Abstract
Interest in plant-based diets has been on the rise in recent years owing to the potential health benefits of their individual components and the notion that plant-based diets might reduce the incidence of several diseases. Egyptian dukkah and Syrian za’atar are two of the most historic and famous Middle Eastern herbal blends used for their anti-inflammatory, hypolipidemic, and antidiabetic effects. Headspace SPME-GCMS and HPLC-DAD were adopted for characterizing the aroma profile and phenolic compounds of both herbal blends, respectively. Further, vapor-phase minimum inhibitory concentration was employed for assessing each blend’s antibacterial potential, while their antioxidant potential was estimated via in vitro antioxidant assays. SPME headspace analysis indicated the abundance of ethers and monoterpene hydrocarbons, while HPLC revealed the presence of several phenolics including rosmarinic acid, ferulic acid, and rutin. Biological investigations affirmed that vapor-phase of the tested blends exhibited antibacterial activities against Gram-positive and Gram-negative pathogens, while the antioxidant potential of the blends was investigated and expressed as Trolox (125.15 ± 5.92 to 337.26 ± 13.84 μM T eq/mg) and EDTA (18.08 ± 1.62 to 51.69 41 ± 5.33 μM EDTA eq/mg) equivalent. The presented study offers the first insight into the chemical profile and biological activities of both dukkah and za’atar.
Keywords: dukkah, za’atar, SPME-GCMS, HPLC-DAD, VP-MIC, DPPH, FRAP, ABTS, ORAC
1. Introduction
Chronic illnesses have increased dramatically during the last few decades. Obesity, diabetes, cardiovascular disease, respiratory illnesses, and cancer account for 63 percent of worldwide death each year. Furthermore, chronic illnesses account for almost 45.9% of all disorders globally [1]. People’s health has deteriorated in recent decades, a condition that can be attributed to an unhealthy lifestyle, improper diet, and excessive intake of unrestricted foods and beverages [1]. People consume more than one and a half tons of food per capita during their lifetimes, the composition of which is crucial because nutrition influences 40–60% of diseases [1]. Lifestyle modifications, particularly dietary changes, can be extremely beneficial in preventing, treating, and even reversing a variety of chronic diseases, including coronary artery disease and diabetes [2,3,4]. A plant-based diet consists totally of plant-based foods with a wide range of components. i.e., vegetables, fruits, whole grains, nuts, and seeds [4,5]. Among plant-based diets, Egyptian dukkah and Syrian za’atar are two common examples made entirely of plant ingredients and of potential food (food ingredient, snack, crust; or topping for meals and seasoning for salad) and health benefits (anti-inflammatory, hypolipidemic, and anti-diabetic effects) [6,7].
The Mediterranean diet is regarded as one of the healthiest eating practices. It is considered the most evidence-based diet, where it is capable of preventing and/or treating a variety of ailments. The examination of historic, popular foods, which are widely used as a part of local culture and history, is of particular importance in this context [6]. The Middle Eastern condiments dukkah and za’atar could be regarded as nutritious dietary ingredients owing to the presence of various bioactive components with potentially positive effects on human health.
Because of the growing consumer demand for chemical-free products, researchers have conducted extensive research to assess the feasibility of other preservation techniques, such as the use of essential oils, to improve the microbial quality and safety of food products during their shelf life [8]. Considering their ability to suppress the growth of microorganisms in foods, essential oils obtained from the individual components of dukkah and za’atar (thyme, sumac, coriander, etc.) [9,10,11] have been proposed as food preservatives. Despite their high efficacy against food-borne pathogens, essential oils only have an effect at concentrations exceeding the acceptable flavor thresholds; this may imply an organoleptic impact. Few approaches to lower concentrations have been proposed, such as plant combinations and the use of essential oils in the vapor phase. Essential oils in the vapor phase have potential antibacterial actions against food-borne pathogens, with advantages over essential oils in the liquid phase, such as greater activity at lower concentrations and with no changes in sensory properties of food products [12].
Dukkah, also known as du’ah, do’a, or duqqa, is a traditional Egyptian combination of herbs, nuts, seeds, and spices. Its name is derived from the Arabic word “to pound,” which refers to the ancient practice of pounding spices using a mortar and pestle. It dates back to ancient Egypt but is today extremely popular throughout the Middle East. In Egypt, the term “eeish we dua’ah” is commonly denoted, which literally translates to bread and dukkah, i.e., if someone is experiencing hunger, he or she can always depend on a loaf of bread and some dukkah for dipping [7]. In addition to its nutritional value as a condiment or spice, dukkah has demonstrated a hypoglycemic impact and effectiveness in delaying diabetic complications [7]. While there is no single “traditional” recipe for dukkah, there are some widely accepted foundational ingredients. Seeds (sesame seeds, coriander) and nuts (hazelnuts are the most common frequently combined with almonds, peanuts, and walnuts) are popular garnishes.
Za’atar is another unique flavorful Middle Eastern seasoning blend composed of several blended plants and spices [6]. The Arabic word za’atar describes both a type of herb and a seasoning blend. The za’atar seasoning blend is popular throughout the Middle East, especially in Palestine, Jordan, and Syria. No two recipes are alike, and there are almost certainly many variations following countries’ own cuisines. Nonetheless, three key ingredients define za’atar: sesame seeds, thyme, and sumac [6]. Other herbs and spices found in za’atar include oregano, marjoram, coriander, and cumin. Two varieties of za’atar are available, namely, green za’atar and red za’atar, the primary difference being in the ratio of green herbs (mainly thyme) to red sumac.
Despite the fact that dukkah and za’atar are two of the most famous Middle Eastern herbal blends, and that the beneficial effects of their individual components have been well reported, no studies have defined the chemical profile or bioactivity of the entire herbal mixtures so far in literature. Consequently, this study’s main goal was to characterize the chemical and biological effects of these famous blends, with two varieties of each herbal blend being selected for comparison. Thyme-based Egyptian dukkah (EDT) and nut-based Egyptian dukkah (EDN) were selected as examples for the dukkah mixture, while the za’atar mixture was represented by the green variety, thyme-based Syrian za’atar (SZT), as well as the red variety, sumac-based Syrian za’atar (SZS). Currency inflation and importing restrictions have driven consumers to seek low-cost alternatives; therefore, peanuts have taken the place of more expensive nuts in all commercially available EDN, in contrast to the original recipe. As a result, a common traditional recipe was used to prepare the traditional EDN for examination. The main purpose of this research was to: (1) determine the aroma profile of both dukkah and za’atar; (2) characterize the phenolic profile of the selected blends; (3) screen their antibacterial activity against various resistant bacterial strains; and (4) assess their antioxidant potential via various in vitro assays.
2. Results and Discussion
2.1. Volatile Components and Their Influence on the Aroma of Egyptian Dukkah and Syrian Za’atar via SPME Analysis
Aroma analysis of Egyptian dukkah and Syrian za’atar was performed to investigate the aroma profile and key aroma compounds of both dukkah and za’atar and determine how the volatile components of the Egyptian dukkah differ from that of Syrian za’atar. Both dukkah’s and za’atar’s aroma compounds consist of several chemical classes; 58 volatiles were identified as belonging to aldehydes, alcohols, carboxylic acids, aromatics, esters, furans, ethers, ketones, nitrogen-containing compounds, oxides, phenols, pyrans/pyrroles, and monoterpene and sesquiterpene hydrocarbons. Quantitatively, the most abundant classes in thyme-based (53.14%) and nut-based dukkah (37.60%) as well as the red variety of za’atar (34.71%) were ethers, confirming their antimicrobial and antioxidant effects, while monoterpene hydrocarbons accounted for the major volatile class in the two za’atar varieties (31.7%). The complete list of identified volatiles in dukkah and za’atar are presented in Table 1.
Table 1.
Relative percentage of volatile components in Egyptian dukkah and Syrian za’atar analyzed using headspace SPME-GCMS. Each presented value is a mean ± SD (n = 3).
