Abstract
Preterm infants have an increased incidence of infection, which is principally due to deficiencies in neonatal host defense mechanisms. Monocyte adherence is important in localizing cells at sites of infection and is associated with enhanced antimicrobial functions. We isolated cord blood monocytes from preterm and full-term infants to study their adhesion and immune functions, including superoxide (O2−) generation, degranulation, and cytokine secretion and their adhesion receptors. O2− production and degranulation were significantly diminished, by 28 and 37%, respectively, in adherent monocytes from preterm infants compared to full-term infants (P < 0.05); however, these differences were not seen in freshly isolated cells. We also observed a significant decrease of 35% in tumor necrosis factor alpha secretion by lipopolysaccharide-stimulated adherent monocytes from preterm infants compared to full-term infants (P < 0.05); however, this difference was not observed in interleukin-1β or interleukin-6 production by the monocytes. The cell surface expression of the CD11b/CD18 adhesion receptor subunits was significantly decreased (by 60 and 52%, respectively) in monocytes from preterm infants compared to full-term infants (P < 0.01). The cascade of the immune response to infection involves monocyte upregulation and adherence via CD11b/CD18 receptors followed by cell activation and the release of cytokines and bactericidal products. We speculate that monocyte adherence factors may be important in the modulation of immune responses in preterm infants.
Despite advances in antimicrobial therapy, immunotherapy, and neonatal intensive care, preterm infants have a 10-fold-higher risk of mortality from infection than full-term infants (7, 11, 13). The increased susceptibility to infection is multifactorial, but it is principally due to deficiencies in neonatal host defense mechanisms. These functions have been well studied in monocytes derived from adults and full-term infants but not in monocytes from preterm infants (5, 20, 23).
The monocyte is crucial in the immune response to infection, playing a role in antigen presention, phagocytosis, bactericidal activity, and secretory function (23). In response to inflammatory stimuli, monocytes first localize at sites of infection. Localization is mediated by adherence via the CD11b/CD18 receptor on the surfaces of monocytes and intercellular adhesion molecule 1 on the endothelium. These activated monocytes transport additional CD11b/CD18 receptors from intracellular compartments to the cell surface membrane to enhance adhesion and upregulate immune function (19). The intravascular monocytes then migrate from the endothelium to the extravascular site of infection. At the site of infection, bactericidal activity includes the production of oxygen radicals via the respiratory burst, specifically O2−, and degranulation with the release of granule contents, including lysozyme (2, 12, 20). These functions are further enhanced by the secretion of proinflammatory cytokines, including tumor necrosis factor (TNF), interleukin-1 (IL-1), and IL-6 from activated monocytes at the site of infection.
We hypothesized that adherent monocytes from preterm infants may have reduced O2− generation, degranulation, and cytokine secretion. These diminished immune responses may be related to deficiencies in adhesion factors at the sites of infection, such as the decreased expression of the CD11b/CD18 molecule. The purposes of this study were (i) to compare O2− production and lysozyme release of adherent and freshly isolated monocytes from preterm and full-term infants, (ii) to determine TNF-α, IL-1β, and IL-6 secretion by adherent monocytes in response to lipopolysaccharide (LPS), and (iii) to examine the cell surface expression of the CD11b/CD18 adhesion receptor in monocytes from preterm and full-term infants.
MATERIALS AND METHODS
Monocyte isolation.
Cord blood was collected in heparinized syringes from placentas of preterm and full-term infants at delivery. Monocytes were isolated and purified as previously described by Hassan et al. (10). Whole blood was layered on lymphocyte separation medium (Organon Teknika, Durham, N.C.), followed by centrifugation at 500 × g for 45 min at room temperature. The band containing the peripheral blood mononuclear cells was carefully aspirated and then transferred to gelatin-coated flasks for monocyte adherence. After 45 min in 5% CO2 at 37°C, the flasks were washed with Dulbecco’s modified Eagle medium (DMEM; GIBCO Laboratories, Grand Island, N.Y.) to remove the lymphocytes and nonadherent cells. Monocytes were then detached by exposure to 10 mM EDTA in DMEM containing 20% fetal calf serum (HyClone Laboratories, Logan, Utah) for 15 min. Monocytes were recovered by aspirating the flasks, followed by centrifugation for 10 min at 4°C. The pellet was resuspended in DMEM containing 20% fetal calf serum and 1% penicillin–streptomycin–l-glutamine.
