVC surface (Fig. 3A and B). Macrophages adherent to the POF surface were evenly distributed over the surface as a monolayer in contrast to macrophages adherent to the PVC surface, which showed only isolated, focal areas of cell adhesion. Macrophages on the POF surface were well spread out, and the central area of cells showed raised, closely packed membranous (veils and ruffles) projections. Macrophages adherent to the PVC surface, however, showed flat cell surfaces without membranous projections, although the cells were spread out on the surface. Macrophages adherent to the POF surface exhibited smooth edges, and many fine filopodial processes were present. These filopodial processes made contact with similar processes on adjacent cells. Some cells showed extending or stout cytoplasmic processes interconnecting with other cells. Macrophages adherent to the PVC surface, however, showed rounded edges without filopodial processes or pinocytotic pits.
FIG. 2.
Representative scanning electron micrographs of human monocytes/macrophages adherent to POF (A) and PVC (B) surfaces. Isolated human MCs were allowed to adhere to discs of each polymer surface for 5 days in a 5% CO2 incubator at 37°C. Magnification, ×438.
FIG. 3.
Representative scanning electron micrographs of human monocytes/macrophages adherent to POF (C and D) and PVC (A and B) surfaces. Isolated human MCs were allowed to adhere to discs of each polymer surface for 5 days in a 5% CO2 incubator at 37°C. Magnification, ×146 (A and C) and ×730 (B and D).
Cytokine assays.
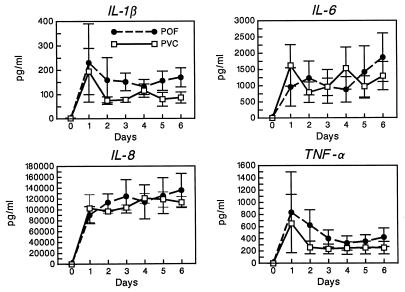
Cytokine levels in the culture wells peaked early and exhibited consistently higher values for IL-1β and TNF-α when incubated with POF than when incubated with PVC. There was less consistency in the IL-6 and IL-8 levels in the wells (Fig. 4). Cytokine levels from the storage bags peaked later than in the wells and exhibited, in three of four instances and for nearly all determinations, markedly higher values for IL-1β, TNF-α, IL-6, and IL-8 when platelets were stored in bags made of POF than when they were stored in PVC bags (Fig. 5). In the fourth instance, stimulation in both the POF and PVC bags was minimal throughout the storage period.
FIG. 4.
Proinflammatory cytokine (IL-1β, TNF-α, IL-6, and IL-8) levels in culture when incubated on discs of POF and PVC. Depicted are the mean values ± 1 standard error of the mean (n = 4 donors).
FIG. 5.
Proinflammatory cytokine (IL-1β, TNF-α, IL-6, and IL-8) levels in platelet storage bags composed of POF and PVC. Depicted are the mean values ± 1 standard error of the mean (n = 4 donors).
Water contact angles.
Advancing water contact angles were similar for POF (94.3°; standard deviation [SD], ±0.58) and PVC (90.0°; SD, ±0.0). The receding water contact angles demonstrated that the PVC surface was more hydrophilic in character (90.0° [SD, ±0.0] to 56.0° [SD, ±0.0]) than the POF surface (94.3° [SD, ±0.58] to 79.0° [SD, ±2.8]). These results are consistent with the hypothesis that hydrophilic surfaces minimize cell adhesion and activation.
DISCUSSION
We have observed, and others have previously reported, a higher incidence of FNHTRs in patients receiving RDP pools than in those receiving SDP units, despite total leukocyte content (9). Traditional but unsubstantiated explanations for this apparently paradoxical finding include (i) a greater age at transfusion of RDP pools versus SDP units, leading to a greater age-associated accumulation of cytokines in the older units; (ii) differences in collection techniques and in the time platelets are exposed to whole blood prior to separation; and (iii) a greater likelihood of HLA-antigen disparity, and thus a greater chance of an HLA-antibody-mediated reaction, in recipients of an RDP pool. We developed and tested an alternative hypothesis, namely, that differential MC adhesion to platelet storage bag polymers, resulting in differences in cytokine production and accumulation, may account for differences in the observed incidence of FNHTRs. While this is the first study to investigate the adhesive interactions of blood bag plastic polymers with MCs, the findings are consistent with those of prior investigations, establishing MC adhesion to a biological (endothelium) (15) or nonbiological (plastic) (4) surface as essential to the production and subsequent elaboration of cytokines.