| No. | RT (min) | Name | RI | EDT a | EDN b | SZT c | SZS d |
|---|---|---|---|---|---|---|---|
| 1 | 5.84 | 3-Hexen-1-ol | 807 | 0.01 ± 0.02 | 0.02 ± 0.01 | 0.02 ± 0.00 | 0.04 ± 0.01 |
| 2 | 8.01 | Linalool | 1083 | 0.32 ± 0.12 | 0.25 ± 0.12 | 0.17 ± 0.04 | 1.78 ± 0.32 |
| 3 | 8.78 | Borneol | 1162 | 0.00 ± 0.01 | 0.04 ± 0.02 | - | - |
| 4 | 8.96 | 4-Terpineol | 1170 | 0.73 ± 0.09 | 0.34 ± 0.08 | 0.17 ± 0.00 | 0.07 ± 0.01 |
| 5 | 9.03 | p-Cymene-8-ol | 1175 | 0.24 ± 0.05 | 0.24 ± 0.03 | 0.03 ± 0.01 | 0.07 ± 0.03 |
| Alcohols | Total | 1.30 | 0.89 | 0.40 | 1.97 | ||
| 6 | 4.17 | Hexanal | 734 | 0.04 ± 0.02 | 0.02 ± 0.01 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| 7 | 6.70 | Benzaldehyde | 937 | 0.75 ± 0.04 | 1.43 ± 0.50 | 0.93 ± 0.03 | 0.49 ± 0.04 |
| 8 | 7.24 | n-Octanal | 984 | 0.42 ± 0.15 | 0.74 ± 0.16 | 1.98 ± 0.11 | 1.67 ± 0.29 |
| 9 | 8.09 | Nonanal | 1091 | 0.02 ± 0.00 | 0.05 ± 0.01 | - | 0.02 ± 0.00 |
| 10 | 9.59 | Cumin aldehyde | 1234 | 1.94 ± 0.36 | 1.04 ± 0.16 | 0.04 ± 0.03 | 0.54 ± 0.15 |
| 11 | 9.73 | p-Anisaldehyde | 1247 | 0.62 ± 0.15 | 0.23 ± 0.06 | 1.63 ± 0.11 | 7.51 ± 2.12 |
| Aldehydes | Total | 3.79 | 3.50 | 4.59 | 10.24 | ||
| 12 | 6.48 | Styrene | 853 | 0.18 ± 0.14 | 0.36 ± 0.11 | 0.20 ± 0.12 | 0.46 ± 0.10 |
| 13 | 7.27 | β-Cymene | 1003 | 0.02 ± 0.01 | 0.02 ± 0.02 | 0.05 ± 0.01 | 0.06 ± 0.03 |
| 14 | 7.99 | p-Dimethylstyrene | 1080 | 0.42 ± 0.16 | 0.22 ± 0.01 | 0.11 ± 0.00 | 0.22 ± 0.05 |
| 15 | 11.81 | α-Curcumene | 1461 | 0.85 ± 0.36 | 0.56 ± 0.10 | 0.20 ± 0.05 | 0.13 ± 0.04 |
| Aromatics | Total | 1.46 | 1.17 | 0.56 | 0.88 | ||
| 16 | 6.87 | n-Caproic acid | 955 | 0.72 ± 0.11 | 1.66 ± 0.09 | 0.43 ± 0.04 | 0.34 ± 0.03 |
| 17 | 8.74 | n-Caprylic acid | 1149 | 0.11 ± 0.04 | 0.21 ± 0.02 | 0.06 ± 0.01 | 0.04 ± 0.01 |
| Carboxylic acids | Total | 0.83 | 1.87 | 0.50 | 0.37 | ||
| 18 | 9.49 | Fenchyl acetate | 1226 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.02 | 0.01 ± 0.00 |
| 19 | 10.60 | Terpinyl acetate | 1339 | 0.24 ± 0.08 | 1.96 ± 0.25 | 0.01 ± 0.01 | 0.13 ± 0.03 |
| 20 | 10.84 | Geranyl acetate | 1364 | 0.19 ± 0.07 | 1.09 ± 0.00 | 0.01 ± 0.01 | 0.11 ± 0.02 |
| 21 | 11.36 | Ethyl linoleate | 1453 | 0.32 ± 0.06 | 0.16 ± 0.03 | 0.11 ± 0.01 | 0.03 ± 0.01 |
| 22 | 11.79 | Methyl linolelaidate | 1459 | 0.20 ± 0.09 | 0.06 ± 0.09 | 0.06 ± 0.01 | 0.03 ± 0.00 |
| Esters | Total | 0.97 | 3.30 | 0.21 | 0.31 | ||
| 23 | 7.35 | Cineole | 1020 | 0.25 ± 0.09 | 0.60 ± 0.06 | 0.03 ± 0.02 | 0.21 ± 0.03 |
| 24 | 9.15 | Estragole | 1188 | 35.45 ± 1.17 c,d | 28.97 ± 4.26 c,d | 2.84 ± 0.97 a,b | 9.10 ± 2.45 a,b |
| 25 | 9.42 | O-Methylthymol | 1222 | 0.01 ± 0.00 | 0.51 ± 0.40 | - | - |
| 26 | 10.02 | Anethole | 1277 | 17.43 ± 1.99 | 7.51 ± 1.94 | 13.64 ± 0.10 | 25.40 ± 14.22 |
| Ethers | Total | 53.14 | 37.60 | 16.51 | 34.71 | ||
| 27 | 4.69 | Furfural | 777 | 0.01 ± 0.02 | 0.13 ± 0.10 | 0.54 ± 0.07 | 0.52 ± 0.03 |
| 28 | 6.71 | 5-Methyl-2-Furaldehyde | 942 | 1.62 ± 0.08 | 3.07 ± 0.42 | 1.67 ± 0.16 | 0.89 ± 0.04 |
| 29 | 7.64 | Furaneol | 1043 | 0.04 ± 0.01 | 0.07 ± 0.04 | 0.36 ± 0.08 | 0.12 ± 0.03 |
| 30 | 7.83 | 5-formylfurfural | 1065 | 0.37 ± 0.00 | 2.11 ± 1.13 | 0.12 ± 0.02 | 0.13 ± 0.03 |
| 31 | 9.38 | 5-Hydroxymethylfurfural | 1211 | 0.44 ± 0.18 | 4.59 ± 3.86 | 0.08 ± 0.04 | 0.12 ± 0.02 |
| Furans | Total | 2.48 | 9.96 | 2.76 | 1.78 | ||
| 32 | 8.00 | Fenchone | 1082 | 0.03 ± 0.02 | 0.01 ± 0.01 | 0.01 ± 0.00 | 0.17 ± 0.01 |
| 33 | 8.19 | Maltol | 1103 | 0.38 ± 0.06 | 0.83 ± 0.42 | 0.03 ± 0.01 | 0.20 ± 0.01 |
| 34 | 8.64 | Camphor | 1141 | 0.47 ± 0.00 | 1.24 ± 0.17 | 0.02 ± 0.00 | 0.26 ± 0.04 |
| 35 | 9.16 | Dihydrocarvone | 1197 | 0.62 ± 0.06 | 0.48 ± 0.16 | 0.03 ± 0.02 | 0.19 ± 0.08 |
| 36 | 9.63 | Carvone | 1237 | 12.09 ± 3.30 c,d | 14.83 ± 5.58 c,d | 1.06 ± 0.30 a,b,d | 4.77 ± 1.15 a,b,c |
| Ketones | Total | 13.58 | 17.38 | 1.16 | 5.58 | ||
| 37 | 6.52 | α-Thujene | 906 | 0.10 ± 0.00 | 0.43 ± 0.35 | 0.41 ± 0.37 | 0.99 ± 0.33 |
| 38 | 6.69 | Camphene | 925 | 0.04 ± 0.04 | 0.25 ± 0.35 | 0.07 ± 0.02 | 0.05 ± 0.04 |
| 39 | 6.93 | β-Myrcene | 961 | 6.95 ± 1.89 c,d | 7.62 ± 0.57 c,d | 19.49 ± 1.01 a,b | 16.15 ± 2.79 a,b |
| 40 | 7.23 | β-Pinene | 971 | 1.89 ± 0.47 | 2.08 ± 0.17 | 5.44 ± 0.27 | 4.54 ± 0.78 |
| 41 | 7.26 | α-Terpinene | 1002 | 0.21 ± 0.06 | 0.23 ± 0.02 | 0.60 ± 0.03 | 0.51 ± 0.08 |
| 42 | 7.31 | D-Limonene | 1016 | 6.44 ± 0.76 d | 6.06 ± 1.65 d | 5.55 ± 0.56 d | 9.06 ± 0.71 a,b,c |
| 43 | 7.35 | β-cis-Ocimene | 1027 | 0.18 ± 0.02 | 0.49 ± 0.00 | 0.04 ± 0.00 | 0.15 ± 0.02 |
| 44 | 7.45 | β-Ocimene | 1033 | 0.16 ± 0.04 | 0.37 ± 0.04 | 0.04 ± 0.00 | 0.17 ± 0.05 |
| 45 | 7.58 | γ-Terpinene | 1042 | 0.24 ± 0.11 | 0.27 ± 0.06 | 0.15 ± 0.02 | 0.06 ± 0.06 |
| 46 | 8.45 | Neo-allo-ocimene | 1117 | 0.03 ± 0.02 | 0.04 ± 0.02 | 0.01 ± 0.00 | 0.02 ± 0.00 |
| Monoterpene hydrocarbons | Total | 16.23 | 17.84 | 31.78 | 31.70 | ||
| 47 | 4.68 | 2-Methylpyrazine | 763 | 0.05 ± 0.08 | 0.09 ± 0.08 | 0.05 ± 0.03 | 0.03 ± 0.00 |
| 48 | 6.49 | 2,5-Dimethylpyrazine | 876 | 0.06 ± 0.02 | 0.48 ± 0.23 | 0.47 ± 0.18 | 0.71 ± 0.46 |
| 49 | 7.70 | α-Aminoxypropionic acid | 1056 | 0.04 ± 0.06 | 0.07 ± 0.08 | 0.08 ± 0.00 | 0.03 ± 0.03 |
| Nitrogen-containing compounds | Total | 0.16 | 0.64 | 0.60 | 0.77 | ||
| 50 | 7.82 | Linalool oxide | 1062 | 0.22 ± 0.01 | 1.26 ± 0.68 | 0.07 ± 0.01 | 0.10 ± 0.01 |
| Oxides | Total | 0.22 | 1.26 | 0.07 | 0.10 | ||
| 51 | 10.10 | Carvacrol | 1287 | 3.91 ± 1.18 c | 2.67 ± 0.53 c | 37.43 ± 4.33 a,b,d | 8.84 ± 1.76 c |
| Phenols | Total | 3.91 | 2.67 | 37.43 | 8.84 | ||
| 52 | 8.57 | Pyranone | 1133 | 0.02 ± 0.00 | 0.31 ± 0.25 | 0.01 ± 0.00 | 0.02 ± 0.01 |
| Pyrans | Total | 0.02 | 0.31 | 0.01 | 0.02 | ||
| 53 | 7.25 | Pyrrole-2-aldehyde | 991 | 0.61 ± 0.16 | 0.63 ± 0.30 | 2.94 ± 0.13 | 2.49 ± 0.44 |
| 54 | 8.24 | 2-Formyl-1-methylpyrrole | 1110 | 0.03 ± 0.02 | 0.18 ± 0.04 | 0.08 ± 0.01 | 0.10 ± 0.03 |
| Pyrroles | Total | 0.65 | 0.81 | 3.02 | 2.58 | ||
| 55 | 10.53 | δ-EIemene | 1332 | 0.03 ± 0.01 | 0.11 ± 0.02 | 0.02 ± 0.00 | 0.02 ± 0.00 |
| 56 | 10.98 | Bourbonene | 1383 | 0.13 ± 0.05 | 0.18 ± 0.03 | 0.02 ± 0.01 | 0.04 ± 0.00 |
| 57 | 11.33 | β-Caryophyllene | 1416 | 1.03 ± 0.21 | 0.49 ± 0.11 | 0.36 ± 0.03 | 0.11 ± 0.02 |
| 58 | 11.34 | Himachalene | 1443 | 0.06 ± 0.02 | 0.02 ± 0.01 | 0.02 ± 0.00 | - |
| Sesquiterpene hydrocarbons | Total | 1.25 | 0.80 | 0.42 | 0.17 | ||
Different letters (a, b, c, d) indicate significant differences of the major characteristic constituents between groups.
Alcohols (0.40–1.97%): Five alcohols were detected, with linalool (1.78%) and 4-terpineol (0.73%) being the most abundant, particularly in sumac-based Syrian za’atar and thyme-based Egyptian dukkah, respectively. Linalool, previously detected in fennel [13], and different types of thyme [14] demonstrated significant antibacterial action against P. fluorescens [15]. Linalool and terpineol were effective against several tested organisms except Pseudomonas aeruginosa [16].
