Abstract
Experiments were conducted to evaluate the effects of amphetamine (0.4 mg/kg of body weight/day) on the development of oral candidiasis in Sprague-Dawley rats. Animals were submitted to surgical hyposalivation in order to facilitate the establishment and persistence of Candida albicans infection. Treatment with drugs (placebo or amphetamine) was initiated 7 days before C. albicans inoculation and lasted until the end of the experiments, day 15 postinoculation. Establishment of C. albicans infection was evaluated by swabbing the inoculated oral cavity with a sterile cotton applicator on days 2 and 15 after inoculation, followed by plating on YEPD (yeast extract-peptone-dextrose) agar. Tissue injury was determined by the quantification of the number and type (normal or abnormal) of papillae on the dorsal tongue per microscopic field. A semiquantitative scale was devised to assess the degree of colonization of the epithelium by fungal hyphae. Our results show that amphetamine exacerbates C. albicans infection of the tongues of rats. Significant increases in Candida counts, the percentage of the tongue’s surface covered with clinical lesions, the percentage of abnormal papillae, and the colonization of the epithelium by fungal hyphae were found in amphetamine-treated rats compared to those found in the rats injected with a placebo. The last two parameters increased in rats treated with the placebo compared to the parameters of the untreated control rats.
Candida albicans is a major cause of oral and esophageal infections in immunocompromised patients (21). This opportunistic human pathogen preferentially causes invasive and disseminated infections in patients with defective phagocytic defenses and serious mucocutaneous infection in patients with deficiencies in T-cell function. Phagocytes appear to protect the host from fungal colonization even in the absence of adaptive immune mechanisms, while as-yet-undefined T-cell-dependent factors seem necessary for the control of C. albicans on body surfaces (21).
In our previous research we had observed adverse effects of amphetamine on the immune systems of rodents that may lead to more severe C. albicans infections. Both defective T-cell-mediated immunity and qualitative or quantitative defects of phagocytes have been found in mice repeatedly injected with amphetamine (0.4 mg/kg of body weight/day). They showed a reduction in thymus and spleen cellularities, the peripheral T-lymphocyte population, the blastogenic response of T- and B-lymphocyte mitogenic functions (11, 25), the natural killer (NK) cell activity, and the capacity of T cells to generate cytotoxic T lymphocytes in mixed lymphocyte cultures and in vivo (23). A decrease in in vitro and in vivo phagocytoses (12), measured by the zymosan particle uptake method and the carbon clearance test, respectively, and a delayed-type hypersensitivity response in mice (13) were also observed. These adverse effects of amphetamine appeared after 4 days of administration and lasted until the end of the experiments (day 20), with a maximum intensity at 8 to 12 days of administration. Nevertheless, there is little data on the effects of this compound on the development of C. albicans infection. This is important since drug addiction is commonly associated with candidiasis, especially in human immunodeficiency virus patients, and since the potential immunosuppressive properties of drugs like amphetamine are not always taken into account in the pathogenicity of C. albicans. To further elucidate this relationship, we studied the effects of repeated injections of amphetamine on the development of oral candidiasis in rats.
MATERIALS AND METHODS
Animals.
Two-month-old male pathogen-free rats of the Sprague-Dawley strain (Interfauna Ibérica, S.A., Barcelona, Spain) weighing 180 to 200 g were used. They were housed individually in filter-top cages and screened for the presence of C. albicans by plating oral swabs on YEPD (yeast extract-peptone-dextrose; Sigma Chemical Co., St. Louis, Mo.) agar (21). The cages were kept in a temperature-controlled (22 to 24°C) and humidity-controlled animal room, with an alternating light-dark cycle (lights on at 0600 and lights off at 1800) and with food (diet A.03; Panlab, Barcelona, Spain) and sterile water ad libitum.
Procedure.
After verifying that the rats were free of C. albicans, we randomly divided them into three experimental groups of four animals each according to the treatment they were to be submitted to: either no treatment (i.e., no placebo or amphetamine) (control), placebo, or amphetamine. Treatment with drugs started 7 days before C. albicans inoculation and lasted until the end of the experiments, day 15 postinoculation.
