Abstract
Improving plant resilience to changing environmental conditions is the primary focus of today’s scientific research globally. It is essential to find various strategies for the better survival of plants with higher resistance potential to climate change. Strigolactones (SLs) are multifunctional β-carotene derivative molecules that determine a range of plant growth and development aspects, such as root architecture, shoot branching, chlorophyll synthesis, and senescence. SLs facilitate strong defense responses against drought, salinity, heavy metal, nutrient starvation, and heat stress. The SLs trigger other hormonal-responsive pathways and determine plant resilience against stressful environments. This review focuses on the mechanisms regulated by SLs and interaction with other plant hormones to regulate plant developmental processes and SLs’ influence on the mitigation of plant damage under abiotic stresses. A better understanding of the signaling and perception of SLs may lead to the path for the sustainability of plants in the changing environmental scenario. The SLs may be considered as an opening door toward sustainable agriculture.
Keywords: abiotic stress, phytohormones, strigolactones, sustainable agriculture
1. Introduction
Climate change is a significant threat and will worsen in the future. The consequences of climate change include flooding, drought, higher temperature, irregular rainfall patterns, and others [1,2]. Humans are mobile and therefore easily adaptable to the environment through avoidance; however, sessile plants rely more on resistance than avoidance responses. The global food system has an ample environmental footprint, and agriculture is the most significant sector with the most influential after-effects of ecological imbalance. Researchers are getting too strategic in mitigating crop and nutrient loss. Feeding the growing population and protecting the environment are the ways toward sustainable development. Therefore, the human race is busy finding alternative resources and hidden pathways to reduce the damage and contribute to sustainable development. Plant research these days mainly focuses on strengthening plant immunity against climate change. For this purpose, work is focused on exploring hormonal signaling pathways, gene manipulation, and other proteomic strategies. Abiotic stress also causes alterations in plant morphology and physiology related to plant hormone systems [3]. Strigolactones (SLs) are one of the emerging hormones with much scope in plant resilience. Initially reported as a germination stimulant in parasitic plants, SLs are now in demand for improving plant growth and development [4]. As per the studies, SLs govern the overall plant architecture. SLs influence shoot branching and root structure, modify the phenotypic output of PIN-FORMED (PIN) auxin transporters by inhibiting the formation of auxin-conducting channels after wounding or from artificial auxin sources [5], and monitor secondary growth [6]. SLs also mediate plant response to nutrient deficiency of nitrogen (N) and phosphorous (P) [7]. SLs’ role in improving mycorrhizal colonization in plants makes them distinctive and captivates workers to unravel the complete mechanism of action in living organisms. There are instances where SLs have played an influential role in mitigating plant impairment when exposed to abiotic or biotic stress. This review will focus on pointing out the SL’s role in curbing the extreme environmental condition without much loss of productivity and elucidating the pathways involved in this regulation.
2. Strigolactone: History and Background
Strigol is the first characterized SL as a germination stimulant for the root parasitic plant Striga lutea [4]. Since then, SLs are mainly known for host–parasitic plant interaction. SLs are βcarotene-derived molecules synthesized in terrestrial plants. In root parasitic plants such as Striga, Phelipanche, and Orobanche spp. SLs act as germination stimulants released from the host and some non-host plants [8,9]. SLs also enhance arbuscular mycorrhizal fungi (AMF) colonization and act as hyphal branching factors for fungi. This in turn helps in nutrient uptake by fungi in symbiotic association with a plant. This mechanism, in many ways, improves plant nutrient content, growth, and development [10,11]. Moreover, SLs have a pivotal role in certain non-mycotrophic plants by improving stress responses and general plant growth [12].
SLs play key roles in several developmental pathways. The analysis demonstrated that SLs synthesis is reported in liverworts, mosses, lycophytes, gymnosperms, and angiosperms with the core set of SL biosynthesis enzymes [13]. On the contrary, core synthesis enzymes are absent in some species such as Marchantia polymorpha, Marchantia paleacea, and Physcomitrella patens but can synthesize SL through a non-canonical pathway [14,15,16]. For algae, it is suggested that predecessors of Suppressor of MAX2 1-Like (SMXL) are present in charophytes, but specific SMXL are absent [17,18] (Figure 1). So far, it is hypothesized that SLs are produced only in land plants, with consistent evolution of true SL biosynthesis enzymes at the base of land plants.
Figure 1.
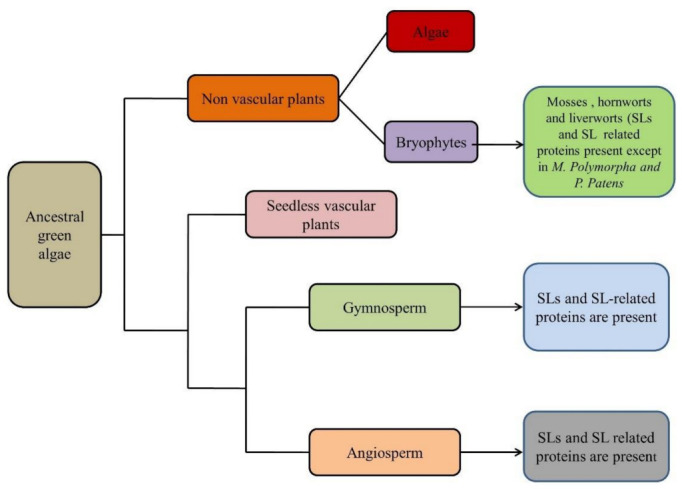
Evolutionary scheme showing common ancestry (presence of either SLs (strigolactones) or SLs-related proteins in land plants).
3. Strigolactone Biosynthesis and Signal Perception
SLs are tricyclic lactone structures (containing rings as ABC), with different carbon A-ring sizes and substitution patterns on AB-rings. An enol ether bridge connects the core to an α, β-unsaturated furanone moiety (the D-ring). So far, more than 30 SLs have been identified as canonical and non-canonical SLs based on the complete ABC-ring system’s presence or absence [19,20]. Twenty naturally occurring SLs have been identified and characterized so far in root exudates of various land plants. They are separated into two major groups–(a) strigol and orobanchol (ORO) (canonical SLs)–based on the stereochemistry of the B–C-ring junction, with both having a conserved R-configuration at the C-2′ position. The region that connects the D-ring to the core is responsible for the various SLs bioactivities, which can differ according to SL type [21]. In contrast, non-canonical SLs lack typical ABC-rings but possess an enol-ether bridge and D-ring moieties such as methyl carlactonoate (MeCLA) and avenaol [22,23,24].
A series of recessive mutants with increased shoot branching responses helped to understand SLs biosynthesis. The mutants include Arabidopsis more axillarygrowth (max), pea ramosus (rms), petunia decreased apical dominance (dad), and rice dwarf/high tillering dwarf (d/htd). At first, Arabidopsis max3 and max4, the pea rms5 and rms1, and the rice d17 and d10 mutants, defective in CAROTENOID CLEAVAGE DIOXYGENASE 7 (CCD7) and CCD8, respectively, were identified to be SL-deficient mutants [25,26]. Later on, the role of DWARF27 (D27) in rice and AtD27 in Arabidopsis was also reported in SLs biosynthesis [27,28].
SLs biosynthesis occurs in plastids with trans β-carotene and carlactone (CL) as the ultimate precursor for other SLs [29,30] (Figure 2). The specific enzymes such as carotenoid isomerase, (D27), which has been characterized so far in rice and Arabidopsis (At27) [27,28], can convert all-trans-β-carotene into 9-cis-β-carotene [31].
Figure 2.
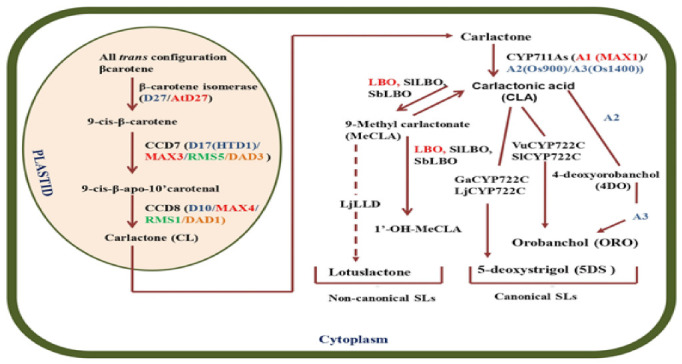
Strigolactone biosynthesis in a plant cell. Proposed SL biosynthesis pathway. D27, CCD7, and CCD8 are plastid localized enzymes that form CL from all-trans β-carotene. CL is then oxidized by the CYP711A family to yield CLA. GaCYP722C and LjCYP722C are involved in the production of strigol-type canonical SL, 5DS. For non-canonical SLs, MeCLA was shown to be synthesized from CLA in Arabidopsis. It has recently been demonstrated that Arabidopsis, tomato, and sorghum LBOs convert MeCLA into 1-OH-MeCLA and CLA. Moreover, LjLLD, encoding a novel 2OGD, was shown to be involved in the biosynthesis of lotuslactone (non-canonical SL). Enzymes of rice, Arabidopsis, pea, petunia, and other plants are shown in blue, red, green, and orange, respectively. Solid arrows indicate the confirmed pathways, whereas dashed arrows indicate the pathways which are not fully established. LBO (LATERAL BRANCHING OXIDOREDUCTASE) Vu, Vigna unguiculata (cowpea); Sl, Solanum lycopersicum (tomato); Ga, Gossypium arboreum (cotton); Lj, Lotus japonicus; Sb, Sorghum bicolor.
9-cis-β-carotene then serves as a substrate for carotenoid cleavage dioxygenase, CCD7, forming 9 cis β-apo-10′-carlactone, which directly precedes CCD8 in the strigolactone pathway [31]. In rice, the partial loss of function of SL biosynthesis genes (High TILLERING AND DWARF 1/DWARF 17) increases tiller number and grain yield. These genes are essential in determining plant architecture [32]. Through a series of oxygenation steps in the presence of cytochrome P450 (MAX1), carlactone, a butenolide ring-like structure is formed, followed by carlactonic acid (CLA) [33], which eventually gives rise to other types of SLs and SL-like compounds. The CYP711As subfamily of cytochrome P450 oxygenases functions in converting CL into both canonical and non-canonical SLs in vascular plants. CYP711A involvement in SLs biosynthesis was first implicated in Arabidopsis. As per the report, the Arabidopsis max1 mutant, defective in CYP711A1, exhibited the hyper-branching phenotype as max3 and max4 [34]. MAX 1 functions downstream of MAX3 and MAX4 as suggested by grafting experiments [35]. Moreover, the drastic increase in the endogenous CL level in max1 mutant suggested that CL is a substrate for MAX1 [36]. In rice, five homologs of MAX1 with diversified functions have been reported. One of these, Os01g0700900, functions as a carlactone oxidase that converts carlactone to 4-deoxyorobanchol, the precursor for orobanchol-type SLs [29]. A second MAX1 homolog, Os01g0701400, catalyzes the conversion of 4-deoxyorobanchol to orobanchol [29].
According to recent studies, for canonical SLs CYP722C subfamily plays a role in synthesizing both strigol and ORO [37,38,39]. Moreover, the study of Wakabayashi et al. [37] demonstrated the conversion of CLA to ORO in the presence of VuCYP722C and SlCYP722C via18-hydroxy-CLA in cowpea (Vigna unguiculata) and tomato (Solanum lycopersicum), respectively. The conversion of CLA to ORO by CYP722Cs needs factor(s) controlling the stereospecificity; stereochemistry of the C ring can be determined by LOW GERMINATION STIMULANT1, a sulfotransferase with unknown function in sorghum (Sorghum bicolor) [40]. GaCYP722C was shown to catalyze the reaction from CLA to 5-deoxystrigol (5DS) in cotton (Gossypium arboreum) [38]. Moreover, in Lotus japonicus, LjCYP722C was reported to function in 5DS biosynthesis downstream of CYP711A9/LjMAX1, which produces 18-hydroxy-CLA via CLA [39,41].
For non-canonical SLs, methyl carlactonoate (MeCLA) is the key intermediate and is reported to be produced from CLA [33]. The metabolic action of LATERAL BRANCHING OXIDOREDUCTASE (LBO), a 2-oxoglutarate-dependent dioxygenase (2OGD) in Arabidopsis converts MeCLA to such a product which inhibited shoot branching compared to the lbo mutant but showed a weaker hyper-branching phenotype compared with the max4 mutant [42]. According to Wakabayashi et al. [43], the SABATH methyltransferase in the clade to which At4g36470 belongs may be involved in the carboxymethylation of CLA and the biosynthesis of these non-canonical SLs in Arabidopsis. The identified At4g36470 revealed high substrate specificity for (11R)-CLA, suggesting the enzyme and its orthologs in other plant species may be involved in non-canonical SL biosynthesis. Recently, hydroxymethyl-carlactonoate (1’-OH-MeCLA) has been identified as an unstable LBO product and readily converted to CLA enzymatically or non-enzymatically [44]. The LBO homologs have shown similar reactions in maize, sorghum, and tomato [42]. In lotus, MeCLA or 18-hydroxy-CLA was converted to lotuslactone via LOTUSLACTONE-DEFECTIVE (LLD) because the LLD-defective mutant could not produce lotuslactone [39,41].