Aldehydes (3.5–10.24%): Aldehydes are commonly identified compounds in the aroma of thyme containing mixtures, and a significant aldehyde increase is observed in most volatiles after the addition of thyme [17]. Aldehydes and their derivatives demonstrated considerable antibacterial activity by mechanically destroying bacterial cell membranes [18]. Cuminaldehyde (0.04–1.94%), one of the major aldehydes in cumin [19], improved antibacterial and anti-biofilm properties in S. aureus and E. coli when used in conjunction with ciprofloxacin [20]. Anisaldehyde (0.23–7.51%) reported in sumac [21] synergistically improved nisin antimicrobial activity against several resistant food borne pathogens [22].
Esters (0.21–3.3%): Esters are considered to be one of the most important biologically active ingredients in different essential oils compositions [23]. Five esters were found in the four herbal mixes under investigation.
Ethers (16.51–53.14%): Anethole was one of the major identified ethers (7.51–25.40%) that was previously reported as a volatile oil component in thyme [13,24] and was detected as a major constituent in coriander [25]. Anethole-rich volatile oils exerted significant antioxidant and antimicrobial activities [26].
Ketones (1.16–17.38%): Ketones were most abundant in nut-based and thyme-based Egyptian dukkah and were represented by carvone (1.06–14.83%) as the major form. It exceeded 14% of the total volatiles in nut-based Egyptian dukkah. Carvone is the major component of the volatile oil of the family Lamiaceae (including thyme) [27] and was identified as a component of coriander volatile oil [28]. Carvone was proposed to be the most responsible for mentha antibacterial activity against various foodborne pathogenic bacteria [29].
A previous study on 12 fennel accessions demonstrated that anethole and estragole were the main volatiles present at various combinations reaching maximum abundance at 98.4% and 72.4%, respectively. Subsp. piperitum from Minia, Egypt had the highest average estragole exposure at ca. 35 mg/kg body weight for 5 g fennel consumption per day, warranting cautious use in sensitive groups, i.e., nursing mothers and infants [13]. However, our findings indicated much lower estragole level (2.84–35.45%), suggestive of the safe daily use of dukkah and za’atar for prolonged periods. Anethole was also reported as the major volatile in coriander fruits at 85.47% [30]. Moreover, linalool was detected as the main volatile in mature coriander fruits amounting to 87.54% [30]. Our results showed that linalool was detected in sumac-based Syrian za’atar (SZS) at 10-fold higher levels compared to thyme-based Syrian za’atar (SZT), indicative of higher ratios of coriander fruits incorporated in the former herbal blend. Hexanal (56.3%), nonanal (8.7%), and 3-hexen-1-ol (4.4%) were documented as major volatiles in chickpea [31]. Furthermore, hexanal (72.16%) and nonanal (3.24%) constituted the main volatiles in sesame seed [32]. Thus, the content of the aforementioned components in dukkah and za’atar were suggested for a low ratio of chick-pea and sesame incorporated in various herbal blends. Carvacrol (48.5%) and camphor (13.1%) were abundant volatiles previously detected in four thyme accessions [33]. Therefore, SZT encompassed a higher carvacrol content compared to SZS. Likewise, EDT contained more carvacrol compared to EDN. β-Cymene (7.7%), β-ocimene (7.5%), and limonene (7.3%) were reported as chief components in fresh sumac fruit [21], with SZS encompassing higher levels of the aforementioned components compared to SZT.
2.2. Phenolic Profile in Egyptian Dukkah and Syrian Za’atar (ug/g)
To complement the profile of volatiles using GC–MS, HPLC was further employed for phenolics profiling in these spice blends to account more for their health benefits and food preservative actions. HPLC revealed that Egyptian dukkah as well as Syrian za’atar were rich in phenolics (Table 2). The thyme-based Egyptian dukkah (EDT) showed the highest levels of identified phenolics such as rosmarinic acid (338.30 ug/g), ferulic acid (325.79 ug/g), and rutin (302.06 ug/g), respectively.
Table 2.
Phenolic compound contents (ug/g) of Egyptian dukkah and Syrian za’atar.
| Compound | Rt (min) | EDT | EDN | SZT | SZS | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Gallic acid | 4.1 | 0.9 D | 0.00 | 2.0 C | 0.03 | 9.6 B | 0.03 | 21.3 A | 0.09 |
| Protocatechuic acid | 7 | 13.6 A | 0.22 | 5.2 B | 0.10 | 5.2 B | 0.00 | 2.8 C | 0.06 |
| p-Hydroxybenzoic acid | 10.3 | 11.3 A | 0.24 | ND | ND | 1.9 C | 0.04 | 5.6 B | 0.05 |
| (Epi)Catechin | 11.9 | 14.7 A | 0.16 | ND | ND | 14.8 A | 0.04 | 3.9 B | 0.53 |
| Chlorogenic acid | 12.8 | 2.37 B | 0.04 | ND | ND | 1.3 C | 0.00 | 2.5 A | 0.02 |
| Caffeic acid | 13.9 | 194.4 A | 0.19 | 31.0 D | 0.07 | 89.4 B | 0.28 | 63.6 C | 1.61 |
| Syringic acid | 14.9 | 23.1 A | 0.14 | 0.3 C | 0.00 | 1.5 B | 0.02 | 1.4 B | 0.09 |
| Vanillic acid | 16.7 | 2.8 B | 0.01 | 0.6 D | 0.02 | 3.9 A | 0.07 | 1.2 C | 0.05 |
| Ferulic acid | 21 | 325.8 A | 0.23 | 18.9 D | 0.07 | 91.3 B | 0.14 | 31.7 C | 0.26 |
| Sinapic acid | 21.8 | 28.8 A | 0.11 | 2.6 D | 0.05 | 5.4 B | 0.01 | 3.8 C | 0.11 |
| Rutin | 24.4 | 302.1 A | 0.89 | 12.5 D | 0.59 | 68.6 B | 0.43 | 29.0 C | 0.13 |
| p-Coumaric acid | 27.1 | 2.4 C | 0.06 | 4.1 B | 0.11 | 2.7 C | 0.09 | 10.5 A | 0.32 |
| Apigenin-7-O-glucoside | 28.8 | 7.8 | 0.29 | ND | ND | ND | ND | ND | ND |
| Rosmarinic acid | 30.2 | 338.3 A | 1.62 | 22.6 D | 0.38 | 149.6 B | 0.06 | 34.7 C | 0.33 |
| Daidzein | 34 | 26.8 A | 0.22 | 1.4 C | 0.11 | ND | ND | 5.1 B | 0.13 |
| Cinnamic acid | 35.6 | 11.0 A | 0.03 | 1.1 C | 0.02 | 1.3 B | 0.02 | 0.3 D | 0.03 |
| Quercetin | 36.4 | 3.9 C | 0.00 | 4.4 B | 0.04 | 5.6 A | 0.17 | 5.7 A | 0.13 |
| Genistin | 39 | 9.7 A | 0.04 | 3.2 C | 0.08 | 4.9 B | 0.01 | ND | ND |
| Kaempferol | 40.8 | 2.8 A | 0.07 | ND | ND | 1.1 B | 0.06 | 1.1 B | 0.00 |
Means with the same letter (superscript) in raw are not significantly different at (p > 0.05). ND: not detected.
It was obvious that the quantities of these compounds in the various herbal combinations under study varied and accounted for differences in antioxidant activities. Catechin was found at significant levels in EDT and SZT (14.7 and 14.8 ug/g, respectively), but not in EDN. Catechin has very potent antioxidant effects through different mechanisms [34], which could be related to the high antioxidant activity of EDT and SZT using diverse assay techniques.
Caffeic and sinapic acids are two hydroxycinnamates found at substantially higher levels in EDT than in other samples (194.4 and 28.8 ug/g, respectively). They are among the most important hydroxycinnamic acids with strong antioxidant properties [35].
Chlorogenic acid is a polyphenol and an ester of caffeic acid and quinic acid found in the four samples under investigation. Chlorogenic acid and its isomers were shown to have substantial antioxidant and DNA-protective properties [36].
Ferulic acid was found in the four samples with much higher concentrations in EDT, followed by SZT (325.8 and 91.3 ug/g, respectively). Ferulic acid has low toxicity and possesses numerous biological activities (anti-inflammatory, antioxidant, antimicrobial activity, anticancer, and antidiabetic effects) [37].
Rutin, a flavonoid glycoside of quercetin, was detected at 302.1, 12.5, 68.6, and 29 ug/g in EDT, EDN, SZT, and SZS, respectively. Rutin exerts potential antioxidant, anti-inflammatory, and neuroprotective properties, implying a substantial role in the treatment of neurodegenerative and other disease conditions [38].
Apigenin-7-O-glucoside is a flavone O-glycoside that was detected only in EDT (7.76 ug/g).
Rosmarinic acid, a polyphenol (an ester of caffeic acid and 3-(3,4-dihydroxyphenyl) lactic acid), was found at high levels in both EDT and SZT (338.3 and 149.6 ug/respectively). It has potent antioxidant effects and showed significant cardioprotective activity in diabetic rats [39].
Daidzein and genistin are examples of isoflavonoids, with daidzein found in all samples except SZT, while genistin was detected in all blends except SZS.
