Abstract
South Africa has a rich history of medicinal plant species and their documented uses as traditional medicines, and is also home to three well-known, blue-flowered sage species of ethnobotanical importance. The Namaqualand bloublomsalie (Salvia dentata) has so far remained unstudied and apparently overlooked. Our study is the first to report on the essential oil chemistry of this medicinally relevant species and provide a comparison with the other two (well-studied) closely related Cape bloublomsalies (Salvia africana and S. chamelaeagnea). The data, generated from three geographically isolated populations comprised of 13 individual plants of S. dentata, revealed diagnostically high levels of camphor (14.37%), α-pinene (11.43%), camphene (10.18%), 1,8-cineole (eucalyptol) (9.42%) and bornyl acetate (8.56%) which provide a distinct chemical profile from the other two species.
Keywords: Lamiaceae, Salvia, essential oils, GC-MS, camphor, Salvia africana, Salvia chamelaeagnea, Salvia dentata, medicinal plants, South Africa
1. Introduction
The genus Salvia L. is the largest genus within the Lamiaceae (ca. 1010 spp. [1]) with various species used worldwide in traditional and folk medicines, treating ailments such as angiogenesis, bacterial and viral infections, inflammation and oxidative stress [2]. Although the genus was recognized by both Egyptian and the Greek civilizations, the name Salvia is derived from the Latin word “salvēre”, meaning “to feel healthy, to heal” given by the Roman Empire [3,4]. Many members of this genus are well known, such as S. officinalis L. (common sage), S. rosmarinus Spenn. (rosemary; hitherto better known as Rosmarinus officinalis L. [5]), S. officinalis subsp. lavandulifolia (Vahl) Gams (Spanish sage) and S. fruticosa Mill. (Greek sage) [6], largely for their therapeutic effects owed to their bioactive constituents [7]. These constituents include compounds such as 1,8-cineole (eucalyptol), α-thujone, camphor and camphene found in their essential oil (EO), as well as phenolic compounds such as caffeic-, rosmarinic- and salvianolic acid. In terms of flavonoids, 6-hydroxyflavones are characteristic of Salvia species and can be used as a chemosystematic/chemophenetic marker [8]. Being the largest genus within the Lamiaceae, the species have a wide geographical distribution, with the greatest concentration in Mexico and the Mediterranean region. In Asia, more than 40 species of Salvia are indigenous to China, with S. miltiorrhiza Bunge (red sage) being a popular herb in traditional Chinese medicine locally referred to as ‘Danshen’ or ‘Tanshen’ which is used to treat cardiovascular and renal ailments [7]. Salvia hispanica L., native to Mexico and Ecuador, is used both medicinally and as a source of protein where the seeds of this species are used as the notable superfood popularly known as ‘chia’ [9].
Southern Africa is home to 28 Salvia species, 14 of which are endemic to the region [10]. The Cape region of South Africa houses, amongst others, three closely related species of blue-flowered sages that are used in traditional medicines and are locally referred to as bloublomsalies (“blue flower sages”), and are Salvia africana L., S. chamelaeagnea Berg. and S. dentata Aiton (Figure 1A–C). The trichomes (hairs and glands) on the calyces are of diagnostic value used to distinguish between the three species [11]. Salvia africana and S. chamelaeagnea have been well studied, both in terms of their ethnobotanical records, pharmacological effects and chemistry [12,13]. The third species however, which is centered in Namaqualand (Figure 2)—is less well known and requires further investigation. Some traditional uses of S. africana and S. chamelaeagnea include the treatment of coughs, colds, influenza, chest troubles, headaches and fever [14,15]. Salvia dentata is used for the treatment of coughs, colds, influenza and stomach complaints [16,17]. Furthermore, an overlap exists between the traditional uses of these three medicinal Salvia species by various cultural groups of the Western and Northern Cape provinces, especially in the treatment of respiratory and gastrointestinal ailments. No publications could be found regarding the chemistry and essential oil composition of S. dentata. The aim of this paper is to present a review of all recorded ethnobotanical information about these three medicinally important species and to report for the first time the volatile components of S. dentata and compare the essential oil chemistry to that of the other two well-studied species.
Figure 1.
Three closely related, blue-flowered South African medicinal Salvia species. (A) Salvia dentata, (B) S. africana and (C) S. chamelaeagnea. All photographs taken by B.-E. Van Wyk.
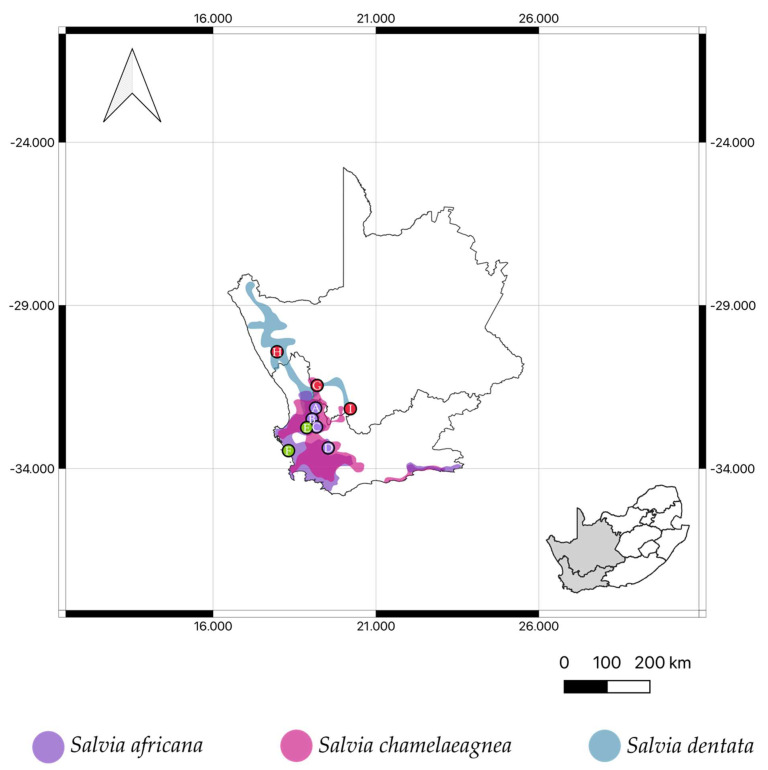
Figure 2.
Distribution of the three medicinal, blue-flowered Salvia species in the Western and Northern Cape provinces of South Africa. Localities where plant materials were collected for analysis are numbered as in the material and methods section (data points A–I).
2. Results and Discussion
2.1. Morphological Characteristics
Although the three species of Salvia appear to be morphologically similar, subtle differences exist by which the species can be distinguished from each other. The most useful and obvious diagnostic character is the pubescence of the calyx. The following characters and character states have been extracted from Codd [11] and can be used in identifying each species:
2.1.1. Salvia dentata
A twiggy erect shrub ca. 2 m tall with greyish-tomentulose, gland-dotted stems (Figure 3A). Inflorescence of 2–9 spaced or crowded verticils, 2–6-flowered (Figure 3B,C). The calyx is somewhat funnel shaped, hispid and generally copiously red gland-dotted and occasionally hispid-villous (Figure 3D). The corolla is light blue or whitish to violet-blue to purple (Figure 3B–D).
Figure 3.
Morphology and diagnostic characters of Salvia dentata. (A) habit (near Garies); (B) inflorescence (near Garies); (C) leaves and flowers (Nieuwoudtville; note dentate leaf margins); (D) leaves and flower (near Middelpos; note dentate leaves and glandular calyx). All photographs taken by B.-E. Van Wyk.
2.1.2. Salvia africana
A shrub ca. 2 m tall which often branches at the base (Figure 4A) with several erect, usually sparingly branched stems of which are greyish-tomentulose to hispidulous and gland-dotted with occasional glandular hairs. Inflorescences are often dense or spaced below with 5–12 verticils which are 2–6-flowered (Figure 4B). The calyx is somewhat funnel-shaped, glandular-villous and purple tinged (Figure 4B). The corolla is light blue to bluish purple or pinkish with the lower lip usually with a paler blue margin and white to yellowish in the center (Figure 4B).
Figure 4.
Morphology and diagnostic characters of Salvia africana. (A) habit (Cederberg); (B) inflorescence and leaves (Malmesbury); (C) flowers (Cederberg; note hirsute calyx). All photographs taken by B.-E. Van Wyk.
2.1.3. Salvia chamelaeagnea
A much-branched shrub 0.6–2 m tall (Figure 5A) with stems that are scabrid to pilose and gland-dotted. Inflorescences are large panicles, 100–300 mm long, with verticils two-flowered (Figure 5B). The calyx is reddish purple, glandular-hispid and gland-dotted. The corolla is blue or purplish blue often with white on the lower lip (Figure 5C,D).
Figure 5.
Morphology and diagnostic characters of Salvia chamelaeagnea. (A) habit (Piketberg); (B) inflorescence and leaves (Piketberg); (C) leaves and flowers (Darling); (D) flower (note glandular calyx). All photographs taken by B.-E. Van Wyk.
2.2. Ethnobotanical Data
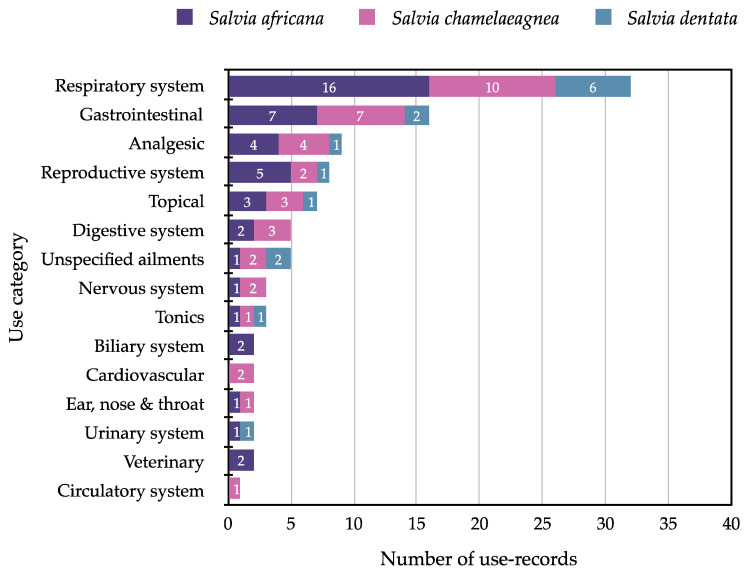
For the three Salvia species, the overall use categories with the highest number of use-records are respiratory system (32 records), gastrointestinal (16 records), analgesic (9 records), reproductive system (8 records) and topical applications (7 records; Figure 6). The ethnobotanical data show that Salvia dentata is mainly used for the treatment of respiratory ailments (colds, coughs and influenza), topical application, gastrointestinal (stomach complaints and diarrhoea) and unspecified medicinal uses (Table 1). Interestingly all three of the species are documented to be used as treatment for similar ailments (colds, coughs, influenza, stomach complaints and woman’s ailments) by different cultural groups across the Western and Northern Cape provinces.
Figure 6.
Number of medicinal use-records per use category for the three Cape blue-flowered Salvia species.
Table 1.
