Abstract
The seroreactivities of both naturally and experimentally infected cats to Bartonella henselae was examined. Serum samples collected weekly from nine cats experimentally infected with B. henselae LSU16 were tested by enzyme-linked immunosorbent assay (ELISA) and Western blot analysis. The magnitude and isotype of the antibody response were investigated by ELISA. Western blot analysis allowed the identification of at least 24 Bartonella-specific antigens recognized by the cats during infection. Antibody titers to specific antigens, as determined by Western blot analysis, ranged from 10 to 640 and varied among the different antibody-antigen interactions. Absorption of sera from an experimentally infected cat, using whole cells and cell lysates of various Bartonella species and other bacteria that commonly colonize cats, supported the identification of those Bartonella-specific antigens recognized by the experimentally infected cats. Furthermore, a number of possible species- and type-specific antigens were identified. Finally, sera obtained from cats at local animal shelters were screened for the presence of antibodies directed against the Bartonella-specific bands identified in the experimentally infected cats. A number of Bartonella-specific antigens have been identified to which strong antibody responses are generated in both experimentally and naturally infected cats, some of which may be useful in diagnosing species- and/or type-specific infections. In addition, the results from these experiments will lead to the development of monoclonal antibodies targeted against those genus-, species-, and type-specific antigens.
Bartonella henselae is the causative agent of human cat scratch disease (CSD) (2, 13, 31, 35) and has been associated with bacillary angiomatosis, bacillary peliosis, recurrent bacteremia (26), and endocarditis (14). Epidemiological evidence indicates that cats serve as vectors for the transmission of B. henselae to people (27, 40, 42, 43). The cat flea (Ctenocephalides felis) may also be involved in transmission of this organism; B. henselae was found in fleas from an infected cat (26), and it was reported that fleas (12) and flea feces (19) transferred from B. henselae-bacteremic cats to specific-pathogen-free cats were capable of transferring the infection. Although cats are the natural reservoir for B. henselae, they appear to be asymptomatic even during bacteremic episodes (29, 35). There is, however, recent evidence to indicate that experimentally infected cats do present with mild clinical signs, such as fever, anorexia, lethargy, and peripheral lymphadenopathy (1, 22), which dissipate within a short time. Naturally infected cats may develop similar clinical signs, but these signs may not be noticed by cat owners.
A strong humoral response to infection with B. henselae has been seen; whether this immune response is protective against future infection is debated (12, 20, 36, 37). Furthermore, some cats with B. henselae bacteremia have high levels of circulating antibodies, and thus the role of the humoral immune response in Bartonella infections is not clear (12). Seroprevalence studies suggest that 3.7 to 65.4% of cats within the United States are positive for antibodies to B. henselae (9, 10, 25). Serologically positive cats are often also bacteremic (7, 11, 29). The mechanism by which this organism is able to survive and replicate within the cat and not cause overt symptoms is unclear. Similarly, the immunological response of the cat to this pathogen is not understood.
At this time, pet cats are not routinely screened for Bartonella infections; screening, however, could be of particular benefit to those owners who are immunocompromised. The most widely used serodiagnostic tool for Bartonella infections in cats are immunofluorescence assays (IFA) (35). The IFA, however, although specific and sensitive, has a number of drawbacks. This assay lends itself poorly to large numbers of samples and is time-consuming and costly. Furthermore, quantitation of IFA requires that titrations be performed, which increases the cost of the test. More recently enzyme-linked immunoabsorbent assays (ELISAs) have become available for diagnosis of Bartonella infections in humans (30) and in cats (21). While the ELISA is similar to the IFA in regard to reported sensitivity (86.2 versus 88%) and specificity (95.9 versus 94%) (21, 36), use of an ELISA has some inherent advantages. For instance, these assays are particularly useful because large numbers of samples can be screened at one time, and the tests are relatively inexpensive.
If the risk of contracting CSD from a pet cat is to be reduced, a better understanding of the history of the feline Bartonella infection must be obtained. This understanding will further aid in the improvement of diagnostic tools used in screening for Bartonella infections. The purpose of this study was to define the feline humoral immunological response to infection with Bartonella species. The hypothesis of this research was that characterization of the feline humoral immune response to infection with B. henselae will lead to the identification of possible genus-, species-, and type-specific antigens recognized by the cat’s immune system. These antigens could serve as targets for the generation of monoclonal antibodies that could be used to improve the sensitivities and specificities of currently available serodiagnostic tools.
The objectives of this research were (i) to utilize an ELISA to quantify the magnitude of antibody responses in nine cats experimentally infected with B. henselae LSU16 and to examine the isotype of these responses; (ii) to perform Western blot analysis on both unabsorbed and absorbed sera from experimentally infected cats in order to identify immunodominant proteins recognized by these cats and to identify those antigens which are possibly Bartonella genus, species, and type specific; and (iii) to examine, by Western blot analysis, sera obtained from cats at local animal shelters for seroreactivity to B. henselae species-specific antigens and to compare the reactivity patterns of naturally infected cats to those of the experimentally infected cats. These results will assist in the identification of those antigens that would be the most valid targets in the development of Bartonella-specific diagnostic screening tests and in determining the usefulness of Western blot analysis as a confirmatory test.
MATERIALS AND METHODS
Bacteria and culture conditions.
B. henselae Houston-1 (ATCC 49882), Bartonella quintana (ATCC VR-358), and Bartonella clarridgeiae (ATCC 700095) were obtained from the American Type Culture Collection (Rockville, Md.). B. henselae LSU16 was isolated at Louisiana State University from a naturally infected cat. B. quintana and B. henselae Houston-1 and LSU16 were grown on chocolate agar under 5% CO2 at 37°C. B. clarridgeiae was grown on rabbit blood agar at 35°C under 5% CO2. The bacteria were scraped from the plates after 5 to 8 days of culture, suspended in heart infusion broth with 25% glycerol, and stored at −70°C until they were used. Bordetella bronchiseptica, Pasteurella multocida, and Escherichia coli were cultivated in Luria-Bertani (39) broth at 37°C for 24 h.
Cats.
Six 12- to 16-week-old kittens (cats 37, 40, 58, 39, 50, and 54) were obtained from animal shelters and underwent conditioning at the Division of Laboratory Animal Medicine, which included vaccination and treatment for internal and external parasites. Three additional cats (cats 182, 184, and 223) were obtained from Harlan-Sprague-Dawley, Inc. (Indianapolis, Ind.). At the commencement of the study all nine cats were 6 to 8 months old and negative for B. henselae by culture and serology. The cats were maintained at the Division of Laboratory Animal Medicine, Louisiana State University. All cats were allowed water and food ad libitum and were housed in individual cages according to the policies outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Sera for the Bartonella seroprevalence study were obtained from cats at animal shelters in East Baton Rouge, West Baton Rouge, Plaquemine, and Lafayette parishes from May through August 1996.
Experimental infection.
All cats were sedated with Telazol (Tiletamine HCL and Zolazepam HCL [Fort Dodge Animal Health, Fort Dodge, Iowa]; 7 mg/kg) prior to venous puncture and experimental infection. Nine cats were infected intradermally with 5 × 107 CFU of B. henselae LSU16 in phosphate-buffered saline (1.9 mM NaH2PO4, 8.1 mM Na2HPO4 [pH 7.2], 154 mM NaCl). All cats were monitored on a daily basis for fever, lymphadenopathy, malaise, anorexia, and any abnormal behavior. Blood was collected by jugular vein puncture every week for 12 weeks (cats 182, 184, and 223), 16 weeks (cats 39, 50, and 54), and 26 weeks (cats 37, 40, and 58) and cultured for bacterial growth. Serum samples were analyzed by ELISA and Western blot analysis.
Blood culture.
Blood for culture was collected in 1.5-ml pediatric lysis-centrifugation isolator tubes (Wampole Laboratories, Cranbury, N.J.). A 10-μl aliquot of blood was removed from the isolator tube and 10-fold serially diluted. The dilutions were then inoculated onto chocolate agar plates. The plates were incubated for 1 to 2 weeks at 37°C under 5% CO2 and checked regularly for bacterial growth. The number of colonies observed was recorded as the number of CFU per milliliter of blood. Isolates were periodically examined microscopically with a Gram stain.
