Abstract
We measured the capacity to opsonize Streptococcus pneumoniae serotype 6B and estimated the concentration of immunoglobulin G anti-6B capsular polysaccharide (PS) antibodies in 25 pre- and postimmune sera from adults immunized with a pneumococcal PS vaccine. We first studied two postvaccination serum samples displaying less opsonophagocytic capacity than expected. The majority of anti-6B antibodies in the two samples reacted with the capsular PSs of several unrelated serotypes (2, 4, 9V, 19F, and 23F) and with the lysate of noncapsulated S. pneumoniae bacteria but not with C-PS. The non-type-specific antibodies accounted for at least one-half of anti-6B antibodies in 40% of prevaccination sera and 10% of postvaccination sera from adults. The non-type-specific antibodies could be demonstrated in the enzyme-linked immunosorbent assays (ELISAs) for pneumococcal antibodies to other serotypes (4, 9V, 18C, 19F, and 23F). The nonspecific antibodies appear to bind a contaminant(s) in the current preparations of capsular PS. ELISA for antibodies to pneumococcal capsules may not be serotype specific for some samples.
Streptococcus pneumoniae has been classified into 90 different serotypes based on the structure of its polysaccharide (PS) capsule (7). S. pneumoniae is a major causative agent for pneumonia, meningitis, and sepsis among young children and older adults (4). Antibiotic treatment has become less effective since the prevalence of antibiotic-resistant S. pneumoniae has become very high (1). Thus, there is a great need for pneumococcal vaccines effective among young children and older adults.
Antibodies to capsular PS provide protection against S. pneumoniae expressing the homologous or cross-reactive capsular serotypes, and pneumococcal vaccines are designed to induce antibodies to the capsular PS. The currently available vaccines contain the capsular PSs from 23 common serotypes of S. pneumoniae (12). Because many PSs in the 23-valent vaccine are not immunogenic in young children, PS-protein conjugate vaccines containing several serotypes (e.g., serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F) are being developed (16).
The development of new pneumococcal vaccines would be simplified greatly if there was a simple serologic assay for vaccine-induced protective immunity. Previous studies suggested that the concentration of antibodies in the serum correlates with in vivo animal protection (8) and in vitro opsonic activity (14, 17), the key step of protection in vivo. Yet, a recent epidemiological study suggested that the antibody concentrations may not predict vaccine efficacy against homologous serotypes (18). Also, the correlation between antibody concentration and opsonic activity can be low (r = 0.5) (10, 13). We have therefore examined the antigen-binding properties of several sera with less opsonic activity than expected on the basis of antibody concentration.
(Part of this material has been submitted as an abstract for the 199 meeting of the Society of Pediatric Research in New Orleans.)
MATERIALS AND METHODS
Human sera and bacteria.
Twenty-five healthy adults were immunized once with a 23-valent pneumococcal vaccine (PNU-IMMUNE 23) from Lederle Laboratories (Pearl River, N.Y.). Serum samples were collected before and 1 month after vaccination and were stored frozen at −20°C until analysis. The following strains of S. pneumoniae were used: DS2214 (G. Carlone, Atlanta, Ga.), a serotype 14 strain; WU2 (Janet Yother, Birmingham, Ala.), a serotype 3 strain; JY1119 (David Briles, Birmingham, Ala.) and JD908 (Janet Yother), WU2 variants lacking PspA and the PS capsule, respectively; Tre-108 (David Briles), a variant of D39 (serotype 2) lacking PspC (3); and CSR-SCS2 (G. Schiffman, Brooklyn, N.Y.), a variant lacking the capsule (15).
ELISA.
The amount of anti-capsular PS antibody was determined by “sandwich-type” enzyme-linked immunosorbent assay (ELISA). Briefly, the wells of Maxisorb plates (Nunc, Roskilde, Denmark) were coated at 37°C with 10 μg of the capsular PS (6B serotype)/ml overnight in phosphate-buffered saline, which was prepared fresh with water from a Milli-Q UF water purification system (Millipore, Bedford, Mass.) to minimize the background signal. All pneumococcal capsular PSs were purchased from the American Type Culture Collection (Rockville MD).
