Abstract
Cancer is a global public health problem that is related to different environmental and lifestyle factors. Although the combination of screening, prevention, and treatment of cancer has resulted in increased patient survival, conventional treatments sometimes have therapeutic limitations such as resistance to drugs or severe side effects. Oriental culture includes herbal medicine as a complementary therapy in combination with chemotherapy or radiotherapy. This study aimed to identify the bioactive ingredients in Kalanchoe pinnata, a succulent herb with ethnomedical applications for several diseases, including cancer, and reveal its anticancer mechanisms through a molecular approach. The herb contains gallic acid, caffeic acid, coumaric acid, quercetin, quercitrin, isorhamnetin, kaempferol, bersaldegenin, bryophyllin a, bryophyllin c, bryophynol, bryophyllol and bryophollone, stigmasterol, campesterol, and other elements. Its phytochemicals participate in the regulation of proliferation, apoptosis, cell migration, angiogenesis, metastasis, oxidative stress, and autophagy. They have the potential to act as epigenetic drugs by reverting the acquired epigenetic changes associated with tumor resistance to therapy—such as the promoter methylation of suppressor genes, inhibition of DNMT1 and DNMT3b activity, and HDAC regulation—through methylation, thereby regulating the expression of genes involved in the PI3K/Akt/mTOR, Nrf2/Keap1, MEK/ERK, and Wnt/β-catenin pathways. All of the data support the use of K. pinnata as an adjuvant in cancer treatment.
Keywords: Kalanchoe pinnata, phytochemicals, antitumor activity, adjuvant agent
1. Introduction
Cancer is a genetic disease that displays a variety of molecular alterations in the genome of somatic cells [1]. Cancer cells’ traits, such as sustained proliferation, resistance to cell death, angiogenesis capacity, invasion, evasion of immune surveillance, and metastasis, could be the targets of bioactive compounds of medicinal plants. These alterations include small or large structural variations due to epigenetic changes characterized by the addition or removal of chemical groups from/to DNA, histones [2], or RNA [3]. As such, inherited or sporadic mutations cannot only activate oncogenes or deactivate tumor suppressor genes, but may even lead to reversible chemical modifications such as the methylation of DNA, histones, or RNA, which can affect gene expression without changing DNA sequences. Plants from the genus Kalanchoe (Fam: Crassulaceae) have a global distribution in warm climates, where they are used as ornamental plants. Some of the 200 Kalanchoe species are known for their curative uses in different diseases, including cancer. Natural remedies are used on a large scale worldwide, and herbal extracts are obtained from a great variety of plants. At the level of chemical composition, Kalanchoe species include flavonoids, bufadienolides, fatty acids, triterpenoids, alkaloids, phenolic acids, saponins, tannins, glycosides, and kalanchosides [4,5,6]; many of these constituents have remarkable anticancer potential. Numerous studies have evaluated the anticarcinogenic efficacy of these natural bioactive molecules. For example, quercetin has been reported to inhibit the proliferation of human breast cancer cells [7]. Bufadienolides have been shown to have antiangiogenic activity, cause inhibition of cell growth and proliferation, and induce cell death [8]. Antiproliferative activity has also been reported for triterpenes [9] and kalanchosides [5]. Important issues in cancer treatment are drug-related cumulative toxicity and chemoresistance. Hence, combination therapy offers good results due to the use of different targets and reduced adverse effects. Alternative therapy options may include the use of phytochemicals. For example, Lin et al. [10] suggested that the use of natural compounds could offer benefits such as sensitization of tumor cells to drugs, decreased drug efflux from tumor cells, and promotion of repair in normal cells, keeping in mind the potential for herbal–drug interactions. Recent research has also considered the anticancer efficacy of phytochemicals in epigenetic regulation pathways.
This work reviews the features of Kalanchoe pinnata (Figure 1) and its phytochemicals, with a focus on the mechanisms underlying its chemopreventive and therapeutic properties.
Figure 1.
Taxonomic classification of K. pinnata. Source: Photos by authors, image created with BioRender.com.
2. Phytochemical Constituents of Kalanchoe pinnata
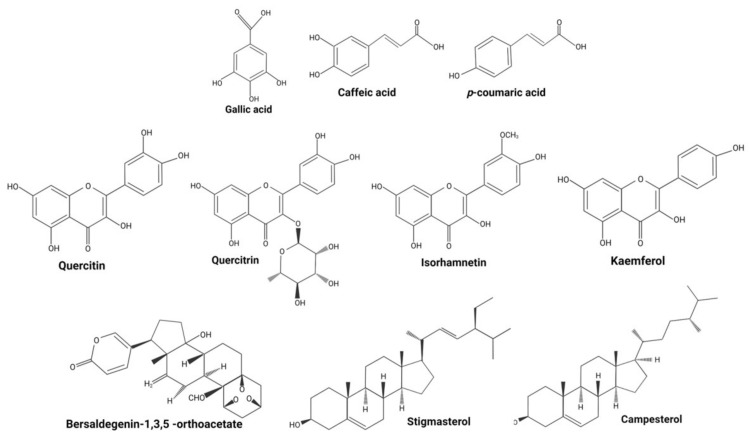
Kalanchoe pinnata (Lam) Pers. (syn. Bryophyllum pinnatum Lam.), belonging to the genus Kalanchoe, is a succulent plant cultivated in gardens [11], which can be found as a herb or shrub. The plant has opposite, simple, and compound leaves, with a red to dark purple crenate margin. It reproduces from seeds and vegetatively through leaves [12], presenting clusters of reddish-purple pendulous flowers [13]. K. pinnata is used in traditional medicine to treat different diseases including cancer [6]. Mora-Pérez and Hernández-Medel [14] analyzed the ingredients of K. pinnata and found alkaloids and sterols in methanolic root extract, and terpenes, sterols, flavonoids, chlorides, nitrates, and potassium in methanolic stem extract. The leaves of this plant contain phenols (gallic acid), flavonoids (quercetin) (in methanol extract), lycopenes, and β-carotenes (in petroleum ether) [15], as well as tannins and alkaloids [16]. Jaiswal et al. [17] found that the phenolic content of leaves was 28.4 ± 2 µg mg−1 and suggested that this was responsible for their antioxidant capacity. El Abdellaoui et al. [18] detected three phenolic acids (gallic, caffeic, and coumaric acids), three flavanol glycosides (quercetin, isorhamnetin, and kaempferol), 4′,5-dihydroxy-3′,8-dimethoxyflavone 7-O-β-D-glucopyranoside, and quercitrin [19]. Bufadienolides such as bersaldegenin acetate-2, -3, -4, -5, bryophyllin a, bryophyllin c, and bersaldegenin-1,3,5-orthoacetate [20] have also been found. Reported phenanthrene derivatives include Ψ-taraxasterol and 18-α-oleanane, and other ingredients include sterols; bryophynol, bryophyllol, and bryophollone [21]; stigmasterol [22]; campesterol, 24-epiclerosterol, (24R)-5α-stigmasta-7,25-dien-3β-ol, 5 α-stigmast-24-en-3β-ol, and 25-methyl-5α-ergost-24(28)-en-3β-ol [23] (Figure 2). The leaves of K. pinnata are rich in ascorbic acid (vitamin C) and contain riboflavin, thiamine, niacin, magnesium, calcium, potassium, phosphorus, sodium, and microelements such as iron and zinc [16]. K. pinnata flowers contain a higher concentration of glycosides [24], similar to other Kalanchoe species.
Figure 2.
Structures of major phytochemicals reported in K. pinnata. Source: Image created with BioRender.com.
3. Activity of Kalanchoe pinnata in Cancer
Several plant components have been identified as sources of anticancer therapeutics. However, those components should be investigated in clinical trials to confirm their pharmacokinetic effects. Members of the Kalanchoe genus (Crassulaceae) have remedial properties for a wide range of diseases such as gastric ulcers, urolithiasis, bacterial, viral, and parasitic infections, skin diseases, cold, memory improvement, or even improvement in sleep quality when undergoing cancer treatment. Based on ethnobotanical evidence, the Kalanchoe genus has been evaluated on various cancer cell lines (Table 1). The phytochemicals present in K. pinnata have been analyzed in a variety of studies on cancer.
Table 1.
Anticancer properties found in the Kalanchoe genus.
| Kalanchoe | Subject | Effect | Study Type | Cell Line | Reference |
|---|---|---|---|---|---|
| K. daigremontiana Raym.-Hamet and H. Perrier | Ovarian, cervical, breast cancer, and melanoma | Antiproliferative, cytotoxic, and antioxidant activity. Cell cycle arrest and caspase-independent cell death |
In vitro | SCOV-3, HaCaT, HeLa, MCF-7, A375 | [8,20,26,27,28] |
| K. integra var. crenata (Andr.) | Cardiotoxicity by doxorubicin Colorectal adenocarcinoma, lung cancer, mesothelioma, hepatocarcinoma, breast cancer |
Cardio-protection against cardiotoxicity by cancer therapy Apoptosis |
In vivo (rats) In vitro |
DLD-1, A549, SPC212, HepG2, MCF-7 |
[9,29] |
| K. tubiflora (Harvey) | Lung cancer Lung adenocarcinoma, oral adenosquamous carcinoma, melanoma, and leukemia cell lines |
Induction of autophagy Cell cycle arrest and senescence Cell cycle arrest and apoptosis Cytoprotective autophagy |
In vitro In vitro, in vivo (mice) |
CL1-5 A549, Cal-27, A2058, HL-60 |
[30,31,32] |
| K. gastonis-bonnieri Raym.-Hamet | Benign prostatic hyperplasia Prostate cancer |
Antiproliferative activity and apoptosis induction Antiproliferative activity, apoptosis induction, and androgen receptor degradation |
In vitro | Stromal cells LAPC-4, LNCaP, PC-3, DU145 |
[33,34] |
| K. flammea | Prostate cancer | Apoptosis induction and cell cycle arrest | In vitro | PC-3, LNCaP, PrEC | [35] |
| K. laetivirens | Lung cancer | Reversion of etoposide resistance | In vitro | A549, A549RT-eto | [36] |
| K. gracilis (L.) DC | Murine macrophage and human hepatocarcinoma | Antiproliferative, antioxidant, and anti-inflammatory activity | In vitro | RAW264.7, HepG2 | [37] |
| K. beharensis | Acute myeloid leukemia | Apoptosis induction, inhibition of NF-κB | In vitro | HL-60, HL60R | [38] |
| K. brasiliensis | Kidney carcinoma | Cytotoxic activity | In vitro | 3T3, 786-0 | [39] |
| K. laciniata | Baby hamster kidney cell line | Cytotoxic activity | In vitro in vivo (mice) | BHK-21 | [40] |
Plant extracts by themselves do not produce a significant effect against cancer, but they may enhance therapeutic efficacy when combined with chemotherapeutic drugs. The most studied natural compounds are polyphenols, which are widely distributed in plant tissues. These compounds are secondary metabolites produced for protection against bacteria, fungi, and insects. Natural phenols more studied for their properties are curcumin, epigallocatechin-3-gallate, resveratrol, quercetin, and myricetin [25], of these compounds, quercetin is found in K. pinnata.