ELISA.
B. henselae Houston-1 was incubated in 50% (vol/vol) methanol for 4 days to kill the bacteria. The killed bacteria were lyophilized, resuspended at 1 mg (dry weight) per ml, and treated with 1% (wt/vol) sodium dodecyl sulfate (SDS). The bacterial suspension was diluted to 1 μg/ml in sodium carbonate buffer (0.1 M Na2CO3, 0.1 M NaHCO3, pH 9.6) and applied to polystyrene Immulon-4 96-well plates (100 μl/well; 1 μg of protein and 0.001% [wt/vol] SDS per 100 μl). The plates were incubated overnight at room temperature and then washed five times with ELISA wash buffer (50 mM Tris, 1 M EDTA, 250 mM NaCl [pH 7.4], and 0.05% [vol/vol] Tween 20). Feline serum samples and positive and negative controls were diluted 1:200 in ELISA sample buffer (50 mM Tris, 1 M EDTA, 250 mM NaCl, pH 7.4), and 100 μl was added to the plates in triplicate. The plates were incubated for 30 min at room temperature and then washed five times with ELISA wash buffer. One hundred microliters of a 1:12,800 dilution of peroxidase-conjugated, affinity-purified goat anti-cat immunoglobulin G (IgG) (Fc fragment; Bethyl Laboratories, Inc., Montgomery, Tex.) was used for IgG testing. One hundred microliters of a 1:6,500 dilution of peroxidase-conjugated, affinity-purified goat anti-cat IgM (μ chain specific; Bethyl Laboratories, Inc.) was used for IgM testing. The conjugate was incubated in the wells for 30 min, and they were washed as before. Next, 100 μl of TMB (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.) mixed according to the manufacturer’s instructions was added to the wells and incubated for 10 min, and the incubation was stopped by addition of 100 μl of 1 M H2SO4. The plates were read at 410 nm with an ELISA plate reader (Dynatech Laboratories, Inc., Chantilly, Va.). The optical densities (OD) of the triplicate wells were averaged in order to obtain an average OD for each test serum.
Western blot analysis.
Bartonella species cultured as described above were adjusted to a final OD at 600 nm of 1, and a bacterial lysate for each species was prepared. The cells were subjected to centrifugation at 12,000 × g for 10 min, and then the pelleted cells were resuspended in a half volume of sample buffer (62.5 mM Tris, 10% [vol/vol] glycerol, 5.0% [vol/vol] 2-mercaptoethanol, 2.3% [wt/vol] SDS), vortexed for 3 min, and boiled for 10 min. Western blot analysis was performed with modifications as described for other bacteria (38). Briefly, 75 μl of prepared lysate were electrophoresed on a 12% (wt/vol) polyacrylamide two-well preparation minigel (Bio-Rad Laboratories, Richmond, Calif.) at 100 V for 60 to 75 min. Electrophoretically separated antigens were then transferred to pure nitrocellulose protein transfer membrane (Schleicher & Schuell, Keene, N.H.) at 100 V for 90 min with an electrophoretic transfer cell (Mini-Trans-Blot; Bio-Rad Laboratories). The blotted membranes were blocked overnight with 10% (wt/vol) nonfat dry milk in NET (150 mM NaCl, 1.0 M EDTA, 50 mM Tris, pH 7.4) (milk-NET) and washed in NET with 0.05% (vol/vol) Tween 20 (NET-T) for 20 min. Feline sera diluted 1:10 in milk-NET were applied to the blots with a 28-chamber miniblotter (Miniblotter 28; Immunetics, Cambridge, Mass.) and incubated at room temperature for 1 h. The blots were then washed with NET-T and incubated with peroxidase-conjugated, affinity-purified F(ab′) fragment goat anti-cat IgG (H + L) (Jackson Immunoresearch Inc., Avondale, Pa.) diluted at 1:5,000 in milk-NET for 1 h. Antibody-bound conjugate was detected by enhanced chemiluminescence (ECL Western blot detection system; Amersham Life Science, Inc., Arlington Heights, Ill.). The enhanced chemiluminescence reagents were diluted 1:2 in distilled water.
Antibody titers for three cats (cats 58, 39, and 182) were examined by Western blot analysis. Sera obtained from weeks 0, 4, 8, 12, 16, and 20 were each diluted 1:10, 1:40, 1:160, 1:640, 1:2,560, and 1:10,240 with milk-NET. Upon dilution, the serum samples were processed in the manner described above.
Absorptions.
Cells from B. henselae Houston-1 and LSU16, B. clarridgeiae, and B. quintana were scraped from 20 to 30 chocolate agar plates, subjected to centrifugation at 15,000 × g for 20 min, and resuspended in lysis buffer (50 mM Tris-HCL [pH 7.5], 50 mM NaCl, 5% [vol/vol] glycerol, 1 mM phenylmethylsulfonyl fluoride [PMSF; Sigma Chemical Co., St. Louis, Mo.], and 1% [vol/vol] aprotinin [Sigma]). One-liter cultures of B. bronchiseptica, P. multocida, and E. coli TB1 were propagated in Luria-Bertani broth, subjected to centrifugation at 15,300 × g for 30 min, and resuspended in lysis buffer. Each suspension was sonicated (Sonic Dismembrator model 300; Fischer Scientific, Pittsburgh, Pa.) four times for 30 s each time at 30% maximum power. After sonication, each lysate was subjected to centrifugation at 27,000 × g for 20 min. The supernatant was used as a cell lysate, and the remaining pellet was used as a whole organism. The protein concentration was determined for each cell lysate with the bicinchoninic acid protein detection system (Pierce Chemical Co., Rockford, Ill.) and was adjusted to 2.0 mg/ml. Twenty micrograms of whole organism was resuspended in 500 μl of undiluted feline serum with 0.5 mM PMSF and incubated overnight on a rocker at room temperature. The whole-cell-absorbed serum was then subjected to centrifugation at 27,000 × g for 10 min, and the supernatant was collected. Two hundred fifty microliters of whole-cell-absorbed serum was diluted 1:10 with lysis buffer and stored at −20°C until it was used, and an additional 200 μl of whole-cell-absorbed serum was used for the cell lysate absorption. Six hundred microliters of cell lysate was added to 200 μl of whole-cell-absorbed serum. This was incubated on a rocker at room temperature for approximately 5 h; then, 1 ml of cell lysate was added and incubated overnight on a rocker at room temperature. Finally, 200 μl of cell lysate (for a final serum dilution of 1:10) was added and incubated for 3 h. As a control, unabsorbed serum was treated in the same manner as the whole-cell- and whole-cell- and cell lysate-absorbed sera, with the exception that lysis buffer alone, as opposed to cell lysate, was added at the specified times. Unabsorbed, whole-cell-absorbed, and whole-cell- and cell lysate-absorbed sera were examined by Western blot analysis.
RESULTS
ELISA for the detection of B. henselae-specific antibodies.
