Abstract
Cydonia oblonga is a medicinal plant that is used to treat a number of health complications in traditional medication systems. The objective of this study was to evaluate the phytochemical composition, and antibacterial, antioxidant, and ant-diabetic potentials of methanolic extracts of Cydonia oblonga bark. The Cydonia oblonga bark extraction was fractionated through HPLC and seven purified fractions labeled as F1, F2, F3, F4, F5, F6, and F7 were obtained. The HPLC-UV analysis of methanolic extract showed the presence of a number of possible compounds. The GC-MS and HPLC analysis confirmed the presence of the following bioactive compounds in the crude extract and purified fractions: malic acid, mandelic acid, quercetin, caffeic acid, catechin hydrate, as morin (HPLC analysis), BIS-(2-ethylhexyl)phthalate and diisooctyl phthalate (F1), carbamide (F2, used as fertilizer), octasiloxane and dimethylsiloxanecyclictrimer (F3), silicic acid and cyclotrisiloxane (F4), 6-AH-cAMP, 4H-cyclopropa[5′,6′]benz[1′,2′,7,8]azule, and 4-(4-chlorophenyl)-3-morpholinepyrol-2-yl)-butenedioic acid (F5), isopropyamine (F6), and 1-propylhydrazine (F7). The extract and purified fractions were then tested for biological activities. All the purified fractions and methanolic extract showed effective antibacterial activity; however, the highest activity was recorded for methanolic extract against Staphylococcus aureus and Streptococcus pneumonia. Antioxidant evaluation of methanolic extract and purified fractions against DPPH showed strong % inhibition of the synthetic free radical. The methanolic extract exhibited 87.41 ± 0.54% inhibition whereas fractions showed: F1, 85.45 ± 0.85; F2, 65.78 ± 0.68; F3, 58.61 ± 0.58; F4, 80.76 ± 0.59; F5, 571.29 ± 0.49; F6, 85.28 ± 0.94; and F7, 48.45 ± 0.62% inhibition. Ascorbic acid (standard) was used as a control with 94.88 ± 0.56% inhibition at a maximum concentration of 1000 µg/mL. The α-glucosidase inhibition assay of methanolic extract and purified fractions at a maximum concentration of 1000 µg/mL showed activities as: methanolic extract, 78.21 ± 0.67; F1, 55.01 ± 0.29; F2, 56.10 ± 0.24; F3, 62.44 ± 1.03; F4, 70.52 ± 0.15; F5, 62.18 ± 0.92; F6, 72.68 ± 0.2; and F7, 57.33 ± 0.05% inhibition. α-Amylase % inhibition of methanolic extract and purified fractions were noted as: methanolic extract, 77.98 ± 0.57; F1, 79.72 ± 0.02; F2, 79.72 ± 0.02; F3, 82.16 ± 0.48; F4, 77.37 ± 0.28; F5, 72.14 ± 0.30; F6, 74.24 ± 0.29; and F7, 56.58 ± 0.10 at the highest concentration of 1000 µg/mL. Acarbose (standard) showed 87.65 ± 0.71% inhibition of α-glucosidase and 85.99 ± 0.44% inhibition of α-amylase at the highest concentration of 1000 µg/mL. It was found that all biological activities of methanolic extract and purified fractions might be attributed to the fact that they are rich sources of phenolic and flavonoids along with other bioactive compounds. The total phenolic and flavonoid contents of methanolic extract were recorded higher as compared to purified fractions (TPC = 70% and TFC = 69%). Amongst the purified fractions, fraction 6 exhibited the highest TPC value (64%), and purified fraction 1 exhibited the highest value of TFC (58%). Recent research demonstrated that Cydonia oblonga may be considered an antibacterial medicinal plant. The result of the present study revealed that it might be utilized for the isolation of bioactive phytochemicals that can lead to new opportunities in the discovery of new antibiotics.
Keywords: HPLC analysis, GC-MS analysis, antioxidant activity, antibacterial activity
1. Introduction
Much work has been carried out on studying and isolating natural compounds with pharmacological, pesticidal, and dietary benefits. Natural products have attracted a lot of attention in research. Since the earliest days, natural products have been employed as therapeutic agents for curing diseases [1]. The WHO estimates that more than 80% of the world’s population uses herbal medicines [2]. Herbal medicine is still incorporated into the basic healthcare system despite being replaced by conventional pharmaceuticals. The potential health and pharmacological benefits of phytochemicals have been highlighted, including their antioxidant, antibacterial, anti-cancer, cardioprotective, immune system-boosting, UV radiation-protective [3], and anti-inflammatory [3,4] properties. Additionally, medicinal plants are thought to be an important source in the development of new drugs. Synthetic medicines are constantly replacing effective pharmacological phytochemicals. However, the value of natural products cannot be emphasized enough, as herbal medicine offers a wide range of therapeutic benefits and may be used to treat a wide range of illnesses [5], including chronic diseases such as diabetes and related health complications of oxidative stress. Diabetes is considered to be the third most life-threatening health complication after cancer and heart attacks. Oxidative stress is caused by a number of causative agents and leads to number of health issues including diabetes. The most important causative agent of oxidative stress in the human body is the production of free radicals, also called reactive oxygen species, which cause the breaking of biologically important molecules such as protein and DNA, thus leading to a number of health issues. They are produced in normal oxidation processes in the body. Phenolic compounds, by virtue of a benzene ring, can stabilize these free radicals, which is why vegetables and fruits are important to be ingested in a person’s diet [1,2,3,4,5].
Secondary metabolites are important components of the human diet as they are present in daily food products such as coffee, tea, soybean, fruits, and vegetables [6]. Due to their advantages in human health, including the treatment and prevention of many illnesses, phenolic compounds and flavonoids are well known as antioxidants and other significant bioactive agents. Various studies have revealed that the consumption of polyphenols has a protective effect against inflammation and cancer [7]. Because they are known to scavenge and stop the generation of reactive oxygen and nitrogen species, the anti-inflammatory actions of polyphenols have been attributed exclusively to their antioxidant activity [8]. Moreover, phenolic-rich plants have anti-tumor [9], anti-mutagenic, anti-HIV [10], and anti-diabetic [11] properties.
Antimicrobial resistance has caused serious concern for both human health and the control of bacterial infectious illnesses [12]. Due to the intensive misuse of antibiotics to treat infections, bacterial resistance has increased in recent years [13]. A number of common pathogenic strains already bear antibiotic-resistant genes, and, presumably, more antibiotic-resistant pathogens will emerge in the future, if suitable measures are not taken to prevent this [14]. Antimicrobial resistance may be handled in two different ways, either by developing new active materials, which has growing bench-to-market costs and is time consuming [15], or by looking for ways to make existing antimicrobials more effective [16]. As phytochemicals have antibacterial potential [3], they may be potential candidates to counter antibacterial resistance. Diabetes mellitus is a metabolic illness with numerous etiologies marked by a lack of glucose homeostasis as well as abnormalities in carbohydrate, lipid, and protein metabolism as a result of deficiencies in insulin production and/or action [17]. Raised blood glucose is one of the most significant public health issues in the world; after high blood pressure and cigarette smoking, it is the third most significant risk factor for early mortality globally, according to a study by the International Diabetes Federation [18]. Well-known anti-inflammatory phytochemicals such as flavonoids, polyphenols, and tannins, which function as free radical scavengers, may have anti-diabetic effects [19,20]. These antioxidants are considered to have an insulin-mimetic impact on peripheral tissues, either by promoting tissue regeneration or by causing existing cells to secrete pancreatic insulin. The phytochemicals may also speed up filtration and renal excretion, improving metabolism, or integrating glucose into fat deposits, which improves the efficiency of the pancreas to make insulin [19].
