Abstract
In this paper, the confusion of the sources of medicinal materials was briefly expounded, and the differences among the varieties were pointed out. At the same time, the chemical components and pharmacological properties of Elsholtzia ciliata (Thunb.) Hyland (E. ciliata) were reviewed. The structures of 352 compounds that have been identified are listed. These mainly include flavonoids, terpenoids, phenylpropanoids, alkaloids, and other chemical components. They have antioxidant, anti-inflammatory, antimicrobial, insecticidal, antiviral, hypolipidemic, hypoglycemic, analgesic, antiarrhythmic, antitumor, antiacetylcholinesterase, and immunoregulator activities. At present, there are many researches using essential oil and alcohol extract, and the researches on antioxidant, anti-inflammatory, anti-microbial, and other pharmacological activities are relatively mature. This paper aims to summarize the existing research, update the research progress regarding the phytochemicals and pharmacology of E. ciliate, and to provide convenience for subsequent research.
Keywords: Elsholtzia ciliata (Thunb.) Hyland, phytochemistry composition, pharmacological activities
1. Introduction
Elsholtzia ciliata (Thunb.) Hyland belongs to the genus Elsholtzia, family Lamiaceae. In the clinical application of traditional Chinese medicine, the aerial parts of Mosla chinensis Maxim (MCM) and Mosla chinensis Maxim cv. Jiangxiangru (JXR) are used as E. ciliata. MCM is mostly wild, and JXR is the cultivated product of MCM, which was often confused with Elsholtzia splendens Nakai ex F. Maek. before [1]. However, Ganpei Zhu believes that JXR has obvious plant morphological differences from MCM. The plant height of JXR can reach 25–66 cm. The stem has gray, white curly pubescence. The leaf blade is broadly lanceolate to lanceolate, and the leaf margin is obviously serrate. The bracts are obovate and ovate. Calyx lobes are triangular lanceolate in shape. There is a hair ring at the base of the crown tube. Nutlets are yellowish brown and nearly round, with lightly carved surface, reticulate and flattened inside. MCM plants are shorter. Stem inversely pilose. Leaf blade linear to linear lanceolate, leaf margin serrate, inconspicuous. Bracts ovate orbicular. Calyx lobes subulate. There is no hairy ring at the base of the crown. Nutlets are nearly spherical, brown, with deep carving on the surface, and uneven in the mesh [2]. Therefore, JXR should be listed as an independent variety [3].
E. ciliata is a herbaceous plant distributed in Russia (Siberia), Mongolia, Korea, Japan, India, the Indochina peninsula, and China, while in Europe and North America it was also introduced and cultivated. In China, it is produced almost all over the country, except Xinjiang and Qinghai. It has low requirements for growth environment, a short growth cycle, flowering period from July to October, and harvest in summer and autumn [4,5,6].
Traditional Chinese medicine theory believes that E. ciliata has a spicy flavour and a lukewarm nature. It also has the effect of inducing diaphoresis and relieving superficies, removing dampness for regulating the stomach and inducing diuresis for removing edema. The following is a review of chemical compositions and pharmacological activities.
2. Chemical Constituents
