Abstract
Pregnancy can exert suppressive effects on chronic inflammatory conditions. We have previously demonstrated a depression in polymorphonuclear leukocyte (PMN) respiratory burst during pregnancy which could explain this amelioration. To elucidate the biochemical mechanism, we have examined PMN phospholipase A2 (PLA2) activity and its relationship to cellular and circulating fatty acids in pregnant women (30 to 34 weeks) and nonpregnant controls. PMN PLA2 activity was determined by arachidonic acid (AA) and leukotriene B4 (LTB4) release, respiratory burst activity was determined by lucigenin-enhanced chemiluminescence, and total serum and PMN fatty acid levels were determined by gas-liquid chromatography. AA release was significantly reduced for pregnancy PMNs in response to N-formyl-met-leu-phe (fMLP) under unprimed and tumor necrosis factor alpha (TNF-α)- or interleukin 8-primed conditions. Similarly, LTB4 liberation was significantly reduced in response to fMLP and phorbol myristate acetate in unprimed and TNF-α-primed pregnancy PMNs. All major fatty acid classes were altered in the pregnant state. Of these differences in PMNs, oleic acid and α-linolenic acid showed a significant increase (13 and 26%, respectively) and stearic acid and AA showed a significant decrease (8 and 30%, respectively). The stearic acid, oleic acid, and AA compositions of all cells analyzed correlated with their corresponding changes in serum fatty acid levels. Crossover serum incubations modified both fatty acid profiles and the PMN respiratory burst accordingly, while individual fatty acid incorporation studies highlighted the importance of polyunsaturated fatty acids for NADPH oxidase efficiency. These findings indicate that the attenuation of PMN function in pregnancy may originate from a reduction in the available pool of cellular fatty acids. Furthermore, this reduction arises as a direct result of a pregnancy-induced shift in circulating fatty acids from polyunsaturated to monounsaturated forms.
For more than a century, clinical observations have highlighted the ameliorating effect of pregnancy on certain inflammatory disorders. Since the first detailed account of this phenomenon published by Hench in 1938 (21), the beneficial effect of pregnancy on rheumatoid arthritis (RA) has been continually reaffirmed. Most authors agree that the activity of RA is significantly altered during pregnancy, with approximately 70% of patients experiencing a substantial resolution of pain, swelling, and stiffness (for a review, see reference 39). This symptomatic relief becomes apparent from the first trimester and then progresses throughout gestation, often enabling patients to reduce or completely interrupt the use of medication (13). Unfortunately, this remission is short-lived; more than 90% of the improved patients will relapse within 8 to 9 months postpartum, and the majority will relapse within 6 weeks of delivery (31).
A steadily increasing number of theories have been proposed to explain this dramatic gestational improvement in RA. The majority of these proposals invoke one or more mechanisms of pregnancy-induced immunosuppression. Although humoral immunity during pregnancy remains unchanged (7), a marked depression in cell-mediated immunity is suggested by a diminished skin reaction to tuberculin (17), by prolonged skin graft survival (1), and by an increased susceptibility to specific intracellular infections (27, 36). Polymorphonuclear leukocytes (PMN) are thought to be of central importance in the eradication of invasive pathogens and in the tissue damage associated with connective-tissue disorders. Previous studies of functional differences during pregnancy have emphasized a reduction in PMN chemotaxis (24), adherence (5), and microbial killing (15). Pregnancy sera have been shown to suppress bacterial killing (34) and to diminish PMN phagocytosis (33) and enzyme release (20). Recently, we have reported a depression in PMN function in pregnancy characterized by a reduction in receptor-mediated respiratory burst activity (11). A significant decline in NADPH oxidase activity, as measured by superoxide anion release, was observed for pregnancy PMN in response to formyl-peptide and to zymosan-activated serum. A longitudinal study showed that this effect begins in the first trimester and gradually progresses to term before returning to preconception and control levels within 6 weeks of delivery (11).