Surgical hyposalivation.
As in humans, xerostomia in rats facilitates the establishment and persistence of C. albicans infection in the mouth; therefore, it constitutes a suitable animal model for the study of oral candidiasis (17). Sialoadenectomy in rats causes intense xerostomia, but the minor salivary glands, the main producers of mucin, an important barrier for mucosal permeability and a major source of immunoglobulin A, were preserved. In our experiment, xerostomia was surgically provoked in all rats 1 month before treatment with drugs was initiated. The rats were anesthetized with 44 mg of ketamine (Ketolar; Parke-Davis, Barcelona, Spain) per kg of body weight and 1 mg of diazepam (Valium; Roche, Madrid, Spain) per kg (31). The parotid salivary ducts of the animals were ligated, and the submandibular and sublingual salivary glands were surgically removed according to procedures previously described (6, 20, 21).
Source and culture of C. albicans.
The C. albicans organisms used to inoculate the rats were obtained from a patient with erythematous oral candidiasis. The Candida strain was grown on YEPD agar plates at room temperature (27). The isolated organisms were identified as C. albicans by a germ tube test and chlamydospore production as described by Schaar et al. (28).
Inoculation of C. albicans.
The C. albicans isolates were prepared for inoculation by suspending colonies in sterile buffered saline, washing and centrifuging them twice in the saline, and then resuspending them in the saline. The concentration of organisms was adjusted to 3 × 108/ml (1) by hemocytometer count. The tongues of the animals were swabbed on two successive days with a cotton-tipped applicator (21) saturated with 0.1 ml of fresh inoculum.
Quantification of C. albicans cells.
Establishment of C. albicans infection was evaluated by swabbing the inoculated oral cavity with a sterile cotton applicator, followed by plating on YEPD agar (17). Samples were collected 2 days after inoculation and at the end of the experiment. The cotton applicator was immediately immersed in 0.99 ml of sterile isotonic saline to obtain a dilution of 10−2, and it was agitated for 2 min. This dilution was considered to be 10−2. Dilutions up to 10−5 (0.1 ml) were cultured in duplicate in Sabouraud’s dextrose agar at 37°C for 48 h. Candida colonies were counted in plates exhibiting between 30 and 300 colonies. Plates with fewer than 30 colonies in the 10−2 dilution were considered to have 101 cells (17).
Clinical lesions.
At the end of the experiment, the animals were killed by asphyxiation in a CO2 atmosphere and were then decapitated. The dorsal tongue was photographed in situ at a magnification of ×10 (1). Clinical lesions were measured with a digital imaging system (Técnicas Médicas MAB, Barcelona, Spain) and were expressed as the percentage of the surface area of the tongue (percent area) that was covered with the lesions.
Tissue handling.
The tongues from the rats were hemisected in the sagittal plane, with half of the lesion immersed in 10% buffered formalin for routine processing and the other half placed in 2.5% glutaraldehyde with 0.1 M Sorensen’s phosphate buffer at 4°C (1).
Light microscopy.
Both hematoxylin and eosin stain and periodic acid-Schiff stain were used. C. albicans infection was assessed by evidence of lesions and by hyphal colonization on the dorsal tongue (1, 24) with a digital imaging system. Tissue injury was determined by the quantification of the number and type (normal, atrophic, and hypertrophic) of papillae per microscopic field (magnification, ×46). A semiquantitative scale was devised to assess the degree of colonization of the epithelium by fungal hyphae. In this scale, the absence of colonization was given a score of 0, while maximal colonization, where in excess of 50 hyphae could be seen in each high-power field (magnification, ×400), was assigned a score of 4 (24). The scores given were 1 for 1 to 5 hyphae, 2 for 6 to 15 hyphae, and 3 for 16 to 50 hyphae. The specimens were examined by one of us, who was blinded as to the source.
Scanning electron microscopy.
