Abstract
Background: Atrial fibrillation (AF) and heart failure (HF) often coexist and synergistically contribute to an increased risk of hospitalization, stroke, and mortality. Objective: To compare the efficacy of catheter ablation (CA) versus medical therapy (MT) in HF patients with AF. Methods: Electronic databases were queried for randomized controlled trials (RCTs) of CA versus MT of AF in patients with HF. Risk ratios (RRs), mean differences (MDs), and 95% confidence intervals (CIs) were measured using the Mantel–Haenszel method. Results: A total of nine RCTs enrolling 2155 patients met the inclusion criteria. Compared to MT, CA led to a significant reduction in the composite of all-cause mortality and HF hospitalization (24.6% vs. 37.1%; RR: 0.65 (95% CI: 0.53–0.80); p < 0.0001), all-cause mortality (8.8% vs. 13.6%; RR: 0.65 (95% CI: 0.51–0.82); p = 0.0005), HF hospitalization (15.4% vs. 22.4%; (RR: 0.67 (95% CI: 0.54–0.82); p = 0.0001), AF recurrence (31.8% vs. 77.0%; RR: 0.36 (95% CI: 0.24–0.54); p < 0.0001), and cardiovascular (CV) death (4.9% vs. 8.4%; RR: 0.58 (95% CI: 0.39–0.86); p = 0.007). CA improved the left ventricular ejection fraction (MD:4.76% (95% CI: 2.35–7.18); p = 0.0001), 6 min walk test (MD: 20.48 m (95% CI: 10.83–30.14); p < 0.0001), peak oxygen consumption (MD: 3.1 2mL/kg/min (95% CI: 1.01–5.22); p = 0.004), Minnesota Living with Heart Failure Questionnaire score (MD: −6.98 (95% CI: −12–03, −1.93); p = 0.007), and brain natriuretic peptide levels (MD:−133.94 pg/mL (95% CI: −197.33, −70.55); p < 0.0001). Conclusions: In HF patients, AF catheter ablation was superior to MT in reducing CV and all-cause mortality. Further significant benefits occurred within the ablation group in terms of HF hospitalizations, AF recurrences, the systolic function, exercise capacity, and quality of life.
Keywords: atrial fibrillation, heart failure, catheter ablation, medical therapy, randomized controlled trials, recurrence
1. Introduction
Atrial fibrillation (AF) and heart failure (HF) are closely interlinked by pathophysiological mechanisms synergistically contributing to atrial and ventricular myopathy and dysfunction [1,2,3,4].
AF prevalence ranges between <10% and 50%, according to the clinical severity of HF [5]. When AF and HF coexist, their natural history is further complicated by an increased risk of hospitalization, stroke, and all-cause and cardiovascular (CV) mortality [6,7,8]. Therefore, treating AF in HF patients poses several challenges due to the complexity of this population [9,10,11].
As earlier trials have provided conflicting results regarding the best treatment strategy for AF, it is still debated whether rhythm control should be preferred over rate control. Recently, the Early Treatment of Atrial Fibrillation for Stroke Prevention Trial (EAST-AFNET 4) showed the significant benefits of a rhythm control strategy compared to usual care among patients with AF diagnosed within 1 year and other concomitant CV conditions [12].
These results, although encouraging, were not corroborated by two other recent trials [13,14] showing no significant clinical advantage provided by catheter ablation (CA), with both trials being terminated early due to their apparent futility. To explain these uncertainties, we performed a systematic review and meta-analysis of the randomized controlled trials (RCTs), aiming to compare CA versus the medical therapy (MT) of AF in HF patients.
2. Methods
2.1. Data Sources and Searches
We systematically searched the Medline, Cochrane, Journals@Ovid, and Scopus electronic databases for RCTs published from the time of inception to 30 May 2022 and focusing on CA versus MT in HF patients with AF. Three investigators (A.P, G.V., and M.M.) independently performed searches including the following terms: atrial fibrillation, heart failure, left ventricular dysfunction, and catheter ablation. Detailed information of our literature search strategy is available in the expanded methods.
2.2. Study Selection and Outcomes
The preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement for reporting systematic reviews and meta-analyses was used in this study [15].
All titles and full-text versions of the selected relevant RCTs were screened to identify those comparing AF ablation with rate or rhythm control therapy in HF patients, which had at least a 6-month follow-up period, included adults aged 18 years or older, and reported 1 or more clinical outcomes.
Observational studies, nonrandomized trials, editorials, case reports, reviews, expert opinions, and non-English studies were excluded.
2.3. Data Extraction and Quality Appraisal
Three investigators (A.P., G.V., and M.M.) extracted data from each study using standardized protocols and reporting forms and independently assessed the quality items. Disagreements were resolved by consensus. The quality of the individual studies was assessed by three investigators (A.P, G.V., and M.M.) using the Cochrane risk of bias Tool [16], as reported in the Supplementary Materials, Figure S1.
2.4. Study Endpoints
The primary endpoint was a composite of all-cause mortality and HF hospitalization. These endpoints were also assessed independently. Other secondary endpoints were CV death, AF recurrence rate, changes in the left ventricular ejection fraction [(LVEF) ΔLVEF], changes in quality of life (assessed via the Minnesota Living with Heart Failure Questionnaire (ΔMLHFQ)), changes in peak oxygen consumption (ΔVO2max), changes in distance walked during a 6-min walk test (Δ6MWT), and changes in brain natriuretic peptide (ΔBNP) levels.
The safety endpoints were CA-related periprocedural adverse events (e.g., access site complications (femoral bleeding or hematoma), pericardial complications (with and without tamponade), pulmonary vein stenosis, and procedural stroke) and antiarrhythmic drug therapy-related side effects (e.g., proarrhythmic effects, pulmonary, liver, and thyroid toxicity).
2.5. Statistical Analysis
Descriptive statistics are presented as means and standard deviations (SD) for the continuous variables or a number of cases (n) and percentages (%) for the dichotomous and categorical variables. The Mantel–Haenszel risk ratio (RR) model was used to summarize the data among the treatment arms. Summary estimates and 95% confidence intervals (CI) were reported for the continuous variables as the standardized mean difference. Freeman–Tukey double arcsine transformation was used to establish the variance of raw proportions. We used the Hartung–Knapp–Sidik–Jonkman method with the random effect model to combine the transformed proportions. The heterogeneity across studies was evaluated by using the Chi2, Tau2, and Higgins-I2 statistics, while random effects models of DerSimonian and Laird or fixed effects models were used in cases of I2 > 25% or ≤25%, respectively. Sensitivity analyses comparing CA and drug therapy were performed including patients with reduced LVEF (<50%) or persistent AF or with a pharmacological rhythm control strategy. The publication bias was assessed using the funnel plot and Egger’s test. The statistical analysis was performed using Review Manager (RevMan) (computer program) Version 5.4.1, Copenhagen, Denmark: Nordic Cochrane Centre, the Cochrane Collaboration, 2020.
