Abstract
Statins are lipid-lowering medications used for the prevention of cardiovascular disease (CVD), but the pleiotropic effects of statins might be beneficial in other chronic diseases. This meta-analysis investigated the association between statin use and mortality in different chronic conditions. Eligible studies were real-world studies that compared all-cause mortality over at least 12 months between propensity score-matched statin users and non-users. Overall, 54 studies were included: 21 in CVD, 6 in chronic kidney disease, 6 in chronic inflammatory diseases, 3 in cancer, and 18 in other diseases. The risk of all-cause mortality was significantly reduced in statin users (hazard ratio: 0.72, 95% confidence interval: 0.66–0.76). The reduction in mortality risk was similar in CVD studies (0.73, 0.66–0.76) and non-CVD studies (0.70, 0.67–0.79). There were no significant differences in the risk reduction between cohorts with different diseases (p = 0.179). The greatest mortality reduction was seen in studies from Asia (0.61, 0.61–0.73) and the lowest in studies from North America (0.78, 0.73–0.83) and Australia (0.78, 0.62–0.97). There was a significant heterogeneity (I2 = 95%, tau2 = 0.029, p < 0.01). In conclusion, statin use was associated with a significantly reduced risk of all-cause mortality in real-world cohorts with CVD and non-CVD.
Keywords: statins, all-cause mortality, cardiovascular disease, non-cardiovascular disease
1. Introduction
Statins are lipid-lowering medications used for the primary and secondary prevention of cardiovascular disease (CVD) [1,2]. Apart from lowering blood lipid levels, statins might reduce cardiovascular risk through antithrombotic and anti-inflammatory effects, atherosclerotic plaque regression, or improved endothelial function [3]. Some of these pleiotropic effects might be beneficial also in patients without CVD. The benefits of statin use were reported for patients with nephropathy, head injury, rheumatoid arthritis, neurodegenerative diseases, cancer, or infections [4,5].
A reduction in all-cause mortality is a reliable measure of treatment efficacy in various populations. Therefore, all-cause mortality seems to be an adequate outcome to compare the benefits of statins between patients with different diseases. Some meta-analyses of randomized controlled trials (RCTs) found that statins reduced all-cause mortality when used in the primary and secondary prevention of CVD [6,7], heart failure [8], atrial fibrillation [9], and chronic kidney disease (CKD) requiring dialysis [10]. In contrast, other meta-analyses did not find any significant effect of statins on all-cause mortality in CKD [11] or the primary prevention of CVD [12,13]. To date, no RCTs have evaluated the effects of statins on mortality in other chronic diseases.
Considering the inconsistent evidence from RCTs on the association between statin use and all-cause mortality in patients with CVD, and the lack of evidence for numerous other diseases, we reviewed nonrandomized studies that used propensity score matching to reduce confounding. Such quasi-experimental studies provide real-world evidence, which helps assess the efficacy of treatments in nonhomogeneous populations that are characteristic of clinical practice. Moreover, these studies can provide relevant information when RCTs are unavailable [14]. The purpose of this study was to analyze the association between statin use and all-cause mortality in different chronic diseases in studies that used propensity score matching to match statin users and non-users in a real-world setting.
2. Methods
2.1. Protocol and Registration
The systematic review protocol was developed in accordance with the Preferred Reporting Items for Systematic Review and Meta-analysis Protocols (PRISMA) guidance [15]. The protocol is available in supplement 1.
2.2. Data Sources and Searches
This was a systematic review and meta-analysis. The PubMed electronic database was searched for articles published in English from February 2012 to February 2022. Last search was performed 20th February 2022. Relevant keywords were applied alone or in combination to identify data. The search strategy with the number of hits is presented in the study protocol. For abstracts potentially meeting the inclusion criteria, full-text publications were retrieved. Each study was assessed for eligibility by two independent reviewers, according to the criteria presented in the study protocol. Reasons for exclusion were briefly documented.
2.3. Study Selection
Studies were eligible that reported adjusted hazard ratios (HR) for all-cause mortality over at least 12 months in statin users vs. non-users in real-world cohorts matched with propensity score matching. Only studies carried out among adults were included.
2.4. Data Extraction and Quality Assessment
Two independent investigators (MMN and MN) extracted the following variables: adjusted HR, sample size, percentage of men, mean age, average follow-up (mean or median), and number of deaths. Disagreements were resolved by consensus. The total duration of follow-up was obtained from publications or calculated by multiplying the average follow-up by cohort size (patient-years). The number of deaths per 1000 patient-years was obtained from publications or calculated by dividing the number of deaths by the total follow-up duration.
Risk of bias assessment of the included studies was performed by two independent authors using the Newcastle-Ottawa Scale (NOS). NOS consists of three domains: (1) selection, (2) comparability, and (3) outcome [16]. Discrepancies were resolved by discussion. The certainty of evidence was assessed based on Grading of Recommendations, Assessment, Development and Evaluations (GRADE) framework.
2.5. Data Synthesis and Analysis
We conducted a meta-analysis of adjusted HRs for changes in all-cause mortality in statin users. Standard errors were calculated from 95% confidence intervals or from P values [17]. The log-transformed values of point estimates and of standard errors were used in an inverse variance random-effects meta-analysis, with the restricted maximum-likelihood estimator for tau2 and the Q-profile method for the confidence interval of tau2 and tau. Heterogeneity was expressed with the I2 statistic and evaluated with the Cochran’s Q test. Prediction intervals were calculated to aid the interpretation of the estimates, with consideration of heterogeneity [18]. Influential studies with the greatest impact on the estimate and heterogeneity were explored by a visual inspection of the Baujat plot [19]. Subgroup analyses were performed to compare the cohorts of patients with CVD vs. those with other diseases. CVD was defined as coronary artery disease, ischemic stroke, peripheral artery disease, heart failure, atrial fibrillation, and valvular disease. Additional subgroup analyses compared cohorts with CVD, CKD, inflammatory diseases (autoimmune diseases, gout), cancer, and other diseases. Studies including patients with both CVD and CKD were classified as CVD cohorts. We also compared studies conducted on different continents. A restricted maximum-likelihood random-effects meta-regression analysis was used to explore heterogeneity, with the following covariates assessed: percentage of men, mean age, publication year, average follow-up, and number of deaths per 1000 person-years. A funnel plot and the Egger’s test were used to assess publication bias [20]. A P value of less than 0.05 was considered statistically significant. The R software (version 4.1.2) and the meta and dmetar packages were used for all analyses [21,22]. The study did not receive any funding.
3. Results
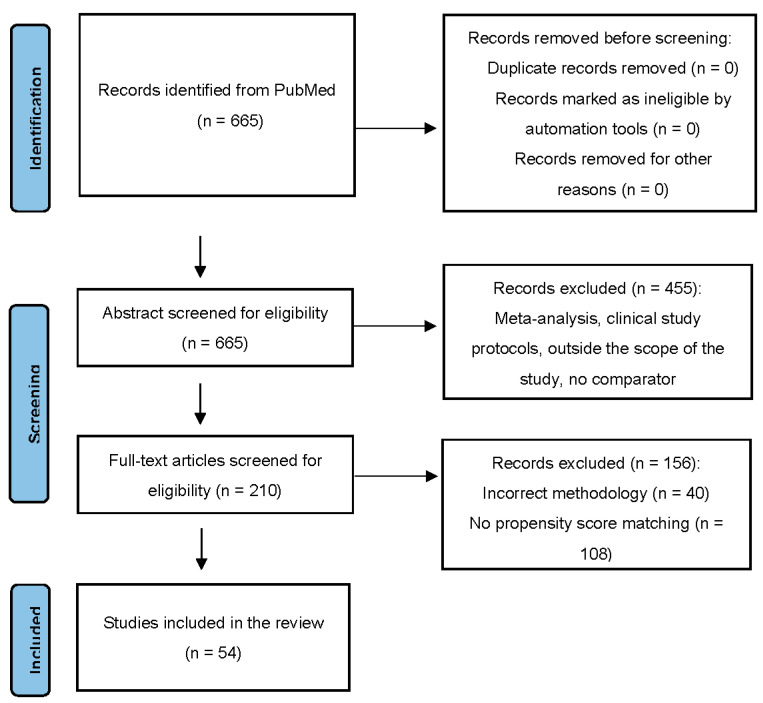
A total of 665 citations were identified, and 210 potentially eligible articles were retrieved in full text. Overall, 54 studies were included in the review (Figure 1) [23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76]. Risk of bias assessment using NOS showed that the studies have low risk of bias. The characteristics of included studies are summarized in Table 1. In the analysis, we included 3 cohorts from the study by Lee et al. [74] The mean age of patients was 67.3 years (range, 33.4–85.2), and men constituted 58% of the population. A total of 21 studies (23 cohorts) were focused on CVD. Among the remaining 33 non-CVD studies, 6 included patients with CKD; 6 with inflammatory diseases; 3 with cancer; and 18 with other diseases. Almost half of the studies (n = 25) were conducted in Asia; 17 in North America; 12 in Europe; and 2 in Australia.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart of the study selection process.