Following fixation for 24 h, the tissue was rinsed three times in buffer and postfixed in 1% phosphate-buffered osmium tetroxide (pH 7.4) for 1 h. After two buffer rinses, the specimens were dehydrated in ascending concentrations of ethanol, followed by critical point dehydration in a Denton DCP-1 critical point drying apparatus with liquid CO2. The tissue samples were affixed on aluminum stubs with silver conductive paint and were sputter-coated with gold-palladium by using a Hummer VI sputter-coating apparatus (Anatech Electronics). Specimens were viewed with a Zeiss 910 electron microscope (Zeiss, Oberkochen, Germany) operated at 20 kV (2).
Treatment with drugs.
Racemic amphetamine sulfate (Sigma Chemical Co.) was subcutaneously injected at a dose of 0.4 mg/kg in a volume of 1 ml of 0.9% saline solution per kg. The basis for employing this low dose of amphetamine is based on previous dose-response assays that proved to affect the immune system (12, 13, 23). Rats in the placebo group were subcutaneously injected with 1 ml of 0.9% saline solution per kg. Drugs were administered daily at 0930.
Statistical analysis.
Statistical analysis of the quantitation of C. albicans cells in oral tissue was performed by using the one-way analysis of variance, followed by Bonferroni’s t test. Percent areas of clinical lesions were analyzed by Student’s t test (17). The Wilcoxon signed-rank sum test for paired comparisons and the Kruskal-Wallis test for multiple comparisons were used to determine the degree of colonization of the epithelium by fungal hyphae (24). Differences were considered significant at a P value of <0.05.
RESULTS
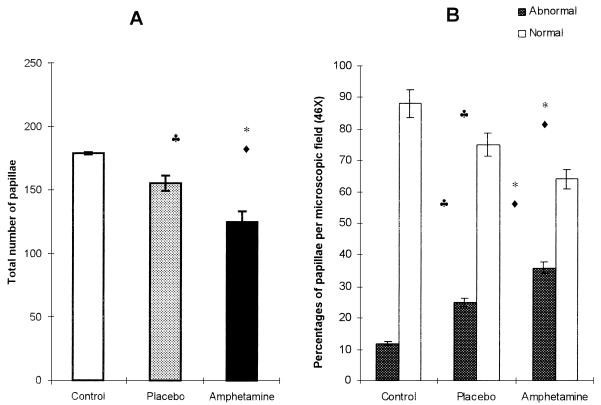
C. albicans counts 2 and 15 days after inoculation were greater in amphetamine-treated rats than they were in control and placebo-treated rats (Table 1). The mean percent areas of clinical legions ± standard deviations in the dorsal tongue were also greater in amphetamine-treated rats (68.308 ± 5.8) than they were in control (4.160 ± 0.4) and placebo-treated (4.424 ± 0.8) rats. A decrease in the total number of papillae and an increase in the percentage of abnormal (atrophic and hypertrophic) papillae (Fig. 1) were observed in rats injected with amphetamine compared with the total numbers and percentages observed in the control and placebo-treated rats (differences, P < 0.01). Significant differences (P < 0.05) between untreated controls and placebo-injected rats in the latter parameter were observed.
TABLE 1.
C. albicans counts from the tongues of rats
| Day postinoculation | Mean Candida count
(104 CFU/ml) ± SD in rats
receivinga:
|
||
|---|---|---|---|
| No treatment (control) | Placebo | Amphetamine | |
| 2 | 60.75 ± 2.04 | 60.39 ± 2.75 | 65.11 ± 3.16*† |
| 15 | 4.53 ± 1.04 | 4.72 ± 1.75 | 6.33 ± 0.08*† |
Establishment of C. albicans infection was evaluated by swabbing the inoculated oral cavity with a sterile cotton applicator, followed by plating on YEPD agar. ∗, differences between control and amphetamine-treated rats were considered significant at a P value of < 0.05; †, differences between placebo- and amphetamine-treated rats were considered significant at a P value of <0.05.
FIG. 1.