Similar to biosynthesis, certain SL-insensitive mutants are characterized, such as max2 in Arabidopsis, d3 in rice, and rms4 in pea for SLs perception. SLs signaling cascade consists of three important components: (a) an α/β fold hydrolase called DWARF 14/DECREASED APICAL DOMINANCE 2 (D14/At D14/DAD2 in rice, Arabidopsis, and petunia, respectively) [45]. DAD2 catalyzes the hydrolysis of the synthetic SL analogue GR24 (b), an F-box leucine-rich protein called MAX2/D3 [34,46], and (c) a repressor protein called D53 belonging to the SMXL protein family [47]. The SL receptor protein D14 is activated after ligand binding, leading to its interaction with other molecules to form a signaling complex; hormonal signal transduction is followed by subsequent hydrolysis of the bound SL, deactivating the hormone [48]. α/β-fold hydrolase, D14 (D14/AtD14/DAD2), and F-box protein (MAX2/D3/RMS4) act as a recognition subunits in an SKP1-CUL1-F-box-protein (SCF)-type ubiquitin ligase complex [49]. This complex further activates the 26S proteasome and degrades transcription repressors, such as the Suppressor of MAX2 1-Like (SMXLs 6,7, and 8 in Arabidopsis [50] and D53 in rice [51]). The signal transduction process begins with the binding of SL to the “open state” pocket of D14/AtD14, and the α/β-hydrolase receptor. The interaction with SL induces deletion of the ABC ring (ABC formyltricyclic lactone) and enabled the D ring to remain tightly and permanently attached to the D14/AtD14 (hydroxymethylbutenolide (HMB)). This closed state conformation of D14/AtD14 triggers interaction with the D3/MAX2-based SCF complex (SKP1-CUL1-F-box-protein (SCF)-type ubiquitin ligase complex) [52]. SCF complex targets the D53 and D53-like SMXL repressor proteins for proteasomal degradation, followed by activation of SL signal transduction and responses [47,51,53] (Figure 3). SLs in the roots are acropetally relocated from the rhizosphere to shoot through the PDR1 transporter [54]. The hydrolytic degradation of SLs was confirmed to be a common reaction catalyzed by the D14 family proteins [55,56,57].
Figure 3.
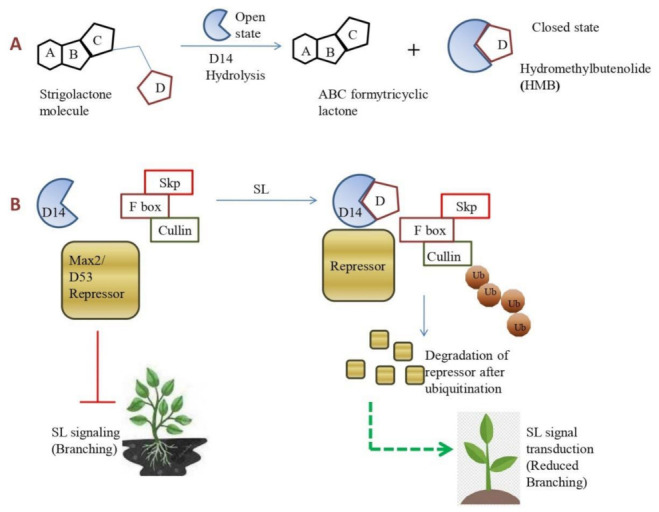
Signal transduction pathway of SLs and protein interaction. (A) Degradation of D14 into ABC-FTL and HMB. The figure also shows the open (inactive) and closed (active) conformation of D14 with the “D” ring. (B) Interaction of D14 (DWARF 14) with F-box protein D3 and a repressor D53 mediating the signaling pathway. In the absence of SLs, D53 arrests SLs transduction, however, SLs repressor is degraded through controlled ubiquitination followed by SLs release and successful transduction. SLs (Strigolactones); D14 (DWARF 14); ABC-FTL (ABC formlytricyclic lactone); HMB (Hydroxymethylbutenolide); D3 (DWARF 3); D53 (DWARF 53).
In support of the above view, Yao et al. [52] demonstrated a covalently linked intermediate molecule (CLIM) model for SLs perception (Figure 4a). AtD14, four α-helices form a V-shaped lid structure, and a loop containing the aspartate (Asp) residue of the catalytic triad (serine, Ser; histidine, His; and Asp (Asp loo)) exists between this V-shaped structure; Ser is present at the bottom of the pocket in the open conformation. It was expected that the methyl butenolide part (D-ring) of SL would be the target of the nucleophilic attack by the Ser residue [58]. According to the author, the SL-derived D-ring part is covalently linked to form a bridge between the Ser and His residues which is necessary for the complex formation with D3. However, Carlsson et al. [59] reanalyzed the reported D14–D3–ASK1 complex structural data, and they found that the electron density found in the pocket was too small to accommodate the proposed intermediate molecule [59]. The hydrolysis of D14 was reported to be extremely slow compared to the degradation of the repressor proteins D53/SMXLs [47,51]. Thus, the CLIM model seemed to be inconsistent with this rapid response because this model requires the hydrolysis reaction by D14 to transmit the signal.
Figure 4.
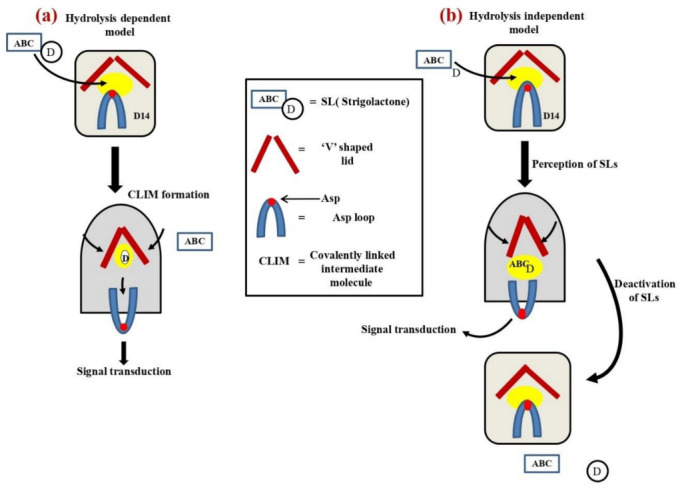
Two different models of SL perception showing the conformational change in D14. The figures are modified from Mashiguchi et al. [30]. (a) The hydrolysis intermediate derived from the D-ring part of SLs covalently linked with the receptor, D14, and resulted in a conformational change [52]. (b) The intact SL molecules cause a conformational change in D14, and D14 then returns to its catalytically active form [57].
Seto et al. [57] proposed the hydrolysis independent model of SL perception (Figure 4b). The catalytically inactive AtD14D218A mutant was able to complement the atd14 mutant phenotype in an SL-dependent manner, demonstrating that the hydrolytic degradation of SL by D14 is not necessary for its signal transduction. The presence of the enlarged pocket was observed in the AtD14-D3 protein complex, and the pocket was shown to have enough capacity to accommodate the intact SL molecules. The induction of the D14 active state is triggered by an intact SL molecule, not by the hydrolysis intermediate or products. The intact SL, D14, initially adopts a destabilized conformation due to the disruption of the catalytic triad formation. This form is the active state for SL signaling. In this state, the conformationally altered D14 protein interacts with its signaling partners, D53/SMXLs and D3/MAX2, to transmit the SL signal. The complex D53/SMXLs may bind around the Asp loop region. After the signal transduction, D14 returns to the apo form, which is enzymatically active and deactivates the SL molecules by hydrolytic degradation. The signaling mechanisms of SLs are mediated by a catalytically active α/β-hydrolase (D14) responsible for both the perception and deactivation of bioactive hormone signals. Moreover, yet another model based on the conformations of the D3 F-box protein was proposed by Shabek et al. [60]. Here they showed that the C terminal helix (CTH) of D3 has a more flexible structure, and this part interacts with D14 in an SL-dependent manner. The hydrolysis of D14 is inhibited in the D14-D3 CTH complex. However, it is reactivated in the presence of D53, and the crystal structure of D14-D3CTH induced by SL showed the presence of SL in the active site of D14. This allowed them to conclude that the signal transduction does not require SL hydrolysis and the signaling complex formation process does not require the conformational change of D14, which was different from Seto et al. [57] findings. Burger and Chory [61] reanalyzed the structural data of the AtD14–D3–ASK1 complex reported by Yao et al. [52] and proposed another model in which the D-ring part is covalently attached to only the His residue. It is difficult to integrate all these models and obtain consistent signaling and perception at this point. The SLs perception model is still under discussion and needs further research to understand the mechanism completely.
4. Strigolactone Is an Essential Plant Hormone in Regulating Plant Functions
SLs have been popularly known for their germination property in parasitic plants. However, in recent years, several other aspects of plant growth and development have been associated with it. Some of them are listed below.
4.1. Root Architecture
A robust root system provides proper anchorage to plants and efficient mineral transport acropetally. Moreover, plants also show some modification and particular root structures depending upon the adaption in parallel with their habitat. Plant hormones, such as auxin, play critical roles in regulating root growth [62]. Similarly, SLs influence various features and plants’ overall root system development. The significant role of SLs has been discovered in primary root formation, lateral and adventitious root development, and root hair elongation. For instance, in Arabidopsis, exogenous application of GR24 (synthetic SL) resulted in longer primary roots. The response was because of increased cell number in transition and meristematic zone [63]. Exogenous application of GR24 suppressed adventitious root formation in Arabidopsis and cut pea stem [64]. Auxin gradient is the chief regulator of root system architecture. For instance, the auxin concentration gradient determines the cell size in transition, meristematic zone, and adventitious root formation [64]. In the presence of auxin, the plant was reported with CCD8 (SL biosynthesis gene) in root cortical and epidermal cells of transition and elongation zone. Moreover, auxin can promote adventitious rooting even in the absence of SLs, and SLs can suppress adventitious rooting even in the presence of high auxin content. This means both are independently regulated. An auxin response mutant (axr1) and SL mutant (max 3) double mutant lines tested resulted in no roots similar to axr1 mutant lines, suggesting an AXR1-dependent pathway of MAX action. Root hair elongation after SLs application in wild line and SL-deficient line were reported, but not in SL response mutant max 2 proves that this elongation is facilitated via MAX 2 gene [64]. In contrast, SLs promote crown root elongation in rice by increasing cell numbers [65].
4.2. Shoot Branching
SLs play a significant role in affecting shoot branching. The Branching Inhibiting Signal (BIS) and SLs are similar in their function as they are significant players in apical dominance and are carotene derivatives; thus, they could be at least related [66]. D14 is the first receptor in branch signaling. The signaling is further preceded by the degradation of SMXLs 6, 7, and 8 [51]. Loss of function of the SMXL genes causes a reduction in shoot branching and promotes auxin transport and PIN1 accumulation in the shoot. This suggests that SLs mediate shoot branching in coordination with auxin via SMXLs [67]. BRC1 is a repression factor on auxiliary bud, and SMXL is known to release this suppression and promote branching. Target degradation of SMXL by SLs reverses this effect. BRC1 has been reported in pea, Arabidopsis, and rice species acting downstream of the SLs pathway [68,69]. In maize, the BRC1 homolog TEOSINTE BRANCHED1 (TB1) is reported with a similar mode of action and function [70]. The loss of function mutant and use of SL deficient strains supported the above findings and proved that SLs down-regulate shoot branching.
4.3. Leaf Senescence and Photosynthesis
Leaf senescence takes place by the sequence of events such as chloroplast degradation and denaturation of RuBisCo and chlorophyll (Chl) a/b binding protein (CAB), then leaf colour changes to yellow [71]. The role of SL in leaf senescence is proved by the application of GR24 in SL deficient (d27, d17, and d10 in Oryza sativa as well as max1, max3, and max4 in Arabidopsis thaliana) and SL insensitive mutants (d3 and d14 of Oryza sativa, and max2 and atd14 of Arabidopsis thaliana) [71,72]. The normal course of senescence was restored regarding leaf colour, Chl content, and electrolyte leakage in SL-deficient Oryza sativa mutants, but not SL-insensitive mutants after GR24 application. The delayed senescence and increased senescence in the presence of GR24 were observed in SL-related mutants, indicating the role of SLs in leaf senescence.
