Abstract
Opsonophagocytosis is the primary mechanism for clearance of pneumococci from the host, and the measurement of opsonophagocytic antibodies appears to correlate with vaccine-induced protection. We developed a semiautomated flow cytometric opsonophagocytosis assay using HL-60 granulocytes as effector cells and nonviable 5,6-carboxyfluorescein, succinimidyl ester-labeled Streptococcus pneumoniae (serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F) as bacterial targets. The flow cytometric opsonophagocytosis assay was highly reproducible (for 87% of repetitive assays the titers were within 1 dilution of the median titer) and serotype specific, with ≥97% inhibition of opsonophagocytic titer by addition of homologous serotype-specific polysaccharide. In general, opsonophagocytic titers were not significantly inhibited by the presence of either heterologous pneumococcal polysaccharide or penicillin in the serum. The flow cytometric assay could reproducibly measure functional antibody activity in prevaccination (n = 28) and postvaccination (n = 36) serum specimens from healthy adult volunteers vaccinated with the 23-valent pneumococcal polysaccharide vaccine. When compared with a standardized manual viable opsonophagocytic assay, a high correlation (r = 0.89; P ≤ 0.01) was found between the two assays for the seven serotypes tested. The flow cytometric assay is rapid (∼4 h) with high throughput (∼50 serum samples per day per technician) and provides a reproducible measurement of serotype-specific functional antibodies, making it a highly suitable assay for the evaluation of the immune responses elicited by pneumococcal vaccines.
Serologic correlates of protection for Streptococcus pneumoniae (pneumococcal) vaccine evaluation are not well established (6). Immune responses to pneumococcal vaccines have been evaluated by using assays that measure total binding antibodies, such as radioimmunoassays or enzyme-linked immunosorbent assays (ELISAs) (15, 19, 23). Other measurements of host immune responses to pneumococcal vaccines have been considered, most notably, opsonophagocytic assays, which measure functional antibody activity (20, 26). Opsonophagocytic assays are more attractive than other measures of in vitro protective immunity because they more closely resemble the mechanism of natural immunity, do not require the use of animal models, and appear to provide a closer correlation with serotype-specific vaccine efficacy than ELISAs (27).
Opsonophagocytic assays have traditionally used polymorphonuclear cells (PMNs) as effector cells in a variety of radioisotopic, flow cytometric, microscopic, and bacterial viability assays (4, 5, 8, 10, 14, 17, 25, 26, 28). A standardized viable opsonophagocytic assay with culturable granulocytes (differentiated HL-60 cells) has been described for the measurement of functional opsonophagocytic antibodies against S. pneumoniae (20). Standardization of assay components is essential for comparison of results between laboratories. Most of these reported assays require considerable technical expertise, the use of cumbersome, labor-intensive steps such as isolation of phagocytes from whole blood, the use of radioisotopes or differential centrifugation, and quantitation by microscopic counting of bacteria or colony-forming units.
Pneumococcal conjugate vaccines will eventually be licensed after favorable results from phase III efficacy trials. After licensure, new conjugate vaccines will most likely be licensed primarily on the basis of immunogenicity. In anticipation of the need for large-scale immunogenicity testing, we developed and standardized a simple, rapid, and semiautomated flow cytometric opsonophagocytic assay that minimized handling of viable bacteria, used culturable effector cells, demonstrated high reproducibility, was insensitive to penicillin in the serum, and was easily adapted for automation. We tested seven serotypes found in the 23-valent polysaccharide vaccine, although the assay is adaptable to other serotypes as well. The flow cytometric opsonophagocytic assay can be used for large immunogenicity studies, as part of the evaluation of existing or new pneumococcal vaccines, or for the study of immune responses with a high degree of reproducibility.
MATERIALS AND METHODS
Serum samples.
All serum samples (28 prevaccination and 36 postvaccination serum samples) were collected after informed consent was obtained from healthy adult volunteers, 16 serum samples were collected through the Emory University Donor Services (Atlanta, Ga.), and 24 paired serum samples previously used in a multilaboratory ELISA validation study (18) were collected through the National Blood Service (Oxford Centre, Oxford, England). Postvaccination serum was collected 4 to 6 weeks after immunization with the 23-valent pneumococcal polysaccharide vaccine (Lederle Laboratories [Praxis-American Cyanamid Co.], Pearl River, N.Y.). All serum samples were stored at −70°C and were heated to 56°C for 30 min just prior to testing to inactivate endogenous complement activity.
Bacterial growth and labeling.