Freshly isolated monocytes.
Freshly isolated monocytes were defined as cells that were stimulated immediately after isolation. Cells at a density of 0.5 × 106 monocytes per well in 96-well plates were used for measurement of O2− production, and cells at a concentration of 1 × 106 monocytes per ml in polystyrene tubes were used for degranulation assays.
Adherent monocytes.
Adherent monocytes were defined as monocytes that were cultured for 18 h in a humidified atmosphere containing 5% CO2 at 37°C prior to the O2− production and degranulation assays. Cells were plated at a concentrations of 0.5 × 106 monocytes per well in 96-well plates for O2− production and 1.0 × 106 monocytes per well in 48-well plates for degranulation.
O2− generation.
Triplicate samples of 0.5 × 106 cells per well were stimulated with phorbol myristate acetate (PMA) (1.0 μg/ml) or N-formyl methionyl leucyl phenylalanine (FMLP) (10−6 M) for both freshly isolated and adherent monocytes. O2− generation was measured as superoxide dismutase-inhibitable cytochrome c reduction by a continuous recording method over 15 min starting at the time of stimulation (14). O2− generation was expressed as nanomoles of O2− per 106 cells.
Degranulation.
Monocytes (106 cells per well) were analyzed immediately after isolation (freshly isolated cells) or after incubation for 18 h in tissue culture wells (adherent cells). Freshly isolated cells were distributed in polystyrene tubes and pretreated with cytochalasin B (50 μg/ml). Triplicate samples were stimulated with FMLP (10−6 M), and a second group of triplicate samples were treated with Triton X-100 (0.2% final concentration; Sigma, St. Louis, Mo.). Both groups were placed in a 37°C shaking bath for 30 min. Tubes were centrifuged at 1,200 rpm for 5 min, and the supernatant was collected. For adherent monocytes, the medium was replaced after 18 h of incubation with fresh medium, and then cells were treated with cytochalasin B (50 μg/ml) in tissue culture wells. Triplicate samples were stimulated with FMLP (10−6 M) or treated with Triton X-100 (0.2% final concentration) in tissue culture wells for 30 min in 5% CO2 at 37°C. Supernatants were collected from both freshly isolated and adherent monocytes and then incubated with Micrococcus lysodeikticus. Lysozyme release was measured as the decrease in absorbance at 450 nm. Percent degranulation was calculated as the change in absorption of FMLP-treated samples (stimulated degranulation) divided by the change in absorption of Triton X-100-treated samples (total degranulation).
TNF-α, IL-1β, and IL-6 production by monocytes.
Adherent monocytes were stimulated with LPS (10 ng/ml; Sigma) and then cultured in 5% CO2 at 37°C for 18 h. Supernatants were collected and stored at −70°C until analyzed. TNF-α (Genzyme, Cambridge, Mass.), IL-1β (Cistron, Pine Brook, N.J.), and IL-6 (Genzyme) levels were determined by enzyme-linked immunosorbent assay with kits from the above-mentioned companies.
Adhesion receptor expression.
Whole blood was collected in heparinized syringes from preterm and full-term placentas. Fluorescein isothiocyanate-conjugated monoclonal antibodies to CD11b (Immunotech, Westbrook, Maine), CD18 (Caltag, San Francisco, Calif.), and CD14 (Immunotech) cell surface receptors were added to 100 μl of whole blood and incubated for 30 min at 4°C in the dark. After ammonium chloride lysis, cells were fixed in 1% paraformaldehyde, and fluorescence intensity was measured by flow cytometry (Epics Elite flow cytometer; Coulter, Miami, Fla.). Expression of the monocyte-specific marker CD14 was used as a gating criterion to identify monocytes in whole-blood preparations. Instrument calibration was performed prior to the evaluation of patient and control specimens with DNA check microspheres (Coulter Immunotech, Hialeah, Fla.). Two thousand cells were counted for each antibody, and cell surface receptor expression was quantitated as mean channel fluorescence (MCF). Whole blood from healthy adult donors was used for reference points to standardize each assay.