3.1. Phenolic Acids
3.1.1. Gallic Acid
Gallic acid (3,4,5-trihydroxybenzoic acid, GA) is a phenolic acid belonging to the tannin family. It is found in a variety of fruits and vegetables, either in a free form or in the form of tannins [41]. Acute oral administration of GA at a concentration of 500 mg/kg body weight is nontoxic, and subacute administration at a dose of 1000 mg/kg body weight has not shown toxic side effects in mice [42]. Reproductive toxicity in rats was also evaluated, and no evidence of toxicity at 430 mg/kg was found [43]. In addition to the antioxidant and anti-inflammatory effects, several reports have supported the anticancer function of GA. GA affects cancer cells by regulating angiogenesis, proliferation, and apoptosis. Mirvish et al. [44] reported a strong inhibition of lung adenomas in mice by GA added to the food. Inoue et al. [45] proposed that GA triggers apoptosis in HL-60RG cells through the formation of reactive oxygen species (ROS) and calmodulin activation. ROS are involved in the regulation of many processes by up- or downregulation of critical protein kinase activities and are the cause of intracellular oxidative stress. Prostate, stomach, liver, skin, leukemia, lymphoma, colon, breast, cervical, esophageal, and endometrial cancer cell lines have been investigated for GA activity as reviewed elsewhere [46]. Studies on cancer cell lines have found not only apoptosis induction by the release of cytochrome c from mitochondria and the activation of caspases but also ferroptotic cell death and necroptotic pathways [47]. Tang and Cheung [47] identified enrichment of AMPK, TNF, and mTOR signaling pathways in GA-induced HeLa cells. Using an in vivo mouse model, Ko et al. [48] found that GA regulated the PI3K/Akt pathway in lung cancer cell line A549-derived tumors. When comparing the effects of GA and cisplatin, the authors found that GA inhibited cell proliferation and colony formation by inhibiting the PI3K/Akt pathway and upregulating p53. According to Isuzugawa et al. [49], GA induces apoptosis through the generation of hydrogen peroxide. Moreover, they found a great difference in catalase levels between hepatocellular carcinoma cells and normal hepatocytes, suggesting that this difference is key to the GA response. Indeed, catalase is important to hydrogen peroxide provision, which is why GA is more effective against tumor cells than against nontumor cells. Due to its low cytotoxic effects, GA is a promising chemopreventive agent. The use of nanoparticle-based drug delivery systems has achieved many advances in recent years. For example, hyaluronic acid (HA) is an attractive biopolymer as it has the advantage of affinity for CD44—a receptor highly expressed on various cancer cells [50]. Nanoparticles of HA conjugated with polyethylene glycol-distearoyl-phosphoethanolamine co-loaded with doxorubicin and GA have been tested in doxorubicin-resistant human HL promyelocytic leukemia cells, showing good results in inhibiting tumor growth [51]. DNA and RNA methylation and histone modifications are other key elements in the etiology of cancer. Phytochemicals can modulate the gene expression of writer and reader proteins, which are involved in the addition and removal of epigenetic marks.
3.1.2. Caffeic Acid
Unlike GA, caffeic acid (CA), or hydroxycinnamic acid [(E)-3-(3,4-dihrydroxyphenyl) prop-2-enoic acid], is an antiapoptotic polyphenol. It is almost fully absorbed in the small intestine and is nontoxic even at daily doses of 0.5–1 g [52]. Rosendahl et al. [53] reported that CA was associated with a moderate reduction in estrogen receptor-α (ER)+ and ER− human breast cancer cell proliferation via ER and insulin-like growth factor-I receptor (IGFIR). Neuroblastoma SH-SY5Y cells are protected from cyclophosphamide toxicity by CA; specifically, cell death is suppressed through a decrease in lipid peroxidation levels [54]. The concomitant action of CA and cisplatin in ovarian carcinoma cell line A2780 enhances cisplatin cytotoxicity, but the use of CA before cisplatin treatment causes resistance through phase II induction of glutathione S-transferase (GST) and glutathione reductase (GR) by induction of the Nrf2/Keap1 pathway [55]. Additionally, some studies have found that CA can inhibit the migration of cancer cells [56,57].
3.1.3. P-coumaric Acid
p-coumaric acid (p-CA) is a hydroxycinnamic acid derivative of cinnamic acid. It has been shown that p-CA protects against oxidative stress and DNA and chromosome breakage in HT-29 cells [58]. p-CA has an antiproliferative effect on Caco-2 cells [59]. In an induced model of colon carcinogenesis, it was shown that p-CA reduced preneoplastic changes and stimulated apoptosis [60]. Gastaldello et al. [61] reported a reduction in angiogenesis and the presence of intratumoral neutrophils in rats inoculated with B16F10 melanoma cells.
3.2. Flavonoids
3.2.1. Quercetin
Quercetin (3,5,7,3′,4′-pentahydroxyflavone, Qu) is an important bioflavonoid found in vegetables in its glycoside form. Like phenolic acids, it is absorbed in the small intestine. Oral administration of 1 g/day is safe [62]. Wätjen et al. [63] suggested a dose of 10–25 µmol/L Qu acted against H2O2-induced cytotoxicity, DNA strand breaks, and caspase-3 activation; the authors also suggested that Qu induced cytotoxicity at concentrations between 50 and 250 µmol/L. Qu protects cells from oxidative stress, thereby reducing the levels of ROS. Many studies have shown that Qu represses the proliferation of different cancer cell lines. In endometrial cells, 100 µM Qu-inhibited DNA synthesis and cell growth by suppressing cyclin D and EGF [64]. In addition, Qu exhibited an antiangiogenic effect by suppressing VEGF-induced phosphorylation of VEGF receptor 2 and signaling pathways that involve protein kinases AKT, mTOR, and ribosomal protein S6 in human umbilical vein endothelial cells (HUVECs) and prostate tumor cells [65]. Qu can be used as an antimetastatic agent as it is structurally homologous to LY 294002 (LY), the commercially available inhibitor of PI3K; both agents are effective in suppressing the Akt/PKB pathway through PIP3 regulation [66]. In combination with cisplatin, 5 µM Qu sensitizes osteosarcoma 143B cells to cisplatin, while in concentrations ≥ 10 µM, Qu inhibits the proliferation of these cells [67]. While Qu inhibits cell proliferation and invasion and increases apoptosis and PTEN expression, it downregulates the expression of p-Akt in MCF-7 cells [68]. Using mouse breast tumor cells (EMT6), Liu et al. [69] reported that Qu plus cisplatin synergistically inhibited tumor growth, reduced renal toxicity induced by cisplatin, and increased the cytotoxicity of cisplatin. The co-delivery of Qu and paclitaxel reduced multidrug resistance (MDR) in breast cancer cells with multidrug-resistance MDA-MB-231/MDR1 and activated mitochondria-dependent apoptosis [70]. In glioblastoma multiforme, Qu combined with temozolomide, using HA as a nanocarrier, showed a synergistic effect on temozolomide efficacy. The CD44 receptor was the preferred target, enhancing the anti-inflammatory mechanism and decreasing interleukin secretion [71]. As for drugs used to treat advanced carcinomas, Qu enhanced the efficacy of gemcitabine and doxorubicin, and it reduced resistance to chemotherapy by S-phase arrest, apoptosis induction, and increased p53 expression [72].
3.2.2. Quercitrin
Quercitrin (quercetin 3-O-α-L-rhamnopyranoside, Qi) is a glycoside of Qu [73]. It acts as an antioxidant by scavenging free radicals [74]. Similar to Qu, Qi has antiproliferative and apoptotic effects on different cell lines, such as colorectal and lung cancer cell lines, where it has been shown to increase caspase-3 activity and decrease mitochondrial membrane potential [75,76]. Tumor initiation, promotion, and progression are prompted by different transcription factors such as AP-1 and NF-κB; these are activated by mitogen-activated protein kinases (MAPKs), c-jun NH-2 terminal kinase, or extracellular signal-regulated kinases. It has been suggested that Qi inhibits NF-κB and AP-1-MAPKs signaling and induces the activity of phase II detoxifying enzymes such as GST and NQO1 in JB6 and JB6 P+ mouse epidermal cell lines, which gives Qi potential as an anticancer agent [77].
3.2.3. Isorhamnetin
Isorhamnetin (isorhamnetin-3-O-methylquercetin, Iso) is a flavonoid metabolite of quercetin. It has been found that Iso participates in the PI3K/AKT pathway, thereby suppressing proliferation and metastasis and triggering G2/M phase arrest in gallbladder cancer [78]. Inhibition of Src activity and inhibition of β-catenin nuclear translocation by induction of C-terminal Src kinase expression were the results of Iso incorporation into the diet of a mouse model of colorectal cancer and in HT-29 colon cancer cells [79]. Recently, it has been found that Iso promotes apoptosis of gastric cancer MKN-45 cells in a hypoxic environment by inhibiting PI3K/AKT/mTOR-mediated adaptive autophagy [80]. In breast cancer cell lines, Iso inhibits the AKT/mTOR and MEK/ERK pathways [81]. Considering that the PI3K/AKT/mTOR pathway has been associated with tamoxifen resistance, Iso has an antitumor action [82]. In gastric cancer, the combination of Iso with capecitabine enhanced the apoptotic effect of capecitabine by avoiding NF-κB activation in MKN-45 cells [83]. Zhang et al. [84] found that non-small cell lung cancer cell line A-549 pretreated with Iso had a better response to radiotherapy, probably thanks to increased expression of IL-3 and inhibited activation of NF-κB.
3.2.4. Kaempferol
Similarly to Iso, kaempferol 3,5,7-trihydroxy-2-(4-hydroxyphenyl, Kae)-4H-1-benzopyran-4-one [85] belongs to the flavanols. Kae has been reported to induce apoptosis of breast cancer cell line MCF-7 intrinsically via apoptotic downregulation of Bcl2, upregulation of Bax expression, and cleavage of PARP [86]. In ovarian cancer cells A2780/CP70, Kae stimulated the extrinsic apoptosis pathway through death receptors/FADD/caspase-8; moreover, Kae induced G2/M cell cycle arrest via two pathways, Chk2/Cdc25C/Cdc2 and Chk2/p21/Cdc2. Doxorubicin induces cellular apoptosis, but it has significant side effects, and its therapeutic effect is limited by acquired drug resistance. In combination with Kae, doxorubicin inhibits viability, colony formation, cell cycle progression, DNA damage response, and mitochondrial function in liver cancer cells. The combined treatment has a stronger inhibitory effect on the PI3K/mTOR/MMP pathway than either alone [87]. In the same way, Kae plus erlotinib is more efficient at inhibiting PI3K/AKT in pancreatic cancer cells PANC-1 and BxPC-3 targeting EGFR and promoting apoptosis [88]. In vitro data reveal that the actions of many flavonoids converge on the PI3k/Akt/mTOR pathway, making them good antitumor candidates. Flavonoids can inhibit the efflux function of ATP-binding cassette (ABC) transporters, such as ABCB1 and ABCG2, thereby increasing the bioavailability of poorly available drugs; yet, they can potentiate the toxicity of some ABC transporter substrates, such as chemotherapeutic drugs [89].
3.3. Sterols
3.3.1. Bufadienolides
Bufadienolides are made up of polyhydroxy C-24 steroids and their glycosides, which have an α-pyrone ring at the C-17β position [90]. Their name comes from the genus Bufo, given that toad skin contains poison glands with these compounds. Bufadienolides show insecticidal, antimicrobial, anti-inflammatory, cardiotonic, anesthetic, sedative, and antitumoral activities [91]. Bufadienolides are involved in diverse processes such as apoptosis, autophagy, cell cycle arrest, angiogenesis, epithelial–mesenchymal transition, and metastasis [92].