GR24 application restored the level of Chl in drought stress. Parallel observations were also observed in wheat under drought stress [73] and rapeseed under salinity stress [74]. The Chl a/b ratio, an indicator of a plant’s ability to use light energy of different wavelengths [75], was reduced in SL-deficient mutants (d27, d17, d10, and max1, max3, and max4) [71,72]. The value of electron transport rate through PSII, Y(II), and NPQ was lower than that in wild type, indicating that SLs could modulate the capacity of leaves for capturing light energy by altering the components of photosynthetic pigments.
4.4. Hyphal Branching and Nodulation
AMF formed a symbiotic association with most land plants [76,77,78]. Studies supported that SLs help in inducing hyphal growth in AMF, thereby increasing mycorrhizal colonization in plants and consequently improving nutrient absorption and resource transport. Studies on rhizosphere, the mutant of pea and tomato deficient in SLs production, showed reduced AMF hyphae branches compared to wild type plants [79]. However, in the SL-biosynthesis mutant, lower colonization rates were observed in the wild type. The differences were minimized when the plants were inoculated with spores and hyphae or infected roots [79].
SLs play essential roles in regulating nodules under nitrogen deficiency in legumes [80,81]. In pea, SLs and brassinosteroids biosynthesis genes promote nodulation independently of the autoregulation of nodulation (AON) system [80]. Furthermore, a low concentration of GR24 in alfalfa can significantly increase nodulation number [82]. Likewise, in SL-deficient pea and lotus, GR24 helped maintain nodule formation. Additionally, the SL-deficient rms4 mutant in pea carries the maximum number of nodules than the wild type in comparison to rms1 plants [80,81,83]. Moreover, soybean with the over-expression of GmMAX2a has a higher number of nodules. In contrast, knockdown strains of the same gene have fewer nodules [84]. Therefore, we can say SLs positively control nodulation in legumes and play an essential role in nitrogen acquisition, nutrient access, and yield in legumes.
5. Strigolactones and Abiotic Stress
Generally, plants growing in standard conditions in the laboratory have deficient strigolactone levels. This primary hormone level allows some branching for maximal light capture and limits root growth for sufficient nutrient uptake and structural stability. On the other hand, when the plant encounters specific environmental difficulties, such as suboptimal nutrient availability or abiotic stress, strigolactone levels rise to optimize and adapt the plant’s growth strategy to fit the conditions [85,86].
The plant needs both morphological and physiological changes to maintain homeostasis. Researchers have documented SLs’ behaviour under unusual conditions. Some of them are mentioned here.
5.1. Nutrient Starvation
SLs have been suggested to plant architecture regulators concerning P regulation. Lateral root formation is repressed during sufficient P availability, whereas it is promoted when P is at low levels in the surroundings [87]. The phosphorus starvation-induced (PSI) genes are associated with plants responding to low P conditions and are used as markers. The expression of the PSI gene is not controlled by P but through SLs. SLs increase P mobility and absorption during severe deficiency. P and N deficiencies induce SLs deposition in root tissues, which activates signaling pathways all through nutrient stress. The pathway involves the expression of D10, D17, and D27, while suppressing D3, D14, and D53, as recorded in rice [88]. Further, in the case of Arabidopsis thaliana, the increased transcription of MAX3 and MAX4 in N deficiency suggests SLs accumulation in root tissues [7]. The establishment of AMF is one of the most remarkable contributions of SLs in P-deficient soil. The intimacy between the host and AMF is necessary for an influential symbiotic association and efficient P absorption. This association increases mycorrhizal colonization and hyphal growth [89]. Low soil P triggers SLs synthesis in Trifolium pratense and induction of CCD7 in Zea mays [90,91]. Similarly, in the case of leguminous plants, nutrient uptake is promoted via excessive nodulation, and in non-leguminous plants, P and N deficiencies facilitate SLs exudation and adopt AM association for nutrient absorption [92].
5.2. Drought
In drought stress, the chief avoidance responses include stomatal closure, lower transpiration, reactive oxygen species (ROS) scavenging, reduced lipid peroxidation, controlled Chl content, and photosynthetic rate. SLs have been shown to mitigate drought resulting in damage to many plants. Reduced H2O2, malondialdehyde (MDA), and electrolyte leakage levels and improved water content, photosynthesis, and membrane stability in the drought-exposed wheat and Vitis vinifera after SLs application suggest that it triggers ROS scavenging machinery [93,94]. Likewise, in Arabidopsis, max mutant strains showed a higher transpiration rate than the wild type. SLs also regulate Chl components and photosynthetic rate under drought stress [95]. SLs can also alter stomatal closure through abscisic acid (ABA) regulation indirectly. Reduced ABA biosynthesis in the rice strain mutant in the OsD27 gene suggested some correlation. In the d27 mutant line, reduced SL and ABA synthesis were recorded with higher drought sensitivity [96]. Similarly, the D14 mutant of Hordeum vulgare was hypersensitive to drought stress. In the mutant, lower relative water content, disorganized chloroplast structure, impaired photosynthesis, altered stomatal density, slower stomatal closure, and disrupted ABA metabolism were reported, unlike wild type [97]. Moreover, SLs proved beneficial in improving oil content in Dracocephalum kotschyi under drought conditions [98].
5.3. Salinity
SLs have also been found effective in controlling damage caused by salinity stress. SL-deficient (max3 and max4) and SL-signaling (max2) mutants showed hypersensitivity to salinity in Arabidopsis thaliana and Malus domestica during the germination and vegetative phase. Moreover, applying SLs in salt-exposed rice [99] and Salvia nemorosa [100] improved ROS scavenging responses, such as reduced H2O2 and MDA content and lesser cellular damage. The findings of Ren et al. [101] state that a combination of salt stress, ABA, and H2O2 stimulates SL-biosynthesis genes, including CCD7, CCD8 (in root) as well as MAX2 (in the shoot) in AMF-associated Sesbania cannabina. In the presence of H2O2 and SL inhibitor (TIS108, SL biosynthesis inhibitor), ABA-induced SL synthesis was blocked. Thus, we can conclude that ABA is essential for AMF-induced SL synthesis under salt stress. Likewise, in AMF inoculated Lactuca sativa roots SLs and ABA biosynthetic genes showed a positive correlation under salinity stress [102].
5.4. Heavy Metals
Heavy metal toxicity is one of the significant challenges in the agriculture sector. It retards growth, photosynthetic rate, Chl content, and antioxidant activities and increases plants’ reactive oxygen species (ROS) production. For instance, Cd stress in Panicum virgatum causes suppression in growth attributes, photosynthetic rate, Chl content, and antioxidant activities while enhancing levels of ROS. However, SLs have been shown to reverse all these symptoms in Panicum virgatum against Cd toxicity [103]. In the case of arsenic (As) toxicity, transcript levels of antioxidant enzymes including OsCuZnSOD1, OsCuZnSOD2, OsAPX1, OsAPX2, and OsCATA were noted to be relatively higher in wild type roots than SLs mutant (d10 and d17) roots in rice plants. The expression of transporter genes (OsPT1, OsPT2, OsPT4, and OsPT8) was seen to be higher in mutant plants than in wild type [104]. Structural similarity between As and P causes competitive inhibition of the P transporter, leading to P deficiency in the plant [105]. As stated earlier in this review, P deficiency triggers SL biosynthesis and signaling. Thus, we can assume that the SLs may be involved in the mitigation of heavy metal stress in plants. However, we need more detailed molecular pathways by which SLs act on heavy metal-stressed plants to reduce their harmful effects. Some important genes associated with nutrient deficiency are D10, D17, D27, and OsMAX1, which stimulate SL biosynthesis.
5.5. Heat Stress
Global temperature rise data are alarming to all the developed and under-developing countries. The hike is showing a devastating effect on crop productivity and longevity. Heat stress can sabotage plants’ physiological and morphological processes within a short period if the temperature goes beyond the threshold. During the early stages of plant development, the impact of heat stress is evident as decreased seed germination potential, poor germination, reduced seedling vigour, and, in extreme cases, complete loss of viability [106,107]. Photosynthetic damage, excessive ROS generation, electrolyte leakage, membrane permeability, and chlorosis are the ways by which heat stress can stun plant growth and productivity. To cope with the extreme temperature conditions, plants adopt several strategies, such as the synthesis of heat shock factors and heat shock proteins [108,109], involvement of hormones [110,111,112], activation of enzymatic and non-enzymatic antioxidant systems [113,114], and the accumulation of osmolytes, such as proline and betaine and glyoxylate. SLs bring noticeable changes in plants such as root and shoot architecture patterning [7], responses to nutrient (N and P) deficiency [7], and leaf senescence [115]. Studies showed that cold and heat stress resulted in higher CCD7 and CCD8 gene transcription in tomatoes suggesting that SLs positively regulate tomato heat and cold tolerance responses [116].
To have a clear view of SLs signaling and regulation, gene silencing experiments were performed with CCD7, CCD8, MAX1, and MAX2. According to the results, silent lines were prone to water loss under dehydration and higher stomatal conductance. The increased stomatal conductance and sensitivity of plants transformed with empty vector (pTRV) pTRV-MAX2 leaves to dehydration relative to pTRV-CCD7, pTRV-CCD8, and pTRV-MAX1 leaves are probably due to the role of MAX2 link not only to the strigolactone pathway but also to the (KARRIKIN INSENSITIVE2) KAI2-dependent signaling [117]. The ccd7 mutant in tomatoes showed increased lateral branches, reduced plant height, and higher stomatal conductance than the wild type. However, then the roots of these plants were treated with GR24 under heat stress, and heat-induced wilting was alleviated in both plants [116]. Heat stress resulted in a decrease in pigment system (PS) II efficiency, quantum yield, and increased REL was overcome in wild type and ccd mutants. Accumulation of HSP70 after GR24 exposure has been found to increase, improving heat tolerance responses [116].
SLs regulation under abiotic stress has been the main focus of current studies. Its role under drought and salinity stress has been extensively studied; however, its modulation under heat stress is still emerging. A thermo-inhibition study on Arabidopsis seed revealed the promotive role of SLs in seed germination [118]. The possible coordination between hormones suggested that SL application reduces the ABA/GA ratio, which alleviates seed thermo-inhibition. SLs act upstream of these essential seed hormones to inhibit ABA synthesis and stimulate gibberellin (GA) accumulation. It is the ABA/GA ratio that SLs regulate to break seed dormancy in both parasitic and non-parasitic plants. SLs may suppress the expression of ABA biosynthesis gene 9-cis-epoxycarotenoid dioxygenase (NCED9), resulting in a lower ABA/GA ratio. SLs application also enhanced the level of cytokinin (CK). In parasitic plant findings, CK and ethylene (ET) can stimulate seed germination in some Striga spp. including S. asiatica and S. hermonthica, even without SLs, which means they work downstream of SLs [119]. In the case of Arabidopsis, CK-induced Et biosynthesis facilitates seed germination under heat stress [118].
Another germination experiment was performed on lupine seeds under normal and heat-stressed conditions, and different physiological responses were recorded after SLs application. SLs effectuate specific changes such as higher germination indices, enhanced proline content, and reduced lipid peroxidation. GR24 also enhanced antioxidant enzyme activity and glyoxalase systems in lupine seedlings. The Chl, a fluorescence transient analysis (JIP-test), indicated that rac-GR24 provides strength to the oxygen evolution complex and prevents the inactivation of PSII reaction centres, thus conferring heat stress resistance in lupine seedlings [120].
SLs regulation on root development under different temperature conditions was proved by Hu et al. [121] in tall fescue. The result showed crown root elongation, increase in cell numbers, higher transcript of cell cycle-related genes, and down-regulation of auxin transport-related genes in crown root tips of tall fescue. Regulation of cell cycle and auxin transport are the primary targets of GR24 in these plants. Former studies demonstrated root elongation after SLs application by stimulating cell division in the root meristem zone [63,122]. According to this study, SL played a notable role under heat stress in overcoming damage associated with cell cycle genes such as proliferating cell nuclear antigen (PCNA), Cyclin D (CycD2), and Cyclin dependent kinase (CDKB). The expression level of these genes is accelerated after strigolactone exposure under heat stress compared to normal conditions [121].
SLs have shown visible effects in determining leaf morphology in the case of both monocots and dicots. Synthetic SLs have shown elongation of internodes [123], mesocotyl [124], hypocotyl [125], and roots [126] through active cell division and proper regulation. SL deficient mutants (max 1,3,4) plants are recorded to have reduced petiole and shorter leaf blades than wild type in Arabidopsis [127]. Strigolactone application resulted in increased leaf area under normal conditions; however, its application conferred resistance to heat stress on leaf elongation as explained in the case of root elongation in tall fescue plants [128]. Table 1 provides a summary of the effects of SL on plant functions under abiotic stress. Figure 5 provides the summary of SLs’ response under abiotic stress.
