Abstract
A Leishmania donovani species-specific monoclonal antibody (monoclonal antibody D2) was evaluated for its diagnostic and prognostic potential by a competitive enzyme-linked immunosorbent assay (C-ELISA) in sera from Indian patients with visceral leishmaniasis (VL) and seven patients with post-kala-azar dermal leishmaniasis (PKDL). These results were compared with those obtained by microscopy with Giemsa-stained tissue smears and a direct enzyme-linked immunosorbent assay (direct ELISA) with crude parasite antigen. Of 121 patients with clinically diagnosed VL examined, 103 (85.1%) were positive and 11 (9.1%) were negative by all three methods. An additional 7 (5.8%) who were negative by microscopy were positive by both C-ELISA and direct ELISA. Seven PKDL patients were also examined and were found to be positive by all three methods. Analysis of the chemotherapeutic response to sodium antimony gluconate of these 110 serologically positive VL patients showed that 57 (51.8%) were drug responsive and 53 (48.2%) were drug resistant. The C-ELISA with sera from 20 longitudinally monitored VL patients before and after chemotherapy showed a significant decrease in percent inhibition of monoclonal antibody D2 in drug-responsive patients. However, in drug-unresponsive patients, the percent inhibition of D2 was unchanged or was slightly increased. Our results therefore indicate (i) the applicability of L. donovani species-specific monoclonal antibody D2 for sensitive and specific serodiagnosis by C-ELISA, (ii) that the C-ELISA is more sensitive than microscopy, especially for early diagnosis, (iii) that L. donovani is still the main causative agent of VL, irrespective of the chemotherapeutic response, and (iv) that the C-ELISA can be used to evaluate the success of drug treatment.
Visceral leishmaniasis (VL) or kala-azar is a major public health problem in eastern India, where it is endemic and where every 10 to 15 years it assumes epidemic proportions (5). In 1993, 30 districts in Bihar Province with a target population of 71.4 million were seriously affected (23). In the present epidemic (early 1990s to date), the incidence of unresponsiveness to antimonials has increased dramatically to over 60% (21), whereas it was only 8.6% in the epidemic that occurred in the early 1980s (22).
Considering the cyclical reemergence of the disease, a vital aspect of disease control is early diagnosis. The “gold standard” for diagnosis is still the direct demonstration of the Leishmania parasite in Giemsa-stained smears from tissue aspirates. However, due to the insensitivity of this method (1) coupled with the potential risks of the procedure, most patients in rural areas are empirically treated.
High titers of specific and nonspecific antibodies are a hallmark of patients presenting with kala-azar. This has allowed the development of several antibody-based serological tests in India, namely, the direct agglutination test (DAT) (17), indirect immunofluorescence (3), antigen detection (19), and the enzyme-linked immunosorbent assay (ELISA) (4, 6, 13). These tests have yet to gain widespread acceptability due to cross-reactivity with specimens from patients with coendemic diseases or antigen instability in the case of DAT (15). Introduction of PCR has obviated these problems (20), but its application as a routine diagnostic method has been limited by the mandatory technical expertise and equipment required. A major failing with most diagnostic methods presently available in India is their inability to identify the parasite species. In light of the increasing incidence of unresponsiveness of patients with VL and post-kala-azar dermal leishmaniasis (PKDL) to antimonial treatment in the current epidemic (1990s to date) and recent reports that Leishmania tropica causes VL in India (18), a pertinent unanswered question is whether a variation in the causative Leishmania parasite species may be responsible for the refractoriness to treatment with antimonials. If a causal relationship does exist, then early species identification may provide guidelines for the modification of clinical treatment.
A previous study (10) with sera primarily from patients in South America and Africa showed that a competitive ELISA (C-ELISA) with species-specific monoclonal antibodies (MAbs) directed against Leishmania donovani (MAb D2) could be used to diagnose VL specifically. In this study we demonstrate that the C-ELISA with MAb D2 can be used to diagnose both specifically and sensitively Indian leishmaniasis and that it can also be used for the prognostic evaluation of the disease and the success of drug treatment.
MATERIALS AND METHODS
Study population.