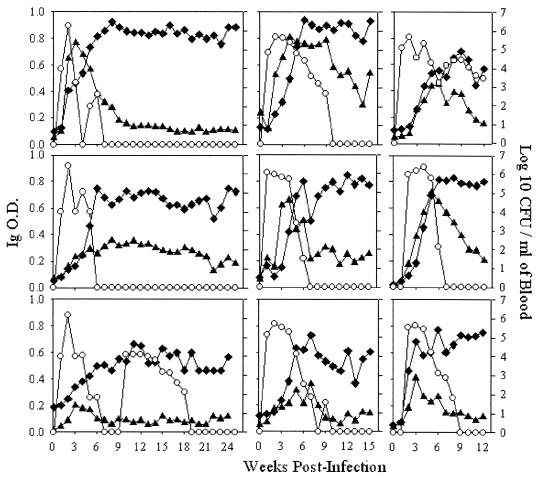
ELISA analysis was performed in order to examine the magnitude of antibody response elicited in cats experimentally infected with B. henselae LSU16 and to examine the isotype of the antibody response. B. henselae-specific IgM and IgG responses, as well as decreasing primary bacteremia with concurrent increasing IgG levels, were seen in all experimentally infected cats. The IgG response in these experimentally infected cats did not fall over time. While the magnitude of the antibody response varied among the cats, a similar pattern, in which peak IgM levels were followed by a rise in IgG antibodies, was observed. Three types of antibody responses were seen in these cats: one in which a strong peak in IgM antibodies occurred within 3 to 5 weeks and was closely followed and surpassed by a strong IgG antibody response (cats 37, 39, 50, and 184); another in which the IgM peak did not occur until between 6 and 8 weeks and was lower than that seen in cats 37, 39, 50, and 184 but was closely followed with a peak in IgG levels (cats 40, 54, and 182); and finally, an IgM peak that occurred at 3 weeks but was much lower than that in the other cats with IgM peaks at 3 weeks (cats 58 and 223). Interestingly, cat 58 underwent a secondary bacteremia that occurred without an additional peak in IgM levels or a rise in IgG levels as seen during primary bacteremia. Furthermore, the magnitude of the IgG response was lower in cat 58 and developed much more slowly than those in the other cats. Bacteremias lasted for 5 to 7 weeks in cats 37, 40, 50, and 184 and for 9 to 13 weeks in cats 39, 54, 182, and 223; bacteremia was recurrent in cat 58 (Fig. 1).
FIG. 1.
Bacteremias and antibody responses of nine cats experimentally infected with B. henselae LSU16. The OD (primary y axis) for IgM (▴) and IgG (⧫) were determined by ELISA. The OD value at each time point are the averages of triplicate wells. Bacteremia levels (○); secondary y axis) are also shown. Bacteremia levels and antibody responses were followed for 25 weeks postinfection in cats 37 and 40; 24 weeks postinfection in cat 58; 15 weeks postinfection in cats 39, 50, and 54; and 12 weeks postinfection in cats 182, 184, and 223.
Western blot analysis for the identification of immunodominant antigens recognized by experimentally infected cats.
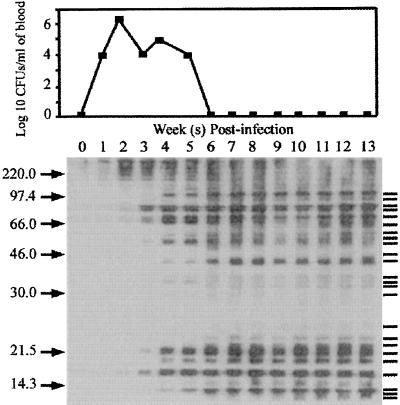
Serum samples collected on a weekly basis were examined by Western blot analysis to identify immunodominant antigens recognized by the experimentally infected cats and to determine if there was a predominant pattern of seroreactivity in these cats (Fig. 2). Sera from all nine experimentally-infected cats exhibited similar patterns of immunoreactivity, the exception being the kinetics of the appearance of antibody to some of the Bartonella-specific antigens recognized by the cats’ immune systems (Fig. 3). At least 24 unique antigens that were recognized by the immune system of each experimentally infected cat were identified. A representative blot is shown in Fig. 2 (cat 40). A strong response was elicited to the following antigens: 97.0, 76.0, 69.0, 65.0, 57.0, 54.0, 45.0, 16.9, 13.3, and 11.3 kDa. This response was evident by no later than four weeks postinfection and was maintained throughout the course of observation for each cat. A strong response to the 7.0-, 8.0-, 18.0-, 19.0-, 21.7-, and 51.0-kDa antigens was also elicited in each cat, but antibodies to these antigens were not detected in all cats until between 6 and 8 weeks postinfection. Antibodies to these antigens were also maintained throughout the course of observation. Antibody responses to the 15.0-kDa band were relatively weak in cats 39, 54, 182, and 223. Responses to the 28.2-kDa band were also weak in all cats examined. Immune responses to the 15.0-, 28.2-, 30.0-, 33.7-, 36.0-, 48.0-, 60.5-, and 81.0-kDa antigens were weak, and they waxed and waned throughout the course of infection.
FIG. 2.
Representative immunoblot from the analysis of antigen-specific seroreactivity in an experimentally infected cat (cat 40). B. henselae LSU16 antigen preparations were electrophoretically separated on 12% polyacrylamide gels, transferred to nitrocellulose, and reacted with 1:10 dilutions of cat sera from each week postinfection. The twenty-four Bartonella-specific antigens identified are indicated on the right ( ); the approximate molecular masses of those antigens are (from top to bottom) 97.0, 81.0, 76.0, 69.0, 60.5, 57.0, 54.0, 51.0, 48.0, 45.0, 36.0, 33.3, 30.0, 28.7, 21.7, 19.0, 18.0, 16.9, 15.0, 13.3, 11.3, 8.0, and 7.0 kDa. Molecular mass markers (in kilodaltons) are shown on the left. The top panel is a plot of the log 10 CFU/ml of blood determined at each week postinfection.
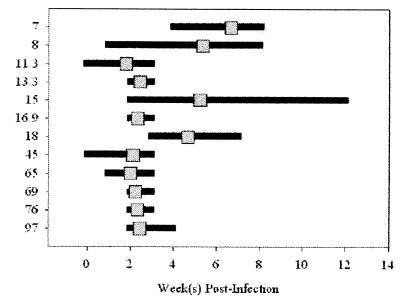
FIG. 3.
Kinetics of Bartonella-specific antibody appearance. Serum from each time point postinfection for each of the nine cats was examined by Western blot analysis for the first appearance of B. henselae antigen-specific antibodies. Specifically, the serum was analyzed for seroreactivity to those molecular mass antigens indicated on the left (in kilodaltons). The horizontal bars indicate the range of weeks postinfection in which antibodies to a particular molecular mass antigen first appeared in each of the nine cats. The gray squares represent the average week postinfection for each of the nine cats in which antibodies to a particular antigen were first detectable.
In order to further examine the magnitude of the feline immune response to infection with B. henselae, antibody titers to the 97.0-, 76.0-, 69.0-, 65.0-, 45.0-, 16.9-, 15.0-, 13.3-, and 11.3-kDa antigens were examined by Western blot analysis at 0, 4, 8, 12 (cats 39, 58, and 182), 16 (cats 39 and 58), and 20 (cat 58) weeks postinfection (Table 1). One cat from each pattern of bacteremia was chosen for titer determination. The peak magnitude of antibody titers to the 97.0-, 76.0/69.0-, and the 65-kDa antigens reached 640 for cats 39 and 182 and 160 for cat 58. Peak titers of antibody to the 16.9-, 13.3-, and 11.3-kDa antigens reached 160 for cats 39 and 182 and 40 for cat 58. Cat 39 had a titer of 160 of antibody to the 45-kDa protein, while cats 58 and 182 had a peak titer of 40. The peak titers of antibody to the 15-kDa antigen were low: 40 in cats 58 and 39 and 10 in cat 182. In the cat with recurrent bacteremia (cat 58), the titers of antibody to the 11.3-, 45.0-, 65.0-, and 76.0/69.0-kDa antigens rose and fell with the bacteremic state of the cat. Furthermore, the titers of antibody to the 11.3-, 13.3-, 15.0-, 16.9-, and 45.0-kDa antigens waned in cat 58.
TABLE 1.