All strains of S. pneumoniae were recent clinical isolates used in the standardized viable opsonophagocytic assay reported previously (20) and were stored at −70°C. Briefly, the bacteria were incubated overnight on blood agar plates (Life Technologies, Grand Island, N.Y.) at 37°C in 5% CO2. The isolated colonies were then inoculated into Todd-Hewitt broth with 0.5% yeast extract and were incubated without shaking for 3 to 4 h at 37°C in 5% CO2. The bacteria were harvested by centrifugation at 800 × g for 10 min at room temperature and were resuspended in 5 ml of bicarbonate buffer (0.1 M NaHCO3 [pH 8.0]). Fifty microliters of 5,6-carboxyfluorescein, succinimidyl ester (FAM-SE; Molecular Probes, Eugene, Oreg.), solution (10 mg/ml in dimethyl sulfoxide [Fisher Scientific Co., Fair Lawn, N.J.]) was added, and the mixture was incubated for 1 h without shaking at 37°C in 5% CO2 (2). Finally, 1 ml of 2% paraformaldehyde (Sigma Chemical Co., St. Louis, Mo.) was added, and fixation was allowed to proceed overnight at 37°C without shaking. To confirm that the labeled bacteria were nonviable, 0.1 ml of bacterial suspension was cultured on a blood agar plate and the plate was incubated overnight as before. The labeled bacteria were washed by centrifugation six times in 20 ml of opsonophagocytosis buffer (Hanks balanced salt solution with Ca2+ and Mg2+ [Life Technologies], 0.2% bovine serum albumin [Sigma], and 1× penicillin-streptomycin [Life Technologies]) until no free dye was observed in the supernatant. FAM-SE-labeled bacteria were counted with the BacCount kit (Molecular Probes). FAM-SE-labeled bacteria were stored at 4°C under protection from light and were stable for a minimum of 3 months. Bacterial concentrations were adjusted to 4 × 105 bacteria in 20 μl prior to use. The presence of a capsule was verified by the Quellung (16) reaction before and after FAM-SE labeling, and no significant differences were observed.
Cell line growth and differentiation.
HL-60 (human promyelocytic leukemia cells; CCL240; American Type Culture Collection, Rockville, Md.) were grown to a cell density of 4 × 105 to 6 × 105 cells/ml in 80% RPMI 1640 medium that contained 1% l-glutamine but no phenol red (Life Technologies) and that was supplemented with 20% heat-inactivated fetal bovine serum (HyClone Laboratories, Logan, Utah) and 1× penicillin-streptomycin. These cells were differentiated into granulocytes by culturing in the same medium supplemented with 100 mM N,N-dimethylformamide (99.8% purity; Fisher Scientific) for a period of 5 days (20). The flow cytometric opsonophagocytosis assay required differentiated HL-60 granulocytes with high degrees of viability (≥90%, as judged by 0.4% trypan blue exclusion staining). Such a high degree of cell viability was obtained by daily feeding or division of the undifferentiated HL-60 cell line stock. Adequate phagocytosis was observed in differentiated HL-60 cells through at least 230 passages.
Differentiated HL-60 cells were harvested by centrifugation at 160 × g for 10 min and were washed twice in 15 ml of wash buffer containing Hanks balanced salt solution without Ca2+ and Mg2+, 0.2% bovine serum albumin, and 1× penicillin-streptomycin. The cells were then washed once in opsonophagocytosis buffer and were resuspended in 4 ml of opsonophagocytosis buffer and counted in a hemocytometer. The cell concentration was adjusted to 105 cells per 40-μl volume, resulting in an effector cell/target cell ratio of 1:4.
Flow cytometric opsonophagocytic assay.
Eight twofold serum dilutions were made in opsonophagocytosis buffer from 10 μl of test serum. A 20-μl aliquot of bacterial suspension containing 4 × 105 bacteria was added to each well, and the plate was incubated for 30 min at 37°C in room air with horizontal shaking (200 rpm). Then, 10 μl of 3- to 4-week-old, sterile baby rabbit serum (Pel-Frez, Brown Deer, Wis.) was added to each well with the exception of the HL-60 cell control wells as a source of complement; HL-60 cell control wells received 10 μl of opsonophagocytosis buffer. After incubation at 37°C in room air for 15 min with shaking, 40 μl of washed, differentiated HL-60 cells (105 cells) was added to each well and the plates were incubated with shaking at 37°C in air for 15 min. The final well volume was 80 μl. An additional 80 μl of opsonophagocytosis buffer was added to each well to provide sufficient volume for flow cytometric analysis, and the well contents were resuspended and transferred to titer tubes (Bio-Rad Laboratories, Richmond, Calif.). The titer tubes were placed inside polystyrene disposable tubes (12 by 75 mm; Fisher) for flow cytometric analysis. The samples were stored in the dark and on ice until they were analyzed. Samples were typically analyzed within 3 to 4 h without affecting the results and were held for as long as 6 h without affecting the observed titer. The tubes were vortexed for 3 s before sampling in the flow cytometer.
Three controls were included per assay for each serotype assayed: (i) an HL-60 cell control containing only cells and bacteria, (ii) three complement controls containing all test reagents except antibody source, and (iii) a positive quality control serum sample, which was a postvaccination serum sample with a known opsonophagocytic titer. The positive quality control serum sample was included on every microtiter plate. Manual opsonophagocytic assays were performed as described previously (20). Using the manual assay, we did not observe any difference between human and baby rabbit serum as a complement source. Since we were attempting to develop a flow opsonophagocytic assay using readily available reagents, we used only the rabbit complement for the development and standardization of the flow cytometric assay.
Flow cytometric analysis.