Table 1.
Study characteristics.
| Author and Year | Continent | Population | Mean Age (Years) |
Sex (% Male) | Adjusted HR for All-Cause Mortality | Mean/Median Follow-up (Months) | NOS |
|---|---|---|---|---|---|---|---|
| Ozen 2022 [23] | North America | Inflammatory disease | 65.7 | 33.7 | 0.46 | 15.0 | 7 |
| Kim 2021 [24] | Asia | CKD | 57.0 | 55.4 | 0.72 | 35.7 | 8 |
| Yashima 2021 [25] | Asia | CVD | 84.2 | 29.6 | 0.76 | 22.0 | 7 |
| Cheng 2021 [26] | Asia | CKD | 33.4 | 45.6 | 0.88 | 60.2 | 8 |
| Cheung 2021 [27] | Asia | Other | 61.2 | 71.3 | 0.21 | 94.8 | 8 |
| Chhibber 2021 [28] | North America | Inflammatory disease | 60.1 | 34.1 | 0.72 | 36.0 | 8 |
| Jung 2021 [29] | Asia | Other | 59.2 | 45.1 | 0.71 | 72.0 | 8 |
| Shavadia 2021 [30] | North America | CVD | 69.0 | 48.4 | 0.96 | 20.7 | 7 |
| Kim 2020 [31] | Asia | Other | 78.0 | 34.3 | 0.83 | 104.4 | 8 |
| Tan 2020 [32] | North America | Cancer | 74.0 | 100 | 0.89 | 42.0 | 8 |
| Lin 2018 [33] | Asia | CVD | 61.2 | 56.1 | 0.54 | 60.0 | 8 |
| Köhler-Forsberg 2019 [34] | Europe | Other | 65.7 | 49.5 | 0.90 | 162.2 | 8 |
| Jung 2019 [35] | Asia | Other | 60.5 | 46.7 | 0.61 | 78.0 | 8 |
| Huang 2018 [36] | Asia | CKD | 59.4 | 36.2 | 0.59 | 63.6 | 8 |
| Jorge 2018 [37] | North America | Inflammatory disease | 64.4 | 23.1 | 0.84 | 61.2 | 8 |
| Keller 2017 [38] | North America | Inflammatory disease | 64.9 | 80.8 | 0.84 | 60.0 | 8 |
| Orkaby 2017 [39] | North America | Other | 76.0 | 100 | 0.82 | 84.0 | 8 |
| Kim 2017 [40] | Asia | CVD | 69.8 | 52.9 | 0.80 | 12.0 | 7 |
| Oza 2017 [41] | North America | Inflammatory disease | 61.4 | 79.5 | 0.63 | 63.6 | 8 |
| Fung 2017 [42] | Asia | Other | 64.8 | 41.8 | 0.38 | 50.5 | 8 |
| Chung 2017 [43] | Asia | CKD | 63.3 | 44.5 | 0.73 | 44.4 | 8 |
| Hsu 2017 [44] | Asia | CVD | 62.2 | 44.0 | 0.72 | 68.4 | 8 |
| Holzhauser 2017 [45] | North America | Other | 72.0 | 38.0 | 0.42 | 12.0 | 7 |
| Cho 2017 [46] | Asia | CKD | 53.9 | 53.3 | 0.54 | 30.0 | 8 |
| Sanfilippo 2016 [47] | North America | Cancer | 68.6 | 97.9 | 0.78 | 34.0 | 8 |
| Tanaka 2017 [48] | Asia | CVD | 71.0 | 95.0 | 0.14 | 24.0 | 8 |
| Rothschild 2016 [49] | North America | CVD | 85.2 | 46.0 | 0.88 | 37.2 | 8 |
| Ble 2017 [50] | Europe | CVD | 76.4 | 54.5 | 0.62 | 120.0 | 8 |
| Ramos 2016 [51] | Europe | Other | 66.9 | 55.9 | 0.81 | 43.2 | 8 |
| Woo 2015 [52] | Asia | CVD | 66.7 | 28.0 | 1.42 | 11.2 | 7 |
| Vedel-Krogh 2015 [53] | Europe | Other | 71.0 | 64.0 | 0.73 | 91.2 | 8 |
| Sun 2015 [54] | Asia | Cancer | 68.5 | 100 | 0.65 | 93.0 | 8 |
| Yu 2015 [55] | Asia | Inflammatory disease | 37.9 | 11.1 | 0.67 | 100.8 | 8 |
| Schoenfeld 2016 [56] | North America | Inflammatory disease | 65.3 | 34.4 | 0.79 | 54.1 | 8 |
| Alehagen 2015 [57] | Europe | CVD | 77.0 | 47.0 | 0.80 | 21.3 | 7 |
| Smith 2015 [58] | North America | Other | 72.0 | 50.9 | 0.79 | 12.0 | 7 |
| Chen 2015 [59] | Asia | CVD | 59.0 | 60.4 | 0.87 | 24.0 | 8 |
| Alehagen 2015 [60] | Europe | CVD | 73.0 | 71.0 | 0.81 | 24.9 | 7 |
| De Blois 2015 [61] | Europe | CVD | 70.2 | 71.0 | 0.68 | 120.0 | 8 |
| Yang 2014 [62] | Asia | Other | 64.9 | 52.0 | 0.68 | 12.0 | 7 |
| Carlsson 2014 [63] | Europe | CVD | 77.2 | 100 | 0.66 | 40.8 | 8 |
| Wändell 2014 [64] | Europe | CVD | 76.3 | 55.0 | 0.56 | 44.4 | 8 |
| Lawes 2012 [65] | Australia | Other | 71.6 | 58.4 | 0.69 | 22.8 | 7 |
| Orkaby 2020 [66] | North America | Other | 81.1 | 97.3 | 0.75 | 81.6 | 8 |
| Zhou 2020 [67] | Australia | Other | 74.2 | 39.4 | 0.87 | 56.4 | 8 |
| Jung 2020 [68] | Asia | CKD | 64.9 | 58.8 | 0.65 | 40.8 | 8 |
| van Dongen 2019 [69] | Europe | CVD | 45.0 | 67.6 | 0.38 | 99.6 | 8 |
| Rusnak 2019 [70] | Europe | CVD | 68.0 | 77.0 | 0.44 | 36.0 | 8 |
| Al-Gobari 2019 [71] | Europe | CVD | 74.9 | 58.0 | 0.86 | 12.0 | 7 |
| Marume 2019 [72] | Asia | CVD | 76.0 | 34.0 | 0.21 | 25.0 | 8 |
| Wu 2018 [73] | Asia | Other | 66.3 | 51.6 | 0.79 | 12.0 | 7 |
| Lee 2018a [74] | North America | CVD | 73.0 | 50.6 | 0.73 | 30.0 | 8 |
| Lee 2018b [74] | North America | CVD | 72.1 | 65.1 | 0.76 | 30.0 | 8 |
| Lee 2018c [74] | North America | CVD | 68.8 | 66.7 | 0.86 | 30.0 | 8 |
| Chung 2017 [75] | Asia | CVD | 66.0 | 58.0 | 0.76 | 48.0 | 8 |
| Tsujimoto 2017 [76] | Asia | Other | nd | 58.6 | 0.62 | 62.4 | 8 |
CKD, chronic kidney disease; CVD, cardiovascular disease; DM, diabetes mellitus; HR, hazard ratio; nd, no data; NOS, Newcastle-Ottawa Scale. Inflammatory diseases consist of rheumatoid arthritis, systemic lupus, gout, and ankylosing spondylitis. Other include the elderly, hepatitis, lung diseases, diabetes mellitus, and healthy subjects.