Recorded ethnobotanical uses for the three medicinal, blue-flowered sages.
| Species | Ethnobotanical Uses | Reference |
|---|---|---|
| Salvia africana | leaves: coughs, colds, chest troubles, uterine troubles, whooping cough; steam: infusion mixed with Epsom salts, lemon for abdominal troubles, diarrhoea, colic, flatulence, indigestion, veterinary (given to cows to expel placenta). | [20] |
| leaves: coughs, colds, women’s ailments, abdominal troubles, fevers, measles, | [21] | |
| leaves: cough, colds, women’s ailments, chest ailments, fever, measles, stomach ailments, topical (bed sores, verrucose veins), | [22] | |
| leaves: coughs, colds, chest troubles, convulsions, stomach pain, flatulence, colic, woman’s ailments, diarrhoea | [14] | |
| leaves: coughs, colds, menstrual cramps, diarrhoea | [15] | |
| unspecified medicinal use | [23] | |
| leaves: tea, flavourant; flowers: nectar (snack) | [24] | |
| leaves: tea, flavourant; flowers: nectar (snack) | [25] | |
| leaves: burns, chest complaints, influenza, headache, fever, stomachache, stomach tonic, earache | [26] | |
| Salvia chamelaeagnea | leaves: coughs, colds, bronchitis, diarrhoea, diaphoretic, female ailments, convulsions | [20] |
| “also used medicinally” | [21] | |
| leaves: coughs, colds, chest troubles, convulsions, stomach pain, flatulence, colic, woman’s ailments, diarrhoea | [14] | |
| leaves: burns, chest complaints, influenza, headache, fever, stomachache, earache, dialarhoea | [15] | |
| leaves: colds, influenza, pain, inflammation, stroke, stomachache, topical (wash) | [27] | |
| unspecified medicinal use | [23] | |
| leaves: burns, chest complaints, influenza, headache, fever, stomachache, stomach tonic, earache | [26] | |
| Salvia dentata | leaves and flowers: influenza | [28] |
| leaves: influenza | [29] | |
|
Salvia dentata Cont. |
leaves: leaf decoctions used for various ailments, often mixed with other herbs, general tonic, colds, backache, kidney diseases | [30] |
| leaves: colds, influenza | [16] | |
| leaves: leaf decoctions used for coughs, colds, women’s ailments, diarrhoea | [31] | |
| leaves: colds, influenza, stomach complaints, unspecified medicinal use, measles; other: firewood | [17] | |
| unspecified medicinal use | [23] |
In South America Salvia oppositiflora Ruiz & Pav., S. tubiflora Sm., S. dombeyi Epling and S. revoluta Ruiz & Pav. are used by indigenous people primarily for the treatment of respiratory ailments. Secondary uses include analgesic, stomach concerns and topical uses [18]. Similarly, in the East Mediterranean, S. fruticosa Mill. [=S. libanotica Boiss. & Gaill.] is a popular medicinal plant that is widely used in the region. In Lebanon, Syria and Jordan a tea is made from the leaves which are commonly sold at the market and drunk for abdominal pain, headaches, stomach complaints and several other disorders. In Palestine it used for the treatment of stomach and cardiovascular issues. Furthermore, in Turkey, the plant is used to treat colds, coughs, influenza, and for gall bladder and kidney stones [19].
There seems to be a cultural overlap regarding the ethnobotanical uses across the globe for Salvia species in the treatment for certain conditions (i.e., respiratory, gastrointestinal, and topical).
2.3. Chemical Data
For comparative reasons, EOs were distilled from fresh material of Salvia africana, S. chamelaeagnea and S. dentata collected from several localities within the Northern and Western Cape provinces of South Africa (Figure 2; data points A–I). The mean percentage yield of EOs obtained for each group was 1.23% for S. dentata, 0.56% for S. africana and 0.54% for S. chamelaeagnea. The mean percentage yield obtained for S. africana was higher than results reported by other studies (0.17% [12], 0.13% [32] and 0.14% [33]), whereas the mean percent yield for S. chamelaeagnea were similar to those reported by Kamatou et al. [32] (0.39%) and Lim Ah Tock et al. [34] (0.43%). Higher EO yields are likely due to factors such as vegetative state, temperature, climate, geology and time of harvest [35,36]. Furthermore, the higher yield of S. dentata EO could be as a direct result of the species having a higher density of glandular trichomes on the leaf surface, which was observed for S. africana and S. chamelaeagnea by Kamatou et al. [32]. The essential oil obtained from S. dentata, S. africana and S. chamelaeagnea presented a pale-yellow color and all of the samples were aromatic. Further, S. dentata presented a highly aromatic odor distinct from the other two species.
Essential oils were analyzed by gas chromatography (GC) and gas chromatography-mass spectrometry (GC-MS). A total of 89 compounds were identified across three species (Table 2, Table 3 and Table 4) with S. dentata having the range 76.66–98.62% of compounds identified, S. africana with 81.45–94.29% of compounds identified and S. chamelaeagnea having 79.75–91.89% of compounds identified. Oxygenated monoterpenes were the dominant class of constituents in S. dentata (mean value of 35.21%), followed by monoterpene hydrocarbons, sesquiterpene hydrocarbons, oxygenated sesquiterpenes and hydrocarbons (mean values of 33.7%, 10.28%, 10.25% and 2.42% respectively). The S. africana oils were dominated by monoterpene hydrocarbons, oxygenated sesquiterpenes, oxygenated monoterpenes and sesquiterpene hydrocarbons (mean values of 36.02%, 24.92%, 16.08% and 11.58% respectively) and S. chamelaeagnea were dominated by oxygenated sesquiterpenes, oxygenated monoterpenes, sesquiterpene hydrocarbons and oxygenated monoterpenes (mean values of 30.78% 30.47%, 13.61% and 10.22% respectively). The percentage of grouped components for S. africana differed to a previous study by Kamatou et al. [32] where the authors reported 8.8% monoterpene hydrocarbons, 3.0% oxygenated monoterpenes, 7.5% sesquiterpene hydrocarbons and 58.7% oxygenated sesquiterpenes. Further, for S. chamelaeagnea, the same study reported 9.0% monoterpene hydrocarbons, 6.2% oxygenated monoterpenes, 16.6% sesquiterpene hydrocarbons and 47.9% oxygenated sesquiterpenes, ratios which are comparable to grouped compounds for S. chamelaeagnea EO identified in this study.
Table 2.
Identified essential oil components (%) of Salvia dentata.
| Salvia dentata | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nieuwoudtville | Spoegrivier Road | Tankwa | ||||||||||||||
| Sd1 | Sd2 | Sd3 | Sd4 | Sd5 | Sd6 | Sd7 | Sd8 | Sd9 | Sd10 | Sd11 | Sd12 | Sd13 | Mean | |||
| Components/Yield (% dry weight) | RRI | ID | 0.99 | 0.71 | 1.38 | 1.25 | 1.14 | 1.30 | 1.20 | 1.56 | 1.76 | 0.95 | 1.70 | 1.06 | 1.01 | 1.23 ± 0.31 |
| tricyclene | 921 | 1, 2 | 0.33 | 0.00 | 0.00 | 0.61 | 0.45 | 0.54 | 0.00 | 0.64 | 0.00 | 0.78 | 0.72 | 0.50 | 0.66 | 0.4 ± 0.3 |
| α-thujene | 927 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.13 | 0.16 | 0.13 | 0.13 | 0.17 | 0.00 | 0.00 | 0.00 | 0.00 | 0.12 | 0.06 ± 0.07 |
| α-pinene | 934 | 1, 2 | 9.81 | 5.87 | 15.44 | 12.16 | 10.74 | 11.17 | 12.70 | 12.88 | 13.35 | 8.98 | 13.30 | 9.93 | 12.27 | 11.43 ± 2.42 |
| camphene | 948 | 1, 2 | 8.52 | 6.08 | 14.06 | 11.17 | 8.62 | 10.44 | 10.51 | 11.51 | 11.33 | 6.27 | 12.32 | 9.64 | 11.88 | 10.18 ± 2.32 |
| verbenene | 963 | 1, 2 | 0.00 | 0.00 | 5.41 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.42 ± 1.5 |
| sabinene | 973 | 1, 2 | 0.00 | 0.00 | 0.06 | 0.05 | 0.07 | 0.06 | 0.04 | 0.00 | 0.07 | 0.12 | 0.05 | 0.06 | 0.05 | 0.05 ± 0.03 |
| β-pinene | 976 | 1, 2 | 2.19 | 1.39 | 2.58 | 2.50 | 2.18 | 2.37 | 2.17 | 2.64 | 3.38 | 3.14 | 3.50 | 3.02 | 2.74 | 2.6 ± 0.57 |
| myrcene | 990 | 1, 2 | 0.57 | 0.64 | 0.76 | 0.93 | 1.12 | 0.59 | 0.77 | 0.62 | 0.86 | 0.71 | 0.46 | 0.49 | 0.35 | 0.68 ± 0.21 |
| α-phellandrene | 1002 | 1, 2 | 0.00 | 0.27 | 0.16 | 0.37 | 0.27 | 0.26 | 0.00 | 0.31 | 0.49 | 0.04 | 0.06 | 0.36 | 0.07 | 0.2 ± 0.16 |
| δ-3-carene | 1009 | 1, 2 | 0.00 | 3.67 | 0.00 | 4.90 | 2.52 | 3.03 | 2.22 | 3.80 | 3.55 | 3.46 | 0.00 | 3.36 | 0.28 | 2.37 ± 1.72 |
| α-terpinene | 1015 | 1, 2 | 0.46 | 0.37 | 0.56 | 0.54 | 0.55 | 0.49 | 0.54 | 0.60 | 0.53 | 0.48 | 0.32 | 0.34 | 0.00 | 0.45 ± 0.16 |
| para-cymene | 1023 | 1, 2 | 0.53 | 0.98 | 0.70 | 0.75 | 0.30 | 0.77 | 0.67 | 0.99 | 0.49 | 1.19 | 0.51 | 0.85 | 0.39 | 0.7 ± 0.26 |
| sylvestrene | 1025 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.45 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | t |
| limonene | 1028 | 1, 2 | 2.12 | 2.90 | 0.00 | 3.24 | 2.38 | 2.55 | 2.29 | 2.73 | 2.76 | 2.54 | 1.91 | 2.28 | 1.65 | 2.26 ± 0.8 |
| β-phellandrene | 1029 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| 1,8-cineole (eucalyptol) | 1031 | 1, 2 | 13.49 | 3.59 | 7.42 | 11.86 | 12.05 | 6.74 | 13.87 | 10.19 | 12.95 | 6.21 | 9.22 | 9.58 | 5.30 | 9.42 ± 3.35 |
| cis-β-ocimene | 1037 | 1, 2 | 0.34 | 1.01 | 0.70 | 0.00 | 0.20 | 0.00 | 0.48 | 0.36 | 0.00 | 0.00 | 0.00 | 0.00 | 0.22 | 0.25 ± 0.32 |
| trans-β-ocimene | 1047 | 1, 2 | 0.45 | 1.43 | 0.90 | 0.00 | 0.30 | 0.00 | 0.55 | 0.52 | 0.00 | 0.00 | 0.49 | 0.00 | 0.22 | 0.37 ± 0.43 |
| γ-terpinene | 1059 | 1, 2 | 1.11 | 0.94 | 1.06 | 1.01 | 1.11 | 0.97 | 1.07 | 1.16 | 1.09 | 0.92 | 0.67 | 0.69 | 0.58 | 0.95 ± 0.19 |
| cis-sabinene hydrate | 1068 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.28 | 0.00 | 0.00 | 0.07 | 0.08 | 0.23 | 0.18 | 0.08 | 0.03 | 0.00 | 0.07 ± 0.1 |
| terpinolene | 1088 | 1, 2 | 0.34 | 0.41 | 0.43 | 0.38 | 0.33 | 0.39 | 0.35 | 0.43 | 0.43 | 0.56 | 0.00 | 0.39 | 0.18 | 0.36 ± 0.14 |