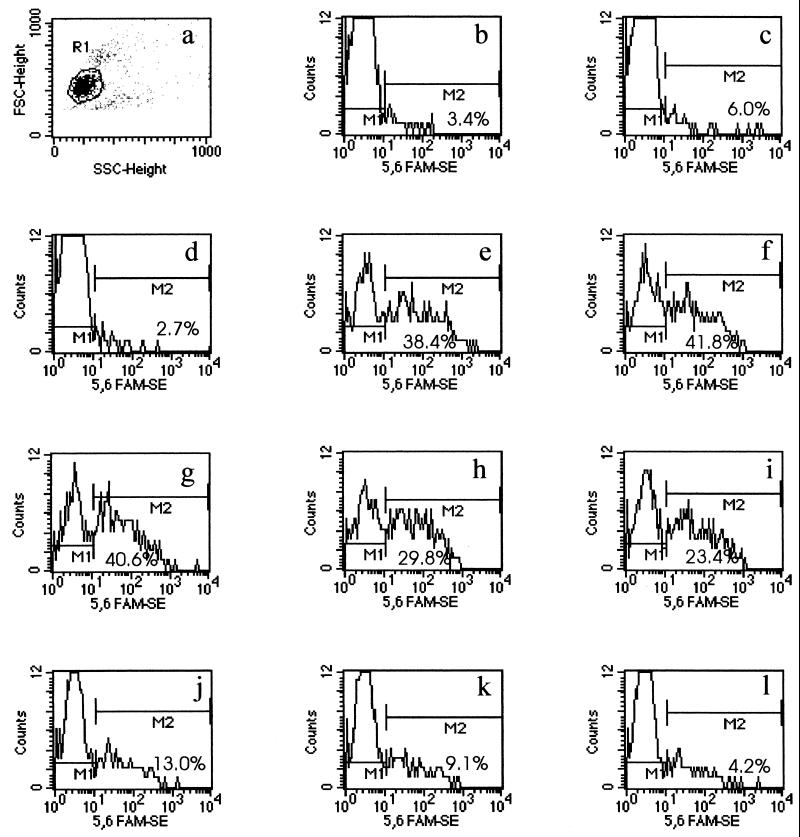
Samples were assayed with a FACSCalibur immunocytometry system (Becton Dickinson and Co., Paramus, N.J.) and were analyzed with CELLQuest software (version 1.2 for Apple system 7.1; Becton Dickinson). A minimum of 3,000 gated HL-60 granulocytes were analyzed per tube. FAM-SE was excited at a wavelength of 488 nm, and the FAM-SE fluorescence signals of gated viable HL-60 cells were measured at 530 nm. The upper limit of the background fluorescence was measured in the HL-60 cell controls and consisted of autofluorescence of HL-60 cells, nonspecific adherence of bacteria, and bacterial clumps. A marker region (M1) was superimposed above the cell control fluorescence peak to include 98% of the population. A second marker region (M2) was used to determine the percentage of differentiated HL-60 cells with fluorescence greater than that of M1 for each serum dilution. The cells in this region had phagocytosed S. pneumoniae. Titers were reported as the reciprocal of the highest serum dilution yielding ≥50% of the maximum phagocytic uptake. Samples with a maximum phagocytic uptake of <20% were considered negative and were reported to have a titer of 4.
Competitive inhibitions.
A panel of prevaccination serum samples (n = 5), postvaccination serum samples (n = 5), and Sandoglobulin, a pooled immunoglobulin G (IgG) antibody preparation (Sandoz Pharmaceuticals Co., East Hanover, N.J.) at a concentration of 6%, was tested for opsonophagocytic antibodies to seven pneumococcal serotypes (serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F) after preabsorption for 30 min at room temperature with equal volumes of either homologous or heterologous polysaccharide (American Type Culture Collection) diluted to a final concentration of 0.5 mg/ml. Competitive inhibition with homologous polysaccharide was performed only with the postvaccination sera. The samples were competitively inhibited and were tested in triplicate. The results were analyzed by the Wilcoxon rank sum test for statistical differences.
Statistical analysis.
Pearson’s product moment correlation coefficient for normally distributed data was determined and Wilcoxon rank sum tests for nonparametric data were performed with the SigmaStat software program, version 2.0 (Jandel Scientific, San Rafael, Calif.). Significance levels were set at P values of <0.05. Differences between paired data were determined by paired t test. The geometric 95% confidence interval (G95% CI) was estimated as the geometric mean titer (GMT) ± (geometric standard error × 1.96).
RESULTS
Specificity of flow cytometric opsonophagocytosic assay.
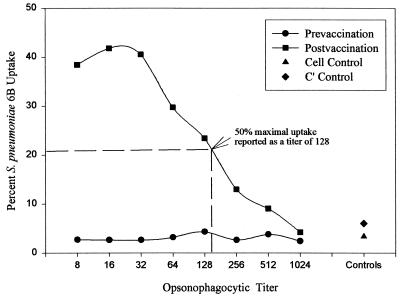
In the flow cytometric opsonophagocytosic assay, FAM-SE-labeled pneumococci were opsonized by type-specific anticapsular antibodies in an antibody concentration- and complement-dependent manner. We measured functional antibody activity against seven pneumococcal serotypes (serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F) in pre- and postvaccination serum samples. Measurement of functional antibody activity was demonstrated by increased fluorescence of HL-60 PMNs containing phagocytosed FAM-SE-labeled pneumococci (Fig. 1). The opsonophagocytosis, i.e., fluorescence, was dependent upon the amount of functional antibody present in each sample and behaved in an antibody concentration-dependent manner, as illustrated in Fig. 1e to l. The percentage of HL-60 PMNs containing phagocytosed pneumococci decreased as the amount of functional antibody was decreased by dilution. The percentage of HL-60 PMNs containing fluorescent pneumococci could then be plotted for each sample’s dilution series to determine an opsonophagocytic titer for each sample. The opsonophagocytic titer is defined as the reciprocal of the dilution with 50% of the maximal percent uptake for each sample. Figure 2 shows the differences in the percent uptake between a pre- and a postvaccination serum sample. Figure 2 also shows the opsonophagocytic titer for the postvaccination serum sample. Competitive inhibitions with homologous polysaccharide and with a panel of quality control serum samples resulted in ≥97% inhibition for all seven serotypes tested (Table 1).