Materials.
PMA was purchased from Sigma, stored as a concentrated stock solution in dimethyl sulfoxide (1 mg/ml), and diluted before use. Cytochalasin B (Sigma) was stored in a stock solution of 10 mg/ml in dimethyl sulfoxide at −70°C. FMLP (Sigma) was stored in a stock solution of ethanol (10−2 M) and diluted in buffer before use. M. lysodeikticus was stored at 4°C in 0.1 M KH2PO4.
Statistical analysis.
Cytokine levels, O2− production, percent degranulation, MCF, and other variables were compared between patient groups by using the independent t test. A probability of less than 0.05 was considered significant.
RESULTS
Patient population.
The study subjects were different for each assay, secondary to cell number as a limiting factor. In each assay there was a significant difference between preterm and full-term infant populations in both gestational age (GA) and birth weight (BW) (P < 0.001). For each assay, the specific patient population is reported.
O2− production.
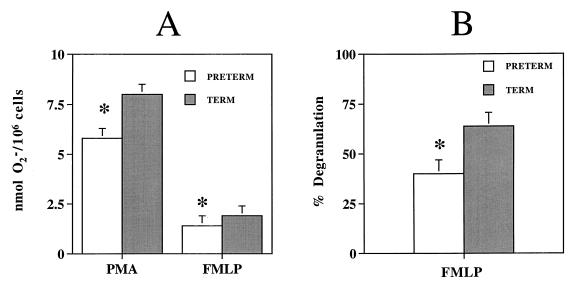
Monocytes were isolated from the cord blood of 21 full-term infants (mean BW ± standard error of the mean [SEM], 3,291 ± 85 g; GA, 39.5 ± 0.5 weeks) and 14 preterm infants (BW, 2,311 ± 148 g [P < 0.001]; GA, 34.7 ± 0.5 weeks [P < 0.001]). The levels of O2− generated by adherent monocytes from full-term infants were 8.0 ± 0.6 nmol of O2−/106 cells after PMA stimulation and 1.9 ± 0.6 nmol of O2−/106 cells after FMLP stimulation. O2− generation was significantly lower for adherent monocytes from preterm infants: 5.8 ± 0.8 nmol of O2−/106 cells (a 28% decrease) with PMA (P < 0.05) and 1.4 ± 0.4 nmol of O2−/106 cells (a 26% decrease) with FMLP (P < 0.05) (Fig. 1).
FIG. 1.
Bactericidal function of adherent cord blood monocytes from preterm and full-term infants. Each value is expressed as mean ± SEM. ∗, significant difference in values between groups (P < 0.05).
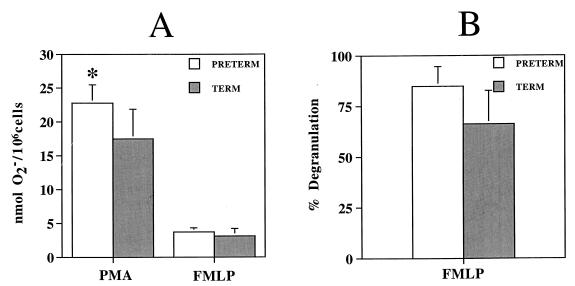
For freshly isolated monocytes from full-term infants, the levels of O2− production were 17.5 ± 4.4 nmol of O2−/106 cells with PMA and 3.1 ± 1.1 nmol of O2−/106 cells after FMLP stimulation. O2− production by freshly isolated monocytes from preterm infants was significantly greater, 22.8 ± 2.7 nmol of O2−/106 cells (a 30% increase) with PMA (P < 0.05), but not significantly different with FMLP (Fig. 2).
FIG. 2.
Bactericidal function of freshly isolated cord monocytes from preterm and full-term infants. Each value is expressed as mean ± SEM. ∗, significant difference in values between groups (P < 0.05).