Quince (Cydonia oblonga) belongs to the family Rosaceae, which is conventionally popular for medicinal, nutritional, and ornamental uses [21,22]. The name of the Cydonia genus is derived from the name of a region Kydonia on the northwestern Coast of Crete, Greece, where this tree has been cultivated since ancient times. In older Greek rituals, the fruit was offered at weddings as they symbolized fertility [23]. The fruits of quince are used in various countries for food, pharmaceutical, perfume, and agricultural purposes [24]. Therefore, this plant has garnered considerable attention due to its economic value. Secondary metabolites (phenolic compounds and essential oils), minerals, and vitamins are found in large amounts in quince fruits [25]. Various studies have revealed that the pulp of quince reduces the risk of diseases such as cardiovascular diseases [26], cancer [27], colon issues, and kidney problems [28]. Quince tannins are used as an antiseptic agent in pulmonary infections as they show activity against bacteria, fungi, and viruses [29]. Furthermore, the seed of quince is used for the treatment of cough, diarrhea, constipation, dysentery, and bronchitis. Numerous secondary metabolites have been identified in the phytochemical screening of quince fruits [30].
The bark of the Cydonia oblonga plant has not been studied to date. Therefore, the aim of the present work was to determine and characterize of the secondary metabolites of Cydonia oblonga bark using high-performance liquid chromatography, and to investigate the antioxidant, antibacterial, and anti-diabetic activity of the bark extract and the seven isolated fractions.
2. Results
2.1. Phytochemical Evaluation
Preliminary phytochemical screening of Met + Ext of Cydonia oblonga showed positive results for major phytochemical constituents as given in Table 1.
Table 1.
Preliminary qualitative phytochemicals screening of Cydonia oblonga Met + Ext.
| Phytochemicals | Reagent | Analyses | Result |
|---|---|---|---|
| Flavonoids | Ferric chloride | Appearance of yellow color and colorless after HCL addition | + |
| Alkaloids | Dragendroff’s | Formation of orange–red color precipitate | + |
| Glycosides | Keller Killiani | Red to brown layer formation | + |
| Triterpenoids | Liebermann Burchard | Reddish–brown boundary | + |
| Tannins | Gelatine | Brownish to green precipitates | + |
The + sign represents the presence of a given phytochemical group.
2.2. TFC and TPC in the Met-Ext and Purified Fractions of Cydonia oblonga
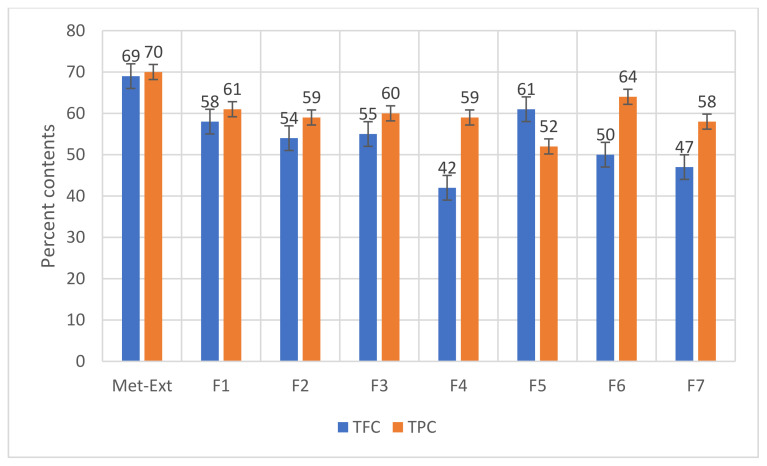
The results of TPC and TFC are presented in Figure 1. Met-Ext has the highest TFC and TPC values of 69% and 70%, respectively, while purified fraction 6 has the highest TPC (64%) and purified F1 has the highest TFC (58%).
Figure 1.
TPC and TFC of Met-Ext and purified fractions of Cydonia oblonga.
2.3. HPLC Analysis of Methanolic Extract of Cydonia oblonga
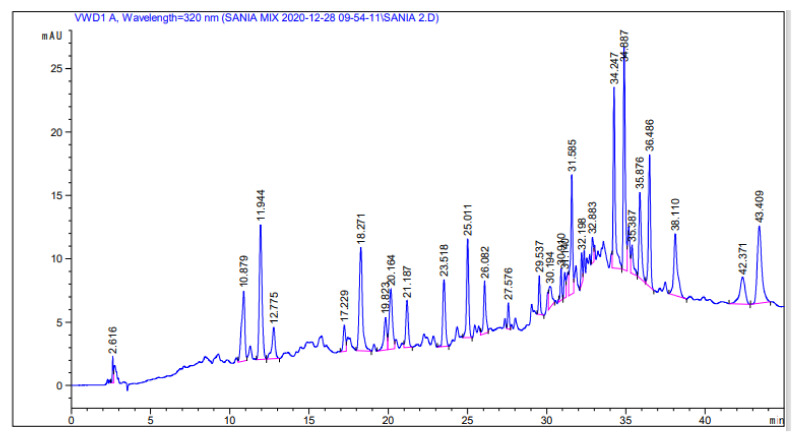
The human health benefits of phenolic compounds are widely documented. HPLC analysis was carried out to determine the phenolic content of the Met + Ext of Cydonia oblonga bark. Figure 2 displays the extract chromatogram and Table 2 displays the possible compounds. Although there are many peaks in the chromatogram, only a few were identified by comparing their retention times with that of available standards. Furthermore, it should be noted that they are only possible compounds as HPLC is not an absolute technique for compound identifications.
Figure 2.
HPLC-UV chromatogram of bioactive compounds of the Cydonia oblonga Met + Ext.
Table 2.
Identification of bioactive compounds in Met + Ext of Cydonia oblonga through HPLC-UV technique.
| Retention Time (min) |
Phenolic Compound Identity | Peak Area of Sample | Identification Reference |
|---|---|---|---|
| 2.616 | Malic acid | 7.64427 | Ref. Stand |
| 10.879 | Mandelic acid | 28.98360 | Ref. Stand |
| 12.373 | Caffeic acid | 21.41109 | Ref. Stand |
| 20.578 | Quercetin | 77.68702 | Ref. Stand |
| 29.537 | Catechin hydrate | 46.64664 | Ref. Stand |
| 30.408 | Morin | 134.41394 | Ref. Stand |
2.4. GC-MS Characterization of Methanolic Extract and Purified Fractions
The results of GC-MS analysis of the fractions are given in the Supporting Materials in Figures S1–S20 (Figures S1, S4, S7, S10, S13, S15 and S18 are the GC chromatograms, Figure S2, S5, S8, S11, S14, S16 and S19 represents structure of compounds identified whereas Figure S3, S6, S9, S12, S17 and S20 represents the mass fragmentation of individual compounds identified in each fraction) whereas Tables S1–S7 correspondingly summarizes the major compounds present in fractions F1 to F7. The identified compounds are depicted in Table 3.
Table 3.