After being coated with the antigen, the plates were washed and blocked with phosphate-buffered saline containing 1% bovine serum albumin (Sigma Chemical Co., St. Louis, Mo.) and 0.05% Tween 20. A serum pool (89-SF) (11) from C. Frasch of the Food and Drug Administration (Bethesda, Md.) was used as the standard and was found to contain 16.9 μg of immunoglobulin G (IgG) anti-6B as published previously (11). All samples were absorbed with 10 μg of C-PS (purchased from Statens Seruminstitut, Copenhagen, Denmark) per 20 μl of serum in a total volume of 1 ml of diluent for 30 min at room temperature. The samples were then added to the wells, serially diluted, and incubated for 2 h at room temperature. For the inhibition ELISA, inhibitors were added to each well before the test serum was added. The wells were washed and incubated with alkaline phosphatase-conjugated goat antibody specific for human IgG (Sigma Chemical). The amount of the enzyme immobilized to the well was determined with para-nitrophenyl phosphate substrate (Sigma Chemical) in diethanolamine buffer. The optical density at 405 nm was read with a microplate reader (Cambridge Technology, Watertown, Mass.). The amount of antibody in the sample was determined by comparing the optical density of the sample to the curve that was constructed by a linear interpolation of the data of the standard sample at multiple dilutions.
ELISA with a chaotropic agent.
ELISA was performed as described above with the following modifications. The serum samples at various dilutions were added to the ELISA wells for 2 h and incubated at 37°C. The ELISA plates were washed before 0.1 ml of buffer containing various concentrations of NaSCN was added. After 15 min of incubation, the plates were washed and alkaline phosphatase-conjugated goat antibody against human IgG (Sigma Chemical) was added.
Opsonophagocytic-killing assay.
The opsonophagocytic activities of the samples were determined by the method of B. Gray (5), with minor modifications. Briefly, 10 μl of bacterial suspension (about 2,000 CFU) was incubated with 40 μl of appropriately diluted antibody for 30 min at room temperature with shaking. Pneumococci of serotype 6B (strain L82016) (2) were obtained from D. Briles and were grown in Todd-Hewitt broth with 0.1% yeast extract and kept frozen in aliquots in Hank’s buffer with 15% glycerol. After the 30-min incubation, this mixture was incubated with 40 μl of phagocytic cell suspension (about 1,000,000 HL-60 cells) and 10 μl of baby rabbit complement (Pelfreeze, Brown Deer, Wis.) for 1 h at 37°C with shaking. HL60 cells (a human promyelocytic cell line from the American Type Culture Collection) were differentiated in a medium containing 0.8% dimethyl formamide for 5 days (13, 14).
At the end of the 1-h incubation, 100 μl of normal saline was added to each well and mixed. Ten microliters of the reaction mixture was sampled and applied to a THY agar plate. The plates were incubated overnight at 37°C in a candle jar, and the number of colonies of surviving bacteria was determined. The opsonization titer of the serum was determined as the dilution of the serum that results in half as many viable bacteria as are seen with no antiserum.
RESULTS
IgG antibodies in some sera bind a novel epitope that is found in capsular PS from multiple serotypes.