Degranulation.
Cord blood monocytes were isolated from 11 full-term infants (BW, 3,340 ± 75 g; GA, 40.0 ± 0.9 weeks) and 4 preterm infants (BW, 2,432 ± 134 g [P < 0.001]; GA, 32.0 ± 1.5 weeks [P < 0.001]). Degranulation by adherent monocytes from full-term infants was 63.8% ± 6.9%. Degranulation by adherent monocytes from preterm infants was significantly lower, 40.1% ± 13.5% (a 37% decrease) (P < 0.05) (Fig. 1). There was no significant difference in the degranulation of lysozyme by freshly isolated monocytes from preterm infants (84.9% ± 9.7%) and full-term infants (66.3% ± 16.4%) (Fig. 2).
TNF-α, IL-1β, and IL-6 production.
Cord blood monocytes were isolated from 21 full-term infants (BW, 3,291 ± 85 g; GA, 39.5 ± 0.5 weeks) and 14 preterm infants (BW, 2,311 ± 148 g [P < 0.001]; GA, 34.7 ± 0.5 weeks [P < 0.001]) to determine levels of cytokine production. The level of TNF-α production by adherent monocytes from full-term infants in response to LPS was 10,548 ± 996 pg/ml. TNF-α production was significantly lower in adherent monocytes from preterm infants, 6,875 ± 798 pg/ml (a decrease of 35%) (P < 0.05) (Table 1). The levels of IL-1β and IL-6 production by LPS-stimulated adherent monocytes from full-term infants were 2,649 ± 168 and 8,515 ± 427 pg/ml, respectively. In contrast to TNF-α production, IL-1β and IL-6 production was not significantly different for preterm and full-term infants (Table 1).
TABLE 1.
Cytokine production by monocytes from preterm and full-term infants
| Infant group | Mean cytokine level ± SEM, pg/ml
(no. of infants)
|
||
|---|---|---|---|
| TNF-α | IL-1β | IL-6 | |
| Preterm | 6,875 ± 798a (14) | 2,310 ± 346 (8) | 8,189 ± 696 (14) |
| Full-term | 10,548 ± 996 (21) | 2,649 ± 168 (11) | 8,515 ± 427 (21) |
TNF-α production by LPS-stimulated monocytes from preterm infants was significantly lower (P < 0.05) than that of full-term infants.
Adhesion receptor expression.
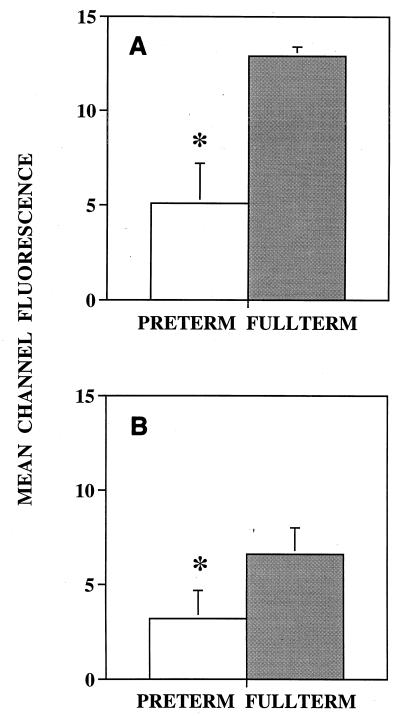
Cord blood samples were collected from 14 full-term infants (BW, 3,213 ± 442 g; GA, 39.0 ± 1.4 weeks [P < 0.001]) and 11 preterm infants (BW, 1,359 ± 505 g; GA, 29.5 ± 3.0 weeks). MCF intensities in monocytes from full-term infants were 12.9 ± 0.5 for CD11b and 6.6 ± 1.4 for CD18. CD11b expression in monocytes from preterm infants was significantly lower, 5.1 ± 2.1 (a decrease of 60%) (P < 0.01), and CD18 expression was also significantly lower, 3.2 ± 1.5 (a decrease of 52%) (P < 0.01) (Fig. 3).