2.3. Vapor-Phase Minimum Inhibitory Concentration (VP-MIC)
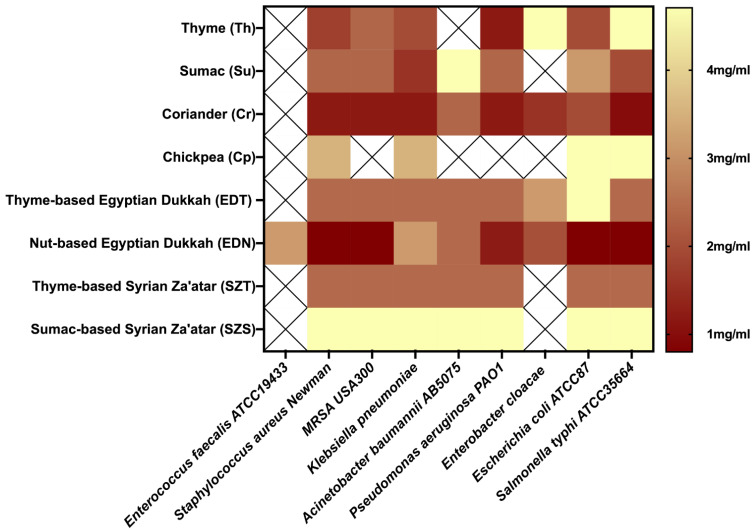
Natural antimicrobial compounds have a wide range of applications, particularly in food and biomedical applications [40]. Essential oils’ vapor phase antimicrobial potential has numerous applications in food preservation [41]. As a result, several research studies have been conducted to examine the components and antibacterial activities of essential oils in the vapor phase, owing to the advantages of VP antibacterial assays over direct-contact assays (diffusion or dilution methods) [42]. As an example, EO components are partitioned across the agar in diffusion assays, while in dilution methods, low water solubility must be overcome by the use of emulsifiers or solvents, which may change the action [42]. The vapor-phase of the four mixtures as well as their major components (thyme (Th), sumac (Su), coriander (Cr), chickpeas (Cp)) showed varied antibacterial activity against the highly virulent and resistant tested bacterial strains (Table 3/Figure 1). Interestingly, the vapor-phase of the tested mixtures recorded antibacterial activity against both Gram-positive and Gram-negative pathogens, justifying the use of these herbal blends in food preservation. The tested individuals showed differences in antimicrobial effects against the selected bacterial strains. Coriander was the most potent with VP-MIC ranging from 1.2 ± 0 to 2.4 ± 2 mg/mL (Table 3). Regarding the mixtures, EDN recorded the highest antibacterial activity with the least VP-MIC value against tested pathogens (0.8 ± 0.3 mg/mL) (Table 3; Figure 1). Mixture EDN was the only tested mixture with a vapor-phase that showed antibacterial activity against Enterococcus faecalis ATCC19433, with a VP-MIC value of 3.1 ± 1.4 mg/mL. It is worth mentioning that the antimicrobial potential of the EDN exceeded that of individuals against the tested bacterial strains, with the exception of coriander, which showed the lowest VP-MIC (1.2 ± 0 mg/mL) against Klebsiella pneumoniae (Table 3). The vapor-phase of mixture SZS recorded the lowest antibacterial activity, with no detected activity against Enterococcus faecalis ATCC19433 or Enterobacter cloacae. The essential oils of the four herbal blends had effective antibacterial effects in the vapor phase, which was attributed to volatile component dispersion. The presence of carvone [29], furfural, and furfural derivatives [43] could explain EDN’s potential antimicrobial activity. Together with estragole [44] and the other minor constituents, these components could be, at least in part, responsible for the antibacterial activities of EDN. While thyme and coriander have previously been reported to prevent foodborne diseases [45], the synergistic effect between them and the other components of the herbal blends shown in EDN and the other tested mixtures may be a viable option to improve their antimicrobial potential, suggesting the use of Egyptian dukkah and Syrian za’atar in food preservation.
Table 3.
Vapor-phase antibacterial activity of the tested herbal mixtures and their major individuals.
| Vapor-Phase Minimum Inhibitory Concentration (VP-MIC) mg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mix |
Enterococcus faecalis
ATCC19433 |
Staphylococcus aureus Newman | MRSA USA300 | Klebsiella pneumoniae ATCC13883 | Acinetobacter baumannii AB5075 | Pseudomonas aeruginosa PAO1 | Enterobacter cloacae | Escherichia coli ATCC87 |
Salmonella typhi ATCC
35664 |
| Th | * | 1.8 ± 1 | 2.4 ± 0 | 2 ± 0.7 | * | 1.2 ± 1 | 4.7 ± 0 | 2 ± 0.7 | 4.7 ± 0 |
| Su | * | 2.4 ± 0 | 2.4 ± 0 | 1.6 ± 0.7 | 4.7 ± 0 | 2.4 ± 2 | * | 3.1 ± 1.4 | 2 ± 0.7 |
| Cr | * | 1.2 ± 0 | 1.2 ± 0 | 1.2 ± 0 | 2.4 ± 2 | 1.2 ± 0 | 1.6 ± 0.7 | 2 ± 0.7 | 1 ± 0.3 |
| Cp | * | 3.5 ± 2 | * | 3.5 ± 2 | * | * | * | 4.7 ± 0 | 4.7 ± 0 |
| EDT | * | 2.4 ± 0 | 2.4 ± 0 | 2.4 ± 0 | 2.4 ± 0 | 2.4 ± 0 | 3.1 ± 1.4 | 4.7 ± 0 | 2.4 ± 0 |
| EDN | 3.1 ± 1.4 | 0.8 ± 0.3 | 0.8 ± 0.3 | 3.1 ± 1.4 | 2.4 ± 0 | 1.2 ± 0 | 2.0 ± 0.7 | 0.8 ± 0.3 | 0.8 ± 0.3 |
| SZT | * | 2.4 ± 0 | 2.4 ± 0 | 2.4 ± 0 | 2.4 ± 0 | 2.4 ± 0 | * | 2.4 ± 0 | 2.4 ± 0 |
| SZS | * | 4.7 ± 0 | 4.7 ± 0 | 4.7 ± 0 | 4.7 ± 0 | 4.7 ± 0 | * | 4.7 ± 0 | 4.7 ± 0 |
*—Indicates that there was no antibacterial activity detected within the tested concentrations.
Figure 1.
Heatmap showing the vapor-phase antibacterial activity of the tested herbal mixtures and their major individuals. The antibacterial activity is represented by means of vapor-phase minimum inhibitory concentration (VP-MIC). Dark red and yellow represent the lowest and highest VP-MIC (mg/mL), respectively. Symbol “X” means that there was no antibacterial activity detected within the tested concentrations.
2.4. Antioxidant Potential of Egyptian Dukkah and Syrian Za’atar
There are various in vitro chemical models to evaluate the antioxidant potential of the tested mixtures and that are likely to be mediated via phenolic compositions of these herbal blends. The results are shown in Table 4. Radical scavenging capabilities were revealed using DPPH, ABTS, and ORAC and expressed as Trolox equivalent (μM T eq/mg).
Table 4.
Antioxidant potential of the tested herbal mixtures.
| Tested Mixtures | DPPH Assay | ABTS Assay | ORAC Assay | FRAP Assay | Ferrozine Iron Metal Chelation Assay |
|---|---|---|---|---|---|
| μM T eq/mg | μM T eq/mg | μM T eq/mg | μM T eq/mg | μM EDTA eq/mg | |
| EDT | 59.93 ± 1.99 | 225.32 ± 6.15 | 1577.86 ± 50.14 | 145.26 ± 4.32 | 51.69 ± 5.33 |
| EDN | 26.92 ± 0.75 | 125.15 ± 5.92 | 1303.68 ± 92.02 | 59.89 ± 2.33 | 22.26 ± 2.38 |
| SZS | 86.79 ± 1.38 | 263.52 ± 9.7 | 1138.11 ± 83.03 | 144.81 ± 7.95 | 19.30 ± 0.52 |
| SZT | 104.10 ± 1.68 | 337.26 ± 13.84 | 1479.09 ± 88.19 | 212.75 ± 12.85 | 18.08 ± 1.62 |
The DPPH radical scavenging activities of herbal blend extracts varied from 59.93 to 104.10 μM T eq/mg (Table 4). All tested herbal mixtures exerted scavenging activity, indicating that the components of the herbal mixtures have proton donating ability as well as free radical scavenging ability. STZ showed the highest antioxidant capacity (104.10 μM T eq/mg), followed by SZS (86.79 μM T eq/mg), while EDN showed the lowest antioxidant capacity (26.92 μM T eq/mg). The high concentration of phenolic compounds in SZT (catechin, vanillic acid, rosmarinic acid, and quercetin) may account for its strong DPPH scavenging activity [46].
Regarding the ABTS assay, values varied from 125.15 to 337.26 μM T eq/mg (Table 4). SZT possessed the highest antioxidant capacity (337.26 μM T eq/mg) followed by EDT (225.32 μM T eq/mg). In accordance with the DPPH assay, EDN showed the lowest antioxidant capacity (125.15 μM T eq/mg). Catechin and rosmarinic acids are the major constituents in SZT, detected at 14.8 and 149.6 ug/g, respectively, suggesting that they play a significant role in the antioxidant activity in various assays [47,48].
ORAC values varied from 1138.11 to 1577.86 μM T eq/mg (Table 4). The herbal blend that showed the highest antioxidant capacity was EDT (1577.86 μM T eq/mg) followed by SZT (1479.09 μM T eq/mg). The ORAC assay also confirmed the presence of polyphenols in various blends with significant antioxidant activity [49]. EDT encompassed high levels of caffeic acid, ferulic acid, rutin, and rosmarinic acid (194.4, 325.8, 302.1, and 338.3 ug/g, respectively), demonstrating the strongest antioxidant effect and ROS scavenging activity [48,50]. In this assay, SZS (1138.11 μM T eq/mg) showed the lowest antioxidant potential compared to other tested herbal mixtures.
To confirm the antioxidant potential of herbal blends, ferric reducing antioxidant power (FRAP) was also performed considering its slightly different action mechanism targeting metal chelation and likely mediated via flavonoids found in abundance using HPLC analysis (Table 2). The results did not show much differences from DPPH and ABTS scavenging activities. Similar to the results attained for radical scavenging assays, SZT (212.75 μM T eq/mg) exhibited the highest ferric ion reducing potential versus the lowest, which was exhibited by EDN (59.89 μM T eq/mg).
The ferrozine iron metal chelation power assay was performed to determine the chelation ability of the four herbal blends. Unlike the other assays, the varieties of dukkah exhibited the highest capacities to chelate ferrous ions in comparison to za’atar varieties. EDT (51.69 μM EDTA eq/mg) showed the highest chelation capacity followed by EDN (22.26 μM EDTA eq/mg). The observed iron chelating activity of dukkah’s varieties may be explained by the chelating activity of nut components (hazelnuts, almonds, peanuts, and walnuts) [51]. The chelating activity is attributed to the fact that transition metal ions contribute to oxidative damage in neurodegenerative disorders such as Parkinson’s and Alzheimer’s [52].
It was concluded that Egyptian dukkah and Syrian za’atar are rich in polyphenolics, which accounts for their potential antioxidant and free radical scavenging activities as measured by various assays. The presence of other components in mixtures, as well as differences in the types of major phenolics among blends, resulted in different antioxidant activities. Polyphenolic compounds containing herbs have well-known antioxidant properties [46]. Major phenolics included phenolic acids (caffeic acid, sinapic acid, chlorogenic acid, ferulic acid, and rosmarinic acid), flavonoid aglycones (quercetin and kaempferol), flavonoid glycosides (rutin and apigenin-7-O-glucoside), and isoflavonoids (daidzein and genistin). The content of various phenolic components varied across types of Egyptian dukkah and Syrian za’atar, which may be the reason for differences in antioxidant responses depending on the mechanism of compounds present in each sample.