3.1. Statin Use and All-Cause Mortality
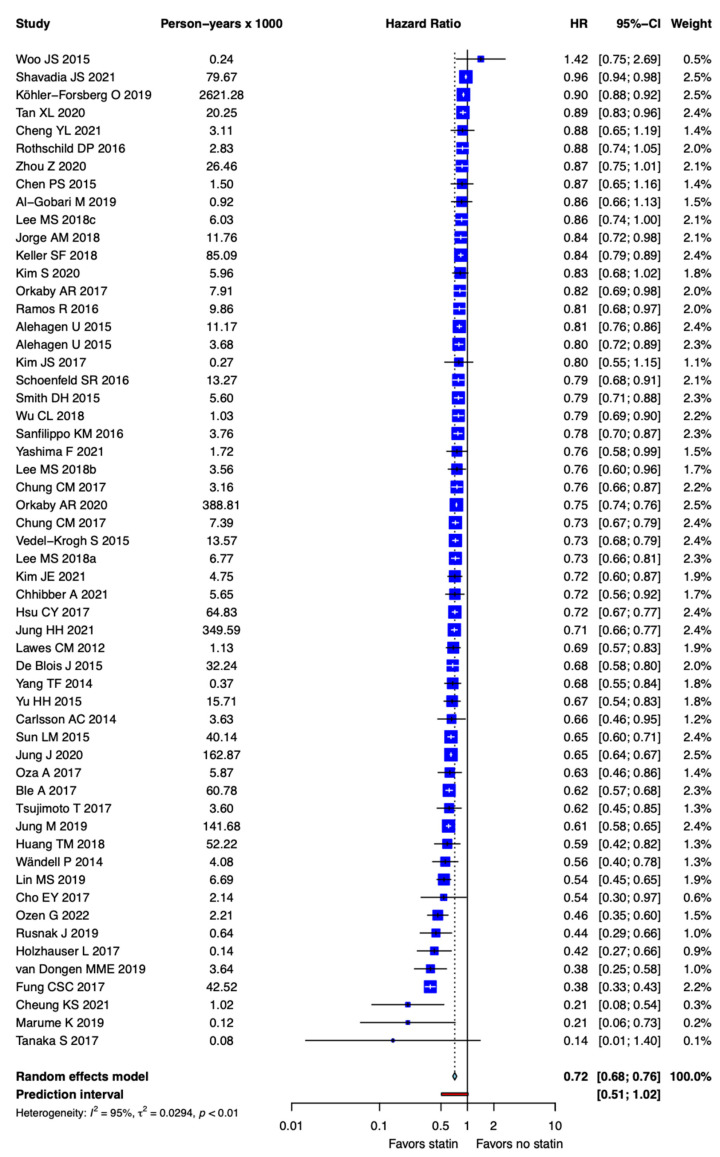
The pooled estimate showed that statin use was associated with a significant reduction in all-cause mortality (HR = 0.72; 95% CI, 0.68–0.76), but there was significant heterogeneity (I2 = 95%, tau2 = 0.0294, p < 0.01; prediction interval, 0.51–1.02; Figure 2).
Figure 2.
Forest plot showing the association between statin use and all-cause mortality.
3.2. Publication Bias
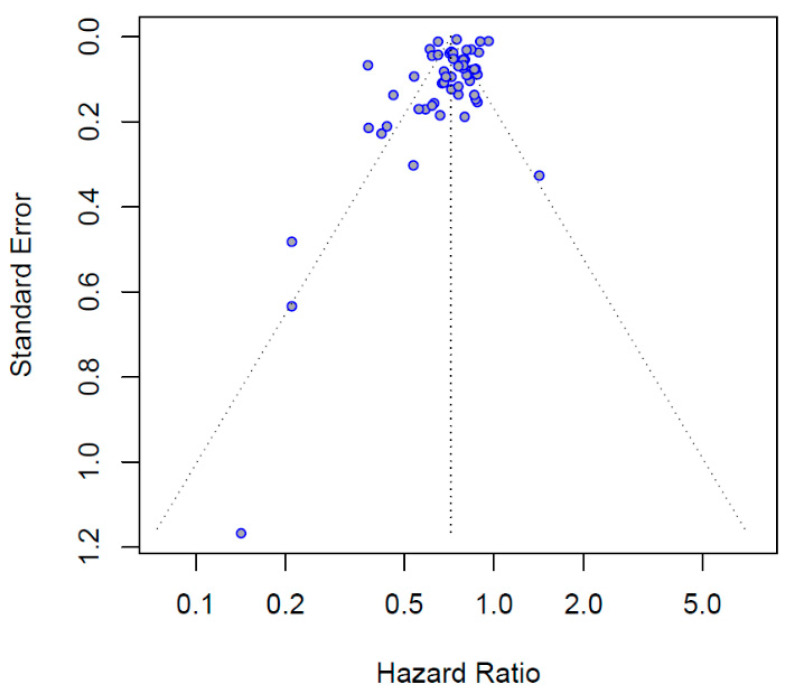
The funnel plot was asymmetrical, with more studies reporting estimates that were lower than the pooled estimate (Figure 3). The Egger’s test showed a trend for a publication bias (p = 0.096).
Figure 3.
Funnel plot for publication bias.
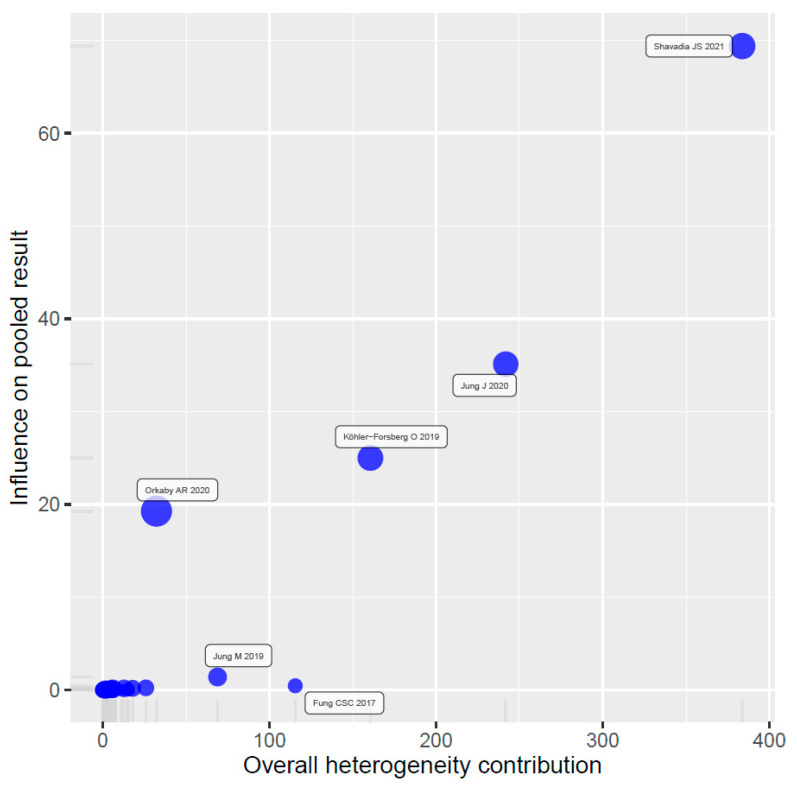
3.3. Influential Studies
The Baujat plot (Figure 4) revealed six studies with a substantial contribution to the overall heterogeneity and influence on the pooled result. After excluding these studies, the pooled effect estimate was 0.73 (95% CI, 0.70–0.76), and the residual heterogeneity was lower but significant (I2 = 72%, tau2 = 0.0123, p < 0.01; prediction interval, 0.58–0.92).
Figure 4.
Baujat plot for the analysis of influential studies.
3.4. Sensitivity Analyses
There was no significant difference in statin-related reduction in the risk of mortality between CVD and non-CVD cohorts (Table 2). The estimate was lower in cohorts with inflammatory diseases and cancer than in the remaining subgroups, but the difference was not significant (Table 2). The estimates were lower in Asian and European studies than in those conducted in Australia and North America (Table 2, p = 0.044).
Table 2.
Sensitivity analyses of the association between statin use and all-cause mortality.
| All Studies (n = 56) | Without Influential Studies (n = 50) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subgroup | No. of Studies | Estimate (HR) | 95% CI | Prediction Interval | I2 (%) | p Value | No. of Studies | Estimate (HR) | 95% CI | Prediction Interval | I2 (%) | p Value |
| CVD | 23 | 0.73 | 0.66–0.76 | 0.51–1.04 | 92 | 0.598 | 22 | 0.72 | 0.66–0.78 | 0.53–0.97 | 73 | 0.497 |
| Non-CVD | 33 | 0.70 | 0.67–0.79 | 0.49–1.02 | 95 | 28 | 0.74 | 0.71–0.78 | 0.60–0.91 | 71 | ||
| CVD | 23 | 0.73 | 0.67–0.79 | 0.51–1.04 | 92 | 0.179 | 22 | 0.72 | 0.66–0.77 | 0.53–0.97 | 73 | 0.525 |
| CKD | 6 | 0.69 | 0.63–0.75 | 0.55–0.85 | 61 | 5 | 0.72 | 0.67–0.77 | 0.64–0.81 | 1.2 | ||
| Inflammatory disease | 6 | 0.78 | 0.72–0.85 | 0.63–0.96 | 39 | 6 | 0.78 | 0.72–0.85 | 0.63–0.96 | 39 | ||
| Cancer | 3 | 0.77 | 0.64–0.92 | 0.07–7.52 | 94 | 3 | 0.77 | 0.64–0.92 | 0.07–7.52 | 94 | ||
| Other | 18 | 0.68 | 0.60–0.76 | 0.40–1.13 | 96 | 14 | 0.72 | 0.66–0.78 | 0.54–0.95 | 67 | ||
| Asia | 25 | 0.67 | 0.61–0.73 | 0.45–0.98 | 83 | 0.044 | 22 | 0.71 | 0.67–0.74 | 0.62–0.80 | 51 | 0.126 |
| Australia | 2 | 0.78 | 0.62–0.97 | - | 73 | 2 | 0.78 | 0.62–0.97 | - | 73 | ||
| Europe | 12 | 0.71 | 0.62–0.79 | 0.46–1.06 | 92 | 11 | 0.69 | 0.61–0.77 | 0.47–1.00 | 79 | ||
| North America | 17 | 0.78 | 0.73– 0.83 | 0.60–1.02 | 96 | 15 | 0.78 | 0.72–0.83 | 0.61–0.98 | 68 | ||
CI, confidence interval; CKD, chronic kidney disease; CVD, cardiovascular disease; HR, hazard ratio. p values were derived from the Q test for subgroup differences.