Bersaldegenin-1,3,5-orthoacetate plays a role in cell cycle arrest and caspase-independent cell death in HeLa cells [8]. Xiuzhen et al. [93] reported its potent cytotoxicity in human lung carcinoma A-59 and colon HTC-8 cell lines, which could be attributed to the presence of bersaldegenin-1,3,5-orthoacetate in chloroform extract from a whole fresh plant of Bryophyllum pinnatum collected in Taipei, Taiwan.
Bryophyllin a is a bufadienolide-1,3,5-orthoacetate with potent cytotoxicity against KB, A-549, and HCT-8 cells [94]. Bryophyllin c [95] and a have been reported to inhibit tetradecanoylphorbol acetate-induced Epstein Barr virus activation in Raji cells [28]. Other bufadienolides present in K. pinnata, such as bryophynol, bryophyllol, and bryophollone, or the sterol 24-epiclerosterol, have not been examined in terms of their antitumor activity.
3.3.2. Stigmasterol
Stigmasterol (3beta-hydroxystigmastane with double bonds at the 5,6- and 22–23 positions, St) is a phytosterol [85]. Li et al. [96] demonstrated that St-inhibited A278 and SKOV3 ovarian cancer cell proliferation and migration, reduced mRNA expression of PI3K/Akt, and increased the expression of PTEN. In SGC-7901 and MGC-803 gastric cancer cells, St-inhibited viability and proliferation and induced autophagy by blocking the Akt/mTOR pathway, with autophagy playing a cryoprotective role against apoptosis induced by St [97]. When Nrf2, a transcription factor involved in the induction of phase II enzymes and endogenous antioxidants, is overexpressed, chemoresistance is observed in many cancers [98]. Liao et al. [99] analyzed the role of St in the chemoresistance of the endometrial cancer cell line Ishikawa overexpressing Nrf2; they found that the overexpression of Nrf2 reduced sensitivity to cisplatin and that St-sensitized cells to chemotherapy by suppressing Nrf2 expression. In another study, St-reduced the activity of P-glycoprotein efflux, a membrane transporter, in drug-resistant human leukemia CEM/ADR5000 cells at nontoxic concentrations, and it increased the sensitivity to doxorubicin in drug-resistant cancer colon cells Caco-2, thereby reestablishing doxorubicin cytotoxicity [100]. Furthermore, St was successfully used in a phyto-liposome with hyaluronic acid-modified PEGylated doxorubicin in breast cancer MDA-MB-231 cells, especially in CD44-overexpressing cells [101].
3.3.3. Campesterol
Campesterol (Cam) is a phytosterol, a 3-beta-sterol, and a 3beta-hydroxy-Delta(5)-steroid [85]. The analysis of its effect on ovarian cancer ES2 and OV90 cells provided information on its participation in the activation of cell death signals, reduced mitochondrial membrane function, and apoptosis. Similar to other sterols, Cam affects the PI3K/MAPK pathway and inhibits cell cycle proliferation by targeting PCNA. Additionally, Cam enhances the effects of cisplatin and paclitaxel [102].
4. Participation of K. pinnata in Epigenetic Regulation
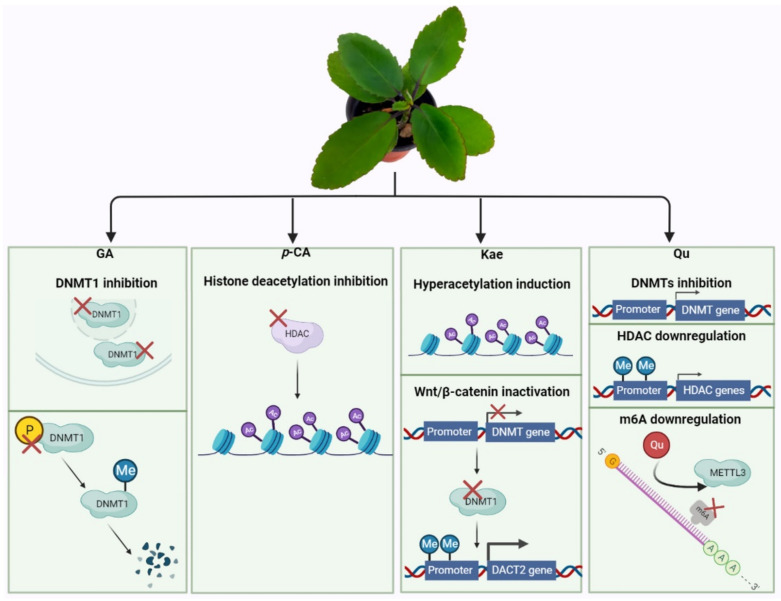
Gene expression is regulated by diverse factors, including epigenetic regulation; major epigenetic mechanisms include DNA methylation, histone modifications, chromatin remodeling, microRNA, and RNA methylation (Figure 3). Research has accumulated on several of these mechanisms, while epitranscriptomics is an area that is currently being intensively investigated. Weng et al. [103] demonstrated that GA inhibited nuclear and cytoplasmic DNA methyltransferase DNMT1 in lung cancer cell line H1299 after seven days of treatment; they also found that GA reduced phosphorylation of DNMT1, thereby allowing for its methylation and destabilization, and leading to degradation. p-CA acts as histone deacetylases (HDAC) inhibitor [104] in breast cancer MCF-7 and cervical cancer HeLa cells; altering histone acetylation leads to increased p21 expression. Qu can also decrease the transcript levels of DNA methyltransferases (DNMTs) DNMT1, 3A, and 3B, along with the enzymatic activity of DNMTs. It has been suggested that Qu binds to the polycomb repressor protein EZH2, thereby suppressing its ability to recruit DNMT. Furthermore, Qu could reduce the expression of class II HDACs, degrade class I HDACs that induce apoptosis, block the cell cycle and angiogenesis, and reduce methylation of the tumor suppressor genes’ promoter in cancer cell lines [105,106]. Treatment of leukemia cell lines HL60 and U937 with Qu-induced demethylation of BCL2L11 and DAPK1 genes, inhibition of HDAC, enrichment of H3ac and H4ac in the promoter of genes involved in apoptosis, and downregulation of DNMT via a STAT3-dependent mechanism; thus, Qu enhanced apoptosis through epigenetic regulation [107]. Kae, similar to Qu, has an epigenetic role, acting as an HDAC inhibitor in hepatoma cell lines HepG2 and Hep3B and colon cancer cell line HCT-116. Kae induces hyperacetylation of histone complex H3 [108]. Lu et al. (2018) found that Kae increased DACT2 expression in colorectal cancer cells HCT116, HT29, and YB5 by DACT2 demethylation through the suppression of DNMT1 and DNMT3b expression, thereby inhibiting the nuclear β-catenin expression and deactivating Wnt/β-catenin pathway proteins [109].
Figure 3.
Epigenetic regulation by K. pinnata phytochemicals. Some bioactive chemical constituents have a role in epigenetic mechanisms in DNA and RNA. GA: Gallic acid; p-CA: p-coumaric acid; Kae: kaempferol; Qu: quercetin; Me: methyl; Ac: acetyl. Source: Image created with BioRender.com.
RNA presents numerous modifications but N6-methyladenosine (m6A) is the most abundant (110). m6A is located by a ~900 kDa methylosome complex that contains METTL3, METTL14, WTAP, KIA1429, RBM15, KIAA0853, and HAKAI proteins. The m6A mark is erased by proteins FTO and ALKBH5, and it is decoded by proteins that contain the YTH domain [110]. Du et al. [111] recently published a docking-based high-throughput screening report, in which they found that Qu decreased the m6A level in a dose-dependent manner in MIA PaCa-2 pancreatic cancer cells. Qu inhibited the proliferation in MIA PaCa-2 and Huh7 cells. They found that Qu took the place of METTL3 and formed a stable protein-ligand complex, thereby avoiding the METTL3 function and decreasing the m6A/A ratio. Here, it is important to point out that epigenetic modifications of both DNA and RNA are reversible, unlike mutations, which makes them a promising target in the development of therapies.
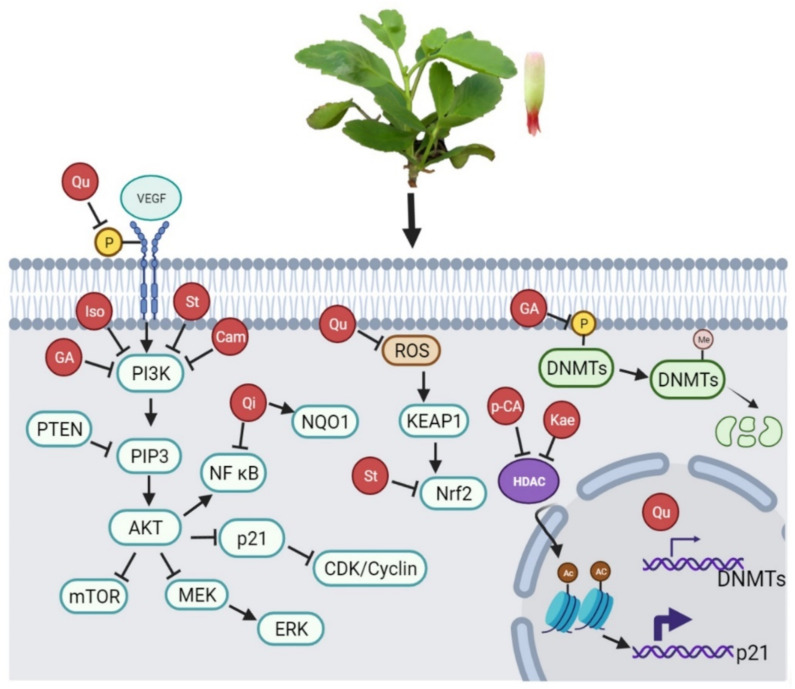
Cancer remains an important cause of death worldwide. While new drugs have contributed to improved treatment options, it is important that we find more efficient approaches with lower toxicity for patients. Considering the above information, Qu is one of the most promising phytochemicals present in K. pinnata, with well-known pharmacokinetics and a lack of systemic toxicity. The use of herbs from traditional medicine is a promising alternative already used in Oriental culture. In a meta-analysis, Sun et al. [112] summarized the clinical efficacy of Kang-ai injection (an extract from three Chinese herbs) in combination with conventional treatment of hepatocellular carcinoma. Based on the results obtained from its bioactive components, the potential is noted for K. pinnata to act as an antioncogenic adjuvant agent. K. pinnata may regulate the expression of genes, especially by targeting the PI3K/Akt/mTOR, Nrf2/Keap1, MEK/ERK, and Wnt/β-catenin pathways (Figure 4).
Figure 4.
Pathways in which K. pinnata regulates gene expression. Phytochemicals of K. pinnata are involved in processes such as proliferation, apoptosis, cell migration, angiogenesis, metastasis, promoter methylation, inhibition of nuclear and cytoplasmic DNMTs, oxidative stress, and autophagy (see text for details). Source: Image created with BioRender.com.
5. Pathways Involved in Cancer and Potential Targets to K. pinnata Phytochemicals
Cellular metabolism is regulated by various signaling pathways which widely crosstalk. Aberrant regulation of signaling pathways induces disturbance in cellular metabolism and causes diseases. Cancer cells have a complex interplay among different pathways, some components commonly associated with carcinogenesis are Mitogen-Activated Protein Kinase (MAPK) or Extracellular-signal-Regulated Kinase (ERK), Wnt/β-catenin, Phosphatidylinositol 3-kinase (PI3K)/AKT, mammalian Target of Rapamycin (mTOR), and Nuclear factor-E2 p45-related factor (Nrf2).