3. Results
3.1. Study Selection
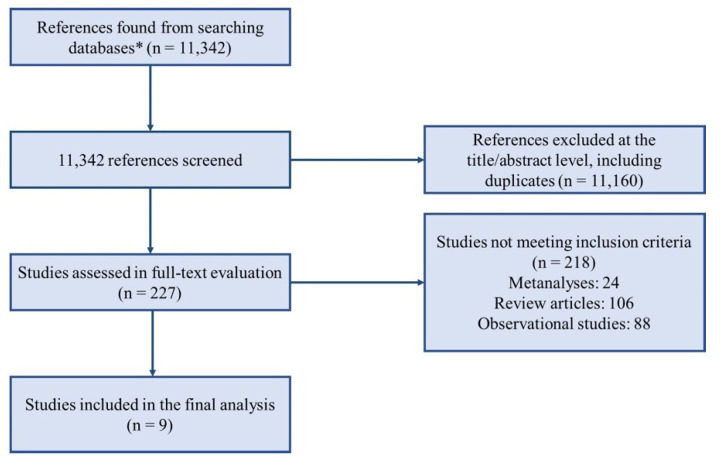
We screened 11,342 articles, from which 229 full texts were retrieved and reviewed for possible inclusion. A total of nine RCTs [13,14,17,18,19,20,21,22,23,24] comprising 2155 patients were identified (Figure 1).
Figure 1.
Evidence search and selection of the preferred reporting items for systematic reviews and meta-analyses (PRISMA). * Medline, Cochrane, Journals@Ovid, Scopus.
3.2. Baseline Characteristics
Baseline clinical characteristics are reported in Table 1. The nine RCTs enrolled 2155 patients. Among them, 1077 were assigned to CA and 1078 to drug therapy. Overall, 69.9% (n = 1507) of patients were male, with an average age of 63.5 years (95% CI: 62.1–64.9), and the mean LVEF was 37.9% (95% CI: 32.3–43.6). Further details on the baseline clinical characteristics are reported in Table 1 and Supplementary Materials, Table S1.
Table 1.
Study Baseline Characteristics of Patients Included in the Analysis.
| Study | MacDonald et al., 2011 [23] | ARC-HF, 2013 [21] | CAMTAF, 2014 [20] | AATAC, 2016 [19] | CAMERA-MRI, 2017 [24] | CASTLE-AF, 2018 [18] | AMICA, 2019 [13] | CAMERA LATE OUTCOMES, 2020 [22] | CABANA, 2021 [17] | RAFT-AF, 2022 [14] | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Monocentric or multicentric | Multicentric | Multicentric | Monocentric | Multicentric | Multicentric | Multicentric | Multicentric | Multicentric | Multicentric | Multicentric | |
| Comparison | Ablation vs. medical rate control | Ablation vs. medical rate control | Ablation vs. medical rate control | Ablation vs. amiodarone | Ablation vs. medical rate control | Ablation vs. medical rhythm and rate control | Ablation vs. medical rhythm and rate control | Ablation vs. medical rate control | Ablation vs. medical rhythm and rate control | Ablation vs. medical rate control | |
| HF inclusion criteria | NYHA class II or greater and optimal HF treatment for at least 3 months | NYHA class II or greater and optimal HF treatment for at least 1 month | NYHA class II or greater and optimal HF treatment for at least 3 months | NYHA class II to III | NYHA class ≥ II | NYHA class ≥ II | NYHA class II or greater and optimal HF treatment for at least 1 months | NYHA class ≥ II | NYHA class ≥ II | NYHA class II/III HF on optimal guideline directedmedical therapy and elevated NT-proBNP | |
| LVEF inclusion criterion | ≤35% (RNVG) | ≤35% | <50% | <40% | ≤45% | ≤35% | ≤35% | ≤45% | No LVEF inclusion criterion | No LVEF inclusion criterion | |
| Type of AF | Persistent | Persistent | Persistent | Persistent | Persistent | Paroxysmal or persistent | Persistent | Persistent | Paroxysmal or persistent | Paroxysmal or persistent | |
| Patients at randomization, n | Ablation | 22 | 26 | 26 | 102 | 34 | 200 | 104 | 34 | 378 | 214 |
| Drug | 19 | 26 | 24 | 101 | 34 | 197 | 98 | 34 | 400 | 197 | |
| Mean age, years (SD or IQR) | Ablation | 62.3 ± 6.7 | 64 ± 10 | 55 ± 12 | 62 ± 10 | 59 ± 11 | 64 (56–71) | 65 ± 8 | 60.5 ± 10.7 | 68 (62, 73) | 65.9 ± 8.6 |
| Drug | 64.4 ± 8.3 | 62 ± 9 | 60 ± 10 | 60 ± 11 | 62 ± 9.4 | 64 (56–73.5) | 65 ± 8 | 65.5 ± 7.2 | 67 (62, 73) | 67.5 ± 8.0 | |
| LVEF Baseline (SD or IQR), % | Ablation | 36.1 ± 11.9 (MRI) 16.1 ± 7.1 (RNVG) | 22 ± 8 (RNVG) | 31.8 ± 7.7 | 29 ± 5 | 35 ± 9.8 (MRI) | 32.5 (25.0–38.0) | 27.8 ± 9.5 | 36.1 ± 9.6 (MRI) | 55 (50-60) | EF ≤ 45%: 30.1 ± 8.5 EF > 45%: 55.9 ± 6.7 |
| Drug | 42.9 ± 9.6 (MRI) 19.6 ± 5.5 (RNVG) | 25 ± 7 (RNVG) | 33.7 ± 12.1 | 30 ± 8 | 35 ± 9.3 (MRI) | 31.5 (27.0–37.0) | 24.8 ± 8 | 34.6 ± 9.1 (MRI) | 56 (50-62) | EF ≤ 45%: 30.3 ± 9.2 EF > 45%: 54.6 ± 7.3 | |
| Mean baseline 6MWT (SD), meters | Ablation | 317.5 ± 125.8 | 416 ± 78 | NA | 348 ± 111 | 491 ± 147 | NA | NA | NA | NA | 363.1 ± 101.4 |
| Drug | 351.8 ± 117.1 | 411 ± 109 | NA | 350 ± 130 | 489 ± 132 | NA | NA | NA | NA | 344.4 ± 107.1 | |
| Mean baseline VO2 max (SD), mL/kg per min | Ablation | NA | 16.3 ± 5.3 | 22 | NA | NA | NA | NA | NA | NA | NA |
| Drug | NA | 18.2 ± 4.8 | 19.5 | NA | NA | NA | NA | NA | NA | NA | |
| Mean baseline MLHFQ score (SD) | Ablation | 55.8 ± 19.8 | 42 ± 23 | 42 | 52 ± 24 | NA | NA | NA | NA | NA | NA |
| Drug | 59.2 ± 22.4 | 49 ± 21 | 48 | 50 ± 27 | NA | NA | NA | NA | NA | NA | |
| Mean baseline BNP(SD or IQR), pg/mL | Ablation | NA | 412 ± 324 | NA | NA | 266 ± 210 | NA | NA | NA | NA | NA |
| Drug | NA | 283 ± 285 | NA | NA | 256 ± 208 | NA | NA | NA | NA | NA | |
| Follow-up | 6 mo | 12 mo | 6 and 12 mo | 24 mo | 6 mo | 60 mo | 12 mo | 4.0 ± 0.9 years | 48.5 mo | 24 mo |
Note: 6MWT: 6 min walk test; AATAC: ablation versus amiodarone for treatment of atrial fibrillation in patients with congestive heart failure and an implanted ICD; AF: atrial fibrillation; AMICA: atrial fibrillation management in congestive heart failure with ablation; ARC-HF: a randomized trial to assess catheter ablation versus rate control in the management of persistent atrial fibrillation in chronic heart failure; BNP: brain natriuretic peptide; CABANA: catheter ablation vs. antiarrhythmic drug therapy for atrial fibrillation; CAMERA-MRI: catheter ablation versus medical rate control in atrial fibrillation and systolic dysfunction; CAMTAF: a randomized controlled trial of catheter ablation versus medical treatment of atrial fibrillation in heart failure; CASTLE-AF: catheter ablation versus standard conventional therapy in patients with left ventricular dysfunction and atrial fibrillation; HF: heart failure; IQR: interquartile range; LVEF: left ventricular ejection fraction; MLHFQ: Minnesota Living with Heart Failure Questionnaires; mo: months; MRI: magnetic resonance imaging; NA: not available; NYHA: New York Heart Association; RAFT: randomized ablation-Based rhythm control versus rate control; RNVG: radionuclide ventriculography; SD: standard deviation; VO2 max: peak oxygen consumption.