Titers in three experimentally-infected cats of antibody to eight B. henselae LSU16 antigens
| Molecular mass band (kDa) | Cat no. | Titer
|
|||||
|---|---|---|---|---|---|---|---|
| 0a | 4 | 8 | 12 | 16 | 20 | ||
| 58 | <10 | 40 | 40 | 160 | 160 | 40 | |
| 97.0 | 39 | <10 | 160 | 160 | 160 | 160 | |
| 182 | <10 | <10 | 160 | 160 | |||
| 58 | <10 | 160 | 40 | 160 | 160 | 40 | |
| 76.0/69.0 | 39 | <10 | 640 | 640 | 640 | 160 | |
| 182 | <10 | 160 | 640 | 640 | |||
| 58 | 10 | 160 | 40 | 160 | 160 | 40 | |
| 65.0 | 39 | <10 | 640 | 640 | 160 | 160 | |
| 182 | <10 | 160 | 640 | 640 | |||
| 58 | <10 | 40 | 10 | 40 | 10 | <10 | |
| 45.0 | 39 | <10 | 40 | 160 | 40 | 40 | |
| 182 | <10 | 10 | 40 | 40 | |||
| 58 | <10 | 40 | 40 | 40 | 10 | <10 | |
| 16.9 | 39 | <10 | 40 | 160 | 40 | 40 | |
| 182 | <10 | 10 | 160 | 160 | |||
| 58 | <10 | 10 | 10 | 40 | <10 | <10 | |
| 15.0 | 39 | <10 | <10 | 40 | <10 | <10 | |
| 182 | <10 | <10 | <10 | 10 | |||
| 58 | <10 | 40 | 40 | 40 | <10 | <10 | |
| 13.3 | 39 | <10 | 40 | 160 | 40 | 40 | |
| 182 | <10 | 10 | 40 | 160 | |||
| 58 | <10 | 40 | 10 | 40 | 10 | <10 | |
| 11.3 | 39 | <10 | <10 | 160 | 160 | 160 | |
| 182 | 10 | 10 | 40 | 160 | |||
| CFU/ml of blood | 58 | 0 | 1.0 × 104 | 0 | 1.2 × 104 | 1.3 × 103 | 0 |
| 39 | 0 | 2.4 × 105 | 1.6 × 103 | 0 | 0 | ||
| 182 | 0 | 2.2 × 105 | 2.6 × 104 | 3.0 × 103 | |||
Week postinfection.
Absorptions.
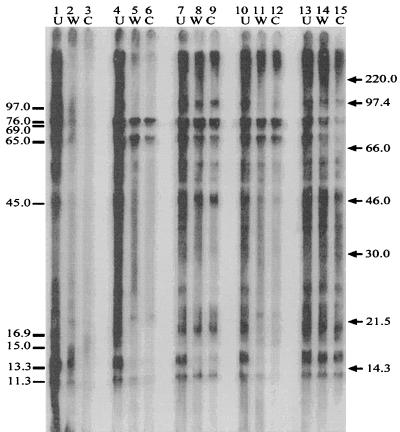
In order to confirm the Bartonella specificity of those antigens recognized by the experimentally infected cats, absorptions with whole cells and cell lysates of B. bronchiseptica, P. multocida, and E. coli, as well as various species of Bartonella, including B. clarridgeiae, B. quintana, B. henselae Houston-1, and B. henselae LSU16, were performed. Whole-cell absorption with B. henselae LSU16 removed a majority of the seroreactivity, indicating that in these experimentally infected cats, most of the antibodies generated were against external antigens; absorption with bacterial cell lysate removed the remaining reactive antibodies (Fig. 4, lanes 1 to 3). Whole-cell and cell lysate absorption with B. bronchiseptica, P. multocida, and E. coli did not appear to have any effect on the Western blot antibody-antigen profile (Fig. 4, lanes 13 to 15). These results suggest that most of the seroreactive antigens identified by Western blot analysis are likely Bartonella-specific, since they do not cross-react with the other bacteria that commonly colonize cats. Absorptions performed with other species of Bartonella that are known to be cross-reactive to some extent support these results. B. henselae Houston-1 absorption (Fig. 4, lanes 4 to 6) and B. quintana absorption (Fig. 4, lanes 10 to 12) presented with antibody profiles similar to that seen with B. henselae LSU16 with the exception of three antibody-antigen interactions; B. henselae Houston-1 and B. quintana absorption did not remove antibodies to the 76.0-, 69.0-, and 65.0-kDa antigens. These antibodies were also not removed by absorption with B. clarridgeiae (Fig. 4, lanes 7 to 9). Although absorption with B. clarridgeiae did remove most seroreactivity to the 13.3- and 21.7-kDa antigens, B. clarridgeiae absorption had little effect on the overall antibody-antigen profile.
FIG. 4.
Immunoabsorption. Cat serum (cat 40) that was previously absorbed with whole cells (W) and cell lysates (C) of B. henselae LSU16 (lanes 2 and 3), B. henselae Houston-1 (lanes 5 and 6), B. clarridgeiae (lanes 8 and 9), B. quintana (lanes 11 and 12), or B. bronchiseptica, P. multocida, and E. coli (lanes 14 and 15) is shown. Unabsorbed serum (U) (lanes 1, 4, 7, 10, and 13) from cat 40 (B. henselae LSU16 infected) was treated in the same manner as the absorbed sera. The sera were allowed to react with B. henselae LSU16 antigen. Bartonella-specific antigens which were focused upon in this experiment are indicated on the left (in kilodaltons). Molecular mass markers are shown on the right (in kilodaltons).
Bartonella antigen-specific seroreactivity in cats from local animal shelters.
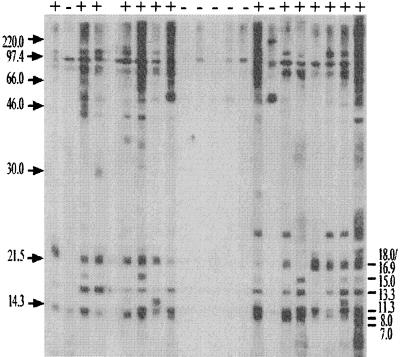
Sera from 601 cats at local animal shelters were screened for B. henselae Houston-1 seroreactivity by Western blot analysis. These blots were examined for the predominance of those Bartonella immunoreactive antigens recognized by the experimentally infected cats (Fig. 5). Slight variations from blot to blot required that those bands which separated as doublets or triplets be considered as one band. Thus, all sera were examined for reactivity to 16 different proteins with the following molecular masses: 7.0, 8.0, 11.3, 13.3, 15.0, 16.9/18.0, 19.0, 21.7, 28.2, 30.0/36.0, 45.0, 48.0, 51.0/57.0, 60.5/65.0, 69.0/81.0, and 97.0 kDa (Table 2). Reactivity was observed most often with the 11.3-, 13.3-, 16.9/18.0-, 51.0-/57.0-, 60.5/65.0-, 69.0-/81.0-, and 97.0-kDa antigens. There were 279 different banding patterns observed, most representing one or two animals; however, 21 patterns which were each found in at least four cats accounted for 43% of the cats. Sera from 72 cats were reactive with all 16 bands. Forty cats showed no reactivity to the 16 bands. The most common patterns of Western blot reactivity are shown in Table 3.
FIG. 5.
Representative immunoblot used in the determination of the prevalence in 601 cats of Bartonella-specific antibodies to B. henselae Houston-1 antigen. Sera which reacted to two of the six molecular mass bands indicated on the right (in kilodaltons) were considered seropositive (+) for Bartonella-specific antibodies. Whether a cat was considered seropositive or seronegative (−) is shown at the top. The sera were also examined for antibodies to the following Bartonella-specific bands: 19.0, 21.7, 28.7, 30.0 to 36.0, 45.0, 48.0, 51.0 to 57.0, 60.5/65.0, 69.0 to 81.0, and 97.0 kDa.
TABLE 2.
Summary of Bartonella antibody reactivity in 601 shelter cats
| Molecular mass (kDa) | No. reactive |
|---|---|
| 7.0 | 303 |
| 8.0 | 350 |
| 11.3 | 462 |
| 13.3 | 463 |
| 15.0 | 255 |
| 16.9/18.0 | 452 |
| 19.0 | 331 |
| 21.7 | 290 |
| 28.2 | 173 |
| 30.0/36.0 | 269 |
| 45.0 | 388 |
| 48.0 | 327 |
| 57.0 | 461 |
| 60.5/65 | 482 |
| 69.0/81.0 | 515 |
| 97.0 | 470 |
TABLE 3.