Table 1.
Effects of strigolactone application at different concentrations on plant functions under abiotic stresses in various plant species.
| Plant Specimen | Type of Stress | Mode of Application | Concentration | Effects on Plant | References |
|---|---|---|---|---|---|
| Triticum aestivum | Drought | Foliar spray | 10 μM GR24 (synthetic SL analogue) |
Increased: Relative water content, membrane stability index, activities of POD, CAT, and APX Decrease: Electrolyte leakage, MDA |
[73] |
| Vitis vinifera | Drought | Foliar spray | 1, 3, and 5 μM rac-GR24 | Increase: Relative water content, Chl content, and rate of photosynthesis, antioxidant capacity, activate transcriptions of VvHYD1, VvHYD2, VvCCD7, VvCCD8, and VvNCED1 Decrease: Electrolyte leakage, stomatal opening, ROS, and MDA content |
[93] |
| Triticum aestivum | Drought | Foliar spray | 10 μM GR24 (synthetic SL analogue) |
Increase: Proline and soluble sugar content, stomatal conductance, photosynthetic rate, osmotic adjustment Decrease: Water potential, transpiration rate, H2O2 |
[94] |
| Dracocephalum kotschyi | Drought | Spray | 10 μM rac-GR24 |
Increase: Fresh and dry weights, essential oil content, and yield Decrease: Electrolyte leakage, MDA, H2O2 |
[98] |
| Triticum aestivum | Salinity | Solution | 0.001, 0.01, and 0.1 mg L−1 GR24 (synthetic SL analogue) | Increase: CO2 assimilation rate, non-photochemical quenching and photochemical quenching, stomatal conductance Decrease: Total leaf area, root length, root fresh and dry weights |
[129] |
| Brassica napus | Salinity | Solution | 0.18 μM GR24 (synthetic SL analogue) |
Increase Leaf Chl content, net photosynthetic rate, stomatal conductance, intercellular CO2 concentration and transpiration rate, POD and SOD activities, expression of DEGs Decrease: H2O2 level, MDA content |
[74] |
| Oryza sativa | Salt | Hoagland nutrient solution |
0.1, 0.2, 1, and 5 μM GR24 (synthetic SL analogue) |
Increase: Plant height, root length, SOD and POD activities, intercellular CO2 concentration, net photosynthetic rate Decrease Lateral buds outgrowth, MDA, ROS |
[86] |
| Salvia nemorosa | Salinity | Spray | 0.1, 0.2, 0.3, and 0.4 μM GR24 | Increase: Net photosynthetic rate, stomatal conductance, intracellular CO2 and gas-exchange, essential oil yield, Chl content Decrease: MDA, electrolyte leakage, H2O2, activities of SOD, POD, CAT, and glutathione Reductase |
[100] |
| Malus domestica and Arabidopsis thaliana | Salt, drought, and low temperature | MS medium | 5, 10, and 20 μΜ GR24 (synthetic SL analogue) |
Increase: MdD14 degradation Decrease: Hypocotyl elongation, shoot branching, MdD14-His protein |
[130] |
| Helianthus annuus | Salinity | MS medium | 0.001, 0.01, and 0.1 mg L−1 GR24 (synthetic SL analogue) |
Increase: Activities of CAT and SOD, callus biomass, glycine betaine and protein content Decrease: MDA, Na+ content, H2O2 |
[131] |
| Lotus japonicas | Osmotic and phosphorous | Solution |
5.0 μM GR24 (synthetic SL analogue) |
Increase: LjPDR1-295a and LjPDR1-345 expression Decrease: ABA level, transcript level of ABA biosynthetic gene LjNCED2, and ABA catabolic gene LjAAO3 |
[132] |
| Oryza sativa | N | Nutrient solution |
2 μM GR24 (synthetic SL analogue) |
Increase: FC1 expression in tiller buds, OsIPT transcript level, expression of OsCKX Decrease: Tiller bud outgrowth, auxin transport capacity, IAA level, expression of OsPIN9 |
[133] |
| Festuca arundinacea | Heat | Foliar spray | 0.01 μM GR24 (synthetic SL analogue) |
Increase: Cell cycle-related genes, cell number, crown root elongation, expressions of D3 and D14 Decrease: Auxin transport related genes (TIR1, PIN1, PIN2, and PIN5) |
[121] |
| Festuca arundinacea | Heat | Foliar spray | 0.01 μM GR24 (synthetic SL analogue) |
Increase: leaf elongation, Cell cycle-related genes, cell number expressions of D3 and D14 Decrease: Auxin transport-related genes (TIR1, PIN1, PIN2, and PIN5) |
[128] |
| Arabidopsis thaliana | Heat | Solution | 20, 0.1 µM GR24 (synthetic SL analogue) |
Increase: Seed germination, P level, GA, and CK accumulation Decrease: ABA/GA ratio ABA levels, secondary dormancy |
[118] |
| Solanum lycopersicum | Heat and cold | Solution | 1, 3 and 9 µM GR24 (synthetic SL analogue) |
Increase: Hsp70, ABA synthesis, transcription of CBF1, CBF3, SOD, APX, GR, MDAR, and DHAR activity Decrease: heat sensitivity, REL level, MDA content, H2O2 content |
[116] |
| Lupinus angustifolius | Heat stress | Petri plate treatment | 3 µM rac-GR24 | Increase: seed resilience to high temperature, SOD activity, proline content, glyoxalase I and II activity PIabs, ROS scavenging mechanism Decrease: peroxidase activity, lipid peroxidation, ABS/RC ratio |
[120] |
Figure 5.
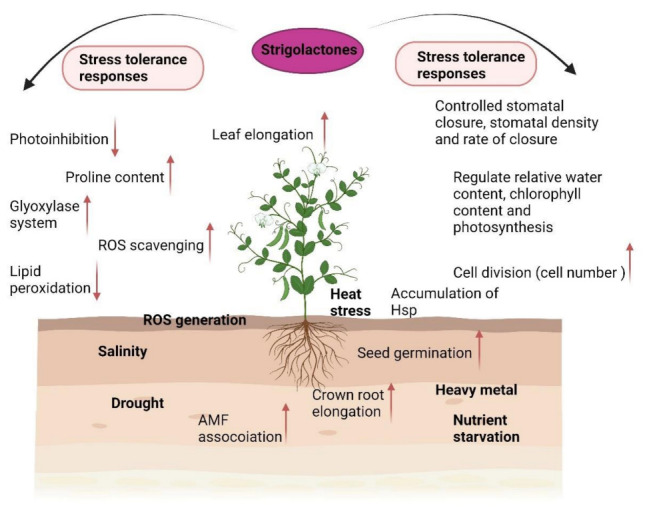
Involvement of strigolactones in plant adaptation to a range of abiotic stresses. ROS (Reactive oxygen species); AMF (Arbasscular Mychorrizal Fungi). Arrows:  Increase;
Increase;  Decrease.
Decrease.
6. Interaction of Strigolactones with Other Hormones
6.1. Auxin
Auxin regulation in root elongation through SLs has been well documented by workers [63,126]. In tall fescue plants, the combination of SLs and 1-N--naphthylphthalamic acid (NPA, auxin transport inhibitor) enhanced root elongation under heat stress. The combined GR24 and naphthaleneacetic acid (NAA) treatment did not enhance root elongation under non-stress or heat stress. These results suggested that SLs could not directly reverse the adverse effects of NAA on crown root elongation in tall fescue but through inhibition of NAA transport. The transcriptional study of the PIN protein family of auxin transporters states suppression of TIR1, PIN1, PIN2, and PIN5 under non-stress and heat stress conditions and positive regulation of auxin on SLs signaling via the D3 gene, similar is the case with leaf elongation [121,128].
SLs inhibit shoot branching by regulating auxin transport. Auxin transport inhibitor NPA prevents bud out-growth in max mutants in Arabidopsis and dwarf mutants in rice [27,134]. SLs application reduced basipetal auxin transport and PIN1 accumulation in the plasma membrane in biosynthesis mutants but not in max2 [135]. This proves that SLs dampen the PAT stream in a MAX2-dependent manner [135]. SLs modulated PIN cycling between the plasma membrane and endosome [136]. SLs action was simulated to increase the PIN1 removal rate from the plasma membrane in shoot branching mutants. The study concluded showed that SLs’ action in shoot stimulation depends upon auxin transport status and SLs concentration [137].
6.2. Cytokinin and Ethylene
CKs and SLs have generally been found to behave antagonistically in plant systems. Auxin, CK, and SLs have shown a significant role in bud formation. Here auxin and SL inhibit lateral bud growth, whereas CK promotes it. Therefore, tight regulation of the three is needed for standard plant architecture. SLs inhibit bud growth by suppressing CK biosynthesis, as reported in Zantedeschia aethiopica and rice. The transcription factors such as AXR1 and BRC1, which upregulate SL synthesis and are found in less branched varieties, have been shown to suppress CK synthesis. On the other hand, the concentration of CK biosynthesis enzymes in peas is higher in SL mutant (rms 1,2) plants than in normal. This suggests the antagonist mechanism of CK and SL regulation in plants. In Arabidopsis and pea, BRC1 is suggested to be expressed in axillary buds and act downstream of SL signaling during shoot branching inhibition [68,69,138]. BRC1 expression is upregulated by SLs application whereas downregulated by CK [69,138].
Parasitic plant seed germination is initiated when they are close to the host. The complex hormonal exchange takes place to break this dormancy. CK and ET can promote this seed germination process in parasitic plants in the absence of SLs [119,139,140], which means they act downstream of SLs; however, CK-induced seed germination is mainly due to enhanced ET biosynthesis [139].
6.3. Gibberellins
Both SLs and GAs regulate plant structure and function. According to some workers, GA negatively regulates endogenous SL levels in plants. The crosstalk between SLs and GAs could be linked with SLENDER1 (SLR1), a representative of DELLA proteins that negatively regulate GA signaling because SLR1 might be degraded in an SL-dependent manner, similar to how it occurs in the GA signaling pathway [141]. The GA receptor GIBBERELLIC ACID INSENSITIVE1 (GID1) and GA molecule together stimulate the interaction of the GID1 and DELLA proteins and cause its degradation by the 26S proteasome complex. In GA insensitive rice mutant higher level of SL is recorded with semi-dwarf phenotype and increased tillers [142]. According to Ito et al. [143], SL biosynthesis is regulated by GA through GA receptor GID1 and F-box protein GID2 dependent manner also, GA treatment reduced the infection of rice plants by the parasitic plant witchweed (Striga hermonthica) [143].
Moreover, the SL level was reduced after the application of active GA in the same plant. This indicates that SLs probably regulate shoot branching in cooperation with GAs. In Arabidopsis thaliana, promoter regions of SL biosynthesis genes contain fewer motifs recognized by GA-dependent transcription factors. Microarray data analysis has shown that treatment with GA3 resulted in varied expression of Arabidopsis thaliana SL-biosynthesis genes but in a dose-dependent manner [144]. Final confirmation of the crosstalk between SLs and GAs needs genetic analysis of the hormone and interactions of the SL receptor with single DELLA proteins. SLs application could alleviate thermo-inhibition by decreasing ABA levels by suppressing NCED9 transcript accumulation, increasing GA accumulation through MAX2, and breaking secondary dormancy in Arabidopsis [118]. SLs reduce the ABA:GA ratio, which amplifies germination activity, and GA is sufficient to counteract thermo inhibition in Arabidopsis seeds but is not sufficient to do so in parasitic plant seeds [118].