3.5. Meta-Regression Analysis
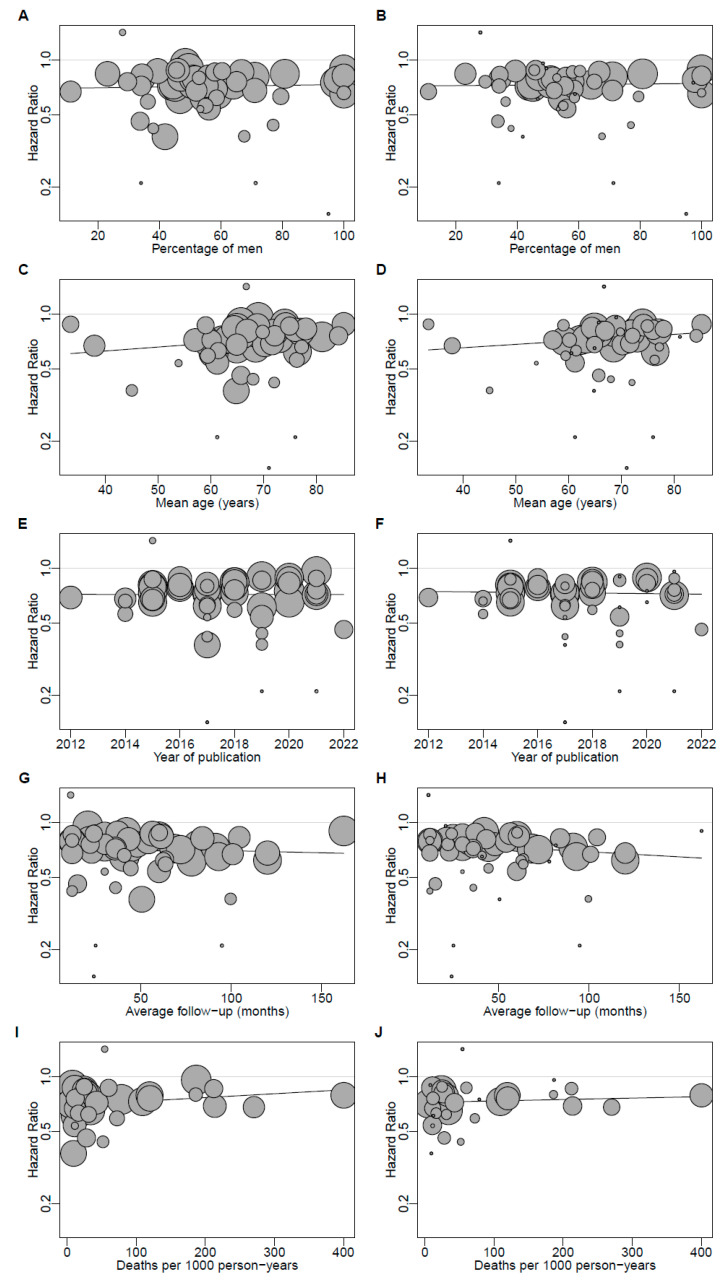
After the exclusion of influential studies, the HRs for the association between statin use and mortality tended to increase with mean age (p = 0.075) and to decrease with average follow-up (p = 0.051; Table 3, Figure 5). The HRs were not associated with the percentage of men, publication year, and number of deaths per 1000 patient-years (Table 3, Figure 5). None of the covariates accounted for heterogeneity (Table 3).
Table 3.
Meta-regression models for the association between statin use and all-cause mortality.
| Primary Meta-Analysis | Omitting Influential Studies | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of Studies | Estimate | 95% CI | p Value | Residual I2 (%) | No. of Studies | Estimate | 95% CI | p Value | Residual I2 (%) | |
| Percentage of men | 56 | 0.0005 | −0.0021–0.0031 | 0.698 | 95 | 50 | 0.0003 | −0.0017–0.0024 | 0.738 | 72 |
| Mean age (years) | 56 | 0.0050 | −0.0009–0.0109 | 0.094 | 95 | 55 | 0.0042 | −0.0004–0.0089 | 0.075 | 70 |
| Publication year | 56 | −0.0003 | −0.0240–0.0235 | 0.983 | 96 | 50 | −0.0034 | −0.0228–0.0160 | 0.729 | 73 |
| Average follow-up (months) | 56 | −0.0005 | −0.0021–0.0011 | 0.520 | 96 | 50 | −0.0013 | −0.0026–0.0000 | 0.051 | 67 |
| Deaths per 1000 person-years | 35 | 0.0005 | −0.0003–0.0013 | 0.204 | 96 | 30 | 0.0002 | −0.0004–0.0007 | 0.514 | 68 |
CI, confidence interval.
Figure 5.
Bubble plots for the association between statin use and all-cause mortality regressed against the percentage of men (A,B), mean age (C,D), year of publication (E,F), average follow-up (G,H), and number of deaths per 1000 patient-years (I,J). Plots on the left represent the primary meta-analysis, whereas plots on the right represent the meta-analysis after the exclusion of influential studies. Bubble size represents study weight.
4. Discussion
This systematic review evaluated evidence from real-world studies assessing statin use, with a total of over 4 million patient-years of follow-up. All studies except one reported lower all-cause mortality in statin users vs. matched cohorts. The meta-analysis showed that statin use was associated with a significant reduction in all-cause mortality, with the risk lower by about 30% in patients on statins. There was no substantial difference in mortality reduction between studies including CVD and non-CVD cohorts. Substantial heterogeneity was noted in the main analysis, subgroup analyses, and after the exclusion of influential studies. None of the covariates assessed in meta-regression models accounted for heterogeneity. The funnel plots suggested possible publication bias towards studies reporting favorable effects of statins, which may be study limitation, although this effect was not significant. Therefore, although the included studies used propensity score matching and adjusted the estimates for potential confounders, the overall evidence on the association of statins with all-cause mortality in real-world clinical practice is of suboptimal quality.
Statins are potent antiatherogenic agents that reduce blood lipid concentrations and prevent atherosclerosis progression [77]. These effects likely translate into a lower risk of cardiovascular events, including death. There is evidence that statins might also have nephroprotective effects and slow the progression of CKD (a slower decline in glomerular filtration rate among statin users) [78]. However, other studies found that high-dose statin therapy, as compared with low-dose therapy, was associated with an increased risk of kidney disease [79]. The anti-inflammatory and antioxidant effects of statins can be beneficial in patients with autoimmune diseases [80]. Statins might also slow the progression of certain types of cancer by inhibiting cancer cell proliferation and tumor angiogenesis and by inducing apoptosis and stimulating immune surveillance of cancer cells [81].
In our meta-analysis, the reduction in all-cause mortality in statin users was similar among those with or without CVD. Assuming there was a lower cardiovascular risk in non-CVD than CVD cohorts, a similar risk reduction in mortality in the two cohort types, which we found in this meta-analysis, may suggest that statins reduce the risk of mortality through non-cardiovascular effects as well. Among patients with CKD, the reduction in all-cause mortality in statin users was similar to that observed in CVD patients. CVD is common in patients with CKD and constitutes the most frequent cause of death in this population [82]. Thus, statin-associated reduction in morality risk in CKD is likely due to a lower incidence of cardiovascular events. Further subgroup analyses suggested that statin-associated reduction in mortality risk could be lower in inflammatory diseases and cancer. In previous studies, statin use was associated with lower disease severity scores in rheumatoid arthritis [83]. However, it remains unknown whether this disease-modifying effect translates into lower mortality, because CVD is a common cause of death in patients with rheumatoid arthritis [84]. In our study, the lower survival benefit in statin users among patients with cancer or inflammatory disease, compared with other subgroups, could be due to a lower cardiovascular risk in these patients. Similar to our study, a meta-analysis of studies enrolling over 1 million patients with cancer found that statin use was associated with a significant decrease in all-cause mortality (HR = 0.70) [85]. Notably, statin use was also associated with reduced cancer-specific mortality (HR = 0.60) [85].