3. Material and Methods
3.1. Material
3.1.1. Herbal Blends Preparation
Two varieties of each herbal blend were selected for comparison. Except for the nut-based Egyptian dukkah (EDN), all of the tested blends were commercially available. The composition of each blend is listed below:
Thyme-based Egyptian dukkah (EDT): thyme, peanut, chickpea, wheat, and lemon salt.
Nut-based Egyptian dukkah (EDN): chickpea, coriander, sesame, mixture of nuts and apricot seeds, thyme (low percentage), and spices (cumin, salt). An equal proportion of chickpea, coriander, sesame, and a mixture of nuts and apricot seeds was utilized. The nut mixture itself was composed of equal proportions of hazelnuts, almonds, peanuts, walnuts, and apricot seeds. All ingredients were subjected to roasting at 60 °C for 1 h, with stirring before blending.
Thyme-based Syrian za’atar (SZT): sumac, thyme (high percentage), sesame, fennel, anise, coriander, chickpea (also called leblebi or qudamah), and spices (cumin, salt).
Sumac-based Syrian za’atar (SZS): sumac (high percentage), thyme, sesame, fennel, anise, coriander, chickpea (also called leblebi or qudamah), and spices (cumin, salt).
3.1.2. Bacterial Strains
Nine standard strains were tested: Enterococcus faecalis ATCC19433, Staphylococcus aureus Newman, Klebsiella pneumoniae ATCC13883, Methicillin-resistant Staphylococcus aureus (MRSA USA300), Pseudomonas aeruginosa PAO1, Acinetobacter baumannii AB5075, Escherichia coli ATCC87, Enterobacter cloacae, and Salmonella typhi ATCC35664 [53,54,55].
3.2. SPME Volatiles Analysis
Headspace volatiles analysis using SPME was adopted from Farag et al. (2021) [56], with a few modifications. Three grams of each sample were placed inside 20 mL clear glass vials. Vials were then immediately capped and placed on a temperature-controlled tray for 30 min at 50 °C with the SPME fiber coated with (DVB/CAR/PDMS, 50/30 μm) 203 divinylbenzene/carboxen/polydimethylsiloxane inserted into the headspace above the sample. A system blank containing no plant material was run as a control.
3.3. GCMS Analysis
SPME fiber was desorbed in the injection port of a Shimadzu Model GC-17A gas chromatograph interfaced with a Shimadzu Model QP-5000 mass spectrometer for 1 min at 210 °C (Kyoto, Japan). A DB5-MS column was used to separate volatiles (J&W Scientific, Santa Clara, CA, USA). For 30 s, injections were performed in the splitless mode. The gas chromatograph was used in the manner described by Farag et al. (2021) [56]. At 70 eV, the HP quadrupole mass spectrometer was set to electron ionization mode. The scanning speed was set to 40–500 m/z. Peaks were deconvoluted using AMDIS software (www.amdis.net; accessed on 12 July 2022) and identified by their retention indices (RI) relative to n-alkanes (C6–C20), mass spectra matching to NIST, the WILEY library database, and authentic standards when possible [57]. For quantification, the relative percentile based on peak area was used as previously reported by Farag et al. (2022) [58].
3.4. Determination of Phenolic Compounds Using HPLC-DAD
After placing the sample (0.5 g) in a quick fit conical flask, 20 mL of 2 M NaOH was added, the flasks were flushed with N2, and the stopper was replaced. The samples were stored at room temperature for 4 h. With 6 M HCl, the pH was adjusted to 2. The samples were centrifuged for 10 min at 5000 rpm, and the supernatant was collected. Phenolic compounds were extracted twice with 50 mL of a 1:1 mixture of diethyl ether and ethyl acetate. The organic phase was separated, collected, and evaporated under vacuum at 40 °C using a rotary evaporator before being reconstituted in 2 mL of pure methanol.
A liquid chromatography model 1100 series instrument (Agilent Technologies, CA, USA) equipped with an auto sampler, quaternary pump, and diode-array detector was used for HPLC analysis and phenolics profiling. The phenolic compounds were separated using an Eclipse XDB-C18 analytical column (4.6 × 150 mm; 5 µm) with a Zorbax C18 guard column (4.6 × 12.5 mm; 5 µm). The mobile phase was made up of acetonitrile (solvent A) and 2% acetic acid in water (v/v) (solvent B). The flow rate was kept at 1 mL/min for a total run time of 65 min, and the gradient program was as follows: 100% B to 87% B in 15 min, 87% B to 85% B in 5 min, 85% B to 78% B in 10 min, 78% B to 60% B in 10 min, 60% B to 40% B in 15 min, 40% B to 0% B in 5 min, and back to initial percent of B in 5 min. The injection volume was 50 µL, and peaks for the benzoic acid and cinnamic acid derivatives, as well as the flavonid, were monitored simultaneously at 280, 320, and 360 nm, respectively. Before injection, all samples were filtered through a 0.45 m syringe filter. Targeted peaks were identified and quantified by matching them with standard materials.
3.5. Vapor-Phase Antibacterial Activity of the Tested Mixtures
A group of Gram-negative and Gram-positive highly virulent and multidrug resistant bacterial pathogens was selected to be used as model organisms in testing the vapor-phase antibacterial activity of the studied mixtures. The selected pathogens are members of the "ESKAPE pathogens" that are attracting research attention, being the top leading causes of life-threatening infections [53].
Vapor-Phase Minimum Inhibitory Concentration (VP-MIC)
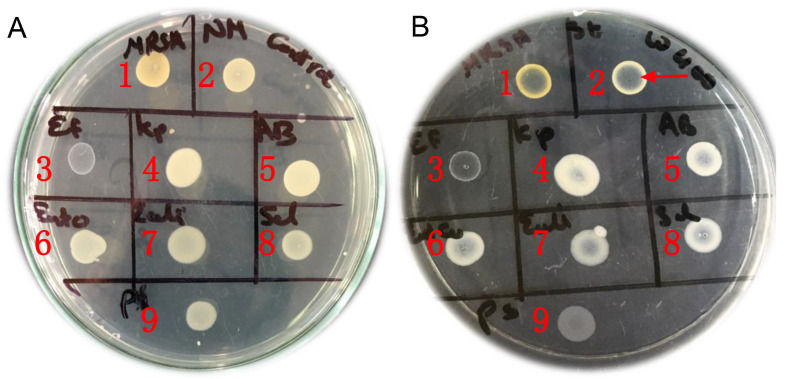
The vapor-phase antibacterial activity of the tested mixtures was investigated by determination of the vapor-phase minimum inhibitory concentration (VP-MIC) adopting the principles and approaches described before [12,59]. The previously established methods for the determination of VP-MIC used either the disc volatilization assay or the airtight box assay [12,59]. The disc volatilization assay was not applicable to our samples since we were dealing with mixtures of powders/solid particles instead of dealing with essential oils (liquid samples). The airtight box assay was thus found more convenient with our samples but with the drawback of consuming a large number of materials (culture media, inoculum, samples, etc.). The previously established method was thus slightly modified to develop a cost-efficient method that could test a large number of microorganisms using the least amount of samples using the conventional petri dish and without the need for any specialized tools. The VP-MIC of the tested mixtures was determined as follows: 15 mL of sterile Mueller–Hinton agar (MHA) was pipetted in a 10 cm diameter glass petri dish. The solidified MHA surface was then surface inoculated by spotting 10 μL of the tested bacterial suspension (106 CFU/mL). Since nine microorganisms were tested in each assay, 10 μL of each bacterial inoculum was spotted side by side in the same petri dish, as shown in Figure 2. The tested mixture was then placed on the cover of the petri dish, and the plate was kept inverted so that the inoculated agar was upward and the cover with the tested mixture was downward. Later, the petri dish was sealed by parafilm and incubated at 37 °C for 24 h. The control plate was prepared in the same way but keeping the cover of the petri dish empty without adding any samples. For each mixture, several plates were prepared with different concentrations (4.7, 2.4, 1.8, 0.6 mg/mL). The concentration of the tested mixture was calculated by dividing the weight (mg) of the mixture (placed on the cover of the petri dish) by the volume (mL) of the airspace in the petri dish. After incubation, the bacterial growth in the sample (mixture) plates and control plate was compared, and the VP-MIC was determined. The VP-MIC was identified as the least concentration of the tested mixture that caused apparent growth suppression of the tested microorganism when compared to the control (Figure 2). The assay was repeated at least three independent times, and the VP-MIC was reported as mean ± SD (standard deviation).
Figure 2.
Vapor-phase minimum inhibitory concentration assay (VP-MIC). (A) Photo of the control plate showing the bacterial growth of the nine selected microorganisms. (B) Photo of the sample plate showing inhibition of bacterial growth. The numbers 1–9 on photos (A) and (B) correspond to the tested microorganisms as follows: 1. Methicillin-resistant Staphylococcus aureus (MRSA USA300), 2. Staphylococcus aureus Newman, 3. Enterococcus faecalis ATCC19433, 4. Klebsiella pneumoniae ATCC13883, 5. Acinetobacter baumannii AB5075, 6. Enterobacter cloacae, 7. Escherichia coli ATCC87, 8. Salmonella typhi ATCC35664, and 9. Pseudomonas aeruginosa PAO1. The arrow in photo B2 shows growth suppression of the tested microorganism. The VP-MIC is identified as the least concentration of the tested mixture that causes growth suppression of the tested microorganism when compared to the control.
3.6. Antioxidant Potential of the Tested Herbal Mixtures
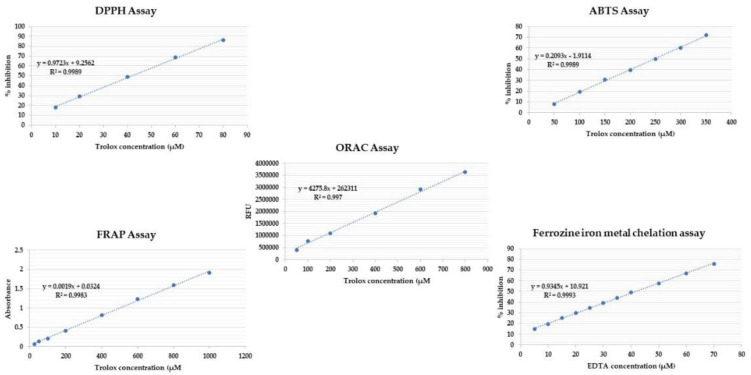
Several in vitro antioxidant assays were conducted on the crude methanolic extract of each blend and expressed as Trolox and EDTA. The calibration curve for the standard of each assay is shown in Figure 3.