| trans-sabinene hydrate | 1098 | 1, 2 | 0.00 | 0.00 | 0.11 | 0.00 | 0.19 | 0.25 | 0.21 | 0.11 | 0.00 | 0.35 | 0.00 | 0.00 | 0.32 | 0.12 ± 0.13 |
| linalool | 1099 | 1, 2 | 0.00 | 0.00 | 0.10 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.19 | 0.12 | 0.00 | t |
| 2-methylbutyl 2-methylbutyrate | 1002 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.10 | 0.00 | 0.00 | 0.08 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | t |
| solusterol | 1103 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.05 | 0.40 | 0.28 | 0.49 | 0.00 | 0.11 | 0.00 | 0.08 | 0.00 | 0.04 | 0.11 ± 0.17 |
| n-amyl isovalerate | 1107 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.28 | 0.00 | 0.00 | 0.00 | 0.32 | 0.53 | 0.27 | 0.20 | 0.22 | 0.12 | 0.15 ± 0.17 |
| α-thujone | 1118 | 1, 2 | 0.26 | 0.00 | 0.03 | 0.21 | 0.00 | 0.09 | 0.06 | 0.00 | 0.21 | 0.37 | 0.21 | 0.26 | 0.16 | 0.14 ± 0.12 |
| cis-para-menth-2-en-1-ol | 1123 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.04 | 0.00 | 0.10 | 0.06 | 0.06 | 0.00 | t |
| α-campholene aldehyde | 1128 | 1, 2 | 0.00 | 0.00 | 0.06 | 0.00 | 0.00 | 0.05 | 0.06 | 0.07 | 0.07 | 0.08 | 0.09 | 0.05 | 0.05 | t |
| terpinen-1-ol | 1142 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.06 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.10 | 0.00 | 0.00 | 0.00 | t |
| trans-verbenol | 1142 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.06 | 0.00 | 0.00 | 0.00 | 0.06 | 0.04 | 0.00 | 0.09 | 0.00 | 0.08 | t |
| camphor | 1148 | 1, 2 | 17.70 | 14.57 | 8.93 | 19.16 | 15.15 | 18.07 | 18.46 | 18.66 | 18.06 | 7.36 | 12.31 | 9.87 | 8.45 | 14.37 ± 4.43 |
| camphene hydrate | 1152 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.06 | 0.00 | 0.06 | 0.00 | 0.07 | 0.06 | 0.00 | 0.00 | 0.00 | 0.00 | t |
| exo-methyl-camphenilol | 1153 | 1, 2 | 0.00 | 0.00 | 0.07 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.10 | 0.09 | 0.08 | 0.06 | t |
| borneol | 1170 | 1, 2 | 1.40 | 1.94 | 1.45 | 0.31 | 0.29 | 0.88 | 0.53 | 0.55 | 0.42 | 1.41 | 1.05 | 1.02 | 1.14 | 0.95 ± 0.51 |
| terpinen-4-ol | 1180 | 1, 2 | 0.00 | 1.00 | 0.85 | 0.56 | 0.54 | 0.59 | 0.69 | 0.70 | 0.76 | 1.04 | 0.72 | 0.85 | 0.60 | 0.69 ± 0.26 |
| α-terpineol | 1194 | 1, 2 | 0.00 | 0.00 | 0.09 | 0.18 | 0.08 | 0.05 | 0.21 | 0.25 | 0.08 | 0.14 | 0.08 | 0.11 | 0.09 | t |
| myrtenol | 1199 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.05 | 0.00 | 0.00 | nd |
| nopol | 1206 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| cis-piperitol | 1211 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| β-cyclocitral | 1224 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| cis-3-hexenyl isovalerate | 1235 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.11 | 0.00 | 0.00 | 0.00 | t |
| linalyl acetate | 1255 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.05 | 0.05 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | t |
| phellandral | 1282 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| bornyl acetate | 1284 | 1, 2 | 11.90 | 21.68 | 6.34 | 0.00 | 2.33 | 3.67 | 0.00 | 3.75 | 3.47 | 8.47 | 14.39 | 15.41 | 19.88 | 8.56 ± 7.39 |
| α-fenchyl acetate | 1298 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 3.98 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.31 ± 1.1 |
| myrtenyl acetate | 1328 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| isoledene | 1382 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.15 | 0.24 | 0.04 | 0.15 | 0.19 | 0.00 | 0.20 | 0.11 | 0.10 | 0.23 | 0.11 ± 0.09 |
| α-cubebene | 1384 | 1, 2 | 0.00 | 0.00 | 0.05 | 0.18 | 0.20 | 0.00 | 0.00 | 0.00 | 0.39 | 0.15 | 0.00 | 0.00 | 0.06 | 0.08 ± 0.12 |
| α-copaene | 1385 | 1, 2 | 0.00 | 0.32 | 0.21 | 0.00 | 0.00 | 0.29 | 0.14 | 0.15 | 0.00 | 0.00 | 0.07 | 0.00 | 0.37 | 0.12 ± 0.14 |
| α-ylangene | 1387 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.07 | 0.00 | t |
| cis-jasmone | 1403 | 1, 2 | 0.00 | 0.00 | 0.13 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | t |
| cis-β-caryophyllene | 1417 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.10 | 0.09 | 0.05 | 0.05 | t |
| α-gurjunene | 1420 | 1, 2 | 0.62 | 0.00 | 0.00 | 0.85 | 1.49 | 2.20 | 0.30 | 0.65 | 1.17 | 0.96 | 0.20 | 0.53 | 0.58 | 0.74 ± 0.62 |
| β-caryophyllene | 1431 | 1, 2 | 4.48 | 9.00 | 6.13 | 0.00 | 4.65 | 4.04 | 2.38 | 1.71 | 0.96 | 5.88 | 8.44 | 6.41 | 5.34 | 4.57 ± 2.74 |
| clovene | 1439 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| α-bergamotene | 1443 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| calarene | 1444 | 1, 2 | 0.00 | 0.00 | 0.18 | 0.21 | 0.26 | 0.17 | 0.54 | 0.00 | 0.12 | 0.11 | 0.15 | 0.06 | 0.23 | 0.16 ± 0.15 |
| aromadendrene | 1451 | 1, 2 | 1.93 | 0.00 | 0.06 | 2.76 | 0.00 | 2.54 | 2.50 | 2.11 | 0.00 | 0.00 | 1.89 | 0.00 | 4.29 | 1.39 ± 1.45 |
| valencene I | 1452 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 3.62 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.13 | 0.00 | 0.37 ± 1.03 |
| trans-β-farnesene | 1458 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| α-humulene | 1466 | 1, 2 | 0.54 | 1.01 | 0.00 | 0.00 | 0.46 | 0.41 | 0.00 | 0.18 | 0.13 | 0.00 | 1.00 | 0.86 | 0.78 | 0.41 ± 0.4 |
| allo-aromadendrene | 1473 | 1, 2 | 0.00 | 0.00 | 1.90 | 0.54 | 0.78 | 0.71 | 0.40 | 0.00 | 0.55 | 3.74 | 0.00 | 0.95 | 0.00 | 0.74 ± 1.05 |
| γ-gurjunene | 1484 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| α-amorphene | 1486 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.25 | 0.00 | 0.13 | 0.00 | 0.28 | 0.00 | 0.06 | 0.00 | 0.06 ± 0.1 |
| β-ionone | 1491 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| β-selinene | 1495 | 1, 2 | 0.00 | 0.00 | 0.37 | 0.00 | 0.00 | 0.27 | 0.00 | 0.00 | 0.00 | 0.22 | 0.00 | 0.00 | 0.00 | 0.07 ± 0.13 |
| germacrene-D | 1499 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.08 | t |
| γ-himachalene | 1501 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.20 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | t |
| valencene | 1506 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| ledene | 1507 | 1, 2 | 0.73 | 0.00 | 0.93 | 1.11 | 1.88 | 1.24 | 0.87 | 0.00 | 1.00 | 1.01 | 0.60 | 0.52 | 1.16 | 0.85 ± 0.51 |
| β-bisabolene | 1513 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.07 | 0.00 | 0.15 | 0.09 | 0.03 | 0.00 | t |
| γ-cadinene | 1524 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.23 | 0.19 | 0.00 | 0.00 | 0.00 | 0.25 | 0.63 | 0.27 | 0.31 | 0.00 | 0.14 ± 0.19 |
| β-cadinene | 1532 | 1, 2 | 0.36 | 0.00 | 0.70 | 0.53 | 0.49 | 0.77 | 0.24 | 0.36 | 0.64 | 0.06 | 0.00 | 0.03 | 1.30 | 0.42 ± 0.38 |
| nerolidol | 1565 | 1, 2 | 0.00 | 0.00 | 2.89 | 1.95 | 1.35 | 1.20 | 1.48 | 1.54 | 1.58 | 2.75 | 0.76 | 1.50 | 1.48 | 1.42 ± 0.86 |
| epi-globulol | 1573 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| palustrol | 1583 | 1, 2 | 0.64 | 0.00 | 0.00 | 2.22 | 2.87 | 6.18 | 0.00 | 1.80 | 2.94 | 2.96 | 0.00 | 2.15 | 0.00 | 1.67 ± 1.84 |
| spathulenol | 1590 | 1, 2 | 0.32 | 0.00 | 0.29 | 0.24 | 0.16 | 0.16 | 0.17 | 0.17 | 0.22 | 0.23 | 0.00 | 0.17 | 0.28 | 0.18 ± 0.1 |
| caryophyllene oxide | 1597 | 1, 2 | 0.00 | 0.39 | 1.69 | 0.00 | 0.00 | 0.00 | 0.00 | 0.48 | 0.00 | 1.81 | 0.12 | 0.00 | 0.00 | 0.35 ± 0.64 |
| globulol | 1598 | 1, 2 | 0.00 | 0.00 | 0.18 | 0.57 | 0.52 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.1 ± 0.2 |
| viridiflorol | 1607 | 1, 2 | 1.28 | 0.00 | 8.14 | 10.82 | 13.12 | 1.77 | 15.33 | 12.44 | 7.10 | 0.00 | 2.20 | 3.31 | 1.79 | 5.95 ± 5.48 |
| γ-eudesmol | 1647 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.09 | 0.86 | 0.50 | 0.19 ± 0.38 |
| 1(5),6-guaiadiene | 1662 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| β-eudesmol | 1665 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.08 | 0.78 | 0.52 | 0.18 ± 0.37 |
| α-eudesmol | 1669 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 2.28 | 0.00 | 0.00 | 0.18 ± 0.63 |
| τ-muurolol | 1666 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| α-bisabolol | 1692 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.06 | 0.00 | 0.00 | 0.00 | 0.17 | 0.11 | t |
| tetracosane | 2300 | 1, 2 | 12.24 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.94 ± 3.4 |
| heneicosane | 2264 | 1, 2 | 0.00 | 19.17 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.47 ± 5.32 |
| % Identification | 94.66 | 98.62 | 92.07 | 94.91 | 94.70 | 86.63 | 97.56 | 96.46 | 92.33 | 76.66 | 93.65 | 89.53 | 87.11 | 91.91 ± 5.88 | ||
| Grouped components: | ||||||||||||||||
| Monoterpene hydrocarbons | 26.77 | 25.96 | 42.80 | 39.19 | 31.29 | 33.75 | 34.44 | 39.36 | 38.34 | 29.14 | 34.30 | 31.91 | 31.70 | 33.77 ± 5.08 | ||
| Oxygenated monoterpenes | 44.76 | 42.78 | 25.43 | 33.35 | 31.14 | 30.69 | 38.63 | 34.89 | 36.95 | 26.29 | 38.91 | 37.61 | 36.25 | 35.21 ± 5.77 | ||
| Sesquiterpene hydrocarbons | 8.64 | 10.32 | 10.53 | 6.57 | 14.25 | 12.88 | 7.51 | 5.74 | 5.21 | 13.48 | 12.91 | 11.07 | 14.48 | 10.28 ± 3.27 | ||
| Oxygenated sesquiterpenes | 2.24 | 0.39 | 13.31 | 15.79 | 18.02 | 9.31 | 16.98 | 16.48 | 11.84 | 7.75 | 7.53 | 8.94 | 4.69 | 10.25 ± 5.74 | ||
| Hydrocarbons | 12.24 | 19.17 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 2.42 ± 6.07 | ||
RRI: Relative retention indices calculated against n-alkanes, ID: identification method—1 = comparison of retention index; 2 = comparison of mass spectra with MS libraries, nd: not detected, t: trace, major compounds (% > 5) highlighted in bold.