We observed that the reduction in all-cause mortality was lower in the United States and Australia than in Europe and Asia. Pharmaceutical sales data show that statins are used more often in the United States and Australia than in Europe and Asia [86]. This is likely due to a higher number of prescriptions in primary prevention, that is, among patients with lower cardiovascular risk in whom the survival benefit is lower than in patients requiring secondary prevention [87]. This could explain a lower mortality reduction in American and Australian studies.
The existing evidence from observational studies shows that statins are associated with a significantly reduced all-cause mortality, whereas this effect is not always significant in RCTs. Limited sample size and shorter follow-up in RCTs may partly explain this observation. Indeed, we found that the reduction in all-cause mortality in statin users tended to increase with longer follow-up. Moreover, among observational studies, we found a trend for a publication bias towards studies reporting favorable outcomes.
Our study is limited by substantial heterogeneity, with the upper limit of the prediction interval above 1. However, the sensitivity analysis after exclusion of influential studies was characterized by reduced heterogeneity, with the upper limit of the prediction interval below 1. Heterogeneity in meta-analyses of large registry-based studies is typically greater than that in meta-analyses of RCTs [88,89,90,91]. It is possible that in our meta-analysis substantial heterogeneity occurred because individual studies included participants who differed in terms of statin type, statin dose, adherence, treatment duration, and comorbidities. In RCTs, such confounding factors are balanced by random allocation to treatment, but they are difficult to be accounted for in observational studies, even when propensity score matching is used. From a statistical standpoint, large studies have a low sampling error (high precision), and therefore, the differences in effect size between such studies result in high heterogeneity. Although the covariates assessed in meta-regression models did not account for heterogeneity, we observed that the reduction in all-cause mortality in statin users tended to be greater in studies with longer follow-up and to be lower with the increasing age of participants. This observation is consistent with previous reports [90,92] and underscores the importance of conducting real-world studies that reflect clinical practice, have a much longer follow-up, and have a greater proportion of elderly patients than that in most clinical trials. The strengths of our study include sensitivity analyses performed after the exclusion of influential studies and in different subgroups. Moreover, we carried out meta-regression analyses and used all-cause mortality as an outcome that is unlikely to be biased. Searching for eligible studies in the PubMed database only is another limitation.
In conclusion, statin use was associated with a significant reduction in all-cause mortality in various populations treated in real-world clinical practice in an analysis of over 4 million patient-years. It remains unclear whether the reduction in mortality risk associated with statins is solely due to a reduced incidence of cardiovascular death or other effects of these medications. Substantial heterogeneity limits the available evidence on the association between statin use and all-cause mortality in real-world practice.
Acknowledgments
Editorial assistance was provided by Proper Medical Writing, Warsaw, Poland.
Author Contributions
Conceptualization, L.P. and M.M.N.; methodology, L.P. and M.M.N.; software, M.M.N. and M.F.; validation, M.M.N., M.N. and L.P.; formal analysis M.M.N., M.N.; investigation, M.M.N.; resources, M.K. and L.P.; data curation, M.K. and L.P.; writing—original draft preparation, M.M.N.; writing—review and editing, M.M.N., M.N and M.F.; visualization, M.F.; supervision, M.K. and L.P.; project administration, M.K. and L.P.; funding acquisition, M.N. and M.K. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Arnett D.K., Blumenthal R.S., Albert M.A., Buroker A.B., Goldberger Z.D., Hahn E.J., Himmelfarb C.D., Khera A., Lloyd-Jones D., McEvoy J.W., et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019;74:e177–e232. doi: 10.1016/j.jacc.2019.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mach F., Baigent C., Catapano A.L., Koskinas K.C., Casula M., Badimon L., Chapman M.J., De Backer G.G., Delgado V., Ference B.A., et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020;41:111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 3.Diamantis E., Kyriakos G., Quiles-Sanchez L.V., Farmaki P., Troupis T. The Anti-Inflammatory Effects of Statins on Coronary Artery Disease: An Updated Review of the Literature. Curr. Cardiol. Rev. 2017;13:209–216. doi: 10.2174/1573403X13666170426104611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohammad S., Nguyen H., Nguyen M., Abdel-Rasoul M., Nguyen V., Nguyen C.D., Nguyen K.T., Li L., Kitzmiller J.P. Pleiotropic Effects of Statins: Untapped Potential for Statin Pharmacotherapy. Curr. Vasc. Pharmacol. 2019;17:239–261. doi: 10.2174/1570161116666180723120608. [DOI] [PubMed] [Google Scholar]
- 5.Soulaidopoulos S., Nikiphorou E., Dimitroulas T., Kitas G.D. The Role of Statins in Disease Modification and Cardiovascular Risk in Rheumatoid Arthritis. Front. Med. 2018;5:24. doi: 10.3389/fmed.2018.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brugts J.J., Yetgin T., Hoeks S.E., Gotto A.M., Shepherd J., Westendorp R.G., de Craen A.J., Knopp R.H., Nakamura H., Ridker P., et al. The benefits of statins in people without established cardiovascular disease but with cardiovascular risk factors: Meta-analysis of randomised controlled trials. BMJ. 2009;338:b2376. doi: 10.1136/bmj.b2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung B.M., Lauder I.J., Lau C.P., Kumana C.R. Meta-analysis of large randomized controlled trials to evaluate the impact of statins on cardiovascular outcomes. Br. J. Clin. Pharmacol. 2004;57:640–651. doi: 10.1111/j.1365-2125.2003.02060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lipinski M.J., Cauthen C.A., Biondi-Zoccai G.G., Abbate A., Vrtovec B., Khan B.V., Vetrovec G.W. Meta-analysis of randomized controlled trials of statins versus placebo in patients with heart failure. Am. J. Cardiol. 2009;104:1708–1716. doi: 10.1016/j.amjcard.2009.07.055. [DOI] [PubMed] [Google Scholar]
- 9.Pastori D., Baratta F., Di Rocco A., Farcomeni A., Del Ben M., Angelico F., Violi F., Pignatelli P., Lip G.Y.H. Statin use and mortality in atrial fibrillation: A systematic review and meta-analysis of 100,287 patients. Pharmacol. Res. 2021;165:105418. doi: 10.1016/j.phrs.2021.105418. [DOI] [PubMed] [Google Scholar]
- 10.Barylski M., Nikfar S., Mikhailidis D.P., Toth P.P., Salari P., Ray K.K., Pencina M.J., Rizzo M., Rysz J., Abdollahi M., et al. Statins decrease all-cause mortality only in CKD patients not requiring dialysis therapy—A meta-analysis of 11 randomized controlled trials involving 21,295 participants. Pharmacol. Res. 2013;72:35–44. doi: 10.1016/j.phrs.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Strippoli G.F., Navaneethan S.D., Johnson D.W., Perkovic V., Pellegrini F., Nicolucci A., Craig J.C. Effects of statins in patients with chronic kidney disease: Meta-analysis and meta-regression of randomised controlled trials. BMJ. 2008;336:645–651. doi: 10.1136/bmj.39472.580984.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ray K.K., Seshasai S.R., Erqou S., Sever P., Jukema J.W., Ford I., Sattar N. Statins and all-cause mortality in high-risk primary prevention: A meta-analysis of 11 randomized controlled trials involving 65,229 participants. Arch. Intern. Med. 2010;170:1024–1031. doi: 10.1001/archinternmed.2010.182. [DOI] [PubMed] [Google Scholar]