According to the current knowledge of PMN regulation, a conceivable and relevant mechanism explaining these variations in oxidative metabolism could be differences in cell membrane composition. Modulating agents which can affect lipid composition, order, and mobility, such as cholesterol, cholesterol esters, and certain saturated and unsaturated fatty acids, have been shown to modify the neutrophil superoxide anion response (10, 16, 25, 35). In addition, certain unsaturated fatty acids, most notably arachidonic acid (AA), can have a direct effect on the assembly and activation of the NADPH oxidase system (12). The mobilization and liberation of free AA from membrane phospholipids by phospholipase A2 (PLA2) is one of the earliest events in PMN activation. Once liberated, AA can be further metabolized by 5-lipoxygenase or by cyclooxygenase to yield the inflammatory metabolites leukotriene B4 (LTB4) and prostaglandin E2. To investigate the relationships among PMN activity, cell lipid composition, and the oxidative metabolic response in pregnancy, we have examined the PLA2 activity of PMN from pregnant and nonpregnant subjects. PLA2was measured by the release of AA and the formation of LTB4 following either direct stimulation or priming, an induced condition of increased readiness which can enhance cell activation upon stimulation. In addition, in response to the suggestion that the levels of plasma free fatty acids are considerably altered during pregnancy (37), we have examined their relative amounts in the sera and isolated PMN of patients from both study groups and have confirmed a relationship between PMN fatty acid composition and cell function. Finally, by incubating normal PMN in pregnancy serum, we have produced changes in both cellular fatty acids and cell responses equivalent to those observed for pregnancy PMN.
MATERIALS AND METHODS
Reagents.
Ficoll-Hypaque was purchased from Flow Laboratories, Hertfordshire, United Kingdom. Gentran 70 (6% dextran 70 in 0.9% NaCl) was supplied by Baxter Healthcare Ltd., Norfolk, United Kingdom. EDTA (analytical grade) and 2,6-di-ter-p-cresol were obtained from BDH, Dorset, United Kingdom. [3H]AA was purchased from Amersham Plc, Buckingham, United Kingdom. Tumor necrosis factor alpha (TNF-α), interleukin 1β (IL-1β), and IL-8 were obtained from Genzyme Corporation, Kent, United Kingdom. All other reagents used were supplied by Sigma Chemical Company Ltd., Poole, United Kingdom.
Study subjects.
Venous blood was obtained from healthy pregnant women during the third trimester of pregnancy (30 to 34 weeks of gestation) and placed into EDTA-dipotassium at a final concentration of 3 mM. Subjects with any preexisting medical disorders or taking any medication apart from vitamin or iron supplementation were excluded from the study. Blood was simultaneously obtained from healthy nonpregnant women of comparable age and who were not taking a contraceptive pill or any other form of medication. The age range of the pregnancy group was 18 to 37 years, with a mean of 28 years; that of the control group was 19 to 39 years, with a mean of 27 years. There were no demographic differences between the two groups. Informed consent was obtained from all subjects before inclusion in the study, which was approved by the local ethical committee.
Preparation of human PMN.
Human peripheral blood PMN were prepared by standard methods (6). Erythrocytes were sedimented on dextran, and the leukocyte-rich plasma was further purified by centrifugation over Histopaque 1077. The contaminating erythrocytes were lysed with 0.2% (wt/vol) NaCl, and the osmolality was restored with an equal volume of 1.6% (wt/vol) NaCl. Once isolated, cells were washed twice in phosphate-buffered saline (pH 7.2) (PBS), and their viability was assessed by trypan blue dye exclusion. PMN were regularly obtained with a purity greater than 97% and a viability greater than 99%. Cells were resuspended in PBS and used immediately.
Cytokine priming.
Prior to stimulation, PMN were preincubated with priming agents for the following times and concentrations: TNF-α 250 pg/ml, 30 min; IL-1β, 1 ng/ml, 60 min; IL-8, 20 ng/ml, 30 min; and cytochalasin B, 5 μg/ml, 30 min. When necessary, initial dilutions were made with dimethyl sulfoxide. All priming agents were further diluted in PBS containing 1 mM CaCl2, 0.7 mM MgCl2, and 0.1% (wt/vol) endotoxin-free bovine serum albumin (BSA) (PBS/Ca/Mg/BSA). Final concentrations of dimethyl sulfoxide did not exceed 0.01%, and solvent controls were included for all experiments. Preincubations were conducted at 37°C with end-over-end rotation.
Respiratory burst activity.
Extracellular PMN superoxide anion production was measured by lucigenin-enhanced chemiluminescence with a Labsystems (Basingstoke, United Kingdom) Luminoskan plate-reading luminometer. Briefly, 140 μl of PBS/Ca/Mg/BSA, 20 μl of 250 μM lucigenin (bis-N-methylacridinium nitrate), and 20 μl of PMN suspension (107/ml) were added to triplicate wells of a 96-well Immunofluor microtiter plate (Dynatech, Billinghurst, United Kingdom). The plate was warmed in the luminometer to 37°C before the addition of 10 μM N-formyl-met-leu-phe (fMLP). Chemiluminescence light output was monitored every 60 s for 30 min, and the integral over this period was expressed as relative light units.
Measurement of PLA2 activity.