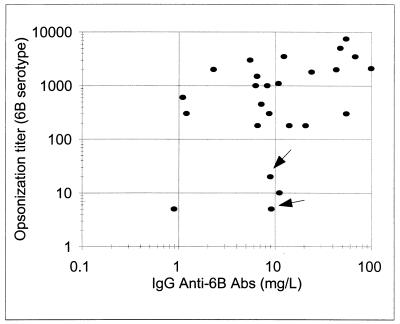
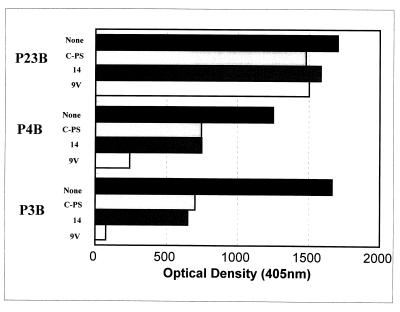
While comparing the opsonophagocytic-killing capacity with the concentration of antibodies to serotype 6B, we observed that some postvaccination sera have less opsonic capacity than expected on the basis of the concentrations of IgG anti-6B PS antibody (Fig. 1). To investigate the epitopes recognized by the antibodies in such “ineffective” antisera, we chose two serum samples (P3B and P4B [Fig. 1]) and measured their anti-6B antibodies before and after preabsorbing the sera with C-PS or other PS (Fig. 2). We found that preabsorption with the conventional amount of C-PS (10 mg/liter) reduced the signal as expected (Fig. 2) and that preabsorption with an additional (10-fold) amount of C-PS did not reduce the signal (data not shown). Also, additional preabsorption of the two sera (P3B and P4B) with serotype 14 pneumococcal PS (10 mg/liter) (Fig. 2) or with other poly-anionic PSs, such as heparin (50 U/ml) or Hib-PS (10 mg/liter) (data not shown), did not reduce the signal. However, to our surprise, an additional preabsorption with 9V PS (10 mg/liter) reduced the IgG antibody binding to 6B PS by about 70% for P4B and about 100% for P3B (Fig. 2). In contrast, when a control serum (P23B) with an opsonic activity commensurate to its antibody concentration was preabsorbed with 9V, the preabsorption did not reduce the amount of IgG antibody binding to 6B PS. This finding suggested that the ineffective antisera may have IgG antibodies binding a “novel epitope(s),” which is present in the preparations of capsular PS of many serotypes.
FIG. 1.
Opsonization titer (y axis) versus IgG antibody concentration (x axis) for serotype 6B. P3B and P4B serum samples are indicated with arrows. The correlation coefficient is 0.4. The regression line is as follows: log (opsonization titer) = 0.65 × log(IgG anti-6B) + 2. Abs, antibodies.
FIG. 2.
Amounts of IgG antibody binding to 6B-coated ELISA plates in various serum samples following preabsorption with various materials. The serum samples (P23B, P4B, and P3B) are identified on the left. The preabsorbents, identified to the left of each bar, are none, C-PS (10 mg/liter), capsular PS of serotype 14 (10 mg/liter), and capsular PS of serotype 9V (10 mg/liter).
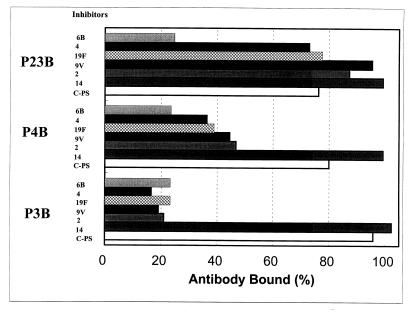
To further investigate this novel epitope, we preabsorbed the three postvaccination sera (P23B, P4B, and P3B) with C-PS and then performed the ELISA for anti-6B antibody in the presence of varying concentrations of different PSs in solution (Fig. 3). With the control serum sample (P23B), the amount of IgG antibody binding to 6B PS-coated ELISA plates could be almost completely inhibited at 0.5 mg of 6B PS/liter. No other PS could reduce the P23B signal, even at 50 mg/liter. These observations indicate that IgG anti-6B antibodies in P23B are 6B specific. However, when IgG anti-6B antibodies in P3B were analyzed, the optical density could be reduced by pneumococcal capsular PSs of serotypes 2, 4, 9V, 19F (Fig. 3), and 3 (data not shown), as well as 6B PS itself (Fig. 3). Pneumococcal capsular PS of serotype 14 could not reduce P3B signal (Fig. 3). When P4B was analyzed, the capsular PS from all the serotypes (except serotype 14) listed above inhibited more than half of the binding. In all cases, the ELISA signal was not reduced even in the presence of very high concentrations of C-PS. Thus, the novel epitope is clearly distinct from C-PS, and the antibodies binding the novel epitope account for almost 100% of IgG anti-6B for the P3B sample, more than half for the P4B sample, and a negligible amount for the P23B sample.
FIG. 3.
Amounts of IgG antibodies in three serum samples which bind to the ELISA plate coated with 6B PS in the presence (5 mg/liter) of various inhibitors.