Attachment of monocytes/macrophages to a fixed substrate is an important process of cell activation (7, 19, 23). In our study, macrophages adherent to the POF surface were well spread out, with many ruffled edges and filopodial processes, indicating that the macrophages were activated. In contrast, macrophages adherent to the PVC surface showed round edges without any ruffling or filopodial processes, suggesting that macrophages may exhibit marked adhesion and spreading without any activation (7, 19, 23). Since macrophages would be attracted to chemoattractants (or inflammatory sites) as they become activated, macrophages adherent to the POF surface may be activated, resulting in increased cytokine production. In contrast, macrophages adherent to the PVC surface may not be activated, as suggested by the adhesion and spreading of a few cells without filopodial processes. This is further corroborated by the demonstration that the PVC surface is more hydrophilic than the POF surface, which supports the hypothesis that hydrophilic surfaces minimize cell adhesion and activation (6, 7, 14).
To facilitate our investigations, we developed an in vitro model system utilizing discs of platelet bag plastic polymers: the culture well method. This model system avoided the difficulties of attempting to identify and quantitate MCs adherent to the inner surfaces of the platelet storage bags. Adherent cells are more difficult to locate and study in the bags themselves because of (i) the vast surface areas of the bags (337.5 cm2 for POF bags; 681.5 cm2 for PVC bags) compared to those of the discs and (ii) the tendency for the cells in the storage bags to strip away from the substrate during fixation and processing. The latter is attributed to interactions between plasma proteins and the plastic surfaces (4). These problems were circumvented with the in vitro model, which (i) limited the surface area to 201.6 mm2, facilitating cell quantitation; and (ii) largely avoided, by the use of a 10% serum medium, the adherence of cells to surface proteins, thus inhibiting cellular peeling.
While the plate culture model enables the study of MC adhesion to plastic substrates, it necessarily does not perfectly simulate the storage bag environment. Thus, there are differences in storage temperature (room temperature for platelet bags; 37°C for plate cultures), atmosphere (room air for platelet bags; 5% CO2 for plate cultures), incubation (continuous gentle agitation for platelet bags; stationary for plate cultures), and surface area available for cell adhesion, as noted above. And for plastic surfaces in particular, since adhesion is mediated via adsorption of proteins on the polymer surface which interact with MC receptors, with subsequent activation and secretion of cytokines (17, 23), such protein absorption is intentionally greatly reduced in the in vitro model system. These differences may account for the difference in kinetics and amounts of cytokine accumulation in the culture wells compared to those in the storage bags proper. Most notably, for example, the cytokine levels peaked much earlier (1 to 3 days) in the culture wells than in the storage bags (fifth day and beyond). In addition, it is perplexing that in one of the four donors, elaboration of cytokines during bag storage was minimal for both the POF and PVC bags. This suggests an element of donor-associated idiosyncrasy in the stimulation and elaboration of cytokines during platelet storage. Previous investigations of the interaction and cytokine response of monocytes/macrophages with biopolymers has revealed the response capacity of fresh human leukocytes to be highly variable (3, 4, 18, 20). Despite these limitations, the combined trends of culture well and platelet storage bag cytokine data strongly implicate differences in MC adhesion to different plastic polymers as likely to be pivotal in the differential production and release of cytokines.
Our observations may help to unravel the puzzling, although now largely historical, clinical observation of a higher incidence of FNHTRs with transfusions of RDP pools with a smaller number of contaminating leukocytes compared to that with non-leukocyte-reduced SDP units. While additional data, including corroborating clinical observations, on the rates of FNHTRs with storage bags composed of different polymers would help to further define such an association, this work, although preliminary, further supports the importance of cytokine production and accumulation in the pathophysiology of FNHTRs (2, 10, 12, 22, 24). The potential relevance of this work to current clinical practice may be questioned, since most SDP units and an increasing number of RDP units are rendered profoundly leukocyte reduced prior to storage and are thus unable to generate clinically significant quantities of cytokines. However, the basic pathophysiological mechanism explored in this study, namely, differential mononuclear and macrocytic cellular adhesion to plastic polymers, merits particular attention for any biopolymer material undergoing human cellular interactions. In addition, information on the differential adhesion of MCs to plastic substrates used in the manufacture of platelet (and blood) storage bags may be important in optimizing the methods and timing of leukocyte reduction strategies. Finally, it further suggests that a possibly novel alternative approach to abrogating such reactions might be further refinement in the manufacture of plastic polymers for platelet storage bags to reduce MC substrate adhesion and cellular activation.