Table 3.
Identified essential oil components (%) of Salvia africana.
| Salvia africana | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cederberg | Citrusdal | Elandskloof | Theronsberg Pass | ||||||
| Sa1 | Sa2 | Sa3 | Sa4 | Sa5 | Sa6 | Mean | |||
| Components/Yield (% dry weight) | RRI | ID | 0.36 | 0.33 | 0.28 | 1.40 | 0.34 | 0.62 | 0.56 ± 0.43 |
| tricyclene | 921 | 1, 2 | 0.00 | 0.00 | 0.08 | 0.00 | 0.00 | 0.30 | 0.06 ± 0.12 |
| α-thujene | 927 | 1, 2 | 0.32 | 0.56 | 0.19 | 0.15 | 0.00 | 0.47 | 0.28 ± 0.21 |
| α-pinene | 934 | 1, 2 | 5.61 | 9.64 | 11.45 | 8.34 | 4.95 | 9.53 | 8.25 ± 2.52 |
| camphene | 948 | 1, 2 | 0.17 | 0.11 | 1.70 | 3.20 | 0.20 | 5.30 | 1.78 ± 2.11 |
| verbenene | 963 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| sabinene | 973 | 1, 2 | 0.08 | 0.09 | 0.05 | 0.00 | 0.09 | 0.13 | 0.07 ± 0.04 |
| β-pinene | 976 | 1, 2 | 1.86 | 2.78 | 2.81 | 2.20 | 3.26 | 3.03 | 2.66 ± 0.53 |
| myrcene | 990 | 1, 2 | 1.50 | 1.18 | 1.25 | 1.27 | 0.81 | 0.90 | 1.15 ± 0.26 |
| α-phellandrene | 1002 | 1, 2 | 0.10 | 0.00 | 0.03 | 0.69 | 1.08 | 1.68 | 0.60 ± 0.68 |
| δ-3-carene | 1009 | 1, 2 | 0.07 | 0.08 | 0.06 | 0.00 | 7.83 | 1.69 | 1.62 ± 3.11 |
| α-terpinene | 1015 | 1, 2 | 0.33 | 0.08 | 0.11 | 0.46 | 0.42 | 0.68 | 0.35 ± 0.23 |
| para-cymene | 1023 | 1, 2 | 3.24 | 0.56 | 2.03 | 0.24 | 0.08 | 1.66 | 1.30 ± 1.23 |
| sylvestrene | 1025 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 1.21 | 0.00 | 0.20 ± 0.50 |
| limonene | 1028 | 1, 2 | 3.68 | 1.11 | 1.53 | 21.96 | 18.60 | 0.00 | 7.81 ± 9.79 |
| β-phellandrene | 1029 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| 1,8-cineole (eucalyptol) | 1031 | 1, 2 | 4.18 | 5.65 | 1.08 | 17.39 | 0.29 | 7.55 | 6.02 ± 6.2 |
| cis-β-ocimene | 1037 | 1, 2 | 0.00 | 4.59 | 6.36 | 0.34 | 0.11 | 0.20 | 1.93 ± 2.8 |
| trans-β-ocimene | 1047 | 1, 2 | 6.78 | 8.53 | 11.45 | 0.75 | 0.00 | 0.09 | 4.6 ± 4.97 |
| γ-terpinene | 1059 | 1, 2 | 3.60 | 0.41 | 0.95 | 0.75 | 1.01 | 3.32 | 1.67 ± 1.4 |
| cis-sabinene hydrate | 1068 | 1, 2 | 0.13 | 0.12 | 0.00 | 0.09 | 0.00 | 0.00 | 0.06 ± 0.06 |
| terpinolene | 1088 | 1, 2 | 0.16 | 0.18 | 0.37 | 0.18 | 0.33 | 0.33 | 0.26 ± 0.09 |
| trans-sabinene hydrate | 1098 | 1, 2 | 0.00 | 0.19 | 0.29 | 0.00 | 0.24 | 0.61 | 0.22 ± 0.23 |
| linalool | 1099 | 1, 2 | 0.34 | 0.00 | 0.00 | 0.24 | 0.37 | 0.00 | 0.16 ± 0.18 |
| 2-methylbutyl 2-methylbutyrate | 1002 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| solusterol | 1103 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| n-amyl isovalerate | 1107 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.08 | t |
| α-thujone | 1118 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.13 | t |
| cis-para-menth-2-en-1-ol | 1123 | 1, 2 | 0.04 | 0.00 | 0.00 | 0.29 | 0.08 | 0.15 | 0.09 ± 0.11 |
| α-campholene aldehyde | 1128 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.03 | t |
| terpinene-1-ol | 1142 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.07 | 0.11 | t |
| trans-verbenol | 1142 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| camphor | 1148 | 1, 2 | 0.15 | 0.08 | 2.46 | 6.63 | 0.27 | 5.39 | 2.5 ± 2.89 |
| camphene hydrate | 1152 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| exo-methyl-camphenilol | 1153 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.04 | t |
| borneol | 1170 | 1, 2 | 0.06 | 0.00 | 0.00 | 0.22 | 0.10 | 0.59 | 0.16 ± 0.22 |
| terpinene-4-ol | 1180 | 1, 2 | 0.40 | 0.29 | 0.51 | 0.47 | 0.95 | 0.77 | 0.56 ± 0.25 |
| α-terpineol | 1194 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.17 | 0.12 | 0.19 | 0.08 ± 0.09 |
| myrtenol | 1199 | 1, 2 | 1.09 | 1.17 | 0.96 | 0.00 | 3.66 | 1.21 | 1.35 ± 1.22 |
| nopol | 1206 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.20 | 0.00 | 0.00 | t |
| cis-piperitol | 1211 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.07 | 0.07 | t |
| β-cyclocitral | 1224 | 1, 2 | 0.00 | 0.00 | 0.07 | 0.07 | 0.04 | 0.03 | t |
| cis-3-hexenyl isovalerate | 1235 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| linalyl acetate | 1255 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.04 | 0.00 | 0.00 | t |
| phellandral | 1282 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.04 | 0.00 | t |
| bornyl acetate | 1284 | 1, 2 | 0.12 | 0.09 | 6.14 | 0.00 | 0.00 | 7.81 | 2.36 ± 3.62 |
| α-fenchyl acetate | 1298 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| myrtenyl acetate | 1328 | 1, 2 | 0.75 | 0.83 | 1.73 | 1.73 | 0.13 | 0.22 | 0.9 ± 0.7 |
| isoledene | 1382 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| α-cubebene | 1384 | 1, 2 | 0.00 | 0.06 | 0.00 | 0.06 | 0.19 | 0.06 | 0.06 ± 0.07 |
| α-copaene | 1385 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.13 | 0.05 | 0.30 | 0.08 ± 0.12 |
| α-ylangene | 1387 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| cis-jasmone | 1403 | 1, 2 | 0.17 | 0.00 | 0.11 | 0.00 | 0.00 | 0.00 | 0.05 ± 0.08 |
| cis-β-caryophyllene | 1417 | 1, 2 | 0.00 | 0.06 | 0.03 | 0.00 | 0.00 | 0.03 | t |
| α-gurjunene | 1420 | 1, 2 | 0.00 | 0.00 | 0.67 | 0.00 | 0.09 | 0.41 | 0.2 ± 0.28 |
| β-caryophyllene | 1431 | 1, 2 | 10.89 | 16.38 | 13.35 | 0.72 | 5.29 | 6.15 | 8.8 ± 5.78 |
| clovene | 1439 | 1, 2 | 0.11 | 0.00 | 0.06 | 0.00 | 0.00 | 0.00 | t |
| α-bergamotene | 1443 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| calarene | 1444 | 1, 2 | 0.03 | 0.04 | 0.00 | 0.00 | 0.06 | 0.06 | t |
| aromadendrene | 1451 | 1, 2 | 0.00 | 0.00 | 0.28 | 0.00 | 0.31 | 0.83 | 0.24 ± 0.33 |
| valencene I | 1452 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| trans-β-farnesene | 1458 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.62 | 0.00 | 0.00 | 0.1 ± 0.25 |
| α-humulene | 1466 | 1, 2 | 1.58 | 1.84 | 1.72 | 0.45 | 0.61 | 0.83 | 1.17 ± 0.61 |
| allo-aromadendrene | 1473 | 1, 2 | 0.02 | 1.64 | 0.32 | 0.03 | 1.64 | 0.00 | 0.61 ± 0.81 |
| γ-gurjunene | 1484 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| α-amorphene | 1486 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.35 | 0.00 | 0.06 ± 0.14 |
| β-ionone | 1491 | 1, 2 | 0.03 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | t |
| β-selinene | 1495 | 1, 2 | 0.00 | 0.05 | 0.00 | 0.00 | 0.00 | 0.00 | t |
| germacrene-D | 1499 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| γ-himachalene | 1501 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| valencene | 1506 | 1, 2 | 0.56 | 0.00 | 0.00 | 0.00 | 0.11 | 0.00 | 0.11 ± 0.22 |
| ledene | 1507 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.82 | 0.73 | 0.26 ± 0.4 |
| β-bisabolene | 1513 | 1, 2 | 0.03 | 0.19 | 0.00 | 0.00 | 0.06 | 0.08 | 0.06 ± 0.07 |
| γ-cadinene | 1524 | 1, 2 | 1.13 | 0.00 | 0.00 | 0.00 | 0.00 | 0.12 | 0.21 ± 0.45 |
| β-cadinene | 1532 | 1, 2 | 0.00 | 0.00 | 0.23 | 0.00 | 0.00 | 0.00 | 0.04 ± 0.09 |
| nerolidol | 1565 | 1, 2 | 1.37 | 1.52 | 0.84 | 0.00 | 0.82 | 0.99 | 0.92 ± 0.53 |
| epi-globulol | 1573 | 1, 2 | 0.00 | 0.10 | 0.00 | 0.00 | 0.33 | 0.00 | 0.07 ± 0.13 |
| palustrol | 1583 | 1, 2 | 0.00 | 0.00 | 4.24 | 0.00 | 0.00 | 1.32 | 0.93 ± 1.71 |
| spathulenol | 1590 | 1, 2 | 0.00 | 0.49 | 0.18 | 0.00 | 1.06 | 0.37 | 0.35 ± 0.4 |
| caryophyllene oxide | 1597 | 1, 2 | 3.89 | 2.90 | 2.98 | 0.00 | 0.00 | 0.99 | 1.79 ± 1.68 |
| globulol | 1598 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| viridiflorol | 1607 | 1, 2 | 16.47 | 29.00 | 11.69 | 23.12 | 24.98 | 13.19 | 19.74 ± 6.97 |
| γ-eudesmol | 1647 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| 1(5),6-guaiadiene | 1662 | 1, 2 | 13.97 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 2.33 ± 5.7 |
| β-eudesmol | 1665 | 1, 2 | 0.00 | 0.69 | 0.21 | 0.00 | 0.00 | 0.42 | 0.22 ± 0.28 |
| α-eudesmol | 1669 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| τ-muurolol | 1666 | 1, 2 | 0.20 | 0.00 | 0.00 | 0.00 | 0.34 | 0.37 | 0.15 ± 0.18 |
| α-bisabolol | 1692 | 1, 2 | 0.22 | 1.10 | 0.06 | 0.00 | 0.12 | 0.09 | 0.27 ± 0.42 |
| tetracosane | 2300 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| heneicosane | 2264 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| % Identification | 85.28 | 94.29 | 90.58 | 93.14 | 83.56 | 81.45 | 88.05 ± 5.34 | ||
| Grouped components: | |||||||||
| Monoterpene hydrocarbons | 27.52 | 29.91 | 40.38 | 40.55 | 39.97 | 29.30 | 34.61 ± 6.29 | ||
| Oxygenated monoterpenes | 7.22 | 8.41 | 13.23 | 27.49 | 6.36 | 24.91 | 14.6 ± 9.33 | ||
| Sesquiterpene hydrocarbons | 28.22 | 20.17 | 16.65 | 1.98 | 9.59 | 9.51 | 14.35 ± 9.28 | ||
| Oxygenated sesquiterpenes | 22.32 | 35.80 | 20.32 | 23.12 | 27.64 | 17.74 | 24.49 ± 6.44 | ||
| Hydrocarbons | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd | ||
RRI: Relative retention indices calculated against n-alkanes, ID: identification method—1 = comparison of retention index; 2 = comparison of mass spectra with MS libraries, nd: not detected, t: trace, major compounds (% > 5) highlighted in bold.