A total of 352 compounds have been identified from E. ciliata. Among the chemical components of E. ciliata, flavonoids and terpenoids are the main components, which make E. ciliata have more obvious antimicrobial, anti-inflammatory, and antioxidant effects. Terpenoids such as 3-carene and some aromatic compounds such as carvacrol exhibit antimicrobial activity. Some polysaccharides can inhibit the proliferation of tumor cells, and show positive effects in immunoregulation.
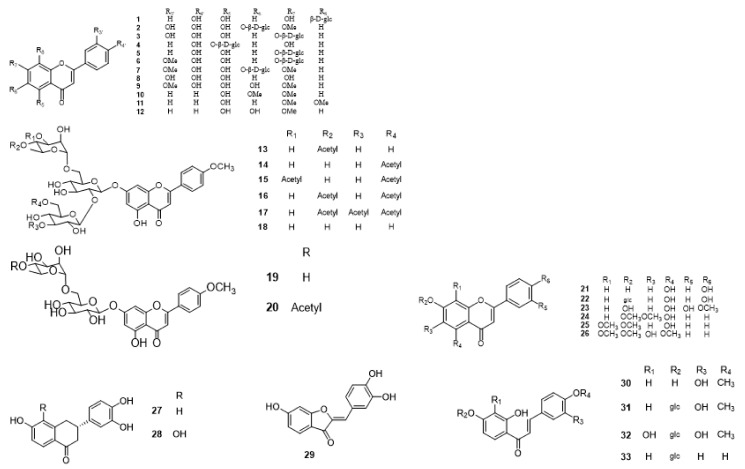
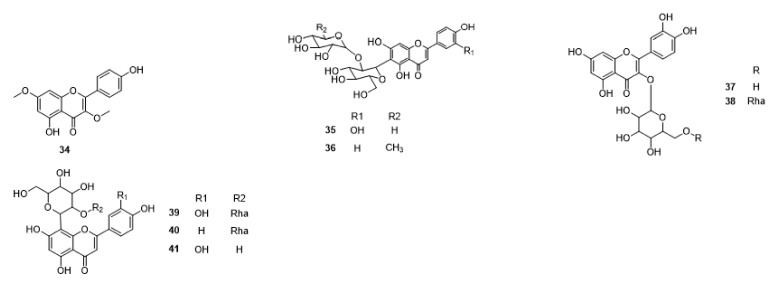
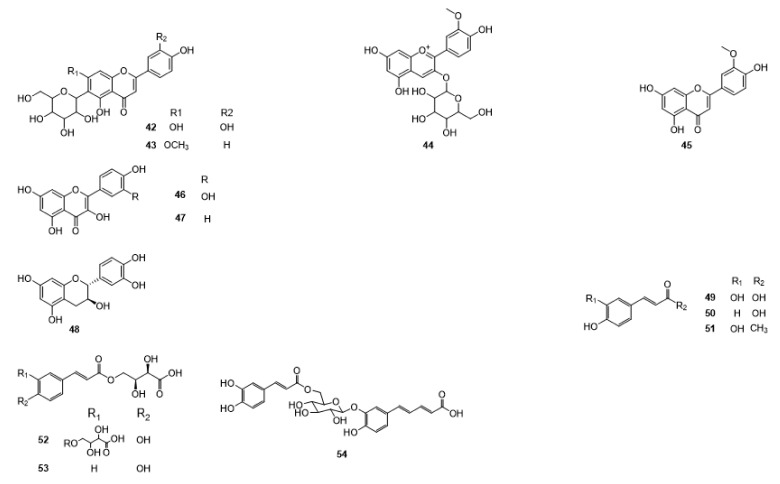
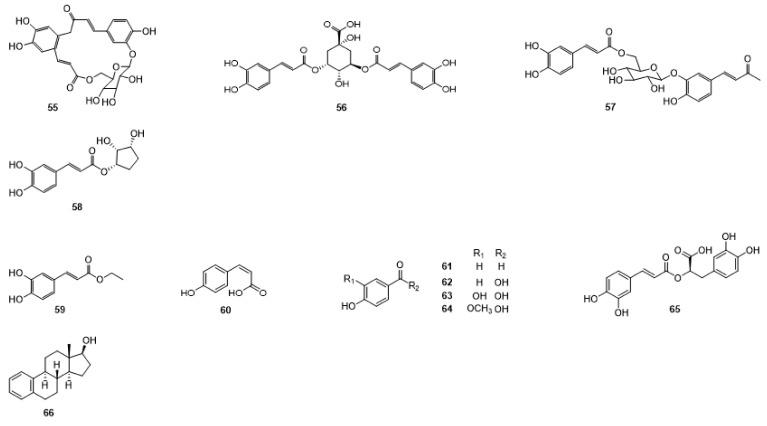
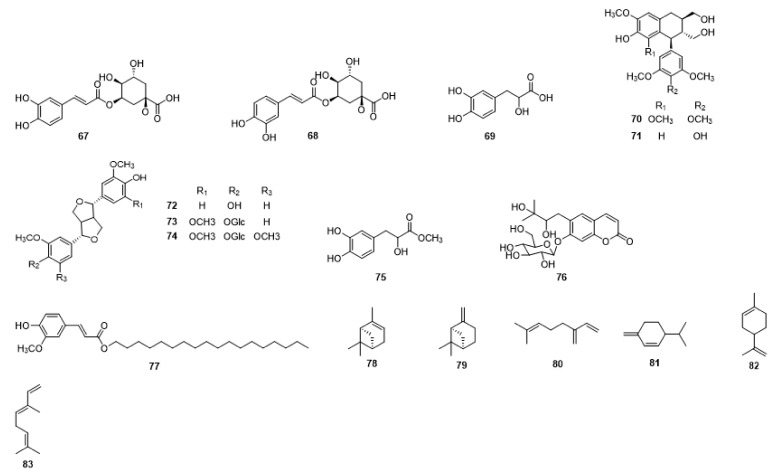
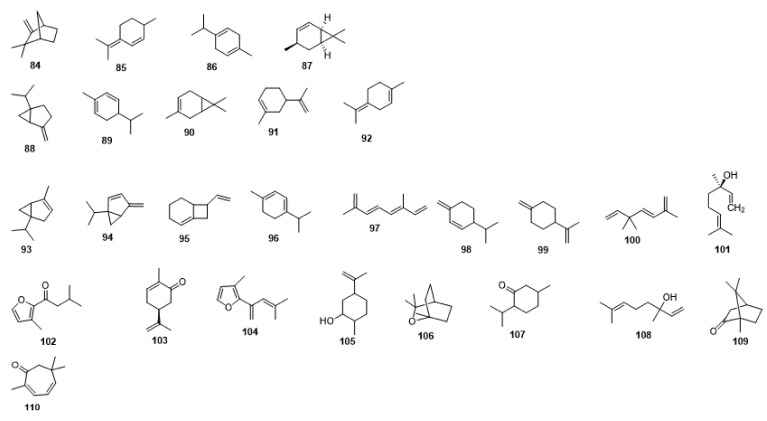
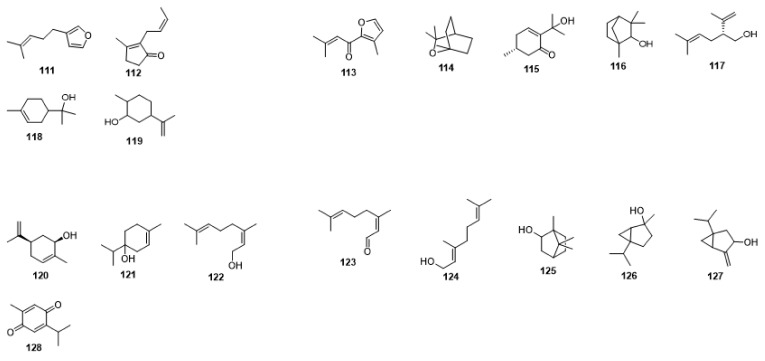
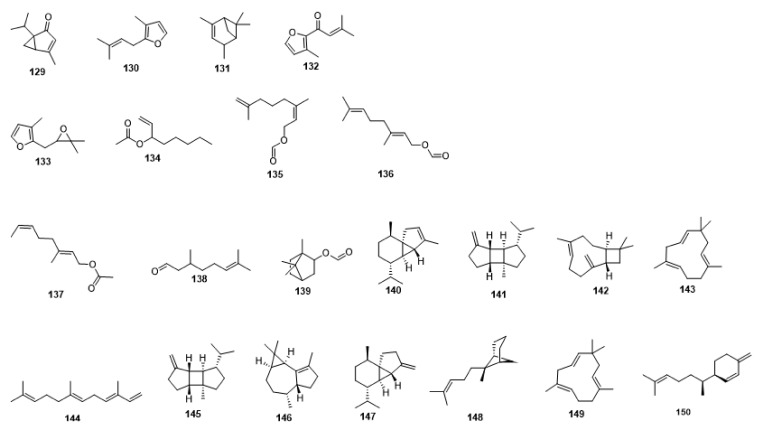
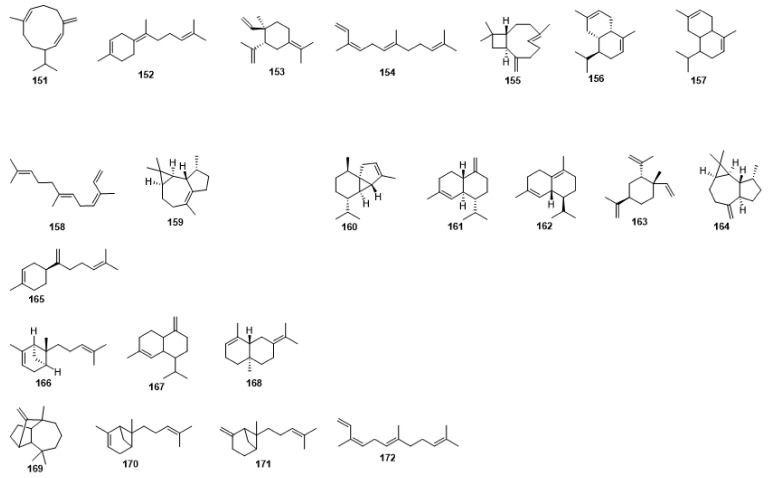
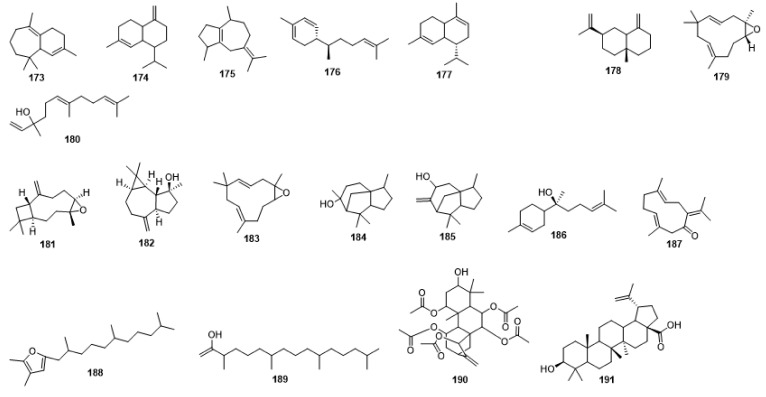
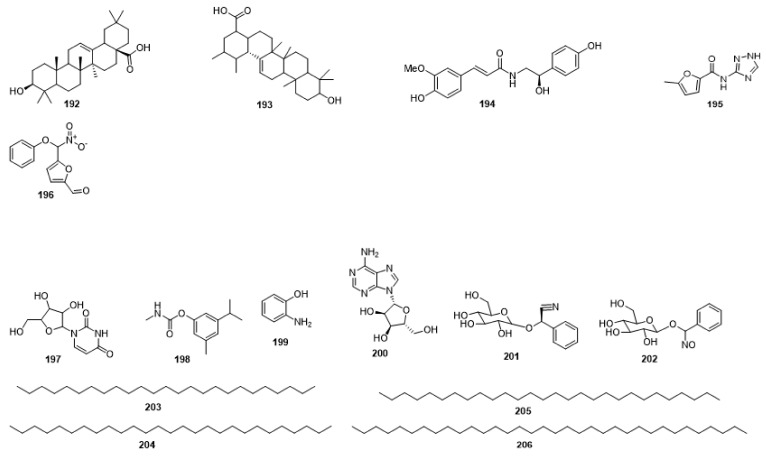
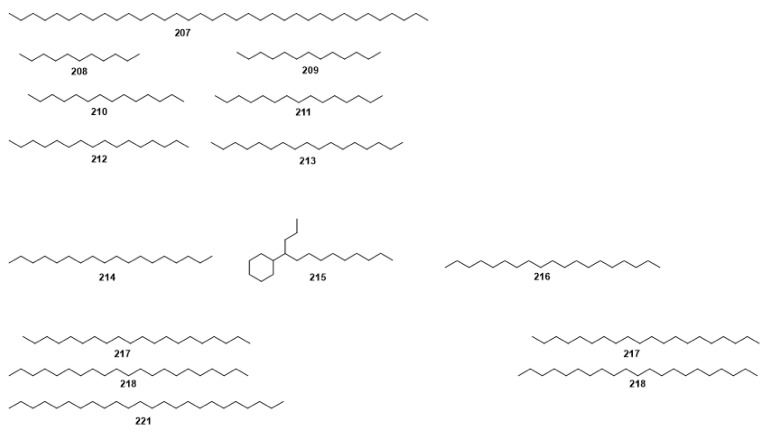
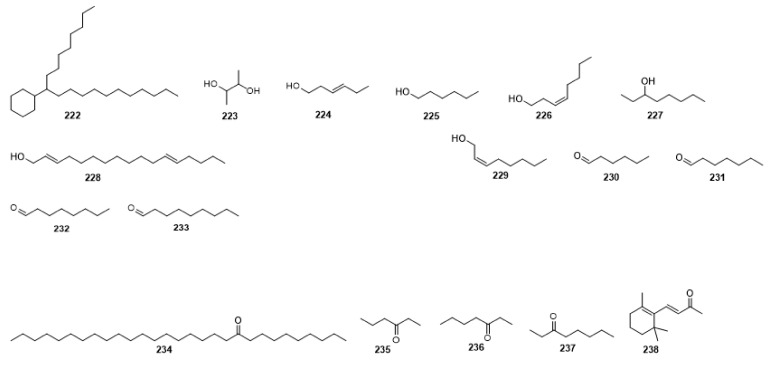
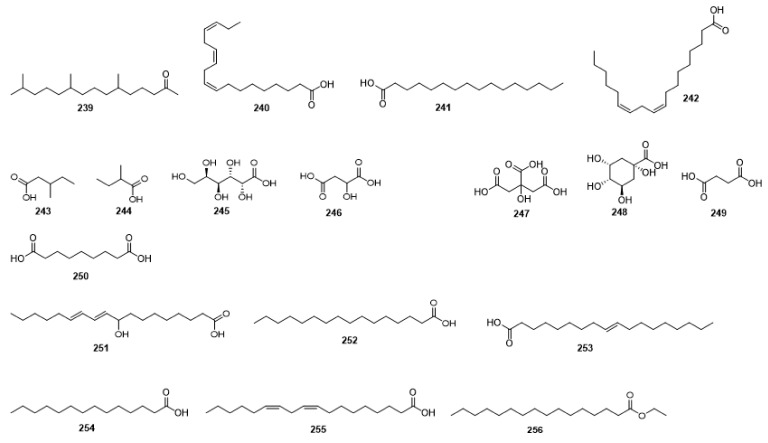
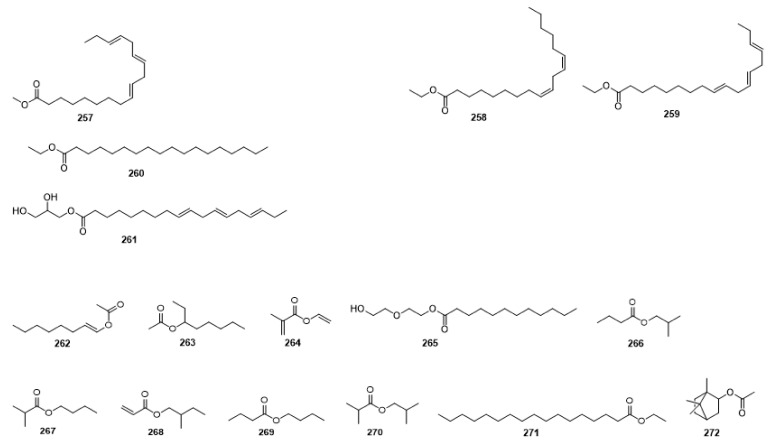
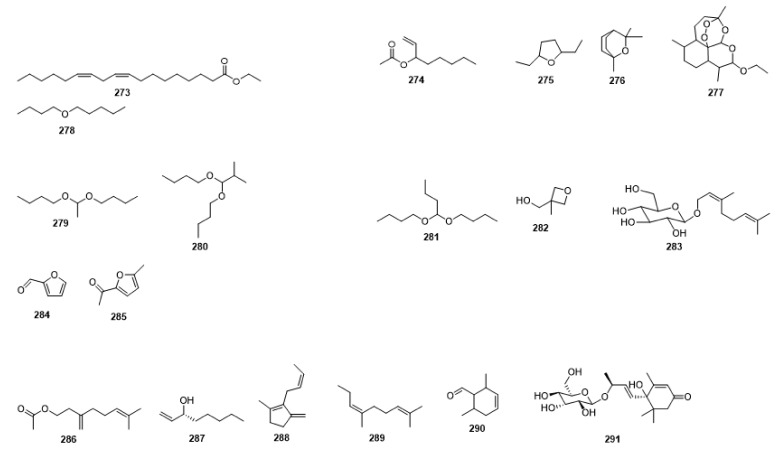
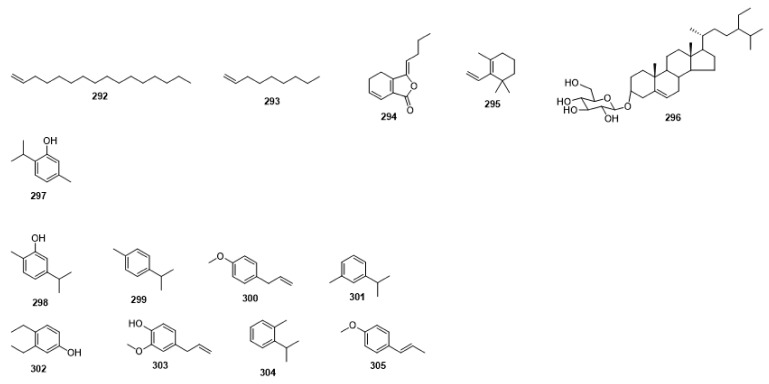
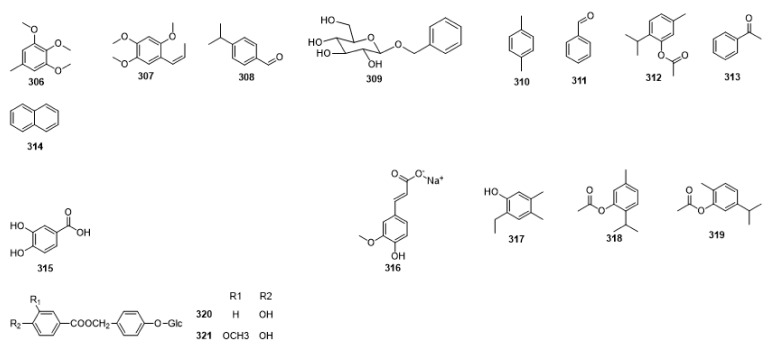
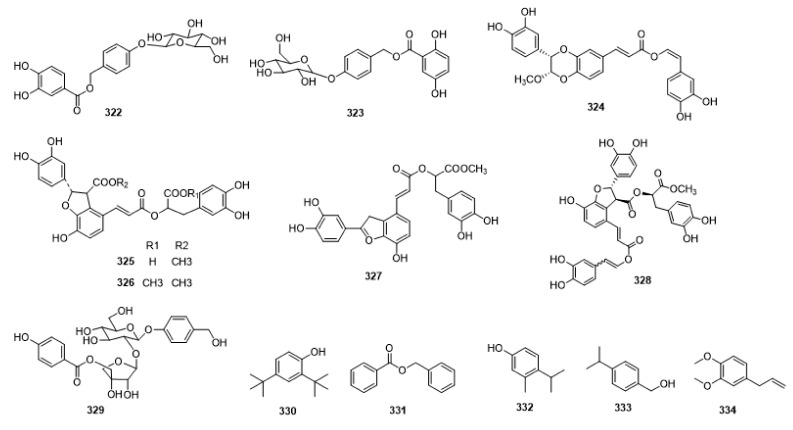
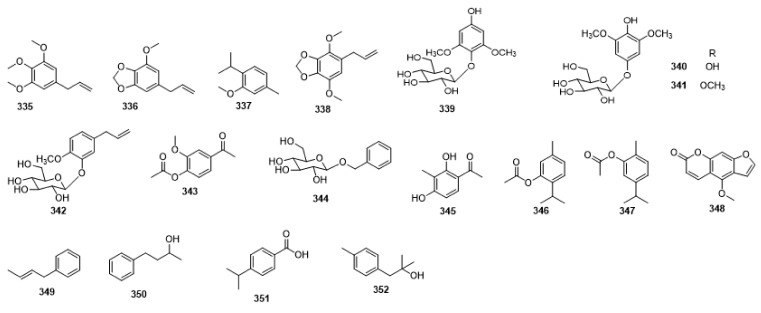
Compounds 1–48 are flavonoids, 49–77 are phenylpropanoids, 78–193 are terpenoids, 194–202 are alkaloids compounds, and 203–352 are other compounds. Compounds 1–352 are listed in Table 1 and the structures 1–352 are listed in Figure 1.
3. Pharmacological Activities
In the traditional application of Chinese medicine, E. ciliata is mainly used for the treatment of summer cold, cold aversion and fever, headache without sweat, abdominal pain, vomiting and diarrhea, edema, and poor urination. Modern pharmacological studies show that E. ciliata has antioxidant, anti-inflammatory, antimicrobial, insecticidal, antiviral, hypolipidemic, hypoglycemic, analgesic, antiarrhythmic, antitumor, antiacetylcholinesterase, and immunoregulator activities.
3.1. Antioxidant Activity
Oxidative stress refers to a state of imbalance between oxidation and antioxidant effects in vivo. It is a negative effect caused by free radicals in the body and is considered to be an important factor in aging and disease. It was reported that the essential oil of E. ciliata could increase catalase (CAT) activity in brain of mice by 26.94%, which may be related to the decomposition of hydrogen peroxide by CAT to reduce oxidative stress [7]. There is a phenolic substance osmundacetone in E. ciliata ethanol extract. In DPPH experiment, the IC50 value of osmundacetone was 7.88 ± 0.02 µM, indicating a certain antioxidant capacity. The inhibitory effect of osmundacetone on glutamate-induced oxidative stress in HT22 cells was studied by reactive oxygen species (ROS) method. The results showed that osmundacetone significantly reduced the accumulation of ROS and could be used as a potential antioxidant [8]. By studying the effect of E. ciliata methanol extract on J774A.1 murine macrophage, the evaluation of antioxidant activity showed that all the tested compounds had significant effects on ROS release under oxidative stress at the highest concentration (10 M), especially luteolin-7-O-β-D-glucopyranoside, luteolin, and 5,6,4’-trihydroxy-7,3’-dimethoxyflavone [9].