List of the identified compounds in Cydonia oblonga bark fractions (F1-F7) determined through GC-MS analysis.
| S. No | Fraction | Compounds |
|---|---|---|
| 1 | F1 | BIS-(2-ethylhexyl)phtjalate, diisooctyl phthlate |
| 2 | F2 | carbamide |
| 3 | F3 | octasiloxane, dimethylsiloxanecyclictrimer |
| 4 | F4 | silicic acid, cyclotrisiloxane |
| 5 | F5 | 6-AH-cAMP, 4H-cyclopropa[5′,6′]benz[1′,2′,7,8]azule, 4-(4-chlorophenyl)-3-morpholinepyrol-2-yl)-butenedioic acid |
| 6 | F6 | isopropyamine |
| 7 | F7 | 1-propylhydrazine |
2.5. Evaluation of Antibacterial Activity of Methanolic Extract and Fractions
Using the disc diffusion methodology, the antibacterial activities of the Met + Ext and purified fractions were assessed against several microbial strains. The zones of inhibition were noted and the results are presented in Table 4.
Table 4.
Antibacterial activity of Met + Ext and purified fractions of Cydonia oblonga using agar well diffusion method.
| Sample | Zone of Inhibition (mm) | |||||
|---|---|---|---|---|---|---|
| Microbial Strains | ||||||
| Escherichia coli | Salmonella typhi | Klebsiella pneumonia | Bacillus subtilis | Staphylococcus aureus | Streptococcus pneumonia | |
| Crude | 18 ± 0.7 | 19 ± 2.0 | 18 ± 0.6 | 19 ± 0.2 | 25 ± 1.8 | 20 ± 2.2 |
| F1 | 10 ± 0.6 | 10 ± 0.9 | 10 ± 0.8 | 10 ± 0.8 | 15± 0.4 | 12 ± 1.1 |
| F2 | 11 ± 0.2 | 14 ± 0.4 | 10 ± 1.3 | 15 ± 1.5 | 15 ± 1.1 | 20 ± 2.0 |
| F3 | 11 ± 1.9 | 10 ± 1.3 | 15 ± 0.9 | 10 ± 1.2 | 10 ± 1.2 | 16 ± 1.6 |
| F4 | 15 ± 1.4 | 13 ± 0.8 | 11 ± 1.0 | 12 ± 1.5 | 11 ± 0.5 | 13 ± 1.4 |
| F5 | 9 ± 1.6 | 10 ± 1.6 | 12 ± 0.6 | 16 ± 1.0 | 14 ± 0.3 | 11 ± 0.8 |
| F6 | 9 ± 08 | 12 ± 1.2 | 12 ± 1.9 | 10 ± 1.1 | 9 ± 1.7 | 11 ± 1.3 |
| F7 | 10 ± 0.4 | 9 ± 0.5 | 12 ± 1.3 | 11 ± 0.8 | 10 ± 1.3 | 12 ± 1.4 |
2.6. Antioxidant Activity of Crude and Purified Fractions of Cydonia oblonga
DPPH scavenging activity was investigated to find the antioxidant potential of the Met + Ext and the purified fractions and results are displayed in Table 5. The standard ascorbic acid caused scavenging up to 94.88 ± 0.96% with the lowest IC50 value of 30 µg/mL at 1000 µg/mL against DPPH. Met + Ext exhibited 87.41 ± 0.54 with the lowest IC50 value of 120, while among the fractions the highest % inhibition value was recorded for F1, as 85.45 ± 0.85, with the lowest IC50 value of 115.
Table 5.
DPPH free radical scavenging potential of Cydonia oblonga at various concentrations.
| S. No | Sample | Concentration (µg/mL) | % DPPH Scavenging | IC50 |
|---|---|---|---|---|
| 1 | Met-Ext | 1000 | 87.41 ± 0.54 | 120 |
| 500 | 85.12 ± 0.76 | |||
| 250 | 68.27 ± 0.85 | |||
| 125 | 52.78 ± 0.25 | |||
| 62.5 | 36.98 ± 0.63 | |||
| 2 | F1 | 1000 | 85.45 ± 0.85 | 115 |
| 500 | 74.23 ± 0.75 | |||
| 250 | 67.12 ± 0.91 | |||
| 125 | 57.34 ± 0.77 | |||
| 62.5 | 38.65 ± 0.48 | |||
| 3 | F2 | 1000 | 65.78 ± 0.68 | 200 |
| 500 | 60.27 ± 0.63 | |||
| 250 | 57.92 ± 0.78 | |||
| 125 | 46.74 ± 0.89 | |||
| 62.5 | 36.04 ± 0.43 | |||
| 4 | F3 | 1000 | 58.61 ± 0.58 | 380 |
| 500 | 52.05 ± 0.49 | |||
| 250 | 48.93 ± 0.75 | |||
| 125 | 44.21 ± 0.48 | |||
| 62.5 | 34.96 ± 0.81 | |||
| 5 | F4 | 1000 | 80.76 ± 0.59 | 110 |
| 500 | 68.23 ± 0.67 | |||
| 250 | 64.04 ± 0.83 | |||
| 125 | 54.92 ± 0.79 | |||
| 62.5 | 42.71 ± 0.58 | |||
| 6 | F5 | 1000 | 71.29 ± 0.49 | 200 |
| 500 | 67.61 ± 0.59 | |||
| 250 | 57.93 ± 0.59 | |||
| 125 | 46.97 ± 0.59 | |||
| 62.5 | 37.12 ± 0.69 | |||
| 7 | F6 | 1000 | 85.28 ± 0.94 | 105 |
| 500 | 81.60 ± 0.96 | |||
| 250 | 70.26 ± 0.86 | |||
| 125 | 55.82 ± 0.89 | |||
| 62.5 | 36.05 ± 0.99 | |||
| 8 | F7 | 1000 | 48.45 ± 0.62 | 110 |
| 500 | 40.38 ± 0.38 | |||
| 250 | 34.18 ± 0.85 | |||
| 125 | 31.41 ± 0.45 | |||
| 62.5 | 29.34 ± 0.63 | |||
| 10 | Ascorbic acid | 1000 | 94.88 ± 0.56 | 30 |
| 500 | 86.59 ± 0.45 | |||
| 250 | 78.64 ± 0.76 | |||
| 125 | 69.14 ± 0.25 | |||
| 62.5 | 62.87 ± 0.53 |
2.7. In Vitro Antidiabetic Potential of Met + Ext and Purified Fractions
2.7.1. In Vitro α-Glucosidase Enzyme Inhibition Activity
The IC50 values of the purified fractions and Met + Ext were calculated at different concentrations as presented in Table 6. Met + Ext was the most potent inhibitor with the lowest IC50 value. Acarbose (standard) at the maximum concentration 1000 µg/mL showed 87.65 ± 0.71 α-glucosidase inhibition with 30 µg/mL IC50 value.
Table 6.