5.1. MEK/ERK
The most studied of the four MAPK pathways include ERK1/2, which is involved in the transmission of mitogenic signals. ERK1/2 acts downstream of Ras, and it involves phosphorylation and sequential activation of kinases. The activation begins with a membrane receptor (receptor tyrosine kinase, G-protein-coupled receptor, or other) which transmits the signal by recruiting adaptor proteins such as Grb2. Recruitment of adaptor proteins induces the activation of Ras, followed by activation of Raf-1, B-Raf, and A-Raf. Upon the activation of Raf proteins, MEK1/2 is phosphorylated, and they finally activate ERK1/2, which are the executors of the upstream signals. Phosphorylation of ERK1/2 on threonine and tyrosine residues allows their nuclear translocation to regulate targets such as Elk1, c-Fos, and c-Jun [113]. The treatment of cell lines with the phytochemicals mentioned above has made it possible to determine that the main effect on the ERK pathway is the phosphorylation of key components such as the ERK1/2 effectors. In HeLa and HTB-35 cells, it was demonstrated that GA reduces the phosphorylation levels of ERK protein suppressing cancer progression [114]. On the other hand, GA can inhibit ERK through the phosphorylation of EGFR, thereby decreasing the up-regulation of metalloproteinase 9 (MMP-9) and reducing the invasive capacity of tumoral cells [115]. CA and p-CA also phosphorylate ERK to promote translocation of Nrf2 from the cytoplasm to the nucleus, activating the expression of detoxification enzymes HO-1, GCLC, and GLCM as protection against oxidative damage in HepG2 cells [116]. Qu phosphorylates ERK and c-Jun thus promoting the cleavage of caspase-3 and inducing apoptosis in A549 cells [117]. In MDA-MB-231, HeLa, and SiKa cells, Qu induces changes in ERK1/2 phosphorylation leading to induction of apoptosis, inhibition of cell survival [118,119], and inhibition of colon cancer cell migration and invasion. Qi showed the same effects [120]. Iso suppressed EGF-induced phosphorylation of ERKs, Akt, and p70 by inhibition of MEK1 [121]. Kae also suppresses the phosphorylation of ERK and induces autophagy and apoptosis in ovarian cancer cells [122].
5.2. Wnt/β-catenin
Cysteine-rich glycoprotein Wnt is a ligand that can trigger intracellular signal transduction pathways such as the Wnt/β-catenin pathway. Wnt binds to the Frizzled (FZD) receptor and LRP-5/6 co-receptors. This activates Disheveled and in turn, this causes recruitment of the complex (Axin, GSK3 β, APC, CK1) to the receptor. The Wnt-Frizzled-Axin-LRP-5/6 complex sequesters cytosolic GSK3 β rendering it incapable of phosphorylating β-catenin. Accumulated non-phosphorylated β-catenin in the cytosol migrates to the nucleus, there β-catenin functions as a transcriptional co-activator interacting with T cell-specific factor (TCF)/lymphoid enhancer-binding factor (LEF) and binding to the promoters of target genes. Without Wnt signaling, cytoplasmic β-catenin is degraded by the destruction complex (Axin-APC-GSK3 β) [123,124]. Experiments using GA have shown that it suppresses the expression of β-catenin at mRNA and protein levels. Such downregulation suppresses downstream target genes such as VEGF, Oct3/4, surviving, and CCND1 in hepatocellular carcinoma cells.
Furthermore, in a xenograft model, E-cadherin and Bax were up-regulated, and vimentin, MMP9, Bcl2, and Bcl-xl were down-regulated. Authors hypothesized that GA inhibits the epithelial-mesenchymal transition polarization and blocks the potential of metastatic dissemination and induces apoptosis [125]. In human teratocarcinoma cells NT2/D1, Qu inhibits β-catenin translocation, this avoided the expression of genes involved in proliferation, migration, and invasion in diverse cell lines [126,127]. Liu et al. [128] found recently that Qu increases Sirtuin 6, this could reduce transcripts levels of FZD4 and prevent β-catenin cytoplasmic accumulation and translocation.
5.3. PI3K/AKT/mTOR
PI3K belongs to a family of lipid kinases that phosphorylate the 3′OH group of phosphatidylinositol, PI3K activation initiates a signal transduction cascade. Akt is a serine-threonine kinase that is directly activated in response to PI3K due to its amino-terminal PH domain which is bound by PIP3. Akt is one of the PI3K effectors, its activation results in cell survival, proliferation, growth, and angiogenesis. There are many downstream effectors, such as GSK3 β, FOXO, and MDM2, which in turn are controlled by compensatory pathways. One of the major effectors downstream of Akt is the serine/threonine kinase mTOR which is a key regulator of cell growth [129,130]. GA downregulates protein levels of p-AKT, PI3K, and mTOR affecting cell viability, proliferation, invasion, and angiogenesis in lung cancer, ovarian cancer, and glioma cells [48,131,132]. p-Akt was significantly decreased by CA on colorectal cancer stem cells [133]. In prostate cancer cells, breast cancer cells, and pancreatic cells, Qu binds PI3K suppressing AKT phosphorylation and inducing apoptosis by regulation of the Bax, Bcl2, and p53 expression [119,134]. In the same way, Qi, Sti, and Kae induce apoptosis by inhibition of PI3K phosphorylation [88,135,136], and Iso down-regulated the expression of p-PI3K, p-Akt, and p-mTOR inhibiting cell migration and invasion by matrix metalloproteinases regulation in prostate cancer [137] and skin cancer cells [121].
5.4. Nuclear Factor-E2 p45-Related Factor (Nrf2)
Nrf2 belongs to the cap’n’collar subfamily of transcription factors with a basic leucine zipper domain and plays a key role in the cellular stress response against oxidative insults such as electrophiles or cytotoxic agents. Nrf2 persists in an inactivated status, it binds to Kelch ECH associating protein 1 (Keap1), a repressor protein cysteine-rich that promotes Nrf2 degradation by the ubiquitin-proteasome pathway. The oxidative stress causes Nrf2 dissociation and its subsequent translocation into the nucleus to form heterodimers with small Maf proteins and bind to the antioxidant response element (ARE) and induce transcription of phase II enzymes, GST, NQO1, HO-1, GSC, and GCL [138,139]. CA induces Nrf2 translocation to the nucleus and oxidation of the Keap1 protein. CA possesses a dual mechanism of action, acts as a GSTP1 and GSR1 inducer and as their inhibitor in ovarian carcinoma cells A2780 [140], and CA in these cells before cisplatin treatment induces GST and GR expression [55]. Qu and Kae were found could be used as Keap1 inhibitors and Nrf2 activators [141]. Qu increased nuclear localization of Nrf2, enhanced ARE-binding complexes, and caused NQO1 transcription in HepG2 cells. Besides, Qu reduced the level of Keap1 by inhibiting the ubiquitination and destruction of Nrf2 [142] and increased the expression of HO-1 and NOS1 in a neuroblastoma cell line [143].
As we observed, the components of K. pinnata converge and affect pathways involved in various processes of cancer development and progression, and they also affect key genes such as p53 and p21. As recently reviewed by Ng et al. [144], there is a large amount of experimental data with plant extracts or fractions, but cancer characteristics and interactions among components in the extracts or in the human body upon administration need more analysis. There is not enough clinical data concerning the application of phytochemicals for cancer treatment, but their potential use as co-treatment is promising.
6. Conclusions
This review demonstrated that the use of phytochemicals in combination with oncologic treatments can give promising results in reducing the drug dose. Phytochemicals have been tested in many in vitro studies with cell lines; as such, it is necessary to test them in animal models as possible adjuvants in chemotherapeutic treatment. The perennial herb widely distributed in tropical areas, K. pinnata, has components that could repress hallmarks of cancer, such as proliferation, apoptosis, cell migration, angiogenesis, or metastasis, and regulate processes such as oxidative stress or autophagy. They could also act as epigenetic drugs, reverting the acquired epigenetic changes associated with tumor resistance to therapy such as the promoter methylation of suppressor genes, inhibition of DNMTs activity, and HDAC regulation through methylation.
Acknowledgments
We thank José Luis Martínez, Andres Vargas and Uriel Vazquez by the location, collection and photographies of K. pinnata.