Figure 3.
Standard calibration curves of the antioxidant assays expressed as Trolox and EDTA.
3.6.1. DPPH Free Radical Scavenging Activity
Scavenging of free radicals such as DPPH is the most employed antioxidant assay to determine the antioxidant capacity of the plant extracts [60]. Except for sample EDN, which was prepared at a concentration of 1 mg/mL, all extracts were prepared in 100% methanol at a concentration of 0.5 mg/mL. In methanol, a stock solution of 100 μM Trolox was obtained, from which 7 concentrations of 80, 60, 40, 20, and 10 μM were prepared (Figure 3). The DPPH (2,2-diphenyl-1-picryl-hydrazyl-hydrate) free radical assay was conducted using the method described by Boly [61]. In brief, 100 μL of freshly prepared DPPH reagent (0.1 percent in methanol) was mixed with 100 μL of sample in a 96-well plate (n = 6), and the reaction was incubated at room temperature for 30 min in the dark. The reduction in DPPH color intensity was measured at 540 nm at the end of the incubation time. Data are represented as means ± SD according to the following equation: percentage inhibition = ((average absorbance of blank − average absorbance of the test)/(average absorbance of blank)) × 100. The results were recorded using a FluoStar Omega microplate reader (BMG LABTECH, Berlin, Germany).
3.6.2. ABTS Assay
The ABTS assay, also known as the Trolox equivalent antioxidant capacity (TEAC) assay, compares the ability of antioxidants to scavenge ABTS in the aqueous phase to a Trolox standard [62]. The extracts were prepared at the concentration of 0.5 mg/mL in methanol, except for sample EDN, which was prepared at the concentration of 1 mg/mL. A Trolox stock solution of 1 mM in methanol was prepared, and 5 serial dilutions were prepared in the concentrations of 400, 350, 300, 250, 200, 150, 100, and 50 μM (Figure 3). The assay was carried out according to the method of Arnao [63], with minor modifications carried out in the microplates. Briefly, 192 mg of ABTS was dissolved in distilled water and transferred to a 50 mL volumetric flask, and then the volume was completed with distilled water. Then 1 mL of the previous solution was added to 17 μL of 140 mM potassium persulphate, and the mixture was left in the dark for 24 h. After that, 1 mL of the reaction mixture was completed to 50 mL with methanol to obtain the final ABTS dilution used in the assay. Then 190 μL of the freshly prepared ABTS reagent was mixed with 10 μL of the sample/compound in 96-well plates (n = 6), and the reaction was incubated at room temp. for 30 min in dark. At the end of the incubation time, the decrease in ABTS color intensity was measured at 734 nm. Data were represented as means ± SD according to the following equation: percentage inhibition = ((average absorbance of blank − average absorbance of the test)/(average absorbance of blank)) × 100.
3.6.3. Oxygen Radical Absorbance Capacity (ORAC) Assay
ORAC provides a tool for preliminary evaluation and screening of antioxidant potential of herbal extracts [64]. The extracts were prepared at the concentration of 0.4 mg/mL in methanol. A Trolox stock solution of 1 mM in MeOH was prepared, and 6 serial dilutions were prepared in the concentrations of 800, 600, 400,200, 100 and 50 μM (Figure 3). The assay was carried out according to the method of Liang [65], with modifications; briefly, 10 μL of the prepared sample(s) was incubated with 30 μL fluorescein (100 nM) for 10 min at 37 °C. Fluorescence measurement (485 EX, 520 EM, nm) was carried out for three cycles (cycle time, 90 s) for background measurement. Afterward, 70 μL of freshly prepared 2,2′-azobis(2-amidinopropane) dihydrochloride (AAPH) (300 mM) was added immediately to each well. Fluorescence measurement (485 EX, 520 EM, nm) was continued for 60 min (40 cycles, each 90 sec). Data were represented as means (n = 3) ± SD, and the antioxidant effect of the compound/extract was calculated as μM Trolox equivalents by substitution in the linear regression equation y = 4275.8x + 262311. The results were recorded using a FluoStar Omega microplate reader (BMG LABTECH, Germany).
3.6.4. The Ferric Reducing Ability of Plasma (FRAP Assay)
The ferric reducing antioxidant power (FRAP) assay is an antioxidant capacity assay that measures antioxidants’ ability to reduce ferric ion (Fe3+)–ligand complexes to ferrous (Fe2+) complexes in an acidic medium [66] and provide a reliable tool to measure the combined activity of redox-active antioxidants [67]. In methanol, extracts were prepared at a concentration of 5 mg/mL. A Trolox stock solution in methanol was prepared at a concentration of 2 mM, and the following dilutions were prepared: 1000, 800, 600, 400, 200, 100, 50, and 25 M (Figure 3). The assay was carried out using Benzie’s method [68], with minor modifications to be carried out in microplates. A freshly prepared TPTZ reagent (300 mM acetate buffer (pH = 3.6), 10 mM TPTZ in 40 mM HCl, and 20 mMFeCl3, respectively, in a ratio of 10:1:1 v/v/v) was used. In a 96-well plate (n = 3), 190 uL of freshly prepared TPTZ reagent were mixed with 10 uL of sample, and the reaction was incubated at room temperature for 30 min in the dark. At the end of the incubation period, the resulting blue colored sample was measured at 593 nm. The ferric reducing ability of the samples was presented as μM TE/mg sample using the linear regression equation extracted from the linear dose–response curve of Trolox: y = 0.0014x + 0.1639. Data are presented as means ± SD recorded using a FluoStar Omega microplate reader (BMG LABTECH, Germany).
3.6.5. Ferrozine Iron Metal Chelation Assay
The transition metal ion Fe2+ has the ability to sustain the formation of free radicals through electron gain or loss. As a result, the chelation of metal ions with chelating agents can reduce the formation of reactive oxygen species [69]. Sample EDT was prepared at the concentration of 0.5 mg/mL in methanol, while the other samples were prepared at 2.5 mg/mL. An EDTA stock solution of 0.1 mM was prepared in water, and 11 serial dilutions were prepared in the concentrations of 5, 10,15, 20, 25, 30, 35, 40, 50, 60, and 70 μM (Figure 3). The assay was carried out according to the method of Santos [70], with minor modifications to be carried out in microplates; briefly, 20 μL of the freshly prepared ferrous sulphate (0.3 mM) were mixed with 50 μL of the sample/compound in 96-well plates (n = 6). Afterwards, 30 μL of ferrozine (0.8 mM) was added to each well. The reaction mixture was incubated at room temperature for 10 min. At the end of incubation time, the decrease in the produced color intensity was measured at 562 nm. Data were represented as means ± SD according to the following equation: (1) percentage inhibition = ((average absorbance of blank − average absorbance of the test)/(average absorbance of blank)) × 100. The results were recorded using a FluoStar Omega microplate reader (BMG LABTECH, Germany).
4. Conclusions
The aroma profiles, phenolic compounds, and antibacterial and antioxidant effects of Egyptian dukkah and Syrian za’atar were evaluated in this study. Attempts to characterize essential oil compositions of the herbal blends revealed the abundance of ethers and monoterpene hydrocarbons in dukkah and za’atar, respectively. Alcohols, aldehydes, aromatics, carboxylic acids, esters, furans, ketones, nitrogen-containing compounds, oxides, phenols, pyrans, pyrroles, and sesquiterpene hydrocarbons were also detected. HPLC further revealed that both dukkah and za’atar were rich in phenolics, with rosmarinic and ferulic acids as the most abundant, especially in the thyme-based Egyptian dukkah (EDT). Regarding the vapor-phase of the four herbal blends, blends showed variable antibacterial activity, with nut-based Egyptian dukkah (EDN) recording the highest antibacterial activity against the tested pathogens, and sumac-based Syrian za’atar (SZS) recording the lowest antibacterial activity. The in vitro assays affirmed the antioxidant potential of the four herbal blends. Thyme-based za’atar (SZT) showed the highest DPPH and ABTS scavenging activities as well as the highest ferric ion reducing potential, while thyme-based Egyptian dukkah (EDT) showed the highest ORAC capacity followed by the highest chelation capacity. The presence of thyme may explain za’atar’s enhanced free radical scavenging antioxidant activities, while the presence of nuts may explain the chelation activity as well as the potentiation of dukkah’s antimicrobial activities. Further studies are needed to provide the complete chemical characterization of Egyptian dukkah and Syrian za’atar, as well as to demonstrate the beneficial effects of the two herbal blends as typical examples for plant-based diets. Further research is also required to investigate the herbal blends for other potential biological activities and to examine how much a synergized effect is observed compared to herbal blends of individual components regarding other effects as revealed from antimicrobial assays.
Acknowledgments
The authors would like to extend their sincere appreciation to Taif University Researchers Supporting Project number (TURSP-2020/56), Taif University, Taif, Saudi Arabia. Additionally, the authors would like to thank the Deanship of Scientific Research at Umm Al-Qua University for supporting this work by grant code (22UQU4290565DSR89). Mohamed A. Farag would like to thank the Alexander von Humboldt foundation, Germany, for financial support. Authors are grateful to Mohamed Reda for assistance in running GCMS analysis at Global institute of health, American University in Cairo, Egypt.