Pretreatment sera from 121 patients clinically diagnosed with kala-azar or VL were examined. Their salient clinical features were fever of known duration, hepatosplenomegaly, either a resident in or recent travel to a region where kala-azar is endemic, and no prior antileishmanial treatment. On the basis of the duration of fever, VL patients were broadly classified into the following three groups: group A, short duration (<30 days); group B, intermediate duration (1 to 3 months); and group C, long duration (>3 months). PKDL patients (n = 7) were also included.
A longitudinal study was also carried out. In that study serum was collected on admission and again on completion of a single course of sodium antimony gluconate (SAG) treatment (20 mg/kg of body weight for 4 to 6 weeks) from 20 patients with biopsy-confirmed kala-azar. On the basis of their clinical and parasitological responses to treatment, they were grouped as either drug responsive (remission of fever, regression of liver and spleen showed, and absence of parasites in Giemsa-stained tissue smears) or drug unresponsive (persistence of fever and hepatosplenomegaly along with persistence of parasites in Giemsa-stained tissue smears).
Control sera (n = 60) were obtained from patients with malaria (n = 10), tuberculosis (n = 10), and leprosy (n = 10) as well as healthy controls from both areas of endemicity (n = 10) and areas of nonendemicity (n = 20).
Study design.
Coded blood samples were sent to the Indian Institute of Chemical Biology, Calcutta, India, for serodiagnosis (C-ELISA and ELISA). Giemsa-stained biopsy smears were reviewed independently in Muzaffarpur, Patna, and Calcutta, India, according to the recommendations of the World Health Organization (24). The C-ELISA was initially performed with 20 specimens from patients with biopsy-proven VL, i.e., microscopic demonstration of amastigotes. Subsequently, samples were collected from patients who were clinically diagnosed with VL, and on completion of the assays the results were compared to ensure “blindness” in the protocol.
Bone marrow or splenic aspirates were routinely investigated for all patients in this study who were clinically diagnosed with kala-azar. Informed consent was obtained from patients or their parents and volunteers for collection of 1 ml of peripheral blood; serum was separated from whole blood and was stored at −20°C.
MAbs.
The species-specific MAbs directed against L. donovani (MAb D2) and L. tropica (MAb T11) were prepared by fusing the NS-1 (P3-X63/Ag8) plasmacytoma cell line with spleen cells obtained from BALB/c mice immunized with crude membranes of L. donovani (12) and L. tropica (11). MAb D2 reacts with L. donovani-specific antigenic determinants of 70 and 72 kDa (9), whereas MAb T11 recognizes an L. tropica-specific glycoconjugate corresponding to 32 to 44 kDa and also reacts with additional antigenic components of 55, 80, 92, and 130 kDa (11). Ascitic fluid containing either MAb D2 or MAb T11 was prepared by injecting the hybridoma cell line into pristane-primed BALB/c mice and was used in this study.
Preparation of parasite antigen.
Crude parasite antigen for ELISA and C-ELISA was prepared from an Indian L. donovani strain (MHOM/IN/83/AG83) (2) and an L. tropica strain (MHOM/SU/74/K27) recommended for use by the World Health Organization. Promastigotes were harvested with phosphate-buffered saline (PBS), and the cell pellet was resuspended in lysis buffer (20 mM Tris-HCl, 40 mM NaCl [pH 7.4]) containing 2 mM phenylmethylsulfonyl fluoride, 1 mg of leupeptin per ml, 5 mM EDTA, and 5 mM iodoacetamide (10).
C-ELISA and ELISA.
Immobilized crude Leishmania antigen prepared as described above was used to coat 96-well flat-bottom microtiter plates (0.1 mg/ml, 50 μl/well, in 0.02 M phosphate buffer [pH 7.8]). Following an overnight incubation, the wells were washed thrice with 0.1% Tween 20 in PBS (PBS-T; pH 7.2) washing buffer. The wells were then blocked with 2% fetal calf serum in PBS for 8 h at 4°C. Patient serum, diluted 1:50 for C-ELISA and 1:500 for ELISA, was incubated overnight at 4°C in duplicate and was washed with PBS-T. In the C-ELISA, the wells were then incubated with MAb D2 or MAb T11 (dilution, 1:1,000) for 2 h at 4°C and washed with PBS-T. Binding was assayed colorimetrically with either horseradish peroxidase-conjugated rabbit anti-mouse immunoglobulin G (IgG; 1:5,000; Cappel) for C-ELISA or anti-human IgG (1:10,000; Sigma) for ELISA and 100 μl of 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) ammonium salt as the substrate. The optical density (OD) at 405 nm was measured. Negative control samples from asymptomatic individuals from areas of endemicity were included on each plate. MAb (D2 and T11) binding following incubation of the plates with control normal human serum was equated to 100% binding, i.e., no MAb D2 or MAb T11 inhibition. The percent MAb D2 or MAb T11 binding of a sample was then calculated as follows: [(OD with 100% binding − OD of the sample)/OD with 100% binding] × 100.