6.4. Abscisic Acid
The coordinating role of SLs with ABA during heat stress has already been discussed in the review; however, ABA helps overcome other stress. Both SLs and ABA are stress hormones and carotenoid derivatives. Changes in ABA levels also induce transcription of protein-encoding genes, including dehydrins, osmoprotectants, salinity, and drought-related genes that boost plant stress tolerance. The de novo synthesis of ABA has also been reported in stressed leaves and roots [145]. A series of physiological mechanisms are controlled via ABA and SLs under normal and uncontrolled conditions [101]. ABA helps protect mycorrhizal roots from stress, a crucial function for symbiosis development, completion of arbuscular formation, and promotion of sustainable plant root colonization. According to Lopez-Raez [146], a higher level of ABA in mycorrhiza, associated with stressed plants, would help to foster stress tolerance while at the same time enhancing and sustaining AM symbiosis. Under salt stress, SLs and ABA are essential for regulating and establishing symbiotic relationships among host plants and AMF. As per reports, exogenously applied ABA under stress may increase the accumulation of SLs. ABA is well known for its accumulation in plants under abiotic stress, especially in dehydration events [147]. In tomato leaves, ABA content increased after heat or cold stress in wild type and ccd7 plants, and the effect was more evident with the application of GR24. According to the study, heat and cold stress selectively regulated the transcript levels of ABA biosynthesis gene NCED6, Lycopersicon esculentum DEHYDRIN 4 (Le4), and ABA-RESPONSIVE ELEMENT BINDING (ABF4) in wild type and ccd7 plants. The transcription level of Le4 in ccd7 plants under hot conditions and ABF4 in ccd7 plants under cold conditions showed smaller peaks; however, treatment with GR24 increased the transcript levels of all the genes in wild type and ccd7 plants in both the temperature conditions. SLs also mediate the activity of the antioxidant enzyme system under heat stress. Increased transcript levels of Cu/Zn-superoxide dismutase (SOD), ascorbate peroxidase (APX), glutathione reductase (GR), monodehydroascorbate reductase (MDAR), and dehydroxyascorbate reductase (DHAR) under optimal or stress conditions in both wild and ccd7 in the presence of SL prove its role in activating ROS scavenging responses [116].
Figure 6 shows the possible crosstalk between SLs and other hormones and the relatable outcome after this interaction.
Figure 6.
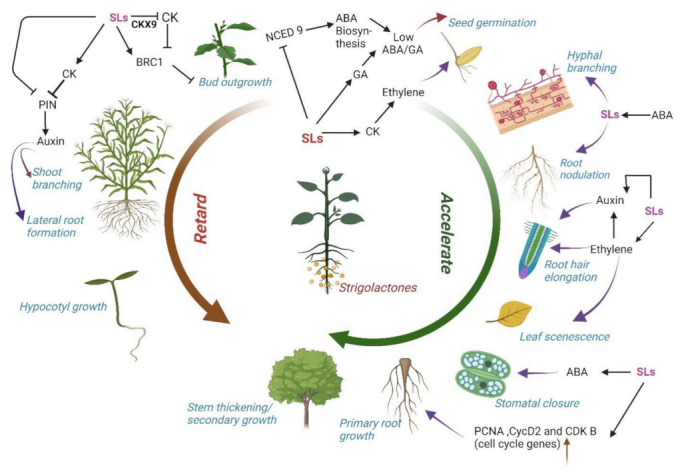
Coordinated role of strigolactones (SLs) with different hormones in regulating plant functions. There are certain phenomena that SLs either retard or accelerate. It needs coordinated crosstalk of different hormones and involvement of various genes to accomplish the outcome. Sometimes SLs may stop the synthesis or signaling of one hormone while promoting the other. In this way, homeostasis is maintained in the plant system in normal and stressed conditions. CKs (Cytokinins); ABA (Abscisic acid); gibberellins (GAs, BRC1 (BRANCH 1); CKX9 (Cytokinin Dehydrogenase); NCED 9 (9); Cyc D (Cyclin D); CDK (Cyclin dependent kinase); PCNA (Proliferating cell nuclear antigen); PIN (PIN FORMED; transporter protein).  Promotes
Promotes  Stops.
Stops.
7. Conclusions
SLs are essential for proper plant growth and vigour being associated with plant architecture and development, such as seed germination, shoot branching, leaf senescence, root development, and many more. Moreover, they have gained popularity for their significant role in plants’ adaptions to several abiotic stresses, such as drought, salinity, nutrient deficiency, heat, chilling and heavy metals, and in controlling several physiological and molecular processes. Stress may affect SLs biosynthesis, signaling, and crosstalk with other plant hormones. Several hints have recently been reported concerning SLs biosynthesis, but the bioactive form of SLs that regulates various aspects of plant growth and development is still uncertain. There are still many gaps in SLs signaling and perception that must be resolved for sustainable usage in agriculture. Identification of genetic variation and favourable alleles of genes involved in SLs’ diversification and downstream signaling processes would be a valuable asset to future breeding operations. It would aid in fine-tuning to maximize agricultural production. A clear understanding of this will open new doors toward plant resistance and higher yields.
Author Contributions
Conceptualization, N.A.K. and A.F.A.; software, A.F.A. and Z.S.; resources, Z.S., M.F. and A.M.; writing—original draft preparation, A.F.A.; writing—review and editing, Z.S., A.M. and N.A.K.; supervision, N.A.K. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Markham A. Potential impacts of climate change on ecosystems: A review of implications for policymakers and conservation biologists. Clim. Res. 1996;6:179–191. doi: 10.3354/cr006179. [DOI] [Google Scholar]
- 2.Hughes L. Biological consequences of global warming: Is the signal already apparent? Trends Ecol. Evol. 2000;15:56–61. doi: 10.1016/S0169-5347(99)01764-4. [DOI] [PubMed] [Google Scholar]
- 3.Morgan P.W. Effects of abiotic stresses on plant hormone systems. In: Alscher R.G., Cumming J.R., editors. Stress Responses in Plants: Adaptation and Acclimation Mechanism. Wiley-Liss; New York, NY, USA: 1990. pp. 113–146. [Google Scholar]
- 4.Cook C.E., Whichard L.P., Turner B., Wall M.E., Egley G.H. Germination of witchweed (Striga lutea Lour.): Isolation and properties of a potent stimulant. Science. 1966;154:1189–1190. doi: 10.1126/science.154.3753.1189. [DOI] [PubMed] [Google Scholar]
- 5.Zhang J., Mazur E., Balla J., Gallei M., Kalousek P., Medveďová Z., Friml J. Strigolactones inhibit auxin feedback on PIN-dependent auxin transport canalization. Nat. Commun. 2000;11:3580. doi: 10.1038/s41467-020-17252-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marzec M., Melzer M. Regulation of root development and architecture by strigolactones under optimal and nutrient deficiency conditions. Int. J. Mol. Sci. 2018;197:1887. doi: 10.3390/ijms19071887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito S., Ito K., Abeta N., Takahashi R., Sasaki Y., Yajima S. Effects of strigolactone signaling on Arabidopsis growth under nitrogen deficient stress condition. Plant Signal. Behav. 2016;11:e1126031. doi: 10.1080/15592324.2015.1126031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hauck C., Müller S., Schildknecht H. A germination stimulant for parasitic flowering plants from Sorghum bicolor, a genuine host plant. J. Plant Physiol. 1992;139:474–478. doi: 10.1016/S0176-1617(11)80497-9. [DOI] [Google Scholar]
- 9.Yokota T., Sakai H., Okuno K., Yoneyama K., Takeuchi Y. Alectrol and orobanchol, germination stimulants for Orobanche minor, from its host red clover. Phytochemistry. 1998;49:1967–1973. doi: 10.1016/S0031-9422(98)00419-1. [DOI] [Google Scholar]
- 10.Latef A.A.H.A., Hashem A., Rasool S., Abd_Allah E.F., Alqarawi A.A., Egamberdieva D., Jan S., Anjum N.A., Ahmad P. Arbuscular mycorrhizal symbiosis and abiotic stress in plants: A review. J. Plant Biol. 2016;59:407–426. doi: 10.1007/s12374-016-0237-7. [DOI] [Google Scholar]
- 11.Pozo M.J., Jung S.C., López-Ráez J.A., Azcón-Aguilar C. Impact of arbuscular mycorrhizal symbiosis on plant response to biotic stress: The role of plant defense mechanisms. In: Koltai H., Kapulnik Y., editors. Arbuscular Mycorrhizas: Physiology and Function. Springer; Dordrecht, The Netherlands: 2010. pp. 193–207. [Google Scholar]
- 12.Goldwasser Y., Yoneyama K., Xie X., Yoneyama K. Production of strigolactones by Arabidopsis thaliana responsible for Orobanche aegyptiaca seed germination. Plant Growth Regul. 2008;55:21–28. doi: 10.1007/s10725-008-9253-z. [DOI] [Google Scholar]
- 13.Walker C.H., Siu-Ting K., Taylor A., O’Connell M.J., Bennett T. Strigolactone synthesis is ancestral in land plants, but canonical strigolactone signalling is a flowering plant innovation. BMC Biol. 2019;17:70. doi: 10.1186/s12915-019-0689-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waters M.T., Gutjahr C., Bennett T., Nelson D. Strigolactone signalling and evolution. Ann. Rev. Plant Biol. 2017;68:298–322. doi: 10.1146/annurev-arplant-042916-040925. [DOI] [PubMed] [Google Scholar]
- 15.Waldie T., McCulloch H., Leyser O. Strigolactones and the control of plant development: Lessons from shoot branching. Plant J. 2014;79:607–622. doi: 10.1111/tpj.12488. [DOI] [PubMed] [Google Scholar]
- 16.Kodama K., Rich M.K., Yoda A., Shimazaki S., Xie X., Akiyama K., Mizuno Y., Komatsu A., Luo Y., Suzuki H., et al. An ancestral function of strigolactones as symbiotic rhizosphere signals. Nat. Commun. 2022;13:3974. doi: 10.1038/s41467-022-31708-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delaux P.M., Xie X., Timme R.E., Puech-Pages V., Dunand C., Lecompte E., Delwiche C.F., Yoneyama K., Bécard G., Séjalon-Delmas N. Origin of strigolactones in the green lineage. New Phytol. 2014;195:857–871. doi: 10.1111/j.1469-8137.2012.04209.x. [DOI] [PubMed] [Google Scholar]
- 18.Nishiyama T., Sakayama H., de Vries J., Buschmann H., Saint-Marcoux D., Ullrich K.K., Haas F.B., Vanderstraeten L., Becker D., Lang D., et al. The Chara Genome: Secondary complexity and implications for plant terrestrialization. Cell. 2018;174:448–464. doi: 10.1016/j.cell.2018.06.033. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y., Bouwmeester H.J. Structural diversity in the strigolactones. J. Exp. Bot. 2018;69:2219–2230. doi: 10.1093/jxb/ery091. [DOI] [PubMed] [Google Scholar]
- 20.Jia K.P., Baz L., Al-Babili S. From carotenoids to strigolactones. J. Exp. Bot. 2018;69:2189–2204. doi: 10.1093/jxb/erx476. [DOI] [PubMed] [Google Scholar]
- 21.Zwanenburg B., Pospíšil T. Structure and activity of strigolactones: New plant hormones with a rich future. Mol. Plant. 2013;6:38–62. doi: 10.1093/mp/sss141. [DOI] [PubMed] [Google Scholar]
- 22.Al-Babili S., Bouwmeester H.J. Strigolactones, a novel carotenoid-derived plant hormone. Ann. Rev. Plant Biol. 2015;66:161–186. doi: 10.1146/annurev-arplant-043014-114759. [DOI] [PubMed] [Google Scholar]
- 23.Tokunaga T., Hayashi H., Akiyama K. Medicaol, a strigolactone identified as a putative didehydro-orobanchol isomer, from Medicago truncatula. Phytochemistry. 2015;111:91–97. doi: 10.1016/j.phytochem.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 24.Kim H.I., Kisugi T., Khetkam P., Xie X., Yoneyama K., Uchida K., Yokota T., Nomura T., McErlean C.S., Yoneyama K. Avenaol, a germination stimulant for root parasitic plants from Avena strigosa. Phytochemistry. 2014;103:85–88. doi: 10.1016/j.phytochem.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 25.Gomez-Roldan V., Fermas S., Brewer P.B., Puech-Pagès V., Dun E.A., Pillot J.P., Letisse F., Matusova R., Danoun S., Portais J.C., et al. Strigolactone inhibition of shoot branching. Nature. 2008;455:189–194. doi: 10.1038/nature07271. [DOI] [PubMed] [Google Scholar]
- 26.Umehara M., Hanada A., Yoshida S., Akiyama K., Arite T., Takeda-Kamiya N., Magome H., Kamiya Y., Shirasu K., Yoneyama K., et al. Inhibition of shoot branching by new terpenoid plant hormones. Nature. 2008;455:195–200. doi: 10.1038/nature07272. [DOI] [PubMed] [Google Scholar]