The novel epitope is not on the capsular PS.
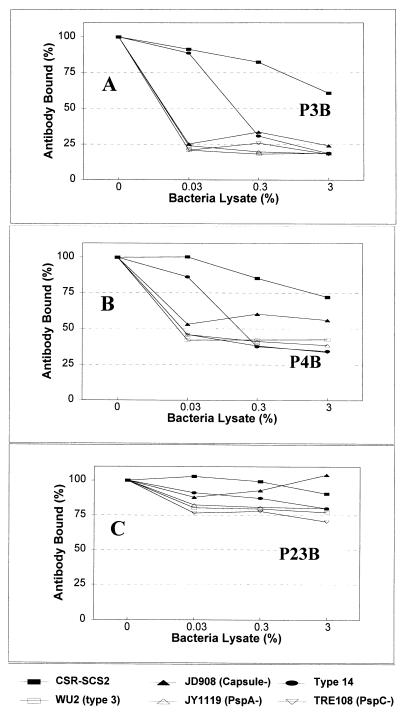
The capsular PS used for our experiments is not chemically synthesized but is isolated from the bacteria; thus, it may contain a contaminant(s) other than C-PS. Our observations could be explained if the novel epitope is an unidentified contaminant whose structure is conserved among different serotypes of S. pneumoniae. To investigate this possibility, we performed an ELISA with the three serum samples in the presence of the lysates of various S. pneumoniae strains (Fig. 4). The binding of IgG antibodies in P3B to 6B PS-coated plates could be inhibited with the lysates (0.03% [vol/vol]) of various bacterial strains lacking either the capsular PS, PspA (3), or PspC (3), although the inhibition by SCR-SCS2, a noncapsular variant derived from S. pneumoniae serotype 2 (15) was not complete. For this experiment, the bacterial lysate was prepared by repeatedly freezing and thawing a bacterial suspension. Even though the purified capsular PS of serotype 14 was not inhibitory (Fig. 3), the lysate of serotype 14 pneumococci could efficiently inhibit the binding of IgG antibodies in P3B (Fig. 4A). Unlike S. pneumoniae, lysate of a Streptococcus pyogenes strain was not significantly inhibitory (data not shown). Consistent with the fact that only a part of the antibodies in P4B bind the novel epitope, about 40% of antibody remained bound even at the high concentration of bacterial lysates (Fig. 4B). In contrast to P3B and P4B, no bacterial lysates could inhibit the binding of IgG antibodies in P23B to 6B PS-coated ELISA plates by more than 25% (Fig. 4C). Our findings taken together strongly support the conclusion that the novel epitope(s) recognized by IgG antibodies in P3B or P4B is not part of the capsular PS but an unidentified bacterial antigen.
FIG. 4.
Amounts of IgG antibodies in three serum samples (P3B, P4B, and P23B) which bind to the ELISA plate coated with 6B PS in the presence of various bacterial lysates. The bacterial strains used were CSR-SCS2; WU2, a serotype 3 strain; JD908, a WU2 variant expressing no capsular PS; JY1119, a WU2 variant lacking PspA; Tre108, a variant lacking PspC; and DS2214, a serotype 14 strain.
Antibodies can bind the novel epitope in the presence of a chaotropic agent.
Some antibodies against pneumococcal PSs have very low avidity for 6B PS and do not opsonize well. Since these low-avidity antibodies do not bind the antigen in the presence of a chaotropic agent such as NaSCN, it is often added to the buffer used for ELISA in order to measure only pneumococcal antibodies with high avidity (6). It is possible that the antibody to the novel epitope may have low avidity and may not bind to 6B PS in the modified ELISA protocol employing NaSCN. When we directly examined whether NaSCN prevents the binding of this antibody to immobilized 6B PS, to our surprise, we repeatedly observed P3B to be more resistant to NaSCN than P23B or 89-SF. The binding of P23B is reduced by more than 50% with 0.25 M NaSCN, whereas the binding of P3B is reduced by less than 20%, even at 1 M NaSCN. Thus, the antibodies to the novel epitope can bind the immobilized pneumococcal capsular PS even in the presence of a chaotropic agent.