Pulmonary vein isolation (PVI) was the mainstay ablation strategy used for all AF patients randomized to CA. Additional ablation outside the pulmonary veins (e.g., left atrial roof, mitral isthmus and/or cavotricuspid isthmus, posterior wall, left atrial appendage, superior vena cava, complex fractionated atrial electrograms) is specified in the Supplementary Materials, Table S2 [4,25,26,27].
Among the patients randomized to MT, a rate control strategy was pursued in five studies [14,20,21,22,23] and rhythm was pursued control therapy in one, while a combined treatment of both strategies was pursued in three trials (the AMICA (atrial fibrillation management in congestive heart failure with ablation) [13], CASTLE-AF [18], and CABANA trials [17] had 38%, 30%, and 29% of patients on rhythm control, respectively).
3.3. Composite Endpoint, All-Cause Mortality, HF Hospitalizations
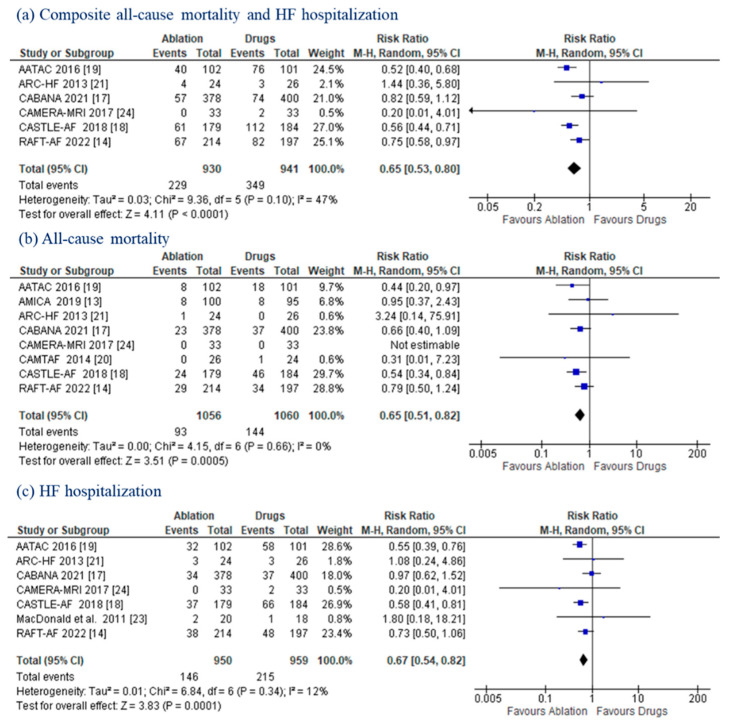
All trials reported data on all-cause mortality and/or HF hospitalization. AF ablation led to a significant reduction in the composite endpoint (24.6% vs. 37.1%; RR: 0.65 (95% CI: 0.53–0.80); p < 0.00001; I2: 47%]) (Figure 2a). CA also contributed to a significantly lower incidence of all-cause mortality (8.8% vs. 13.6%; RR: 0.65; (95% CI: 0.51–0.82); p = 0.0005) and HF hospitalization (15.4% vs. 22.4%; RR: 0.67 (95% CI: 0.54–0.82); p = 0.0001) (Figure 2b,c). No statistically significant heterogeneity was documented (I2 = 0% and 12%, respectively).
Figure 2.
Composite Endpoint, All-Cause Mortality, HF Hospitalizations. Forest plots displaying a decrease in the composite endpoint (a), all-cause mortality (b), and HF hospitalizations (c) in patients with AF and HF undergoing CA versus MT. CI: confidence interval; HF: heart failure.
3.4. Other Secondary Endpoints
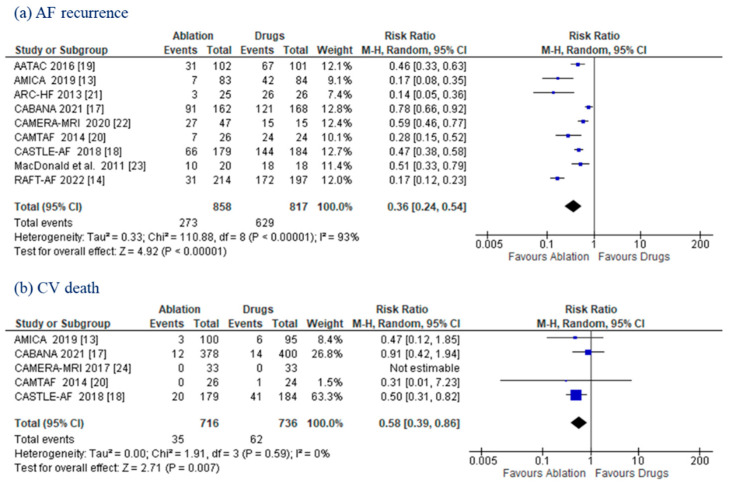
AF relapse was higher in the MT population (77.0% vs. 31.8%), with CA promoting significantly higher freedom from AF (RR: 0.36; (95% CI: 0.24–0.54); p < 0.00001) (Figure 3a). Five RCTs [13,17,18,20,24] reported data on CV death, which was less frequent in the CA group than in the MT group (4.9% vs. 8.4%; RR: 0.58; (95% CI: 0.39–0.86); p = 0.007). No heterogeneity (I2 = 0%) was observed (Figure 3b).
Figure 3.
AF Recurrence and CV Death. Forest plots displaying risk ratio in AF recurrence (a) and cardiovascular death (b) between the ablation and drug groups. AF: atrial fibrillation; CI: confidence interval; CV: cardiovascular; LVEF: left ventricular ejection fraction.