Author Contributions
Conceptualization, M.E.H.-C.; writing—original draft preparation, M.E.H.-C.; writing—review and editing, M.E.H.-C., J.A.S.-R., R.V.-V., and E.S.-M.; visualization, R.V.-V., J.A.S.-R., and E.S.-M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
This research was funded by the Benemérita Universidad Autónoma de Puebla.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vogelstein B., Kinzler K.W. Cancer genes and the pathways they control. Nat. Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 2.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Desrosiers R., Friderici K., Rottman F. Identification of Methylated Nucleosides in Messenger RNA from Novikoff Hepatoma Cells. Proc. Natl. Acad. Sci. USA. 1974;71:3971–3975. doi: 10.1073/pnas.71.10.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nielsen A.H., Olsen C.E., Møller B.L. Flavonoids in flowers of 16 Kalanchoë blossfeldiana varieties. Phytochemistry. 2005;66:2829–2835. doi: 10.1016/j.phytochem.2005.09.041. [DOI] [PubMed] [Google Scholar]
- 5.Wu P.-L., Hsu Y.-L., Wu T.-S., Bastow K.F., Lee K.-H. Kalanchosides A−C, New Cytotoxic Bufadienolides from the Aerial Parts of Kalanchoe gracilis. Org. Lett. 2006;8:5207–5210. doi: 10.1021/ol061873m. [DOI] [PubMed] [Google Scholar]
- 6.Kamboj A., Saluja A. Bryophyllum pinnatum (Lam.) Kurz.: Phytochemical and Pharmacological Profile: A Review. Phcog. Rev. 2009;3:364–374. [Google Scholar]
- 7.Tao S.-F., He H.-F., Chen Q. Quercetin inhibits proliferation and invasion acts by up-regulating miR-146a in human breast cancer cells. Mol. Cell. Biochem. 2015;402:93–100. doi: 10.1007/s11010-014-2317-7. [DOI] [PubMed] [Google Scholar]
- 8.Stefanowicz-Hajduk J., Gucwa M., Moniuszko-Szajwaj B., Stochmal A., Kawiak A., Ochocka J.R. Bersaldegenin-1,3,5-orthoacetate induces caspase-independent cell death, DNA damage and cell cycle arrest in human cervical cancer HeLa cells. Pharm. Biol. 2021;59:54–65. doi: 10.1080/13880209.2020.1866025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuete V., Fokou F.W., Karaosmanoğlu O., Beng V.P., Sivas H. Cytotoxicity of the methanol extracts of Elephantopus mollis, Kalanchoe crenata and 4 other Cameroonian medicinal plants towards human carcinoma cells. BMC Complement. Altern. Med. 2017;17:280. doi: 10.1186/s12906-017-1793-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin S.R., Chang C.H., Hsu C.F., Tsai M.J., Cheng H., Leong M.K., Sung P.J., Chen J.C., Weng C.F. Natural compounds as potential adjuvants to cancer therapy: Preclinical evidence. Br. J. Pharmacol. 2020;177:1409–1423. doi: 10.1111/bph.14816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oliver-Bever B. Medicinal plants in tropical west africa III. Anti-infection therapy with higher plants. J. Ethnopharmacol. 1983;9:1–83. doi: 10.1016/0378-8741(83)90028-4. [DOI] [PubMed] [Google Scholar]
- 12.Sandoval M.C., Martínez J.L. El uso de Kalanchoe pinnata (Lam.) Pers. en el estado de Veracruz. Cienc. Hombre. 1994;16:49–56. [Google Scholar]
- 13.Majaz Q., Tatiya A.U., Khurshid M., Nazim S., Siraj S. The miracle plant (Kalanchoe pinnata): A phytochemical and pharmacological review. Int. J. Res. Ayurveda Pharm. 2011;2:1478–1482. [Google Scholar]
- 14.Mora-Pérez A., Hernández-Medel M. Actividad anticonvulsivante del extracto metanólico de tallo y raíz de Kalanchoe pinnata Lam. en ratones: Comparación con diazepam|Elsevier Enhanced Reader. Neurología. 2016;31:161–168. doi: 10.1016/j.nrl.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Mohan S.C., Balamurugan V., Salini S.T., Rekha R. Metal ion chelating activity and hydrogen peroxide scavenging activity of medicinal plant Kalanchoe pinnata. J. Chem. Pharm. Res. 2012;4:197–202. [Google Scholar]
- 16.Okwu D.E., Josiah C. Evaluation of the chemical composition of two Nigerian medicinal plants. Afr. J. Biotechnol. 2006;5:357–361. [Google Scholar]
- 17.Jaiswal S., Chawla R., Sawhney S. Kalanchoe pinnata—A Promising Source of Natural Antioxidants. Eur. J. Med. Plants. 2014;4:1210–1222. doi: 10.9734/EJMP/2014/11374. [DOI] [Google Scholar]
- 18.El Abdellaoui S., Destandau E., Toribio A., Elfakir C., Lafosse M., Renimel I., André P., Cancellieri P., Landemarre L. Bioactive molecules in Kalanchoe pinnata leaves: Extraction, purification, and identification. Anal. Bioanal. Chem. 2010;398:1329–1338. doi: 10.1007/s00216-010-4047-3. [DOI] [PubMed] [Google Scholar]
- 19.Muzitano M.F., Tinoco L.W., Guette C., Kaiser C.R., Rossi-Bergmann B., Costa S.S. The antileishmanial activity assessment of unusual flavonoids from Kalanchoe pinnata. Phytochemistry. 2006;67:2071–2077. doi: 10.1016/j.phytochem.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 20.Stefanowicz-Hajduk J., Hering A., Gucwa M., Hałasa R., Soluch A., Kowalczyk M., Stochmal A., Ochocka R. Biological activities of leaf extracts from selected Kalanchoe species and their relationship with bufadienolides content. Pharm. Biol. 2020;58:732–740. doi: 10.1080/13880209.2020.1795208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siddiqui S., Faizi S., Siddiqui B.S., Sultana N. Triterpenoids and phenanthrenes from leaves of Bryophyllum pinnatum. Phytochemistry. 1989;28:2433–2438. doi: 10.1016/S0031-9422(00)97999-8. [DOI] [Google Scholar]
- 22.Anjoo K., Saluja A.K. Isolation of stigmasterol from petroleum ether extract of aerial parts of Bryophyllum pinnatum (Crassulaceae) J. Pharm. Res. 2010;3:2802–2803. [Google Scholar]
- 23.Akihisa T., Kokke W.C.M.C., Tamura T., Matsumoto T. Sterols ofKalanchoe pinnata: First report of the isolation of both C-24 epimers of 24-alkyl-Δ25-sterols from a higher plant. Lipids. 1991;26:660–665. doi: 10.1007/BF02536432. [DOI] [Google Scholar]
- 24.McKENZIE R.A., Dunster P.J. Hearts and flowers: Bryophyllum poisoning of cattle. Aust. Vet. J. 1986;63:222–227. doi: 10.1111/j.1751-0813.1986.tb03000.x. [DOI] [PubMed] [Google Scholar]
- 25.Leri M., Scuto M., Ontario M.L., Calabrese V., Calabrese E.J., Bucciantini M., Stefani M. Healthy Effects of Plant Polyphenols: Molecular Mechanisms. Int. J. Mol. Sci. 2020;21:1250. doi: 10.3390/ijms21041250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stefanowicz-Hajduk J., Hering A., Gucwa M., Sztormowska-Achranowicz K., Kowalczyk M., Soluch A., Ochocka J.R. An In Vitro Anticancer, Antioxidant, and Phytochemical Study on Water Extract of Kalanchoe daigremontiana Raym.-Hamet and H. Perrier. Molecules. 2022;27:2280. doi: 10.3390/molecules27072280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stefanowicz-Hajduk J., Asztemborska M., Krauze-Baranowska M., Godlewska S., Gucwa M., Moniuszko-Szajwaj B., Stochmal A., Ochocka J.R. Identification of Flavonoids and Bufadienolides and Cytotoxic Effects of Kalanchoe daigremontiana Extracts on Human Cancer Cell Lines. Planta Med. 2020;86:239–246. doi: 10.1055/a-1099-9786. [DOI] [PubMed] [Google Scholar]
- 28.Supratman U., Fujita T., Akiyama K., Hayashi H., Murakami A., Sakai H., Koshimizu K., Ohigashi H. Anti-tumor Promoting Activity of Bufadienolides from Kalanchoe pinnata and K. daigremontiana × butiflora. Biosci. Biotechnol. Biochem. 2001;65:947–949. doi: 10.1271/bbb.65.947. [DOI] [PubMed] [Google Scholar]
- 29.Asiedu-Gyekye I.J., Arhin E., Arthur S.A., N’Guessan B.B., Amponsah S.K. Genotoxicity, nitric oxide level modulation and cardio-protective potential of Kalanchoe Integra Var. Crenata (Andr.) Cuf Leaves in murine models. J. Ethnopharmacol. 2022;283:114640. doi: 10.1016/j.jep.2021.114640. [DOI] [PubMed] [Google Scholar]
- 30.Huang H.-C., Chang W.-T., Lee M.-S., Chen H.-Y., Chen Y.-H., Lin C.-C., Lin M.-K. Three bufadienolides induce cell death in the human lung cancer cell line CL1-5 mainly through autophagy. Bioorganic Med. Chem. Lett. 2021;31:127715. doi: 10.1016/j.bmcl.2020.127715. [DOI] [PubMed] [Google Scholar]
- 31.Hsieh Y.-J., Huang H.-S., Leu Y.-L., Peng K.-C., Chang C.-J., Chang M.-Y. Anticancer activity of Kalanchoe tubiflora extract against human lung cancer cells in vitro and in vivo. Environ. Toxicol. 2016;31:1663–1673. doi: 10.1002/tox.22170. [DOI] [PubMed] [Google Scholar]
- 32.Huang H.-C., Lin M.-K., Yang H.-L., Hseu Y.-C., Liaw C.-C., Tseng Y.-H., Tsuzuki M., Kuo Y.-H. Cardenolides and Bufadienolide Glycosides from Kalanchoe tubiflora and Evaluation of Cytotoxicity. Planta Med. 2013;79:1362–1369. doi: 10.1055/s-0033-1350646. [DOI] [PubMed] [Google Scholar]
- 33.Palumbo A., Casanova L., Corrêa M.F.P., Da Costa N.M., Nasciutti L.E., Costa S.S. Potential Therapeutic Effects of Underground Parts of Kalanchoe gastonis-bonnieri on Benign Prostatic Hyperplasia. Evid.-Based Complement. Altern. Med. 2019;2019:6340757. doi: 10.1155/2019/6340757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shamaladevi N., Araki S., Lyn D.A., Ayyathurai R., Gao J., Lokeshwar V.B., Navarrete H., Lokeshwar B.L. The andean anticancer herbal product BIRM causes destabilization of androgen receptor and induces caspase-8 mediated-apoptosis in prostate cancer. Oncotarget. 2016;7:84201–84213. doi: 10.18632/oncotarget.12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arias-González I., García-Carrancá A.M., Cornejo-Garrido J., Ordaz-Pichardo C. Cytotoxic effect of Kalanchoe flammea and induction of intrinsic mitochondrial apoptotic signaling in prostate cancer cells. J. Ethnopharmacol. 2018;222:133–147. doi: 10.1016/j.jep.2018.04.038. [DOI] [PubMed] [Google Scholar]
- 36.Kaewpiboon C., Srisuttee R., Malilas W., Moon J., Kaowinn S., Cho I.-R., Johnston R.N., Assavalapsakul W., Chung Y.-H. Extract of Bryophyllum laetivirens reverses etoposide resistance in human lung A549 cancer cells by downregulation of NF-κB. Oncol. Rep. 2014;31:161–168. doi: 10.3892/or.2013.2844. [DOI] [PubMed] [Google Scholar]
- 37.Lai Z.-R., Ho Y.-L., Huang S.-C., Huang T.-H., Lai S.-C., Tsai J.-C., Wang C.-Y., Huang G.-J., Chang Y.-S. Antioxidant, Anti-inflammatory and Antiproliferative Activities of Kalanchoe gracilis (L.) DC Stem. Am. J. Chin. Med. 2011;39:1275–1290. doi: 10.1142/S0192415X1100955X. [DOI] [PubMed] [Google Scholar]