Most common patterns of Western blot reactivity from sera of 601 shelter cats
| Mass (kDa) | Reactivitya
pattern:
|
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | |
| 7.0 | + | + | + | + | + | + | + | + | − | + | − | + | + | − | − | − | − | − | − | − | − |
| 8.0 | + | + | + | + | + | + | + | + | − | + | − | + | + | − | + | + | − | − | − | − | − |
| 11.3 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | − | − | − | − | − |
| 13.8 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | − | − | − | − | − |
| 15.0 | + | + | + | − | + | + | − | − | − | + | + | + | − | − | + | + | − | − | − | − | − |
| 16.8/18.0 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | − | − | − | − | − |
| 19.0 | + | + | + | + | + | + | + | + | − | + | + | + | − | − | + | + | − | − | − | − | − |
| 21.7 | + | + | + | + | + | − | + | + | − | + | + | + | − | − | + | + | − | − | − | − | − |
| 28.2 | + | − | − | + | + | + | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − |
| 30.0/36.0 | + | + | − | + | + | + | + | − | − | − | + | + | − | − | − | + | − | − | − | − | − |
| 45.0 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | − | − | + | − | − |
| 48.0 | + | + | + | + | − | + | + | + | − | − | + | − | + | + | + | + | − | − | − | − | − |
| 51.0/57.0 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | − | − | − | − |
| 60.5/65.0 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | − | − | − | − |
| 69.0/81.0 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | − | − | + | − |
| 97.0 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | − | + | − | − | − |
| Overall reactivity | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | − | − | − | − | − |
| No. with patternb | 72 | 30 | 14 | 9 | 9 | 8 | 7 | 7 | 7 | 6 | 6 | 5 | 5 | 4 | 4 | 4 | 4 | 5 | 5 | 9 | 40 |
+, positive; −, negative.
Totals represent 197 of 482 (40.9%) seropositive cats and 63 of 119 (52.9%) seronegative cats.
The goal of this experiment was to compare the patterns of reactivity seen in the naturally infected cats with that seen in the experimentally infected cats. Because the cats from the animal shelters could have been at any week postinfection when serum was collected and because they could have been infected with one or more species or types of Bartonella, and considering the kinetics of antibody appearance and antibody persistence in the experimentally infected cats, six antigens were chosen for use in defining a positive cat: 7.0, 8.0, 11.3, 13.3, 15.0, and 16.9/18.0 kDa. Further, to include the detection of early infections, the requisite that the serum only needed to react with two of the six molecular mass antigens was established. Therefore, in this study, a cat was considered Bartonella-seropositive if it reacted with two of the following six antigens: 7.0, 8.0, 11.3, 13.3, 15.0, and 16.9/18.0 kDa.
Using the above definition, the prevalence of Bartonella seroreactivity in this population of cats from the local animal shelters was determined to be 80% (482 of 601). As would be expected, the naturally infected cats had a wider variability of banding patterns than did the experimentally infected cats. More variable patterns were seen in the positive cats than in the negative cats (Table 3). The 16 most common positive patterns represent 32.8% of all the cats (197 of 601) and 40.9% (197 of 482) of the positive cats. The five most common negative patterns represent 10.5% of all the cats (63 of 601) and 53.9% (63 of 119) of the negative cats. The six defining bands are absent (0 of 63) in the five most common negative patterns. Table 4 summarizes the most common banding patterns with the selected antigens. There were 234 different banding patterns observed among the 482 seropositive cats, most representing one or two animals; however, 13 patterns which were each found in at least five cats accounted for 92.7% (447 of 482) of the positive cats. There were 45 different banding patterns observed among the 119 seronegative cats, with the 4 most common negative patterns accounting for 94.1% (112 of 119) of all the negative cats. Thirty-eight percent (182 of 482) of the positive cats had antibodies to all six bands, and 83% (400 of 482) of the positive cats had antibodies to the 11.3-, 13.3-, and 16.9/18.0-kDa bands.
TABLE 4.
Most common Western blot reactivity patterns of sera from 601 shelter cats with selected antigens
| Mass (kDa) | Reactivitya
pattern:
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | |
| 11.3 | + | + | + | + | + | + | + | − | − | + | + | + | + | − | − | + | − |
| 13.8 | + | + | + | + | + | + | + | + | + | − | + | + | + | + | − | − | − |
| 16.9 | + | + | + | + | + | + | − | + | + | + | + | + | − | − | + | − | − |
| 7 | + | + | − | − | − | − | − | − | + | + | + | + | + | − | − | − | − |
| 8 | + | + | − | − | + | + | − | − | + | + | − | − | + | − | − | − | − |
| 15 | + | − | − | + | + | − | − | − | − | − | − | + | − | − | − | − | − |
| Overall reactivity | + | + | + | + | + | + | + | + | + | + | + | + | + | − | − | − | − |
| No. with patternb | 182 | 77 | 53 | 28 | 26 | 21 | 18 | 11 | 9 | 6 | 6 | 5 | 5 | 5 | 6 | 15 | 86 |
+, positive; −, negative.
Totals represent 447 of 482 (92.7%) seropositive cats and 112 of 119 (94.1%) seronegative cats.
DISCUSSION
Immunofluorescent-antibody tests are currently the most widely used serological tests for the diagnosis of CSD in humans. Regnery et al. (35) and Zangwill et al. (44) report an 88% sensitivity and a 94% specificity for the IFA. ELISAs, which are inherently less subjective, less expensive, and less time-consuming than the IFA, are also being developed. Litwin et al. (30) reported on an enzyme immunoassay (EIA) to detect IgM and IgG antibodies to B. henselae based on an outer membrane preparation of B. henselae; the sensitivity and specificity of this test were indicated to be 94.1 and 99.2%, respectively, for IgM and 86.2 and 95.9%, respectively, for IgG. Western blot analysis of sera from patients with CSD has also been performed in order to identify critical antigens involved in eliciting an immune response in humans. A 17-kDa antigen has been identified that, when used as an EIA test antigen for Bartonella seroreactivity, showed an agreement of 92% with IFA-positive serum samples and 88% with IFA-negative serum samples (3). The outer membrane preparation used by Litwin et al. (30) consisted of several antigens that reacted with sera from patients with CSD: 209-, 208.5-, 208-, 116-, 80.0-, 30.0-, 23.0-, 10.0-, and 8.0-kDa bands, along with a cluster of proteins with molecular masses ranging from 35.0 to 49.0 kDa. A strong correlation between reactivity to the 8.0-kDa band and a positive result by IFA and EIA was reported (30). The approximate molecular masses of other Bartonella-immunoreactive antigens that have been reported include 97.0-, 69.0-, 45.0-, and 23.0-kDa (41) and 48.5-kDa (32). Identification of these immunodominant antigens is leading to the development of monoclonal antibodies targeted to those specific antigens. For instance, it has recently been reported that B. henselae species-specific monoclonal antibodies to a 40.0- and a 30.0-kDa antigen have been generated (33). Identification of immunodominant antigens and the generation of monoclonal antibodies to these antigens should lead to a greater understanding of the human immune response to CSD and to improved diagnostic tests.
While a great deal of progress has been made in studying the human humoral immune response to CSD, less is known about the feline humoral immune response to Bartonella infections. Furthermore, the role of antibody in the pathogenesis of Bartonella infections in both humans and cats has not been determined. Feline serological surveys to date have been done by IFA (10, 11, 23, 25, 35). EIAs with outer membrane preparations for detecting serum antibodies in experimentally infected cats have also been utilized (21). Currently, there are no reports on Western blot analysis of feline sera for the purpose of screening for Bartonella-reactive antibodies. Thus, the aims of this study were (i) to examine the feline humoral immune response to infection with B. henselae by ELISA; (ii) to identify, using Western blot analysis and absorption assays, those antigens which are recognized by the cat’s immune system and possible species- and type-specific immunoreactive antigens; and (iii) to determine the seroprevalence of Bartonella in cats from local animal shelters based on the presence of antibodies to those Bartonella-specific antigens identified in step 2 and to examine the sensitivities and specificities of individual bands against an internal standard.