- 27.Lin H., Wang R.X., Qian Q., Yan M.X., Meng X.B., Fu Z.M., Yan C.Y., Jiang B., Su Z., Li J.Y., et al. DWA RF27, an iron-containing protein required for the biosynthesis of strigolactones, regulates rice tiller bud outgrowth. Plant Cell. 2009;21:1512–1525. doi: 10.1105/tpc.109.065987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Waters M.T., Brewer P.B., Bussell J.D., Smith S.M., Beveridge C.A. The Arabidopsis ortholog of rice DWARF27 acts upstream of MAX1 in the control of plant development by strigolactones. Plant Physiol. 2012;159:1073–1085. doi: 10.1104/pp.112.196253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y., van Dijk A.D.J., Scaffidi A., Flematti G.R., Hofmann M., Charnikhova T., Verstappen F., Hepworth J., van der Krol S., Leyser O., et al. Rice cytochrome P450 MAX1 homologs catalyze distinct steps in strigolactone biosynthesis. Nat. Chem. Biol. 2014;10:1028–1033. doi: 10.1038/nchembio.1660. [DOI] [PubMed] [Google Scholar]
- 30.Mashiguchi K., Seto Y., Yamaguchi S. Strigolactone biosynthesis, transport and perception. Plant J. 2021;105:335–350. doi: 10.1111/tpj.15059. [DOI] [PubMed] [Google Scholar]
- 31.Alder A., Jamil M., Marzorati M., Bruno M., Vermathen M., Bigler P., Ghisla S., Bouwmeester H., Beyer P., Al-Babili S. The path from β-carotene to carlactone, a strigolactone- like plant hormone. Science. 2012;335:1348–1351. doi: 10.1126/science.1218094. [DOI] [PubMed] [Google Scholar]
- 32.Cardinale F., Korwin K.P., Schubert A., Visentin I. Strigolactones: Mediators of osmotic stress responses with a potential for agrochemical manipulation of crop resilience. J. Exp. Bot. 2018;69:2291–2303. doi: 10.1093/jxb/erx494. [DOI] [PubMed] [Google Scholar]
- 33.Abe S., Sado A., Tanaka K., Kisugi T., Asami K., Ota S., Nomura T. Carlactone is converted to carlactonoic acid by MAX1 in Arabidopsis and its methyl ester can directly interact with AtD14 in vitro. Proc. Natl. Acad. Sci. USA. 2014;111:18084–18089. doi: 10.1073/pnas.1410801111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stirnberg P., van De Sande K., Leyser H.M. MAX1 and MAX2 control shoot lateral branching in Arabidopsis. Development. 2002;129:1131–1141. doi: 10.1242/dev.129.5.1131. [DOI] [PubMed] [Google Scholar]
- 35.Booker J. MAX1 encodes a cytochrome P450 family member that acts downstream of MAX3/4 to produce a carotenoid-derived branch-inhibiting hormone. Dev. Cell. 2005;8:443–449. doi: 10.1016/j.devcel.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 36.Seto Y., Sado A., Asami K., Hanada A., Umehara M., Akiyama K., Yamaguchi S. Carlactone is an endogenous biosynthetic precursor for strigolactones. Proc. Natl. Acad. Sci. USA. 2014;111:1640–1645. doi: 10.1073/pnas.1314805111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wakabayashi T., Hamana M., Mori A., Akiyama R., Ueno K., Osakabe K., Suzuki H., Takikawa H., Mizutani M., Sugimoto Y. Direct conversion of carlactonoic acid to orobanchol by cytochrome P450 CYP722C in strigolactone biosynthesis. Sci. Adv. 2019;5:eaax9067. doi: 10.1126/sciadv.aax9067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wakabayashi T., Shida K., Kitano Y., Takikawa H., Mizutani M., Sugimoto Y. CYP722C from Gossypium arboreum catalyzes the conversion of carlactonoic acid to 5-deoxystrigol. Planta. 2020;251:97. doi: 10.1007/s00425-020-03390-6. [DOI] [PubMed] [Google Scholar]
- 39.Mori N., Nomura T., Akiyama K. Identification of two oxygenase genes involved in the respective biosynthetic pathways of canonical and non-canonical strigolactones in Lotus japonicus. Planta. 2020;251:40. doi: 10.1007/s00425-019-03332-x. [DOI] [PubMed] [Google Scholar]
- 40.Gobena D., Shimels M., Rich P.J., Ruyter-Spira C., Bouwmeester H., Kanuganti S., Mengiste T., Ejeta G. Mutation in sorghum LOW GERMINATION STIMULANT 1 alters strigolactones and causes Striga resistance. Proc. Natl. Acad. Sci. USA. 2017;114:4471–4476. doi: 10.1073/pnas.1618965114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mori N., Sado A., Xie X., Yoneyama K., Asami K., Seto Y., Nomura T., Yamaguchi S., Yoneyama K., Akiyama K. Chemical identification of 18-hydroxycarlactonoic acid as an LjMAX1 product and in planta conversion of its methyl ester to canonical and non-canonical strigolactones in Lotus japonicus. Phytochemistry. 2020;174:112–349. doi: 10.1016/j.phytochem.2020.112349. [DOI] [PubMed] [Google Scholar]
- 42.Brewer P.B., Yoneyama K., Filardo F., Meyers E., Scaffidi A., Frickey T., Akiyama K., Seto Y., Dun E.A., Cremer J.E., et al. LATERAL BRANCHING OXIDOREDUCTASE acts in the final stages of strigolactone biosynthesis in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2016;113:6301–6306. doi: 10.1073/pnas.1601729113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wakabayashi T., Yasuhara R., Miura K., Takikawa H., Mizutani M., Sugimoto Y. Specific methylation of (11R)-carlactonoic acid by an Arabidopsis SABATH methyltransferase. Planta. 2021;254:88. doi: 10.1007/s00425-021-03738-6. [DOI] [PubMed] [Google Scholar]
- 44.Yoneyama K., Akiyama K., Brewer P.B., Mori N., Kawano-Kawada M., Haruta S., Nishiwaki H., Yamauchi S., Xie X., Umehara M., et al. Hydroxyl carlactone derivatives are predominant strigolactones in Arabidopsis. Plant Direct. 2020;4:219. doi: 10.1002/pld3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arite T., Umehara M., Ishikawa S., Hanada A., Maekawa M., Yamaguchi S., Kyozuka J. d14, a strigolactone-insensitive mutant of rice, shows an accelerated outgrowth of tillers. Plant Cell Physiol. 2009;50:1416–1424. doi: 10.1093/pcp/pcp091. [DOI] [PubMed] [Google Scholar]
- 46.Johnson X., Brcich T., Dun E.A., Goussot M., Haurogné K., Beveridge C.A., Rameau C. Branching genes are conserved across species. Genes controlling a novel signal in pea are coregulated by other long-distance signals. Plant Physiol. 2006;142:1014–1026. doi: 10.1104/pp.106.087676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang L., Liu X., Xiong G., Liu H., Chen F., Wang L., Meng X., Liu G., Yu H., Yuan Y., et al. DWARF 53 acts as a repressor of strigolactone signalling in rice. Nature. 2013;504:401–405. doi: 10.1038/nature12870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marzec M., Gruszka D., Tylec P., Szarejko I. Identification and functional analysis of the HvD14 gene involved in strigolactone signaling in Hordeum vulgare. Physiol. Plant. 2016;158:341–355. doi: 10.1111/ppl.12460. [DOI] [PubMed] [Google Scholar]
- 49.Bhoi A., Yadu B., Chandra J., Keshavkant S. Contribution of strigolactone in plant physiology, hormonal interaction and abiotic stresses. Planta. 2021;254:28. doi: 10.1007/s00425-021-03678-1. [DOI] [PubMed] [Google Scholar]
- 50.Wang L., Wang B., Jiang L., Liu X., Li X., Lu Z., Meng X., Wang Y., Smith S.M., Li J. Strigolactone signaling in Arabidopsis regulates shoot development by targeting D53-like SMXL repressor proteins for ubiquitination and degradation. Plant Cell. 2015;27:3128–3142. doi: 10.1105/tpc.15.00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou F., Lin Q., Zhu L., Ren Y., Zhou K., Shabek N., Wan J. D14–SCFD3-dependent degradation of D53 regulates strigolactone signalling. Nature. 2013;504:406–410. doi: 10.1038/nature12878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yao R., Ming Z., Yan L., Li S., Wang F., Ma S., Yu C., Yang M., Chen L., Chen L., et al. DWARF14 is a non-canonical hormone receptor for strigolactone. Nature. 2016;536:469–473. doi: 10.1038/nature19073. [DOI] [PubMed] [Google Scholar]
- 53.Bennett T., Liang Y., Seale M., Ward S., Müller D., Leyser O. Strigolactone regulates shoot development through a core signalling pathway. Biol. Open. 2016;5:1806–1820. doi: 10.1242/bio.021402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kretzschmar T., Kohlen W., Sasse J., Borghi L., Schlegel M., Bachelier J.B., Reinhardt D., Bours R., Bouwmeester H.J., Martinoia E.A. petunia ABC protein controls strigolactone-dependent symbiotic signalling and branching. Nature. 2012;483:341–344. doi: 10.1038/nature10873. [DOI] [PubMed] [Google Scholar]
- 55.Zhao L.H., Zhou X.E., Wu Z.S., Yi W., Xu Y., Li S., Xu T.-H., Liu Y., Chen R.-Z., Kovach A., et al. Crystal structures of two phytohormone signal-transducing alpha/beta hydrolases: Karrikin-signaling KAI2 and strigolactone-signaling DWARF14. Cell Res. 2013;23:436–439. doi: 10.1038/cr.2013.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Saint Germain A., Clave G., Badet-Denisot M.A., Pillot J.P., Cornu D., Le Caer J.P., Burger M., Pelisser F., Retailleau P., Turnbull C., et al. An histidine covalent receptor and butenolide complex mediates strigolactone perception. Nat. Chem. Biol. 2016;12:787–794. doi: 10.1038/nchembio.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seto Y., Yasui R., Kameoka H., Tamiru M., Cao M., Terauchi R., Sakurada A., Hirano R., Kisugi T., Hanada A., et al. Strigolactone perception and deactivation by a hydrolase receptor DWARF14. Nat. Commun. 2019;10:191. doi: 10.1038/s41467-018-08124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kagiyama M., Hirano Y., Mori T., Kim S.Y., Kyozuka J., Seto Y., Yamaguchi S., Hakoshima T. Structures of D14 and D14L in the strigolactone and karrikin signaling pathways. Genes Cells. 2013;18:147–160. doi: 10.1111/gtc.12025. [DOI] [PubMed] [Google Scholar]
- 59.Carlsson G.H., Hasse D., Cardinale F., Prandi C., Andersson I. The elusive ligand complexes of the DWARF14 strigolactone receptor. J. Exp. Bot. 2018;69:2345–2354. doi: 10.1093/jxb/ery036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shabek N., Ticchiarelli F., Mao H., Hinds T.R., Leyser O., Zheng N. Structural plasticity of D3–D14 ubiquitin ligase in strigolactone signalling. Nature. 2018;563:652–656. doi: 10.1038/s41586-018-0743-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Burger M., Chory J. The many models of Strigolactone signaling. Trends Plant Sci. 2020;25:395–405. doi: 10.1016/j.tplants.2019.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jiang K., Feldman L.J. Root Meristem Establishment and Maintenance: The Role of Auxin. J. Plant Growth Regul. 2002;21:432–440. doi: 10.1007/s00344-002-0037-9. [DOI] [Google Scholar]
- 63.Ruyter-Spira C., Kohlen W., Charnikhova T., van Zeijl A., van Bezouwen L., de Ruijter N., Cardoso C., Lopez-Raez J.A., Matusova R., Bours R., et al. Physiological effects of the synthetic strigolactone analog GR24 on root system architecture in Arabidopsis: Another belowground role for strigolactones? Plant Physiol. 2011;155:721–734. doi: 10.1104/pp.110.166645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rasmussen A., Mason M.G., De Cuyper C., Brewer P.B., Herold S., Agusti J., Beveridge C.A. Strigolactones suppress adventitious rooting in Arabidopsis and pea. Plant Physiol. 2012;158:1976–1987. doi: 10.1104/pp.111.187104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arite T., Kameoka H. Strigolactone Positively Controls Crown Root Elongation in Rice. J. Plant Growth Regul. 2012;31:165–172. doi: 10.1007/s00344-011-9228-6. [DOI] [Google Scholar]