- 13.Thavendiranathan P., Bagai A., Brookhart M.A., Choudhry N.K. Primary prevention of cardiovascular diseases with statin therapy: A meta-analysis of randomized controlled trials. Arch. Intern. Med. 2006;166:2307–2313. doi: 10.1001/archinte.166.21.2307. [DOI] [PubMed] [Google Scholar]
- 14.Li M., Chen S., Lai Y., Liang Z., Wang J., Shi J., Lin H., Yao D., Hu H., Ung C.O.L. Integrating Real-World Evidence in the Regulatory Decision-Making Process: A Systematic Analysis of Experiences in the US, EU, and China Using a Logic Model. Front. Med. 2021;8:669509. doi: 10.3389/fmed.2021.669509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moher D., Shamseer L., Clarke M., Ghersi D., Liberati A., Petticrew M., Shekelle P., Stewart L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015;4:1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lo C.K.-L., Mertz D., Loeb M. Newcastle-Ottawa Scale: Comparing reviewers’ to authors’ assessments. BMC Med. Res. Methodol. 2014;14:45. doi: 10.1186/1471-2288-14-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Altman D.G., Bland J.M. How to obtain the confidence interval from a P value. BMJ. 2011;343:d2090. doi: 10.1136/bmj.d2090. [DOI] [PubMed] [Google Scholar]
- 18.IntHout J., Ioannidis J.P., Rovers M.M., Goeman J.J. Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open. 2016;6:e010247. doi: 10.1136/bmjopen-2015-010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baujat B., Mahé C., Pignon J.P., Hill C. A graphical method for exploring heterogeneity in meta-analyses: Application to a meta-analysis of 65 trials. Stat. Med. 2002;21:2641–2652. doi: 10.1002/sim.1221. [DOI] [PubMed] [Google Scholar]
- 20.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balduzzi S., Rücker G., Schwarzer G. How to perform a meta-analysis with R: A practical tutorial. Evid. Based Ment. Health. 2019;22:153–160. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harrer M., Cuijpers P., Furukawa T., Ebert D.D. Dmetar: Companion R Package For The Guide ’Doing Meta-Analysis in R’, R Package Version 0.0.9000. Chapman and Hall/CRC; London, UK: 2019. [Google Scholar]
- 23.Ozen G., Dell’Aniello S., Pedro S., Michaud K., Suissa S. Reduction of Cardiovascular Disease and Mortality versus Risk of New Onset Diabetes with Statin Use in Patients with Rheumatoid Arthritis. Arthritis Care Res. 2022 doi: 10.1002/acr.24866. Accepted . [DOI] [PubMed] [Google Scholar]
- 24.Kim J.E., Park S., Kim M.S., Kang S.J., Lee J.W., Kim K.S., Kim Y.C., Kim D.K., Joo K.W., Kim Y.S., et al. Statin initiation and all-cause mortality in incident statin-naïve dialysis patients. Atherosclerosis. 2021;337:59–65. doi: 10.1016/j.atherosclerosis.2021.08.026. [DOI] [PubMed] [Google Scholar]
- 25.Yashima F., Hara M., Inohara T., Jinzaki M., Shimizu H., Fukuda K., Tanaka M., Yamamoto M., Watanabe Y., Naganuma T., et al. Statin therapy for patients with aortic stenosis who underwent transcatheter aortic valve implantation: A report from a Japanese multicentre registry. BMJ Open. 2021;11:e044319. doi: 10.1136/bmjopen-2020-044319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng Y.L., Yang H.Y., Wu C.Y., Tsai C.Y., Chen C.Y., Hsiao C.C., Hsu H.H., Tian Y.C., Yen C.L. Does Statin Therapy Reduce the Risks of Mortality and Major Adverse Cardiac and Cerebrovascular Events in Young Adults with End-Stage Renal Disease? Population-Based Cohort Study. J. Clin. Med. 2021;10:2097. doi: 10.3390/jcm10102097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheung K.S., Mak L.Y., Lam L.K., Fung J., Liu F., Seto W.K., Yuen M.F. Statins associate with better clinical outcomes in chronic hepatitis B patients with HBsAg seroclearance. Hepatol. Int. 2021;15:881–891. doi: 10.1007/s12072-021-10197-4. [DOI] [PubMed] [Google Scholar]
- 28.Chhibber A., Hansen S., Biskupiak J. Statin use and mortality in rheumatoid arthritis: An incident user cohort study. J. Manag. Care Spec. Pharm. 2021;27:296–305. doi: 10.18553/jmcp.2021.27.3.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jung H.H. Statin use and outcome risks according to predicted CVD risk in Korea: A retrospective cohort study. PLoS ONE. 2021;16:e0245609. doi: 10.1371/journal.pone.0245609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shavadia J.S., Wilson J., Edmonston D., Platt A., Ephraim P., Hall R., Goldstein B.A., Boulware L.E., Peterson E., Pendergast J., et al. Statins and atherosclerotic cardiovascular outcomes in patients on incident dialysis and with atherosclerotic heart disease. Am. Heart J. 2021;231:36–44. doi: 10.1016/j.ahj.2020.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim S., Choi H., Won C.W. Effects of Statin Use for Primary Prevention among Adults Aged 75 Years and Older in the National Health Insurance Service Senior Cohort (2002–2015) Ann. Geriatr. Med. Res. 2020;24:91–98. doi: 10.4235/agmr.20.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan X.L., E J.Y., Lin Y., Rebbeck T.R., Lu S.E., Shang M., Kelly W.K., D’Amico A., Stein M.N., Zhang L., et al. Individual and joint effects of metformin and statins on mortality among patients with high-risk prostate cancer. Cancer Med. 2020;9:2379–2389. doi: 10.1002/cam4.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin M.S., Lin Y.S., Chang S.T., Wang P.C., Chien-Chia Wu V., Lin W.Y., Chung C.M. Effect of initiating statin therapy on long-term outcomes of patients with dyslipidemia after intracerebral hemorrhage. Atherosclerosis. 2019;288:137–145. doi: 10.1016/j.atherosclerosis.2019.07.009. [DOI] [PubMed] [Google Scholar]
- 34.Köhler-Forsberg O., Gasse C., Petersen L., Nierenberg A.A., Mors O., Østergaard S.D. Statin treatment and the risk of depression. J. Affect. Disord. 2019;246:706–715. doi: 10.1016/j.jad.2018.12.110. [DOI] [PubMed] [Google Scholar]
- 35.Jung M., Lee S. Effects of Statin Therapy on the Risk of Intracerebral Hemorrhage in Korean Patients with Hyperlipidemia. Pharmacotherapy. 2019;39:129–139. doi: 10.1002/phar.2211. [DOI] [PubMed] [Google Scholar]
- 36.Huang T.M., Wu V.C., Lin Y.F., Wang J.J., Shiao C.C., Chen L., Chueh S.J., Chueh E., Yang S.Y., Lai T.S., et al. Effects of Statin Use in Advanced Chronic Kidney Disease Patients. J. Clin. Med. 2018;7:285. doi: 10.3390/jcm7090285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jorge A.M., Lu N., Keller S.F., Rai S.K., Zhang Y., Choi H.K. The Effect of Statin Use on Mortality in Systemic Autoimmune Rheumatic Diseases. J. Rheumatol. 2018;45:1689–1695. doi: 10.3899/jrheum.171389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keller S.F., Rai S.K., Lu N., Oza A., Jorge A.M., Zhang Y., Choi H.K. Statin use and mortality in gout: A general population-based cohort study. Semin. Arthritis Rheum. 2018;48:449–455. doi: 10.1016/j.semarthrit.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 39.Orkaby A.R., Gaziano J.M., Djousse L., Driver J.A. Statins for Primary Prevention of Cardiovascular Events and Mortality in Older Men. J. Am. Geriatr. Soc. 2017;65:2362–2368. doi: 10.1111/jgs.14993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim J.S., Kim W., Park J.Y., Woo J.S., Lee T.W., Ihm C.G., Kim Y.G., Moon J.Y., Lee S.H., Jeong M.H., et al. Effects of statin therapy on clinical outcomes after acute myocardial infarction in patients with advanced renal dysfunction: A propensity score-matched analysis. PLoS ONE. 2017;12:e0183059. doi: 10.1371/journal.pone.0183059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oza A., Lu N., Schoenfeld S.R., Fisher M.C., Dubreuil M., Rai S.K., Zhang Y., Choi H.K. Survival benefit of statin use in ankylosing spondylitis: A general population-based cohort study. Ann. Rheum. Dis. 2017;76:1737–1742. doi: 10.1136/annrheumdis-2017-211253. [DOI] [PubMed] [Google Scholar]