Total number of papillae and percentages of normal and abnormal (atrophic and hypertrophic) papillae on the tongues of rats. The results are the means ± standard deviations of four animals. Values were analyzed by Bonferroni’s t test. ∗, differences between control and amphetamine-treated rats were considered significant at a P value of <0.01; ⧫, differences between placebo- and amphetamine-treated rats were considered significant at a P value of <0.01; ♣, differences between control and placebo- and amphetamine-treated rats were considered significant at a P value of <0.05.
On the semiquantitative scale of colonization of the epithelium by fungal hyphae (Table 2), both placebo- and amphetamine-treated rats scored higher than untreated controls (differences, P < 0.05). Nevertheless, the scores were higher in amphetamine-injected rats than they were in placebo-injected rats (differences, P < 0.01).
TABLE 2.
Degree of colonization of the epithelium by fungal hyphae
| Rat | Colonization score for rats
receivinga:
|
||
|---|---|---|---|
| No treatment (control) | Placebo | Amphetamine | |
| 1 | 1 | 2 | 4 |
| 2 | 1 | 2 | 3 |
| 3 | 2 | 2 | 3 |
| 4 | 1 | 2 | 3 |
A semiquantitative scale was devised to assess the degree of colonization of the epithelium by fungal hyphae. In this scale the absence of colonization was given a score of 0, while maximal colonization, where in excess of 50 hyphae could be seen in each high-powered field (magnification, ×400), was assigned a score of 4. The other scores were 1 for 1 to 5 hyphae, 2 for 6 to 15 hyphae, and 3 for 16 to 50 hyphae.
Clinically evident lesions and inflammatory changes of the underlying connective tissue were observed 15 days after C. albicans inoculation. The latter were found in all experimental groups, but they were most evident in the amphetamine-treated rats. Animals showed macroscopic focal patchy atrophy of the dorsal tongue papillae. Light microscopy showed localized dense zones of hyphal penetration of the keratin layer in the giant conical papillae and filiform papillae of the dorsal tongue. Microabscesses in the keratin and the superficial spinous layers were observed in association with hyphal invasion. The underlying connective tissue showed a mild chronic inflammatory cell infiltrate. Those papillae which supported the candidal growth appeared shorter and blunter than the surrounding uninfected papillae.
Scanning electron microscopy of the dorsal tongues from rats injected with amphetamine and infected with C. albicans showed a higher loss of papillae in the giant conical and filiform areas of the specimens than the control and placebo-treated rats showed, together with an increase in the size of the flat central portion of the lesion in comparison with the control and placebo-treated rats.
DISCUSSION
Our results show that amphetamine exacerbates C. albicans infection of the tongues of rats. Significant increases in Candida counts, the percent area of clinical lesions, the percentage of abnormal papillae, and the colonization of the epithelium by fungal hyphae were found in amphetamine-treated rats compared with those found in rats injected with the placebo. The last two parameters increased in the placebo-treated rats compared with the parameters of the untreated control rats, possibly as a consequence of stress-induced effects of animal handling on the immune system (23). In previous investigations, we had observed that amphetamine did not statistically affect these two parameters in noninoculated animals.
C. albicans is an opportunistic human pathogen which preferentially causes invasive and disseminated infections in patients with defective phagocytic defenses as well as serious mucocutaneous infection in patients with deficiencies in T-cell function (19). Clinical and experimental observations indicate that the opportunistic proclivities of this fungus vary considerably, depending on the nature of the immunological defect of the victim. Patients with qualitative or quantitative defects of phagocytes are mainly prone to the invasive form of this mycosis (8, 9, 30). In contrast, defective T-cell-mediated immunity has been specifically associated with thrush and other forms of candidiasis limited to mucocutaneous surfaces (10, 15, 18). In this regard, Krause and Schaffner (19) demonstrated that cyclosporine, a relatively selective suppressor of T-cell-mediated immunity and NK cell activity, promoted the formation of thrushlike lesions in artificial pneumatized cyst surfaces and impeded the elimination of C. albicans from such lesions, but it had no effect on systemic candidiasis induced by intravenous inoculation.