Table 4.
Identified essential oil components (%) of Salvia chamelaeagnea.
| Salvia chamelaeagnea | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Piketberg | Darling | ||||||||
| Sc1 | Sc2 | Sc3 | Sc4 | Sc5 | Sc6 | Mean | |||
| Components/Yield (% dry weight) | RRI | ID | 0.35 | 0.13 | 1.60 | 0.55 | 0.22 | 0.40 | 0.54 ± 0.54 |
| tricyclene | 921 | 1, 2 | 0.56 | 0.00 | 0.29 | 0.00 | 0.00 | 0.00 | 0.14 ± 0.24 |
| α-thujene | 927 | 1, 2 | 7.16 | 0.29 | 0.61 | 0.05 | 0.13 | 0.00 | 1.37 ± 2.84 |
| α-pinene | 934 | 1, 2 | 0.13 | 7.16 | 7.07 | 2.61 | 4.70 | 2.54 | 4.03 ± 2.79 |
| camphene | 948 | 1, 2 | 0.00 | 0.10 | 6.40 | 0.24 | 0.09 | 0.10 | 1.16 ± 2.57 |
| verbenene | 963 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| sabinene | 973 | 1, 2 | 0.16 | 0.10 | 0.15 | 0.00 | 0.08 | 0.00 | 0.08 ± 0.07 |
| β-pinene | 976 | 1, 2 | 2.19 | 2.29 | 2.99 | 0.75 | 1.48 | 0.53 | 1.71 ± 0.96 |
| myrcene | 990 | 1, 2 | 2.80 | 1.62 | 1.18 | 0.95 | 1.95 | 0.79 | 1.55 ± 0.75 |
| α-phellandrene | 1002 | 1, 2 | 13.76 | 0.54 | 2.12 | 0.26 | 0.22 | 0.30 | 2.87 ± 5.39 |
| δ-3-carene | 1009 | 1, 2 | 0.07 | 0.11 | 0.09 | 0.00 | 0.17 | 0.00 | 0.07 ± 0.07 |
| α-terpinene | 1015 | 1, 2 | 2.50 | 0.43 | 0.00 | 0.18 | 0.35 | 0.17 | 0.61 ± 0.94 |
| para-cymene | 1023 | 1, 2 | 0.00 | 0.00 | 0.36 | 0.31 | 0.31 | 0.24 | 0.2 ± 0.16 |
| sylvestrene | 1025 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| limonene | 1028 | 1, 2 | 0.00 | 7.85 | 0.00 | 20.88 | 15.36 | 19.38 | 10.58 ± 9.36 |
| β-phellandrene | 1029 | 1, 2 | 0.00 | 0.00 | 12.68 | 0.00 | 0.00 | 0.00 | 2.11 ± 5.18 |
| 1,8-cineole (eucalyptol) | 1031 | 1, 2 | 3.29 | 1.22 | 1.80 | 7.64 | 8.14 | 4.95 | 4.51 ± 2.93 |
| cis-β-ocimene | 1037 | 1, 2 | 1.63 | 2.17 | 0.26 | 0.00 | 0.00 | 0.36 | 0.74 ± 0.93 |
| trans-β-ocimene | 1047 | 1, 2 | 2.88 | 1.09 | 0.15 | 1.98 | 7.13 | 0.93 | 2.36 ± 2.52 |
| γ-terpinene | 1059 | 1, 2 | 0.30 | 1.09 | 0.93 | 0.41 | 0.97 | 0.32 | 0.67 ± 0.36 |
| cis-sabinene hydrate | 1068 | 1, 2 | 0.06 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | t |
| terpinolene | 1088 | 1, 2 | 0.41 | 0.35 | 0.38 | 0.00 | 0.27 | 0.11 | 0.25 ± 0.17 |
| trans-sabinene hydrate | 1098 | 1, 2 | 0.00 | 0.00 | 1.14 | 0.00 | 0.00 | 0.00 | 0.19 ± 0.47 |
| linalool | 1099 | 1, 2 | 0.23 | 0.15 | 0.00 | 0.32 | 0.20 | 0.14 | 0.17 ± 0.11 |
| 2-methylbutyl 2-methylbutyrate | 1002 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| solusterol | 1103 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| n-amyl isovalerate | 1107 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| α-thujone | 1118 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| cis-para-menth-2-en-1-ol | 1123 | 1, 2 | 0.07 | 0.00 | 0.14 | 0.09 | 0.00 | 0.09 | 0.06 ± 0.06 |
| α-campholene aldehyde | 1128 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| terpinen-1-ol | 1142 | 1, 2 | 0.00 | 0.00 | 0.09 | 0.07 | 0.00 | 0.00 | t |
| trans-verbenol | 1142 | 1, 2 | 0.06 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | t |
| camphor | 1148 | 1, 2 | 0.06 | 0.11 | 7.61 | 0.14 | 0.06 | 0.08 | 1.34 ± 3.07 |
| camphene hydrate | 1152 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| exo-methyl-camphenilol | 1153 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| borneol | 1170 | 1, 2 | 0.00 | 0.00 | 10.90 | 0.00 | 0.00 | 0.00 | 1.82 ± 4.45 |
| terpinen-4-ol | 1180 | 1, 2 | 1.02 | 0.00 | 0.80 | 0.28 | 0.35 | 0.25 | 0.45 ± 0.38 |
| α-terpineol | 1194 | 1, 2 | 0.23 | 0.34 | 0.23 | 0.40 | 0.21 | 0.17 | 0.26 ± 0.09 |
| myrtenol | 1199 | 1, 2 | 0.47 | 2.03 | 4.91 | 0.00 | 0.00 | 0.00 | 1.23 ± 1.96 |
| nopol | 1206 | 1, 2 | 0.00 | 0.09 | 0.06 | 0.00 | 0.00 | 0.00 | t |
| cis-piperitol | 1211 | 1, 2 | 0.00 | 0.00 | 0.11 | 0.00 | 0.00 | 0.00 | t |
| β-cyclocitral | 1224 | 1, 2 | 0.11 | 0.09 | 0.06 | 0.00 | 0.00 | 0.00 | t |
| cis-3-hexenyl isovalerate | 1235 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| linalyl acetate | 1255 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| phellandral | 1282 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| bornyl acetate | 1284 | 1, 2 | 0.00 | 0.00 | 0.29 | 0.00 | 0.00 | 0.00 | 0.05 ± 0.12 |
| α-fenchyl acetate | 1298 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| myrtenyl acetate | 1328 | 1, 2 | 0.00 | 0.04 | 0.00 | 0.00 | 0.00 | 0.00 | t |
| isoledene | 1382 | 1, 2 | 0.08 | 0.05 | 0.06 | 0.00 | 0.30 | 0.21 | 0.12 ± 0.11 |
| α-cubebene | 1384 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.19 | 0.10 | 0.05 ± 0.08 |
| α-copaene | 1385 | 1, 2 | 0.40 | 0.94 | 0.00 | 0.07 | 0.00 | 0.00 | 0.24 ± 0.38 |
| α-ylangene | 1387 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| cis-jasmone | 1403 | 1, 2 | 0.36 | 0.00 | 0.06 | 0.00 | 0.00 | 0.00 | 0.07 ± 0.14 |
| cis-β-caryophyllene | 1417 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.03 | 0.00 | 0.00 | t |
| α-gurjunene | 1420 | 1, 2 | 0.16 | 0.20 | 0.07 | 0.76 | 0.40 | 1.03 | 0.44 ± 0.38 |
| β-caryophyllene | 1431 | 1, 2 | 0.00 | 14.76 | 3.17 | 2.85 | 5.32 | 4.53 | 5.1 ± 5.07 |
| clovene | 1439 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| α-bergamotene | 1443 | 1, 2 | 0.00 | 0.24 | 0.00 | 0.00 | 0.00 | 0.00 | t |
| calarene | 1444 | 1, 2 | 0.17 | 0.15 | 0.04 | 0.31 | 0.27 | 0.36 | 0.22 ± 0.12 |
| aromadendrene | 1451 | 1, 2 | 0.00 | 0.73 | 0.00 | 3.79 | 0.00 | 1.09 | 0.93 ± 1.47 |
| valencene I | 1452 | 1, 2 | 0.00 | 0.73 | 1.43 | 0.00 | 0.00 | 4.05 | 1.03 ± 1.58 |
| trans-β-farnesene | 1458 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| α-humulene | 1466 | 1, 2 | 0.00 | 2.07 | 0.00 | 1.60 | 1.11 | 2.71 | 1.25 ± 1.1 |
| allo-aromadendrene | 1473 | 1, 2 | 5.75 | 0.00 | 0.00 | 0.89 | 5.25 | 0.00 | 1.98 ± 2.75 |
| γ-gurjunene | 1484 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.15 | 0.21 | 0.06 ± 0.1 |
| α-amorphene | 1486 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 1.12 | 0.19 ± 0.46 |