Using nine cats experimentally infected with B. henselae, we were able to follow the development of the feline humoral immune response to B. henselae. All nine cats developed IgM and IgG responses that, although variable in magnitude between cats, were characterized by a peak in IgM levels followed by a rise in IgG levels. In each cat, primary bacteremia decreased as IgG levels increased. Decreasing bacteremia with increasing IgG levels has been reported in other studies (5). Three patterns of bacteremia were seen in the nine experimentally infected cats: one pattern in which cats became abacteremic by week 7 (cats 37, 40, 50, and 184), a second pattern in which cats became abacteremic between weeks 9 and 13 (cats 39, 54, 182, and 223), and a third pattern in which the cat underwent a secondary bacteremia (cat 58). Interestingly, in the one cat (58) that underwent a secondary bacteremia the IgM levels remained low throughout the course of infection, the IgG response developed much more slowly than those in the other eight cats, and virtually no change in the IgM levels was seen from the end of the primary bacteremia to the commencement of the secondary bacteremia. The apparent abacteremic state of cat 37 in week 4, cat 54 in week 8, and cat 58 in weeks 7 to 9 may represent bacteremias below the level of detection (<33 CFU/ml of blood) in our assay rather than true abacteremia.
Western blot analysis of the sera from the experimentally infected cats identified at least 24 Bartonella-specific bands that were immunogenic in all nine cats. We observed detectable levels of antibodies to some of these antigens (11.3-, 13.3-, 16.0/18.0-, 45.0-, 65.0-, 69.0-, and 76.0-kDa bands) by 3 weeks postinfection, while others did not appear until 4 to 8 weeks postinfection (7.0-, 8.0-, 19.0-, 21.7-, 30.0-, 33.3-, 36.0-, 48.0-, 51.0-, 54.0-, 57.0-, 60.5-, 81.0-, and 97.0-kDa bands). The different kinetics for antigen-specific antibody responses seen with each cat suggest that the antigenicity of B. henselae LSU16 is dependent upon the individual cat’s immune system.
While it is difficult to compare bands of reactivity in Western blots from one study to another because of variations in protein preparations and electrophoretic conditions, many of the same molecular mass antigens reported in this study have been found in other studies with human sera. However, it has not been determined whether any of the antigens identified in this study are equivalent to those identified in the human serum studies. Those antigens identified in this study that have not been found in other Western blotting studies may reflect differences between the immune responses of humans and cats. In addition, these differences could be a result of antigenic variability among B. henselae strains.
The titer in serum from one cat with each pattern of bacteremia was determined in order to examine the magnitude of the antibody responses to particular antigens and to determine if there was any relationship between the titer of the antibody response and the bacteremic state of the cat. Cat 58, which underwent a secondary bacteremia, generated an antibody titer lower than that generated by cats 39 and 182 to all of the bands examined. Furthermore, the titer of the antibody response generated by cat 182 increased much more slowly than that generated by cat 39, even though the levels of bacteremia were approximately the same throughout the course of infection (except that the bacteremia in cat 182 persisted for 3 weeks longer than that in cat 39). Thus, it appears that the duration of bacteremia may be related to the magnitude of the antibody response (titer) generated. Chomel et al. (11) reported a similar association, in which titers were higher in bacteremic cats than in nonbacteremic cats. Together, these results may suggest a role for antibodies in the clearance of B. henselae. It has not been determined if antibodies are protective; however, passive-transfer studies could be useful in addressing these questions. Although the titer numbers for the low-molecular-mass bands are generally lower than those for the higher-molecular-mass bands, this may not truly represent a difference in immunoreactivity. The difference could be a result of more epitopes being available on higher-molecular-mass antigens for interaction with specific antibodies. These results warrant further investigation.
DNA hybridization studies comparing the interrelatedness of various species of Bartonella indicate that B. henselae Houston-1 and B. quintana are 71% related (8) while B. henselae Houston-1 and B. clarridgeiae are only 47% related (28). DNA hybridization studies have not been performed for B. henselae LSU16. Comparative seroreactive studies of cats, rabbits, and humans have been described in the literature (4, 19, 34). It has been shown by IFA that absorption of cat serum with B. henselae is able to distinguish species-specific reactivity to B. henselae from cross-reactivity to B. quintana antigen (4). Immunoelectrophoretic studies with rabbit sera and antigen preparations of B. henselae and B. quintana indicate that rabbits produce antibodies to seven unique antigenic proteins when immunized with B. henselae compared to immunization with B. quintana (17).
In our study B. henselae Houston-1 absorption and B. quintana absorption of serum from a cat experimentally infected with B. henselae LSU16 yielded antibody profiles that were indistinguishable from each other. However, these two absorption antibody profiles were different from that of the absorption performed with B. henselae LSU16 in that antibodies to the 76.0-, 69.0-, and 65.0-kDa antigens were not removed. Two variants (type 1 and type 2) of B. henselae have been identified based on sequence differences in the 16S rRNA (6). B. henselae LSU16 has not been typed at this time, and it is possible that B. henselae LSU16 could represent a type 2 variant, whereas B. henselae Houston-1 has been identified as a type 1 variant. Alternatively, the difference in absorption antibody profiles may represent the loss of these immunodominant antigens through multiple passage of the Houston-1 strain; B. henselae LSU16 is a new isolate that has not been extensively passaged. Cross-reactivity with the 13.3-kDa antigen was found for each of the Bartonella species examined. While some antibody profiles did not appear to be affected by absorption, antigen-specific titers could have decreased; this experiment does not directly address this question.
The high degree of diversity among Bartonella species sheds some doubt on the sensitivities of various diagnostic procedures. A prevalence study of B. clarridgeiae based on blood culture estimates a 16% prevalence in stray cats (24). Considering the relatively low DNA relatedness and the lack of cross-reactivity between B. henselae Houston-1 and B. clarridgeiae, it is likely that IFA seroprevalence studies utilizing a B. henselae antigen preparation would underestimate the number of cats with antibodies to Bartonella. Evidence for this supposition is provided in a study in which a patient with CSD was seronegative for B. henselae, B. quintana, and Bartonella elizabethae but was seropositive for B. clarridgeiae (28). Furthermore, a number of patients with CSD who tested negative by IFA for B. henselae Houston-1 were determined to be seropositive for B. henselae Marseille (15). Some studies have indicated that 60% of patients with CSD test negative in standard IFA (16, 42). Development of a test that includes antigens specific to the various species and types of Bartonella is critical in improving the sensitivities of the IFA and other diagnostic tests.
In examining the sera from the 601 cats at local animal shelters for Bartonella seroreactivity, a number of antigens were identified that could aid in the improvement of Bartonella serology tests. The Western blotting and titration results indicated that a strong antibody response to the 13.3-kDa antigen was generated and that the response persisted throughout the course of infection. In addition, this antigen was present in a high percentage of shelter cats in association with other bands which appear to be Bartonella specific. Together, these results suggest that the utilization of this antigen as a test antigen may be beneficial in improving serological tests. The use of the 11.3- or 16.9/18.0-kDa antigens as test antigens could help to increase the sensitivity of serological tests; antibodies to these antigens and to the 13.3-kDa antigen were not detected in the most common negative patterns of seroreactivity (Table 3). On the other hand, the 76.0-, 69.0-, and 65.0-kDa antigens may be extremely useful in distinguishing between seroreactivity with B. henselae LSU16 and cross-reactivity with B. henselae Houston-1. The 97.0-kDa protein may also serve as a useful test antigen. However, these antigens were not present in as many animals and thus the number of false negatives with these antigens may be higher. Grouping of some of the higher-molecular-mass antigens may have affected the usefulness of each individual antigen. In the prevalence determination, the higher-molecular-mass antigens were not initially considered because of the difficulty in determining the molecular mass of each antigen from gel to gel (due probably to slight variations in protein preparation and electrophoretic conditions). Future studies utilizing a decreased percentage of polyacrylamide will result in greater separation of the higher-molecular-mass antigens. Greater separation of these antigens should yield a better picture of the immune response to them. In a situation where a highly specific test is desired, the use of the 21.7- and 28.0-kDa antigens as test antigens would be beneficial. The 7.0-, 8.0-, and 15.0-kDa antigens were used as screen test antigens because, although these antigens were not detected in as many positive cats, the antibody responses to the antigens when seen produced a signal that was easy to detect by Western blot analysis. We would expect that the sensitivities associated with these antigens will be lower, given that the antibody responses to the antigens waxed and waned throughout the course of infection. While these three antigens may not be useful in improving the sensitivities of Bartonella serology tests, they may be used to differentiate between an acute infection and a chronic infection. However, this has not been determined and warrants further investigation.