α-Glucosidase and α-amylase inhibitions of Cydonia oblonga of methanolic extract and subsequent purified fractions at different concentrations.
| Sample | Concentration (µg/mL) |
% α-Glucosidase Inhibition | IC50 (µg/mL) |
% α-Amylase Inhibition | IC50 (µg/mL) |
|---|---|---|---|---|---|
| Mean ± SEM | Mean ± SEM | ||||
| Met-Ext | 1000 | 78.21 ± 0.67 | 57 | 77.98 ± 0.57 | 52 |
| 500 | 73.51 ± 0.56 | 76.29 ± 0.36 | |||
| 250 | 64.79 ± 0.14 | 74.32 ± 0.25 | |||
| 125 | 60.28 ± 0.38 | 68.38 ± 0.68 | |||
| 62.5 | 52.51 ± 1.06 | 57.28 ± 0.52 | |||
| F1 | 1000 | 55.01 ± 0.29 | 500 | 79.72 ± 0.02 | 57 |
| 500 | 50.97 ± 0.39 | 78.22 ± 0.72 | |||
| 250 | 43.13 ± 0.34 | 74.02 ± 0.76 | |||
| 125 | 47.24 ± 0.29 | 67.97 ± 0.80 | |||
| 62.5 | 32.41 ± 0.74 | 52.58 ± 0.10 | |||
| F 2 | 1000 | 56.10 ± 0.24 | 494 | 79.97 ± 0.30 | 45 |
| 500 | 51.88 ± 0.38 | 79.27 ± 0.28 | |||
| 250 | 47.62 ± 0.91 | 76.22 ± 0.62 | |||
| 125 | 47.09 ± 0.01 | 75.82 ± 0.56 | |||
| 62.5 | 30.89 ± 1.99 | 70.18 ± 0.48 | |||
| F3 | 1000 | 62.44 ± 1.02 | 200 | 82.16 ± 0.48 | 49 |
| 500 | 57.61 ± 0.28 | 80.41 ± 0.92 | |||
| 250 | 55.88 ± 0.18 | 78.06 ± 0.65 | |||
| 125 | 48.19 ± 0.15 | 76.72 ± 0.53 | |||
| 62.5 | 43.27 ± 1.02 | 74.67 ± 0.59 | |||
| F4 | 1000 | 70.52 ± 0.15 | 370 | 77.37 ± 0.28 | 54 |
| 500 | 63.86 ± 0.03 | 76.57 ± 0.04 | |||
| 250 | 55.04 ± 0.08 | 74.47 ± 0.92 | |||
| 125 | 49.04 ± 0.71 | 68.88 ± 0.38 | |||
| 62.5 | 43.25 ± 0.90 | 57.18 ± 0.05 | |||
| F5 | 1000 | 62.18 ± 0.92 | 180 | 72.14 ± 0.30 | 220 |
| 500 | 59.92 ± 0.49 | 61.50 ± 0.58 | |||
| 250 | 52.92 ± 0.32 | 53.44 ± 0.28 | |||
| 125 | 48.84 ± 0.73 | 46.16 ± 0.74 | |||
| 62.5 | 36.73 ± 0.07 | 38.58 ± 0.48 | |||
| F6 | 1000 | 72.68 ± 0.22 | 125 | 74.24 ± 0.29 | 59 |
| 500 | 68.05 ± 0.52 | 67.38 ± 1.62 | |||
| 250 | 57.39 ± 0.99 | 62.67 ± 0.25 | |||
| 125 | 50.92 ± 0.27 | 56.52 ± 0.49 | |||
| 62.5 | 46.35 ± 0.89 | 51.81 ± 0.76 | |||
| F7 | 1000 | 57.33 ± 0.05 | 495 | 56.58 ± 0.10 | 500 |
| 500 | 51.61 ± 0.12 | 50.42 ± 0.46 | |||
| 250 | 46.84 ± 0.83 | 44.39 ± 0.78 | |||
| 125 | 39.60 ± 0.39 | 38.28 ± 0.60 | |||
| 62.5 | 34.68 ± 0.06 | 31.09 ± 1.05 | |||
| Acarbose | 1000 | 87.65 ± 0.71 | 30 | 85.99 ± 0.44 | 32 |
| 500 | 83.05 ± 0.65 | 83.61 ± 0.58 | |||
| 250 | 78.90 ± 1.02 | 76.85 ± 0.96 | |||
| 125 | 71.83 ± 0.99 | 70.47 ± 0.78 | |||
| 62.5 | 65.15 ± 0.75 | 64.89 ± 0.71 |
2.7.2. In Vitro α Amylase Enzyme Inhibition Activity
The IC50 values of the purified fractions and Met + Ext were calculated at different concentrations along with percent inhibition values and are depicted in Table 6. The crude extract was recorded to be the most potent α-amylase inhibitor l with the lowest IC50 value. Standard acarbose at the maximum concentration 1000 µg/mL showed 85.99 ± 0.44 α-glucosidase inhibition with 32 µg/mL IC50 values.
3. Discussion
The analysis of the present study indicated that Met-Ext had the highest TFC and TPC values of 69% and 70%, respectively. Purified fraction 6 had the highest TPC of 64% and purified fraction 1 had the highest TFC of 58%. According to Erdoğan et al. [25], the leaves of Cydonia oblonga are a rich source of phenolic compounds, which confirms our observed results. Scientific interest in the standardization and characterization of herbal medications is ongoing in the herbal drug sector. Simple, rapid, practical, and affordable standardization approaches are becoming more and more desirable as sophisticated chromatographic techniques are introduced [27]. For the standardization of Met + Ext from plant bark, HPLC was used as it is a sensitive and accurate technique that is widely used for the evaluation of plant extracts and their derived products/formulations [31]. The HPLC-UV analysis of Met + Ext showed the presence of a number of possible compounds such as malic acid, mandelic acid, quercetin, morin, catechin hydrate, and caffeic acid, which were identified through comparison of the retention time of the respective peak in the chromatogram with that of the available standards. As mentioned in the Section 2, this is not a standard technique for the identification of compounds and thus must be used in conjunction with MS in the form of GC-MS or LC-MS and/or NMR investigations in order to fully identify the compounds present and comprehend their structure [32]. The GC-MS technique was successfully employed for Cydonia oblonga bark isolated fractions, and is one of the most extensively used techniques for separating phytoconstituents. About 25 phytochemical substances in the leaves and rhizome of Amomum nilgiricum species have been identified previously through GC-MS [33]. The GC-MS analysis of the purified fractions also confirmed the presence of certain valuable phytochemicals. F1 contains bis(2-ethylhexyl) phthalate and diisooctyl phthalate with a retention time of 22.90 min. Carbamide was found in F2 with a retention time of 22.2 min. Octasiloxane and dimethylsiloxanecyclictrimer were confirmed in F3 with their retention time of 25.57 min. Silicic acid and cyclotrisiloxane with retention times of 25.67 min were found in F4. 6-AH-cAMP, 4H-cyclopropa[5′,6′]benz[1′,2′.7,8]azure 4-(4-chlorphenyl)-3-morpholino-pyrrol-2-yl)-butenedioic acid were active compounds of F5 with retention times of 25.61 min. Isopropylamine was found in F6 with a retention time of 2.30 min. 1-Propylhydrazine was found as an active compound in F7 with a retention time of 1.04 min. Being purified fractions, few bioactive compounds were present in each fraction.
Antibiotics are widely used to address a variety of infections brought on by bacteria, which can be multidrug resistant. These infections are common, which raises treatment resistance and impedes the research and development of new drugs [34]. Analysis has revealed that Gram-positive bacteria are more vulnerable than Gram-negative bacteria to the tested extract and fractions. Our research is consistent with the reported studies where it has been discovered that quince extract has a significant antibacterial effect against Gram-positive bacteria but is less effective against Gram-negative bacteria [35,36]. The outer membranes of Gram-negative bacteria contain lipopolysaccharide, which keeps phenolics from adhering to the surface of the bacterial strain [37], which is why lower activity has been observed against each Gram-negative strain. In general, Gram-positive bacteria appear to be more sensitive to bactericidal polyphenols than Gram-negative species [38]. The DPPH scavenging activity of methanolic and the purified fractions were measured, and the results exhibited potential antioxidant activity. The leaf extract of quince has depicted a concentration-dependent antioxidant potential of 120 µg/mL [39] previously. In another study, methanolic extracts of edible peel and pulp of quince showed IC50 values of 600 and 800 µg/mL, respectively, whereas seed extracts were reported to have a lower antioxidant potential [40]. The Cydonia obolonga extract and the isolated fractions showed effectiveness against α-glucosidase and α-amylase. A previous study showed that plant extracts (methanolic and n-hexane) display remarkable anti-diabetic activity against α-glucosidase and α-amylase [41]. The antidiabetic activity of Cydonia oblonga leaves in hydro-ethanolic extract was studied in normal and streptozotocin-induced diabetic rats. There was no significant effect on normal rat glucose, while a significant reduction in blood glucose levels was recorded in diabetic rats at the time period of 0 to 3 h [42,43]. These reported findings confirm our observations on the selected plant.