FIG. 1.
Flow cytometric analysis of serum with functional antibody activity by differentiated HL-60 cells (granulocytes). (a) Scattergram of the forward light scatter (FSC) versus the side light scatter (SSC). Gated cells (dark gray) represent the viable singlet population of differentiated HL-60 cells. (b) Histogram representation of the gated HL-60 cells with various degrees of fluorescence caused by uptake of FAM-SE-labeled pneumococci in the HL-60 cell control. M1 was adjusted to encompass 98% of the gated HL-60 cells; M2 defines the fluorescent gated HL-60 cell population. The percentage of cells in M2 is shown. (c) Histogram representation of uptake in the complement control. (d) Histogram representation of a prevaccination serum sample diluted 1:8 (serotype 6B). Similar profiles were obtained at higher dilutions of the prevaccination serum samples shown. (e to l) Fluorescence profiles of HL-60 granulocytes (n = 3,000) in the presence of a postvaccination serum sample diluted 1/8, 1/16, 1/32, 1/64, 1/128, 1/256, 1/512, and 1/1,024, respectively.
FIG. 2.
Dilution curve of the functional opsonophagocytic activity in a postvaccination serum sample (percent uptake of FAM-SE-labeled pneumococci serotype 6B by differentiated HL-60 cells, as shown in Fig. 1e through l). The opsonophagocytic titer was the reciprocal of the dilution with ≥50% maximum uptake observed in each serum sample, in this case, a titer of 128 (arrow). A dilution curve of the opsonophagocytic activity in the corresponding prevaccination serum sample (Fig. 1d) is shown for comparison. The titer for this nonimmune serum was <8. C′, complement.
TABLE 1.
Competitive inhibition of opsonophagocytic activity with type-specific polysaccharide
| Serotypea | Opsonophagocytic
activityb
|
Avg % inhibitionc | ||
|---|---|---|---|---|
| Median titer | Titer range | Median titer postabsorption | ||
| 4 | 2048 | 512–2,048 | 4 | 99.7 |
| 6B | 128 | 64–512 | 4 | 97.0 |
| 9V | 512 | 256–2,048 | 4 | 99.3 |
| 14 | 512 | 256–512 | 4 | 98.9 |
| 18C | 256 | 128–512 | 4 | 98.9 |
| 19F | 128 | 64–256 | 4 | 96.9 |
| 23F | 128 | 64–512 | 4 | 96.9 |
Serotype of S. pneumoniae.
All titers in the presence of 0.5 mg of homologous polysaccharides per ml were <1:8 and were reported as a titer of 4 for analysis purposes.
Percent inhibition of opsonophagocytic activity after addition of type-specific polysaccharide.
The maximum percent uptake of FAM-SE-labeled pneumococci by differentiated HL-60 cells in postvaccination serum was similar for all serotypes tested, with a mean ± 1 standard deviation uptake for all serotypes of 40% ± 10.6%. The maximum percent uptake was serum dependent. The range of percent uptake observed in the complement controls was also similar for each serotype tested, with a mean of 9% ± 1.2%. Similar percent uptakes were observed for PMNs isolated from different donors. For example, in a representative experiment, maximum uptakes (reported titer) for serotype 14 were 49.6% (titer, 256) and 48.7% (titer, 512) for PMNs isolated from whole blood and HL-60 PMNs, respectively. Measurement of ingested bacteria as opposed to adherent bacteria was confirmed by trypan blue quenching of the fluorescent FAM-SE signal. No appreciable reduction in the signal was observed in the fluorescent FAM-SE signal with the addition of 0.4% trypan blue (9). We examined different HL-60:bacterium ratios, from 4:1 to 1:100 HL-60 cells/bacteria. HL-60/bacterium ratios between 1:2 and 1:10 resulted in maximal percent phagocytosis in postvaccination serum, with minimal increases (≤10% uptake) in phagocytosis in the complement-containing control (data not shown).
Cross-reactivity of antibodies to heterologous polysaccharides.
The serotype specificity of the flow cytometric opsonophagocytic assay for type 4, 6B, 9V, 14, 18C, 19F, or 23F was evaluated in five postvaccination serum samples by preincubation with heterologous polysaccharide in a checkerboard design. Of 42 combinations of heterologous preabsorption, only 1 produced a significant reduction in mean titer compared to that for the unabsorbed serum sample. Preabsorption of serum with a type 9V polysaccharide produced a mean titer inhibition of 17.4% in the assay for serotype 4 (P < 0.001).
By contrast, when a pooled antibody preparation, Sandoglobulin, was cross-absorbed with heterologous polysaccharide, a significant reduction in flow cytometric opsonophagocytic titers was observed by the Wilcoxon rank sum test for antibodies against serotypes 4 (24% decrease; P = 0.02), 9V (58% decrease; P = 0.03), and 14 (22% decrease; P = 0.02). The reductions in heterologous polysaccharide-absorbed Sandoglobulin titers were not significant for serotypes 6B (22% decrease; P = 0.08), 18C (38% decrease; P = 0.06), and 23F (25% decrease; P = 0.06).
Reproducibility of the flow cytometric opsonophagocytic assay.