Various scholars studied different polarity extracts of E. ciliata. According to the free radical scavenging experiment of Huynh Xuan Phong, the result showed that E. ciliata extract had certain scavenging ability against 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2’-azino-bis (3-ethylbenzothiazoline -6-sulfonic acid) (ABTS), with IC50 values of 495.80 ± 17.16 and 73. 59 ± 3.18 mg/mL. [10]. In DPPH experiment, the EC50 values of dichloromethane extract, crude ethanol extract and n-hexane extract were 0.041 µg/µg, 0.15 µg/µg and 0.46 µg/µg, respectively, showing strong antioxidant activity. Such antioxidant capacity may be related to non-polar flavonoids and phenols contained in E. ciliata, among which the total phenol content of dichloromethane is 96.68 ± 0.0010 µg GAEs/mg Extract, and the total flavonoid content is 71.5 ± 0.0089 µg QEs/mg Extract. Therefore, it has the strongest antioxidant capacity [11]. Jing-en Li et al. extracted JXR ethanol extract with petroleum ether, ethyl acetate and water-saturated n-butanol, respectively, and studied the antioxidant activities of the three parts and water phase. The results indicated that ethyl acetate showed good antioxidant activities in ferric reducing antioxidant power (FRAP), DPPH, and β-carotene assay, which may be related to the higher flavonoid content in this extract [12]. The antioxidant ability of mytilus polysaccharide-I (MP-I) contained in JXR water extract was concentration-dependent. When the concentration was 16 mg/mL, the chelation rate of MP-I and Fe2+ was 87.80%. When the concentration was 20 mg/mL, the scavenging rate of DPPH free radical was 81.32%. The scavenging rate of hydroxyl radical was 81.94% [13]. The DPPH test IC50 of MCM essential oil and methanol extract were 1230.4 ± 12.5 and 1482.5 ± 10.9 μg/mL, respectively. Reducing power test EC50 were 105.1 ± 0.9 and 313.5 ± 2.5 μg/mL, respectively. β-Carotene bleaching assay EC50 were 588.2 ± 4.2 and 789.4 ± 1.3 μg/ml, respectively. The total phenolic content of the essential oil was about 1.7 times that of methanol extract, which further verified the stronger antioxidant capacity of the essential oil [14].
Different parts of E. ciliata have different antioxidant capacity. Lauryna Pudziuvelyte et al. used DPPH, ABTS, FRAP, and cupric ion reducing antioxidant capacity (CUPRAC) to evaluate the antioxidant activity of different parts of E. ciliata DPPH and ABTS results showed that total phenolics content (TPC) and total flavonoids content (TFC) amounts of ethanol extracts from E. ciliata flower, leaf and whole plant were the highest and had the strongest antioxidant activity. The results of FRAP and CUPRAC test showed that the ethanol extract of E. ciliata flower had the highest antioxidant activity. Among different parts of the ethanol extract, the content of quercetin glycosides, phenolic acids, TPC, and TFC in stem extract was the lowest, and the antioxidant activity was the lowest [4]. The ethyl acetate fraction of E. ciliata was purified by macroporous resin with 80% ethanol to obtain fraction E. In the DPPH experiment, the EC50 value of fraction E was 0.09 mg/mL, which showed the strongest antioxidant and free radical scavenging ability. The EC50 value of fraction E was higher than positive control butylated hydroxytoluene (0.45), butylated hydroxyanisole (0.21), and vitamin C (0.41). Hence, it can be seen that E. ciliata has the potential to prevent cardiovascular diseases, cancer, and other diseases caused by excess free radicals [15].
3.2. Anti-Inflammatory Activity
Compounds pedalin, luteolin-7-O-β-D-glucopyranoside, 5-hydroxy-6,7-dimethoxyflavone, and α-linolenic acid in the essential oil of E. ciliata were investigated under a lipopolysaccharide (LPS)-induced inflammatory reaction. It can inhibit ROS release, but its mechanism deserves further study [9]. LPS-induced inflammation was evaluated by the number of inflammatory mediators, i.e., tumor necrosis factor-α (TNF-α), interleukin (IL)-6, and prostaglandin E2 (PGE2). E. ciliata ethanol extract could significantly inhibit the secretion of inflammatory mediators, where TNF-α and IL-6 factors could be effectively inhibited in the stem and flower part, and the PGE2 pathway could be inhibited in the leaf part [4]. The effect of E. ciliata on inflammation can be further verified by studying pyretic rats caused by LPS and mononuclear macrophage RAW264.7 induced by LPS. E. ciliata essential oil and water decoction can reduce the contents of PGE2, TNF-α and other inflammatory factors to different degrees, and can reduce the content of nitric oxide (NO) in serum [16]. Excessive NO can induce the production of pro-inflammatory factors, such as TGF-α and IL-1β, and aggravate the inflammatory response [17]. JXR alleviates dextran sulfate sodium induced intestinal knot inflammation in mice by affecting the release of NO, PGE2 and other inflammatory mediators and cytokines [18]. Carvacrol in MCM can inhibit the expression of pro-inflammatory cytokines interferon-γ (IFN-γ), IL-6, and IL-17 and up-regulate the expression of anti-inflammatory factors TGF-β, IL-4, and IL-10, thus reducing the level of inflammatory factors, reducing the damage to cells, and achieving anti-inflammatory effects [19].
In the formalin-induced licking response test, the licking time of E. ciliata crude ethanol extract and dichloromethane extract is shortened at the late phase under 100 mg/kg dose, and the licking time of n-hexane extract is shortened at the early phase under 100 mg/kg dose, which may be related to its anti-inflammatory effect [11]. Water extract of E. ciliata has anti-allergic inflammatory activity and may be related to the inhibition of calcium, P38 mitogen-activated protein kinase, and nuclear factor-κB expression in the human mast cell line [20].
3.3. Antimicrobial Activity
Different polar extracts of E. ciliata demonstrated significant differences in inhibition ability with regard to microorganism. The results showed that dichloromethane fraction had the strongest inhibitory activity on Candida albicans with minimum inhibitory concentration (MIC) of 62.5 µg/mL, while n-hexane fraction had the strongest inhibitory effect on Escherichia coli with MIC of 250 µg/mL [11]. The ethyl acetate extract of JXR had strong inhibitory effect on Rhizopus oryzae, with the inhibition zone diameter of 13.7 ± 2.7 mm, MIC of 5 mg/mL and minimum bactericidal concentration (MBC) of 5 mg/mL [12]. The MIC of JXR petroleum ether extract, n-butanol extract and ethanol extract against Escherichia coli, Staphylococcus aureus and Bacillus subtilis were 31.25 μg/mL, and the MIC of ethyl acetate extract was 15.60 μg/mL [21]. The carbon dioxide extract of E. ciliata demonstrated a certain inhibitory effect on Staphylococcus aureus, Salmonella paratyphoid, and other microorganisms. When the concentration of the extract was 0.10 g/mL, the inhibitory effect on Staphylococcus aureus was the most obvious, and the diameter of the inhibition zone is 19.7 ± 0.1 mm [22].
According to existing research reports, E. ciliata is rich in essential oil, which contains abundant antibacterial ingredients and can inhibit a variety of microorganisms, so it has research significance and value. The main antibacterial active components of the essential oil of E. ciliata are thymol, carvacrol, and p-Cymene, which have inhibitory effect on Staphylococcus aureus, Methicillin-resistant Staphylococcus aureus and Escherichia coli. MIC were 0.39 mg/mL, 3.12 mg/mL and 1.56 mg/mL, and the diameters of inhibition zone were 21.9 ± 0.1230, 18.2 ± 0.0560, and 16.7 ± 0.0115 nm, respectively [23]. The essential oil in E. ciliata flowers, stems, and leaves had inhibitory effects on Escherichia coli, Staphylococcus aureus, Salmonella typhi, Klebsiella pneumoniae, and Pseudomonas aeruginosa. Both of them had the strongest inhibitory effect on Staphylococcus aureus with the inhibitory zone diameter of 12.2 ± 0.4 and 11.2 ± 0.1 mm, respectively [24]. Other relevant findings suggest that JXR essential oil may affect the formation of Staphylococcus aureus biofilm, so as to achieve bacteriostatic effect on its growth. The MIC of JXR essential oil to Staphylococcus aureus was 0.250 mg/mL. When the concentration was 4MIC, the inhibition rate of essential oil to Staphylococcus aureus biofilm formation could reach 91.3%, and the biofilm clearance rate was 78.5%. The MIC of carvacrol, thymol, and carvacrol acetate against Staphylococcus aureus were 0.122, 0.245, and 0.195 mg/mL, respectively, which were the effective antibacterial components of essential oil. Carvacrol, carvacryl acetate, α-cardene, and 3-carene had strong inhibitory effects on the formation of Staphylococcus aureus biofilm, and the inhibition rates were more than 80% at 1/4 MIC (0.0305, 1.4580, 0.1267 and 2.5975 mg/mL, respectively) [25,26]. In another study, Li Cao et al. studied the inhibitory effect of MCM essential oil on 17 kinds of microorganisms, among which, It significantly inhibited Chaetomium globosum, Aspergillus fumigatus and Candida rugosa. The antibacterial zone diameters were 16.3 ± 0.58, 15.0 ± 1.00, 16.0 ± 0.00 mm, and MIC were 31.3, 62.5, 62.5 μg/mL, respectively [14]. It also has obvious inhibitory effect on Bacillus subtilis and Salmonella enteritidis, which might be related to the terpenes contained, but this opinion remains to be verified [27]. Thymol and carvacrol are the main antibacterial components of MCM. Caryophyllene oxide can be used in the treatment of dermatomycosis, especially in the short-term treatment of mycosis ungualis [28]. The bactericidal mechanism of essential oil may be due to the fact that active components such as carvacrol can damage cell membranes and alter their permeability [29].
The extract of MCM had a significant inhibitory effect on the spore germination of Aspergillus flavus and could significantly change the morphology of Aspergillus flavus mycelia, podocytes, and sporophytes, with a MIC of 0.15 mg/mL [30]. The germination rate of Penicillium digitorum treated with carvacrol significantly decreased, the mechanism may be that carvacrol can change the surface morphology of mycelia, and the cavity rate of mycelia increased with the increase of carvacrol concentration. The permeability of the cell membrane of bacteria increases, causing an electrolyte imbalance in bacteria. As a result, the sugar content and nutrients in bacteria are reduced, so as to achieve bacteriostasis. The MIC and MBC of carvacrol against Penicillium digitorum were 0.125 and 0.25 mg/mL, respectively [31].
3.4. Insecticidal Activity
Some studies have shown that E. ciliata has an insecticidal effect. The repellency rate of E. ciliata essential oil to Blattella germanica was 64.50%, no significant difference from positive control diethyltoluamide (DEET) (p < 0.05). RD50 of E. ciliata essential oil was 218.634 µg/cm2, which was better than DEET (650.403 µg/cm2) [32]. Contact toxicity IC50 of E. ciliata essential oil to Liposcelis bostrychophila was 145.5 μg/cm2, and fumigation toxicity IC50 was 475.2 mg/L. (R)-carvone. Dehydroelsholtzia ketone and elsholtzia ketone are the active components of E. ciliata essential oil against Liposcelis bostrychophila. The IC50 of contact toxicity were 57.0, 151.5, and 194.1 μg/cm2, and those of fumigantion toxicity were 417.4, 658.2, and 547.3 mg/L, respectively [6]. Carvone and limonene are the two main components in E. ciliata essential oil. The ability of E. ciliata essential oil, carvone, and limonene against Tribolium castaneum larvae and adults was evaluated by a contact toxicity test and fumigation assay. Contact toxicity test showed that the LD50 of E. ciliata essential oil, carvone, and limonene to Tribolium castaneum adults were 7.79, 5.08, and 38.57 mg/Adult, respectively, and 24.87, 33.03, and 49.68 mg/Larva to Tribolium castaneum larvae. The results of fumigation toxicity test showed that LC50 of Tribolium castaneum adults were 11.61, 4.34, and 5.52 mg/L Air, respectively, and LC50 of Tribolium Castaneum larvae were 8.73, 28.71, and 20.64 mg/L Air, respectively [5]. Thymol, carvacrol, and β -thymol contained in JXR essential oil had significant fumigation toxicity against Mythimna Separate, Myzus Persicae, Sitophilus Zeamais, Musca domestica, and Tetranychus cinnabarinus, among which β-thymol has the strongest activity. The IC50 values for the five pests were 10.56 (9.26–12.73). 14.13 (11.84–16.59), 88.22 (78.53–99.18), 10.05 (8.63–11.46), and 7.53 (6.53–8.79) μL/L air, respectively [33]. Determined by the immersion method, the LC50 of MCM essential oil against Aedes albopictus larvae and pupae at four instars were 78.820 and 122.656 μg/mL, respectively. The chemotaxis activity of MCM essential oil was evaluated by the method of effective time of human local skin coating. When the dose was 1.5 mg/cm2, the complete protection time of Aedes albopictus was 2.330 ± 0.167 h [34]. From this point of view, E. ciliata essential oil has the development potential as a natural anti-insect agent. It provides a basis for the development and utilization of pesticide dosage forms.
Leishmania mexicana can cause cutaneous leishmaniasis. E. ciliata essential oil had anti-leishmania activity with IC50 of 8.49 ± 0.32 nL/mL. Leishmania mexicana mexicana was treated with a survival rate of 0.38 ± 0.00 %. Selectivity indices were 5.58 and 1.56 for mammalian cell WI38 and J774, respectively. This provides a reference for the treatment of cutaneous leishmaniasis [35]. E. ciliata water extract has an obvious anti-trichomonas vaginalis effect, i.e., can destroy the insect body structure, to achieve the purpose of killing insects. The results of in vitro experiments showed that the lowest effective concentration of E. ciliata water extract was 62.5 mg/mL, and the lowest effective time was 12 h. When the concentration was 250 mg/mL, all Trichomonas vaginalis could be killed for 4 h. This experiment provides a new idea for the clinical treatment of vaginal trichomoniasis [36].