4. Material and Methods
4.1. Plant Sample Collection
The bark of Cydonia oblonga was collected from Allahdand Dheri (latitude. 34.61012° or 34°36′3″ north; longitude. 72.0187° or 72°1′7″ east), Malakand Khyber Pakhtunkhwa (KP), Pakistan. The taxonomic identity of the plant was authenticated by a botanist in the Department of the Botany University of Swabi.
4.2. Chemicals and Standards
Gallic acid and quercetin were purchased from Sigma-Aldrich France, and nutrient agar was obtained from Oxoid Ltd. England. 2,2-Diphenyle-1 picrylhydrazyl (DPPH) and Folin–Ciocalteu reagent (Sigma-Aldrich CHEMIE GmbH Burlington, MA, USA), aluminum chloride, methanol, iron chloride, sodium hydroxide, sodium nitrite, and ascorbic acid were purchased from Sigma-Aldrich, Germany. All the chemicals utilized in assays were of analytical grade, except HPLC-grade solvents, which were purchased from Dae-Jung, Korea. They were used as such without any further purification.
4.3. Extraction and Fractionation
The fresh bark of the Cydonia oblonga was washed and dried under a hood to avoid deterioration of bioactive active compounds due to sunlight. The dried sample was ground into a powder and mixed well with methanol. After three days, the extract was filtered through Whatman filter paper, and using a rotary evaporator (Rota Vapor R-200 Buchi, Switzerland) the solvent was evaporated from the filtrate under reduced pressure and at 40 °C. HPLC analysis of the crude was carried out using a reported protocol [44]. Then, 7 HPLC fractions were collected in vials which were labeled as F1, F2, F3, F4, F5, F6, and F7 and stored at 4 °C temperature till further analysis. The separated fractions and methanolic extract (Met + Ext) were screened for phytochemical evaluation (TPC and TFC etc.) and various biological activities. The purified fractions were also analyzed using GC-MS for the presence of bioactive compounds.
4.3.1. Phytochemical Analysis
Preliminary phytochemical screening was undertaken to determine the presence of various bioactive constituents such as alkaloids, flavonoids, glycosides, terpenoids, and tannins in Met + Ext of Cydonia oblonga bark using reported assays [45,46,47].
4.3.2. Analysis of Total Phenolic Content and Total Flavonoids Content
The content of phenolics was determined in Cydonia oblonga bark Met + Ext and purified fractions from F1 to F7 using Folin–Ciocalteu assay [45]. For the preparation of the required solution, 5 mg of sample extract was taken and dissolved in 5 mL of methanol. Then, 1 mL of Folin–Ciocalteu reagent was diluted with 10 mL of distilled water to prepare the Folin–Ciocalteu reagent solution. In a volumetric flask, 9 mL of distilled water was added to 1 mL of sample solution and 1 mL of the diluted solution of Folin–Ciocalteu reagent and allowed to stand for 6 min. After 6 min, 10 mL of 7% NaHCO3 (sodium carbonate) solution was successively added to the mixture. The mixture was made up to 25 mL volume with distilled water and thoroughly mixed. After 90 min, the absorbance was measured at 760 nm using a UV spectrophotometer. Gallic acid solution curve (0 to 100 mg/mL) was used as a standard. Total phenolic content was expressed as gallic acid equivalents (mg GAE/g) per gram of dry sample, which is a common reference compound.
The total flavonoid content was determined using the aluminum chloride calorimetry method [46] with little modification. The plant extract was diluted with methanol. The methanolic solution of extract (100 µL) and 500 µL distilled water were added to 10 mL volumetric flasks followed by the addition of 100 µL NaNO2 (5%), 150 µL AlCl3 (10%), and 200 µL NaOH (1 M). A calibration curve was prepared by using the methanolic solution of quercetin in concentrations ranging from 0–100 μg/mL. The resulting mixture was incubated for 5 min; absorbance was measured spectrophotometrically at 510 nm. The total flavonoid content was expressed in terms of Quercetin equivalent to mg of QE/g of dry samples.
4.3.3. HPLC Characterization
Sample Preparation for HPLC Analysis
HPLC analysis was performed according to a previously reported protocol [44] with some modifications. For analysis, a sample extract was prepared by dissolving 1 g of powder sample in a 10 mL mixture of water and methanol (5:5). The mixture was shaken thoroughly for 1 h at 60 °C and then subjected to filtration using a Whatman filter paper with 0.7 µm pore size. The filtrates were then centrifuged for 15 min at 4000 rpm. The sample extract was filtered twice and collected in a HPLC vial, marked with proper code.
HPLC-UV
The sample was analyzed in an Agilent 1260 infinity High-performance Liquid Chromatography (HPLC) instrument equipped with an autosampler, quaternary pump, degasser, and ultraviolet (UV) detector. An Agilent Zorbax Eclipse C18 column was used for separation. The mobile phase was solvent A (acetic acid, methanol, deionized water, 2.10.88, v/v) and solvent B (acetic acid, deionized water, methanol, 2.8.90, v/v). The system was conditioned with the mobile phase at least for 30–40 min. The gradient profile started with 100% solvent A at 0 min, 85% A at 5 min, 50% solvent A at 20 min, at 25 min solvent A decreased to 30%, and finally, 100% solvent B at 30–40 min. The solvents were delivered at 1 mL/min flow rate, and the absorbance of phytochemicals was measured at 280 nm wavelength, by using retention times (chromatographic comparisons), and UV spectra were recorded with the UV detector identification of different compounds. Seven active fractions were obtained (F1 to F7), which were further analyzed by GC-MS.
GC-MS Analysis for Characterization
Phytoconstituents in purified fractions were analyzed through GC-MS (Agilent Technological, Santa Clara, CA, USA) using a previously reported protocol [48]. The system was equipped with an FID detector to identify phytochemicals. Compounds were identified by comparing their mass spectra and retention times with pure compounds (standards) taken from Willy and NIST libraries software.
4.4. Antibacterial Activity
In vitro antibacterial activity was examined for Met + Ext and purified fractions of Cydonia oblonga against Gram-positive (Bacillus subtilis, Staphylococcus aureus, and Streptococcus pneumonia) and Gram-negative bacteria (Escherichia coli, Salmonella typhi, and Klebsiella pneumonia) using the disc diffusion method. Standard bacterial cultures were used in which the viable count in each culture was determined using a surface viable technique. The bacterial cultures (from 108–109 colony forming units per mL) were swabbed uniformly on the surface of the prepared media using a separate sterile cotton swab each time. Sterilized filter paper discs (Whatman No. 2, Whatman plc, Little Chalfont, GB) impregnated with 20 µL of sample solution were placed individually at equal distances on the surface of seeded agar plates, followed by incubation of bacteria at 37 °C for 24 h in an incubator. Respective solvents without plant extracts were taken as a negative control. After incubation, the results were observed, and we measured the zone diameter around each disc. The experiment was performed in triplicate and mean zone of inhibition values were calculated.