- 38.Poma P., Labbozzetta M., McCubrey J.A., Ramarosandratana A.V., Sajeva M., Zito P., Notarbartolo M. Antitumor Mechanism of the Essential Oils from Two Succulent Plants in Multidrug Resistance Leukemia Cell. Pharmaceuticals. 2019;12:124. doi: 10.3390/ph12030124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fonseca A.G., Dantas L.L.S.F.R., Fernandes J.M., Zucolotto S.M., Lima A.A.N., Soares L.A.L., Rocha H.A.O., Lemos T.M.A.M. In Vivo and In Vitro Toxicity Evaluation of Hydroethanolic Extract of Kalanchoe brasiliensis (Crassulaceae) Leaves. J. Toxicol. 2018;2018:6849765. doi: 10.1155/2018/6849765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharif A., Akhtar M.F., Akhtar B., Saleem A., Manan M., Shabbir M., Ashraf M., Peerzada S., Ahmed S., Raza M. Genotoxic and cytotoxic potential of whole plant extracts of Kalanchoe laciniata by Ames and MTT assay. EXCLI J. 2017;16:593–601. doi: 10.17179/excli2016-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hagerman A.E., Butler L.G. Protein precipitation method for the quantitative determination of tannins. J. Agric. Food Chem. 1978;26:809–812. doi: 10.1021/jf60218a027. [DOI] [Google Scholar]
- 42.Rajalakshmi K., Devaraj H., Devaraj S.N. Assessment of the no-observed-adverse-effect level (NOAEL) of gallic acid in mice. Food Chem. Toxicol. 2001;39:919–922. doi: 10.1016/S0278-6915(01)00022-9. [DOI] [PubMed] [Google Scholar]
- 43.Booth A., Amen R.J., Scott M., Greenway F.L. Oral dose-ranging developmental toxicity study of an herbal supplement (NT) and gallic acid in rats. Adv. Ther. 2010;27:250–255. doi: 10.1007/s12325-010-0021-x. [DOI] [PubMed] [Google Scholar]
- 44.Mirvish S.S., Cardesa A., Wallcave L., Shubik P. Induction of Mouse Lung Adenomas by Amines or Ureas Plus Nitrite and by N-Nitroso Compounds: Effect of Ascorbate, Gallic Acid, Thiocyanate, and Caffeine. JNCI J. Natl. Cancer Inst. 1975;55:633–636. doi: 10.1093/jnci/55.3.633. [DOI] [PubMed] [Google Scholar]
- 45.Inoue M., Suzuki R., Koide T., Sakaguchi N., Ogihara Y., Yabu Y. Antioxidant, Gallic Acid, Induces Apoptosis in HL-60RG Cells. Biochem. Biophys. Res. Commun. 1994;204:898–904. doi: 10.1006/bbrc.1994.2544. [DOI] [PubMed] [Google Scholar]
- 46.Verma S., Singh A., Mishra A. Gallic acid: Molecular rival of cancer. Environ. Toxicol. Pharmacol. 2013;35:473–485. doi: 10.1016/j.etap.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 47.Tang H.M., Cheung P.C.K. Gene expression profile analysis of gallic acid-induced cell death process. Sci. Rep. 2021;11:16743. doi: 10.1038/s41598-021-96174-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ko E.-B., Jang Y.-G., Kim C.-W., Go R.-E., Lee H.K., Choi K.-C. Gallic Acid Hindered Lung Cancer Progression by Inducing Cell Cycle Arrest and Apoptosis in A549 Lung Cancer Cells via PI3K/Akt Pathway. Biomol. Ther. 2021;30:151–161. doi: 10.4062/biomolther.2021.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Isuzugawa K., Inoue M., Ogihara Y. Catalase Contents in Cells Determine Sensitivity to the Apoptosis Inducer Gallic Acid. Biol. Pharm. Bull. 2001;24:1022–1026. doi: 10.1248/bpb.24.1022. [DOI] [PubMed] [Google Scholar]
- 50.Banerji S., Wright A.J., Noble M., Mahoney D.J., Campbell I.D., Day A., Jackson D.G. Structures of the Cd44–hyaluronan complex provide insight into a fundamental carbohydrate-protein interaction. Nat. Struct. Mol. Biol. 2007;14:234–239. doi: 10.1038/nsmb1201. [DOI] [PubMed] [Google Scholar]
- 51.Shao Y., Luo W., Guo Q., Li X., Zhang Q., Li J. In vitro and in vivo effect of hyaluronic acid modified, doxorubicin and gallic acid co-delivered lipid-polymeric hybrid nano-system for leukemia therapy. Drug Des. Dev. Ther. 2019;13:2043–2055. doi: 10.2147/DDDT.S202818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Olthof M.R., Hollman P.C.H., Katan M.B. Chlorogenic Acid and Caffeic Acid Are Absorbed in Humans. J. Nutr. 2001;131:66–71. doi: 10.1093/jn/131.1.66. [DOI] [PubMed] [Google Scholar]
- 53.Rosendahl A.H., Perks C.M., Zeng L., Markkula A., Simonsson M., Rose C., Ingvar C., Holly J.M., Jernström H. Caffeine and Caffeic Acid Inhibit Growth and Modify Estrogen Receptor and Insulin-like Growth Factor I Receptor Levels in Human Breast Cancer. Clin. Cancer Res. 2015;21:1877–1887. doi: 10.1158/1078-0432.CCR-14-1748. [DOI] [PubMed] [Google Scholar]
- 54.Ayna A., Özbolat S.N., Darendelioglu E. Quercetin, chrysin, caffeic acid and ferulic acid ameliorate cyclophosphamide-induced toxicities in SH-SY5Y cells. Mol. Biol. Rep. 2020;47:8535–8543. doi: 10.1007/s11033-020-05896-4. [DOI] [PubMed] [Google Scholar]
- 55.Sirota R., Gibson D., Kohen R. The timing of caffeic acid treatment with cisplatin determines sensitization or resistance of ovarian carcinoma cell lines. Redox Biol. 2016;11:170–175. doi: 10.1016/j.redox.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dziedzic A., Kubina R., Kabała-Dzik A., Wojtyczka R.D., Morawiec T., Bułdak R.J. Caffeic Acid Reduces the Viability and Migration Rate of Oral Carcinoma Cells (SCC-25) Exposed to Low Concentrations of Ethanol. Int. J. Mol. Sci. 2014;15:18725–18741. doi: 10.3390/ijms151018725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kabała-Dzik A., Rzepecka-Stojko A., Kubina R., Wojtyczka R.D., Buszman E., Stojko J. Caffeic Acid Versus Caffeic Acid Phenethyl Ester in the Treatment of Breast Cancer MCF-7 Cells: Migration Rate Inhibition. Integr. Cancer Ther. 2018;17:1247–1259. doi: 10.1177/1534735418801521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ferguson L.R., Zhu S.-T., Harris P.J. Antioxidant and antigenotoxic effects of plant cell wall hydroxycinnamic acids in cultured HT-29 cells. Mol. Nutr. Food Res. 2005;49:585–593. doi: 10.1002/mnfr.200500014. [DOI] [PubMed] [Google Scholar]
- 59.Janicke B., Önning G., Oredsson S.M. Differential Effects of Ferulic Acid and p-Coumaric Acid on S Phase Distribution and Length of S Phase in the Human Colonic Cell Line Caco2. J. Agric. Food Chem. 2005;53:6658–6665. doi: 10.1021/jf050489l. [DOI] [PubMed] [Google Scholar]
- 60.Sharma S.H., Chellappan D.R., Chinnaswamy P., Nagarajan S. Protective effect of p-coumaric acid against 1,2 dimethylhydrazine induced colonic preneoplastic lesions in experimental rats. Biomed. Pharmacother. 2017;94:577–588. doi: 10.1016/j.biopha.2017.07.146. [DOI] [PubMed] [Google Scholar]
- 61.Gastaldello G., Cazeloto A., Ferreira J., Rodrigues D., Bastos J., Campo V., Zoccal K., Tefé-Silva C. Green Propolis Compounds (Baccharin and p-Coumaric Acid) Show Beneficial Effects in Mice for Melanoma Induced by B16f10. Medicines. 2021;8:20. doi: 10.3390/medicines8050020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rhodes M.J.C., Price K.R. Analytical problems in the study of flavonoid compounds in onions. Food Chem. 1996;57:113–117. doi: 10.1016/0308-8146(96)00147-1. [DOI] [Google Scholar]
- 63.Wätjen W., Michels G., Steffan B., Niering P., Chovolou Y., Kampkötter A., Tran-Thi Q.-H., Proksch P., Kahl R. Low Concentrations of Flavonoids Are Protective in Rat H4IIE Cells Whereas High Concentrations Cause DNA Damage and Apoptosis. J. Nutr. 2005;135:525–531. doi: 10.1093/jn/135.3.525. [DOI] [PubMed] [Google Scholar]
- 64.Kaneuchi M., Sasaki M., Tanaka Y., Sakuragi N., Fujimoto S., Dahiya R. Quercetin regulates growth of Ishikawa cells through the suppression of EGF and cyclin D1. Int. J. Oncol. 2003;22:159–164. doi: 10.3892/ijo.22.1.159. [DOI] [PubMed] [Google Scholar]
- 65.Pratheeshkumar P., Budhraja A., Son Y.-O., Wang X., Zhang Z., Ding S., Wang L., Hitron A., Lee J.-C., Xu M., et al. Quercetin Inhibits Angiogenesis Mediated Human Prostate Tumor Growth by Targeting VEGFR- 2 Regulated AKT/mTOR/P70S6K Signaling Pathways. PLoS ONE. 2012;7:e47516. doi: 10.1371/journal.pone.0047516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gulati N., Laudet B., Zohrabian V.M., Murali R., Jhanwar-Uniyal M. The antiproliferative effect of Quercetin in cancer cells is mediated via inhibition of the PI3K-Akt/PKB pathway. Anticancer Res. 2006;26:1177–1781. [PubMed] [Google Scholar]
- 67.Zhang X., Guo Q., Chen J., Chen A.Z. Quercetin Enhances Cisplatin Sensitivity of Human Osteosarcoma Cells by Modulating microRNA-217-KRAS Axis. Mol. Cells. 2015;38:638–642. doi: 10.14348/molcells.2015.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li S.-Z., Qiao S.-F., Zhang J.-H., Li K. Quercetin Increase the Chemosensitivity of Breast Cancer Cells to Doxorubicin Via PTEN/Akt Pathway. Anti-Cancer Agents Med. Chem. 2015;15:1185–1189. doi: 10.2174/1871520615999150121121708. [DOI] [PubMed] [Google Scholar]
- 69.Liu H., Lee J.I., Ahn T.-G. Effect of quercetin on the anti-tumor activity of cisplatin in EMT6 breast tumor-bearing mice. Obstet. Gynecol. Sci. 2019;62:242–248. doi: 10.5468/ogs.2019.62.4.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qian J., Liu S., Yang T., Xiao Y., Sun J., Zhao J., Zhang Z., Xie Y. Polyethyleneimine--Tocopherol Hydrogen Succinate/Hyaluronic Acid-Quercetin (PEI-TOS/HA-QU) Core–Shell Micelles Delivering Paclitaxel for Combinatorial Treatment of MDR Breast Cancer. J. Biomed. Nanotechnol. 2021;17:382–398. doi: 10.1166/jbn.2021.3032. [DOI] [PubMed] [Google Scholar]
- 71.Barbarisi M., Iaffaioli R.V., Armenia E., Schiavo L., De Sena G., Tafuto S., Barbarisi A., Quagliariello V. Novel nanohydrogel of hyaluronic acid loaded with quercetin alone and in combination with temozolomide as new therapeutic tool, CD44 targeted based, of glioblastoma multiforme. J. Cell. Physiol. 2018;233:6550–6564. doi: 10.1002/jcp.26238. [DOI] [PubMed] [Google Scholar]
- 72.Liu Z.-J., Xu W., Han J., Liu Q.-Y., Gao L.-F., Wang X.-H., Li X.-L. Quercetin induces apoptosis and enhances gemcitabine therapeutic efficacy against gemcitabine-resistant cancer cells. Anti-Cancer Drugs. 2020;31:684–692. doi: 10.1097/CAD.0000000000000933. [DOI] [PubMed] [Google Scholar]