The reproducibility of the flow opsonophagocytic assay was assessed in 68 replicates (all serotypes included) of a single quality control serum; 65% gave the median titer, the titers for 87% of the assays were within ±1 dilution of the median titer, and the titers for 97% of the assays were within ±2 dilutions of the median titer. The G95% CIs for a panel of quality control serum samples (n = 4) were determined by testing each serum sample three to six times against each serotype. The GMTs and G95% CIs for each serotype were as follows: serotype 4, 675 and 406 to 1,063; serotype 6B, 260 and 169 to 388; serotype 9V, 2,474 and 1,783 to 3,326; serotype 14, 664 and 388 to 1,176; serotype 18C, 546 and 338 to 891; serotype 19F, 276, 194 to 388; and serotype 23F, 659 and 416 to 1,024. These G95% CIs represent less than 1 dilution from the GMT for all serotypes tested.
Comparison between flow cytometric and manual viable opsonophagocytic assays.
Overall, the results of the flow cytometric assay correlated well (r = 0.89 and P ≤ 0.001) with those of the manual viable assay for all seven serotypes tested. The correlations per serotype are given in Table 2. The GMTs obtained by the flow cytometric assay with postvaccination serum samples were higher for serotypes 9V, 14, and 18C and lower for serotype 4 than those obtained by the manual viable assay. These differences were not significant for serotype 4 (P = 0.117), serotype 14 (P = 0.05), or serotype 18C (P = 0.114) by paired t test. A significant difference was only found for serotype 9V (P < 0.001). Prevaccination GMTs were very similar by both methods. Fifty-two percent of the serum samples tested against all serotypes by the flow cytometric assay gave the same titer as the manual viable assay, 75.9% gave titers within ±1 dilution of those of the manual viable assay, 87.2% gave titers within ±2 dilutions of those of the manual viable assay, and 94.6% gave titers within ±3 dilutions of those of the manual viable assay. In general, the flow cytometric assay tended to give the same opsonophagocytic titers or titers 1 dilution higher than those achieved by the manual viable assay (Table 3). For all serotypes the flow cytometric opsonophagocytic assay values were normally distributed around the median value for the manual opsonophagocytic assay. The flow cytometric assay allowed a higher number of serum samples to be analyzed daily (∼50 serum samples per 8 h), as opposed to the ∼25 serum samples that could be analyzed in 18 to 24 h by the manual viable opsonophagocytic assay. The flow cytometric assay was unaffected by the presence of penicillin (0, 100, or 1,000 U/ml) in the assay buffers since no significant differences in opsonophagocytic titer were observed in a panel of six serum samples tested for antibodies to serotypes 6B (P = 0.49) and 18C (P = 0.57).
TABLE 2.
Correlation between the flow cytometric and manual viable opsonophagocytic assays for pre- and postvaccination serum
| S. pneumoniae serotype | GMTa
|
Correlationb
|
|||||
|---|---|---|---|---|---|---|---|
| Flow cytometric assay
|
Manual viable
opsonophagocytic assay
|
r value | P value | Slope | |||
| Pre | Post | Pre | Post | ||||
| 4 | 5 | 157 | 5 | 117 | 0.90 | <0.001 | 0.83 |
| 6B | 12 | 176 | 11 | 98 | 0.85 | <0.001 | 0.80 |
| 9V | 5 | 665 | 6 | 256 | 0.88 | <0.001 | 0.70 |
| 14 | 24 | 562 | 24 | 352 | 0.87 | <0.001 | 0.77 |
| 18C | 6 | 83 | 7 | 63 | 0.89 | <0.001 | 0.84 |
| 19F | 7 | 56 | 7 | 53 | 0.95 | <0.001 | 1.01 |
| 23F | 5 | 20 | 5 | 31 | 0.91 | <0.001 | 0.94 |
Pre and Post, pre- and postvaccination serum samples, respectively.
The Pearson’s product moment correlation coefficient was used for the linear regression analysis between the two methods. The overall correlation between the two assays for all serotypes combined was as follows: r = 0.89, P < 0.001, and slope = 0.81. Twenty-four paired serum samples were tested to determine the correlation between the two assays.
TABLE 3.
Cumulative percentage of serum samples for which the titers by the flow cytometric opsonophagocytic assay and the manual opsonophagocytic assay were in agreementa
| Dilution well difference from median manual assay titer | Cumulative % serum samples by S.
pneumoniae serotype
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 4 | 6B | 9V | 14 | 18C | 19F | 23F | All | |
| 0 | 59.4 | 45.3 | 43.8 | 39.1 | 50 | 65.6 | 60.9 | 52 |
| ±1 | 75 | 70.3 | 59.4 | 76.6 | 78.2 | 96.8 | 75 | 75.9 |
| ±2 | 87.5 | 84.3 | 75.0 | 89.1 | 87.5 | 98.4 | 89.1 | 87.2 |
| ±3 | 98.5 | 92.1 | 87.5 | 95.4 | 95.3 | 98.4 | 95.4 | 94.6 |
A total of 48 serum samples were analyzed.