FIG. 3.
CD11b (A) and CD18 (B) cell surface expression of cord blood monocytes from preterm and full-term infants. Each value is expressed as mean ± SEM. ∗, significant difference in values between groups (P < 0.01).
DISCUSSION
The preterm infant has an increased incidence and severity of infection which is multifactorial in origin (7, 11, 13). In our study, several aspects of the monocyte immune response were deficient in preterm infants, specifically bactericidal function and TNF-α secretion. In vitro we demonstrated diminished TNF-α secretion, O2− generation, and lysosomal degranulation in adherent monocytes from preterm infants compared to full-term infants. These functions were not diminished in freshly isolated monocytes from preterm infants compared to those from full-term infants. We hypothesized that a receptor important in both adhesion and immune function may also be diminished. We observed diminished cell surface expression of the CD11b/CD18 adhesion receptor subunits in monocytes from preterm infants than from full-term infants. This decreased expression is a possible mechanism for the diminished bactericidal functions and cytokine secretion observed in our study. These abnormalities may contribute to the increased incidence of infection in preterm infants in vivo.
Previous studies have linked the β-2 integrin CD11b/CD18 adhesion receptor and immune function in adults, but to date, similar investigations have not been performed with premature infants. β-2 integrins are located on all leukocytes and are involved in receptor-ligand interactions between cells. They mediate leukocyte-leukocyte adhesion, leukocyte-endothelial cell adhesion, and immune responses (phagocytosis, complement binding, degranulation, cytokine secretion, and O2− production). Owen et al. demonstrated that O2− production in activated monocytes from adults was inhibited by monoclonal antibodies blocking the CD11b/CD18 adhesion receptor (18). Studies of CD18-deficient leukocytes from infants with leukocyte adhesion deficiency demonstrated diminished O2− production and degranulation (8, 20). Fan and Edington showed enhanced TNF-α mRNA expression and protein secretion by LPS-stimulated monocytes when adherent to a CD11b/CD18 receptor (6), an effect blocked by anti-CD11b and anti-CD18 monoclonal antibodies (6). These studies demonstrate that when the CD11b/CD18 receptor was blocked or deficient, bactericidal function and TNF-α secretion were diminished. This relationship between immune function and adherence receptors may also be present in preterm monocytes. Further studies are needed to determine if the decreased expression of β-2 integrin (CD11b/CD18) adherence receptors is a possible basis and mechanism for the decreased immune responses in adherent monocytes from preterm infants.
In our investigations of monocyte function and cytokine secretion, we used cells isolated on denatured type I collagen (gelatin). This adherence step allowed the separation of monocytes from the nonadherent lymphocyte population without using β-2 integrins (1, 9, 18). Previous studies have demonstrated that leukocytes adhere to gelatin and collagen derivatives via β-1 integrins and that blocking monoclonal antibodies to the β-2 integrin, CD18, do not affect adherence of monocytes to gelatin (1, 9, 17, 18). Therefore, we do not suspect that our isolation procedures preselected a population of monocytes deficient in β-2 integrins. Furthermore, our findings demonstrating decreased β-2 receptor expression in monocytes from preterm infants were obtained with whole blood, not isolated cells.
In our assays of monocyte function, cells were stimulated with both FMLP and PMA. FMLP binds to its specific receptor, which then triggers protein kinase C activation and calcium mobilization, leading to O2− generation (14). In contrast, PMA directly stimulates protein kinase C, bypassing a cell surface receptor. We observed decreased O2− production by adherent monocytes from preterm infants when the cells were stimulated with either PMA or FMLP. Additionally, decreased cell function was observed when the LPS ligand was used to stimulate cytokine secretion. This decreased cell function observed with a number of different ligands (PMA, FMLP, and LPS) implies that the abnormalities observed are not receptor specific.