Many individuals have significant amounts of antibodies to the novel epitope.
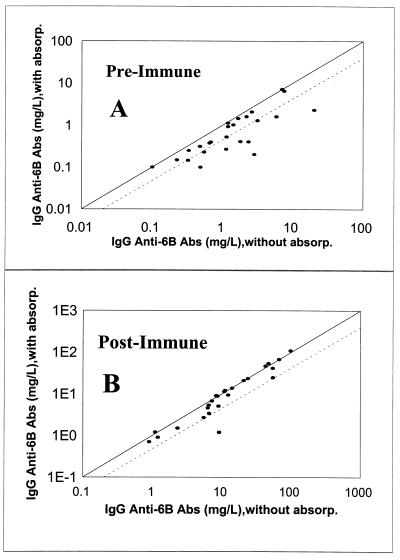
To determine the prevalence of the antibodies binding the novel epitope, we studied 25 pre- and postvaccination serum samples. This was done by performing the ELISA in the absence and presence of 9V PS (10 mg/liter) in the reaction buffer with the immune sera that were already absorbed with C-PS. As can be seen in Fig. 5, preabsorption with 9V reduced the apparent IgG anti-6B antibody concentrations by about twofold in 10 preimmune and in 4 postimmune serum samples. The apparent concentration of anti-6B antibodies in the sample P3B (Fig. 5B) decreased almost 10-fold after absorption with 9V PS. Therefore, a recognizable fraction of IgG “anti-6B antibodies” from adults actually bind the novel epitope. In addition, the antibodies to the novel epitope affect the determination of IgG anti-6B antibody levels more in preimmune sera than in postimmune sera.
FIG. 5.
IgG anti-6B antibody (Ab) concentrations with (y axis) or without (x axis) preabsorption with pneumococcal capsular PS of serotype 9V. (A) Preimmune sera; (B) postimmune sera. The solid line indicates the identity, and the dotted line indicates the 50% reduction in antibody concentrations following preabsorption. absorp., absorption.
Levels of antibodies binding the novel epitope can increase after vaccination.
To determine if the antibodies to the novel epitope increase in concentration following vaccination, we obtained preimmune sera from two individuals who had ineffective antibodies and four randomly chosen individuals who had effective antibodies. Anti-6B antibody concentrations were determined by ELISA with or without 9V PS in solution. Anti-6B antibody concentrations measured in the presence of 9V PS were considered to be 6B-specific antibodies (not cross-reactive with 9V), and the difference in anti-6B antibody concentrations measured without or with 9V PS was considered to represent the antibodies recognizing the novel epitope (Table 1). Concentrations of antibodies to the novel epitope can be negative if most of the anti-6B antibodies are specific for 6B. The 6B-specific antibodies increased 1- to 10-fold with vaccination, and in addition, we also found that the anti-6B cross-reactive with 9V PS increased two- to threefold after vaccination. Thus, the PS vaccine may stimulate the antibodies to the novel epitope as well.
TABLE 1.
Levels of anti-6B antibodies before and after vaccination
| Serum donor | Total IgG anti-6B
Aba (mg/liter)
|
6B-specific IgG
Aba (mg/liter)
|
Polyspecific
Abb (mg/liter)
|
|||
|---|---|---|---|---|---|---|
| Preimmune | Postimmune | Preimmune | Postimmune | Preimmune | Postimmune | |
| P3 | 2 | 6 | 0.7 | 2 | 1.3 | 4 |
| P4 | 6.6 | 7.2 | 2.5 | 3.2 | 4.1 | 4 |
| P11 | 16.3 | 47.9 | 3.3 | 22.2 | 13 | 25.7 |
| P23 | 1.9 | 15.5 | 1 | 10.9 | 0.9 | 4.6 |
| P24 | 1.4 | 7.5 | 1 | 8.4 | 0.4 | −0.9 |
| P26 | 5.8 | 20.9 | 5.7 | 24.2 | 0.1 | −3.3 |
Total IgG anti-6B antibody (Ab) was measured by ELISA as described in Materials and Methods. 6B-specific IgG antibody was measured by ELISA, but 9V PS was present in the buffer.