3.5. Antiviral Activity
T helper 17 (Th17) cells play an important role in maintaining adaptive immune balance, and an excess of Th17 cells can cause inflammation. Carvacrol plays an anti-influenza virus role by reducing the proportion of Th17 cells significantly increased by influenza virus A infection. It can be used as a potential antiviral drug and can also be used to control inflammation caused by influenza virus A infection [19]. Mice with viral pneumonia modeled by A/PR/8/34 (H1N1) virus were treated with low, medium and high dose of MCM total flavonoids. Lung index of the three dose groups were 12.81 ± 3.80, 11.65 ± 2.58, 11.45 ± 2.40 mg/g, respectively, compared with the infection group 16.05 ± 3.87 mg/g, the inhibition rates were 20.18%, 27.41%, 28.66%, respectively [37]. E. ciliata ethanol extract has an inhibitory effect on the proliferation of avian infectious bronchitis virus, which may be related to the increased expression of three antiviral genes suppressor of cytokine signaling 3 (SOCS3), 2′-5′-oligoadenylate synthetase-like (OASL), and signal transducer and activator of transcription 1 (STAT1) in H1299 cells treated with extract, and this inhibitory effect shows a certain concentration dependence. In addition, the extract had no cytotoxicity when the concentration was less than 0.3 g/mL [38]. Above experiments provide new possibilities for the treatment of inflammation caused by the virus.
A/WSN/33/2009 (H1N1) virus was used to infect Madin-Darby canine kidney cells to explore the antiviral activity of phenolic acids from MCM in vitro. The survival rate of the cells treated with the compound 3-(3,4-dihydroxyphenyl) acrylic acid 1-(3,4-dihydroxyphenyl)-2-methoxycarbonylethyl and methyl lithospermate were higher than 80%, and the inhibition rate of virus at 100 μmol/L were 89.28% and 98.61%, respectively [39]. In another study, the lung index of low, medium, and high dose of MCM water extract on mice infected by A/PR8 influenza virus was 1.21 ± 0.22%, 1.12 ± 0.17%, and 0.94 ± 0.21%, respectively. Compared to the virus-infected group 1.80 ± 0.29 %, the inhibition rates were 32.78%, 37.78% and 47.78%, respectively. The extracts of the three groups can increase the amounts of IL-2 and IFN-γ in serum of mice, and promote the antiviral ability of the body indirectly or directly [40]. Fluoranthene is a compound with antiviral activity extracted from E. ciliata. It has a certain inhibitory effect on two enveloped viruses, sindbis virus, and murine cytomegalovirus, with the lowest effective concentrations of 0.01 and 1.0 μg/mL, respectively. However, its biological effects are complex, and its clinical safety and effectiveness need further research [41].
3.6. Hypolipidemic Activity
The hypolipidemic activity of E. ciliata ethanol extract was evaluated by determining the effects on the contents of triglyceride and total cholesterol in serum of mice in vivo and the proliferation of 3T3-L1 preadipocytes in vitro. The results showed that the levels of triglyceride and total cholesterol in serum of mice treated with the extract were decreased, and the differentiation and accumulation of 3T3-L1 preadipocytes were also effectively inhibited. The levels of genes associated with adipogenesis, such as peroxisome proliferator activated receptor γ (PPARγ), fatty acid synthase (FAS), and adipocyte fatty acid-binding protein 2 (aP2) were also significantly reduced. In addition, serum leptin content in E. ciliata ethanol extract treatment group was lower than that in obese mice, which may be due to the reduction of fat content. By this token, the action mechanism of E. ciliata lowering blood lipids may be to inhibit the expression of genes related to fat cell formation. However, the specific mechanism needs further study [42].
3.7. Antitumor Activity
Pudziuvelyte, L. et al. extracted essential oil from E. ciliata fresh herbs, lyophilized herbs, and dried herbs, respectively. In in vitro experiments, three kinds of essential oil presented significant inhibition of proliferation effect on the human glioblastoma (U87), pancreatic cancer (PANC-1), and triple negative breast cancer (MDA-MB231) cells, with EC50 values ranging from 0.017% to 0.021%. However, E. ciliata ethanol extract did not show cytotoxicity in this experiment [43]. The antitumor activity of origin processing integration technology and traditional cutting processing technology of E. ciliata was evaluated by measuring the effect of the decoction and essential oil on the average optical density of TNF-α in rat lung tissue. Average optical densities of water decocted solution and essential oil of traditional cutting E. ciliata were 0.530 ± 0.071 and 0.412 ± 0.038, respectively, and those of integration processing technology of origin were 0.459 ± 0.051 and 0.459 ± 0.051, respectively. Compared with the blank group (0.299 ± 0.028), there were varying degrees of increase [44]. In vitro experiments of JXR pectin polysaccharide (MP-A40) showed that the proliferation of human leukemic cell line K562 was affected by MP-A40. When the concentration of MP-A40 was 500μg/mL, the inhibition rate was 31.32% [45].
3.8. Immunoregulatory Activity
Macrophages can regulate apoptosis by producing NO and other effecting molecules. Macrophage RAW 264.7 cells treated by JXR pectin polysaccharide (MP-A40) showed an obvious increase in NO production. Moreover, it’s concentration-dependent. When the concentration of MP-A40 was as low as 10 μg/mL, NO production was still 15 times that of negative control [45]. Mice treated with cyclophosphamide had elevated levels of free radicals, increasing aggression towards immune organs, and decreased thymus and spleen indices. Polysaccharide MP can scavenge free radicals and promote the proliferation of ConA-induced T cells and LPS-induced B cells. To a certain extent, the immunosuppression induced by cyclophosphamide can be alleviated [13,46]. However, the potential immunomodulatory mechanism of polysaccharide remains to be further studied.
3.9. Others
Different polar ethanol extracts of JXR had different degrees of inhibition on α-glucosidase activity. Therefore, it has certain hypoglycemic activity. When the polar ethanol extract concentration was 4.0 mg/mL, the inhibition rate of petroleum ether extract was 93.8%, IC50 was 0.339 mg/mL, and the inhibition rate of ethyl acetate extract was 92.8%, IC50 was 0.454 mg/mL. The essential oil prepared by steam distillation, petroleum ether cold extraction, and petroleum ether reflux extraction also showed significant inhibition of α-glucosidase at the concentration of 0.25 mg/mL, and the inhibition rates were more than 90% [47].
The results of the formalin-induced Licking test showed that E. ciliata crude ethanol extract has analgesic effect on the early stage of reaction (0–5 min) [11].
THE Langendorff perfused isolated rabbit heart model was used. When E. ciliata essential oil was added into perfusate, QRS interval was increased, QT interval was shortened, AND action potentials upstroke amplitude was decreased, and activation time was prolonged when the concentration of E. ciliata essential oil was increased in the range of 0.01–0.1 μL/mL, and showed concentration dependence. This may be due to the fact that sodium channel block can increase the threshold of action potential generation, prolong the effective refractory period, and inhibit the zero-phase depolarization of late depolarization. The reduction of action potential duration can reduce the occurrence of early depolarization. This experiment provides theoretical basis for E. ciliata in the treatment of arrhythmia [48].
7-O-(6-O-acetyl)-β-D-glucopyranosyl-(1→2)[(4-oacetyl)-α-L-rhamnopyranosyl-(1→6)]-β-D-glucopyranoside in methanol extract of E. ciliata was hydrolyzed to obtain acacetin. The IC50 of acacetin against acetylcholinesterase was 50.33 ± 0.87 μg/mL, which showed a significant inhibitory effect on acetylcholinesterase activity, which may hold promise for Alzheimer’s disease treatment [49].
Table 1.
Compounds [5,6,9,11,18,22,24,26,27,28,33,34,39,43,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69].
| No. | Compound Name | Formula | E. ciliata | JXR | MCM | References |
|---|---|---|---|---|---|---|
| Flavonoids | ||||||
| 1 | vitexin | C21H20O10 | + | - | - | [11] |
| 2 | pedalin | C22H22O12 | + | - | - | [11] |
| 3 | luteolin-7-O-β-D-glucopyranoside | C20H20O11 | + | - | - | [11] |
| 4 | apigenin-5-O-β-D-glucopyranoside | C20H20O10 | + | - | - | [11] |
| 5 | apigenin-7-O-β-D-glucopyranoside | C20H20O10 | + | - | - | [11] |
| 6 | chrysoeriol-7-O-β-D-glucopyranoside | C21H22O11 | + | - | - | [11] |
| 7 | 7,3′-dimethoxyluteolin-6-O-β-D-glucopyranoside | C21H24O12 | + | - | - | [11] |
| 8 | luteolin | C15H10O6 | + | + | - | [11,18] |
| 9 | 5,6,4′-trihydroxy-7,3′-dimethoxyflavone | C17H14O7 | + | - | - | [9] |
| 10 | 5-hydroxy-6,7-dimethoxyflavone | C17H14O5 | + | - | - | [9] |
| 11 | 5-hydroxy-7,8-dimethoxyflavone | C17H14O5 | + | - | - | [9] |
| 12 | negletein | C16H12O5 | - | - | + | [50] |
| 13 | acacetin-7-O-[β-D-glucopyranosyl(1″″→2″)-4‴-O-acetyl-α-L-rhamnopyranosyl(1‴→6″)]-β-D-glucopyranoside | C36H44O20 | + | - | - | [51] |
| 14 | acacetin-7-O-[6″″-O-acetyl-β-D-glucopyranosyl(1″″→2″)-α-L-rhamnopyranosyl(1‴→6″)]-β-D-glucopyranoside | C36H44O20 | + | - | - | [51] |
| 15 | acacetin-7-O-[6″″-O-acetyl-β-D-glucopyranosyl(1″″→2″)-3‴-O-acetyl-α-L-rhamnopyranosyl(1‴→6″)]-β-D-glucopyranoside | C38H46O21 | + | - | - | [51] |
| 16 | acacetin-7-O-[6″″-O-acetyl-β-D-glucopyranosyl(1″″→2″)-4‴-O-acetyl-α-L-rhamnopyranosyl(1‴→6″)]-β-D-glucopyranoside | C38H46O21 | + | - | - | [51] |
| 17 | acacetin-7-O-[3″″,6″″-di-Oacetyl-β-D-glucopyranosyl(1″″→2″)-4‴-O-acetyl-α-L-rhamnopyranosyl(1‴→6″)]-β-D-glucopyranoside | C40H45O25 | + | - | - | [51] |
| 18 | 7-O-β-D-glucopyranosyl-(1 → 2)[α-L-rhamnopyranosyl(1 → 6)]-β-D-glucopyranoside | C34H42O19 | + | - | - | [49] |
| 19 | linarin | C28H32O14 | + | - | - | [51] |
| 20 | acacetin-7-O-[4‴-O-acetyl-α-L-rhamnopyranosyl(1‴→6″)]-β-D-glucopyranoside | C30H34O15 | + | - | - | [51] |
| 21 | apigenin | C15H10O5 | + | - | + | [50,51] |
| 22 | apigetrin | C21H20O10 | + | - | - | [51] |
| 23 | diosmetin | C16H12O6 | + | - | - | [51] |
| 24 | 5-hydroxy-6,7-dimethoxyflavone | C17H14O5 | + | - | - | [51] |
| 25 | 5-hydroxy-7,8-dimethoxyflavone | C17H14O5 | + | - | - | [51] |