These results are in agreement with previous reports on the adverse effects of amphetamine on the immune systems of rodents. Both defective T-cell-mediated immunity (11, 12) and qualitative or quantitative defects of phagocytes (12) have been found in mice repeatedly injected with amphetamine. Moreover, amphetamine was found to decrease the resistance to and the development and passive transfer of immunity to Listeria monocytogenes (11) in C57BL/6 mice and to increase the lethality and pathogenicity of influenza A virus (PR8/34) in CD-1 mice (22).
The mechanism for the action of amphetamine might be either direct (at a target cell) or indirect (affecting neuroendocrine pathways). House et al. (16) found that natural and synthetic amphetamines exhibit direct immunomodulatory activities following in vitro exposure. Amphetamine was found to suppress interleukin 2 production by T lymphocytes, B-lymphocyte proliferation, and NK cell function under in vitro conditions.
The effects of amphetamine can also be secondary to a mediator involved in expressing the drug’s effect. Amphetamine has been shown to have numerous effects on neuronal and endocrine systems. Molecular products of cells of the nervous and immune systems provide a means of communication between the two systems (5). Many of the effects of amphetamine involve the drug modulation of the adrenergic system and mimic stress-like states (3, 7, 14, 29). Cellular immune activity is partially regulated by the adrenergic nervous system (4).
A second point to be considered concerns the neuroendocrinological effects of amphetamine. The stimulatory effect of amphetamine on adrenocorticotropic hormone (ACTH) and adrenocorticoids should be involved (16, 25). Firstly, ACTH from the pituitary gland, and even ir-ACTH of lymphocyte origin, has a direct inhibitory effect on the functional capacities of immune cells. Secondly, the rise in plasma corticosterone concentrations, via ACTH secretion enhancement, suppresses various aspects of immune function (26). Previous investigations have shown a stimulatory effect of chronic amphetamine on ACTH secretion that was proportional to the decrease in the functional activities of spleen cells and the activity of phagocytosis (12). Nevertheless, it was observed that adrenalectomized mice showed less but statistically significant immunosuppression in response to amphetamine administration. This led members of our group to believe that other neuropeptides and neurotransmitters (i.e., prolactin, endorphins, thyrotropin, and dopamine) might be involved in the immunological response to amphetamine. However, the large number of interactions at the molecular, cellular, and functional levels between the nervous and immune systems that characterize the operational compositions and expressions of the neuroimmune network make the isolation of the pathways in which amphetamine may be involved in the regulation of the immune responses complex. Therefore, many questions still need to be addressed in order to understand more fully the immunosuppressive characteristics of amphetamine.
In conclusion, our data at present show that amphetamine, through known and unknown neuroendocrine pathways, should injure the elements of the immunological apparatus, which in turn may leave the subject vulnerable to the action of C. albicans.
ACKNOWLEDGMENT
We express our gratitude to J. A. Veira for his translation services.