The values were obtained by subtracting 6B-specific antibody (Ab) concentration from total antibody concentration. When the polyspecific antibody is much less than the 6B-specific antibody, the polyspecific antibody concentration can become artifactually negative.
The novel epitope affects assays of anti-capsular PS for other serotypes.
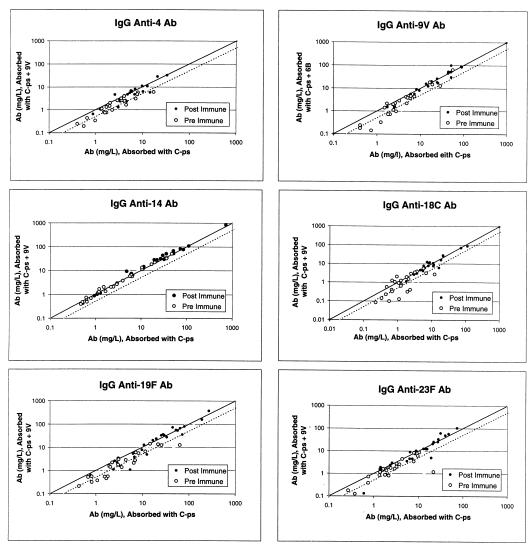
Pneumococcal conjugate vaccines will likely contain at least seven serotypes (serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F). To examine if the antibody to the novel epitope may influence the determination of the concentration of antibodies to capsular PS of serotypes other than 6B, we performed the ELISA for anti-capsular antibodies for five serotypes (4, 14, 18C, 19F, and 23F) with serum samples before and after absorption with 9V PS. For the ELISA assay of serotype 9V, we absorbed the serum samples with 6B PS. As we have seen with antibodies for serotype 6B, most data points were to the right of the identity line, indicating that the concentration estimates became smaller with absorption with an unrelated capsular PS (Fig. 6). For instance, for serotype 18C, 11 of 25 preimmune samples had more than half of the antibodies directed to the novel epitope. Also, the absorption with unrelated PS reduced the estimate of the antibody concentrations more for the preimmune samples than for the postimmune samples. The exception to this general observation is serotype 14, since the antibody concentration estimates for serotype 14 did not change with additional absorption. It is likely that the preparation of capsular PS of serotype 14 used for this study does not contain the novel epitope but capsular PSs of all other serotypes do.
FIG. 6.
Concentrations of IgG antibody (Ab) to capsular PS with (y axis) or without (x axis) additional preabsorption with an irrelevant pneumococcal capsular PS for preimmune sera or for postimmune sera. The serotype specificity of the antibodies is indicated at the top of each panel. The solid line indicates the identity, and the dotted line indicates the 50% reduction in antibody concentrations following preabsorption.
DISCUSSION
In this study we demonstrated that the IgG antibodies to a novel epitope constitute the majority of IgG antibodies to pneumococcal capsule in many prevaccination sera as well as in occasional postvaccination sera. The antibodies to the novel epitope affect estimation of the antibodies not just to 6B PS but to PSs of many other serotypes that are included in the conjugate vaccines, except for serotype 14. The significant effect of the novel epitope was independently observed by the two laboratories collaborating in this project, using different sets of reagents. The presence of the novel epitope was also independently reported by Coughlin et al. in nonimmune sera after our manuscript was prepared for publication (3a). Furthermore, the antibodies to the novel epitope are nonopsonic and may not be protective. Therefore, the presence of antibodies to the novel epitope must be appreciated in evaluating new pneumococcal vaccines.
In several situations, the impact of antibodies to the novel epitope on estimating the concentration of antibodies to the capsular PS may be magnified. The impact could be large in the studies of some populations (e.g., young children) who are poorly responsive to PS. The impact could be magnified when the capsular PS-specific antibody response is weak, such as with serotype 6B. It should be more significant in estimating the antibody levels in preimmune sera than in postimmune sera, which contain generally higher levels of antibodies to capsular PS. Also, the impact could be significant in postimmune sera if the pneumococcal vaccines used were contaminated with the novel epitope more than usual, since the novel epitope appears to be immunogenic.