| 26 | 6-hydroxy-5,7,8-trimethoxyflavone | C18H16O6 | + | - | - | [51] |
| 27 | butin | C16H14O4 | + | - | - | [51] |
| 28 | isookanin | C16H14O5 | + | - | - | [51] |
| 29 | sulfuretin | C15H10O5 | + | - | - | [51] |
| 30 | 3,2′,4′-trihy-droxy-4-methoxychalcone | C16H14O5 | + | - | - | [51] |
| 31 | 3,2′,4′-trihy4′-O-β-D-glucopyranosyl-3,2′-dihydroxy-4-methoxychalcone | C21H24O10 | + | - | - | [51] |
| 32 | okanin-4-methoxy-4′-O-β-D-glucopyranoside | C21H24O11 | + | - | - | [51] |
| 33 | neoisoliquiritin | C20H22O9 | + | - | - | [51] |
| 34 | kumatakenin | C17H14O6 | + | - | - | [52] |
| 35 | isoorientin-2′′-O-rhamnoside | C27H30O15 | - | + | - | [18] |
| 36 | isovitexin-2′′-O-rhamnoside | C27H30O14 | - | + | - | [18] |
| 37 | quercetin-3-O-rutinoside | C27H30O16 | - | + | - | [18] |
| 38 | quercetin-3-O-glucoside | C21H20O12 | - | + | - | [18] |
| 39 | orientin-2′′-O-rhamnoside | C27H30O15 | - | + | - | [18] |
| 40 | vitexin-2′′-O-rhamnoside | C27H30O14 | - | + | - | [18] |
| 41 | orientin | C21H20O11 | - | + | - | [18] |
| 42 | isoorientin | C21H20O11 | - | + | - | [18] |
| 43 | swertisin | C22H22O10 | - | + | - | [18] |
| 44 | peonidin-3-O-glucoside | C22H22O11 | - | + | - | [18] |
| 45 | chrysoeriol | C16H12O6 | - | + | - | [50] |
| 46 | quercetin | C15H10O7 | - | + | - | [50] |
| 47 | kaempferol | C15H10O6 | - | + | - | [53] |
| 48 | catechin | C15H14O6 | + | - | - | [54] |
| Phenylpropanoids | ||||||
| 49 | caffeic acid | C9H8O4 | + | + | - | [9,18] |
| 50 | (E)-p-coumaric acid | C9H8O3 | + | - | - | [9] |
| 51 | osmundacetone | C10H10O3 | + | - | - | [9] |
| 52 | 4-(E)-caffeoyl-L-threonic acid | C17H16O12 | + | - | - | [9] |
| 53 | 4-O-(E)-p-coumaroyl-L-threonic acid | C13H14O7 | + | - | - | [9] |
| 54 | (7E,9E)-3-hydroxyavenalumic acid-3-O-[6′-O-(E)-caffeoyl]-β-D-glucopyranoside | C26H26O12 | + | - | - | [51] |
| 55 | gnaphaliin C | C25H24O11 | + | - | - | [51] |
| 56 | 3,5-di-O-caffeoylquinic acid | C25H24O12 | + | - | - | [51] |
| 57 | everlastoside L | C25H26O11 | + | - | - | [51] |
| 58 | 1-(3′,4′-dihydroxycinnamoyl)cyclopentane2,3-diol | C14H16O6 | + | - | - | [51] |
| 59 | ethyl caffeate | C11H12O4 | + | - | - | [51] |
| 60 | (Z)-p-coumaric acid | C9H8O3 | + | - | - | [51] |
| 61 | p-hydroxybenzaldehyde | C7H6O2 | + | - | - | [51] |
| 62 | p-hydroxybenzoic acid | C7H6O3 | + | + | - | [18,51] |
| 63 | 3,4-dihydroxybenzoic acid | C7H6O4 | + | - | - | [51] |
| 64 | vanillic acid | C8H8O4 | + | - | - | [51] |
| 65 | rosmarinic acid | C18H16O8 | + | + | - | [18,51] |
| 66 | estra-1,3,5(10)-trien-17-β-ol | C18H24O | + | - | - | [55] |
| 67 | 5-caffeoylquinic acid | C16H17O9 | - | + | - | [18] |
| 68 | 4-caffeoylquinic acid | C16H17O9 | - | + | - | [18] |
| 69 | Danshensu | C9H10O5 | - | + | - | [18] |
| 70 | (+)lyoniresinol | C24H38O8 | - | + | - | [53] |
| 71 | (-)-5-methoxyisolariciresinol | C22H30O7 | - | + | - | [53] |
| 72 | pinoresinol | C21H26O6 | - | + | - | [53] |
| 73 | isoeucommin A | C27H34O12 | - | + | - | [53] |
| 74 | episyringaresinol-4-O-β-D-glucopyranoside | C28H36O13 | - | + | - | [53] |
| 75 | methyl-3-(3′,4′dihydroxyphenyl) lactate | C10H12O5 | - | + | - | [56] |
| 76 | (s)-pencedanol-7-O-β-D-glucopyranoside | C20H26O10 | - | + | - | [56] |
| 77 | stearyl ferulate | C28H46O4 | + | - | - | [54] |
| Terpenoids | ||||||
| (1) Monoterpene | ||||||
| 78 | α-Pinene | C10H16 | + | + | + | [5,26,57] |
| 79 | β-Pinene | C10H16 | + | - | + | [5,57] |
| 80 | myrcene | C10H16 | + | - | + | [5,57] |
| 81 | β-Phellandrene | C10H16 | + | - | - | [5] |
| 82 | limonene | C10H16 | + | - | + | [5,57] |
| 83 | β-Ocimene | C10H16 | + | - | + | [5,57] |
| 84 | camphene | C10H16 | + | - | + | [57,58] |
| 85 | isoterpinolene | C10H16 | + | - | - | [58] |
| 86 | γ-terpinene | C10H16 | + | + | - | [26,58] |
| 87 | 4-carene | C10H16 | + | - | - | [6] |
| 88 | sabinene | C10H16 | + | - | + | [57,59] |
| 89 | α-phellandrene | C10H16 | - | + | + | [26,57] |
| 90 | 3-carene | C10H16 | - | + | + | [26,57] |
| 91 | sylvestrene | C10H16 | - | + | - | [26] |
| 92 | terpinolene | C10H16 | - | + | + | [26,57] |
| 93 | α-thujene | C10H16 | - | - | + | [57] |
| 94 | bicyclo [3.1.0]hex-2-ene, 4-methylene-1-(1-methylethyl)- | C10H14 | - | - | + | [57] |
| 95 | bicyclo[4.2.0]oct-1-ene,7-exo-ethenyl- | C10H14 | - | - | + | [57] |
| 96 | α-terpinene | C10H16 | - | - | + | [57] |
| 97 | 2,6-dimethyl-1,3,5,7-octatetraene | C10H14 | - | - | + | [57] |
| 98 | p-mentha-1(7),2-diene | C10H16 | + | - | - | [60] |
| 99 | cyclohexane, 1-methylene-4-(1-methylethenyl)- | C10H16 | + | - | - | [61] |
| 100 | artemisia triene | C10H16 | - | - | + | [28] |
| (2) Oxygenated monoterpene | ||||||
| 101 | linalool | C10H18O | + | - | + | [5,57] |
| 102 | elsholtzia ketone | C10H14O2 | + | - | - | [5] |
| 103 | carvone | C10H14O | + | - | - | [5] |
| 104 | dehydroelsholtzia ketone | C10H12O2 | + | - | - | [5] |
| 105 | neodihydrocarveol | C10H18O | + | - | - | [58] |
| 106 | eucalyptol | C10H18O | + | + | - | [33,58] |
| 107 | menthone | C10H18O | + | - | - | [58] |
| 108 | linalool | C10H18O | + | - | - | [58] |
| 109 | camphor | C10H16O | + | - | - | [58] |
| 110 | eucarvone | C10H14O | + | - | - | [58] |
| 111 | perillene | C10H14O | + | - | - | [58] |
| 112 | jasmone | C10H14O | + | - | - | [58] |
| 113 | dehydroelsholtzia ketone | C10H12O2 | + | - | - | [58] |
| 114 | eucalyptol | C10H18O | + | + | - | [33,58] |
| 115 | (−)-1R-8-hydroxy-p-menth-4-en-3-one | C10H16O2 | + | - | - | [43] |
| 116 | fenchol | C10H18O | + | - | - | [6] |
| 117 | lavandulol | C10H18O | + | - | - | [6] |
| 118 | α-terpineol | C10H18O | + | - | - | [6] |
| 119 | 1,6-dihydrocarveol | C10H18O | + | - | - | [6] |
| 120 | cis-carveol | C10H16O | + | - | - | [6] |
| 121 | terpinen-4-ol | C10H18O | + | + | - | [33,59] |
| 122 | nerol | C10H18O | + | - | - | [59] |
| 123 | neral | C10H16O | + | - | - | [59] |
| 124 | geraniol | C10H18O | + | - | - | [59] |
| 125 | borneol | C10H18O | - | + | - | [33] |
| 126 | trans-4-thujanol | C10H18O | - | + | - | [26] |
| 127 | sabinol | C10H16O | - | + | - | [26] |
| 128 | thymoquinone | C10H12O2 | - | - | + | [57] |
| 129 | umbellulon | C10H14O | - | - | + | [27] |
| 130 | furan,3-methyl-2-(3-methyl-2-buten-1-yl)- | C10H14O | + | - | - | [24] |
| 131 | verbenol | C10H16O | - | - | + | [28] |
| 132 | β-dehydro-elsholtzione | C10H12O2 | + | - | - | [22] |
| 133 | rose furan epoxide | C10H14O2 | + | - | - | [65] |
| 134 | 1-octen-3-yl acetate | C10H18O2 | + | - | - | [24] |
| 135 | neryl formate | C11H18O2 | + | - | - | [59] |
| 136 | geranyl formate | C11H18O2 | + | - | - | [59] |
| 137 | neryl acetate | C12H20O2 | + | - | - | [59] |
| 138 | citronellal | C10H18O | + | - | - | [6] |
| 139 | isobornyl formate | C11H18O2 | - | - | + | [57] |
| (3) Sesquiterpene | ||||||
| 140 | cubebene | C15H24 | + | - | - | [5] |
| 141 | β-bourbonene | C15H24 | + | - | - | [5] |
| 142 | β-caryophyllene | C15H24 | + | + | + | [5,26,57] |
| 143 | α-caryophyllene | C15H24 | + | + | - | [5,33] |
| 144 | α-farnesene | C15H24 | + | + | + | [5,26,57] |
| 145 | β-bourbonene | C15H24 | + | - | - | [58] |
| 146 | β-gurjunene | C15H24 | + | - | - | [58] |
| 147 | π-cubebene | C15H24 | + | - | - | [58] |
| 148 | α-bergamotene | C15H28 | + | + | - | [26,58] |
| 149 | humulene | C15H24 | + | - | - | [58] |
| 150 | π-sesquiphellandrene | C15H24 | + | - | - | [58] |
| 151 | germacrene D | C15H24 | + | + | - | [26,58] |
| 152 | π-bisabolene | C15H24 | + | - | - | [58] |
| 153 | γ-elemene | C15H24 | + | - | - | [58] |
| 154 | (Z, E)-α-farnesene | C15H24 | + | - | - | [58] |
| 155 | caryophyllene | C15H24 | + | + | - | [33,58] |
| 156 | π-muurolene | C15H24 | + | - | - | [58] |
| 157 | π-cadinene | C15H24 | + | - | - | [58] |
| 158 | α-farnesene | C15H24 | + | - | - | [43] |
| 159 | ledene | C15H24 | + | - | - | [43] |
| 160 | α-cubebene | C15H24 | + | - | - | [43] |
| 161 | γ-cadinene | C15H24 | + | - | - | [43] |
| 162 | δ-cadinene | C15H24 | + | - | - | [43] |
| 163 | β-elemene | C15H24 | + | - | - | [6] |
| 164 | aromadendrene | C15H24 | + | - | - | [6] |
| 165 | β-bisabolene | C15H24 | + | - | - | [59] |
| 166 | trans-α-bergamotene | C15H24 | - | + | - | [33] |
| 167 | γ-muurolene | C15H24 | - | + | - | [26] |
| 168 | eudesma-3,7(11)-diene | C15H24 | - | + | - | [26] |
| 169 | longifolene | C15H24 | - | - | + | [57] |
| 170 | trans-α-bergamotene | C15H24 | - | - | + | [57] |
| 171 | trans-β-bergamotene | C15H24 | - | - | + | [57] |
| 172 | (Z,E)-α-farnesene | C15H24 | - | - | + | [57] |
| 173 | β-himachalene | C15H24 | - | - | + | [57] |
| 174 | γ-cadinene | C15H24 | - | - | + | [57] |
| 175 | guaiene | C15H24 | - | - | + | [64] |
| 176 | α-zingiberene | C15H24 | - | - | + | [64] |
| 177 | α-muurolene | C15H24 | - | - | + | [64] |
| 178 | β-selinene | C15H24 | + | - | - | [65] |
| (4) Oxygenated sesquiterpene | ||||||
| 179 | (-)-humulene epoxide II | C15H24O | + | - | - | [5] |
| 180 | nerolidol | C15H26O | + | - | - | [58] |
| 181 | caryophyllene oxide | C15H24O | + | + | - | [33,58] |
| 182 | spathulenol | C15H24O | + | - | - | [6] |
| 183 | humulene-1,2-epoxide | C15H24O | - | + | - | [26] |
| 184 | cedrol | C15H26O | - | - | + | [57] |
| 185 | cedrenol | C15H24O | - | - | + | [64] |
| 186 | levomenol | C15H26O | - | - | + | [64] |
| 187 | germacrone | C15H22O | + | - | - | [22] |
| (5) Oxygenated diterpene | ||||||
| 188 | 2,3-dimethyl-5-(2,6,10trimethylundecyl) furan | C20H36O | + | - | - | [43] |
| 189 | phytol | C20H40O | - | - | + | [28] |
| (6) Triterpene | ||||||
| 190 | forrestin A | C30H42O11 | - | + | - | [18] |
| 191 | betulinic acid | C30H48O3 | - | + | - | [50] |
| 192 | oleanolic acid | C30H48O3 | - | + | - | [50] |
| 193 | ursolic acid | C30H48O3 | - | + | - | [50] |
| Alkaloids | ||||||
| 194 | N-trans-feruloyloctopamine | C18H19NO5 | + | - | - | [51] |
| 195 | 5-methyl-furan-2-carboxylic acid (1H-[1,2,4]triazol3-yl) -amide | C8H8N4O2 | + | - | - | [51] |
| 196 | furane-2-carboxaldehyde, 5 (nitrophenoxymethyl) | C12H9NO5 | + | - | - | [51] |
| 197 | uridine | C9H12N2O6 | - | + | - | [18] |
| 198 | carbamult | C12H17NO2 | - | + | - | [26] |
| 199 | phenol,O-amino- | C6H7NO | + | - | - | [61] |
| 200 | adenosin | C10H13N5O4 | - | + | - | [67] |
| 201 | prunasin | C14H17NO6 | - | + | - | [68] |
| 202 | sambunigrin | C13H17NO7 | - | + | - | [68] |
| Others | ||||||