4.5. DPPH Radical Scavenging Activity
The stock solution was prepared by dissolving 20 mg DPPH in 100 mL of methanol; 3 mL of this solution was taken and its absorbance was measured spectrophotometrically at 517 nm and was considered as a negative control solution. For free radical formation, a stock solution was covered with aluminum foil and incubated at room temperature in dark conditions for 24 h. About 5 mg of extract and each purified fraction were dissolved in 5 mL methanol to obtain the stock solution. Then, the above solution was serially diluted in methanol at the following concentrations: 1000, 500, 250, 125, and 62.5 (µg/mL). Then, 2 mL of each sample solution was mixed with 2 mL solution of DPPH and after reaction for 15 min in dark, the absorbance values of the samples were measured. DPPH radical scavenging activity of plant extract and fractions were calculated by using the following equation:
| (1) |
where A○ is the absorbance of pure DPPH and B○ is the absorbance of samples. IC50 values were determined using ascorbic acid taken as a standard, prepared with a concentration of 5 mg/5 mL. Serial dilutions were performed with different concentrations of ascorbic acid in the samples at 1000, 500, 250, 125, and 62.5 µg/mL. All the experiments were performed in triplicate.
4.6. Antidiabetic Assays
4.6.1. Inhibition of α-Amylase
The α-amylase solution was prepared by dissolving 10 mg enzyme in 100 mL distilled water. Then, 10 µL α-amylase enzyme solution was mixed with each test sample in the concentration range of 30 µL. As a negative control, 30 mL of sodium phosphate buffer was added to 10 µL enzyme solution and incubated at 37 °C for 10 min. Then, 40 mL of starch solution was added to each test tube. Incubation was carried out again at 37 °C for 30 min, then 20 µL of 1 M HCL was added and absorbance was measured at 540 nm using a spectrophotometer. The control samples were prepared without any plant samples. Equation (2) was used to estimate the percent inhibition of the enzyme.
4.6.2. α-Glucosidase Inhibition Activity
The reaction mixture was formulated by adding 100 µL of α-glucosidase enzyme (0.5 units/mL), 0.1 M phosphate buffer (600 µL) at pH 6.9 and 50 µL of each sample dilution (62.4, 125, 250, 500, and 1000 µg/mL). The mixture was incubated for 15 min at 37 °C. The enzymatic reaction began by adding 100 µL p-nitro-phenyl-α-D-glucopyranoside (5 Mm) solution in 0.1 M phosphate buffer at pH 6.9 followed by 15 min incubation at 37 °C. The absorbance of the final reaction mixture was recorded at 405 nm. The reaction mixture with no plant extract was used as positive control while the blank solution was prepared without the enzyme α-glucosidase. The α-glucosidase % inhibition was calculated using the formula:
| (2) |
4.7. Statistical Analysis
To calculate IC50 for % DPPH, α-amylase, and α-glucosidase inhibition, Excel 2007 was used. The standard deviation and means were estimated using Excel.
5. Conclusions
HPLC analysis revealed that the bark of Cydonia oblonga has numerous biologically active compounds such as polyphenols and flavonoids. The crude extract and fractions isolated through HPLC were subjected to GC-MS analysis. A total of twelve compounds were identified through HPLC and GC-MS analysis. The extract and various fractions exhibited potential biological activity such as antioxidant, antibacterial, and anti-diabetic activities. This bark can constitute an alternative therapeutic agent for bacterial infections, oxidative stress, and diabetes, which are major subjects for future research. Pharmacological and toxicological studies are also needed to confirm the hypothesis tested here, which will also further elaborate on the safety profile of the extract.
Acknowledgments
The authors are thankful to the Chairman, department of Biochemistry, University of Malakand for providing access to equipment.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27196360/s1. Figure S1. GC-MS chromatogram of fraction 1 of Cydonia oblonga, Figure S2. Major phytochemical compounds identified in Fraction 1 of Cydonia oblonga, Figure S3. Individual mass fragmentation of each compound, Figure S4. GC-MS chromatogram of Fraction 2 of Cydonia oblonga, Figure S5. Major phytochemical compounds identified in fraction 2 of Cydonia oblonga, Figure S6. Individual mass fragmentation of compound. (A) Carbamide, Figure S7. GC-MS chromatogram of Fraction 3 of Cydonia oblonga, Figure S8: Major phytochemical compounds identified in fraction 3 of Cydonia oblonga, Figure S9. Individual mass fragmentation of Each Compound, Figure S10. GC-MS chromatogram of Fraction 4 of Cydonia oblonga, Figure S11. Major phytochemical compounds identified in fraction 4 of Cydonia oblonga, Figure S12. Individual mass fragmentation of Each Compound, Figure S13. GC-MS chromatogram of Fraction 5 of Cydonia oblonga, Figure S14. Major phytochemical compounds identified in fraction 5 of Cydonia oblonga, Figure S15. GC-MS chromatogram of Fraction 6 of Cydonia oblonga, Figure S16. Major phytochemical compounds identified in fraction 6 of Cydonia oblonga, Figure S17. Individual mass fragmentation of the Compound. (A) Isopropylamine, Figure S18. GC-MS chromatogram of Fraction 7 of Cydonia oblonga, Figure S19. Major phytochemical compounds identified in fraction 7 of Cydonia oblonga, and Figure S20. Individual mass fragmentation of the Compound. (A) 1-Propylhydrazine. Table S1. List of major components and their parameters identified in fraction 1 of Cydonia oblonga, Table S2. List of major components and their parameters identified in Fraction 2 of Cydonia oblonga, Table S3. List of major components and their parameters identified in Fraction 3 of Cydonia oblonga, Table S4. List of major components and their parameters identified in Fraction 4 of Cydonia oblonga, Table S5. List of major components and their parameters identified in Fraction 6 of Cydonia oblonga, Table S6. List of major component and their parameters identified in Fraction 6 of Cydonia oblonga, and Table S7. List of major component and their parameters identified in Fraction 7 of Cydonia oblonga.