| β-ionone | 1491 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| β-selinene | 1495 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| germacrene-D | 1499 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| γ-himachalene | 1501 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| valencene | 1506 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| ledene | 1507 | 1, 2 | 0.91 | 1.30 | 0.85 | 1.25 | 2.34 | 1.93 | 1.43 ± 0.59 |
| β-bisabolene | 1513 | 1, 2 | 0.00 | 0.31 | 0.00 | 0.00 | 0.00 | 0.00 | 0.05 ± 0.12 |
| γ-cadinene | 1524 | 1, 2 | 0.00 | 0.12 | 0.40 | 0.00 | 0.67 | 0.00 | 0.2 ± 0.28 |
| β-cadinene | 1532 | 1, 2 | 0.33 | 1.01 | 0.00 | 0.00 | 0.47 | 0.00 | 0.3 ± 0.4 |
| nerolidol | 1565 | 1, 2 | 2.13 | 3.04 | 3.17 | 2.50 | 2.71 | 3.32 | 2.81 ± 0.45 |
| epi-globulol | 1573 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.48 | 0.08 ± 0.2 |
| palustrol | 1583 | 1, 2 | 0.00 | 0.00 | 0.00 | 3.78 | 0.00 | 4.11 | 1.32 ± 2.04 |
| spathulenol | 1590 | 1, 2 | 2.40 | 0.00 | 0.56 | 0.98 | 0.98 | 0.57 | 0.91 ± 0.81 |
| caryophyllene oxide | 1597 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| globulol | 1598 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| viridiflorol | 1607 | 1, 2 | 39.05 | 24.12 | 15.24 | 25.02 | 23.44 | 20.57 | 24.57 ± 7.93 |
| γ-eudesmol | 1647 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| 1(5),6-guaiadiene | 1662 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| β-eudesmol | 1665 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.51 | 0.00 | 0.00 | 0.09 ± 0.21 |
| α-eudesmol | 1669 | 1, 2 | 0.00 | 0.80 | 0.15 | 0.00 | 1.98 | 2.64 | 0.93 ± 1.13 |
| τ-muurolol | 1666 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| α-bisabolol | 1692 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.04 | 0.00 | t |
| tetracosane | 2300 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| heneicosane | 2264 | 1, 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd |
| % Identification | 91.89 | 79.75 | 88.96 | 81.81 | 87.74 | 80.45 | 85.1 ± 5.08 | ||
| Grouped components: | |||||||||
| Monoterpene hydrocarbons | 34.56 | 25.19 | 35.67 | 28.56 | 33.21 | 25.76 | 30.49 ± 4.58 | ||
| Oxygenated monoterpenes | 5.61 | 4.01 | 28.13 | 8.93 | 8.95 | 5.67 | 10.22 ± 9 | ||
| Sesquiterpene hydrocarbons | 7.79 | 22.59 | 5.97 | 11.52 | 16.47 | 17.32 | 13.61 ± 6.31 | ||
| Oxygenated sesquiterpenes | 43.93 | 27.96 | 19.18 | 32.80 | 29.10 | 31.70 | 30.78 ± 8.04 | ||
| Hydrocarbons | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | nd | ||
RRI: Relative retention indices calculated against n-alkanes, ID: identification method—1 = comparison of retention index; 2 = comparison of mass spectra with MS libraries, nd: not detected, t: trace, major compounds (% > 5) highlighted in bold.
For S. dentata, the most abundant compounds (those with a peak area > 5%) were camphor (14.37%), α-pinene (11.43%), camphene (10.18%), 1,8-cineole (eucalyptol; 9.42%) and bornyl acetate (8.56%).
In S. africana EO the trend of viridiflorol (19.74%) > β-caryophyllene (8.8%) > α-pinene (8.25%) > limonene (7.81%) > 1,8-cineole (eucalyptol) (6.02%) was observed. Salvia chamelaeagnea EO showed to be dominated by viridiflorol (24.57%) > limonene (10.58%) > β-caryophyllene (5.10%). Similar patterns in major compounds have been reported for S. africana by Kamatou et al. [33] and Van Vuuren et al. [37]. Furthermore, Lim Ah Tock et al. [34] observed viridiflorol (33.00%) and limonene (17.30%) being the major compounds for S. chamelaeagnea, where similarly Van Vuuren et al. [37] also noted viridiflorol (32.50%), limonene (14.10%) and 1,8-cineole (14.1%) as the major compounds in their study. The varying content of constituents observed in the extracted EOs among the three species are likely dependent on environmental factors such as altitude, climate, water availability and pedoclimatic conditions [35]. Further, factors such as genetics and phenological period have been shown to have an effect on the yield and content of volatile oils in aromatic plant species [35,38].
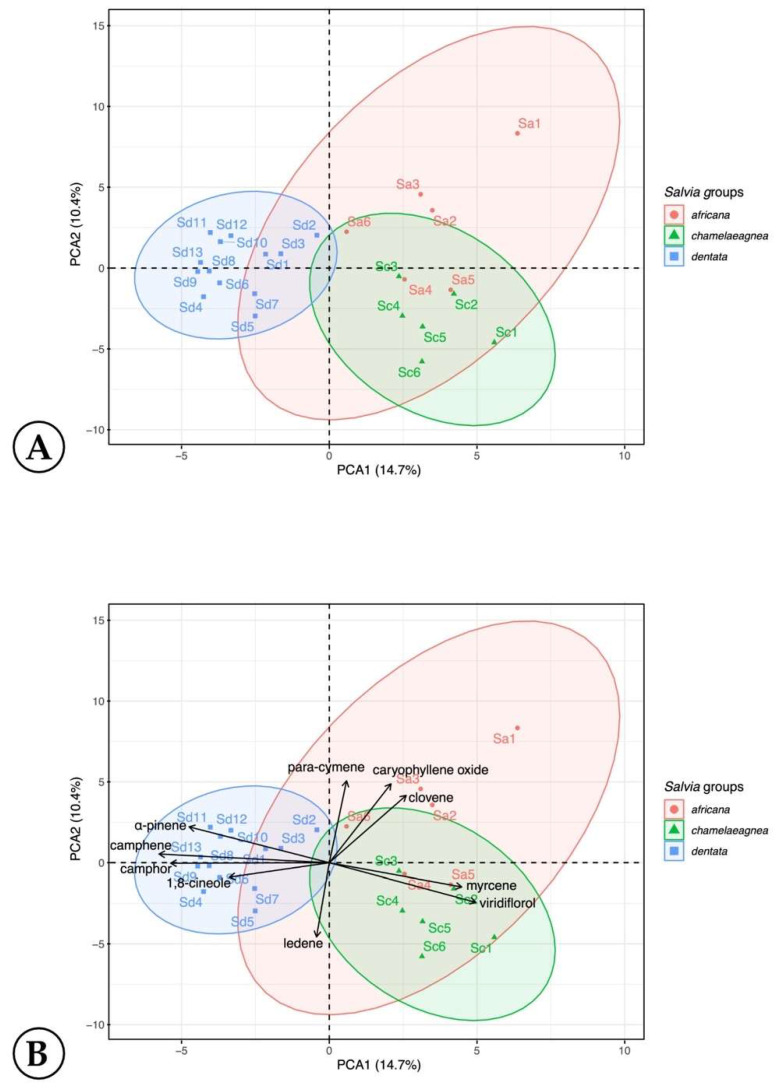
The variability and potential diagnostic value of the essential oil data was investigated, and a principal component analysis (PCA) indicates three groupings for the three species from the first (14.70%) and second (10.40%) principal components (Figure 7). The S. dentata group shows almost no overlap with the S. africana and S. chamelaeagnea groups.
Figure 7.
Principal component analysis (PCA) scatterplot (biplot) of the first (14.70%) and second (10.40%) components; (A) scatterplot of species and (B) scatterplot of the essential oils (loadings plot).
The Salvia dentata plots grouped to the left of the scatterplot due to the higher content of camphene (μ = 10.18 ± 2.32; p < 0.05), camphor (μ = 14.37 ± 4.43; p > 0.05), α-pinene (μ = 11.43 ± 2.42; p Salvia chamelaeagnea samples clustered together due to viridiflorol (μ = 24.57 ± 7.93; p < 0.05) and limonene (μ = 10.58 ± 9.36; p < 0.05). The S. africana group was determined by para-cymene (μ = 1.30 ± 1.23; p > 0.05), caryophyllene oxide (μ = 1.79 ± 1.68; p > 0.05) and clovene (μ = t; p > 0.05).
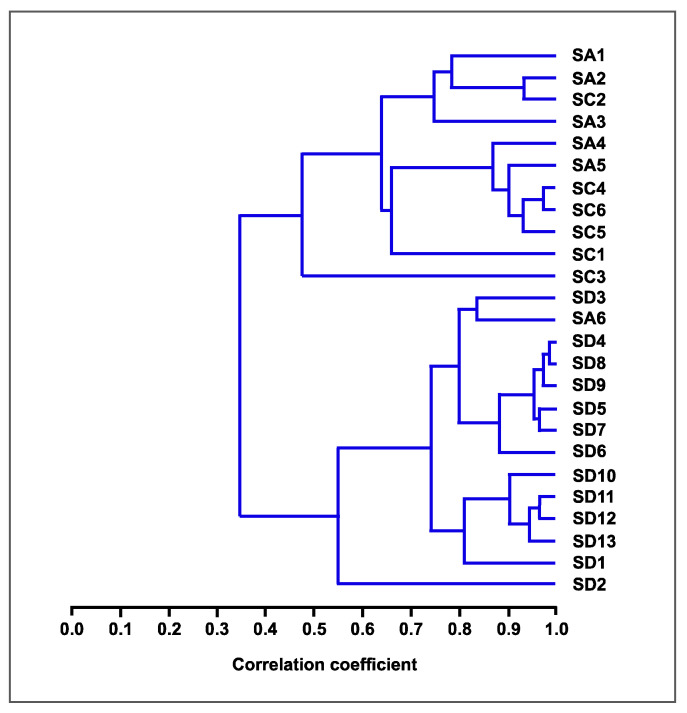
The EO chemistry of S. africana and S. chamelaeagnea are highly similar due to shared major and minor compounds. This is evident from the higher correlation seen between the EOs of S. africana and S. chamelageanea (Scorr > 0.60) as compared with the two against S. dentata EOs (Scorr > 0.35) (Figure 8). This is likely due to S. africana and S. chamelaeagnea EOs containing higher amounts of viridiflorol, limonene and β-caryophyllene whereas S. dentata EOs contain high levels of camphene, camphor, 1,8-cineole, bornyl acetate and α-pinene. The chemical profile of S. dentata is very similar to that of S. officinalis reported from the North of Tunisia in containing notable levels of camphor (33.61%), 1,8-cineole (22.22%), α-thujone (21.43%) and camphene (4.88%) [39].