| (1) Aliphatic | ||||||
| 203 | pentacosane | C25H52 | + | - | - | [55] |
| 204 | heptacosane | C27H56 | + | - | - | [55] |
| 205 | octacosane | C28H58 | + | - | - | [55] |
| 206 | tetratriacontane | C34H70 | + | - | - | [55] |
| 207 | hexatriacontane | C36H74 | + | - | - | [55] |
| 208 | n-dodecane | C11H24 | - | - | + | [57] |
| 209 | n-tridecane | C13H28 | - | - | + | [57] |
| 210 | n-tetradecane | C14H30 | - | - | + | [57] |
| 211 | n-pentadecane | C15H32 | - | - | + | [57] |
| 212 | n-hexadecane | C16H34 | - | - | + | [57] |
| 213 | heptadecane | C17H36 | - | + | - | [63] |
| 214 | octadecane | C18H38 | - | + | - | [63] |
| 215 | tridecane,4-cyclohexyl | C19H38 | - | + | - | [63] |
| 216 | nonadecane | C19H40 | - | + | - | [63] |
| 217 | eicosane | C20H42 | - | + | - | [63] |
| 218 | heneicosane | C21H44 | - | + | - | [63] |
| 219 | docosane | C22H46 | - | + | - | [63] |
| 220 | tricosane | C23H48 | - | + | - | [63] |
| 221 | tetracosane | C24H50 | - | + | - | [63] |
| 222 | 9-cyclohexyl eicosane | C26H52 | - | + | - | [63] |
| 223 | (R, R)-2,3-butanediol | C4H10O2 | + | - | - | [58] |
| 224 | 3-hexen-1-ol | C6H12O | + | - | - | [58] |
| 225 | 1-hexanol | C6H14O | + | - | - | [58] |
| 226 | 3-octen-1-ol | C8H16O | + | - | - | [58] |
| 227 | 3-octanol | C8H18O | + | - | - | [58] |
| 228 | 3,13-octadecadien-1-ol | C18H34O | - | + | - | [63] |
| 229 | 2-octene-1-ol | C8H16O | + | - | - | [66] |
| 230 | hexanal | C6H12O | + | - | - | [58] |
| 231 | heptana | C7H14O | + | - | - | [58] |
| 232 | octanal | C8H16O | + | - | - | [58] |
| 233 | nonanal | C9H18O | + | - | - | [58] |
| 234 | nonacosan-10-one | C29H58O | + | - | - | [55] |
| 235 | 3-hexanone | C6H12O | + | - | - | [58] |
| 236 | 3-heptanone | C7H14O | + | - | - | [58] |
| 237 | 3-octanone | C8H16O | + | - | - | [58] |
| 238 | irisone | C13H20O | + | - | - | [24] |
| 239 | 2-pentadecanone,6,10,14-trimethyl | C18H36O | - | + | - | [63] |
| 240 | α-linolenic acid | C18H30O2 | + | - | - | [9] |
| 241 | n-hexadecanoic acid | C16H32O2 | + | - | - | [55] |
| 242 | 9,12-octadecadienoic acid | C18H32O2 | + | - | - | [55] |
| 243 | 3-methylpentanoic acid | C6H12O2 | + | - | - | [58] |
| 244 | 2-methylbutanoic acid | C5H10O2 | + | - | - | [58] |
| 245 | galactonic acid | C6H12O7 | - | + | - | [18] |
| 246 | malic acid | C4H6O5 | - | + | - | [18] |
| 247 | citric acid | C6H8O7 | - | + | - | [18] |
| 248 | quinic acid | C7H12O6 | - | + | - | [18] |
| 249 | succinic acid | C4H6O4 | - | + | - | [18] |
| 250 | azelaic acid | C9H16O4 | - | + | - | [18] |
| 251 | 9-hydroxy-10,12-octadecadienoic acid | C18H32O3 | - | + | - | [18] |
| 252 | palmitic acid | C16H32O2 | - | + | - | [18] |
| 253 | oleic acid | C18H34O2 | - | + | - | [18] |
| 254 | tetradecanoic acid | C14H28O2 | - | + | - | [63] |
| 255 | linoleic acid | C18H32O2 | + | [66] | ||
| 256 | hexadecanoic acid, ethyl ester | C18H36O2 | + | - | - | [55] |
| 257 | 9,12,15-octadecatrienoic acid methyl ester | C19H32O2 | + | - | - | [55] |
| 258 | linoleic acid ethyl ester | C20H36O2 | + | - | - | [55] |
| 259 | 9,12,15-octadecatrienoic acidethyl ester | C20H34O2 | + | - | - | [55] |
| 260 | octadecanoic acidethyl ester | C20H40O2 | + | - | - | [55] |
| 261 | linolenic acide, 2-hydroxy-1(hydroxymethyl) ethyl ester | C21H36O4 | + | - | - | [55] |
| 262 | octen-1-ol, acetate | C10H18O2 | + | - | - | [58] |
| 263 | 3-octanol, acetate | C10H20O2 | + | - | - | [58] |
| 264 | 2-propenoic acid, 2-methyl-, ethenyl ester | C6H8O2 | + | - | - | [43] |
| 265 | diglycol laurate | C16H32O4 | - | + | - | [18] |
| 266 | butanoic acid, 2-methylpropyl ester | C8H16O2 | - | - | + | [57] |
| 267 | propanoic acid, 2-methyl-,butyl ester | C8H16O2 | - | - | + | [57] |
| 268 | 2-propenoic acid, 2-methyl-,butyl ester | C8H14O2 | - | - | + | [57] |
| 269 | butanoic acid, butyl ester | C8H16O2 | - | - | + | [57] |
| 270 | propanoic acid, 2-methyl,2-methylpropyl ester | C8H16O2 | - | - | + | [57] |
| 271 | heptadecanoic acid, ethylester | C19H38O2 | - | + | - | [63] |
| 272 | acetic acid, bornyl ester | C12H20O2 | - | - | + | [34] |
| 273 | ethyl linoleate | C20H36O2 | + | - | - | [22] |
| 274 | octenylacetate | C10H18O2 | + | - | - | [65] |
| 275 | 2,5-diethyltetrahydrofuran | C8H16O | + | - | - | [58] |
| 276 | 1,8-cineole | C10H18O | + | - | - | [6] |
| 277 | dihydroartemisinin ethyl ether | C17H28O5 | - | + | - | [18] |
| 278 | pentane, 1-butoxy- | C9H20O | - | - | + | [57] |
| 279 | ethane, 1,1-dibutoxy- | C10H22O2 | - | - | + | [57] |
| 280 | 1,1-dibutoxy-isobutane | C12H26O2 | - | - | + | [57] |
| 281 | butane, 1,1-dibutoxy- | C12H26O2 | - | - | + | [57] |
| 282 | 3-methyl-3-oxetanemethanol | C5H10O2 | + | - | - | [43] |
| 283 | neryl-β-D-glucopyranoside | C16H28O6 | + | - | - | [51] |
| 284 | furfural | C5H4O2 | + | - | - | [58] |
| 285 | 2-acetyl-5-methylfuran | C7H8O2 | + | - | - | [58] |
| 286 | geranyl acetate | C12H20O2 | + | - | - | [58] |
| 287 | octen-3-ol | C9H19O | + | - | + | [6,57] |
| 288 | cis-jasmone | C11H16 | + | - | - | [6] |
| 289 | 2,6-octadien-1-ol, 3,7-dimethyl- | C11H20 | - | - | + | [57] |
| 290 | 3,5-dimethylcyclohex-1-ene-4carboxaldehyde | C9H14O | - | + | - | [33] |
| 291 | (6S,9R)-roseoside | C19H30O8 | - | + | - | [67] |
| 292 | 1-hexadecene | C16H32 | - | + | - | [63] |
| 293 | 1-nonene | C9H18 | - | - | + | [34] |
| 294 | ligustilide | C12H14O2 | + | - | - | [22] |
| 295 | cyclohexene, 2-ethenyl-1,3,3-trimethyl | C11H18 | + | - | - | [43] |
| 296 | daucosterol | C35H60O6 | + | - | - | [54] |
| (2) Aromatic | ||||||
| 297 | thymol | C10H14O | + | + | + | [33,57,58] |
| 298 | carvacrol | C10H14O | - | + | + | [33,57] |
| 299 | p-cymene | C10H14 | + | + | + | [6,26,57] |
| 300 | methyl chavicol | C10H12O | + | - | - | [59] |
| 301 | m-cymene | C10H14 | - | - | + | [57] |
| 302 | 3,4-diethylphenol | C10H14O | - | - | + | [57] |
| 303 | p-eugenol | C10H12O2 | - | - | + | [57] |
| 304 | O-cymene | C10H14 | + | - | - | [60] |
| 305 | cis-anethol | C10H12O | - | + | - | [62] |
| 306 | 3,4,5-trimethoxytoluene | C10H14O3 | - | + | - | [62] |
| 307 | cis-asarone | C10H14O2 | - | + | - | [63] |
| 308 | cuminaldehyde | C10H12O | - | - | + | [64] |
| 309 | benzyl-β-D-glucopyranoside | C13H18O6 | + | - | - | [51] |
| 310 | p-xylene | C8H10 | + | - | - | [58] |
| 311 | benzaldehyde | C7H6O | + | - | + | [57,58] |
| 312 | thymol acetate | C12H16O2 | + | + | + | [33,57,58] |
| 313 | acetophenone | C8H8O | + | - | - | [58] |
| 314 | naphthalene | C10H8O | + | - | - | [43] |
| 315 | protocatechuic acid | C7H6O4 | - | + | - | [18] |
| 316 | sodium ferulate | C10H9NaO4 | - | + | - | [18] |
| 317 | 4,5-dimethyl-2-ethylphenol | C10H14O | - | + | - | [33] |
| 318 | thymyl acetate | C12H16O2 | - | + | - | [26] |
| 319 | carvacryl acetate | C12H16O2 | - | + | - | [26] |
| 320 | 4-O-β-D- glucopyranosylbenzyl-4′-hydroxylbenzoate | C20H22O9 | - | - | + | [39] |
| 321 | 4-O-β-D-glucopyranosylbenzyl-3′-hydroxy-4′- methoxybenzoate | C21H24O10 | - | - | + | [39] |
| 322 | amburoside A | C20H22O10 | - | - | + | [39] |
| 323 | 4-[[(2′,5′-Dihydroxybenzoyl)oxy]methyl]phenyl-O-β-D-glucopyranoside | C20H20O10 | - | - | + | [39] |
| 324 | hyprhombin B methyl ester | C26H22O9 | - | - | + | [39] |
| 325 | methyl lithospermate | C28H24O12 | - | - | + | [39] |
| 326 | dimethyl lithospermate | C29H26O12 | - | - | + | [39] |
| 327 | salvianolic acid C methyl ester | C27H23O10 | - | - | + | [39] |
| 328 | sebestenoids C | C36H30O14 | - | - | + | [39] |
| 329 | cucurbitoside D | C25H30O13 | - | - | + | [39] |
| 330 | 2,4-di-tert-butylphenol | C14H22O | - | - | + | [27] |
| 331 | benzyl benzoate | C14H12O2 | + | - | - | [69] |
| 332 | 4-isopropyl-3-methylphenol | C10H14O | + | - | - | [60] |
| 333 | benzenemethanol, 4-(1-methylethyl)- | C10H14O | + | - | - | [61] |
| 334 | methyl eugenol | C11H14O2 | - | + | - | [62] |
| 335 | elemicin | C12H16O3 | - | + | - | [62] |
| 336 | myristicin | C11H12O3 | - | + | - | [62] |
| 337 | methyl thymol ether | C11H16O | - | - | + | [28] |
| 338 | apiole | C12H14O4 | - | - | + | [28] |
| 339 | 4-hydroxy-2,6-dimethoxyphenyl-β-D-glucopyranoside | C14H20O9 | - | + | - | [67] |
| 340 | 4-hydroxy-3,5-dimethoxyphenyl-β-D-glucopyranoside | C14H20O9 | - | + | - | [67] |
| 341 | 3,4,5-dimethoxyphenyl-β-D-glucopyranoside | C15H22O9 | - | + | - | [67] |
| 342 | 3-hydroxyestragole-β-D-glucopyranosides | C16H22O7 | - | + | - | [67] |
| 343 | 4-acetoxy-3-methoxy acetophenone | C11H12O4 | - | + | - | [63] |
| 344 | benzyl-D-glucopyranoside | C13H18O6 | - | + | - | [63] |
| 345 | 2′,4′-dihydroxy-3′methylacetophenone | C9H10O3 | - | - | + | [34] |
| 346 | acetylthymol | C12H16O2 | - | - | + | [64] |
| 347 | carvacrol acetate | C12H16O2 | + | - | - | [66] |
| 348 | bergaptene | C12H8O4 | + | - | - | [66] |
| 349 | 2-butenylbenzene | C10H12 | + | - | - | [22] |
| 350 | α-methyl-benzenepropanol | C10H14O | + | - | - | [22] |
| 351 | cuminic acid | C10H12O | + | - | - | [22] |
| 352 | p-cymen-8-ol | C11H16O | + | - | - | [6] |
“+” Found in plants. “-” Not found in plants.
Figure 1.
Chemical structures isolated from E. ciliata.
4. Conclusions and Prospects
This paper summarizes pharmacological activities of E. ciliata, among which antioxidant, anti-inflammatory, antimicrobial and insecticidal activities are the main activities, but also has antiviral, hypolipidemic, hypoglycemic, anti-tumor activities. Hence, 352 kinds of chemical constituents identified from E. ciliata were summarized. According to their structure types, they can be divided into flavonoids, phenylpropanoids, terpenoids, alkaloids and other compounds.
According to the existing pharmacological experiment results in vivo and in vitro, E. ciliata dichloromethane extract, ethyl acetate extract and essential oil all show good pharmacological activity. Carvacrol contained in E. ciliata is the main active ingredient of antibacterial. At present, researches on pharmacological activity of E. ciliata mainly focus on essential oil, and some researches involve E. ciliata alcohol extract, water extract and polysaccharide, but there are relatively few researches on pharmacological activities of E. ciliata such as analgesia, immune regulation, hypoglycemia and hypolipemia. Whether E. ciliata has potential pharmacological activity still needs further test to prove. The safety study of clinical dose also deserves extensive attention.