- 66.Sorefan K., Booker J., Haurogné K., Goussot M., Bainbridge K., Foo E., Leyser O. MAX4 and RMS1 are orthologous dioxygenase-like genes that regulate shoot branching in Arabidopsis and pea. Genes Dev. 2003;17:1469–1474. doi: 10.1101/gad.256603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Soundappan I., Bennett T., Morffy N., Liang Y., Stanga J.P., Abbas A., Leyser O., Nelson D.C. SMAX1-LIKE/D53 family members enable distinct MAX2- dependent responses to strigolactones and karrikins in Arabidopsis. Plant Cell. 2015;27:3143–3159. doi: 10.1105/tpc.15.00562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aguilar-Martinez J.A., Poza-Carrion C., Cubas P. Arabidopsis BRANCHED1 acts as an integrator of branching signals within axillary buds. Plant Cell Online. 2007;19:458–472. doi: 10.1105/tpc.106.048934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Braun N., de Saint Germain A., Pillot J.P., Boutet-Mercey S., Dalmais M., Antoniadi I., Li X., Maia-Grondard A., Le Signor C., Bouteiller N., et al. The pea TCP transcription factor PsBRC1 acts downstream of strigolactones to control shoot branching. Plant Physiol. 2012;158:225–238. doi: 10.1104/pp.111.182725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kellogg E.A. Plant evolution: The dominance of maize. Curr. Biol. 1997;7:411–413. doi: 10.1016/S0960-9822(06)00204-1. [DOI] [PubMed] [Google Scholar]
- 71.Yamada Y., Umehara M. Possible roles of strigolactones during leaf senescence. Plants. 2015;4:664–677. doi: 10.3390/plants4030664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yamada Y., Furusawa S., Nagasaka S., Shimomura K., Yamaguchi S., Umehara M. Strigolactone signaling regulates rice leaf senescence in response to a phosphate deficiency. Planta. 2014;240:399–408. doi: 10.1007/s00425-014-2096-0. [DOI] [PubMed] [Google Scholar]
- 73.Sedaghat M., Tahmasebi-Sarvestani Z., Emam Y., Mokhtassi-Bidgoli A. Physiological and antioxidant responses of winter wheat cultivars to strigolactone and salicylic acid in drought. Plant Physiol. Biochem. 2017;119:59–69. doi: 10.1016/j.plaphy.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 74.Ma N., Hu C., Wan L., Hu Q., Xiong J., Zhang C. Strigolactones improve plant growth, photosynthesis, and alleviate oxidative stress under salinity in rapeseed (Brassica napus L.) by regulating gene expression. Front. Plant Sci. 2017;8:1671. doi: 10.3389/fpls.2017.01671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dale M.P., Causton D.R. Use of the chlorophyll a/b ratio as a bioassay for the light environment of a plant. Functional Ecol. 1992;6:190–196. doi: 10.2307/2389754. [DOI] [Google Scholar]
- 76.Smith S.E., Read D.J. Mycorrhizal Symbiosis. Volume 611. Elsevier; Amsterdam, The Netherlands: 1997. Mycorrhizal Symbiosis; p. XVIII. [Google Scholar]
- 77.Redecker D., Raab P. Phylogeny of the Glomeromycota (arbuscular mycorrhizal fungi): Recent developments and new gene markers. Mycologia. 2006;98:885–895. doi: 10.1080/15572536.2006.11832618. [DOI] [PubMed] [Google Scholar]
- 78.Akiyama K., Matsuzaki K.I., Hayashi H. Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature. 2005;435:824–827. doi: 10.1038/nature03608. [DOI] [PubMed] [Google Scholar]
- 79.Koltai H., LekKala S.P., Bhattacharya C., Mayzlish-Gati E., Resnick N., Wininger S., Kapulnik Y. A tomato strigolactone-impaired mutant displays aberrant shoot morphology and plant interactions. J. Exp. Bot. 2010;61:1739–1749. doi: 10.1093/jxb/erq041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Foo E., Yoneyama K., Hugill C.J., Quittenden L.J., Reid J.B. Strigolactones and the regulation of pea symbioses in response to nitrate and phosphate deficiency. Mol. Plant. 2013;6:76–87. doi: 10.1093/mp/sss115. [DOI] [PubMed] [Google Scholar]
- 81.Foo E., Davies N.W. Strigolactones promote nodulation in pea. Planta. 2011;234:1073–1081. doi: 10.1007/s00425-011-1516-7. [DOI] [PubMed] [Google Scholar]
- 82.Soto M.J., Fernández-Aparicio M., Castellanos-Morales V., García-Garrido J.M., Ocampo J.A., Delgado M.J., Vierheilig H. First indications for the involvement of strigolactones on nodule formation in alfalfa (Medicago sativa) Soil Biol. Biochem. 2010;42:383–385. doi: 10.1016/j.soilbio.2009.11.007. [DOI] [Google Scholar]
- 83.Liu J., Novero M., Charnikhova T., Ferrandino A., Schubert A., Ruyter-Spira C., Bonfante P., Lovisolo C., Bouwmeester H.J., Cardinale F. Carotenoid cleavage dioxygenase 7 modulates plant growth, reproduction, senescence, and determinate nodulation in the model legume Lotus japonicus. J. Exp. Bot. 2013;64:1967–1981. doi: 10.1093/jxb/ert056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ahmad M.Z., Rehman N.U., Yu S., Zhou Y., Haq B.U., Wang J., Li P., Zeng Z., Zhao J. GmMAX2–D14 and –KAI interaction mediated SL and KAR signaling play essential roles in soybean root nodulation. Plant J. 2020;101:334–351. doi: 10.1111/tpj.14545. [DOI] [PubMed] [Google Scholar]
- 85.Kohlen W., Charnikhova T., Liu Q., Bours R., Domagalska M.A., Beguerie S., Verstappen F., Leyser O., Bouwmeester H., Ruyter-Spira C. Strigolactones are transported through the xylem and play a key role in shoot architectural response to phosphate deficiency in nonarbuscular mycorrhizal host Arabidopsis. Plant Physiol. 2011;155:974–987. doi: 10.1104/pp.110.164640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ling F., Su Q., Jiang H., Cui J., He X., Wu Z., Zhang Z., Liu J., Zhao Y. Effects of strigolactone on photosynthetic and physiological characteristics in salt-stressed rice seedlings. Sci. Rep. 2020;10:6183. doi: 10.1038/s41598-020-63352-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kapulnik Y., Delaux P.M., Resnick N., Mayzlish-Gati E., Wininger S., Bhattacharya C., Koltai H. Strigolactones affect lateral root formation and root-hair elongation in Arabidopsis. Planta. 2011;233:209–216. doi: 10.1007/s00425-010-1310-y. [DOI] [PubMed] [Google Scholar]
- 88.Sun H., Tao J., Liu S., Huang S., Chen S., Xie X., Xu G. Strigolactones are involved in phosphate-and nitrate-deficiency-induced root development and auxin transport in rice. J. Exp. Bot. 2014;65:6735–6746. doi: 10.1093/jxb/eru029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jeffries P., Gianinazzi S., Perotto S., Turnau K., Barea J.M. The contribution of arbuscular mycorrhizal fungi in sustainable maintenance of plant health and soil fertility. Biol. Fertil. Soils. 2003;37:1–16. doi: 10.1007/s00374-002-0546-5. [DOI] [Google Scholar]
- 90.Yoneyama K., Yoneyama K., Takeuchi Y., Sekimoto H. Phosphorus deficiency in red clover promotes exudation of orobanchol, the signal for mycorrhizal symbionts and germination stimulant for root parasites. Planta. 2007;225:1031–1038. doi: 10.1007/s00425-006-0410-1. [DOI] [PubMed] [Google Scholar]
- 91.Pan X., Zheng H., Zhao J., Xu Y., Li X. ZmCCD7/ZpCCD7 encodes a carotenoid cleavage dioxygenase mediating shoot branching. Planta. 2016;243:1407–1418. doi: 10.1007/s00425-016-2479-5. [DOI] [PubMed] [Google Scholar]
- 92.Yoneyama K., Xie X., Kusumoto D., Sekimoto H., Sugimoto Y., Takeuchi Y., Yoneyama K. Nitrogen deficiency as well as phosphorus deficiency in sorghum promotes the production and exudation of 5-deoxystrigol, the host recognition signal for arbuscular mycorrhizal fungi and root parasites. Planta. 2007;227:125–132. doi: 10.1007/s00425-007-0600-5. [DOI] [PubMed] [Google Scholar]
- 93.Min Z., Li R., Chen L., Zhang Y., Li Z., Liu M., Ju Y., Fang Y. Alleviation of drought stress in grapevine by foliar-applied strigolactones. Plant Physiol. Biochem. 2019;135:99–110. doi: 10.1016/j.plaphy.2018.11.037. [DOI] [PubMed] [Google Scholar]
- 94.Sedaghat M., Sarvestani Z.T., Emam Y., Bidgoli A.M., Sorooshzadeh A. Foliar-applied GR24 and salicylic acid enhanced wheat drought tolerance. Russian J. Plant Physiol. 2020;67:733–739. doi: 10.1134/S1021443720040159. [DOI] [Google Scholar]
- 95.Ha C.V., Leyva-González M.A., Osakabe Y., Tran U.T., Nishiyama R., Watanabe Y., Tanaka M., Seki M., Yamaguchi S., Dong N.V., et al. Positive regulatory role of strigolactone in plant responses to drought and salt stress. Proc. Natl. Acad. Sci. USA. 2014;111:851–856. doi: 10.1073/pnas.1322135111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Haider I., Andreo-Jimenez B., Bruno M., Bimbo A., Floková K., Abuauf H., Ntui V.O., Guo X., Charnikhova T., Al-Babili S., et al. The interaction of strigolactones with abscisic acid during the drought response in rice. J. Exp. Bot. 2018;69:2403–2414. doi: 10.1093/jxb/ery089. [DOI] [PubMed] [Google Scholar]
- 97.Marzec M., Daszkowska-Golec A., Collin A., Melzer M., Eggert K., Szarejko I. Barley strigolactone signalling mutant hvd14. d reveals the role of strigolactones in abscisic acid-dependent response to drought. Plant Cell Environ. 2020;43:2239–2253. doi: 10.1111/pce.13815. [DOI] [PubMed] [Google Scholar]
- 98.Bidabadi S.S., Sharifi P. Strigolactone and methyl jasmonate induced antioxidant defense and the composition alterations of different active compounds in Dracocephalum kotschyi Boiss under drought stress. J. Plant Growth Regul. 2021;40:878–889. doi: 10.1007/s00344-020-10157-6. [DOI] [Google Scholar]
- 99.Zhang X., Zhang L., Ma C., Su M., Wang J., Zheng S., Zhang T. Exogenous strigolactones alleviate the photosynthetic inhibition and oxidative damage of cucumber seedlings under salt stress. Sci. Hort. 2022;297:110962. doi: 10.1016/j.scienta.2022.110962. [DOI] [Google Scholar]
- 100.Sharifi P., Bidabadi S.S. Strigolactone could enhances gas exchange through augmented antioxidant defense system in Salvia nemorosa L. plants subjected to saline conditions stress. Ind. Crop. Prod. 2020;151:112460. doi: 10.1016/j.indcrop.2020.112460. [DOI] [Google Scholar]
- 101.Ren C.G., Kong C.C., Xie Z.H. Role of abscisic acid in strigolactone-induced salt stress tolerance in arbuscular mycorrhizal Sesbania cannabina seedlings. BMC Plant Biol. 2018;18:74. doi: 10.1186/s12870-018-1292-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Aroca R., Ruiz-Lozano J.M., Zamarreño Á.M., Paz J.A., García-Mina J.M., Pozo M.J., López-Ráez J.A. Arbuscular mycorrhizal symbiosis influences strigolactone production under salinity and alleviates salt stress in lettuce plants. J. Plant Physiol. 2013;170:47–55. doi: 10.1016/j.jplph.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 103.Tai Z., Yin X., Fang Z., Shi G., Lou L., Cai Q. Exogenous GR24 alleviates cadmium toxicity by reducing cadmium uptake in switchgrass (Panicum virgatum) seedlings. Int. J. Environ. Res. Public Health. 2017;14:852. doi: 10.3390/ijerph14080852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mostofa M.G., Rahman M.M., Nguyen K.H., Li W., Watanabe Y., Tran C.D., Zhang M., Itouga M., Fujita M., Tran L.S.P. Strigolactones regulate arsenate uptake, vacuolar-sequestration and antioxidant defense responses to resist arsenic toxicity in rice roots. J. Hazard. Mater. 2021;415:125589. doi: 10.1016/j.jhazmat.2021.125589. [DOI] [PubMed] [Google Scholar]
- 105.Li N., Wang J., Song W.Y. Arsenic uptake and translocation in plants. Plant Cell Physiol. 2016;57:4–13. doi: 10.1093/pcp/pcv143. [DOI] [PubMed] [Google Scholar]
- 106.Essemine J., Ammar S., Jbir N., Bouzid S. Sensitivity of two wheat species’s seeds (Triticum durum, Variety Karim and Triticum aestivum, Variety Salambo) to heat constraint during germination. Pakistan J. Biol. Sci. 2007;10:3762–3768. doi: 10.3923/pjbs.2007.3762.3768. [DOI] [PubMed] [Google Scholar]