In designing an antibody-based Bartonella screening test, a number of factors, such as the stage of infection, the degree of infection, the immunological state and response of the infected individual, and the serotype of the infecting organism, must be considered; all of these factors can influence the results of the test. An ideal test is one that could handle all the variabilities associated with these factors; however, it is unlikely that a test with only one antigen could meet this criterion. Based on the results of this study, a test with some combination of the 11.3-, 13.3-, and 16.9/18.0-kDa antigens may be prudent.
Absorption assays and Western blot analysis have proven to be useful tools in identifying a number of Bartonella-specific immunodominant antigens recognized by the feline immune system during experimental infection with B. henselae LSU16. The generation and use of multiple monoclonal antibodies against these antigens will aid in improving the sensitivities and specificities of various serological diagnostic tests. Additional studies of the feline humoral immune response to infection with Bartonella should help to identify the roles of these antigens in the immune response. Furthermore, these studies should lead to the advancement of our understanding of the pathogenesis of infections due to this microorganism.
ACKNOWLEDGMENTS
This work was supported by NIH grant 1 R15 AI39720-01.
We thank Patricia Triche, Leslie Birke, Laura Blanke, Tracy Brown, David Good, Victor Goss, Malgorzata Mikoloczyk, Katy Parr, Kenny Ransom, and Jeff Taylor for technical assistance; Melanie Rembert and Laurie Henderson for their assistance with the cats; and Keith Hughes for useful discussions.
REFERENCES
- 1.Abbott R C, Chomel B B, Kasten R W, Floyd-Hawkins K A, Kikuchi Y, Koehler J E, Pedersen N C. Experimental and natural infection with Bartonella henselaein domestic cats. Comp Immunol Microbiol Infect Dis. 1997;20:41–51. doi: 10.1016/s0147-9571(96)00025-2. [DOI] [PubMed] [Google Scholar]
- 2.Adal K A, Cockerell C J, Petri W A., Jr Cat scratch disease, bacillary angiomatosis, and other infections due to Rochalimaea. N Engl J Med. 1994;330:1509–1515. doi: 10.1056/NEJM199405263302108. [DOI] [PubMed] [Google Scholar]
- 3.Anderson B, Lu E, Jones D, Regnery R. Characterization of a 17-kilodalton antigen of Bartonella henselaereactive with sera from patients with cat scratch disease. J Clin Microbiol. 1995;33:2358–2365. doi: 10.1128/jcm.33.9.2358-2365.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baneth G, Kordick D L, Hegarty B C, Breitschwerdt E B. Comparative seroreactivity to Bartonella henselae and Bartonella quintanaamong cats from Israel and North Carolina. Vet Microbiol. 1996;50:95–103. doi: 10.1016/0378-1135(96)00006-5. [DOI] [PubMed] [Google Scholar]
- 5.Bergmans A M. Cat scratch disease: studies on diagnosis and identification of reservoirs and vectors. Ph.D. dissertation. Utrecht, The Netherlands: University of Utrecht; 1996. [DOI] [PubMed] [Google Scholar]
- 6.Bergmans A M, Schellekens J F, van Embden J D, Schouls L M. Predominance of two Bartonella henselaevariants among cat-scratch disease patients in The Netherlands. J Clin Microbiol. 1996;34:254–260. doi: 10.1128/jcm.34.2.254-260.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breitschwerdt E B, Kordick D L, Malarkey D E, Keene B, Hadfield T L, Wilson K. Endocarditis in a dog due to infection with a novel Bartonellasubspecies. J Clin Microbiol. 1995;33:154–160. doi: 10.1128/jcm.33.1.154-160.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brenner D J, O’Connor S P, Winkler H H, Steigerwalt A G. Proposals to unify the genera Bartonella and Rochalimaea, with descriptions of Bartonella quintana comb. nov., Bartonella vinsonii comb. nov., Bartonella henselae comb. nov., and Bartonella elizabethae comb. nov., and to remove the family Bartonellaceae from the order Rickettsiales. Int J Syst Bacteriol. 1993;43:777–786. doi: 10.1099/00207713-43-4-777. [DOI] [PubMed] [Google Scholar]
- 9.Childs J E, Olson J G, Wolf A, Cohen N, Fakile Y, Rooney J A, Bacellar F, Regnery R L. Prevalence of antibodies to Rochalimaeaspecies (cat-scratch disease agent) in cats. Vet Rec. 1995;136:519–520. doi: 10.1136/vr.136.20.519. [DOI] [PubMed] [Google Scholar]
- 10.Childs J E, Rooney J A, Cooper J L, Olson J G, Regnery R L. Epidemiologic observations on infection with Rochalimaeaspecies among cats living in Baltimore, Md. J Am Vet Med Assoc. 1994;204:1775–1778. [PubMed] [Google Scholar]
- 11.Chomel B B, Abbott R C, Kasten R W, Floyd-Hawkins K A, Kass P H, Glaser C A, Pedersen N C, Koehler J E. Bartonella henselaeprevalence in domestic cats in California: risk factors and association between bacteremia and antibody titers. J Clin Microbiol. 1995;33:2445–2450. doi: 10.1128/jcm.33.9.2445-2450.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chomel B B, Kasten R W, Floyd-Hawkins K, Chi B, Yamamoto K, Roberts-Wilson J, Gurfield A N, Abbott R C, Pedersen N C, Koehler J E. Experimental transmission of Bartonella henselaeby the cat flea. J Clin Microbiol. 1996;34:1952–1956. doi: 10.1128/jcm.34.8.1952-1956.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dolan M J, Wong M T, Regnery R L, Jorgensen J H, Garcia M, Peters J, Drehner D. Syndrome of Rochalimaea henselaeadenitis suggesting cat scratch disease. Ann Intern Med. 1993;118:331–336. doi: 10.7326/0003-4819-118-5-199303010-00002. [DOI] [PubMed] [Google Scholar]
- 14.Drancourt M, Birtles R, Chaumentin G, Vandenesch F, Etienne J, Raoult D. New serotype of Bartonella henselaein endocarditis and cat-scratch disease. Lancet. 1996;347:441–443. doi: 10.1016/s0140-6736(96)90012-4. [DOI] [PubMed] [Google Scholar]
- 15.Drancourt M, Birtles R, Chaumentin G, Vandenesch F, Etienne J, Raoult D. New serotype of Bartonella henselaein endocarditis and cat-scratch disease. Lancet. 1996;347:441–443. doi: 10.1016/s0140-6736(96)90012-4. . (Erratum, 347:842. [DOI] [PubMed] [Google Scholar]
- 16.Dupon M, Savin D L A, Brouqui P, Drancourt M, Raoult D, De Mascarel A, Lacut J Y. Evaluation of serological response to Bartonella henselae, Bartonella quintana, and Afipia felisantigens in 64 patients with suspected cat-scratch disease. Scand J Infect Dis. 1996;28:361–366. doi: 10.3109/00365549609037920. [DOI] [PubMed] [Google Scholar]
- 17.Engbaek K, Koch C. Antibody response in rabbits infected with Rochalimaea henselae, Rochalimaea quintana, and Afipia felis. J Pediatr Child Health. 1994;30:467–469. [PubMed] [Google Scholar]
- 18.Engbaek K, Koch C. Immunoelectrophoretic characterization and cross-reactivity of Rochalimaea henselae, Rochalimaea quintana, and Afipia felis. APMIS. 1994;102:931–942. [PubMed] [Google Scholar]
- 19.Foil L, Andress E, Freeland R L, Roy A F, Rutledge R, Triche P C, O’Reilly K L. Experimental infection of domestic cats with Bartonella henselae by inoculation of Ctenocephalides felis(Siphonaptera: Pulicidae) feces. J Med Entomol. 1998;35:625–628. doi: 10.1093/jmedent/35.5.625. [DOI] [PubMed] [Google Scholar]