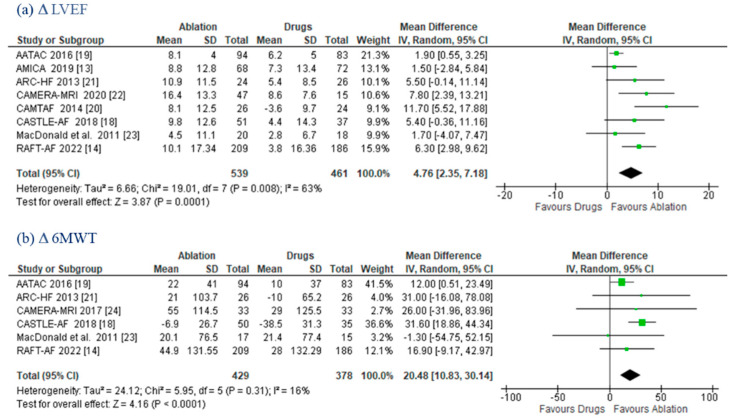
LVEF changes were evaluated with cardiac ultrasound in five trials [13,14,18,19,20], with magnetic resonance imaging (MRI) in the MacDonald et al. [23] trial, with both MRI and echocardiography in CAMERA-MRI [22], and with radionuclide in the ARC-HF trial [21]. The ablation group had a greater increase in ΔLVEF (MD 4.76%; (95% CI: 2.35–7.18); p = 0.0001) compared to MT, with moderate heterogeneity (I2 = 63%) (Figure 4a).
Figure 4.
LVEF and 6MWT. Forest plots displaying mean differences in LVEF (a) and 6MWT (b) between the ablation and drug groups: 6MWT: 6-minute walk test; CI: confidence interval; LVEF: left ventricular ejection fraction; SD: standard deviation.
Δ6MWT data were available for six trials [14,18,19,21,23,24], with AF ablation being associated with a greater improvement at follow-up (MD 20.48 m; (95% CI: 10.83–30.14); p < 0.0001) (Figure 4b).
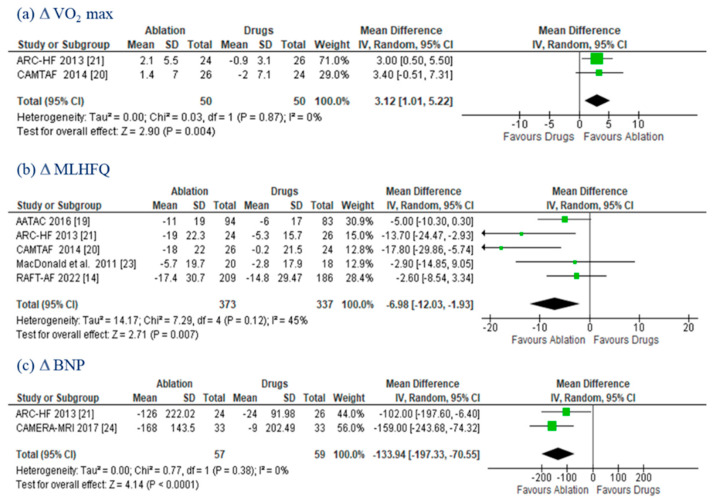
Data on the ΔVO2 max and ΔMLHFQ were provided in two [20,21] and five [14,19,20,21,23] trials, respectively. The improvements promoted by CA were both statistically significant (ΔVO2: MD 3.12 mL/kg/min; (95% CI: 1.01–5.22); p = 0.004; I2 = 0%; and ΔMLHFQ: MD −6.98; (95% CI: −12–03, −1.93); p = 0.007; I2 = 45%) (Figure 5a,b). ΔBNP was evaluated in two RCTs [21,24]. CA patients showed a larger reduction (MD −133.94 pg/mL; (95% CI: −197.33, −70.55); p < 0.0001; I2 = 0%) compared to those on drug therapy (Figure 5c).
Figure 5.
VO2 max, MLHFQ, and BNP. Forest plots displaying mean differences in VO2 max (a), MLHFQ (b), and BNP (c) between the ablation and drug groups. CI: confidence interval; MLHFQ: Minnesota Living with Heart Failure Questionnaires; SD: standard deviation; VO2 Max: peak oxygen consumption.
3.5. Safety Endpoints
Data on CA-related adverse events are summarized in Table 2A. Overall, 62 periprocedural adverse events were reported (5.02% (95% CI: 3.44–0.81); Supplementary Materials, Figure S2a). Among them, access site complications were the most common ones (2.37% (95% CI: 1.42–3.5%), followed by pericardial effusion/tamponade in 0.8% (95% CI: 0.23–1.6%).
Table 2.
Periprocedural Complications of Catheter Ablation (A) and Adverse Events of Antiarrhythmic Drugs (B).
| A. Periprocedural Complications | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study | Access Site Complications, n | Pericardial Effusion/tamponade, n | Esophageal Complications, n | Systemic Embolism, n | Pulmonary Stenosis, n | |||
| MacDonald et al., 2011 [23] | 0 | 2 | 0 | 0 | 0 | |||
| ARC-HF, 2013 [21] | 1 | 1 | 0 | 0 | 0 | |||
| CAMTAF, 2014 [20] | 0 | 1 | 0 | 1 | 0 | |||
| AATAC, 2016 [19] | 2 | 1 | 0 | 0 | 0 | |||
| CAMERA-MRI, 2017 [24] | 1 | 0 | 0 | 0 | 0 | |||
| CASTLE-AF, 2018 [18] | 3 | 3 | 0 | 0 | 1 | |||
| AMICA, 2019 [13] | 2 | 1 | 1 | 0 | 0 | |||
| CABANA, 2021 [17] | 15 | 2 | 4 | 0 | 0 | |||
| RAFT-AF, 2022 [14] | 9 | 6 | 1 | 4 | 0 | |||
| OVERALL, % | 2.37% | 0.8% | 0.07% | 0.01% | 0.001% | |||
| B. Antiarrhythmic Drug Adverse Events | ||||||||
| Study | Thyroid toxicity, n | Liver and Pulmonary toxicity, n | Proarrhythmic effect, n | Unspecified toxicity, n | ||||
| AATAC, 2016 [19] | 4 | 3 | ||||||
| CABANA, 2021 [17] | 9 | 2 | 3 | 5 | ||||
| RAFT-AF, 2022 [14] | 4 | 1 | ||||||
| OVERALL, % | 1.38% | 0.48% | 0.8% | 0.7% | ||||
Anti-arrhythmic drug adverse events are summarized in Table 2B. Only three RCTs (AATAC [19], CABANA [17] and RAFT-AF [14]) reported antiarrhythmic drug-related toxicities, with 31 adverse events being documented (4.28% (95% CI: 2.56–6.39%) Supplementary Materials, Figure S2a).
The stroke risk at follow-up was reported in six RCTs [14,18,20,21,23,24] (Supplementary Materials, Table S3) and was significantly lower after CA compared to MT (0.48% (95% CI: 0–1.61%) vs. 2.60% (95% CI: 1.05–4.62%); p = < 0.01)) (Supplementary Materials, Figure S2b).