Accordingly, the percent inhibition of MAb D2 or MAb T11 binding was extrapolated. Sera which inhibited MAb D2 or MAb T11 binding by >15% were considered positive. This cutoff value of >15% was established on the basis of a pilot study in which the percent inhibition of MAb D2 in 20 positive control serum specimens (i.e., in which amastigotes were found to be present in Giemsa-stained tissue smears) and 20 negative control serum specimens was measured. The mean percent inhibition of MAb D2 for healthy controls was 5.2% ± 1.0%. For ELISA, sera which gave a mean absorbance that was twice that for normal human serum from an area of endemicity were considered positive for VL and PKDL.
Statistical analysis.
The statistical significance of the results of the longitudinal study was compared by Student’s t test (paired and unpaired), and P values of <0.05 were considered significant.
RESULTS
This study included 208 individuals, of whom 121 were suspected cases of VL, and their principal clinical data are given in Table 1. These patients were broadly divided into three groups according to their durations of illness. In all three groups, the ages ranged from 3 to 40 years and the overall ratio of males:females was 78:43, with no differences observed between the three groups. A progressive increase in spleen size was observed with increasing duration of illness. The samples were screened for the presence of antibodies against human immunodeficiency virus (HIV) and were found to be negative.
TABLE 1.
Clinical data for patients with suspected VL included in this study
| Group | Patient no. | Mean ± SD duration of illness (days) | Spleen size
(cm)
|
No. of patients in the indicated response
category/total no. tested (%)a
|
||
|---|---|---|---|---|---|---|
| Avg | Range | SAG resp. | SAG resist. | |||
| A | 25 | 19.0 ± 5.3 | 3.7 | 1.5–10.0 | 12/21 (57.0) | 9/21 (43.0) |
| B | 43 | 63.1 ± 19.6 | 6.2 | 3.0–12.0 | 18/37 (48.6) | 19/37 (51.4) |
| C | 53 | 187.5 ± 95.0 | 8.4 | 4.5–15.0 | 27/52 (52.0) | 25/52 (48.0) |
Patients who were ELISA positive (n = 121) received a single course of SAG. SAG resp., patients cured by chemotherapy; SAG resist., patients refractory to chemotherapy. Data were based on parasitological and clinical criteria evaluated at the conclusion of treatment.
Group A.
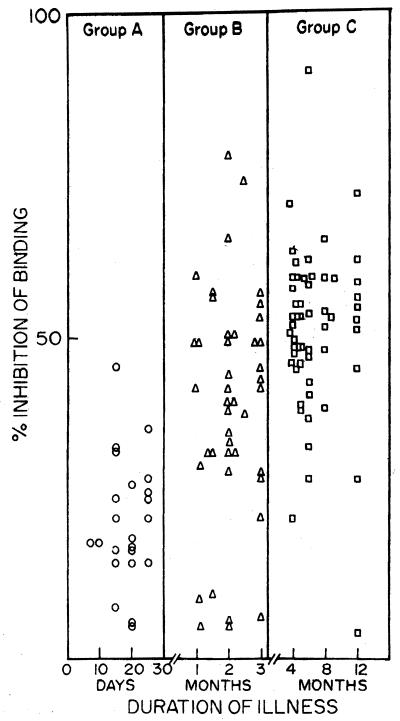
Of 25 patients included in this group, 17 (68%) were positive by microscopic examination, while 21 (84%) were positive by serology (C-ELISA with MAb D2 inhibition and ELISA; Table 2). All 17 smear-positive patients were C-ELISA and ELISA positive, whereas 4 of 8 smear-negative patients were positive by both ELISAs. The 4 (16%) additional patients in whom VL was detected by serology did not have false-positive results because they proved to be have VL on therapeutic grounds. Pairwise comparison of the assays showed 84% concordance between smear and C-ELISA and 100% concordance between ELISA and C-ELISA. Both microscopic and serological methods were able to detect disease as early as 10 days after the first appearance of clinical symptoms. The mean ± standard deviation (SD) percent MAb D2 inhibition and the absorbance at 405 nm by ELISA were 20.7% ± 9.6% and 0.34% ± 0.18%, respectively (Table 2; Fig. 1). The four patients who were negative by all three methods were microscopically confirmed to have malaria by demonstration of the presence of Plasmodium vivax.