- 42.Fung C.S.C., Wan E.Y.F., Chan A.K.C., Lam C.L.K. Statin use reduces cardiovascular events and all-cause mortality amongst Chinese patients with type 2 diabetes mellitus: A 5-year cohort study. BMC Cardiovasc. Disord. 2017;17:166. doi: 10.1186/s12872-017-0599-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chung C.M., Lin M.S., Hsu J.T., Hsiao J.F., Chang S.T., Pan K.L., Lin C.L., Lin Y.S. Effects of statin therapy on cerebrovascular and renal outcomes in patients with predialysis advanced chronic kidney disease and dyslipidemia. J. Clin. Lipidol. 2017;11:422–431.e422. doi: 10.1016/j.jacl.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 44.Hsu C.Y., Chen Y.T., Su Y.W., Chang C.C., Huang P.H., Lin S.J. Statin Therapy Reduces Future Risk of Lower-Limb Amputation in Patients With Diabetes and Peripheral Artery Disease. J. Clin. Endocrinol. Metab. 2017;102:2373–2381. doi: 10.1210/jc.2016-3717. [DOI] [PubMed] [Google Scholar]
- 45.Holzhauser L., Hovnanians N., Eshtehardi P., Mojadidi M.K., Deng Y., Goodman-Meza D., Msaouel P., Ko Y.A., Zolty R. Statin therapy improves survival in patients with severe pulmonary hypertension: A propensity score matching study. Heart Vessels. 2017;32:969–976. doi: 10.1007/s00380-017-0957-8. [DOI] [PubMed] [Google Scholar]
- 46.Cho E.Y., Myoung C., Park H.S., Kim A.J., Ro H., Chang J.H., Lee H.H., Chung W., Jung J.Y. Efficacy of Statin Treatment in Early-Stage Chronic Kidney Disease. PLoS ONE. 2017;12:e0170017. doi: 10.1371/journal.pone.0170017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sanfilippo K.M., Keller J., Gage B.F., Luo S., Wang T.F., Moskowitz G., Gumbel J., Blue B., O’Brian K., Carson K.R. Statins Are Associated With Reduced Mortality in Multiple Myeloma. J. Clin. Oncol. 2016;34:4008–4014. doi: 10.1200/JCO.2016.68.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tanaka S., Ikari Y., Ijichi T., Nakazawa G. Treat-to-target lipid control is effective but highlighted poor prognosis without indication of statin following percutaneous coronary intervention. Cardiovasc. Interv. Ther. 2017;32:358–364. doi: 10.1007/s12928-016-0419-8. [DOI] [PubMed] [Google Scholar]
- 49.Rothschild D.P., Novak E., Rich M.W. Effect of Statin Therapy on Mortality in Older Adults Hospitalized with Coronary Artery Disease: A Propensity-Adjusted Analysis. J. Am. Geriatr. Soc. 2016;64:1475–1479. doi: 10.1111/jgs.14207. [DOI] [PubMed] [Google Scholar]
- 50.Ble A., Hughes P.M., Delgado J., Masoli J.A., Bowman K., Zirk-Sadowski J., Mujica Mota R.E., Henley W.E., Melzer D. Safety and Effectiveness of Statins for Prevention of Recurrent Myocardial Infarction in 12 156 Typical Older Patients: A Quasi-Experimental Study. J. Gerontol. A Biol. Sci. Med. Sci. 2017;72:243–250. doi: 10.1093/gerona/glw082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ramos R., García-Gil M., Comas-Cufí M., Quesada M., Marrugat J., Elosua R., Sala J., Grau M., Martí R., Ponjoan A., et al. Statins for Prevention of Cardiovascular Events in a Low-Risk Population With Low Ankle Brachial Index. J. Am. Coll. Cardiol. 2016;67:630–640. doi: 10.1016/j.jacc.2015.11.052. [DOI] [PubMed] [Google Scholar]
- 52.Woo J.S., Hwang S.J., Kim H.S., Kim J.B., Kim W.S., Kim K.S., Jeong M.H., Kim W. Effects of Statin Therapy on Clinical Outcomes of Survivors of Acute Myocardial Infarction with Severe Systolic Heart Failure. PLoS ONE. 2015;10:e0144602. doi: 10.1371/journal.pone.0144602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vedel-Krogh S., Nielsen S.F., Nordestgaard B.G. Statin Use Is Associated with Reduced Mortality in Patients with Interstitial Lung Disease. PLoS ONE. 2015;10:e0140571. doi: 10.1371/journal.pone.0140571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun L.M., Lin M.C., Lin C.L., Chang S.N., Liang J.A., Lin I.C., Kao C.H. Statin Use Reduces Prostate Cancer All-Cause Mortality: A Nationwide Population-Based Cohort Study. Medicine. 2015;94:e1644. doi: 10.1097/MD.0000000000001644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu H.H., Chen P.C., Yang Y.H., Wang L.C., Lee J.H., Lin Y.T., Chiang B.L. Statin reduces mortality and morbidity in systemic lupus erythematosus patients with hyperlipidemia: A nationwide population-based cohort study. Atherosclerosis. 2015;243:11–18. doi: 10.1016/j.atherosclerosis.2015.08.030. [DOI] [PubMed] [Google Scholar]
- 56.Schoenfeld S.R., Lu L., Rai S.K., Seeger J.D., Zhang Y., Choi H.K. Statin use and mortality in rheumatoid arthritis: A general population-based cohort study. Ann. Rheum. Dis. 2016;75:1315–1320. doi: 10.1136/annrheumdis-2015-207714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alehagen U., Benson L., Edner M., Dahlström U., Lund L.H. Association Between Use of Statins and Mortality in Patients With Heart Failure and Ejection Fraction of ≥50. Circ. Heart Fail. 2015;8:862–870. doi: 10.1161/CIRCHEARTFAILURE.115.002143. [DOI] [PubMed] [Google Scholar]
- 58.Smith D.H., Johnson E.S., Boudreau D.M., Cassidy-Bushrow A.E., Fortmann S.P., Greenlee R.T., Gurwitz J.H., Magid D.J., McNeal C.J., Reynolds K., et al. Comparative Effectiveness of Statin Therapy in Chronic Kidney Disease and Acute Myocardial Infarction: A Retrospective Cohort Study. Am. J. Med. 2015;128:1252.e1–1252.e11. doi: 10.1016/j.amjmed.2015.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen P.S., Cheng C.L., Chang Y.C., Kao Yang Y.H., Yeh P.S., Li Y.H. Early statin therapy in patients with acute intracerebral hemorrhage without prior statin use. Eur. J. Neurol. 2015;22:773–780. doi: 10.1111/ene.12649. [DOI] [PubMed] [Google Scholar]
- 60.Alehagen U., Benson L., Edner M., Dahlström U., Lund L.H. Association between use of statins and outcomes in heart failure with reduced ejection fraction: Prospective propensity score matched cohort study of 21 864 patients in the Swedish Heart Failure Registry. Circ. Heart Fail. 2015;8:252–260. doi: 10.1161/CIRCHEARTFAILURE.114.001730. [DOI] [PubMed] [Google Scholar]
- 61.De Blois J., Fagerland M.W., Grundtvig M., Semb A.G., Gullestad L., Westheim A., Hole T., Atar D., Agewall S. ESC guidelines adherence is associated with improved survival in patients from the Norwegian Heart Failure Registry. Eur. Heart J. Cardiovasc. Pharmacother. 2015;1:31–36. doi: 10.1093/ehjcvp/pvu010. [DOI] [PubMed] [Google Scholar]
- 62.Yang T.F., Chu H., Ou S.M., Li S.Y., Chen Y.T., Shih C.J., Tsai L.W. Effect of statin therapy on mortality in patients with infective endocarditis. Am. J. Cardiol. 2014;114:94–99. doi: 10.1016/j.amjcard.2014.03.064. [DOI] [PubMed] [Google Scholar]
- 63.Carlsson A.C., Wändell P., Sundquist K., Johansson S.E., Sundquist J. Effects of prescribed antihypertensives and other cardiovascular drugs on mortality in patients with atrial fibrillation and hypertension: A cohort study from Sweden. Hypertens. Res. 2014;37:553–559. doi: 10.1038/hr.2014.32. [DOI] [PubMed] [Google Scholar]