REFERENCES
- 1.Allen C M, Paulson R, Duncan R. Clinical, histologic and scanning electron microscopic study of the development of chronic candidiasis of the rat tongue. J Oral Pathol Med. 1989;18:352–359. doi: 10.1111/j.1600-0714.1989.tb01565.x. [DOI] [PubMed] [Google Scholar]
- 2.Allen C M, Blozis G C, Rosen S, Bright J S. Chronic candidiasis of the rat tongue: a possible model for human median rhomboid glossitis. J Dent Res. 1982;61:1287–1291. doi: 10.1177/00220345820610111501. [DOI] [PubMed] [Google Scholar]
- 3.Antelman S M, Eichler A J, Black C A, Kocan D. Interchangeability between stress and amphetamine in sensitization. Science. 1980;207:329–331. doi: 10.1126/science.7188649. [DOI] [PubMed] [Google Scholar]
- 4.Belluardo N, Mudo G, Cardile V, Migliorati G, Riccardi C, Cella S, Bindoni M. Hypothalamic control of the generation of mature natural killer lymphocytes in bone marrow and spleen of the mouse. Nat Immun Cell Growth Regul. 1990;9:26–35. [PubMed] [Google Scholar]
- 5.Blalock J E. Molecular mechanisms of bidirectional communication between the immune and neuroendocrine systems. Int J Neurosci. 1990;51:363–364. doi: 10.3109/00207459008999745. [DOI] [PubMed] [Google Scholar]
- 6.Bowen W H, Pearson S K, Young D A. The effect of desalivation on coronal and root surface caries in rats. J Dent Res. 1988;67:21–23. doi: 10.1177/00220345880670010301. [DOI] [PubMed] [Google Scholar]
- 7.Britton K T, Lee G, Koob G F. Corticotropin releasing factor and amphetamine exaggerate partial agonist properties of benzodiazepine antagonist Ro 15-1788 in the conflict test. Psychopharmacology. 1988;94:306–311. doi: 10.1007/BF00174680. [DOI] [PubMed] [Google Scholar]
- 8.Cohen M S, Isturiz R E, Malech H L, Root R K, Wilfert C M, Gutman L, Buckley R H. Fungal infection in chronic granulomatous disease. Am J Med. 1981;71:59–66. doi: 10.1016/0002-9343(81)90259-x. [DOI] [PubMed] [Google Scholar]
- 9.Edwards J E., Jr Severe Candida infections: clinical perspectives, immune defense mechanisms, and current concept of therapy. Ann Intern Med. 1978;89:91–106. doi: 10.7326/0003-4819-89-1-91. [DOI] [PubMed] [Google Scholar]
- 10.Edwards J E., Jr . Moniliasis of the skin. In: Braude A I, editor. Infectious diseases and medical microbiology. Philadelphia, Pa: Saunders Company; 1986. pp. 50–67. [Google Scholar]
- 11.Freire-Garabal M, Balboa J L, Núñez M J, Castaño M T, Llovo J B, Fernández-Rial J C, Belmonte A. Effects of amphetamine on T-cell immune response in mice. Life Sci. 1991;49:107–112. doi: 10.1016/0024-3205(91)90570-2. [DOI] [PubMed] [Google Scholar]
- 12.Freire-Garabal M, Núñez M J, Balboa J L, Fernández-Rial J C, Belmonte A. Effects of amphetamine on the activity of phagocytosis in mice. Life Sci. 1992;51:145–148. doi: 10.1016/0024-3205(92)90362-s. [DOI] [PubMed] [Google Scholar]
- 13.Freire-Garabal M, Núñez M J, Losada C, Pereiro D, Castro-Bolaño C, Iglesias-Pérez M, Rial-Ruiz R, Fernández-Varela V, Mayán J M, Rey-Méndez M. Effects of amphetamine on delayed type hypersensitivity (DTH) response in stressed mice. Res Commun Subst Abuse. 1995;20:43–48. [Google Scholar]
- 14.Geller I, Seifter J. The effects of meprobamate, barbiturates, d-amphetamine and promazine on experimentally induced conflict in the rat. Psychopharmacology. 1960;1:482–492. [Google Scholar]
- 15.Holmberg K, Meyer R D. Fungal infections in patients with AIDS and AIDS-related complex. Scand J Infect Dis. 1986;18:179–192. doi: 10.3109/00365548609032326. [DOI] [PubMed] [Google Scholar]