TABLE 2.
Results of tissue smear examination and serological tests (ELISA and C-ELISA) for patients clinically diagnosed with VLa
| Group | No. (%) of
patientsb
|
Mean ± SD % inhibition of MAb D2 by C-ELISA | Mean ± SD OD at 405 nm by ELISA | ||
|---|---|---|---|---|---|
| Smear positive, serology positive | Smear negative, serology negative | Smear negative, serology positive | |||
| A (n = 25) | 17 (68.0) | 4 (16.0) | 4 (16.0) | 20.7 ± 9.6 | 0.36 ± 0.18 |
| B (n = 43) | 34 (79.0) | 6 (14.0) | 3 (7.0) | 39.2 ± 17.5 | 0.69 ± 0.36 |
| C (n = 53) | 52 (98.1) | 1 (1.9) | 0 (0.0) | 50.8 ± 13.1 | 0.85 ± 0.24 |
Sera were assayed by C-ELISA and ELISA as described in Materials and Methods.
Smear, microscopic examination of Giemsa-stained splenic or bone marrow aspirates; serology, C-ELISA and ELISA.
FIG. 1.
Percent inhibition of binding of L. donovani-specific MAb D2 by sera from Indian VL patients increases with duration of illness. Patients were divided into three groups on the basis of the length of time that clinical symptoms were apparent: group A, <30 days; group B, 1 to 3 months; and group C, 3 to 12 months.
Group B.
Group B included 43 patients with an intermediate duration of illness (1 to 3 months); 34 (79.0%) were positive by all three methods, and an additional 3 (7.0%) were positive by both ELISAs. The C-ELISA detected antibodies specific for L. donovani (MAb D2) in all smear-positive patients. Concordances were 93.0% between microscopy and C-ELISA and 100% between C-ELISA and ELISA. Compared to group A, there was a 47.2% increase in the inhibition of MAb D2 binding (mean ± SD = 39.2% ± 17.5%) (Fig. 1; Table 2). Similarly, the mean ± SD absorbance at 405 nm by ELISA for this group increased by 50.7% compared to that for group A (0.69 ± 0.36) (Table 2). The six C-ELISA-negative patients were confirmed to have tuberculosis by positive acid-fast staining of sputum specimens.
Group C.
Group C consisted of 53 patients whose duration of illness ranged from 3 months to 1 year. All 52 (98.1%) smear-positive patients were C-ELISA (MAb D2 inhibition) and ELISA positive, with the concordance among the three assays being 100%. The mean ± SD percent inhibition of MAb D2 binding was 50.8% ± 13.1% (Fig. 1; Table 2). The level of inhibition of MAb D2 binding in this group increased by 59.4 and 22.8% compared to those for group A and group B, respectively. The mean ± SD absorbance at 405 nm by ELISA was 0.85 ± 0.24; this was an increase of 60.0 and 19.0% compared to those for group A and group B, respectively (Table 2). A single patient who was negative by all three methods was diagnosed with hypersplenism of unknown etiology.
Evaluation by C-ELISA of drug responsiveness in kala-azar patients.
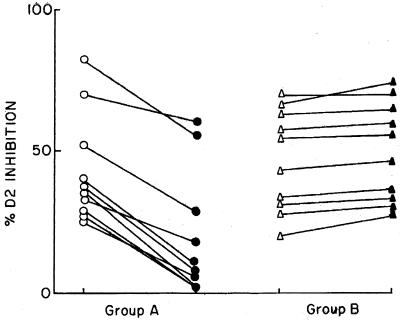
The inhibition of MAb D2 binding was measured in 20 VL patients pre- and posttreatment. Ten patients responded to drug treatment, whereas 10 were drug unresponsive. In the drug-responsive group the mean ± SD percent D2 inhibition decreased from 43.0% ± 18.2% to 20.0% ± 20.0% (P < 0.05) (Fig. 2, group A). This correlated with the observed reductions in their parasite loads. Conversely, in VL patients who were drug unresponsive, their mean ± SD percent MAb D2 inhibition increased from 46.7% ± 17.0% to 50.2% ± 6.3% (Fig. 2, group B).