3.6. Sensitivity Analyses
-
-
Catheter Ablation vs. Rate Control
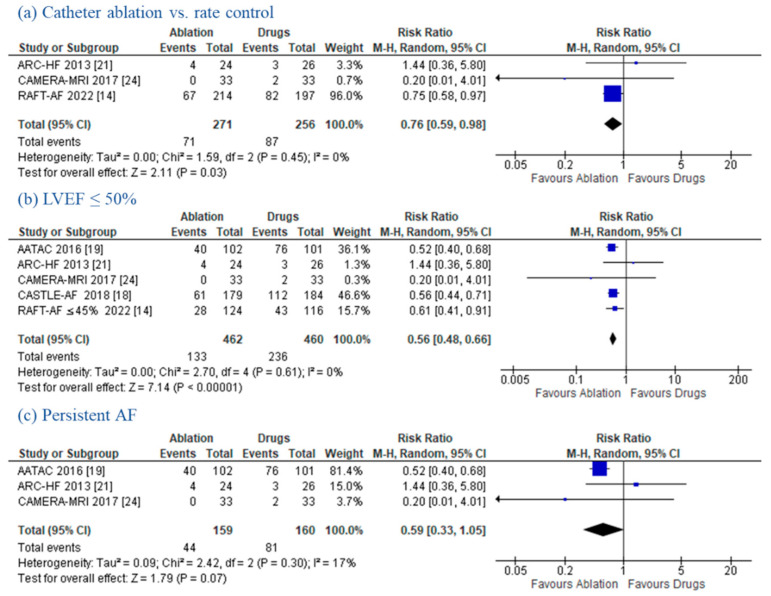
Three RCTs [14,21,24] performed a direct comparison between CA and medical rate control only treatment. A sub-analysis of these trials showed that CA was superior to rate control in reducing the composite endpoint of all-cause death and HF hospitalizations (RR 0.76; (95% CI: 0.59–0.98); p = 0.03; I2 = 0%) (Figure 6a). However, no significant differences were found in the all-cause mortality (RR 0.79; (95% CI: 0.51–1.24); p = 0.31; I2 = 0%), HF hospitalizations (RR 0.75; (95% CI: 0.52–1.07); p = 0.11; I2 = 0%), and CV death (RR 0.31; (95% CI: 0.01–7.23); p = 0.47). Although significant heterogeneity (I2 = 94%) was observed, CA showed a significant reduction in AF recurrence (RR 0.30; (95% CI: 0.14–0.65); p = 0.002). Additional data on ΔLVEF, Δ6MWT, and ΔMLFHQ are reported in the Supplementary Materials, Figure S3.
Figure 6.
Sensitivity Analysis for the Composite Endpoint. Forest plots displaying a decrease in the composite endpoint in the sensitivity analysis: (a) catheter ablation vs. rate control, (b) LVEF ≤ 50%, (c) persistent AF. CI: confidence interval; HF: heart failure.
-
-
LVEF ≤ 50%
The left ventricular systolic function was not an inclusion criterion in the CABANA [17] and RAFT-AF [14] trials. Therefore, we decided to perform a sensitivity analysis of patients with depressed LVEF (cut-off ≤50% or lower as an inclusion criterion). After CABANA [17] and the subpopulation of patients with LVEF > 45% in the RAFT-AF trial were excluded, CA showed a significant reduction in the composite endpoint (RR 0.56; (95% CI: 0.48–0.66); p < 0.00001; I2 = 0%) (Figure 6b), all-cause mortality (RR 0.57; (95% CI: 0.40–0.81); p = 0.002; I2 = 0%), HF hospitalizations (RR 0.57; (95% CI: 0.45–0.72); p < 0.00001; I2 = 0%), CV death (RR 0.49; (95% CI: 0.31–0.78); p = 0.003; I2 = 0%), and AF recurrence (RR 0.39; (95% CI: 0.29–0.53); p < 0.00001; I2 = 75%). Further secondary endpoint analyses (e.g., ΔLVWEF, Δ6MWT, ΔMLFHQ) are depicted in the Supplementary Materials, Figure S4.
-
-
Persistent AF
The CASTLE-AF [18], CABANA [17] and RAFT-AF [14] trials included a mixed population of patients with either paroxysmal or persistent AF. These RCTs were excluded for the purpose of performing a sensitivity analysis focusing on persistent AF patients only (Supplementary Materials, Figure S5). Compared to patients on MT, those undergoing CA showed a trend towards a reduction in the composite endpoint (RR 0.59; (95% CI: 0.33–1.05); p = 0.07; I2 = 17%) (Figure 6c) and a significative reduction in HF hospitalizations (RR 0.57; (95% CI: 0.41–0.78); p = 0.0006; I2 = 0%) and AF recurrence (RR 0.35; (95% CI: 0.22–0.55); p < 0.00001; I2 = 82%). No differences were documented in regard to all-cause mortality (RR 0.63; (95% CI: 0.35–1.12); p = 0.11; I2 = 0%) and CV death (RR 0.44; (95% CI: 0.13–1.54); p = 0.20; I2 = 0%). Other outcome data (e.g., ΔLVEF, Δ6MWT, ΔMLFHQ) in persistent AF patients are reported in the Supplementary Materials, Figure S5.
4. Discussion
Herein, we performed a systematic review and meta-analysis to provide a comprehensive overview of the outcomes of HF patients undergoing CA versus MT of AF.
We observed that CA was associated with a 35% reduction in the composite endpoint (all-cause mortality and HF hospitalization) compared to MT. A significant reduction was observed when the endpoints above were assessed separately (all-cause mortality: −35%; HF hospitalization: −33%).
Regarding other secondary outcomes, CA led to a lower rate of AF recurrence and CV death and promoted a significant improvement in the left ventricular systolic function (ΔLVEF), exercise capacity (Δ6MWT), cardiorespiratory fitness (ΔVO2 max), quality of life (ΔMLHFQ), and HF severity (ΔBNP).
To the best of our knowledge, our systematic review and meta-analysis is the most updated and comprehensive analysis of RCTs thus far. Compared to prior studies [28,29], we included data from the two recent AMICA [13] and RAFT-AF [14] trials, which together account for approximately one third of our study population, and performed additional endpoint/subpopulation analyses.
CA has been demonstrated to be the most effective rhythm control strategy, with several recent trials showing its superiority compared to MT as an early AF therapy in the general population [30]. Nonetheless, it is far more challenging to achieve rhythm control in patients with a high burden of comorbidities (e.g., HF, obstructive sleep apnea, coronary artery disease) [3,26,31,32,33] due to their more diseased atrial substrate. These patients are also more prone to drug-related, as well as ablation-related, complications.
AF and HF often coexist and share several risk factors, which may contribute to arrhythmia progression, as well as worse clinical outcomes, including stroke, hospitalization, and overall and cardiovascular mortality. RCTs investigating the success rate of CA compared to MT in patients with AF and HF have provided conflicting results. Among them, data from the two recent RCTs, AMICA [13] and RAFT [14], were published after the enrolment was closed early due to their apparent futility [13,14].
The RAFT-AF [14] trial enrolled 411 patients with high-burden paroxysmal or persistent AF. Although the initial goal was to recruit 600 patients, a determination of apparent futility by the Data Safety Monitoring Committee after an interim analysis led to the trial’s early termination. A non-significant trend towards improved outcomes with CA was reported, with the HR value of 0.71 (95% CI: 0.49–1.03; p = 0.066). Notably, CA led to a significantly greater improvement in LVEF, exercise capacity, AF and HF symptoms, and quality of life.
The AMICA [13] trial enrolled patients with persistent/longstanding persistent AF and LVEF ≤35%, aiming to assess any LVEF changes after 1 year. The study revealed a similar improvement of the LVEF with CA and MT; however, the comparison between the two treatment arms showed no statistically significant difference. The only significant difference was observed in the device-recorded AF burden, which was significantly higher in the MT group. It can be speculated that the trial included patients with a higher comorbidity rate compared to other similar trials (e.g., CASTLE-AF) [18], who were potentially too sick to show any significant clinical and functional advantages from CA and the resulting better sinus rhythm control. Similar findings were reported in the subpopulation of CASTLE-AF patients with New York Heart Association (NYHA) functional class III symptoms or an LVEF of <25%, who did not show any significant benefits from CA. In the CAMERA-MRI [24] trial, the improvement in LVEF promoted by CA was significantly better, with systolic function normalization being achieved in 58% of patients compared to 9% of those on MT (p = 0.0002). Nonetheless, the absolute LVEF improvement was significantly greater among patients without late gadolinium enhancement [3]. Thus, advanced cardiac remodeling may limit the recovery of the LVEF and also increase the risk of AF relapse in the long term [26,32]. These observations highlight the importance of an early AF ablation strategy.