- 20.Greene C E, McDermott M, Jameson P H, Atkins C L, Marks A M. Bartonella henselaeinfection in cats: evaluation during primary infection, treatment, and rechallenge infection. J Clin Microbiol. 1996;34:1682–1685. doi: 10.1128/jcm.34.7.1682-1685.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guptill L, Slater L, Wu C, Lin T, Glickman L, Welch D, Tobolski J, HogenEsch H. Second International Symposium on Feline Immunology. 1997. Experimental infection of neonatal specific pathogen free cats with Bartonella henselae; p. 8. [Google Scholar]
- 22.Guptill L, Slater L, Wu C C, Lin T L, Glickman L T, Welch D F, HogenEsch H. Experimental infection of young specific pathogen-free cats with Bartonella henselae. J Infect Dis. 1997;176:206–216. doi: 10.1086/514026. [DOI] [PubMed] [Google Scholar]
- 23.Gurfield A N, Boulouis H J, Chomel B B, Heller R, Kasten R W, Yamamoto K, Piemont Y. Coinfection with Bartonella clarridgeiae and Bartonella henselae and with different Bartonella henselaestrains in domestic cats. J Clin Microbiol. 1997;35:2120–2123. doi: 10.1128/jcm.35.8.2120-2123.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heller R, Artois M, Xemar V, de Briel D, Gehin H, Jaulhac B, Monteil H, Piemont Y. Prevalence of Bartonella clarridgeiaein stray cats. J Clin Microbiol. 1997;35:1327–1331. doi: 10.1128/jcm.35.6.1327-1331.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jameson P, Greene C, Regnery R, Dryden M, Marks A, Brown J, Cooper J, Glaus B, Greene R. Prevalence of Bartonella henselaeantibodies in pet cats throughout regions of North America. J Infect Dis. 1995;172:1145–1149. doi: 10.1093/infdis/172.4.1145. [DOI] [PubMed] [Google Scholar]
- 26.Koehler J E, Glaser C A, Tappero J W. Rochalimaea henselaeinfection. A new zoonosis with the domestic cat as reservoir. JAMA. 1994;271:531–535. doi: 10.1001/jama.271.7.531. [DOI] [PubMed] [Google Scholar]
- 27.Koehler J E, Sanchez M A, Garrido C S, Whitfeld M J, Chen F M, Berger T G, Rodriguez-Barradas M C, LeBoit P E, Tappero J W. Molecular epidemiology of Bartonellainfections in patients with bacillary angiomatosis-peliosis. N Engl J Med. 1997;337:1876–1883. doi: 10.1056/NEJM199712253372603. [DOI] [PubMed] [Google Scholar]
- 28.Kordick D L, Hilyard E J, Hadfield T L, Wilson K H, Steigerwalt A G, Brenner D J, Breitschwerdt E B. Bartonella clarridgeiae, a newly recognized zoonotic pathogen causing inoculation papules, fever, and lymphadenopathy (cat scratch disease) J Clin Microbiol. 1997;35:1813–1818. doi: 10.1128/jcm.35.7.1813-1818.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kordick D L, Wilson K H, Hegarty B C, Berkoff H A, Breitschwerdt E B. Program and abstracts of the 34th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1994. Bartonella (Rochalimaea) bacteremia in three feline populations. [Google Scholar]
- 30.Litwin C M, Martins T B, Hill H R. Immunologic response to Bartonella henselaeas determined by enzyme immunoassay and Western blot analysis. Am J Clin Pathol. 1997;108:202–209. doi: 10.1093/ajcp/108.2.202. [DOI] [PubMed] [Google Scholar]
- 31.Margileth A M, Hayden G F. Cat scratch disease. From feline affection to human infection. N Engl J Med. 1993;329:8–13. doi: 10.1056/NEJM199307013290112. [DOI] [PubMed] [Google Scholar]
- 32.Maurin M, Roux V, Stein A, Ferrier F, Viraben R, Raoult D. Isolation and characterization by immunofluorescence, sodium dodecyl sulfate-polyacrylamide gel electrophoresis, Western blot, restriction fragment length polymorphism-PCR, 16S rRNA gene sequencing, and pulsed-field gel electrophoresis of Rochalimaea quintanafrom a patient with bacillary angiomatosis. J Clin Microbiol. 1994;32:1166–1171. doi: 10.1128/jcm.32.5.1166-1171.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGill S, Sjodin-Berglund H, Merza M, Engstand L. Novel species-specific murine monoclonal antibodies against Bartonella henselae: diagnostic implications, abstr. 167. In Program and abstracts of the 98th Annual Meeting of the American Society for Microbiology. Washington, D.C: American Society for Microbiology; 1998. [Google Scholar]
- 34.Raoult D, La Scola B. Serological cross reactions between Bartonella quintana, Bartonella henselae, and Coxiella burnetii. J Clin Microbiol. 1996;34:2270–2274. doi: 10.1128/jcm.34.9.2270-2274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Regnery R L, Olson J G, Perkins B A, Bibb W. Serological response to “Rochalimaea henselae” antigen in suspected cat-scratch disease. Lancet. 1992;339:1443–1445. doi: 10.1016/0140-6736(92)92032-b. [DOI] [PubMed] [Google Scholar]
- 36.Regnery R L, Rooney J A, Johnson A M, Nesby S L, Manzewitsch P, Beaver K, Olson J G. Experimentally induced Bartonella henselaeinfections followed by challenge exposure and antimicrobial therapy in cats. Am J Vet Res. 1996;57:1714–1719. [PubMed] [Google Scholar]
- 37.Rodriguez-Barradas M C, Bandres J C, Hamill R J, Trial J, Clarridge J E, Baughn R E, Rossen R D. In vitro evaluation of the role of humoral immunity against Bartonella henselae. Infect Immun. 1995;63:2367–2370. doi: 10.1128/iai.63.6.2367-2370.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. pp. 18.2–18.88. [Google Scholar]
- 39.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. p. A.1. [Google Scholar]
- 40.Tappero J W, Mohle-Boetani J, Koehler J E, Swaminathan B, Berger T G, LeBoit P E, Smith L L, Wenger J D, Pinner R W, Kemper C A, et al. The epidemiology of bacillary angiomatosis and bacillary peliosis. JAMA. 1993;269:770–775. [PubMed] [Google Scholar]
- 41.Welch D F, Hensel D M, Pickett D A, San Joaquin V H, Robinson A, Slater L N. Bacteremia due to Rochalimaea henselaein a child: practical identification of isolates in the clinical laboratory. J Clin Microbiol. 1993;31:2381–2386. doi: 10.1128/jcm.31.9.2381-2386.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yoshida H, Kusaba N, Omachi K, Miyazaki N, Yamawaki M, Tsuji Y, Nakahara K, Sumino M, Noudomi M, Shimokawa Y, Tanikawa K. Serological study of Bartonella henselaein cat scratch disease in Japan. Microbiol Immunol. 1996;40:671–673. doi: 10.1111/j.1348-0421.1996.tb01126.x. [DOI] [PubMed] [Google Scholar]
- 43.Zangwill K M, Hamilton D H, Perkins B A, Regnery R L, Plikaytis B D, Hadler J L, Cartter M L, Wenger J D. Cat scratch disease in Connecticut. Epidemiology, risk factors, and evaluation of a new diagnostic test. Vet Rec. 1993;133:27–28. doi: 10.1056/NEJM199307013290102. [DOI] [PubMed] [Google Scholar]
- 44.Zangwill K M, Hamilton D H, Perkins B A, Regnery R L, Plikaytis B D, Hadler J L, Cartter M L, Wenger J D. Cat scratch disease in Connecticut. Epidemiology, risk factors, and evaluation of a new diagnostic test. N Engl J Med. 1993;329:8–13. doi: 10.1056/NEJM199307013290102. [DOI] [PubMed] [Google Scholar]