- 107.Iloh A.C., Omatta G., Ogbadu G.H., Onyenekwe P.C. Effects of elevated temperature on seed germination and seedling growth on three cereal crops in Nigeria. Sci. Res. Essays. 2014;9:806–813. doi: 10.5897/SRE2014.5968. [DOI] [Google Scholar]
- 108.Park C.J., Seo Y.S. Heat shock proteins: A review of the molecular chaperones for plant immunity. Plant Pathol. J. 2015;31:323. doi: 10.5423/PPJ.RW.08.2015.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Guo M., Liu J.H., Ma X., Luo D.X., Gong Z.H., Lu M.H. The plant heat stress transcription factors (HSFs): Structure, regulation, and function in response to abiotic stresses. Front. Plant Sci. 2016;7:114. doi: 10.3389/fpls.2016.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ahammed G.J., Li X., Zhou J., Zhou Y.H., Yu J.Q. Plant Hormones under Challenging Environmental Factors. Springer; Dordrecht, The Netherland: 2016. Role of hormones in plant adaptation to heat stress; pp. 1–21. [Google Scholar]
- 111.Gautam H., Fatma M., Sehar Z., Iqbal N., Albaqami M., Khan N.A. Exogenously-sourced ethylene positively modulates photosynthesis, carbohydrate metabolism, and antioxidant defense to enhance heat tolerance in rice. Int. J. Mol. Sci. 2022;23:1031. doi: 10.3390/ijms23031031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sehar Z., Gautam H., Iqbal N., Alvi A.F., Jahan B., Fatma M., Khan N.A. The functional Interplay between ethylene, hydrogen sulfide, and sulfur in plant heat stress tolerance. Biomolecules. 2022;12:678. doi: 10.3390/biom12050678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Caverzan A., Casassola A., Brammer S.P. Antioxidant responses of wheat plants under stress. Genet. Mol. Biol. 2016;39:1–6. doi: 10.1590/1678-4685-GMB-2015-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ergin S., Gülen H., Kesici M., Turhan E., Ipek A., Köksal N. Effects of high temperature stress on enzymatic and non-enzymatic antioxidants and proteins in strawberry plants. Turk. J. Agric. For. 2016;40:908–917. doi: 10.3906/tar-1606-144. [DOI] [Google Scholar]
- 115.Tian M.Q., Jiang K., Takahashi I., Li G.D. Strigolactone-induced senescence of a bamboo leaf in the dark is alleviated by exogenous sugar. J. Pestic. Sci. 2018;43:173–179. doi: 10.1584/jpestics.D18-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chi C., Xu X., Wang M., Zhang H., Fang P., Zhou J., Xia X., Shi K., Zhou Y., Yu J. Strigolactones positively regulate abscisic acid-dependent heat and cold tolerance in tomato. Hortic. Res. 2021;8:237. doi: 10.1038/s41438-021-00668-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.De Cuyper C., Struk S., Braem L., Gevaert K., De Jaeger G., Goormachtig S. Strigolactones, karrikins and beyond. Plant Cell Environ. 2017;40:1691–1703. doi: 10.1111/pce.12996. [DOI] [PubMed] [Google Scholar]
- 118.Toh S., Kamiya Y., Kawakami N., Nambara E., McCourt P., Tsuchiya Y. Thermoinhibition uncovers a role for strigolactones in Arabidopsis seed germination. Plant Cell Physiol. 2012;53:107–117. doi: 10.1093/pcp/pcr176. [DOI] [PubMed] [Google Scholar]
- 119.Logan D.C., Stewart G.R. Thidiazuron stimulates germination and ethylene production in Striga hermonthica–comparison with the effects of GR-24, ethylene and 1-aminocyclopropane-1-carboxylic acid. Seed Sci. Res. 1995;5:99–108. doi: 10.1017/S0960258500002671. [DOI] [Google Scholar]
- 120.Omoarelojie L.O., Kulkarni M.G., Finnie J.F., Pospíšil T., Strnad M., Van Staden J. Synthetic strigolactone (rac-GR24) alleviates the adverse effects of heat stress on seed germination and photosystem II function in lupine seedlings. Plant Physiol. Biochem. 2020;155:965–979. doi: 10.1016/j.plaphy.2020.07.043. [DOI] [PubMed] [Google Scholar]
- 121.Hu Q., Zhang S., Huang B. Strigolactones and interaction with auxin regulating root elongation in tall fescue under different temperature regimes. Plant Sci. 2018;271:34–39. doi: 10.1016/j.plantsci.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 122.Takatsuka H., Umeda M. Hormonal control of cell division and elongation along differentiation trajectories in roots. J. Exp. Bot. 2014;65:2633–2643. doi: 10.1093/jxb/ert485. [DOI] [PubMed] [Google Scholar]
- 123.De Saint Germain A., Ligerot Y., Dun E.A., Pillot J.P., Ross J.J., Beveridge C.A., Rameau C. Strigolactones stimulate internode elongation independently of gibberellins. Plant Physiol. 2013;163:1012–1025. doi: 10.1104/pp.113.220541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hu Z., Yamauchi T., Yang J., Jikumaru Y., Tsuchida-Mayama T., Ichikawa H., Takamure I., Nagamura Y., Tsutsumi N., Yamaguchi S., et al. Strigolactone and cytokinin act antagonistically in regulating rice mesocotyl elongation in darkness. Plant Cell Physiol. 2013;55:30–41. doi: 10.1093/pcp/pct150. [DOI] [PubMed] [Google Scholar]
- 125.Jia K.P., Luo Q., He S.B., Lu X.D., Yang H.Q. Strigolactone-regulated hypocotyl elongation is dependent on cryptochrome and phytochrome signaling pathways in Arabidopsis. Mol. Plant. 2014;7:528–540. doi: 10.1093/mp/sst093. [DOI] [PubMed] [Google Scholar]
- 126.Sun H., Tao J., Hou M., Huang S., Chen S., Liang Z., Xie T., Wei Y., Xie X., Yoneyama K., et al. A strigolactone signal is required for adventitious root formation in rice. Ann. Bot. 2015;115:1155–1162. doi: 10.1093/aob/mcv052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Boerjan W., Cervera M.T., Delarue M., Beeckman T., Dewitte W., Bellini C., Caboche M., Van Onckelen H., Van Montagu M., Inzé D. Superroot, a recessive mutation in Arabidopsis, confers auxin overproduction. Plant Cell. 1995;7:1405–1419. doi: 10.1105/tpc.7.9.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hu Q., Zhang S., Huang B. Strigolactones promote leaf elongation in tall fescue through upregulation of cell cycle genes and downregulation of auxin transport genes in tall fescue under different temperature regimes. Int. J. Mol. Sci. 2019;20:1836. doi: 10.3390/ijms20081836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kausar F., Shahbaz M. Influence of strigolactone (GR24) as a seed treatment on growth, gas exchange and chlorophyll fluorescence of wheat under saline conditions. Int. J. Agric. Biol. 2017;19:321–327. doi: 10.17957/IJAB/15.0283. [DOI] [Google Scholar]
- 130.Yang Y.Y., Ren Y.R., Zheng P.F., Zhao L.L., You C.X., Wang X.F., Hao Y.J. Cloning and functional identification of a strigolactone receptor gene MdD14 in apple. Plant Cell Tissue Organ Cult. 2020;140:197–208. doi: 10.1007/s11240-019-01722-3. [DOI] [Google Scholar]
- 131.Zulfiqar H., Shahbaz M., Ahsan M., Nafees M., Nadeem H., Akram M., Maqsood A., Ahmar S., Kamran M., Alamri S., et al. Strigolactone (GR24) induced salinity tolerance in sunflower (Helianthus annuus L.) by ameliorating morpho-physiological and biochemical attributes under in vitro conditions. J. Plant Growth Regul. 2021;40:2079–2091. doi: 10.1007/s00344-020-10256-4. [DOI] [Google Scholar]
- 132.Liu J., He H., Vitali M., Visentin I., Charnikhova T., Haider I., Schubert A., Ruyter-Spira C., Bouwmeester H.J., Lovisolo C., et al. Osmotic stress represses strigolactone biosynthesis in Lotus japonicus roots: Exploring the interaction between strigolactones and ABA under abiotic stress. Planta. 2015;241:1435–1451. doi: 10.1007/s00425-015-2266-8. [DOI] [PubMed] [Google Scholar]
- 133.Xu J., Zha M., Li Y., Ding Y., Chen L., Ding C., Wang S. The interaction between nitrogen availability and auxin, cytokinin, and strigolactone in the control of shoot branching in rice (Oryza sativa L.) Plant Cell Rep. 2015;34:1647–1662. doi: 10.1007/s00299-015-1815-8. [DOI] [PubMed] [Google Scholar]
- 134.Bennett T., Sieberer T., Willett B., Booker J., Luschnig C., Leyser O. The Arabidopsis MAX pathway controls shoot branching by regulating auxin transport. Curr. Biol. 2006;16:553–563. doi: 10.1016/j.cub.2006.01.058. [DOI] [PubMed] [Google Scholar]
- 135.Crawford S., Shinohara N., Sieberer T., Williamson L., George G., Hepworth J., Müller D., Domagalska M.A., Leyser O. Strigolactones enhance competition between shoot branches by dampening auxin transport. Development. 2010;137:2905–2913. doi: 10.1242/dev.051987. [DOI] [PubMed] [Google Scholar]
- 136.Prusinkiewicz P., Crawford S., Smith R.S., Ljung K., Bennett T., Ongaro V., Leyser O. Control of bud activation by an auxin transport switch. Proc. Natl. Acad. Sci. USA. 2009;106:17431–17436. doi: 10.1073/pnas.0906696106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shinohara N., Taylor C., Leyser O. Strigolactone can promote or inhibit shoot branching by triggering rapid depletion of the auxin efflux protein PIN1 from the plasma membrane. PLoS Biol. 2013;11:e1001474. doi: 10.1371/journal.pbio.1001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dun E.A., de Saint Germain A., Rameau C., Beveridge C.A. Antagonistic action of strigolactone and cytokinin in bud outgrowth control. Plant Physiol. 2012;158:487–498. doi: 10.1104/pp.111.186783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Babiker A.G.T., Butler L.G., Ejeta G., Woodson W.R. Enhancement of ethylene biosynthesis and germination by cytokinins and 1-aminocyclopropane-l-carboxylic acid in Striga asiatica seeds. Physiol. Plant. 1993;89:21–26. doi: 10.1111/j.1399-3054.1993.tb01781.x. [DOI] [Google Scholar]
- 140.Sugimoto Y., Ali A.M., Yabuta S., Kinoshita H., Inanaga S., Itai A. Germination strategy of Striga hermonthica involves regulation of ethylene biosynthesis. Physiol. Plant. 2003;119:137–145. doi: 10.1034/j.1399-3054.2003.00162.x. [DOI] [Google Scholar]
- 141.Nakamura H., Xue Y.L., Miyakawa T., Hou F., Qin H.M., Fukui K., Shi X., Ito E., Ito S., Park S.H., et al. Molecular mechanism of strigolactone perception by DWARF14. Nat. Commun. 2013;4:2613. doi: 10.1038/ncomms3613. [DOI] [PubMed] [Google Scholar]
- 142.Marzec M. Strigolactones and gibberellins: A new couple in the phytohormone world? Trends Plant Sci. 2017;22:813–815. doi: 10.1016/j.tplants.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 143.Ito S., Yamagami D., Umehara M., Hanada A., Yoshida S., Sasaki Y., Yajima S., Kyozuka J., Ueguchi-Tanaka M., Mat-suoka M., et al. Regulation of strigolactone biosynthesis by gibberellin signaling. Plant Physiol. 2017;174:1250–1259. doi: 10.1104/pp.17.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lantzouni O., Klermund C., Schwechheimer C. Largely additive effects of gibberellin and strigolactone on gene expression in Arabidopsis thaliana seedlings. Plant J. 2017;92:924–938. doi: 10.1111/tpj.13729. [DOI] [PubMed] [Google Scholar]
- 145.Fujita Y., Fujita M., Shinozaki K., Yamaguchi-Shinozaki K. ABA-mediated transcriptional regulation in response to osmotic stress in plants. J. Plant Res. 2011;124:509–525. doi: 10.1007/s10265-011-0412-3. [DOI] [PubMed] [Google Scholar]
- 146.López-Ráez J.A. How drought and salinity affect arbuscular mycorrhizal symbiosis and strigolactone biosynthesis? Planta. 2016;243:1375–1385. doi: 10.1007/s00425-015-2435-9. [DOI] [PubMed] [Google Scholar]
- 147.Zhu J.K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002;53:247–273. doi: 10.1146/annurev.arplant.53.091401.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