DISCUSSION
Laboratory correlates of protection that can be used for pneumococcal vaccine development, evaluation, and licensure are needed. Unlike other vaccines with established laboratory correlates of protection, such as the Haemophilus influenzae type b vaccine (7, 11), no standardized laboratory method is used to determine levels of opsonic antibodies which can be used as a correlate of protection for evaluation of pneumococcal vaccines. In a previous report (27), we documented that the measurement of functional antibody activity by opsonophagocytosis appeared to correlate with vaccine point estimates of efficacy and that this correlation was higher than the correlation observed with antibody concentrations measured by ELISA (19). The correlation of opsonophagocytosis and the IgG concentration measured by ELISA varies according to serotype. For example, opsonophagocytosis of serotype 14 has been found to correlate better with the titer obtained by ELISA (r = 0.8 to 0.9) than opsonophagocytosis of serotype 19F does (r = 0.4) (20). A minimum antibody level has not yet been defined for protection against pneumococcal disease. However, it is becoming more evident that measurement of functional antibody activity (opsonophagocytosis or passive protection in animal models) is a better indicator of the immunogenicity in various vaccinated populations than measurement of total binding antibody concentrations (21, 24). In these studies, we have observed a number of serum samples with ELISA-determined IgG antibody concentrations of >2 μg/ml and reduced functional antibody activity (opsonic titers, ≤64).
We describe a flow cytometric opsonophagocytic assay with differentiated HL-60 cells (granulocytes) in an effort to develop a standardized assay that can reproducibly measure the functional antibody activities of large numbers of serum samples. The flow cytometric opsonophagocytosis assay offers a rapid and reproducible alternative to the current manual viable opsonophagocytic assay and has a number of additional advantages. The flow cytometric assay correlated very well with the previously described viable assay (20), and we believe that they have similar sensitivities. This was previously published in Figure 1 of reference 20, in which the opsonophagocytic titer (50% killing) was obtained at ∼0.06 μg/ml. Although the materials and reagents were similar to those used in the manual viable assay, nonviable FAM-SE-labeled pneumococci (target:effector cell ratio, 4:1) were used as targets, whereas viable bacteria (1:400 ratio) were used as targets in the manual viable opsonophagocytic assay. The semiautomation of the flow assay facilitated collection and analysis of larger number of samples in a shorter period of time (∼4 h).
Uptakes of labeled pneumococci were similar when either cultured, differentiated HL-60 granulocytes or isolated donor PMNs from multiple donors were used (data not shown). Although the HL-60 cells have been shown by Jansen et al. (12) to express only the FcγRIIa-R131 allotype, which has a lower affinity for IgG2, these cells are still capable of phagocytosing opsonized pneumococci in the presence of complement. Thus, the C3b receptor appears to play a more important role in opsonophagocytosis in this assay. The assay was optimized to yield maximal uptakes in the presence of specific opsonizing antibodies and to minimize nonspecific uptake in the controls containing only complement. This was done by adjusting the ratio of effector cells to bacterial cell targets to 1:4. This ratio optimized the fluorescent signal observed when HL-60 cells phagocytosed the FAM-SE-labeled bacteria. An increase in the number of bacteria per effector cell resulted in increased bacterial uptakes (they approached 100%); however, there was a concomitant increase in uptake in the control containing only complement but no significant effect on the control containing only cells and bacteria (cell control).
Since the flow cytometric assay used fixed, FAM-SE-labeled pneumococci, the assay was unaffected by the presence of up to 1,000 U of penicillin in the serum sample and, most likely, would be unaffected by other commonly used antibiotics. This is of great importance, since a number of potential study populations may include individuals who have been treated with antibiotics before sample collection, e.g., persons with underlying chronic conditions requiring prophylactic antibiotic therapy. The presence of antibiotics in the serum samples from these patients precludes their use in the standard viability opsonophagocytic assay.
We had previously shown that pneumococcal type-specific functional opsonophagocytic activity could be competitively inhibited by the presence of homologous capsular polysaccharide (20); however, we did not address the possible participation of cross-reactive antibodies in the opsonophagocytic reaction. In this study, we have demonstrated no appreciable difference in the opsonophagocytic titers obtained by the addition of heterologous polysaccharide in pre- and postvaccination serum samples by either the flow cytometric or the manual viable opsonophagocytic assay except when absorption was with the type 9V polysaccharide and testing was performed against serotype 4 bacteria. This group of sera demonstrated significant inhibition. We believe that this represents the presence of cross-reactive antibodies against serotype 9V in one sample in this group. Contrary to opsonophagocytosis, this type of cross-reactivity has been observed in prevaccination serum samples when the consensus ELISA protocol (3) has been used. This ELISA measures total binding of antibodies with various avidities, whereas opsonophagocytosis measures total binding of antibodies with higher aviditities (1, 21). Differences were primarily observed by the flow cytometric assay in a pooled IgG preparation (Sandoglobulin) and were likely due to the large number of nonimmunized donors whose sera were used to generate the preparation and the resulting wide range of antibody specificities and avidities within the preparation. Jansen et al. (13) reported that the growth phase of the pneumococci and the fixation procedure can lead to increased phagocytosis by anti-cell wall polysaccharide antibodies. We used paraformaldehyde-fixed pneumococci grown to the mid-logarithmic phase in which the effect of anti-cell wall polysaccharide antibodies is minimal (data not shown). In addition, we have recently reported that the opsonophagocytic titers of certain serotypes (serotypes 6B and 9V) vary, depending on the amount of capsular polysaccharide present on the target strain (22). The GMTs to serotype 9V for postvaccination serum samples obtained by the flow cytometric assay were higher than those obtained by the manual assay. Several possibilities which may explain these results exist. We observed that serotype 9V polysaccharide absorption decreased the level of antibody activity directed against serotype 4. This suggests that the serotype 9V polysaccharide may have epitopes which may cross-react with antibodies against serotype 4 and perhaps other serotypes to a lesser extent, and this could lead to higher titers for anti-9V antibodies. A more likely explanation for the observed difference in titer is the presence of transparent CFU reported by Romero-Steiner et al. (22). We have observed that the serotype 9V strain used in this study contained approximately equal numbers of opaque and transparent CFU. All other strains used in this study primarily contained the opaque type. The transparent-type organism is more readily phagocytosed. Since uptake rather than killing is being measured in the flow cytometric assay, the transparent form would more likely be phagocytosed, leading to higher titers. Additional studies will be necessary to fully elicidate the cause(s) of this observation.