Figure 8.
A dendrogram generated by cluster analysis of the percentage composition of essential oils from the three Salvia species studied, based on correlation and using the unweighted pair-group method with arithmetic average (UPGMA). SA = S. africana, SC = S. chamelaeagnea and SD = S. dentata.
In another study by Tundis et al. [35], the authors analyzed Mediterranean samples of S. officinalis from several localities and included data from previous studies in their analyses. Their data also observed high levels of camphor (16.16–18.92%), camphene (6.27–8.08%), 1,8-cineole (eucalyptol) (8.80–9.86%), β-pinene (3.08–9.14%) and α-thujone (1.17–9.26%).
Other Salvia species reported with high levels of camphor and 1,8-cineole include S. aytachii Vural & Adigüzel [40] from Turkey and S. fruticosa from Lybia [41]. Alone, each compound has been reported to exhibit potent biological activity. For example, camphor, a natural product derived from the wood of Cinnamomum camphora L., though also present in other aromatic plant species, such as Ocimum kilimandscharicum Gürke (Lamiaceae), is a major source of the compound in Asia [42]. Camphor has been reported to be a counterirritant, rubefacient, mild analgesic, components of liniments for the relief of fibrositis, neuralgia and is a mild expectorant. Camphor oil has been used intramuscular or subcutaneously as a circulatory/respiratory stimulant though these methods are considered hazardous [42,43]. Eucalyptol (1,8-cineole) is a mucolytic monoterpene that has been shown to exhibit anti-inflammatory, bronchodilatory and antimicrobial activity and has been used effectively in the treatment of asthma and associated respiratory ailments [44].
The higher levels of both camphor and 1,8-cineole in S. dentata may suggest to its use as a traditional remedy in the treatment of microbial infections (i.e., respiratory, gastrointestinal and topical) due to the synergistic effect of these two compounds as reported by Viljoen et al. [45].
3. Materials and Methods
3.1. Ethnobotanical Data
Literature searches were conducted by searching several scientific electronic databases, including GoogleScholar (www.scholar.google.com), EBSCOhost (www.ebsco.com), PubMed (www.pubmed.ncbi.nlm.nih.gov), ScienceDirect (www.sciencedirect.com), SciFinder (www.scifinder.cas.org), Springer (www.springer.com) and Wiley Online Library (www.onlinelibrary.wiley.com). Key words were used to search for literature, and this was conducted in the following manner: (“Species name” AND “synonyms” AND “med*”) and (“Species name” AND “synonyms” AND “traditional use”). A collection of scientific papers, books, dissertations and theses, and unpublished sources were also compiled.
3.2. Plant Material and Isolation of Essential Oil
Fresh aerial parts of Salvia africana (n = 6), S. chamelaeagnea (n = 6), and S. dentata (n = 13), were collected from natural populations in the Cape region of South Africa (Table 5). The three species were identified by B.-E. Van Wyk and voucher specimens are housed at the University of Johannesburg Herbarium (JRAU), University of Johannesburg, South Africa.
Table 5.
Sample information.
| Species | Voucher Numbers | Locality | Point on Map (Figure 2) | Dry Weight (g) |
|---|---|---|---|---|
| Salvia africana | RDR and BEVW 0048A | Traveller’s Rest, Cederberg, WC | A | 33.46 |
| RDR and BEVW 0048B | Traveller’s Rest, Cederberg, WC | A | 45.1 | |
| RDR and BEVW 0048C | Traveller’s Rest, Cederberg, WC | A | 60.08 | |
| RDR and BEVW 0076 | Citrusdal, WC | B | 57.1 | |
| RDR and BEVW 0077 | Elandskloof, WC | C | 61.27 | |
| RDR and BEVW 0078 | Theronsberg Pass, WC | D | 101.38 | |
| Salvia chamelaeagnea | RDR and BEVW 0055A | Piketberg, WC | E | 59.97 |
| RDR and BEVW 0055B | Piketberg, WC | E | 39.91 | |
| RDR and BEVW 0055C | Piketberg, WC | E | 46.37 | |
| RDR and BEVW 0073A | Darling, WC | F | 39.64 | |
| RDR and BEVW 0073B | Darling, WC | F | 41.45 | |
| RDR and BEVW 0073C | Darling, WC | F | 30.17 | |
| Salvia dentata | RDR and BEVW 0046A | Nieuwoudtville, NC | G | 89.2 |
| RDR and BEVW 0046B | Nieuwoudtville, NC | G | 71.4 | |
| RDR and BEVW 0046C | Nieuwoudtville, NC | G | 73.2 | |
|
Salvia dentata Cont. |
RDR and BEVW 0047A | Spoegrivier road near Garies, NC | H | 73.43 |
| RDR and BEVW 0047B | Spoegrivier road near Garies, NC | H | 124.45 | |
| RDR and BEVW 0047C | Spoegrivier road near Garies, NC | H | 149.8 | |
| RDR and BEVW 0047D | Spoegrivier road near Garies, NC | H | 66.49 | |
| RDR and BEVW 0047E | Spoegrivier road near Garies, NC | H | 69.31 | |
| RDR and BEVW 0047F | Spoegrivier road near Garies, NC | H | 151.35 | |
| RDR and BEVW 0081A | Tankwa, NC | I | 86.76 | |
| RDR and BEVW 0081B | Tankwa, NC | I | 53.38 | |
| RDR and BEVW 0081C | Tankwa, NC | I | 84.51 | |
| RDR and BEVW 0081D | Tankwa, NC | I | 75.14 |
WC = Western Cape and NC = Northern Cape.
The plant material was airdried at room temperature (indoors, at ca. 18 to 22 °C) for several days until no moisture was present. The essential oils were isolated by subjecting the dried leaf material to hydrodistillation using a Clevenger apparatus for three hours. The resulting EO was stored in amber vials at +4 °C until tested and all results were expressed based on dry matter weight.
3.3. Chemical Analyses
Gas chromatography and gas chromatography coupled to mass spectrometry—A modified method based on Boukhatem, Kameli and Saidi [46] was used where EO samples were prepared by diluting 20 μL EO in 1000 μL acetone. One microliter of the diluted sample was injected onto a Thermo Scientific™ TRACE™ 1300 gas chromatograph (Rodano, Milan-Italy) equipped with a ZB-5Ms capillary column (30 m × 0.25 mm, 0.25 μm), coupled with a Thermo Scientific™ TSQ™ 8000 Triple Quadrupole mass spectrometer (Austin, TX, USA). The GC-MS system was coupled to a Triplus RSH Analytics PAL autosampler (Switzerland). Chromatographic separation of the essential oil was performed on a non-polar ZB-5Ms (30 m, 0.25 mm ID, 0.25 µm film thickness) Zebron capillary column. Helium was used as the carrier gas at a flow rate of 1 mL/min. The injector temperature was maintained at 250 °C. A total of 1µl of the sample was injected in 50:1 split mode. The oven temperature was programmed as follows: initial temperature of 40 °C held for 5 min; and finally ramped up to 250 °C at a rate of 8 °C/min and held for 5 min with a total runtime of 36 min. The MSD was operated in a full scan mode and the source and quad temperatures were maintained at 230 °C and 150 °C, respectively. The transfer line temperature was maintained at 250 °C. The mass spectrometer was operated under electron impact mode at ionization energy of 70 eV, scanning from 35 to 500 m/z. The Salvia EOs constituents were tentatively identified by comparing their Retention Indices (RI) with those from literature such as Adams [47] and the similarity of their mass spectra with those databased in the NIST-MS library. RI were calculated under the same operating conditions in relation to a homologous series of n-alkanes (C8–C24).
3.4. Statistical Analyses
Principle component analysis was done using Rstudio (Rstudio Team (2021). Rstudio: Integrated Development Environment for R. Rstudio, PBC, Boston, MA, USA. http://www.rstudio.com/). The correlation analysis by UPGMA was done using PAST 4.1 software (Copyright Hammer & Harper; free download from: https://www.nhm.uio.no/english/research/infrastructure/past/).
4. Conclusions
Although the three species of blue-flowered sages are closely related, Salvia dentata presents a distinct chemical profile from the other two species (S. africana and S. chamelaeagnea) due to the higher quantities of camphene, camphor, bornyl acetate and 1,8-cineole (eucalyptol) present as highlighted by the data. For the first time, the essential oil chemistry has been reported for this medicinally relevant species and presents opportunities for further investigation within the fields of biological activity and phenolic chemistry.
Acknowledgments
The authors would like to thank the local provincial government agencies (Northern Cape Province and Cape Nature, Western Cape) for issuing the collecting permits used to obtain plant material for this study from the respective provinces (NC permit: FLORA 0075/2021 and WC permit: CN35-28-16005). The authors thank three anonymous reviewers for suggestions which improved the manuscript.
Author Contributions
R.D.R. coordinated the study, did the statistical analyses and wrote the first draft of the paper; L.M. did the GC-MS analyses, wrote up the methods for the analyses and contributed comments to the manuscript; M.A.S. coordinated the chemical analysis and provided comments; B.-E.V.W. conceptualized the study, identified the knowledge gap, funded the study, led the field studies and collection of materials, identified the species and helped to write and edit the final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The geographical data presented in this study are openly available in GBIF (Global Biodiversity Information Facility) at https://doi.org/10.15468/dl.sa4wq9.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Funding Statement
Research was funded by the University of Johannesburg and the National Research Foundation of South Africa (to the National Research in Indigenous Plant Use, NRF grant numbers 84442 and MND200626537046).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.POWO Plants of the World Online Facilitated by the Royal Botanic Gardens, Kew. [(accessed on 1 August 2022)]. Available online: https://powo.science.kew.org.