The exact nature of the novel epitope is unclear. However, it is not part of the capsular PS but is likely a contaminant found in the commercially available vaccine-grade preparations of capsular PS. The novel epitope does not appear to be C-PS, a well known and common contaminant in most pneumococcal capsular PS (9). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis showed several contaminant molecules that varied among different capsular PS preparations, but the magnitude of antibodies to the novel epitope did not correlate with the presence of a specific contaminant visualized in the gel (data not shown). The novel epitope is not expressed on pneumococcal proteins like PspA or PspC, and the novel epitope was also resistant to trypsin or papain (data not shown). Interestingly, the novel epitope appears to be absent in the preparations of capsular PS of serotype 14. Type 14 capsular PS is biochemically quite different from the PSs of other serotypes, and its purification method is different from that of other capsular PSs.
In order to improve our ability to measure vaccine-induced protective immunity, ELISA assays for pneumococcal antibodies have been extensively standardized and are being further modified not to measure low-avidity (and nonopsonic) antibodies. For instance, antibodies to C-PS are routinely neutralized by preabsorption, and a chaotropic agent is often used to measure only the high-avidity antibodies by ELISA (6). The antibodies to the novel epitope not only interfere with the standardized ELISA assays but also bind to the ELISA plates even in the presence of the chaotropic agent. One way of improving the specificity of the standardized ELISA is to preabsorb the serum samples with an unrelated capsular PS in addition to C-PS. While this modification should be evaluated further, the increasing complexity of ELISAs used for estimating the concentrations of protective antibodies further emphasizes the need for standardizing the ELISA and for supplementing the ELISA results with an independent measure of protective immunity (e.g., opsonophagocytosis assay results).
ACKNOWLEDGMENTS
This work was supported by funds from the National Institutes of Health (AI-31473 and AI-85334) and a grant from the World Health Organization (VRD-V23/181/72). M.H.N. is partially supported by NIAID contract N01 AI 45248.
We thank P. Anderson, J. B. Robbins, P. Allen, D. Briles, and J. Treanor for their critical readings. We also thank J. Yother for her generous gift of the bacterial strains and J. Olander and C. Fristschle for their continued interest in this project.
REFERENCES
- 1.Breiman R F, Butler J C, Tenover F C, Elliott J A, Facklam R R. Emergence of drug-resistant pneumococcal infections in the United States. JAMA. 1994;271:1831–1835. [PubMed] [Google Scholar]
- 2.Briles D E, Crain M J, Gray B M, Forman C, Yother J. Strong association between capsular type and virulence for mice among human isolates of Streptococcus pneumoniae. Infect Immun. 1992;60:111–116. doi: 10.1128/iai.60.1.111-116.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Briles D E, Hollingshead S K, Swiatlo E, Brooks-Walter A, Szalai A, Virolainen A, McDaniel L S, Benton K A, White P, Prellner K, Hermansson A, Aerts P C, Van Dijk H, Crain M J. PspA and PspC: their potential for use as pneumococcal vaccines. Microb Drug Resist. 1997;3:401–408. doi: 10.1089/mdr.1997.3.401. [DOI] [PubMed] [Google Scholar]
- 3a.Coughlin R T, White A C, Anderson C A, Carlone G M, Klein D L, Treanor J. Characterization of pneumococcal specific antibodies in healthy unvaccinated adults. Vaccine. 1998;16:1761–1767. doi: 10.1016/s0264-410x(98)00139-x. [DOI] [PubMed] [Google Scholar]
- 4.Fedson D S, Plotkin S A, Mortimer E A., Jr . Pneumococcal vaccine. In: Fedson D S, Plotkin S A, Mortimer E A Jr, editors. Vaccines. W. B. Philadelphia, Pa: Saunders Co.; 1988. pp. 271–299. [Google Scholar]