Author Contributions
M.A.F. and M.K.M. conceptualized the study; M.S.S. and S.M.A. prepared the headspace volatiles using SPME and performed the GC/MS analysis; S.M.A. identified the metabolites; M.A.F. revised the metabolites identification; F.M.M. prepared the samples and performed the HPLC analysis; M.H. tested the antimicrobial activity and wrote her part; M.S.S. prepared the extracts for the antioxidant assays; M.S.S. and M.K.M. managed the resources and prepared the EDN; M.K.M. and M.S.S. wrote the first and the final drafts; M.A.F., supervised the work and revised the final manuscript; I.A.N. and M.A.S.A. funded the work and were responsible for co-writing and revision of the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
Funding Statement
This project received funding from Deanship of Scientific Research at Umm Al-Qua University, Saudi Arabia, for supporting this work by grant code (22UQU4290565DSR89), and Taif University Researchers Supporting Project number (TURSP-2020/56), Taif University, Taif, Saudi Arabia.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fehér A., Gazdecki M., Véha M., Szakály M., Szakály Z. A Comprehensive Review of the Benefits of and the Barriers to the Switch to a Plant-Based Diet. Sustainability. 2020;12:4136. doi: 10.3390/su12104136. [DOI] [Google Scholar]
- 2.Tuso P., Stoll S.R., Li W.W. A plant-based diet, atherogenesis, and coronary artery disease prevention. Perm. J. 2015;19:62. doi: 10.7812/TPP/14-036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McMacken M., Shah S. A plant-based diet for the prevention and treatment of type 2 diabetes. J. Geriatr. Cardiol. 2017;14:342. doi: 10.11909/j.issn.1671-5411.2017.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ostfeld R.J. Definition of a plant-based diet and overview of this special issue. J. Geriatr. Cardiol. 2017;14:315. doi: 10.11909/j.issn.1671-5411.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hemler E.C., Hu F.B. Plant-based diets for personal, population, and planetary health. Adv. Nutr. 2019;10:S275–S283. doi: 10.1093/advances/nmy117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khalil M., Caponio G.R., Diab F., Shanmugam H., Di Ciaula A., Khalifeh H., Vergani L., Calasso M., De Angelis M., Portincasa P. Unraveling the beneficial effects of herbal Lebanese mixture “Za’atar”. History, studies, and properties of a potential healthy food ingredient. J. Funct. Foods. 2022;90:104993. doi: 10.1016/j.jff.2022.104993. [DOI] [Google Scholar]
- 7.Mohamed R.S., Abdel-Salam A.M. Efficiency of a formulated condiment (duqqa) in mitigation of diabetes and its complications induced by streptozotocin-nicotinamide in rats. J. Herbmed Pharmacol. 2021;10:218–225. doi: 10.34172/jhp.2021.24. [DOI] [Google Scholar]
- 8.Goñi P., López P., Sánchez C., Gómez-Lus R., Becerril R., Nerín C. Antimicrobial activity in the vapour phase of a combination of cinnamon and clove essential oils. Food Chem. 2009;116:982–989. doi: 10.1016/j.foodchem.2009.03.058. [DOI] [Google Scholar]
- 9.Tzima K., Makris D., Nikiforidis C.V., Mourtzinos I. Potential use of rosemary, propolis and thyme as natural food preservatives. J. Nutr. Health. 2015;1:6. [Google Scholar]
- 10.Fazeli M.R., Amin G., Attari M.M.A., Ashtiani H., Jamalifar H., Samadi N. Antimicrobial activities of Iranian sumac and avishan-e shirazi (Zataria multiflora) against some food-borne bacteria. Food Control. 2007;18:646–649. doi: 10.1016/j.foodcont.2006.03.002. [DOI] [Google Scholar]
- 11.Silva F., Domingues F.C. Antimicrobial activity of coriander oil and its effectiveness as food preservative. Crit. Rev. Food Sci. Nutr. 2017;57:35–47. doi: 10.1080/10408398.2013.847818. [DOI] [PubMed] [Google Scholar]
- 12.Laird K., Phillips C. Vapour phase: A potential future use for essential oils as antimicrobials? Lett. Appl. Microbiol. 2012;54:169–174. doi: 10.1111/j.1472-765X.2011.03190.x. [DOI] [PubMed] [Google Scholar]
- 13.Afifi S.M., El-Mahis A., Heiss A.G., Farag M.A. Gas chromatography–mass spectrometry-based classification of 12 fennel (Foeniculum vulgare Miller) varieties based on their aroma profiles and estragole levels as analyzed using chemometric tools. ACS Omega. 2021;6:5775–5785. doi: 10.1021/acsomega.0c06188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horváth G., Horváth A., Reichert G., Böszörményi A., Sipos K., Pandur E. Three chemotypes of thyme (Thymus vulgaris L.) essential oil and their main compounds affect differently the IL-6 and TNFα cytokine secretions of BV-2 microglia by modulating the NF-κB and C/EBPβ signalling pathways. BMC Complement. Med. Ther. 2021;21:148. doi: 10.1186/s12906-021-03319-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo F., Chen Q., Liang Q., Zhang M., Chen W., Chen H., Yun Y., Zhong Q., Chen W. Antimicrobial activity and proposed action mechanism of linalool against Pseudomonas fluorescens. Front. Microbiol. 2021;12:562094. doi: 10.3389/fmicb.2021.562094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carson C.F., Riley T.V. Antimicrobial activity of the major components of the essential oil of Melaleuca alternifolia. J. Appl. Bacteriol. 1995;78:264–269. doi: 10.1111/j.1365-2672.1995.tb05025.x. [DOI] [PubMed] [Google Scholar]
- 17.Qi S., Zhan P., Tian H., Wang P., Ma X., Li K. Effects of thyme (Thymus vulgaris L.) addition on the volatile compounds of mutton broth during boiling. Food Sci. Hum. Wellness. 2022;11:305–315. doi: 10.1016/j.fshw.2021.11.025. [DOI] [Google Scholar]
- 18.Aljaafari M.N., Alkhoori M.A., Hag-Ali M., Cheng W.-H., Lim S.-H.-E., Loh J.-Y., Lai K.-S. Contribution of Aldehydes and Their Derivatives to Antimicrobial and Immunomodulatory Activities. Molecules. 2022;27:3589. doi: 10.3390/molecules27113589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morshedi D., Aliakbari F., Tayaranian-Marvian A., Fassihi A., Pan-Montojo F., Pérez-Sánchez H. Cuminaldehyde as the major component of Cuminum cyminum, a natural aldehyde with inhibitory effect on alpha-synuclein fibrillation and cytotoxicity. J. Food Sci. 2015;80:H2336–H2345. doi: 10.1111/1750-3841.13016. [DOI] [PubMed] [Google Scholar]
- 20.Monteiro-Neto V., de Souza C.D., Gonzaga L.F., da Silveira B.C., Sousa N.C.F., Pontes J.P., Santos D.M., Martins W.C., Pessoa J.F.V., Carvalho Junior A.R. Cuminaldehyde potentiates the antimicrobial actions of ciprofloxacin against Staphylococcus aureus and Escherichia coli. PLoS ONE. 2020;15:e0232987. doi: 10.1371/journal.pone.0232987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farag M.A., Fayek N.M., Abou Reidah I. Volatile profiling in Rhus coriaria fruit (sumac) from three different geographical origins and upon roasting as analyzed via solid-phase microextraction. PeerJ. 2018;6:e5121. doi: 10.7717/peerj.5121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi C., Zhao X., Meng R., Liu Z., Zhang G., Guo N. Synergistic antimicrobial effects of nisin and p-Anisaldehyde on Staphylococcus aureus in pasteurized milk. LWT. 2017;84:222–230. doi: 10.1016/j.lwt.2017.05.056. [DOI] [Google Scholar]
- 23.Shin J., Na K., Shin S., Seo S.-M., Youn H.J., Park I.-K., Hyun J. Biological activity of thyme white essential oil stabilized by cellulose nanocrystals. Biomolecules. 2019;9:799. doi: 10.3390/biom9120799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Šegvić Klarić M., Kosalec I., Mastelić J., Piecková E., Pepeljnak S. Antifungal activity of thyme (Thymus vulgaris L.) essential oil and thymol against moulds from damp dwellings. Lett. Appl. Microbiol. 2007;44:36–42. doi: 10.1111/j.1472-765X.2006.02032.x. [DOI] [PubMed] [Google Scholar]
- 25.Wahba H.E., Abd Rabbu H.S., Ibrahim M.E. Evaluation of essential oil isolated from dry coriander seeds and recycling of the plant waste under different storage conditions. Bull. Natl. Res. Cent. 2020;44:192. doi: 10.1186/s42269-020-00448-z. [DOI] [Google Scholar]
- 26.Senatore F., Oliviero F., Scandolera E., Taglialatela-Scafati O., Roscigno G., Zaccardelli M., De Falco E. Chemical composition, antimicrobial and antioxidant activities of anethole-rich oil from leaves of selected varieties of fennel [Foeniculum vulgare Mill. ssp. vulgare var. azoricum (Mill.) Thell] Fitoterapia. 2013;90:214–219. doi: 10.1016/j.fitote.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 27.Bouyahya A., Mechchate H., Benali T., Ghchime R., Charfi S., Balahbib A., Burkov P., Shariati M.A., Lorenzo J.M., Omari N.E. Health benefits and pharmacological properties of carvone. Biomolecules. 2021;11:1803. doi: 10.3390/biom11121803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El-Zaeddi H., Martínez-Tomé J., Calín-Sánchez Á., Burló F., Carbonell-Barrachina Á.A. Volatile composition of essential oils from different aromatic herbs grown in Mediterranean regions of Spain. Foods. 2016;5:41. doi: 10.3390/foods5020041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shahbazi Y. Chemical composition and in vitro antibacterial activity of Mentha spicata essential oil against common food-borne pathogenic bacteria. J. Pathog. 2015;2015:916305. doi: 10.1155/2015/916305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elmassry M.M., Kormod L., Labib R.M., Farag M.A. Metabolome based volatiles mapping of roasted umbelliferous fruits aroma via HS-SPME GC/MS and peroxide levels analyses. J. Chromatogr. B. 2018;1099:117–126. doi: 10.1016/j.jchromb.2018.09.022. [DOI] [PubMed] [Google Scholar]
- 31.Khrisanapant P., Kebede B., Leong S.Y., Oey I. A comprehensive characterisation of volatile and fatty acid profiles of legume seeds. Foods. 2019;8:651. doi: 10.3390/foods8120651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou Q., Geng F., Deng Q., Huang F., Wang J. Dynamic analysis of polar metabolites and volatile compounds in sesame seeds during roasting. Cereal Chem. 2019;96:358–369. doi: 10.1002/cche.10134. [DOI] [Google Scholar]