FIG. 2.
Percent inhibition of binding of L. donovani-specific MAb D2 by sera from Indian VL patients before and after a single course of treatment with pentavalent antimony. Group A, drug-responsive patients before (○) and after (●) treatment; group B, drug-unresponsive patients before (▵) and after (▴) treatment. Sera were diluted 1:50 and assayed by C-ELISA as described in Materials and Methods.
PKDL patients.
Seven patients were microscopically confirmed to have PKDL, and the mean ± SD percent MAb D2 inhibition for these patients was 26.6% ± 5.3% (range, 18 to 34%).
Binding with L. tropica-directed MAb T11.
The binding of MAb T11, when it was tested in conjunction with serum samples from patients with leishmaniasis, was negligible. The mean ± SD percentages of MAb T11 inhibition were 5.3% ± 0.8% and 4.6% ± 1.0% for sera from VL and PKDL patients, respectively.
Correlation between C-ELISA and ELISA.
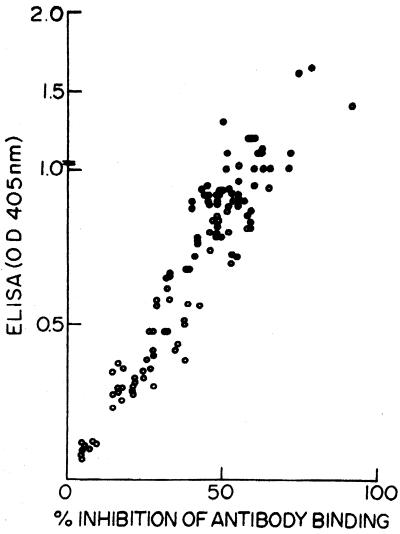
A strong positive correlation between individual antileishmanial antibody titers as measured by ELISA and the percent inhibition of MAb D2 binding as measured by C-ELISA was obtained (r2 = 0.84) (Fig. 3).
FIG. 3.
Correlation between ELISA and percent inhibition of binding of L. donovani-specific monoclonal MAb D2 by C-ELISA of sera from Indian VL patients.
Chemotherapeutic response to SAG.
All patients with clinically diagnosed VL included in this study had not received any prior antileishmanial treatment, and serum was collected prior to pentavalent antimonial treatment. All 110 ELISA-positive patients received a single course of SAG treatment, and on its completion, their chemotherapeutic responses were clinically and parasitologically assessed. They were considered SAG responsive (remission of fever, showed regression of liver and spleen, and absence of parasites in Giemsa-stained tissue smears) or SAG resistant (persistence of fever and hepatosplenomegaly and/or absence of parasites).
The study included 57 of 110 (51.8%) SAG-responsive and 53 of 110 (48.2%) SAG-resistant patients. Groupwise, the distributions of SAG-responsive patients were 57.0, 48.6, and 52.0% in groups A, B, and C, respectively. Accordingly, the distributions of SAG-unresponsive patients were 43.0, 51.4, and 48.0% in groups A, B, and C, respectively (Table 1).
Cross-reactivity studies.
Sera from patients with malaria, tuberculosis, and leprosy as well as healthy controls from areas of endemicity and nonendemicity were checked for cross-reactivity by C-ELISA and ELISA. No false-positive results were observed by either assay. The percent inhibition of MAb D2 binding with sera from healthy controls or patients with malaria or leprosy ranged from 0 to 5% and ranged from 0 to 10% for patients with tuberculosis (mean ± SD = 5.2% ± 1.8%). No differences in MAb D2 inhibition were observed with control sera from patients from areas of endemicity and nonendemicity.