- 64.Wändell P., Carlsson A.C., Sundquist J., Johansson S.E., Bottai M., Sundquist K. Effects of prescribed antithrombotics and other cardiovascular pharmacotherapies on all-cause mortality in patients with diabetes and atrial fibrillation—A cohort study from Sweden using propensity score analyses. Diabetol. Metab. Syndr. 2014;6:2. doi: 10.1186/1758-5996-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lawes C.M., Thornley S., Young R., Hopkins R., Marshall R., Chan W.C., Jackson G. Statin use in COPD patients is associated with a reduction in mortality: A national cohort study. Prim. Care Respir. J. 2012;21:35–40. doi: 10.4104/pcrj.2011.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Orkaby A.R., Driver J.A., Ho Y.L., Lu B., Costa L., Honerlaw J., Galloway A., Vassy J.L., Forman D.E., Gaziano J.M., et al. Association of Statin Use With All-Cause and Cardiovascular Mortality in US Veterans 75 Years and Older. JAMA. 2020;324:68–78. doi: 10.1001/jama.2020.7848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou Z., Ofori-Asenso R., Curtis A.J., Breslin M., Wolfe R., McNeil J.J., Murray A.M., Ernst M.E., Reid C.M., Lockery J.E., et al. Association of Statin Use With Disability-Free Survival and Cardiovascular Disease Among Healthy Older Adults. J. Am. Coll. Cardiol. 2020;76:17–27. doi: 10.1016/j.jacc.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jung J., Bae G.H., Kang M., Kim S.W., Lee D.H. Statins and All-Cause Mortality in Patients Undergoing Hemodialysis. J. Am. Heart Assoc. 2020;9:e014840. doi: 10.1161/JAHA.119.014840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Dongen M.M.E., Aarnio K., Martinez-Majander N., Pirinen J., Sinisalo J., Lehto M., Kaste M., Tatlisumak T., de Leeuw F.E., Putaala J. Use of Statins After Ischemic Stroke in Young Adults and Its Association With Long-Term Outcome. Stroke. 2019;50:3385–3392. doi: 10.1161/STROKEAHA.119.026992. [DOI] [PubMed] [Google Scholar]
- 70.Rusnak J., Behnes M., Schupp T., Lang S., Reiser L., Taton G., Bollow A., Reichelt T., Ellguth D., Engelke N., et al. Statin therapy is associated with improved survival in patients with ventricular tachyarrhythmias. Lipids Health Dis. 2019;18:119. doi: 10.1186/s12944-019-1011-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Al-Gobari M., Agrinier N., Soudant M., Burnand B., Thilly N. Effects of Statins to Reduce All-Cause Mortality in Heart Failure Patients: Findings from the EPICAL2 Cohort Study. Am. J. Cardiovasc. Drugs. 2019;19:497–508. doi: 10.1007/s40256-019-00346-4. [DOI] [PubMed] [Google Scholar]
- 72.Marume K., Takashio S., Nagai T., Tsujita K., Saito Y., Yoshikawa T., Anzai T. Effect of Statins on Mortality in Heart Failure With Preserved Ejection Fraction Without Coronary Artery Disease—Report From the JASPER Study. Circ. J. 2019;83:357–367. doi: 10.1253/circj.CJ-18-0639. [DOI] [PubMed] [Google Scholar]
- 73.Wu C.L., Kor C.T., Chang C.C., Chiu P.F., Tarng D.C., Hsu C.C. Association of Statin Use With Mortality After Dialysis-Requiring Acute Kidney Injury: A Population-Based Cohort Study. Mayo Clin. Proc. 2018;93:1474–1483. doi: 10.1016/j.mayocp.2018.05.034. [DOI] [PubMed] [Google Scholar]
- 74.Lee M.S., Duan L., Clare R., Hekimian A., Spencer H., Chen W. Comparison of Effects of Statin Use on Mortality in Patients With Heart Failure and Preserved Versus Reduced Left Ventricular Ejection Fraction. Am. J. Cardiol. 2018;122:405–412. doi: 10.1016/j.amjcard.2018.04.027. [DOI] [PubMed] [Google Scholar]
- 75.Chung C.M., Lin M.S., Chang C.H., Cheng H.W., Chang S.T., Wang P.C., Chang H.Y., Lin Y.S. Moderate to high intensity statin in dialysis patients after acute myocardial infarction: A national cohort study in Asia. Atherosclerosis. 2017;267:158–166. doi: 10.1016/j.atherosclerosis.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 76.Tsujimoto T., Kajio H., Sugiyama T. Statin Therapy in Patients With Low Serum Levels of Low-Density Lipoprotein Cholesterol. Am. J. Cardiol. 2017;120:1947–1954. doi: 10.1016/j.amjcard.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 77.Elshazly M.B., Stegman B., Puri R. Regression of coronary atheroma with statin therapy. Curr. Opin. Endocrinol. Diabetes Obes. 2016;23:131–137. doi: 10.1097/MED.0000000000000234. [DOI] [PubMed] [Google Scholar]
- 78.Su X., Zhang L., Lv J., Wang J., Hou W., Xie X., Zhang H. Effect of Statins on Kidney Disease Outcomes: A Systematic Review and Meta-analysis. Am. J. Kidney Dis. 2016;67:881–892. doi: 10.1053/j.ajkd.2016.01.016. [DOI] [PubMed] [Google Scholar]
- 79.Citarella A., Linder M., Cammarota S., Sundström A., Kieler H. Influence of statin-potency on the risk of kidney disease—A nationwide cohort study using laboratory data. Pharmacoepidemiol. Drug Saf. 2021;30:210–219. doi: 10.1002/pds.5173. [DOI] [PubMed] [Google Scholar]
- 80.Kim S.W., Kang H.J., Jhon M., Kim J.W., Lee J.Y., Walker A.J., Agustini B., Kim J.M., Berk M. Statins and Inflammation: New Therapeutic Opportunities in Psychiatry. Front. Psychiatry. 2019;10:103. doi: 10.3389/fpsyt.2019.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Di Bello E., Zwergel C., Mai A., Valente S. The Innovative Potential of Statins in Cancer: New Targets for New Therapies. Front. Chem. 2020;8:516. doi: 10.3389/fchem.2020.00516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jankowski J., Floege J., Fliser D., Böhm M., Marx N. Cardiovascular Disease in Chronic Kidney Disease: Pathophysiological Insights and Therapeutic Options. Circulation. 2021;143:1157–1172. doi: 10.1161/CIRCULATIONAHA.120.050686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li G.M., Zhao J., Li B., Zhang X.F., Ma J.X., Ma X.L., Liu J. The anti-inflammatory effects of statins on patients with rheumatoid arthritis: A systemic review and meta-analysis of 15 randomized controlled trials. Autoimmun. Rev. 2018;17:215–225. doi: 10.1016/j.autrev.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 84.Widdifield J., Paterson J.M., Huang A., Bernatsky S. Causes of Death in Rheumatoid Arthritis: How Do They Compare to the General Population? Arthritis Care Res. 2018;70:1748–1755. doi: 10.1002/acr.23548. [DOI] [PubMed] [Google Scholar]
- 85.Mei Z., Liang M., Li L., Zhang Y., Wang Q., Yang W. Effects of statins on cancer mortality and progression: A systematic review and meta-analysis of 95 cohorts including 1,111,407 individuals. Int. J. Cancer. 2017;140:1068–1081. doi: 10.1002/ijc.30526. [DOI] [PubMed] [Google Scholar]
- 86.Blais J.E., Wei Y., Yap K.K.W., Alwafi H., Ma T.T., Brauer R., Lau W.C.Y., Man K.K.C., Siu C.W., Tan K.C.B., et al. Trends in lipid-modifying agent use in 83 countries. Atherosclerosis. 2021;328:44–51. doi: 10.1016/j.atherosclerosis.2021.05.016. [DOI] [PubMed] [Google Scholar]
- 87.Vrecer M., Turk S., Drinovec J., Mrhar A. Use of statins in primary and secondary prevention of coronary heart disease and ischemic stroke. Meta-analysis of randomized trials. Int. J. Clin. Pharmacol. Ther. 2003;41:567–577. doi: 10.5414/CPP41567. [DOI] [PubMed] [Google Scholar]
- 88.Gaudino M., Puskas J.D., Di Franco A., Ohmes L.B., Iannaccone M., Barbero U., Glineur D., Grau J.B., Benedetto U., D’Ascenzo F., et al. Three Arterial Grafts Improve Late Survival: A Meta-Analysis of Propensity-Matched Studies. Circulation. 2017;135:1036–1044. doi: 10.1161/CIRCULATIONAHA.116.025453. [DOI] [PubMed] [Google Scholar]
- 89.Harel Z., Chertow G.M., Shah P.S., Harel S., Dorian P., Yan A.T., Saposnik G., Sood M.M., Molnar A.O., Perl J., et al. Warfarin and the Risk of Stroke and Bleeding in Patients With Atrial Fibrillation Receiving Dialysis: A Systematic Review and Meta-analysis. Can. J. Cardiol. 2017;33:737–746. doi: 10.1016/j.cjca.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 90.Yourman L.C., Cenzer I.S., Boscardin W.J., Nguyen B.T., Smith A.K., Schonberg M.A., Schoenborn N.L., Widera E.W., Orkaby A., Rodriguez A., et al. Evaluation of Time to Benefit of Statins for the Primary Prevention of Cardiovascular Events in Adults Aged 50 to 75 Years: A Meta-analysis. JAMA Intern. Med. 2021;181:179–185. doi: 10.1001/jamainternmed.2020.6084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zein A., Sulistiyana C.S., Khasanah U., Wibowo A., Lim M.A., Pranata R. Statin and mortality in COVID-19: A systematic review and meta-analysis of pooled adjusted effect estimates from propensity-matched cohorts. Postgrad. Med. J. 2022;98:503–508. doi: 10.1136/postgradmedj-2021-140409. [DOI] [PubMed] [Google Scholar]
- 92.Gitsels L.A., Kulinskaya E., Steel N. Survival Benefits of Statins for Primary Prevention: A Cohort Study. PLoS ONE. 2016;11:e0166847. doi: 10.1371/journal.pone.0166847. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.