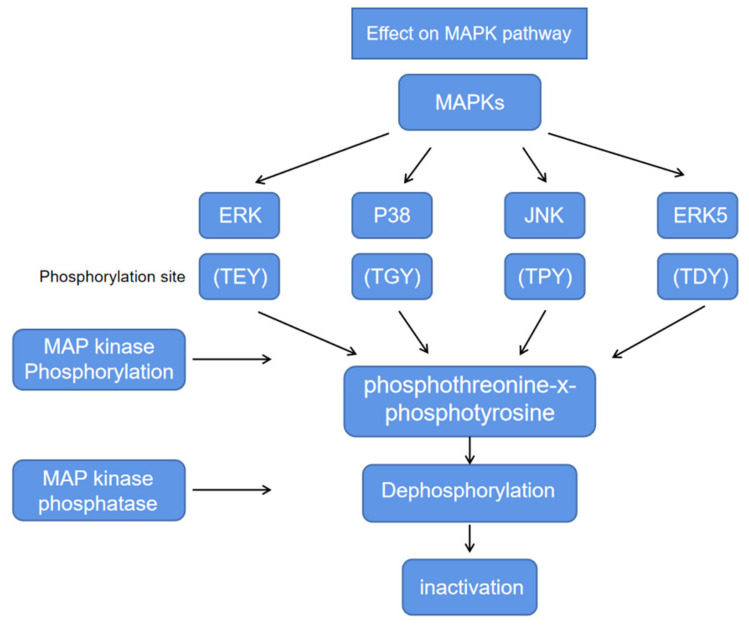
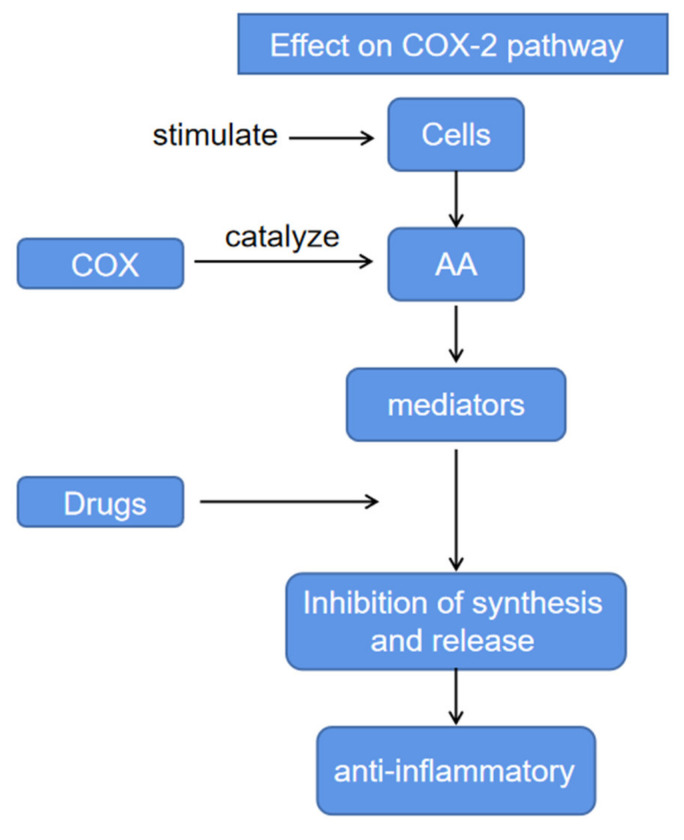
Some representative action mechanisms of E. ciliata were briefly illustrated for the sake of reference. The possible action process is shown in the figure. The mitogen-activated protein kinases (MAPKs) signaling pathway chain consists of three protein kinases, MAP3K–MAP2K–MAPK, which transmit upstream signals to downstream responsive molecules through sequential phosphorylation. MAPK includes four subfamilies: ERK, p38, JNK, and ERK5. MAPK activity is thought to be regulated by the diphosphate sites in the amino acid sequence of the active ring. The active ring contains a characteristic threonine-x-tyrosine (T-x-Y) motif. Mitogen activated protein (MAP) kinase phosphorylates on two amino acid residues, thereby activating MAPK pathway. MAP kinase phosphatase (MKP) can hydrolyze phosphorylated products and inactivate MAPK pathway. The extract inhibited the activation of MAPK signaling pathway by blocking the phosphorylation of p38, JNK and ERK [18]. When stimulated, tissue cells release arachidonic acid (AA). Cyclooxygenase (COX) catalyze AA to produce a series of bioactive substances such as prostaglandins (PGs), causing inflammation. The extract can affect the COX-2 pathway by affecting the release levels of TNF-α, IL-6, and PGE2, which are key mediators released by macrophages during bacterial infection, so as to achieve the purpose of anti-inflammatory [4]. Carvacrol can significantly inhibit the mRNA expression of toll like receptor 7 (TLR7), interleukin-1 receptor associated kinase (IRAK4), TNF receptor associated factor (TRAF6), induced pluripotent stem-I (IPS-I), and interferon regulatory factor 3 (IRF3) in mice, thereby affecting the immunomodulatory signaling pathways of TLR7/RLR and playing an anti-H1N1 influenza virus role [19]. With the deepening of various studies, the gradual clarification of the mechanism of action has created conditions for drugs to play a better role. Two representative mechanisms of action, MAPK and COX-2, are shown in Figure 2 and Figure 3.
E. ciliata has rich resources and low requirements for growth environment. It can be planted artificially and has a short growth cycle. The rich essential oil content makes it possible for E. ciliata to be used as flavor and food additive. Undoubtedly, the development of E. ciliata in new dosage forms and the application to medicine, food, and other fields will provide broad development prospects in the future.
Figure 2.
Effect on Mitogen-activated protein kinases pathway.
Figure 3.
Effect on Cyclooxygenase-2 pathway.
Author Contributions
F.W. and X.L. contributed equally to this work. writing—original draft preparation and investigation, F.W. and X.L.; investigation, Y.C., Y.A., W.Z., L.W. and J.T.; resources, D.K., Y.X. and Y.B.; writing, supervision, review, editing, funding acquisition, H.Z. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was funded by the Project of the Shandong Province Key Research and Development Program (2021CXGC010511), the project of Shandong postgraduate education quality curriculum (SDYKC21051), and the innovation training program for college students of Shandong University of Traditional Chinese Medicine (202255).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lin Q., Lai F.G., Yang S.T. Identification of original plants of Elsholtzia jiangxiang. J. Chin. Med. Mater. 1986;2:22–25. [Google Scholar]
- 2.Zhu G.P., Shi J.L. A new variety of Elsholtzia stone, the original plant of Chinese medicinal herb Elsholtzia Jiang. J. Syst. Evol. 1995;3:305. [Google Scholar]
- 3.Cao H., Zhao W.L., Hao J.D., Li Y.H. Research on latin scientific names of medicinal materials in the 2020 edition of Chinese Pharmacopoeia. J. Chin. Med. Mater. 2022;45:1002–1007. [Google Scholar]
- 4.Pudziuvelyte L., Liaudanskas M., Jekabsone A., Sadauskiene I., Bernatoniene J. Elsholtzia ciliata (Thunb.) Hyl. extracts from different plant parts: Phenolic composition, antioxidant, and anti-Inflammatory activities. Molecules. 2020;25:1153. doi: 10.3390/molecules25051153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liang J.Y., Xu J., Yang Y.Y., Shao Y.Z., Zhou F., Wang J.L. Toxicity and synergistic effect of Elsholtzia ciliata essential oil and its main components against the adult and larval stages of tribolium castaneum. Foods. 2020;9:345. doi: 10.3390/foods9030345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao M.P., Liu X.C., Lai D.W., Zhou L.G., Liu Z.L. Analysis of the essential oil of Elsholtzia ciliate aerial parts and its insecticidal activities against liposcelis bostrychophila. Helv. Chim. Acta. 2016;99:90–94. doi: 10.1002/hlca.201500232. [DOI] [Google Scholar]
- 7.Zienkaitė K., Liekis A., Sadauskienė I., Pudžiuvelytė L., Bernatonienė J., Šimonytė S. The effect of Elsholtzia Ciliata essential oil on catalase (Cat) activity in mice brain; Proceedings of the XIII International Conference of the Lithuanian Neuroscience Association “CONSCIOUSNESS” (LNA conference); Kaunas, Lithuania. 26 November 2021. [Google Scholar]
- 8.Trinh T.A., Seo Y.H., Choi S., Lee J., Kang K.S. Protective effect of osmundacetone against neurological cell death caused by oxidative glutamate toxicity. Biomolecules. 2021;11:328. doi: 10.3390/biom11020328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen D.T., Tran H., Schwaiger S., Stuppner H., Marzocco S. Effect of non-volatile constituents of Elsholtzia ciliata (Thunb.) Hyl. from southern vietnam on reactive oxygen species and nitric oxide release in macrophages. Chem. Biodivers. 2021;18:e2000577. doi: 10.1002/cbdv.202000577. [DOI] [PubMed] [Google Scholar]
- 10.Phong H.X., Viet N.T., Quyen N.T.N., Van Thinh P., Trung N.M., Ngan T.T.K. Phytochemical screening, total phenolic, flavonoid contents, and antioxidant activities of four spices commonly used in Vietnamese traditional medicine. Mater. Today Proc. 2021;56:A1–A5. doi: 10.1016/j.matpr.2021.12.142. [DOI] [Google Scholar]
- 11.Zhang Q., Porto N.M., Guilhon C.C., Giorno T.B.S., Alviano D.S., Agra M.D., Fernandes P.D., Boylan F. Pharmacognostic study on Elsholtzia ciliata (Thumb.) Hyl: Anatomy, phytochemistry and pharmacological activities. Pharmaceuticals. 2021;14:1152. doi: 10.3390/ph14111152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J.E., Fan S.T., Qiu Z.H., Li C., Nie S.P. Total flavonoids content, antioxidant and antimicrobial activities of extracts from Mosla chinensis Maxim. cv. Jiangxiangru. LWT—Food Sci. Technol. 2015;64:1022–1027. doi: 10.1016/j.lwt.2015.07.033. [DOI] [Google Scholar]
- 13.Li J.E., Nie S.P., Xie M.Y., Li C. Isolation and partial characterization of a neutral polysaccharide from Mosla chinensis Maxim. cv. Jiangxiangru and its antioxidant and immunomodulatory activities. J. Funct. Foods. 2014;6:410–418. doi: 10.1016/j.jff.2013.11.007. [DOI] [Google Scholar]
- 14.Cao L., Si J.Y., Liu Y., Sun H., Jin W., Li Z., Zhao X.H., Pan R.L. Essential oil composition, antimicrobial and antioxidant properties of Mosla chinensis Maxim. Food Chem. 2009;115:801–805. doi: 10.1016/j.foodchem.2008.12.064. [DOI] [Google Scholar]
- 15.Liu X.P., Jia J., Yang L., Yang F.J., Ge H.S., Zhao C.J., Zhang L., Zu Y.G. Evaluation of antioxidant activities of aqueous extracts and fractionation of different parts of Elsholtzia ciliata. Molecules. 2012;17:5430–5441. doi: 10.3390/molecules17055430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun D.Y., Gao H., Wang X.T., Yan L., Pang B. Study on the antipyretic and anti-inflammatory effects of producing area processing integrated Mosla chinensis Maxim. Chin. Herb. Med. 2018;49:4737–4742. [Google Scholar]
- 17.Guzik T.J., Korbut R., Adamek-Guzik T. Nitric oxide and superoxide in inflammation and immune regulation. J. Physiol. Pharmacol. 2003;54:469–487. [PubMed] [Google Scholar]
- 18.Wang X., Cheng K., Liu Z., Sun Y., Zhou L., Xu M., Dai X., Xiong Y., Zhang H. Bioactive constituents of Mosla chinensis. cv. Jiangxiangru ameliorate inflammation through MAPK signaling pathways and modify intestinal microbiota in DSS-induced colitis mice. Phytomedicine. 2021;93:153804. doi: 10.1016/j.phymed.2021.153804. [DOI] [PubMed] [Google Scholar]
- 19.Zheng K., Wu S.Z., Lv Y.W., Pang P., Deng L., Xu H.C., Shi Y.C., Chen X.Y. Carvacrol inhibits the excessive immune response induced by influenza virus A via suppressing viral replication and TLR/RLR pattern recognition. J. Ethnopharmacol. 2021;268:113555. doi: 10.1016/j.jep.2020.113555. [DOI] [PubMed] [Google Scholar]
- 20.Kim H.H., Yoo J.S., Lee H.S., Kwon T.K., Shin T.Y., Kim S.H. Elsholtzia ciliata inhibits mast cell-mediated allergic inflammation: Role of calcium, p38 mitogen-activated protein kinase and nuclear factor-kappa B. Exp. Biol. Med. 2011;236:1070–1077. doi: 10.1258/ebm.2011.011017. [DOI] [PubMed] [Google Scholar]
- 21.Li Z.M., Sun Y.M., Wang M., Peng L., Ren P. Study on antibacterial activity of different polar extracts from Mosla chinensis Maxim. cv. Jiangxiangru. Sci. Technol. Food Ind. 2014;35:115–116+120. [Google Scholar]
- 22.Hu H.B., Cao H., Jian Y.F., Zheng X.D. Study on extraction and antibacterial activity of active components from Elsholtzia chinensis. Grassl. Sci. 2007;24:36–39. [Google Scholar]
- 23.Li Y.Q., Ren Y.S., Wang L.J., Ai J., Liang S., Zhang T.P., Liao M.C., Li J. Preparation of nanoemulsion spray from Moslae Herba volatile oil and its antibacterial activity. China J. Chin. Mater. Med. 2021;46:4986–4992. doi: 10.19540/j.cnki.cjcmm.20210408.302. [DOI] [PubMed] [Google Scholar]
- 24.Xiang P., Lou G.Q., Wang S.Y., Chen Q. Analysis of volatile oils in Elsholtzia ciliata and Elsholtzia cypriani and evaluation of their biological activities. Chin. Tradit. Pat. Med. 2017;39:1880–1884. [Google Scholar]
- 25.Li Z.M., Wang M., Peng L. Chemical composition analysis of essential oil from Mosla chinensis Maxim. cv. Jiangxiangru and inhibitory activity of the oil and its major constituents on biofilm formation of staphylococcus aureus. Food Sci. 2016;37:138–143. [Google Scholar]
- 26.Peng L., Xiong Y., Wang M., Han M., Cai W., Li Z. Chemical composition of essential oil in Mosla Chinensis Maxim Cv. Jiangxiangru and its inhibitory effect on Staphylococcus aureus biofilm formation. Open Life Sci. 2018;13:1–10. doi: 10.1515/biol-2018-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu M.T., Luo F.Y., Zeng J.G. Composition analysis of essential oil of Mosla Chinensis Maxim and its antibacterial and antioxidant activity. Chin. Tradit. Pat. Med. 2020;42:3091–3095. [Google Scholar]