- 16.House R V, Thomas P T, Bhargava H N. Comparison of immune functional parameters following in vitro exposure to natural and synthetic amphetamines. Immunopharmacol Immunotoxicol. 1994;16:1–21. doi: 10.3109/08923979409029897. [DOI] [PubMed] [Google Scholar]
- 17.Jorge A O C, Totti M A G, De Almeida O P, Scully C. Effect of sialoadenectomy on the carriage of Candida albicans in the mouths of rats. J Oral Pathol Med. 1993;22:54–56. doi: 10.1111/j.1600-0714.1993.tb01045.x. [DOI] [PubMed] [Google Scholar]
- 18.Klein R S, Harris C A, Small C B, Moll B, Lesser M, Friedland G H. Oral candidiasis in high risk patients as the initial manifestation of the acquired immunodeficiency syndrome. N Engl J Med. 1984;310:354–358. doi: 10.1056/NEJM198408093110602. [DOI] [PubMed] [Google Scholar]
- 19.Krause M W, Schaffner A. Comparison of immunosuppressive effects of cyclosporine A in a murine model of systemic candidiasis and of localized thrushlike lesions. Infect Immun. 1989;57:3472–3478. doi: 10.1128/iai.57.11.3472-3478.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madison K M, Bowen W H, Pearson S K, Young D A. Effect of desalivation and age on susceptibility to infection by Streptococcus sobrinus. Caries Res. 1989;23:70–74. doi: 10.1159/000261159. [DOI] [PubMed] [Google Scholar]
- 21.Meitner S W, Bowen W H, Haidaris C G. Oral and esophageal Candida albicans infection in hyposalivatory rats. Infect Immun. 1990;58:2228–2236. doi: 10.1128/iai.58.7.2228-2236.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Núñez M J, Fernández-Rial J C, Couceiro J, Suárez J A, Gómez-Fernández D E, Rey-Méndez M, Freire-Garabal M. Effects of amphetamine on influenza virus infection in mice. Life Sci. 1993;52:73–78. doi: 10.1016/0024-3205(93)90520-d. [DOI] [PubMed] [Google Scholar]
- 23.Núñez-Iglesias M J, Castro-Bolaño C, Losada C, Pereiro-Raposo M D, Riveiro P, Sánchez-Sebio P, Mayán-Santos J M, Rey-Méndez M, Freire-Garabal M. Effects of amphetamine on cell mediated immune response in mice. Life Sci. 1996;58:29–33. doi: 10.1016/0024-3205(95)02272-4. [DOI] [PubMed] [Google Scholar]
- 24.O’Grady J F, Reade P C. Role of thermal trauma in experimental oral mucosal Candida infections in rats. J Oral Pathol Med. 1993;22:132–137. doi: 10.1111/j.1600-0714.1993.tb01044.x. [DOI] [PubMed] [Google Scholar]
- 25.Pezzone M A, Rush K A, Kusnecov A W, Wood P G, Rabin B S. Corticosterone-independent alteration of lymphocyte mitogenic function by amphetamine. Brain Behav Immun. 1992;6:293–299. doi: 10.1016/0889-1591(92)90050-x. [DOI] [PubMed] [Google Scholar]
- 26.Riley V. Psychoneuroendocrine influences on immunocompetence and neoplasia. Science. 1981;212:1100–1109. doi: 10.1126/science.7233204. [DOI] [PubMed] [Google Scholar]
- 27.Rose M D, Winston F, Hieter P, editors. Methods in yeast genetics: laboratory course manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- 28.Schaar G, Long I, Widra A. A combination rapid and standard method for identification of Candida albicans. Mycopathologia. 1974;52:203–207. doi: 10.1007/BF02198744. [DOI] [PubMed] [Google Scholar]
- 29.Sutton R E, Koob G F, Le Moal M, Rivier J, Vale W. Corticotropin releasing factor produces behavioral activation in rats. Nature. 1982;297:331–333. doi: 10.1038/297331a0. [DOI] [PubMed] [Google Scholar]
- 30.Thaler M, Pastakia B, Shawker H, O’Leary T, Pizzo P R. Hepatic candidiasis in cancer patients: evolving picture of the syndrome. Ann Intern Med. 1988;108:85–100. doi: 10.7326/0003-4819-108-1-88. [DOI] [PubMed] [Google Scholar]
- 31.Weisbroth S H, Fudens J H. The use of ketamine hydrochloride as an anesthetic in laboratory rabbits, rats, mice, and guinea pigs. Lab Anim Sci. 1972;22:904–906. [PubMed] [Google Scholar]