CA, as a first line approach, might be particularly important for HF patients, since arrhythmic recurrences promote worsening cardiomyopathy, arrhythmia progression, and poor outcomes. Once the vicious cycle of AF and HF begins, the success rate of PVI is significantly lower and other sources of triggers outside the PVs may contribute to arrhythmia initiation/relapse [4]. Therefore, early ablation is critical for HF patients, as it is associated with better ablation success. Mechanistic and clinical studies have also highlighted the dynamic interplay between sinus rhythm restoration, cardiac function, symptoms, and clinical outcomes. Specifically, successful rhythm control may subsequently promote positive atrial and ventricular remodeling, which may result in better clinical outcomes. From this perspective, the benefits of CA on the cardiac function may manifest themselves later, and outcome improvements may occur even later. From a functional standpoint, the PABA-CHF [34] trial compared PVI versus atrioventricular-node ablation combined with biventricular pacing in HF patients with EF < 40%. Patients were followed-up over 6 months, showing a steady improvement in the mean LVEF in the CA group, who continued to recover until the end of the study period. From a clinical standpoint, the mortality curves separated only after 2 years in the CASTLE-AF [18] trial. Further studies are necessary in order to understand the best AF ablation strategy for HF patients, as well as the pathophysiological basis of CA-mediated functional and clinical benefits.
Another finding worth highlighting is the safety profile of CA in a population such as the HF population, characterized by a high comorbid profile. Notably, CA appeared to be safe and showed a similar risk of adverse events as MT. The prevalence of procedural complications was 5.02% (95% CI: 3.44–6.81), with access-site complications accounting for approximately half of the events [28,35,36].
5. Limitations
Our study has several limitations that need to be acknowledged. (1) Patients selected for a randomized catheter ablation trial may be healthier than those in real-world situations. (2) The number of patients enrolled in each RCTs was highly variable and may account for the imbalance in the results and heterogeneity. (3) The high heterogeneity regarding the arrhythmia detection techniques during follow-up should be considered. (4) Additional ablation outside the PVs, performed in some RCTs, could have affected the clinical outcomes. (5) The RCTs included here enrolled patients from 2011 to 2022, involving temporal changes in both CA and drug therapy. (6) Because patients and physicians were not blinded to the treatment assignment, it is possible that the post-ablation medical management differed between RCTs.
6. Conclusions
AF ablation was superior to conventional drug therapy in improving the composite of all-cause mortality and HF hospitalization, the all-cause mortality, HF hospitalization, AF recurrence, cardiovascular death, LVEF, 6 min walk test distance, VO2 max, and quality of life.
Abbreviations
| AATAC | Ablation versus Amiodarone for Treatment of Atrial Fibrillation in Patients with Congestive Heart Failure and an Implanted ICD |
| AF | Atrial Fibrillation |
| AMICA | Atrial Fibrillation Management in Congestive Heart Failure with Ablation |
| ARC-HF | A Randomized Trial to Assess Catheter Ablation Versus Rate Control in the Management of Persistent Atrial Fibrillation in Chronic Heart Failure |
| BNP | Brain Natriuretic Peptide |
| CA | Catheter Ablation |
| CABANA | Catheter Ablation vs. Antiarrhythmic Drug Therapy for Atrial Fibrillation |
| CAD | Coronary Artery Disease |
| CAMERA-MRI | Catheter Ablation Versus Medical Rate Control in Atrial Fibrillation and Systolic Dysfunction |
| CAMTAF | A Randomized Controlled Trial of Catheter Ablation Versus Medical Treatment of Atrial Fibrillation in Heart Failure |
| CASTLE-AF | Catheter Ablation versus Standard Conventional Therapy in Patients with Left Ventricular Dysfunction and Atrial Fibrillation |
| CIED | Cardiac Implantable Electronic Device |
| CRT-D | Cardiac Resynchronization Therapy Defibrillator |
| CV | Cardiovascular |
| ECG | Electrocardiogram |
| HF | Heart Failure |
| LVEF | Left Ventricular Ejection Fraction |
| MLHFQ | Minnesota Living with Heart Failure Questionnaire |
| MT | Medical Therapy |
| MRI | Magnetic Resonance Imaging |
| NYHA | New York Heart Association |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PVI | Pulmonary Vein Isolation |
| RAFT | Randomized Ablation-Based Rhythm-Control Versus Rate-Control |
| RCT | Randomized Controlled Trial |
| RR | Risk Ratio |
| SVC | Superior Vena Cava |
| VO2 max | Peak Oxygen Consumption |
| 6MWT | 6-Min Walk Test |
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11195530/s1, Expanded Methods; Full Search Strategy and Search Terms; Data Extraction; Expanded Results; Table S1: Additional Baseline Clinical Characteristics. Table S2: Overview of Ablation Strategies in Each Randomized Controlled Trial. Table S3: Thromboembolic Events during Follow-Up. Figure S1: Methodological evaluation of the included studies. Figure S2: Any Adverse Events and Thromboembolic Events. Figure S3: Catheter Ablation vs Medical Rate Control. Figure S4: Catheter Ablation vs Drug Therapy in patients with LVEF ≤ 50%. Figure S5: Catheter Ablation vs Drug Therapy in patients with Persistent AF.
Author Contributions
Conceptualization, F.M.C.; Data curation, M.M., A.P. and G.V.; Formal analysis, C.G., S.M. and V.M.; Investigation, F.D.V., R.C. and G.-B.C.; Methodology, M.P., L.P., C.M. and P.R.; Project administration, L.D.B.; Writing—original draft, S.B.; Writing—review & editing, C.d.A., A.N. and D.G.D.R. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available in a publicly accessible repository.