Author Contributions
Conceptualization, M.Z.; methodology, S.B., M.J. and M.T.; formal analysis, S.N.A. and F.A.A.-J.; investigation, N.U.I.; resources, S.N.A. and F.A.A.-J.; data curation, S.N.A. and F.A.A.-J.; writing—original draft preparation, M.J., M.Z. and N.U.I.; writing—review and editing, N.U.I. and M.Z.; visualization, S.N.A. and F.A.A.-J.; supervision, M.Z.; project administration, M.Z.; funding acquisition, S.N.A. and F.A.A.-J. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ahmed M., Khan K.-R., Ahmad S., Aati H.Y., Ovatlarnporn C., Rehman M.S., Javed T., Khursheed A., Ghalloo B.A., Dilshad R., et al. Comprehensive Phytochemical Profiling, Biological Activities, and Molecular Docking Studies of Pleurospermum candollei: An Insight into Potential for Natural Products Development. Molecules. 2022;27:4113. doi: 10.3390/molecules27134113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katiyar D., Singh V., Ali M. Phytochemical and Pharmacological Profile of Pterocarpus Marsupium: A Review. Pharma Innov. J. 2016;5:31. [Google Scholar]
- 3.Tungmunnithum D., Thongboonyou A., Pholboon A., Yangsabai A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines. 2018;5:93. doi: 10.3390/medicines5030093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muhammad I., Luo W., Shoaib R.M., Li G.l., Hassan S.S., Yang Z., Xiao X., Tu G., Yan S.K., Ma X., et al. Guaiane-type sesquiterpenoids from Cinnamomum migao H.W. Li: And their anti-inflammatory activities. Phytochemistry. 2021;190:112850. doi: 10.1016/j.phytochem.2021.112850. [DOI] [PubMed] [Google Scholar]
- 5.Bent S., Ko R. Commonly Used Herbal Medicines in the United States: A Review. Am. J. Med. 2004;116:478–485. doi: 10.1016/j.amjmed.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 6.Manach C., Scalbert A., Morand C., Rémésy C., Jiménez L. Polyphenols: Food Sources and Bioavailability. Am. J. Clin. Nutr. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 7.Kang N.J., Shin S.H., Lee H.J., Lee K.W. Polyphenols as Small Molecular Inhibitors of Signaling Cascades in Carcinogenesis. Pharmacol. Ther. 2011;130:310–324. doi: 10.1016/j.pharmthera.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Kim D.O., Lee K.W., Lee H.J., Lee C.Y. Vitamin C Equivalent Antioxidant Capacity (VCEAC) of Phenolic Phytochemicals. J. Agric. Food Chem. 2002;50:3713–3717. doi: 10.1021/jf020071c. [DOI] [PubMed] [Google Scholar]
- 9.Bomser J.A., Singletary K.W., Wallig M.A., Smith M.A.L. Inhibition of TPA-Induced Tumor Promotion in CD-1 Mouse Epidermis by a Polyphenolic Fraction from Grape Seeds. Cancer Lett. 1999;135:151–157. doi: 10.1016/S0304-3835(98)00289-4. [DOI] [PubMed] [Google Scholar]
- 10.He J., Chen Y., Farzan M., Choe H., Ohagen A., Gartner S., Busciglio J., Yang X., Hofmann W., Newman W., et al. CCR3 and CCR5 Are Co-Receptors for HIV-1 Infection of Microglia. Nature. 1997;385:645–649. doi: 10.1038/385645a0. [DOI] [PubMed] [Google Scholar]
- 11.Preuss H.G., Montamarry S., Echard B., Scheckenbach R., Bagchi D. Long-Term Effects of Chromium, Grape Seed Extract, and Zinc on Various Metabolic Parameters of Rats. Mol. Cell. Biochem. 2001;223:95–102. doi: 10.1023/A:1017961029492. [DOI] [PubMed] [Google Scholar]
- 12.Asghar A., Tan Y.C., Zahoor M., Abidin S.A.Z., Yow Y.Y., Khan E., Lahiri C. A Scaffolded Approach to Unearth Potential Antibacterial Components from Epicarp of Malaysian Nephelium lappaceum L. Sci. Rep. 2021;11:13859. doi: 10.1038/s41598-021-92622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown E.D., Wright G.D. Antibacterial Drug Discovery in the Resistance Era. Nature. 2016;529:336–343. doi: 10.1038/nature17042. [DOI] [PubMed] [Google Scholar]
- 14.Oliveira A.P., Pereira J.A., Andrade P.B., Valentão P., Seabra R.M., Silva B.M. Phenolic Profile of Cydonia Oblonge Miller Leaves. J. Agric. Food Chem. 2007;55:7926–7930. doi: 10.1021/jf0711237. [DOI] [PubMed] [Google Scholar]
- 15.Li X., Bai H., Yang Y., Yoon J., Wang S., Zhang X. Supramolecular Antibacterial Materials for Combatting Antibiotic Resistance. Adv. Mater. 2019;31:1805092. doi: 10.1002/adma.201805092. [DOI] [PubMed] [Google Scholar]
- 16.Payne D.J., Gwynn M.N., Holmes D.J., Pompliano D.L. Drugs for Bad Bugs: Confronting the Challenges of Antibacterial Discovery. Nat. Rev. Drug Discov. 2007;6:29–40. doi: 10.1038/nrd2201. [DOI] [PubMed] [Google Scholar]
- 17.Barceló A., Rajpathak S. Incidence and Prevalence of Diabetes Mellitus in the Americas. Rev. Panam. Salud Publica/Pan Am. J. Public Health. 2001;10:300–308. doi: 10.1590/S1020-49892001001100002. [DOI] [PubMed] [Google Scholar]
- 18.International Diabetes Federation IDF . IDF Diabetes Atlas. 7th ed. International Diabetes Federation IDF; Brussels, Belgium: 2015. [Google Scholar]
- 19.Muhtadi, Primarianti A.U., Sujono T.A. Antidiabetic Activity of Durian (Durio Zibethinus murr.) and Rambutan (Nephelium lappaceum L.) Fruit Peels in Alloxan Diabetic Rats. Procedia Food Sci. 2015;3:255–261. doi: 10.1016/j.profoo.2015.01.028. [DOI] [Google Scholar]
- 20.Sharma B., Balomajumder C., Roy P. Hypoglycemic and Hypolipidemic Effects of Flavonoid Rich Extract from Eugenia Jambolana Seeds on Streptozotocin Induced Diabetic Rats. Food Chem. Toxicol. 2008;46:2376–2383. doi: 10.1016/j.fct.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 21.Shemchuk O., Braga D., Grepioni F., Turner R.J. Co-Crystallization of Antibacterials with Inorganic Salts: Paving the Way to Activity Enhancement. RSC Adv. 2020;10:2146–2149. doi: 10.1039/C9RA10353H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marwa S.K., Khan M.A., Ur-Rehman F., Bhat I.U. Aromatic Plant Species Mentioned in the Holy Qura’n and Ahadith and Their Ethnomedicinal Importance. Pak. J. Nutr. 2009;8:1472–1479. doi: 10.3923/pjn.2009.1472.1479. [DOI] [Google Scholar]
- 23.Ganopoulos I., Merkouropoulos G., Pantazis S., Tsipouridis C., Tsaftaris A. Assessing Molecular and Morpho-Agronomical Diversity and Identification of ISSR Markers Associated with Fruit Traits in Quince (Cydonia Oblonga) Genet. Mol. Res. 2011;10:2729–2746. doi: 10.4238/2011.November.4.7. [DOI] [PubMed] [Google Scholar]
- 24.Rop O., Balík J., Řezníček V., Juríková T., Škardová P., Salaš P., Sochor J., Mlček J., Kramářová D. Chemical Characteristics of Fruits of Some Selected Quince (Cydonia oblonga Mill.) Cultivars. Czech J. Food Sci. 2011;29:65–73. doi: 10.17221/212/2009-CJFS. [DOI] [Google Scholar]