- 28.Lin C.L., Cai J.Z., Lin G.Y. Chemical constituents study of volatile oils from the Mosla Chinensis Maxim in zhejiang province. Chin. Arch. Tradit. Chin. Med. 2012;30:197–198. [Google Scholar]
- 29.Nogueira J.O.E., Campolina G.A., Batista L.R., Alves E., Caetano A.R.S., Brandão R.M., Nelson D.L., Cardoso M.D.G. Mechanism of action of various terpenes and phenylpropanoids against Escherichia coli and Staphylococcus aureus. FEMS Microbiol. Lett. 2021;368:fnab052. doi: 10.1093/femsle/fnab052. [DOI] [PubMed] [Google Scholar]
- 30.Jiang H.M., Fang J., Lu X.Y., Peng L.S., Liu J. Preliminary study on inhibition mechanism of Mosla Chinensis Maxim extract on aspergillus flavus. J. Chin. Inst. Food Sci. Technol. 2007;7:47–50. [Google Scholar]
- 31.Qi W.W., Shen Y.T., Chen C.Y., Chen J.Y., Wan C.P. Antifungal mechanisms of the active ingredients carvacrol of Mosla Chinensis ‘Jiangxiangru’ against penicillium digitatum. Mod. Food Sci. Technol. 2018;34:65–69+64. [Google Scholar]
- 32.Huang K., Zhang D., Ren J.J., Dong R., Wu H. Screening of the repellent activity of 12 essential oils against adult german cockroach (Dictyoptera: Blattellidae): Preparation of a sustained release repellent agent of binary oil-γ-CD and its repellency in a small container. J. Econ. Entomol. 2020;113:2171–2178. doi: 10.1093/jee/toaa162. [DOI] [PubMed] [Google Scholar]
- 33.Lu X.P., Weng H., Li C., He J., Zhang X., Ma Z.Q. Efficacy of essential oil from Mosla chinensis Maxim. cv. Jiangxiangru and its three main components against insect pests. Ind. Crops Prod. 2020;147:112237. doi: 10.1016/j.indcrop.2020.112237. [DOI] [Google Scholar]
- 34.Chen F.F., Peng Y.H., Zeng D.Q., Zhang Y., Qin Q.H., Huang Y. Composition and bioactivity of the essential oils from Mosla chinensis against Aedes albopictus. Chin. J. Vector Biol. Control. 2010;21:211–214. [Google Scholar]
- 35.Le T.B., Beaufay C., Nghiem D.T., Mingeot-Leclercq M.P. In vitro anti-leishmanial activity of essential oils extracted from vietnamese plants. Molecules. 2017;22:1071. doi: 10.3390/molecules22071071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng L.L., Cui Y., Qin Y.H., Ren Y.X., Liu X., Tao L., Dai X.D. Effect of Mosla chinensis Maximon Trichomonas vaginalis in vitro. J. Dalian Med. Univ. 2009;31:282–285. [Google Scholar]
- 37.Zhang X.X., Wu Q.F., Yan Y.L., Zhang F.L. Inhibitory effects and related molecular mechanisms of total flavonoids in Mosla chinensis Maxim against H1N1 influenza virus. Inflamm. Res. 2018;67:179–189. doi: 10.1007/s00011-017-1109-4. [DOI] [PubMed] [Google Scholar]
- 38.Li Y., Fang S.G. Effects of elsholtzia chinensis ethanol extract on avian infectious bronchitis virus proliferation. J. Yangtze Univ. (Nat. Sci. Ed.) 2019;16:75–80, 78. [Google Scholar]
- 39.Du J.C., Yang L.Y., Shao L., Yu F., Li R.T., Zhong J.D. Study on phenolic acid compounds from acrial parts of Mosla chinensis and its anti-influenza activity, J. Chin. Med. Mater. 2021;44:2594–2599. [Google Scholar]
- 40.Xu J.L., Jiang W.E. Effect of water extract of Mosla chinensis Maxim against influenza virus. Zhejiang J. Tradit. Chin. Med. 2013;48:273–274. [Google Scholar]
- 41.Lynn Y., Jim H., Towers G. Isolation of the anthropogenic compound fluoranthene in a screening of Chinese medicinal plants for antiviral compounds. Planta Med. 1995;61:187–188. doi: 10.1055/s-2006-958047. [DOI] [PubMed] [Google Scholar]
- 42.Sung Y.Y., Yoon T., Yang W.K., Kim S.J., Kim H.K. Article inhibitory effects of Elsholtzia ciliata extract on fat accumulation in high-fat diet-induced obese mice. Appl. Biol. Chem. 2011;54:388–394. [Google Scholar]
- 43.Pudziuvelyte L., Stankevicius M., Maruska A., Petrikaite V., Ragazinskiene O., Draksiene G., Bernatoniene J. Chemical composition and anticancer activity of Elsholtzia ciliata essential oils and extracts prepared by different methods. Ind. Crops Prod. 2017;107:90–96. doi: 10.1016/j.indcrop.2017.05.040. [DOI] [Google Scholar]
- 44.Sun D.Y., Wang X.Y., Wang X.T., Yan L., Liu X.F., Pang B., Gao H. Comparison of integration processing technology of origin and traditional cutting processing technology of Moslae Herba for lung-Yang deficiency rats. China J. Chin. Mater. Med. 2018;43:2537–2542. doi: 10.19540/j.cnki.cjcmm.20180322.001. [DOI] [PubMed] [Google Scholar]
- 45.Li J.E., Cui S.W., Nie S.P., Xie M.Y. Structure and biological activities of a pectic polysaccharide from Mosla chinensis Maxim. cv. Jiangxiangru. Carbohydr. Polym. 2014;105:276–284. doi: 10.1016/j.carbpol.2014.01.081. [DOI] [PubMed] [Google Scholar]
- 46.Li J.E., Nie S.P., Xie M.Y., Huang D.F., Wang Y.T., Li C. Chemical composition and antioxidant activities in immumosuppressed mice of polysaccharides isolated from Mosla chinensis Maxim cv. Jiangxiangru. Int. Immunopharmacol. 2013;17:267–274. doi: 10.1016/j.intimp.2013.05.033. [DOI] [PubMed] [Google Scholar]
- 47.Wang M., Li Z.M., Peng L. α-Glucosidase Inhibition of Mosla Chinensis Maxim.cv. Jiangxiangru extracts. J. Nanchang Vocat. Tech. Techers’ Coll. 2015;6:40–45. [Google Scholar]
- 48.Macianskiene R., Pudziuvelyte L., Bernatoniene J., Almanaityte M., Navalinskas A., Andriule R., Jurevicius J. Antiarrhythmic properties of Elsholtzia ciliata essential oil on electrical activity of the isolated rabbit heart and preferential inhibition of sodium conductance. Biomolecules. 2020;10:948. doi: 10.3390/biom10060948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nugroho A., Park J.H., Choi J.S., Park K.S., Hong J.P., Park H.J. Structure determination and quantification of a new flavone glycoside with anti-acetylcholinesterase activity from the herbs of Elsholtzia ciliata. Nat. Prod. Res. 2019;33:814–821. doi: 10.1080/14786419.2017.1413556. [DOI] [PubMed] [Google Scholar]
- 50.Shu R.G., Hu H.W., Zhang P.Z., Ge F. Triterpenes and flavonoids from Mosla chinensis. Chem. Nat. Compd. 2012;48:706–707. doi: 10.1007/s10600-012-0359-1. [DOI] [Google Scholar]
- 51.Seo Y.H., Trinh T.A., Ryu S.M., Kim H.S., Choi G., Moon B.C., Shim S.H., Jang D.S., Lee D., Kang K.S., et al. Chemical constituents from the aerial parts of Elsholtzia ciliata and their protective activities on glutamate-induced HT22 cell death. J. Nat. Prod. 2020;83:3149–3155. doi: 10.1021/acs.jnatprod.0c00756. [DOI] [PubMed] [Google Scholar]
- 52.Kim T.W., Kim Y.J., Seo C.S., Kim H.T., Park S.R., Lee M.Y., Jung J.Y. Elsholtzia ciliata (Thunb.) Hylander attenuates renal inflammation and interstitial fibrosis via regulation of TGF-beta and Smad3 expression on unilateral ureteral obstruction rat model. Phytomedicine. 2016;23:331–339. doi: 10.1016/j.phymed.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 53.Zou Y., Pan J.P., Zhou M. Analysis of ethyl acetate constituents of Mosla chinensis Maxim cv. jiangxiangru and their inhibitory activity of α-glucosidase. China Food Saf. Mag. 2021;25:65–68. [Google Scholar]
- 54.Zheng X.D., Hu H.B. Chemical constituents of Elsholtzia ciliata (Thunb.) Hyland. Chem. Res. 2006;17:85–87. [Google Scholar]
- 55.Ma J., Xu R.R., Lu Y., Ren D.F., Lu J. Composition, antimicrobial and antioxidant activity of supercritical fluid extract of Elsholtzia ciliata. J. Essent. Oil-Bear. Plants. 2018;21:556–562. doi: 10.1080/0972060X.2017.1409657. [DOI] [Google Scholar]
- 56.Liu H., Zhang D.M., Luo Y.M. Studies on chemical constituents of Mosla Chinensis Maxim.cv. Jiangxiangru. Chin. J. Exp. Tradit. Med. Formulae. 2010;16:56–59. [Google Scholar]
- 57.Cao H., Li Z., Chen X. QSRR study of GC retention indices of volatile compounds emitted from Mosla chinensis Maxim by multiple linear regression. Chin. J. Chem. 2011;29:2187–2196. doi: 10.1002/cjoc.201180379. [DOI] [Google Scholar]
- 58.Wang X., Gong L., Jiang H. Study on the difference between volatile constituents of the different parts from Elsholtzia ciliata by SHS-GC-MS. Am. J. Anal. Chem. 2017;8:625–635. doi: 10.4236/ajac.2017.810045. [DOI] [Google Scholar]
- 59.Dũng N.X., Van Hac L., Hái L.H., Leclercq P.A. Composition of the essential oils from the aerial parts of Elsholtzia ciliata (Thunb.) Hyland. from vietnam. J. Essent. Oil Res. 1996;8:107–109. doi: 10.1080/10412905.1996.9700569. [DOI] [Google Scholar]
- 60.Zhang Y.X., Gong Q.F., He Y., Zhong L.Y., Yu H., Faan R.N. Optimization of technology of Moslae herba processed with ginger juice and analysis of its volatile components by HS-GC-MS. Chin. J. Exp. Tradit. Med. Formulae. 2019;25:162–167. [Google Scholar]
- 61.Liu X.P., Jing X.M. Research of chemical composition and biological activity of the essential oil from Elsholtzia ciliata. J. Heilongjiang Bayi Agric. Univ. 2018;30:35–39. [Google Scholar]
- 62.Yu H., Hu J., Bai F.P., Qin K.M. Analysis of volatiles from bark of Moslae herba by gas chromatography-mass spectroscopy. Guid. J. Tradit. Chin. Med. Pharm. 2017;23:82–84. [Google Scholar]
- 63.Liu H., Li G.S., Luo Y.M., Chen Z.W., Liu W.Q. Chemical constituents of petroleum spirit part of Mosla chinensis ‘Jiangxiangru’. Chin. J. Exp. Tradit. Med. Formulae. 2011;17:68–70. [Google Scholar]
- 64.Mao Y., Li Z.G., Cao J.L. Analysis of volatile oil composition of Mosla chinensis. J. Zhejiang For. Coll. 2008;25:262–265. [Google Scholar]
- 65.Xiang P. Master’s Thesis. Guizhou University; Guizhou, China: 2018. Study and Application of Chemical Constituents and Biological Activity of Medicinal Plant Elsholtzia ciliata from Guizhou. [Google Scholar]
- 66.Luo G.M., Yang G.Y., Liu H.N., Yang Y.Q., Chen Y., Li X., Zhang X.Y. Comparative study on chemical constituents of volatile oil of Elsholtzia ciliata. China J. Chin. Mater. Med. 2007;32:1483–1485. [Google Scholar]
- 67.Shen J.J., Zhang D.M., Liu H., Luo Y.M. Studies on polar components of Elsholtzia chinensis Ⅱ. China J. Chin. Mater. Med. 2011;36:1779–1781. [PubMed] [Google Scholar]
- 68.Liu H., Shen J.J., Zhang D.M., Luo Y.M. Studies on polar constituents of Mosla Chinensis ‘Jiangxiangru’. Chin. J. Exp. Tradit. Med. Formulae. 2010;16:84–86. [Google Scholar]
- 69.Gao J.P., Li D.D., Qin L., Yang L., Tian H.L. Comparative analysis of volatile components in leaves, flowers, and fruits of three Elsholtzia plants. J. Beijing Univ. Agric. 2020;35:108–114. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.