Decreased cell function in adherent monocytes from preterm infants was not the result of decreased cell number. DNA analysis demonstrated equivalent DNA content between preterm and full-term monocytes (data not shown). In addition to DNA content, secretion of the cytokines IL-1β and IL-6 by adherent monocytes was also equivalent in preterm and term infants. In contrast to our findings for adherent monocytes, O2− production and degranulation were equivalent or enhanced in freshly isolated monocytes from preterm infants compared to full-term infants. These findings suggest that the necessary cellular components of the bactericidal response are present and fully functional in monocytes from preterm infants. When monocytes are placed in culture, the processes of adherence and differentiation induce alterations in protein synthesis and cellular metabolism which modify bactericidal activity (3, 4, 15, 16, 24). Thus, the decreased O2− production and degranulation observed in preterm adherent monocytes appear to be related to regulatory processes rather than defective or absent components of the bactericidal response.
Finally, our observations do not rule out the possibility that there may be subtle alterations in adherence by monocytes from preterm infants which affect signaling pathways that regulate TNF-α secretion. Interestingly, IL-1β and IL-6 production was unaltered in preterm cells following adherence in culture for 18 h, suggesting different regulatory controls than for TNF-α. These findings are similar to the results of other studies (21–23).
The findings in our study demonstrate diminished TNF-α secretion, bactericidal function, and decreased adherence receptor expression in monocytes from preterm infants. These in vitro abnormalities in immune function and adhesion may contribute to the increased incidence and severity of infection in the preterm infant in vivo. Studies of adult monocytes have shown a relationship between the CD11b/CD18 adherence receptor and bactericidal function and cytokine secretion. Our results suggest that diminished expression of the CD11b/CD18 adhesion receptor may be an important factor associated with diminished bactericidal function and cytokine secretion in monocytes from preterm infants in vitro. Further studies are needed to determine the potential association between the CD11b/CD18 receptor and bactericidal function and cytokine secretion in preterm infants.
ACKNOWLEDGMENTS
This work was supported by Wyeth-Ayerst Laboratories, the Howard Heinz endowment, General Clinical Research Center grant MO1-RR 00240 from the National Institutes of Health, and the laboratory of Steven D. Douglas, which is supported by National Institutes of Health grants MH 49981 and UO-1AI32921.
REFERENCES
- 1.Arnaout M A. Structure and function of leukocyte adhesion molecules CD11/CD18. Blood. 1990;75:1037–1050. [PubMed] [Google Scholar]
- 2.Babior B M. Oxygen-dependent microbial killing by phagocytes. N Engl J Med. 1978;298:659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- 3.Cassatella M A, Bazzoni F, Amezaga M A, Rossi F. Studies on the gene expression of several NADPH oxidase components. Biochem Soc Trans. 1991;19:63–67. doi: 10.1042/bst0190063. [DOI] [PubMed] [Google Scholar]
- 4.Cassatella M A, Bazzoni F, Flynn R M, Dusi S, Trinchieri G, Rossi F. Molecular basis of interferon-gamma receptor and lipopolysaccharide enhancement of phagocyte respiratory burst capability. Studies on the gene expression of several NADPH oxidase components. J Biol Chem. 1990;265:20241–20246. [PubMed] [Google Scholar]
- 5.Etzioni A. Adhesion molecules—their role in health and disease. Pediatr Res. 1996;39:191–198. doi: 10.1203/00006450-199602000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Fan S T, Edington T S. Integrin regulation of leukocyte inflammatory functions. CD11b/CD18 enhancement of the tumor necrosis factor-α responses of monocytes. J Immunol. 1993;150:2972–2980. [PubMed] [Google Scholar]