Conflicts of Interest
Dr. Chierchia received compensation for teaching purposes and proctoring from Medtronic, Abbott, Biotronik, Boston Scientific, and Acutus Medical. Dr. de Asmundis receives research grants on behalf of the center from Biotronik, Medtronic, Abbott, LivaNova, Boston Scientific, AtriCure, Philips, and Acutus, and compensation for teaching purposes and proctoring from Medtronic, Abbott, Biotronik, Livanova, Boston Scientific, Atricure, Acutus Medical, and Daiichi Sankyo. Dr. Di Biase is a consultant for Biosense Webster, Boston Scientific, Stereotaxis, and St. Jude Medical. Dr. Di Biase received speaker honoraria/travel support grants from Medtronic, Bristol Meyers Squibb, Pfizer, and Biotronik. Dr. Natale has received speaker honoraria from Boston Scientific, Biosense Webster, St. Jude Medical, Biotronik, and Medtronic, and is a consultant for Biosense Webster, St. Jude Medical, and Janssen. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sanders P., Morton J.B., Davidson N.C., Spence S.J., Vohra J.K., Sparks P.B., Kalman J.M. Electrical remodeling of the atria in congestive heart failure: Electrophysiological and electroanatomic mapping in humans. Circulation. 2003;108:1461–1468. doi: 10.1161/01.CIR.0000090688.49283.67. [DOI] [PubMed] [Google Scholar]
- 2.Martin C.A., Lambiase P.D. Pathophysiology, diagnosis and treatment of tachycardiomyopathy. Heart. 2017;103:1543. doi: 10.1136/heartjnl-2016-310391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Della Rocca D.G., Santini L., Forleo G.B., Sanniti A., Del Prete A., Lavalle C., Di Biase L., Natale A., Romeo F. Novel Perspectives on Arrhythmia-Induced Cardiomyopathy: Pathophysiology, Clinical Manifestations and an Update on Invasive Management Strategies. Cardiol. Rev. 2015;23:135–141. doi: 10.1097/CRD.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 4.Della Rocca D.G., Tarantino N., Trivedi C., Mohanty S., Anannab A., Salwan A.S., Gianni C., Bassiouny M., Al-Ahmad A., Romero J., et al. Non-pulmonary vein triggers in nonparoxysmal atrial fibrillation: Implications of pathophysiology for catheter ablation. J. Cardiovasc. Electrophysiol. 2020;31:2154–2167. doi: 10.1111/jce.14638. [DOI] [PubMed] [Google Scholar]
- 5.Maisel W.H., Stevenson L.W. Atrial fibrillation in heart failure: Epidemiology, pathophysiology, and rationale for therapy. Am. J. Cardiol. 2003;91:2D–8D. doi: 10.1016/S0002-9149(02)03373-8. [DOI] [PubMed] [Google Scholar]
- 6.Dries D.L., Exner D.V., Gersh B.J., Domanski M.J., Waclawiw M.A., Stevenson L.W. Atrial fibrillation is associated with an increased risk for mortality and heart failure progression in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: A retrospective analysis of the SOLVD trials. Studies of Left Ventricular Dysfunction. J. Am. Coll. Cardiol. 1998;32:695–703. doi: 10.1016/s0735-1097(98)00297-6. [DOI] [PubMed] [Google Scholar]
- 7.Wang T.J., Larson M.G., Levy D., Vasan R.S., Leip E.P., Wolf P.A., D’Agostino R.B., Murabito J.M., Kannel W.B., Benjamin E.J. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: The Framingham Heart Study. Circulation. 2003;107:2920–2925. doi: 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 8.The Stroke Prevention in Atrial Fibrillation Investigators Predictors of thromboembolism in atrial fibrillation: I. Clinical features of patients at risk. Ann. Intern. Med. 1992;116:1–5. doi: 10.7326/0003-4819-116-1-1. [DOI] [PubMed] [Google Scholar]
- 9.Bardy G.H., Lee K.L., Mark D.B., Poole J.E., Packer D.L., Boineau R., Domanski M., Troutman C., Anderson J., Johnson G., et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N. Engl. J. Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 10.Flaker G.C., Blackshear J.L., McBride R., Kronmal R.A., Halperin J.L., Hart R.G. Antiarrhythmic drug therapy and cardiac mortality in atrial fibrillation. The Stroke Prevention in Atrial Fibrillation Investigators. J. Am. Coll. Cardiol. 1992;20:527–532. doi: 10.1016/0735-1097(92)90003-6. [DOI] [PubMed] [Google Scholar]
- 11.Kaufman E.S., Zimmermann P.A., Wang T., Dennish G.W., 3rd, Barrell P.D., Chandler M.L., Greene H.L. Risk of proarrhythmic events in the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study: A multivariate analysis. J. Am. Coll. Cardiol. 2004;44:1276–1282. doi: 10.1016/j.jacc.2004.06.052. [DOI] [PubMed] [Google Scholar]
- 12.Kirchhof P., Camm A.J., Goette A., Brandes A., Eckardt L., Elvan A., Fetsch T., van Gelder I.C., Haase D., Haegeli L.M., et al. Early Rhythm-Control Therapy in Patients with Atrial Fibrillation. N. Engl. J. Med. 2020;383:1305–1316. doi: 10.1056/NEJMoa2019422. [DOI] [PubMed] [Google Scholar]
- 13.Kuck K.-H., Merkely B., Zahn R., Arentz T., Seidl K., Schlüter M., Tilz R.R., Piorkowski C., Gellér L., Kleemann T., et al. Catheter Ablation Versus Best Medical Therapy in Patients With Persistent Atrial Fibrillation and Congestive Heart Failure. Circ. Arrhythmia Electrophysiol. 2019;12:e007731. doi: 10.1161/CIRCEP.119.007731. [DOI] [PubMed] [Google Scholar]
- 14.Parkash R., Wells G.A., Rouleau J., Talajic M., Essebag V., Skanes A., Wilton S.B., Verma A., Healey J.S., Sterns L., et al. Randomized Ablation-Based Rhythm-Control Versus Rate-Control Trial in Patients With Heart Failure and Atrial Fibrillation: Results from the RAFT-AF trial. Circulation. 2022;145:1693–1704. doi: 10.1161/CIRCULATIONAHA.121.057095. [DOI] [PubMed] [Google Scholar]
- 15.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P.A., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins J.P.T., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D., Savović J., Schulz K.F., Weeks L., Sterne J.A.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Packer D.L., Piccini J.P., Monahan K.H., Al-Khalidi H.R., Silverstein A.P., Noseworthy P.A., Poole J.E., Bahnson T.D., Lee K.L., Mark D.B., et al. Ablation Versus Drug Therapy for Atrial Fibrillation in Heart Failure. Circulation. 2021;143:1377–1390. doi: 10.1161/CIRCULATIONAHA.120.050991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marrouche N.F., Brachmann J., Andresen D., Siebels J., Boersma L., Jordaens L., Merkely B., Pokushalov E., Sanders P., Proff J., et al. Catheter Ablation for Atrial Fibrillation with Heart Failure. N. Engl. J. Med. 2018;378:417–427. doi: 10.1056/NEJMoa1707855. [DOI] [PubMed] [Google Scholar]
- 19.Di Biase L., Mohanty P., Mohanty S., Santangeli P., Trivedi C., Lakkireddy D., Reddy M., Jais P., Themistoclakis S., Dello Russo A., et al. Ablation Versus Amiodarone for Treatment of Persistent Atrial Fibrillation in Patients With Congestive Heart Failure and an Implanted Device. Circulation. 2016;133:1637–1644. doi: 10.1161/CIRCULATIONAHA.115.019406. [DOI] [PubMed] [Google Scholar]