The flow cytometric opsonophagocytic assay that we describe offers a number of advantages over the manual viable assay. The flow cytometric assay correlates with a standardized viable bacteria assay (20), which appears to correlate with protection (27). This pneumococcal opsonophagocytic assay can be used for the evaluation of new pneumococcal vaccines and pneumococcal vaccines that are being developed since it offers a more rapid and reproducible estimate of the opsonophagocytic activity in pre- and postpneumococcal vaccination human serum samples than the recently reported manual viable assay.
ACKNOWLEDGMENTS
We thank Richard R. Facklam for providing the S. pneumoniae strains used in this study. We also thank Anthony Scott for valuable discussions and critical reading of the manuscript and Brian Plikaytis for assistance with statistical analysis.
REFERENCES
- 1.Anttila M, Eskola J, Åhman H, Käyhty H. Avidity of IgG for Streptococcus pneumoniae type 6B and 23F polysaccharides in infants primed with pneumococcal conjugates and boosted with polysaccharide or conjugate vaccines. J Infect Dis. 1998;177:1614–1621. doi: 10.1086/515298. [DOI] [PubMed] [Google Scholar]
- 2.Brinkley M. A brief survey of methods for preparing protein conjugates with dyes, haptens, and cross-linking reagents. Bioconjugate Chem. 1992;3:2. doi: 10.1021/bc00013a001. [DOI] [PubMed] [Google Scholar]
- 3.Coughlin R T, White A C, Anderson C A, Carlone G M, Klein D L, Treanor J. Characterization of pneumococcal specific antibodies in healthy unvaccinated adults. Vaccine. 1998;16:1761–1767. doi: 10.1016/s0264-410x(98)00139-x. [DOI] [PubMed] [Google Scholar]
- 4.De Velasco A E, Verheul A F M, Van Steijn A M P, Dekker H A T, Feldman R G, Fernandez I M, Karmerling J P, Vliegenthart J F G, Verhoef J, Snippe H. Epitope specificity of rabbit immunoglobulin G (IgG) elicited by pneumococcal type 23F synthetic oligosaccharide and native polysaccharide-protein conjugate vaccines: comparison with human anti-polysaccharide 23F IgG. Infect Immun. 1994;62:799–808. doi: 10.1128/iai.62.3.799-808.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esposito A L, Clark C A, Poirier W J. An assessment of the factors contributing to the killing of type 3 Streptococcus pneumoniae by human polymorphonuclear leukocytes in vitro. APMIS. 1990;98:111–121. [PubMed] [Google Scholar]
- 6.Fedson D S, Musher D M. Pneumococcal vaccine. In: Plotkin S A, Mortimer E A Jr, editors. Vaccines. 2nd ed. Philadelphia, Pa: The W. B. Saunders Co.; 1994. pp. 517–564. [Google Scholar]
- 7.Fothergill L, Wright J. Influenzal meningitis: relation of age incidence to the bactericidal power of blood against the causal organism. J Immunol. 1993;24:273–284. [Google Scholar]
- 8.Guckian J C, Christensen G D, Fine D P. Role of opsonins in recovery from experimental pneumococcal pneumonia. J Infect Dis. 1980;142:175–190. doi: 10.1093/infdis/142.2.175. [DOI] [PubMed] [Google Scholar]
- 9.Hed J, Hallden G, Johansson S G O, Larson P. The use of fluorescence quenching in flow cytofluorometry to measure the attachment and ingestion phases in phagocytosis in peripheral blood without prior cell separation. J Immunol Methods. 1987;101:119–125. doi: 10.1016/0022-1759(87)90224-9. [DOI] [PubMed] [Google Scholar]
- 10.Kaniuk A, Lortan J E, Monteil M A. Specific IgG subclass antibody levels and phagocytosis of serotype 14 pneumococcus following immunization. Scand J Immunol. 1992;36(Suppl. 11):96–98. doi: 10.1111/j.1365-3083.1992.tb01628.x. [DOI] [PubMed] [Google Scholar]
- 11.Käyhty H, Peltola H, Kankako V, Mäkelä P H. The protective level of serum antibodies to the capsular polysaccharide of Haemophilus influenzae type b. J Infect Dis. 1983;147:1100. doi: 10.1093/infdis/147.6.1100. [DOI] [PubMed] [Google Scholar]
- 12.Jansen W, Breukels M, Sanders L, Van Kessel D, Van Houte A J, Horikx P, Van Velzen H, Snipe H, Verheul A, Rijkers G. Program and abstracts of the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1998. The allotype of FcγRIIa receptor determines the extent of phagocytosis of Streptococcus pneumoniae by human polymorphonuclear leukocytes: implications for the evaluation of pneumococcal vaccines, abstr. G-63; p. 302. [Google Scholar]