- 7.Gerdes J S. Clinicopathologic approach to the diagnosis of neonatal sepsis. In: Polin R A, Speck W T, editors. Clinics of perinatology. W. B. Philadelphia, Pa: Saunders; 1991. pp. 361–381. [PubMed] [Google Scholar]
- 8.Goodman E B, Anderson D C, Tenner A J. Clq triggers neutrophil superoxide production by unique CD18-dependent mechanism. J Leukoc Biol. 1995;58:168–176. doi: 10.1002/jlb.58.2.168. [DOI] [PubMed] [Google Scholar]
- 9.Gudewicz P W, Frewin M B, Heinel L A, Minnear F L. Priming of human monocyte superoxide production and arachidonic acid metabolism by adherence to collagen- and basement membrane-coated surfaces. J Leukoc Biol. 1994;55:423–429. doi: 10.1002/jlb.55.4.423. [DOI] [PubMed] [Google Scholar]
- 10.Hassan N F, Campbell D E, Douglas S D. Purification of human monocytes on gelatin coated surfaces. J Immunol Methods. 1986;95:273–276. doi: 10.1016/0022-1759(86)90415-1. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman D J, Harris M C. Diagnosis of neonatal sepsis. In: Spitzer A R, editor. Intensive care of the fetus and neonate. St. Louis, Mo: Mosby-Year Book, Inc.; 1996. pp. 940–950. [Google Scholar]
- 12.Issekutz A C, Chuluyan E, Lopes N. CD11/CD18-independent transendothelial migration of human polymorphonuclear leukocytes and monocytes: involvement of distinct and unique mechanisms. J Leukoc Biol. 1995;57:553–561. doi: 10.1002/jlb.57.4.553. [DOI] [PubMed] [Google Scholar]
- 13.Kilpatrick L, Harris M C. Cytokines and the inflammatory response. In: Polin R A, Fox W W, editors. Fetal and neonatal physiology. W. B. Philadelphia, Pa: Saunders; 1998. pp. 1967–1979. [Google Scholar]
- 14.Korchak H M, Weissmann G. Changes in membrane potential of human granulocytes antecede the metabolic responses to surface stimulation. Proc Natl Acad Sci USA. 1978;75:3818–3822. doi: 10.1073/pnas.75.8.3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Musson R A, McPhail L C, Shafran H, Johnston R B., Jr Differences in the ability of human monocytes and in vitro monocyte-derived macrophages to produce superoxide anion: studies with cells from normals and patients with chronic granulomatous disease. J Reticuloendothel Soc. 1982;32:261–266. [PubMed] [Google Scholar]
- 16.Nakagawara A, Nathan C F, Cohn Z A. Hydrogen peroxide metabolism in human monocytes during differentiation in vitro. J Clin Investig. 1981;68:1243–1252. doi: 10.1172/JCI110370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newman S L, Tucci M A. Regulation of human monocyte/macrophage function by extracellular matrix. J Clin Investig. 1990;86:703–714. doi: 10.1172/JCI114766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Owen C A, Campbell E J, Stockley R A. Monocyte adherence to fibronectin: role of CD11/CD18 integrins and relationship to other monocyte functions. J Leukoc Biol. 1992;51:400–408. doi: 10.1002/jlb.51.4.400. [DOI] [PubMed] [Google Scholar]
- 19.Ruoslahti E. Integrins. J Clin Investig. 1990;87:1–5. doi: 10.1172/JCI114957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Todd R F, Freyer D R. The CD11/CD18 leukocyte glycoprotein deficiency. In: Curnutte J T, editor. Hematology/oncology clinics of North America. W. B. Philadelphia, Pa: Saunders; 1988. pp. 13–31. [PubMed] [Google Scholar]
- 21.Weatherspoon K B, Rich E A. Tumor necrosis factor/cachectin and interleukin-1 secretion by cord blood monocytes from premature and term neonates. Pediatr Res. 1989;25:342–346. doi: 10.1203/00006450-198904000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Wilmott R W, Harris M C, Haines K M, Douglas S D. Interleukin-1 activity from human cord blood monocytes. Diagn Clin Immunol. 1987;5:201–204. [PubMed] [Google Scholar]
- 23.Yoder M C, Hassan H F, Douglas S D. Mononuclear phagocyte system. In: Polin R A, Fox W W, editors. Fetal and neonatal physiology. W. B. Philadelphia, Pa: Saunders; 1992. pp. 1428–1460. [Google Scholar]
- 24.Zuckerman S H, Ackerman S K, Douglas S D. Long-term human peripheral blood monocyte cultures: establishment, metabolism, and morphology of primary monocyte-macrophage cell cultures. Immunology. 1979;38:401–411. [PMC free article] [PubMed] [Google Scholar]