- 2.Hamidpour M., Hamidpour R., Hamidpour S., Shahlari M. Chemistry, pharmacology, and medicinal property of sage (Salvia) to prevent and cure illnesses such as obesity, diabetes, depression, dementia, lupus, autism, heart disease, and cancer. J. Tradit. Complement. Med. 2014;4:82–88. doi: 10.4103/2225-4110.130373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mathew J., Thoppil J.E. Chemical composition and mosquito larvicidal activities of Salvia essential oils. Pharm. Biol. 2011;49:456–463. doi: 10.3109/13880209.2010.523427. [DOI] [PubMed] [Google Scholar]
- 4.Scholey A., Stough C. 11-Neurocognitive effects of herbal extracts. In: Behaviour and Psychiatric IllnessBenton D., editor. Woodhead Publishing Series in Food Science, Technology and Nutrition. Woodhead Publishing; Cambridge, UK: 2011. pp. 272–297. [Google Scholar]
- 5.Drew B.T., González-Gallegos J.G., Xiang C.L., Kriebel R., Drummond C.P., Walker J.B., Sytsma K.J. Salvia united: The greatest good for the greatest number. Taxon. 2017;66:133–145. doi: 10.12705/661.7. [DOI] [Google Scholar]
- 6.Raal A., Orav A., Arak E. Composition of the essential oil of Salvia officinalis L. from various European countries. Nat. Prod. Res. 2007;21:406–411. doi: 10.1080/14786410500528478. [DOI] [PubMed] [Google Scholar]
- 7.Hao D.C., Gu X.-J., Xiao P.G. Medicinal Plants: Chemistry, Biology and Omics. Elsevier GmbH; Amsterdam, The Netherlands: 2015. Phytochemical and biological research of Salvia medicinal resources; pp. 587–639. [Google Scholar]
- 8.Lu Y., Foo L. Polyphenolics of Salvia. Phytochemistry. 2002;59:117–140. doi: 10.1016/S0031-9422(01)00415-0. [DOI] [PubMed] [Google Scholar]
- 9.Orona-Tamayo D., Valverde M.E., Paredes-López O. Chia-The New Golden Seed for the 21st Century: Nutraceutical Properties and Technological Uses. Sustain. Protein Sources. 2017:265–281. doi: 10.1016/B978-0-12-802778-3.00017-2. [DOI] [Google Scholar]
- 10.Rattray R.D., Van Wyk B.-E. The Botanical, Chemical and Ethnobotanical Diversity of Southern African Lamiaceae. Molecules. 2021;26:1–59. doi: 10.3390/molecules26123712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Codd L.E. In: Flora of southern Africa: Part 4 Lamiaceae. Leistner O.A., editor. Botanical Research Institute, Departmet of Agriculture and Water Supply, Republic of South Africa; Pretoria, South Africa: 1985. [Google Scholar]
- 12.Fisher V.L. Mater’s Thesis. University of the Witwatersrand; Johannesburg, South Africa: 2005. Indigenous Salvia Species-an Investigation of the Antimicrobial Activity, Antioxidant Activity and Chemical Composition of Leaf Extracts. [Google Scholar]
- 13.Kamatou G.P.P., Makunga N.P., Ramogola W.P.N., Viljoen A.M. South African Salvia species: A review of biological activities and phytochemistry. J. Ethnopharmacol. 2008;119:664–672. doi: 10.1016/j.jep.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 14.Van Wyk B.-E., Van Oudtshoorn B., Gericke N. In: Medicinal Plants of South Africa. 2nd ed. Smit J., editor. Briza Publications; Pretoria, South Africa: 2009. [Google Scholar]
- 15.Hulley I.M. Medicinal Ethnobotany of the Little Karoo, South Africa. University of Johannesburg; Johannesburg, South Africa: 2018. [Google Scholar]
- 16.Wheat N.M. Ph.D. Thesis. University of Cape Town; Cape Town, South Africa: 2013. An Ethnobotanical, Phytochemical and Metabolomics Investigstion of Plants from the Paulshoek Communal Area, Namaqualand. [Google Scholar]
- 17.Nortje J.M. Ethnobotany of Namaqualand, South Africa. University of Johannesburg; Johannesburg, South Africa: 2019. [Google Scholar]
- 18.Jenks A.A., Kim S.C. Medicinal plant complexes of Salvia subgenus Calosphace: An ethnobotanical study of new world sages. J. Ethnopharmacol. 2013;146:214–224. doi: 10.1016/j.jep.2012.12.035. [DOI] [PubMed] [Google Scholar]
- 19.Gali-Muhtasib H., Hilan C., Khater C. Traditional uses of Salvia libanotica (East Mediterranean sage) and the effects of its essential oils. J. Ethnopharmacol. 2000;71:513–520. doi: 10.1016/S0378-8741(99)00190-7. [DOI] [PubMed] [Google Scholar]
- 20.Watt J.M., Breyer-Brandwijk M.G. The Medicinal and Poisonous Plants of Southern and Eastern Africa. 2nd ed. Livingstone; London, UK: 1962. [Google Scholar]
- 21.Smith C.A. Common Names of South African Plants. Memoirs of the Botanical Survey of South Africa No. 35. Department of Agriculture and Technical Services, Pretoria; Pretoria, South Africa: 1966. [Google Scholar]
- 22.Rood B. Uit Die Veld-Apteek. Tafelberg; Cape Town, South Africa: 1994. [Google Scholar]
- 23.Nortje J.M., Van Wyk B.-E. Useful plants of Namaqualand, South Africa: A checklist and analysis. S. Afr. J. Bot. 2019;122:120–135. doi: 10.1016/j.sajb.2019.03.039. [DOI] [Google Scholar]
- 24.Welcome A.K. Food Plants of Southern Africa. University of Johannesburg; Johannesburg, South Africa: 2019. [Google Scholar]
- 25.Welcome A.K., Van Wyk B.-E. An inventory and analysis of the food plants of southern Africa. S. Afr. J. Bot. 2019;122:136–179. doi: 10.1016/j.sajb.2018.11.003. [DOI] [Google Scholar]
- 26.Mössmer N., David L. Traditional Herbal Remedies. Montagu Museum; Montagu, South Africa: 2019. [Google Scholar]
- 27.Hulley I.M., Van Wyk B.-E. Quantitative medicinal ethnobotany of Kannaland (western Little Karoo, South Africa): Non-homogeneity amongst villages. S. Afr. J. Bot. 2019;122:225–265. doi: 10.1016/j.sajb.2018.03.014. [DOI] [Google Scholar]
- 28.Archer F.M. Planning with people-ethnobotany and African uses of plants in Namaqualand (South Africa); Proceedings of the 12th Plenary Meeting of AEFAT, Mitteilungen aus dem Institut für allgemeine Botanik; Hamburg, Germany. 28–30 September 1990; pp. 959–972. [Google Scholar]
- 29.Archer F.M. Master’s Thesis. University of Cape Town; Cape Town, South Africa: 1994. Ethnobotany of Namaqualand: The Richtersveld. [Google Scholar]
- 30.De Beer J.J.J., Van Wyk B.-E. An ethnobotanical survey of the Agter-Hantam, Northern Cape Province, South Africa. S. Afr. J. Bot. 2011;77:741–754. doi: 10.1016/j.sajb.2011.03.013. [DOI] [Google Scholar]
- 31.Van Wyk B.-E., Gorelik B. The history and ethnobotany of Cape herbal teas. S. Afr. J. Bot. 2017;110:18–38. doi: 10.1016/j.sajb.2016.11.011. [DOI] [Google Scholar]
- 32.Kamatou G.P.P., Van Zyl R.L., Van Vuuren S.F., Viljoen A.M., Figueiredo A.C., Barroso J.G., Pedro L.G., Tilney P.M. Chemical composition, leaf trichome types and biological activities of the essential oils of four related Salvia species indigenous to Southern Africa. J. Essent. Oil Res. 2006;18:72–79. doi: 10.1080/10412905.2006.12067125. [DOI] [Google Scholar]
- 33.Kamatou G.P.P., Van Zyl R.L., Van Vuuren S.F., Figueiredo A.C., Barroso J.G., Pedro L.G., Viljoen A.M. Seasonal variation in essential oil composition, oil toxicity and the biological activity of solvent extracts of three South African Salvia species. S. Afr. J. Bot. 2008;74:230–237. doi: 10.1016/j.sajb.2007.08.002. [DOI] [Google Scholar]
- 34.Lim Ah Tock M.J., Kamatou G.P.P., Combrinck S., Sandasi M., Viljoen A.M. A chemometric assessment of essential oil variation of three Salvia species indigenous to South Africa. Phytochemistry. 2020;172:112249. doi: 10.1016/j.phytochem.2019.112249. [DOI] [PubMed] [Google Scholar]
- 35.Tundis R., Leporini M., Bonesi M., Rovito S., Passalacqua N.G. Salvia officinalis L. from Italy: A Comparative Chemical and Biological Study of Its Essential Oil in the Mediterranean Context. Molecules. 2020;25:5826. doi: 10.3390/molecules25245826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hazrati S., Beidaghi P., Beyraghdar Kashkooli A., Hosseini S.J., Nicola S. Effect of Harvesting Time Variations on Essential Oil Yield and Composition of Sage (Salvia officinalis) Horticulturae. 2022;8:149. doi: 10.3390/horticulturae8020149. [DOI] [Google Scholar]
- 37.Van Vuuren S., Ramburrun S., Kamatou G.P.P., Viljoen A. Indigenous South African essential oils as potential antimicrobials to treat foot odour (bromodosis) S. Afr. J. Bot. 2019;126:354–361. doi: 10.1016/j.sajb.2019.06.019. [DOI] [Google Scholar]
- 38.Çalişkan T., Maral H., Pala C., Kafkas E., Kirici S. Morphogenetic Variation for Essential Oil Content and Composition of Sage (Salvia officinalis L.) In Çukurova Condition. Arab. J. Med. Aromat. Plants. 2019;5:32–38. doi: 10.48347/IMIST.PRSM/ajmap-v5i1.15680. [DOI] [Google Scholar]
- 39.El Euch S.K., Hassine D.B., Cazaux S., Bouzouita N., Bouajila J. Salvia officinalis essential oil: Chemical analysis and evaluation of anti-enzymatic and antioxidant bioactivities. S. Afr. J. Bot. 2019;120:253–260. doi: 10.1016/j.sajb.2018.07.010. [DOI] [Google Scholar]
- 40.Baser K.H.C., Duman H., Vural M., Adigüzel N., Aytaç Z. Essential Oil of Salvia aytachii M. Vural et N. Adigüzel. J. Essent. Oil Res. 1997;9:489–490. doi: 10.1080/10412905.1997.9700760. [DOI] [Google Scholar]
- 41.Giweli A.A., Džamić A.M., Soković M., Ristić M.S., Janaćković P., Marin P.D. The chemical composition, antimicrobial and antioxidant activities of the essential oil of Salvia fruticosa growing wild in Libya. Arch. Biol. Sci. 2013;65:321–329. doi: 10.2298/ABS1301321G. [DOI] [Google Scholar]
- 42.Zuccarini P., Soldani G. Camphor: Benefits and risks of a widely used natural product. Acta Biol. Szeged. 2009;53:77–82. doi: 10.4314/jasem.v13i2.55317. [DOI] [Google Scholar]
- 43.Chen W., Vermaak I., Viljoen A. Camphor—A Fumigant during the Black Death and a Coveted Fragrant Wood in Ancient Egypt and Babylon—A Review. Molecules. 2013;18:5434–5454. doi: 10.3390/molecules18055434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Juergens L.J., Worth H., Juergens U.R. New Perspectives for Mucolytic, Anti-inflammatory and Adjunctive Therapy with 1,8-Cineole in COPD and Asthma: Review on the New Therapeutic Approach. Adv. Ther. 2020;37:1737–1753. doi: 10.1007/s12325-020-01279-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Viljoen A.M., Van Vuuren S., Ernst E., Klepser M., Demirci B., Başer H., Van Wyk B.-E. Osmitopsis asteriscoides (Asteraceae)—The antimicrobial activity and essential oil composition of a Cape-Dutch remedy. J. Ethnopharmacol. 2003;88:137–143. doi: 10.1016/S0378-8741(03)00191-0. [DOI] [PubMed] [Google Scholar]
- 46.Boukhatem M.N., Kameli A., Saidi F. Essential oil of Algerian rose-scented geranium (Pelargonium graveolens): Chemical composition and antimicrobial activity against food spoilage pathogens. Food Control. 2013;34:208–213. doi: 10.1016/j.foodcont.2013.03.045. [DOI] [Google Scholar]
- 47.Adams R.P. Identification of Essential Oil Cimponents by Gas Chromatography/Mass Spectrometry. 4th ed. Allured Publishing Corporation; Carol Stream, IL, USA: 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The geographical data presented in this study are openly available in GBIF (Global Biodiversity Information Facility) at https://doi.org/10.15468/dl.sa4wq9.