ACKNOWLEDGMENT
This work was supported in part by NIH grant HL-33849.
REFERENCES
- 1.Aye M T, Palmer D S, Giulivi A, Hashemi S. Effect of filtration of platelet concentrates on the accumulation of cytokines and platelet release factors during storage. Transfusion. 1995;35:117–124. doi: 10.1046/j.1537-2995.1995.35295125733.x. [DOI] [PubMed] [Google Scholar]
- 2.Blajchman M. Cytokines in transfusion medicine. Transfusion. 1993;33:1–3. doi: 10.1046/j.1537-2995.1993.33193142302.x. [DOI] [PubMed] [Google Scholar]
- 3.Bonfield T L, Colton E, Anderson J M. Plasma protein adsorbed biomedical polymers: activation of human monocytes and induction of interleukin-1. Biomed Mater Res. 1989;23:535–548. doi: 10.1002/jbm.820230602. [DOI] [PubMed] [Google Scholar]
- 4.Bonfield T L, Colton E, Marchant R E, Anderson J M. Cytokine and growth factor production by monocytes/macrophages on protein preadsorbed polymers. J Biomed Mat Res. 1992;26:837–850. doi: 10.1002/jbm.820260702. [DOI] [PubMed] [Google Scholar]
- 5.British Committee for Standards in Haematology; Blood Transfusion Task Force. Guidelines on the clinical use of leucocyte-depleted blood components. Transfus Med. 1998;8:59–71. [PubMed] [Google Scholar]
- 6.Bruil A, Terlingen J G A, Beugeling T, van Aken W G, Feijen J. In vitro leucocyte adhesion to modified polyurethane surfaces. Biomaterials. 1992;13:915–923. doi: 10.1016/0142-9612(92)90114-4. [DOI] [PubMed] [Google Scholar]
- 7.Brunstedt M R, Anderson J M, Spilizewski K L, Marchant R M, Hiltner A. In vivo leucocyte interactions on Pellethane surfaces. Biomaterials. 1990;11:370–378. doi: 10.1016/0142-9612(90)90089-9. [DOI] [PubMed] [Google Scholar]
- 8.Carmen R. The selection of plastic materials for blood bags. Transfus Med Rev. 1993;7:1–10. doi: 10.1016/s0887-7963(93)70027-9. [DOI] [PubMed] [Google Scholar]
- 9.Chambers L A, Kruskall M S, Pacini D G, Donovan L M. Febrile reactions after platelet transfusion: the effect of single versus multiple donors. Transfusion. 1990;30:219–221. doi: 10.1046/j.1537-2995.1990.30390194340.x. [DOI] [PubMed] [Google Scholar]
- 10.Davenport R D, Kunkel S S. Cytokine roles in hemolytic and nonhemolytic transfusion reactions. Transfus Med Rev. 1994;8:157–168. doi: 10.1016/s0887-7963(94)70108-5. [DOI] [PubMed] [Google Scholar]
- 11.Goodnough L T, Riddell J, Lazarus H, Chafel T L, Prince G, Hendrix D, Yomtovian R. Prevalence of platelet transfusion reactions before and after implementation of leukocyte-depleted platelet concentrates by filtration. Vox Sang. 1993;65:103–107. doi: 10.1111/j.1423-0410.1993.tb02124.x. [DOI] [PubMed] [Google Scholar]
- 12.Heddle N M. Febrile nonhemolytic transfusion reactions to platelets. Curr Opin Hematol. 1995;6:478–483. doi: 10.1097/00062752-199502060-00013. [DOI] [PubMed] [Google Scholar]
- 13.Heddle N M, Klama L, Singer J, Richards C, Fedak P, Walker I, Kelton J G. The role of the plasma from platelet concentrates in transfusion reactions. N Engl J Med. 1994;331:625–628. doi: 10.1056/NEJM199409083311001. [DOI] [PubMed] [Google Scholar]