DISCUSSION
In this study with a large number of serum samples, we have evaluated the potential of the C-ELISA as a diagnostic and prognostic tool for the monitoring of patients with VL and PKDL. Our results indicate that the C-ELISA with MAb D2 is an extremely sensitive and specific tool for the screening of patients with VL and PKDL. The C-ELISA is superior to microscopic examination and is comparable to the direct ELISA in detecting early parasite infections, especially in patients with fevers of short duration (group A) and intermediate duration (group B), among whom it identified an additional 16.0 and 7.0% cases of VL, respectively (Table 2).
Because the presenting symptoms of VL, which include fever, progressive weight loss, hepatosplenomegaly, and hypergammaglobulinemia, are common to other coendemic diseases in India, clinicians often face a diagnostic dilemma. The readily available serological tests for VL which use crude antigen frequently show false-positive cross-reactions with other coendemic diseases. Therefore, it is essential to find sensitive, noninvasive diagnostic methods which can specifically identify VL early in the disease process. By the C-ELISA, cross-reactivity with sera from Indian patients with other coendemic diseases, such as malaria, tuberculosis, and leprosy, was not observed.
Longitudinal follow-up of VL patients revealed marked differences in the percent inhibition of MAb D2 binding in the drug-responsive and drug-unresponsive group before and after a single course of treatment with SAG. With elimination of the parasite, the drug-responsive group showed a significant decrease in the percent inhibition of MAb D2 (Fig. 2, group A). However, in patients refractory to chemotherapy, the percent inhibition of MAb D2 either was unchanged or was marginally increased (Fig. 2, group B). Therefore, the C-ELISA indirectly provides circumstantial evidence of the presence or absence of circulating Leishmania parasite because the degree of inhibition of MAb D2 binding depends on the competition by patient antibody for the parasite antigen epitope recognized by the MAb.
In India, VL is generally assumed to be caused by L. donovani, although in recent times L. tropica has been shown to be a causative agent (18). Among four Indian patients with L. tropica infection examined in their study, two were found to be unresponsive to antimonial drugs. Likewise, patients with VL caused by L. tropica in Kenya have also been reported to be nonresponsive to SAG (14). The leishmanial species that cause disease can be characterized either indirectly by culturing the parasites from tissue aspirates or directly by PCR. The former technique is frequently unsuccessful, is disliked by patients, and requires culture of the parasite prior to analysis. The latter technique is still expensive and requires sophisticated laboratory facilities and trained personnel. Unlike both of those techniques, the C-ELISA with MAb D2 is simple, does not require special equipment or training, is performed like a regular ELISA, and is suitable for epidemiological screening studies.
Previous studies have shown that MAb D2 is specific for the L. donovani complex, does not cross-react with other Leishmania species (12), and can be used to identify patients with VL and PKDL (8, 10). The binding specificity of MAb T11 for L. tropica (formerly L. tropica minor) has been demonstrated; it specifically recognizes L. tropica isolates from both cutaneous leishmaniasis and VL patients and does not react with other Leishmania species (11). In fact, this specificity of MAb T11 has been exploited to characterize L. tropica isolates from Indian VL patients (18). Since MAbs D2 and T11 only recognize parasites belonging to either L. donovani or L. tropica and do not cross-react with each other, they provide indirect identification of the leishmanial species causing the disease. Because the 110 serum samples from patients suspected of having VL and 7 serum samples from patients with PKDL tested in this study only inhibited MAb D2 binding, with negligible inhibition of MAb T11, our study indicates that L. donovani still remains the main causative agent of Indian leishmaniasis.
The dramatic increase in unresponsiveness to conventional antimony therapy in the ongoing epidemic has raised questions as to whether an alteration in the causative Leishmania species has a contributory role. Here we have reported on a high incidence of unresponsiveness to antimonial agents, irrespective of the duration of illness (Table 1). However, sera from none of the SAG-resistant patients showed any inhibition of MAb T11 binding, suggesting that the refractoriness of VL patients to SAG is not related to the appearance of L. tropica.