- 13.Jansen W T M, Gootjes J, Zelle M, Madore D V, Verhoef J, Snippe H, Verheul A F M. Use of highly encapsulated Streptococcus pneumoniae strains in a flow cytometric assay for assessment of the phagocytic capacity of serotype-specific antibodies. Clin Diagn Lab Immunol. 1998;5:703–710. doi: 10.1128/cdli.5.5.703-710.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lortan J E, Kaniuk A S, Monteil M A. Relationship of in vitro phagocytosis of serotype 14 Streptococcus pneumoniae to specific class and IgG subclass antibody levels in healthy adults. Clin Exp Immunol. 1993;91:54–57. doi: 10.1111/j.1365-2249.1993.tb03353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nahm M H, Siber G R, Olander J V. A modified Farr assay is more specific than the ELISA for measuring antibodies to Streptococcus pneumoniae capsular polysaccharides. J Infect Dis. 1996;173:113–118. doi: 10.1093/infdis/173.1.113. [DOI] [PubMed] [Google Scholar]
- 16.Neufeld F. Uber die agglutina der Pneumokokken und uber die Theorien der Agglutination. Z Hyg Infecktionskr. 1902;40:54–72. [Google Scholar]
- 17.Obaro S K, Henderson D C, Monteil M A. Defective antibody-mediated opsonisation of S. pneumoniae in high risk patients detected by flow cytometry. Immunol Lett. 1996;49:83–89. doi: 10.1016/0165-2478(95)02487-5. [DOI] [PubMed] [Google Scholar]
- 18.Plikaytis B, Carlone G, Goldblatt D Participating Laboratories. Program and abstracts of the First International Symposium on Pneumococci and Pneumococcal Diseases. 1998. Analytical methods applied to a multi-center pneumococcal ELISA study, abstr. P52; p. 71. [Google Scholar]
- 19.Quataert S A, Kirch C S, Quackenbush Wiedl L J, Phipps D C, Strohmeyer S, Cimino C O, Skuse J, Madore D V. Assignment of weight-based antibody units to a human antipneumococcal standard reference serum, lot 89-S. Clin Diagn Lab Immunol. 1995;2:590–597. doi: 10.1128/cdli.2.5.590-597.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romero-Steiner S, Libutti D, Pais L B, Dykes J, Anderson P, Whitin J C, Keyserling H L, Carlone G M. Standardization of an opsonophagocytic assay for the measurement of functional antibody activity against Streptococcus pneumoniae using differentiated HL-60 cells. Clin Diagn Lab Immunol. 1997;4:415–422. doi: 10.1128/cdli.4.4.415-422.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romero-Steiner S, Pais L B, Groover L, Musher D M, Fiore A, Cetron M, Plikaytis B D, Carlone G M. Abstracts of the 97th General Meeting of the American Society for Microbiology 1997. Washington, D.C: American Society for Microbiology; 1997. Evaluation of functional antibody responses in elderly and middle aged vaccinees to the 23-valent pneumococcal polysaccharide vaccine, abstr. E-64; p. 251. [Google Scholar]
- 22.Romero-Steiner S, Carvalho M, Barnard S, Kim J O, Weiser J, Carlone G M. Program and abstracts of the 38th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1998. Decreased opsonophagocytosis activity in the pneumococcal opaque phenotype is associated with higher capsule polysaccharide content, abstr. G-110; p. 314. [Google Scholar]
- 23.Schiffman G, Douglas R M, Bonner M J, Robins M, Austrian R. A radioimmunoassay for immunologic phenomena in pneumococcal disease and for the antibody response to pneumococcal vaccines. I. Method for the radioimmunoassay of anticapsular antibodies and comparison with other techniques. J Immunol Methods. 1980;33:133–144. doi: 10.1016/s0022-1759(80)80004-4. [DOI] [PubMed] [Google Scholar]
- 24.Shatz D, Schinsky M F, Pais L B, Romero-Steiner S, Kirton O C, Carlone G M. Immune responses of splenectomized trauma patients to the 23-valent pneumococcal polysaccharide vaccine at 1 vs 7 vs 14 days postsplenectomy. J Trauma. 1998;44:760–766. doi: 10.1097/00005373-199805000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Sveum R J, Chused T M, Frank M M, Brown E J. A quantitative fluorescent method for measurement of bacterial adherence and phagocytosis. J Immunol Methods. 1986;90:257–264. doi: 10.1016/0022-1759(86)90083-9. [DOI] [PubMed] [Google Scholar]
- 26.Vióarsson G, Jönsdóttir I, Jonsson S, Valdimarsson H. Opsonization and antibodies to capsular and cell wall polysaccharides of Streptococcus pneumoniae. J Infect Dis. 1994;170:592–599. doi: 10.1093/infdis/170.3.592. [DOI] [PubMed] [Google Scholar]
- 27.Wenger J D, Steiner S R, Pais L B, Butler J C, Perkins B, Carlone G M, Broome C V. Program and abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Laboratory correlates for protective efficacy of pneumococcal vaccines: how can they be identified and validated?, abstr. G37; p. 150. [Google Scholar]
- 28.Wilkelstein J A, Smith M R, Shin H S. The role of C3 as an opsonin in the early stages of infection. Proc Soc Exp Biol Med. 1975;149:397–401. doi: 10.3181/00379727-149-38815. [DOI] [PubMed] [Google Scholar]