- 14.Horbett T A, Waldburger J J, Ratner B D, Hoffman A S. Cell adhesion to a series of hydrophilic-hydrophobic copolymers studied with a spinning disc apparatus. J Biomed Mater Res. 1988;22:383–404. doi: 10.1002/jbm.820220503. [DOI] [PubMed] [Google Scholar]
- 15.Lukacs N W, Strieter R M, Elner V, Evanoff H L, Burdick M D, Kunkel S. Production of chemokines, interleukin-8 and monocyte chemoattractant protein-1 during monocyte:endothelial cell interactions. Blood. 1995;86:2767–2773. [PubMed] [Google Scholar]
- 16.Mangano M M, Chambers L A, Kruskall M S. Limited efficacy of leukopoor platelets for prevention of febrile transfusion reactions. Am J Clin Pathol. 1991;95:733–738. doi: 10.1093/ajcp/95.5.733. [DOI] [PubMed] [Google Scholar]
- 17.McNally A K, Anderson J M. Complement C3 participation in monocyte adhesion to different surfaces. Proc Natl Acad Sci USA. 1994;91:10119–10123. doi: 10.1073/pnas.91.21.10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller K M, Anderson J M. Human monocyte/macrophage activation and interleukin 1 generation by biomedical polymers. J Biomed Mater Res. 1988;22:713–731. doi: 10.1002/jbm.820220805. [DOI] [PubMed] [Google Scholar]
- 19.Miller K M, Huskey R A, Bigby L F, Anderson J M. Characterization of biomedical polymer-adherent macrophages: interleukin 1 generation and scanning electron microscopy studies. Biomaterials. 1989;10:187–196. doi: 10.1016/0142-9612(89)90023-9. [DOI] [PubMed] [Google Scholar]
- 20.Miller K M, Rose-Caprara V, Anderson J M. Generation of IL-1-like activity in response to biomedical polymer implants: a comparison of in vitro and in vivo models. J Biomed Mater Res. 1989;23:1007–1026. doi: 10.1002/jbm.820230904. [DOI] [PubMed] [Google Scholar]
- 21.Muyelle L, Wouters E, DeBock R, Peetermans M E. Reactions to platelet transfusion: the effect of the storage time of the concentrate. Transfus Med. 1992;2:289–293. doi: 10.1111/j.1365-3148.1992.tb00172.x. [DOI] [PubMed] [Google Scholar]
- 22.Muylle L, Wouters E, Peetermans M E. Febrile reactions to platelet transfusion: the effect of increased interleukin 6 levels in concentrates prepared by the platelet-rich plasma method. Transfusion. 1996;36:886–890. doi: 10.1046/j.1537-2995.1996.361097017174.x. [DOI] [PubMed] [Google Scholar]
- 23.Ridley A J. Membrane ruffling and signal transduction. Bioessays. 1994;16:321–327. doi: 10.1002/bies.950160506. [DOI] [PubMed] [Google Scholar]
- 24.Stack G, Berkowicz D. Cytokine production during blood component storage. In: Davenport R D, Snyder E L, editors. Cytokines in transfusion medicine: a primer. Bethesda, Md: AABB Press; 1997. pp. 21–50. [Google Scholar]
- 25.Stack G, Snyder E L. Cytokine generation in stored platelet concentrates. Transfusion. 1994;34:20–25. doi: 10.1046/j.1537-2995.1994.34194098597.x. [DOI] [PubMed] [Google Scholar]
- 26.Yun J K, DeFife K, Colton E, Stack S, Azeez A, Cahalan L, Verhoeven M, Cahalan P, Anderson J M. Human monocyte/macrophage adhesion and cytokine production on surface modified poly(tetrafluoroethylene/hexafluoropropylene) polymers with and without protein preadsorption. J Biomed Mater Res. 1995;29:257–268. doi: 10.1002/jbm.820290217. [DOI] [PubMed] [Google Scholar]