- 25.Erdoğan T., Gönenç T., Hortoğlu Z.S., Demirci B., Başer K.H., Kıvçak B. Chemical composition of the essential oil of quince (Cydonia oblonga Miller) leaves. Med. Aromat. Plants. 2012;1:134. [Google Scholar]
- 26.Yamanaka N., Oda O., Nagao S. Green Tea Catechins Such as (−)-Epicatechin and (−)-Epigallocatechin Accelerate Cu2+-Induced Low Density Lipoprotein Oxidation in Propagation Phase. FEBS Lett. 1997;401:230–234. doi: 10.1016/S0014-5793(96)01455-X. [DOI] [PubMed] [Google Scholar]
- 27.Nomoto H., Iigo M., Hamada H., Kojima S., Tsuda H. Chemoprevention of Colorectal Cancer by Grape Seed Proanthocyanidin Is Accompanied by a Decrease in Proliferation and Increase in Apoptosis. Nutr. Cancer. 2004;49:81–88. doi: 10.1207/s15327914nc4901_11. [DOI] [PubMed] [Google Scholar]
- 28.Carvalho M., Branca M.S., Silva R., Valentão P., Paula B.A. First Report on Cydonia oblonga Miller Anticancer Potential: Differential Antiproliferative Effect against Human Kidney and Colon Cancer Cells. J. Agric. Food Chem. 2010;58:3366–3370. doi: 10.1021/jf903836k. [DOI] [PubMed] [Google Scholar]
- 29.Adel A.M., El-Wahab Z.H.A., Ibrahim A.A., Al-Shemy M.T. Characterization of Microcrystalline Cellulose Prepared from Lignocellulosic Materials. Part I. Acid Catalyzed Hydrolysis. Bioresour. Technol. 2010;101:4446–4455. doi: 10.1016/j.biortech.2010.01.047. [DOI] [PubMed] [Google Scholar]
- 30.Djilali A.B., Mehraz R., Bouacem K., Benseddik A., Moualek I., Nabiev M., Benzara A. Bioactive Substances of Cydonia oblonga Fruit: Insecticidal Effect of Tannins on Tribuliumm Confusum. Int. J. Fruit Sci. 2021;21:721–731. doi: 10.1080/15538362.2021.1926395. [DOI] [Google Scholar]
- 31.Jain M., Kapadia R., Albert S., Mishra S.H. Standardization of Feronia limonia L. Leaves by HPLC, HPTLC, Physico-Chemical and Histological Parameters. Bol. Latinoam. Caribe Plantas Med. Aromat. 2011;10:525–535. [Google Scholar]
- 32.Tripathi I.P., Mishra M.K., Pardhi Y., Dwivedi A., Dwivedi N., Kamal A., Gupta P. HPLC Analysis of Methanolic Extract of Some Medicinal Plant Leaves of Myrtaceae Family. Int. Pharm. Sci. 2012;2:49–53. [Google Scholar]
- 33.Kabra A., Sharma R., Hano C., Kabra R., Martins N., Baghel U.S. Phytochemical Composition, Antioxidant, and Antimicrobial Attributes of Different Solvent Extracts from Myrica Esculenta Buch.-Ham. Ex. d. Don Leaves. Biomolecules. 2019;9:357. doi: 10.3390/biom9080357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blot S., Depuydt P., Vandewoude K., De Bacquer D. Measuring the Impact of Multidrug Resistance in Nosocomial Infection. Curr. Opin. Infect. Dis. 2007;20:391–396. doi: 10.1097/QCO.0b013e32818be6f7. [DOI] [PubMed] [Google Scholar]
- 35.Al-Khazraji S. Phytochemical Screening and Antibacterial Activity of the Crude Extract of Cydonia oblonga Seeds. Glob. Adv. Res. J. Microbiol. 2013;2:137–140. [Google Scholar]
- 36.Benzarti S., Hamdi H., Lahmayer I., Toumi W., Kerkeni A., Belkadhi K., Sebei H. Total Phenolic Compounds and Antioxidant Potential of Quince (Cydonia oblonga Miller) Leaf Methanol Extract. Int. J. Innov. Appl. Stud. 2015;13:518. [Google Scholar]
- 37.Karar M.G.E., Pletzer D., Jaiswal R., Weingart H., Kuhnert N. Identification, Characterization, Isolation and Activity against Escherichia coli of Quince (Cydonia Oblonga) Fruit Polyphenols. Food Res. Int. 2014;65:121–129. doi: 10.1016/j.foodres.2013.10.040. [DOI] [Google Scholar]
- 38.Urbanavičiūte I., Liaudanskas M., Bobinas Č., Šarkinas A., Rezgiene A., Viskelis P. Japanese Quince (Chaenomeles japonica) as a Potential Source of Phenols: Optimization of the Extraction Parameters and Assessment of Antiradical and Antimicrobial Activities. Foods. 2020;9:1132. doi: 10.3390/foods9081132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benzarti S., Belkadhi K., Hamdi H. Biological Activities of Phenolics from Leaves of Tunisian Cydonia oblonga Miller. Allelopath. J. 2018;45:229–242. doi: 10.26651/allelo.j./2018-45-2-1189. [DOI] [Google Scholar]
- 40.Magalhães A.S., Silva B.M., Pereira J.A., Andrade P.B., Valentão P., Carvalho M. Protective Effect of Quince (Cydonia oblonga Miller) Fruit against Oxidative Hemolysis of Human Erythrocytes. Food Chem. Toxicol. 2009;47:1372–1377. doi: 10.1016/j.fct.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 41.Zeb A. A Reversed Phase HPLC-DAD Method for the Determination of Phenolic Compounds in Plant Leaves. Anal. Methods. 2015;7:7753–7757. doi: 10.1039/C5AY01402F. [DOI] [Google Scholar]
- 42.Meda A., Lamien C.E., Romito M., Millogo J., Nacoulma O.G. Determination of the Total Phenolic, Flavonoid and Proline Contents in Burkina Fasan Honey, as Well as Their Radical Scavenging Activity. Food Chem. 2005;91:571–577. doi: 10.1016/j.foodchem.2004.10.006. [DOI] [Google Scholar]
- 43.Edeoga H.O., Okwu D.E., Mbaebie B.O. Phytochemical Constituents of Some Nigerian Medicinal Plants. Afr. J. Biotechnol. 2005;4:685–688. doi: 10.5897/AJB2005.000-3127. [DOI] [Google Scholar]
- 44.Fiuza S.M., Gomes C., Teixeira L.J., Cruz M.T.G.D., Cordeiro M.N.D.S., Milhazes N., Borges F., Marques M.P.M. Phenolic Acid Derivatives with Potential Anticancer Properties—A Structure-Activity Relationship Study. Part 1: Methyl, Propyl and Octyl Esters of Caffeic and Gallic Acids. Bioorg. Med. Chem. 2004;12:3581–3589. doi: 10.1016/j.bmc.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 45.Kielhorn S., Thorngate J.H. Oral Sensations Associated with the Flavan-3-Ols (+)-Catechin and (−)-Epicatechin. Food Qual. Prefer. 1999;10:109–116. doi: 10.1016/S0950-3293(98)00049-4. [DOI] [Google Scholar]
- 46.Belyagoubi-Benhammou N., Belyagoubi L., Gismondi A., Marco G.D., Canini A., Bekkara F.A. GC/MS Analysis, and Antioxidant and Antimicrobial Activities of Alkaloids Extracted by Polar and Apolar Solvents from the Stems of Anabasis Articulata. Med. Chem. Res. 2019;28:754–767. doi: 10.1007/s00044-019-02332-6. [DOI] [Google Scholar]
- 47.Aslan M., Orhan N., Orhan D.D., Ergun F. Hypoglycemic activity and antioxidant potential of some medicinal plants traditionally used in Turkey for diabetes. J. Ethnopharmacol. 2010;128:384–389. doi: 10.1016/j.jep.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 48.Al-Snafi A.E. The medical importance of Cydonia oblonga—A review. IOSR J. Pharm. 2016;6:87–99. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.