- 33.Boga M., Ozkan E.E., Ersoy E., Tuncay E., Canturk Y.Y., Cinar E., Kara E.M., Zengin G. Identification and quantification of phenolic and volatile constituents in five different Anatolian Thyme species using LC–MS/MS and GC-MS, with biological activities. Food Biosci. 2021;43:101141. doi: 10.1016/j.fbio.2021.101141. [DOI] [Google Scholar]
- 34.Bernatoniene J., Kopustinskiene D.M. The role of catechins in cellular responses to oxidative stress. Molecules. 2018;23:965. doi: 10.3390/molecules23040965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gülçin İ. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid) Toxicology. 2006;217:213–220. doi: 10.1016/j.tox.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 36.Xu J.-G., Hu Q.-P., Liu Y. Antioxidant and DNA-protective activities of chlorogenic acid isomers. J. Agric. Food Chem. 2012;60:11625–11630. doi: 10.1021/jf303771s. [DOI] [PubMed] [Google Scholar]
- 37.Zduńska K., Dana A., Kolodziejczak A., Rotsztejn H. Antioxidant properties of ferulic acid and its possible application. Skin Pharmacol. Physiol. 2018;31:332–336. doi: 10.1159/000491755. [DOI] [PubMed] [Google Scholar]
- 38.Enogieru A.B., Haylett W., Hiss D.C., Bardien S., Ekpo O.E. Rutin as a potent antioxidant: Implications for neurodegenerative disorders. Oxid. Med. Cell. Longev. 2018;2018:6241017. doi: 10.1155/2018/6241017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zych M., Wojnar W., Borymski S., Szałabska K., Bramora P., Kaczmarczyk-Sedlak I. Effect of rosmarinic acid and sinapic acid on oxidative stress parameters in the cardiac tissue and serum of type 2 diabetic female rats. Antioxidants. 2019;8:579. doi: 10.3390/antiox8120579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sedeek M.S., Al-Mahallawi A.M., Hussien R.A.A., Ali A.M.A., Naguib I.A., Mansour M.K. Hexosomal dispersion: A nano-based approach to boost the antifungal potential of Citrus essential oils against plant fungal pathogens. Molecules. 2021;26:6284. doi: 10.3390/molecules26206284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu K., Lin Y., Chai X., Duan X., Zhao X., Chun C. Mechanisms of vapor-phase antibacterial action of essential oil from Cinnamomum camphora var. linaloofera Fujita against Escherichia coli. Food Sci. Nutr. 2019;7:2546–2555. doi: 10.1002/fsn3.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kloucek P., Smid J., Frankova A., Kokoska L., Valterova I., Pavela R. Fast screening method for assessment of antimicrobial activity of essential oils in vapor phase. Food Res. Int. 2012;47:161–165. doi: 10.1016/j.foodres.2011.04.044. [DOI] [Google Scholar]
- 43.Chai W.-M., Liu X., Hu Y.-H., Feng H.-L., Jia Y.-L., Guo Y.-J., Zhou H.-T., Chen Q.-X. Antityrosinase and antimicrobial activities of furfuryl alcohol, furfural and furoic acid. Int. J. Biol. Macromol. 2013;57:151–155. doi: 10.1016/j.ijbiomac.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 44.Andrade T.C.B., LIMA S.G., Freitas R.M., Rocha M.S., Islam T., SILVA T.G., Militao G.C.G. Isolation, characterization and evaluation of antimicrobial and cytotoxic activity of estragole, obtained from the essential oil of croton zehntneri (euphorbiaceae) An. Acad. Bras. Cienc. 2015;87:173–182. doi: 10.1590/0001-3765201520140111. [DOI] [PubMed] [Google Scholar]
- 45.Elsharawy N.T. The Inhibition Effect of Thyme and Coriander Essential Oils on of Campylobacter Jejuni and Some of Their Virulence Genes on Chicken Burger and Chicken Shawerma Products. Glob. Adv. Res. J. Microbiol. 2018;7:104–112. [Google Scholar]
- 46.El-Hawary E.A., Zayed A., Laub A., Modolo L.V., Wessjohann L., Farag M.A. How does LC/MS compare to UV in coffee authentication and determination of antioxidant effects? Brazilian and Middle Eastern coffee as case studies. Antioxidants. 2022;11:131. doi: 10.3390/antiox11010131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grzesik M., Naparło K., Bartosz G., Sadowska-Bartosz I. Antioxidant properties of catechins: Comparison with other antioxidants. Food Chem. 2018;241:480–492. doi: 10.1016/j.foodchem.2017.08.117. [DOI] [PubMed] [Google Scholar]
- 48.Adomako-Bonsu A.G., Chan S.L.F., Pratten M., Fry J.R. Antioxidant activity of rosmarinic acid and its principal metabolites in chemical and cellular systems: Importance of physico-chemical characteristics. Toxicol. Vitr. 2017;40:248–255. doi: 10.1016/j.tiv.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 49.Marimoutou M., Le Sage F., Smadja J., Lefebvre d’Hellencourt C., Gonthier M.-P., Silva R.-D. Antioxidant polyphenol-rich extracts from the medicinal plants Antirhea borbonica, Doratoxylon apetalum and Gouania mauritiana protect 3T3-L1 preadipocytes against H2O2, TNFα and LPS inflammatory mediators by regulating the expression of superoxide dismut. J. Inflamm. 2015;12:10. doi: 10.1186/s12950-015-0055-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li N., Jiang L., Liu Y., Zou S., Lu M., An H. Metabolomics Combined with Transcriptomics Analysis Revealed the Amino Acids, Phenolic Acids, and Flavonol Derivatives Biosynthesis Network in Developing Rosa roxburghii Fruit. Foods. 2022;11:1639. doi: 10.3390/foods11111639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gentile C., Tesoriere L., Butera D., Fazzari M., Monastero M., Allegra M., Livrea M.A. Antioxidant activity of Sicilian pistachio (Pistacia vera L. var. Bronte) nut extract and its bioactive components. J. Agric. Food Chem. 2007;55:643–648. doi: 10.1021/jf062533i. [DOI] [PubMed] [Google Scholar]
- 52.Aparadh V.T., Naik V.V., Karadge B.A. Antioxidative properties (TPC, DPPH, FRAP, metal chelating ability, reducing power and TAC) within some Cleome species. Ann. Bot. 2012;2:49–56. [Google Scholar]
- 53.Mulani M.S., Kamble E.E., Kumkar S.N., Tawre M.S., Pardesi K.R. Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: A review. Front. Microbiol. 2019;10:539. doi: 10.3389/fmicb.2019.00539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ibrahim N.M., Fahim S.H., Hassan M., Farag A.E., Georgey H.H. Design and synthesis of ciprofloxacin-sulfonamide hybrids to manipulate ciprofloxacin pharmacological qualities: Potency and side effects. Eur. J. Med. Chem. 2022;228:114021. doi: 10.1016/j.ejmech.2021.114021. [DOI] [PubMed] [Google Scholar]
- 55.Salem M.A., El-Shiekh R.A., Hashem R.A., Hassan M. In vivo antibacterial activity of star anise (Illicium verum Hook.) Extract Using Murine MRSA skin infection model in relation to its metabolite profile. Infect. Drug Resist. 2021;14:33. doi: 10.2147/IDR.S285940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Farag M.A., Fathi D., Shamma S., Shawkat M.S.A., Shalabi S.M., El Seedi H.R., Afifi S.M. Comparative metabolome classification of desert truffles Terfezia claveryi and Terfezia boudieri via its aroma and nutrients profile. LWT. 2021;142:111046. doi: 10.1016/j.lwt.2021.111046. [DOI] [Google Scholar]
- 57.Farag M.A., Khattab A.R., Shamma S., Afifi S.M. Profiling of primary metabolites and volatile determinants in mahlab cherry (Prunus mahaleb L.) seeds in the context of its different varieties and roasting as analyzed using chemometric tools. Foods. 2021;10:728. doi: 10.3390/foods10040728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Farag M.A., Ramadan N.S., Shorbagi M., Farag N., Gad H.A. Profiling of Primary Metabolites and Volatiles in Apricot (Prunus armeniaca L.) Seed Kernels and Fruits in the Context of Its Different Cultivars and Soil Type as Analyzed Using Chemometric Tools. Foods. 2022;11:1339. doi: 10.3390/foods11091339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bueno J. Models of evaluation of antimicrobial activity of essential oils in vapour phase: A promising use in healthcare decontamination. Nat. Volatiles Essent. Oils. 2015;2:16–29. [Google Scholar]
- 60.Chen Z., Bertin R., Froldi G. EC50 estimation of antioxidant activity in DPPH assay using several statistical programs. Food Chem. 2013;138:414–420. doi: 10.1016/j.foodchem.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 61.Faso B. DPPH Free Radical Scavenging Activity of Two Extracts from Agelanthus dodoneifolius (Loranthaceae) Leaves. Int. J. Toxicol. Pharmacol. Res. 2016;8:29–34. [Google Scholar]
- 62.Preedy V.R. Processing and Impact on Antioxidants in Beverages. Elsevier; Amsterdam, The Netherlands: 2014. [Google Scholar]
- 63.Arnao M.B., Cano A., Acosta M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001;73:239–244. doi: 10.1016/S0308-8146(00)00324-1. [DOI] [Google Scholar]
- 64.Schaich K.M., Tian X., Xie J. Hurdles and pitfalls in measuring antioxidant efficacy: A critical evaluation of ABTS, DPPH, and ORAC assays. J. Funct. Foods. 2015;14:111–125. doi: 10.1016/j.jff.2015.01.043. [DOI] [Google Scholar]
- 65.Liang Z., Cheng L., Zhong G.-Y., Liu R.H. Antioxidant and antiproliferative activities of twenty-four Vitis vinifera grapes. PLoS ONE. 2014;9:e105146. doi: 10.1371/journal.pone.0105146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shahidi F. Handbook of Antioxidants for Food Preservation. Woodhead Publishing; Sawston, UK: 2015. [Google Scholar]
- 67.Manivasagan P., Kim S.K. Advances in Food and Nutrition Research. Elsevier; Amsterdam, The Netherlands: 2014. [Google Scholar]
- 68.Benzie I.F.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 69.Sudan R., Bhagat M., Gupta S., Singh J., Koul A. Iron (FeII) Chelation, Ferric Reducing Antioxidant Power, and Immune Modulating Potential of Arisaema jacquemontii (Himalayan Cobra Lily) BioMed Res. Int. 2014;2014:179865. doi: 10.1155/2014/179865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Santos J.S., Brizola V.R.A., Granato D. High-throughput assay comparison and standardization for metal chelating capacity screening: A proposal and application. Food Chem. 2017;214:515–522. doi: 10.1016/j.foodchem.2016.07.091. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.