- 73.Gao Y., Yin J., Rankin G.O., Chen Y.C. Kaempferol Induces G2/M Cell Cycle Arrest via Checkpoint Kinase 2 and Promotes Apoptosis via Death Receptors in Human Ovarian Carcinoma A2780/CP70 Cells. Molecules. 2018;23:1095. doi: 10.3390/molecules23051095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wagner C., Fachinetto R., Corte C.L.D., Brito V.B., Severo D., Dias G.D.O.C., Morel A.F., Nogueira C.W., Rocha J.B. Quercitrin, a glycoside form of quercetin, prevents lipid peroxidation in vitro. Brain Res. 2006;1107:192–198. doi: 10.1016/j.brainres.2006.05.084. [DOI] [PubMed] [Google Scholar]
- 75.Cincin Z.B., Unlu M., Kiran B., Bireller E.S., Baran Y., Cakmakoglu B. Apoptotic Effects of Quercitrin on DLD-1 Colon Cancer Cell Line. Pathol. Oncol. Res. 2015;21:333–338. doi: 10.1007/s12253-014-9825-3. [DOI] [PubMed] [Google Scholar]
- 76.Cincin Z.B., Unlu M., Kiran B., Bireller E.S., Baran Y., Cakmakoglu B. Molecular Mechanisms of Quercitrin-induced Apoptosis in Non-small Cell Lung Cancer. Arch. Med Res. 2014;45:445–454. doi: 10.1016/j.arcmed.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 77.Ding M., Zhao J., Bowman L., Lu Y., Shi X. Inhibition of AP-1 and MAPK signaling and activation of Nrf2/ARE pathway by quercitrin. Int. J. Oncol. 2010;36:59–67. doi: 10.3892/ijo_00000475. [DOI] [PubMed] [Google Scholar]
- 78.Zhai T., Zhang X., Hei Z., Jin L., Han C., Ko A.T., Yu X., Wang J. Isorhamnetin Inhibits Human Gallbladder Cancer Cell Proliferation and Metastasis via PI3K/AKT Signaling Pathway Inactivation. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.628621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Saud S.M., Young M.R., Jones-Hall Y.L., Ileva L., Evbuomwan M.O., Wise J., Colburn N.H., Kim Y.S., Bobe G. Chemopreventive Activity of Plant Flavonoid Isorhamnetin in Colorectal Cancer Is Mediated by Oncogenic Src and β-Catenin. Cancer Res. 2013;73:5473–5484. doi: 10.1158/0008-5472.CAN-13-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li C., Li J., Li Y., Li L., Luo Y., Li J., Zhang Y., Wang Y., Liu X., Zhou X., et al. Isorhamnetin Promotes MKN-45 Gastric Cancer Cell Apoptosis by Inhibiting PI3K-Mediated Adaptive Autophagy in a Hypoxic Environment. J. Agric. Food Chem. 2021;69:8130–8143. doi: 10.1021/acs.jafc.1c02620. [DOI] [PubMed] [Google Scholar]
- 81.Yingchun X., Huang L., Meng L., Sun H., Zhang W., Xu Y. Isorhamnetin inhibits cell proliferation and induces apoptosis in breast cancer via Akt and mitogen-activated protein kinase kinase signaling pathways. Mol. Med. Rep. 2015;12:6745–6751. doi: 10.3892/mmr.2015.4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.De Graffenried L.A., Friedrichs W.E., Russell D.H., Donzis E.J., Middleton A.K., Silva J.M., Roth R.A., Hidalgo M. Inhibition of mTOR Activity Restores Tamoxifen Response in Breast Cancer Cells with Aberrant Akt Activity. Clin. Cancer Res. 2004;10:8059–8067. doi: 10.1158/1078-0432.CCR-04-0035. [DOI] [PubMed] [Google Scholar]
- 83.Manu K.A., Shanmugam M.K., Ramachandran L., Li F., Siveen K.S., Chinnathambi A., Zayed M., Alharbi S.A., Arfuso F., Kumar A.P., et al. Isorhamnetin augments the anti-tumor effect of capeciatbine through the negative regulation of NF-κB signaling cascade in gastric cancer. Cancer Lett. 2015;363:28–36. doi: 10.1016/j.canlet.2015.03.033. [DOI] [PubMed] [Google Scholar]
- 84.Du Y., Jia C., Liu Y., Li Y., Wang J., Sun K. Isorhamnetin Enhances the Radiosensitivity of A549 Cells through Interleukin-13 and the NF-κB Signaling Pathway. Front. Pharmacol. 2020;11:610772. doi: 10.3389/fphar.2020.610772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.U.S. National Library of Medicine PubChem Open Chemistry Database at the National Institutes of Health (NIH) [(accessed on 7 January 2021)]; Available online: https://pubchem.ncbi.nlm.nih.gov/
- 86.Yi X., Zuo J., Tan C., Xian S., Luo C., Chen S., Yu L., Luo Y. Kaempferol, a flavonoid compound from gynura medica induced apoptosis and growth inhibition in mcf-7 breast cancer cell. Afr. J. Tradit. Complement. Altern. Med. 2016;13:210–215. doi: 10.21010/ajtcam.v13i4.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang G., Xing J., Aikemu B., Sun J., Zheng M. Kaempferol exhibits a synergistic effect with doxorubicin to inhibit proliferation, migration, and invasion of liver cancer. Oncol. Rep. 2021;45:32. doi: 10.3892/or.2021.7983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang Z., Guo Y., Chen M., Chen F., Liu B., Shen C. Kaempferol potentiates the sensitivity of pancreatic cancer cells to erlotinib via inhibition of the PI3K/AKT signaling pathway and epidermal growth factor receptor. Inflammopharmacology. 2021;29:1587–1601. doi: 10.1007/s10787-021-00848-1. [DOI] [PubMed] [Google Scholar]
- 89.Amawi H., Ashby C.R., Jr., Tiwari A.K. Cancer chemoprevention through dietary flavonoids: What’s limiting? Chin. J. Cancer. 2017;36:50. doi: 10.1186/s40880-017-0217-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pohl T.S., Crouch N., Mulholland D. Southern African Hyacinthaceae: Chemistry, Bioactivity and Ethnobotany. Curr. Org. Chem. 2000;4:1287–1324. doi: 10.2174/1385272003375806. [DOI] [Google Scholar]
- 91.Kolodziejczyk-Czepas J., Stochmal A. Bufadienolides of Kalanchoe species: An overview of chemical structure, biological activity and prospects for pharmacological use. Phytochem. Rev. 2017;16:1155–1171. doi: 10.1007/s11101-017-9525-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Deng L.-J., Li Y., Qi M., Liu J.-S., Wang S., Hu L.-J., Lei Y.-H., Jiang R.-W., Chen W.-M., Qi Q., et al. Molecular mechanisms of bufadienolides and their novel strategies for cancer treatment. Eur. J. Pharmacol. 2020;887:173379. doi: 10.1016/j.ejphar.2020.173379. [DOI] [PubMed] [Google Scholar]
- 93.Yan X., Lee K., Takashi Y. Isolation and identification of cytotoxic components from bryophyllum pinnatum. Chin. J. Cancer Res. 1992;4:1–3. doi: 10.1007/BF02996394. [DOI] [Google Scholar]
- 94.Yamagashi T., Yan X.-Z., Wu R.-Y., Mcphail D.R., Mcphail A.T., Lee K.-H. Structure and stereochemistry of bryophyllin-A, a novel potent cytotoxic bufadienolide orthoacetate from Bryophyllum pinnatum. Chem. Pharm. Bull. 1988;36:1615–1617. doi: 10.1248/cpb.36.1615. [DOI] [PubMed] [Google Scholar]
- 95.Supratman U., Fujita T., Akiyama K., Hayashi H. New Insecticidal Bufadienolide, Bryophyllin C, from Kalanchoe pinnata. Biosci. Biotechnol. Biochem. 2000;64:1310–1312. doi: 10.1271/bbb.64.1310. [DOI] [PubMed] [Google Scholar]
- 96.Li M., Zhang W., Yang L., Wang H., Wang Y., Huang K. The Mechanism of Xiaoyao San in the Treatment of Ovarian Cancer by Network Pharmacology and the Effect of Stigmasterol on the PI3K/Akt Pathway. Dis. Markers. 2021;2021:4304507. doi: 10.1155/2021/4304507. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 97.Zhao H., Zhang X., Wang M., Lin Y., Zhou S. Stigmasterol Simultaneously Induces Apoptosis and Protective Autophagy by Inhibiting Akt/mTOR Pathway in Gastric Cancer Cells. Front. Oncol. 2021;11:629008. doi: 10.3389/fonc.2021.629008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kwak M.-K., Itoh K., Yamamoto M., Sutter T.R., Kensler T.W. Role of Transcription Factor Nrf2 in the Induction of Hepatic Phase 2 and Antioxidative Enzymes in vivo by the Cancer Chemoprotective Agent, 3H-1, 2-Dithiole-3-thione. Mol. Med. 2001;7:135–145. doi: 10.1007/BF03401947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liao H., Zhu D., Bai M., Chen H., Yan S., Yu J., Zhu H., Zheng W., Fan G. Stigmasterol sensitizes endometrial cancer cells to chemotherapy by repressing Nrf2 signal pathway. Cancer Cell Int. 2020;20:480. doi: 10.1186/s12935-020-01470-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.El-Readi M.Z., Hamdan D., Farrag N., El-Shazly A., Wink M. Inhibition of P-glycoprotein activity by limonin and other secondary metabolites from Citrus species in human colon and leukaemia cell lines. Eur. J. Pharmacol. 2009;626:139–145. doi: 10.1016/j.ejphar.2009.09.040. [DOI] [PubMed] [Google Scholar]
- 101.Gautam M., Thapa R.K., Gupta B., Soe Z.C., Ou W., Poudel K., Jin S.G., Choi H.-G., Yong C.S., Kim J.O. Phytosterol-loaded CD44 receptor-targeted PEGylated nano-hybrid phyto-liposomes for synergistic chemotherapy. Expert Opin. Drug Deliv. 2020;17:423–434. doi: 10.1080/17425247.2020.1727442. [DOI] [PubMed] [Google Scholar]
- 102.Bae H., Park S., Yang C., Song G., Lim W. Disruption of Endoplasmic Reticulum and ROS Production in Human Ovarian Cancer by Campesterol. Antioxidants. 2021;10:379. doi: 10.3390/antiox10030379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Weng Y.-P., Hung P.-F., Ku W.-Y., Chang C.-Y., Wu B.-H., Wu M.-H., Yao J.-Y., Yang J.-R., Lee C.-H. The inhibitory activity of gallic acid against DNA methylation: Application of gallic acid on epigenetic therapy of human cancers. Oncotarget. 2018;9:361–374. doi: 10.18632/oncotarget.23015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Saenglee S., Jogloy S., Patanothai A., Leid M., Senawong T. Cytotoxic effects of peanut phenolics possessing histone deacetylase inhibitory activity in breast and cervical cancer cell lines. Pharmacol. Rep. 2016;68:1102–1110. doi: 10.1016/j.pharep.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 105.Sundaram M.K., Hussain A., Haque S., Raina R., Afroze N. Quercetin modifies 5′CpG promoter methylation and reactivates various tumor suppressor genes by modulating epigenetic marks in human cervical cancer cells. J. Cell. Biochem. 2019;120:18357–18369. doi: 10.1002/jcb.29147. [DOI] [PubMed] [Google Scholar]
- 106.Priyadarsini R.V., Vinothini G., Murugan R.S., Manikandan P., Nagini S. The Flavonoid Quercetin Modulates the Hallmark Capabilities of Hamster Buccal Pouch Tumors. Nutr. Cancer. 2011;63:218–226. doi: 10.1080/01635581.2011.523503. [DOI] [PubMed] [Google Scholar]
- 107.Alvarez M.C., Maso V., Torello C.O., Ferro K.P., Saad S.T.O. The polyphenol quercetin induces cell death in leukemia by targeting epigenetic regulators of pro-apoptotic genes. Clin. Epigenetics. 2018;10:139. doi: 10.1186/s13148-018-0563-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Berger A., Venturelli S., Kallnischkies M., Böcker A., Busch C., Weiland T., Noor S., Leischner C., Weiss T.S., Lauer U.M., et al. Kaempferol, a new nutrition-derived pan-inhibitor of human histone deacetylases. J. Nutr. Biochem. 2013;24:977–985. doi: 10.1016/j.jnutbio.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 109.Lu L., Wang Y., Ou R., Feng Q., Ji L., Zheng H., Guo Y., Qi X., Kong A.-N.T., Liu Z. DACT2 Epigenetic Stimulator Exerts Dual Efficacy for Colorectal Cancer Prevention and Treatment. Pharmacol. Res. 2018;129:318–328. doi: 10.1016/j.phrs.2017.11.032. [DOI] [PubMed] [Google Scholar]