- 20.Hunter R.J., Berriman T.J., Diab I., Kamdar R., Richmond L., Baker V., Goromonzi F., Sawhney V., Duncan E., Page S.P., et al. A Randomized Controlled Trial of Catheter Ablation Versus Medical Treatment of Atrial Fibrillation in Heart Failure (The CAMTAF Trial) Circ. Arrhythmia Electrophysiol. 2014;7:31–38. doi: 10.1161/CIRCEP.113.000806. [DOI] [PubMed] [Google Scholar]
- 21.Jones D.G., Haldar S.K., Hussain W., Sharma R., Francis D.P., Rahman-Haley S.L., McDonagh T.A., Underwood S.R., Markides V., Wong T. A Randomized Trial to Assess Catheter Ablation Versus Rate Control in the Management of Persistent Atrial Fibrillation in Heart Failure. J. Am. Coll. Cardiol. 2013;61:1894–1903. doi: 10.1016/j.jacc.2013.01.069. [DOI] [PubMed] [Google Scholar]
- 22.Sugumar H., Prabhu S., Costello B., Chieng D., Azzopardi S., Voskoboinik A., Parameswaran R., Wong G.R., Anderson R., Al-Kaisey A.M., et al. Catheter Ablation Versus Medication in Atrial Fibrillation and Systolic Dysfunction: Late Outcomes of CAMERA-MRI Study. JACC Clin. Electrophysiol. 2020;6:1721–1731. doi: 10.1016/j.jacep.2020.08.019. [DOI] [PubMed] [Google Scholar]
- 23.MacDonald M.R., Connelly D.T., Hawkins N.M., Steedman T., Payne J., Shaw M., Denvir M., Bhagra S., Small S., Martin W., et al. Radiofrequency ablation for persistent atrial fibrillation in patients with advanced heart failure and severe left ventricular systolic dysfunction: A randomised controlled trial. Heart. 2011;97:740–747. doi: 10.1136/hrt.2010.207340. [DOI] [PubMed] [Google Scholar]
- 24.Prabhu S., Taylor A.J., Costello B.T., Kaye D.M., McLellan A.J.A., Voskoboinik A., Sugumar H., Lockwood S.M., Stokes M.B., Pathik B., et al. Catheter Ablation Versus Medical Rate Control in Atrial Fibrillation and Systolic Dysfunction: The CAMERA-MRI Study. J. Am. Coll. Cardiol. 2017;70:1949–1961. doi: 10.1016/j.jacc.2017.08.041. [DOI] [PubMed] [Google Scholar]
- 25.Mohanty S., Mohanty P., Trivedi C., Gianni C., Della Rocca D.G., Di Biase L., Natale A. Long-Term Outcome of Pulmonary Vein Isolation With and Without Focal Impulse and Rotor Modulation Mapping: Insights From a Meta-Analysis. Circ. Arrhythmia Electrophysiol. 2018;11:e005789. doi: 10.1161/CIRCEP.117.005789. [DOI] [PubMed] [Google Scholar]
- 26.Della Rocca D.G., Di Biase L., Mohanty S., Trivedi C., Gianni C., Romero J., Tarantino N., Magnocavallo M., Bassiouny M., Natale V.N., et al. Targeting non-pulmonary vein triggers in persistent atrial fibrillation: Results from a prospective, multicentre, observational registry. EP Eur. 2021;23:1939–1949. doi: 10.1093/europace/euab161. [DOI] [PubMed] [Google Scholar]
- 27.Mohanty S., Trivedi C., Horton P., Della Rocca D.G., Gianni C., MacDonald B., Mayedo A., Sanchez J., Gallinghouse G.J., Al-Ahmad A., et al. Natural History of Arrhythmia After Successful Isolation of Pulmonary Veins, Left Atrial Posterior Wall, and Superior Vena Cava in Patients With Paroxysmal Atrial Fibrillation: A Multi-Center Experience. JAHA. 2021;10:e020563. doi: 10.1161/JAHA.120.020563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turagam M.K., Garg J., Whang W., Sartori S., Koruth J.S., Miller M.A., Langan N., Sofi A., Gomes A., Choudry S., et al. Catheter Ablation of Atrial Fibrillation in Patients With Heart Failure. Ann. Intern. Med. 2019;170:41–50. doi: 10.7326/M18-0992. [DOI] [PubMed] [Google Scholar]
- 29.Briceño D.F., Markman T.M., Lupercio F., Romero J., Liang J.J., Villablanca P.A., Birati E.Y., Garcia F.C., Di Biase L., Natale A., et al. Catheter ablation versus conventional treatment of atrial fibrillation in patients with heart failure with reduced ejection fraction: A systematic review and meta-analysis of randomized controlled trials. J. Interv. Card. Electrophysiol. 2018;53:19–29. doi: 10.1007/s10840-018-0425-0. [DOI] [PubMed] [Google Scholar]
- 30.Andrade J.G., Wells G.A., Deyell M.W., Bennett M., Essebag V., Champagne J., Roux J.-F., Yung D., Skanes A., Khaykin Y., et al. Cryoablation or Drug Therapy for Initial Treatment of Atrial Fibrillation. N. Engl. J. Med. 2021;384:305–315. doi: 10.1056/NEJMoa2029980. [DOI] [PubMed] [Google Scholar]
- 31.Della Rocca D.G., Mohanty S., Mohanty P., Trivedi C., Gianni C., Al-Ahmad A., Burkhardt J.D., Gallinghouse G.J., Hranitzky P., Sanchez J.E., et al. Long-term outcomes of catheter ablation in patients with longstanding persistent atrial fibrillation lasting less than 2 years. J. Cardiovasc. Electrophysiol. 2018;29:1607–1615. doi: 10.1111/jce.13721. [DOI] [PubMed] [Google Scholar]
- 32.Mohanty S., Trivedi C., Gianni C., Della Rocca D.G., Morris E.H., Burkhardt J.D., Sanchez J.E., Horton R., Gallinghouse G.J., Hongo R., et al. Procedural findings and ablation outcome in patients with atrial fibrillation referred after two or more failed catheter ablations. J. Cardiovasc. Electrophysiol. 2017;28:1379–1386. doi: 10.1111/jce.13329. [DOI] [PubMed] [Google Scholar]
- 33.Natale V., Mohanty S., Trivedi C., Baqai F.M., Gallinghouse J., Della Rocca D.G., Gianni C., MacDonald B., Mayedo A., Burkhardt J.D., et al. Arrhythmia profile and ablation-outcome in elderly women with atrial fibrillation undergoing first catheter ablation. Pacing Clin. Electrophysiol. 2021;44:835–842. doi: 10.1111/pace.14223. [DOI] [PubMed] [Google Scholar]
- 34.Khan M.N., Jaïs P., Cummings J., Di Biase L., Sanders P., Martin D.O., Kautzner J., Hao S., Themistoclakis S., Fanelli R., et al. Pulmonary-Vein Isolation for Atrial Fibrillation in Patients with Heart Failure. N. Engl. J. Med. 2008;359:1778–1785. doi: 10.1056/NEJMoa0708234. [DOI] [PubMed] [Google Scholar]
- 35.Della Rocca D.G., Magnocavallo M., Natale V.N., Gianni C., Mohanty S., Trivedi C., Lavalle C., Forleo G.B., Tarantino N., Romero J., et al. Clinical presentation, diagnosis, and treatment of atrioesophageal fistula resulting from atrial fibrillation ablation. J. Cardiovasc. Electrophysiol. 2021;32:2441–2450. doi: 10.1111/jce.15168. [DOI] [PubMed] [Google Scholar]
- 36.Mohanty S., Trivedi C., Della Rocca D.G., Gianni C., MacDonald B., Quintero Mayedo A., Al-Ahmad A., Burkhardt J.D., Bassiouny M., Gallinghouse G.J., et al. Recovery of Conduction Following High-Power Short-Duration Ablation in Patients With Atrial Fibrillation: A Single-Center Experience. Circ. Arrhythmia Electrophysiol. 2021;14:e010096. doi: 10.1161/CIRCEP.121.010096. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available in a publicly accessible repository.