The humoral response, as measured by the percent inhibition of MAb D2 (C-ELISA) and antileishmanial antibody titers (ELISA), showed increases of 47.2 and 50.7% from a short duration of illness (group A) to an intermediate duration of illness (group B), respectively (Fig. 1; Table 2). Smaller increases in the inhibition of MAb D2 binding (22.8%) and ELISA titers (19%) were seen in patients with a prolonged duration of illness (group C) than in group B patients. Finally, a positive correlation (r2 = 0.84) was found between individual percent inhibition of MAb D2 binding and an increasing reaction of antileishmanial antibodies as measured by ELISA (Fig. 2). Antileishmanial antibody titers measured by DAT have been reported to remain positive for up to 5 years after recovery in >50% of VL patients examined (16). Serological assays for the diagnosis of VL in HIV-positive patients are known to have a high incidence of false negativity, although rK39 has shown promise both for the diagnosis and the monitoring of patients with HIV infection and VL (7). Because the C-ELISA has proved to be a sensitive indicator of the disease status for the evaluation of disease progression and therapeutic effectiveness, its role in patients with HIV infection and VL should be assessed. Additionally, extension of the C-ELISA to samples from a larger population will provide evidence of its clinical applicability; such studies are ongoing.
ACKNOWLEDGMENTS
This work was partially supported by a grant from the United Nations Development Programme and the Department of Biotechnology, Government of India.
REFERENCES
- 1.Adhya S, Chatterjee M, Hassan M Q, Mukherjee S, Sen S. Detection of Leishmania in the blood of early kala-azar patients with the aid of polymerase chain reaction. Trans R Soc Trop Med Hyg. 1995;89:622–624. doi: 10.1016/0035-9203(95)90416-6. [DOI] [PubMed] [Google Scholar]
- 2.Chatterjee M, Manna M, Bhaduri A N, Sarkar D. Recent kala-azar cases in India: isozyme profiles of Leishmania parasites. Indian J Med Res. 1995;101:165–172. [PubMed] [Google Scholar]
- 3.Choudhary A, Guru P Y, Saxena R P, Saxena K C. An indirect fluorescent antibody test for serodiagnosis of kala-azar. J Commun Dis. 1992;24:32–36. [PubMed] [Google Scholar]
- 4.Choudhary A, Guru P Y, Tandon A, Saxena K C. Enzyme linked immunosorbent assay in the diagnosis of kala-azar in Bhadohi (Varanasi) India. Trans R Soc Trop Med Hyg. 1990;84:363–366. doi: 10.1016/0035-9203(90)90319-a. [DOI] [PubMed] [Google Scholar]
- 5.Dye C, Woolport D M. Earthquakes, influenza and cycles of Indian kala-azar. Trans R Soc Trop Med Hyg. 1988;82:843–850. doi: 10.1016/0035-9203(88)90013-2. [DOI] [PubMed] [Google Scholar]
- 6.Gupta S, Srivastava J K, Ray S, Chandra R, Srivastava V K, Katiyar J C. Evaluation of enzyme linked immunosorbent assay in the diagnosis of kala-azar in Malda district (West Bengal) Indian J Med Res. 1993;97:242–246. [PubMed] [Google Scholar]
- 7.Houghton R L, Petrescu M, Benson D R, Skeiky Y A, Scalone A, Badaro R, Reed S G, Gradoni L. A cloned antigen (recombinant rK39) of Leishmania chagasi diagnostic for visceral leishmaniasis in human immunodeficiency virus type 1 patients and a prognostic indicator for monitoring patients undergoing drug therapy. J Infect Dis. 1998;177:1339–1344. doi: 10.1086/515289. [DOI] [PubMed] [Google Scholar]
- 8.Ismail A, Kharazmi A, Permin H, El Hassan A M. Detection and characterization of Leishmania in tissues of patients with post kala-azar dermal leishmaniasis using a species specific monoclonal antibody. Trans R Soc Trop Med Hyg. 1997;91:283–285. doi: 10.1016/s0035-9203(97)90075-4. [DOI] [PubMed] [Google Scholar]