- 110.Roundtree I.A., Evans M.E., Pan T., He C. Dynamic RNA Modifications in Gene Expression Regulation. Cell. 2017;169:1187–1200. doi: 10.1016/j.cell.2017.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Du Y., Yuan Y., Xu L., Zhao F., Wang W., Xu Y., Tian X. Discovery of METTL3 Small Molecule Inhibitors by Virtual Screening of Natural Products. Frontiers in Pharmacology. Front. Media. 2022;13:878135. doi: 10.3389/FPHAR.2022.878135/FULL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sun C., Dong F., Xiao T., Gao W. Efficacy and safety of Chinese patent medicine (Kang-ai injection) as an adjuvant in the treatment of patients with hepatocellular carcinoma: A meta-analysis. Pharm. Biol. 2021;59:470–481. doi: 10.1080/13880209.2021.1915340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wortzel I., Seger R. The ERK Cascade: Distinct Functions within Various Subcellular Organelles. Genes Cancer. 2011;2:195–209. doi: 10.1177/1947601911407328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhao B., Hu M. Gallic acid reduces cell viability, proliferation, invasion and angiogenesis in human cervical cancer cells. Oncol. Lett. 2013;6:1749–1755. doi: 10.3892/ol.2013.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen Y.-J., Lin K.-N., Jhang L.-M., Huang C.-H., Lee Y.-C., Chang L.-S. Gallic acid abolishes the EGFR/Src/Akt/Erk-mediated expression of matrix metalloproteinase-9 in MCF-7 breast cancer cells. Chem. Interactions. 2016;252:131–140. doi: 10.1016/j.cbi.2016.04.025. [DOI] [PubMed] [Google Scholar]
- 116.Yang S.-Y., Pyo M.C., Nam M.-H., Lee K.-W. ERK/Nrf2 pathway activation by caffeic acid in HepG2 cells alleviates its hepatocellular damage caused by t-butylhydroperoxide-induced oxidative stress. BMC Complement. Altern. Med. 2019;19:139. doi: 10.1186/s12906-019-2551-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nguyen T.H., Tran E., Do P., Huynh T., Huynh H. The role of activated MEK-ERK pathway in quercetin-induced growth inhibition and apoptosis in A549 lung cancer cells. Carcinogenesis. 2004;25:647–659. doi: 10.1093/carcin/bgh052. [DOI] [PubMed] [Google Scholar]
- 118.Chen X., Xu P., Zhang H., Su X., Guo L., Zhou X., Wang J., Huang P., Zhang Q., Sun R. EGFR and ERK activation resists flavonoid quercetin-induced anticancer activities in human cervical cancer cells in vitro. Oncol. Lett. 2021;22:754. doi: 10.3892/ol.2021.13015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Safi A., Heidarian E., Ahmadi R. Quercetin Synergistically Enhances the Anticancer Efficacy of Docetaxel through Induction of Apoptosis and Modulation of PI3K/AKT, MAPK/ERK, and JAK/STAT3 Signaling Pathways in MDA-MB-231 Breast Cancer Cell Line. Int. J. Mol. Cell. Med. 2021;10:11–22. doi: 10.22088/IJMCM.BUMS.10.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Trinh N.-T., Nguyen T.M.N., Yook J.-I., Ahn S.-G., Kim S.-A. Quercetin and Quercitrin from Agrimonia pilosa Ledeb Inhibit the Migration and Invasion of Colon Cancer Cells through the JNK Signaling Pathway. Pharmaceuticals. 2022;15:364. doi: 10.3390/ph15030364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kim J.-E., Lee D.-E., Lee K.W., Son J.E., Seo S.K., Li J., Jung S.K., Heo Y.-S., Mottamal M., Bode A.M., et al. Isorhamnetin Suppresses Skin Cancer through Direct Inhibition of MEK1 and PI3-K. Cancer Prev. Res. 2011;4:582–591. doi: 10.1158/1940-6207.CAPR-11-0032. [DOI] [PubMed] [Google Scholar]
- 122.Yang S., Si L., Jia Y., Jian W., Yu Q., Wang M., Lin R. Kaempferol exerts anti-proliferative effects on human ovarian cancer cells by inducing apoptosis, G0/G1 cell cycle arrest and modulation of MEK/ERK and STAT3 pathways. JBUON. 2019;24:975–981. [PubMed] [Google Scholar]
- 123.Koni M., Pinnarò V., Brizzi M.F. The Wnt Signalling Pathway: A Tailored Target in Cancer. Int. J. Mol. Sci. 2020;21:7697. doi: 10.3390/ijms21207697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Komiya Y., Habas R. Wnt signal transduction pathways. Organogenesis. 2008;4:68–75. doi: 10.4161/org.4.2.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shi C.-J., Zheng Y.-B., Pan F.-F., Zhang F.-W., Zhuang P., Fu W.-M. Gallic Acid Suppressed Tumorigenesis by an LncRNA MALAT1-Wnt/β-Catenin Axis in Hepatocellular Carcinoma. Front. Pharmacol. 2021;12:708967. doi: 10.3389/fphar.2021.708967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mojsin M., Vicentic J.M., Schwirtlich M., Topalovic V., Stevanovic M. Quercetin reduces pluripotency, migration and adhesion of human teratocarcinoma cell line NT2/D1 by inhibiting Wnt/β-catenin signaling. Food Funct. 2014;5:2564–2573. doi: 10.1039/C4FO00484A. [DOI] [PubMed] [Google Scholar]
- 127.Srivastava N.S., Srivastava R.A.K. Curcumin and quercetin synergistically inhibit cancer cell proliferation in multiple cancer cells and modulate Wnt/β-catenin signaling and apoptotic pathways in A375 cells. Phytomedicine. 2019;52:117–128. doi: 10.1016/j.phymed.2018.09.224. [DOI] [PubMed] [Google Scholar]
- 128.Liu T., Li Z., Tian F. Quercetin inhibited the proliferation and invasion of hepatoblastoma cells through facilitating SIRT6-medicated FZD4 silence. Hum. Exp. Toxicol. 2021;40:S96–S107. doi: 10.1177/09603271211030558. [DOI] [PubMed] [Google Scholar]
- 129.Vivanco I., Sawyers C.L. The phosphatidylinositol 3-Kinase–AKT pathway in human cancer. Nat. Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 130.Ersahin T., Tuncbag N., Cetin-Atalay R. The PI3K/AKT/mTOR interactive pathway. Mol. BioSyst. 2015;11:1946–1954. doi: 10.1039/C5MB00101C. [DOI] [PubMed] [Google Scholar]
- 131.Han L., Guo X., Bian H., Yang L., Chen Z., Zang W., Yang J. Guizhi Fuling Wan, a Traditional Chinese Herbal Formula, Sensitizes Cisplatin-Resistant Human Ovarian Cancer Cells through Inactivation of the PI3K/AKT/mTOR Pathway. Evid.-Based Complement. Altern. Med. 2016;2016:1–11. doi: 10.1155/2016/4651949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lu Y., Jiang F., Jiang H., Wu K., Zheng X., Cai Y., Katakowski M., Chopp M., To S.-S.T. Gallic acid suppresses cell viability, proliferation, invasion and angiogenesis in human glioma cells. Eur. J. Pharmacol. 2010;641:102–107. doi: 10.1016/j.ejphar.2010.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Park S.-R., Kim S.-R., Hong I.-S., Lee H.-Y. A Novel Therapeutic Approach for Colorectal Cancer Stem Cells: Blocking the PI3K/Akt Signaling Axis with Caffeic Acid. Front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.585987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lu X., Yang F., Chen D., Zhao Q., Chen D., Ping H., Xing N. Quercetin reverses docetaxel resistance in prostate cancer via androgen receptor and PI3K/Akt signaling pathways. Int. J. Biol. Sci. 2020;16:1121–1134. doi: 10.7150/ijbs.41686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Li W., Cheng M., Zhang W., He R., Yang H. New Insights into the Mechanisms of Polyphenol from Plum Fruit Inducing Apoptosis in Human Lung Cancer A549 Cells via PI3K/AKT/FOXO1 Pathway. Mater. Veg. 2021;76:125–132. doi: 10.1007/s11130-021-00882-y. [DOI] [PubMed] [Google Scholar]
- 136.Chen Z., Lin T., Liao X., Li Z., Lin R., Qi X., Chen G., Sun L., Lin L. Network pharmacology based research into the effect and mechanism of Yinchenhao Decoction against Cholangiocarcinoma. Chin. Med. 2021;16:13. doi: 10.1186/s13020-021-00423-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cai F., Zhang Y., Li J., Huang S., Gao R. Isorhamnetin inhibited the proliferation and metastasis of androgen-independent prostate cancer cells by targeting the mitochondrion-dependent intrinsic apoptotic and PI3K/Akt/mTOR pathway. Biosci. Rep. 2020;40 doi: 10.1042/BSR20192826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fan Z., Wirth A.-K., Chen D., Wruck C.J., Rauh M., Buchfelder M., Savaskan N. Nrf2-Keap1 pathway promotes cell proliferation and diminishes ferroptosis. Oncogenesis. 2017;6:e371. doi: 10.1038/oncsis.2017.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kansanen E., Kuosmanen S.M., Leinonen H., Levonen A.-L. The Keap1-Nrf2 pathway: Mechanisms of activation and dysregulation in cancer. Redox Biol. 2013;1:45–49. doi: 10.1016/j.redox.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sirota R., Gibson D., Kohen R. The role of the catecholic and the electrophilic moieties of caffeic acid in Nrf2/Keap1 pathway activation in ovarian carcinoma cell lines. Redox Biol. 2015;4:48–59. doi: 10.1016/j.redox.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.El-Akad R.H., Zeid A.H.A., El-Rafie H.M., Kandil Z.A., Farag M.A. Comparative metabolites profiling of Caryota mitis & Caryota urens via UPLC/MS and isolation of two novel in silico chemopreventive flavonoids. J. Food Biochem. 2021;45:e13648. doi: 10.1111/jfbc.13648. [DOI] [PubMed] [Google Scholar]
- 142.Tanigawa S., Fujii M., Hou D.-X. Action of Nrf2 and Keap1 in ARE-mediated NQO1 expression by quercetin. Free. Radic. Biol. Med. 2007;42:1690–1703. doi: 10.1016/j.freeradbiomed.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 143.Lee Y.-J., Bernstock J., Nagaraja N., Ko B., Hallenbeck J.M. Global SUMOylation facilitates the multimodal neuroprotection afforded by quercetin against the deleterious effects of oxygen/glucose deprivation and the restoration of oxygen/glucose. J. Neurochem. 2016;138:101–116. doi: 10.1111/jnc.13643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ng C.X., Affendi M.M., Chong P.P., Lee S.H. The Potential of Plant-Derived Extracts and Compounds to Augment Anticancer Effects of Chemotherapeutic Drugs. Nutr. Cancer. 2022;74:3058–3076. doi: 10.1080/01635581.2022.2069274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.