- 5.Gray B M. Opsonophagocidal activity in sera from infants and children immunized with Haemophilus influenzae type b conjugate vaccine (meningococcal protein conjugate) Pediatrics. 1990;85:694–697. [PubMed] [Google Scholar]
- 6.Gray B M, Shaw D R. Artifacts with the thiocyanate elution method for estimating relative antibody avidity. J Immunol Methods. 1993;157:269–271. doi: 10.1016/0022-1759(93)90096-p. [DOI] [PubMed] [Google Scholar]
- 7.Henrichsen J. Six newly recognized types of Streptococcus pneumoniae. J Clin Microbiol. 1995;33:2759–2762. doi: 10.1128/jcm.33.10.2759-2762.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Musher D M, Johnson B, Jr, Watson D A. Quantitative relationship between anticapsular antibody measured by enzyme-linked immunosorbent assay or radioimmunoassay and protection of mice against challenge with Streptococcus pneumoniae serotype 4. Infect Immun. 1990;58:3871–3876. doi: 10.1128/iai.58.12.3871-3876.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Musher D M, Luchi M J, Watson D A, Hamilton R, Baughn R E. Pneumococcal polysaccharide vaccine in young adults and older bronchitics: determination of IgG responses by ELISA and the effect of adsorption of serum with non-type-specific cell wall polysaccharide. J Infect Dis. 1990;161:728–735. doi: 10.1093/infdis/161.4.728. [DOI] [PubMed] [Google Scholar]
- 10.Nahm M H, Olander J V, Magyarlaki M. Identification of cross-reactive antibodies with low opsonophagocytic activity for Streptococcus pneumoniae. J Infect Dis. 1997;176:698–703. doi: 10.1086/514093. [DOI] [PubMed] [Google Scholar]
- 11.Quataert S A, Kirch C S, Wiedl L J, Phipps D C, Strohmeyer S, Cimino C O, Skuse J, Madore D V. Assignment of weight-based antibody units to a human antipneumococcal standard reference serum, lot 89-S. Clin Diagn Lab Immunol. 1995;2:590–597. doi: 10.1128/cdli.2.5.590-597.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robbins J B, Austrian R, Lee C J, Rastogi S C, Schiffman G, Henrichsen J, Makela P H, Broome C V, Facklam R R, Tiesjema R H. Considerations for formulating the second-generation pneumococcal capsular polysaccharide vaccine with emphasis on the cross-reactive types within groups. J Infect Dis. 1983;148:1136–1159. doi: 10.1093/infdis/148.6.1136. [DOI] [PubMed] [Google Scholar]
- 13.Romero-Steiner S, Libutti D, Pais L B, Dykes J, Anderson P, Whitin J C, Keyserling H L, Carlone G M. Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against Streptococcus pneumoniae using differentiated HL-60 cells. Clin Diagn Lab Immunol. 1997;4:415–422. doi: 10.1128/cdli.4.4.415-422.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romero-Steiner S, Pais L, Holder P, et al. Abstracts of the 95th General Meeting of the American Society for Microbiology 1995. Washington, D.C: American Society for Microbiology; 1995. Opsonophagocytosis of Streptococcus pneumoniae as an indicator of functional antibody activity in adults vaccinated with a 23-valent polysaccharide vaccine, (abstr. E-42. [Google Scholar]
- 15.Schiffman G, Bornstein D L, Austrian R. Capsulation of pneumococcus with soluble cell wall-like polysaccharide. II. Nonidentity of cell wall and soluble cell wall-like polysaccharides derived from the same and from different pneumococcal strains. J Exp Med. 1971;134:600–617. doi: 10.1084/jem.134.3.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siber G R. Pneumococcal disease: prospects for a new generation of vaccines. Science. 1994;265:1385–1387. doi: 10.1126/science.8073278. [DOI] [PubMed] [Google Scholar]
- 17.Vitharsson G, Jonsdottir I, Jonsson S, Valdimarsson H. Opsonization and antibodies to capsular and cell wall polysaccharides of Streptococcus pneumoniae. J Infect Dis. 1994;170:592–599. doi: 10.1093/infdis/170.3.592. [DOI] [PubMed] [Google Scholar]
- 18.Wenger J D, Steiner S R, Pais L B, Butler J C, Perkins B, Carlone G M, Broome C V. Program and abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Laboratory correlates for protective efficacy of pneumococcal vaccines: how can they be identified and validated? abstr. G37. [Google Scholar]