- 9.Jaffe C L, Zalis M. Purification of two Leishmania donovani membrane proteins recognized by sera from patients with visceral leishmaniasis. Mol Biochem Parasitol. 1988;27:53–59. doi: 10.1016/0166-6851(88)90024-2. [DOI] [PubMed] [Google Scholar]
- 10.Jaffe C L, McMahon-Pratt D. Serodiagnostic assay for visceral leishmaniasis employing monoclonal antibodies. Trans R Soc Trop Med Hyg. 1987;81:587–594. doi: 10.1016/0035-9203(87)90418-4. [DOI] [PubMed] [Google Scholar]
- 11.Jaffe C L, Sarfstein R. Species specific antibodies to Leishmania tropica (minor) recognize somatic antigens and exometabolites. J Immunol. 1987;139:1310–1319. [PubMed] [Google Scholar]
- 12.Jaffe C L, Bennett E, Grimaldi G, Jr, McMahon-Pratt D. Production and characterization of species specific monoclonal antibodies against Leishmania donovani for immunodiagnosis. J Immunol. 1984;133:440–447. [PubMed] [Google Scholar]
- 13.Martin S K, Thuita-Harun L, Adoyo-Adoyo M, Wasunna K M. A diagnostic ELISA for visceral leishmaniasis, based on antigen from media conditioned by Leishmania donovani promastigotes. Ann Trop Med Parasitol. 1998;92:571–577. doi: 10.1080/00034989859267. [DOI] [PubMed] [Google Scholar]
- 14.Mebrahtu Y, Lawyer P, Githure J, Were J B, Muigai R, Hendricks L, Leeuwenburg J, Koech D, Roberts C. Visceral leishmaniasis unresponsive to Pentostam caused by Leishmania tropica in Kenya. Am J Trop Med Hyg. 1989;41:289–294. doi: 10.4269/ajtmh.1989.41.289. [DOI] [PubMed] [Google Scholar]
- 15.Modabber F. The leishmaniases. In: Pearce J M, Pearce A M, editors. Tropical disease research, eighth program report of the UNDP/World Bank/WHO Special Programme for Organization, Geneva, Switzerland. 1987. pp. 77–87. [Google Scholar]
- 16.Okong’O-Odera E A, Wamachi A, Kagai J M, Kurtzhals J A L, Githure J I, Hey A S, Were J B O, Koech D K, Mitema E S, Kharazmi A. Field application of an ELISA using redefined Leishmania antigens in the detection of visceral leishmaniasis. Trans R Soc Trop Med Hyg. 1993;87:423–424. doi: 10.1016/0035-9203(93)90023-j. [DOI] [PubMed] [Google Scholar]
- 17.Pal A, Mukherjee K, Basu D, Naskar K, Mallik K K, Ghosh D K. Evaluation of direct agglutination test (DAT) and ELISA for serodiagnosis of visceral leishmaniasis in India. J Clin Lab Anal. 1991;5:303–306. doi: 10.1002/jcla.1860050502. [DOI] [PubMed] [Google Scholar]
- 18.Sacks D L, Kenney R T, Kreutzer R D, Jaffe C L, Gupta A K, Sharma M C, Sinha S P, Neva F A, Saran R. Indian kala-azar caused by Leishmania tropica. Lancet. 1995;345:959–961. doi: 10.1016/s0140-6736(95)90703-3. [DOI] [PubMed] [Google Scholar]
- 19.Singh S, Gilman-Sachs A, Chang K P, Reed S G. Diagnostic and prognostic value of K39 recombinant antigen in Indian leishmaniasis. J Parasitol. 1995;81:1000–1003. [PubMed] [Google Scholar]
- 20.Smyth A J, Ghosh A, Hassan M Q, Basu D, de Bruijn M H L, Adhya S, Mallik K K, Barker D C. Rapid and sensitive detection of Leishmania kinetoplast DNA from spleen and blood samples of kala-azar patients. Parasitology. 1992;105:183–192. doi: 10.1017/s0031182000074096. [DOI] [PubMed] [Google Scholar]
- 21.Sundar S, Singh V P, Sharma S, Makharia M K, Murray H W. Response to interferon-gamma plus pentavalent antimony in Indian visceral leishmaniasis. J Infect Dis. 1997;176:1117–1119. doi: 10.1086/516526. [DOI] [PubMed] [Google Scholar]
- 22.Thakur C P. Epidemiological, clinical and therapeutic features of Bihar kala-azar (including post kala-azar dermal leishmaniasis) Trans R Soc Trop Med Hyg. 1984;78:391–398. doi: 10.1016/0035-9203(84)90131-7. [DOI] [PubMed] [Google Scholar]
- 23.Thakur C P. Status paper on kala-azar in Bihar. Bihar: Department of Health, Family Welfare and Medical Education; 1993. pp. 1–14. [Google Scholar]
- 24.World Health Organization. The leishmaniases. 1990. p. 154. . Technical report series no. 793. World Health Organization, Geneva, Switzerland. [Google Scholar]