[3H]AA release from PMN was determined by a modification of a previous method (9). PMN at 107/ml were incubated with 1 μCi of [3H]AA per ml at 37°C for 1 h in PBS containing 0.1% (wt/vol) fatty-acid-free BSA. Cells were washed three times and resuspended in PBS containing 1 mM CaCl2, 0.7 mM MgCl2, and 0.1% (wt/vol) fatty acid-free BSA at a concentration of 3 × 107/ml. Cell suspensions were primed as outlined above, and 100-μl aliquots were stimulated with 1 μM fMLP at 37°C. The reactions were stopped by the addition of 0.5 ml of ice-cold 0.9% (wt/vol) NaCl at time zero, immediately after stimulation, and at 20 min. PMN were sedimented by centrifugation, and the [3H]AA release in the supernatant and pellet was determined by liquid scintillation counting.
Determination of LTB4 generation.
The generation of LTB4 was determined with a commercially available enzyme-linked immunosorbent assay (R&D Systems, Oxon, United Kingdom). The reaction mixture, containing PBS/Ca/Mg/BSA (250 μl) and primed or unprimed PMN (107/ml), was preincubated at 37°C for 10 min before the addition of the stimulus. The final concentration of the stimulus was 1 μM fMLP or 10 ng of phorbol myristate acetate (PMA) per ml. The reaction was terminated by the addition of 13 μl of ice-cold citric acid (0.035 M) to reduce the pH to 5.5. PMN were sedimented by centrifugation, and the supernatants were removed and stored at −80°C before being assayed.
Fatty acid composition determination.
Analysis of fatty acids was performed with serum samples (200 μl) and aliquots of PMN preparations (3 × 107 cells) by extraction of total lipids with methanol-benzene (4:1 [vol/vol]) plus 0.5 mM 2,6-di-ter-p-cresol as an antioxidant. Fatty acids were methylated by a direct transesterification technique (26), and the resulting fatty acid methyl esters were separated by a gas-liquid chromatographic method (40) with a Cpsil 88 50-m capillary column (Chrompak; Millharbour, London, United Kingdom) on a 5890 Series II gas chromatograph (Hewlett-Packard, Amsterdam, The Netherlands). Peak identifications were made with commercially available reference fatty acids, and heptanoic acid (C17:0) was used as an internal standard. Fatty acid composition data were expressed as relative molar percentages of fatty acid methyl esters based on peak areas.
Fatty acid incorporation.
Individual fatty acids were complexed with fatty-acid-free BSA in 1:1 molar ratios according to the method of Mahoney et al. (28). The resulting fatty acid-BSA solutions were added to isolated PMN (107/ml) in PBS to give a final fatty acid concentration of 33 μM. Cells were incubated for 5 h at 37°C with continuous end-over-end rotation, washed three times with PBS, and then resuspended in PBS to their original concentration.
Statistical analysis.
Statistical significance of differences was determined by use of the nonparametric Mann-Whitney U test for independent samples and the Wilcoxon rank sum test for paired samples. The relationship between different parameters was investigated with the nonparametric Spearman’s rho correlation coefficient test. The results are presented as the means ± the standard errors of the means (SEM); data were considered significant at a P value of <0.05 (two tailed).
RESULTS
PMN PLA2 activity.
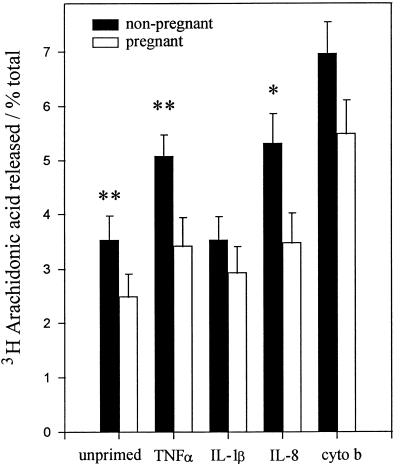
PLA2 catalyzes the hydrolysis of the ester bond between a fatty acid and the hydroxyl group at the sn-2 position of the glycerol backbone of a phospholipid to generate a lysophospholipid and a fatty acid (usually AA). By labelling the phospholipid pool with [3H]AA and measuring its subsequent stimulated release into the extracellular medium (against total incorporation), we were able to determine the general activity of cellular PLA2. Figure 1 shows the contrast between the release of AA from [3H]AA-loaded pregnancy PMN and that from nonpregnancy PMN. In response to fMLP, PLA2 activation was significantly reduced in pregnancy PMN compared to nonpregnancy PMN. Similarly, cells primed with either TNF-α or IL-8 prior to activation showed a marked reduction in responses. Both cytochalasin B, the most potent priming agent used, and IL-1β, the least effective, gave the same alterations in pregnancy PMN activation, although these changes did not appear to reach significance over the experimental time period.
FIG. 1.
Comparison of AA liberation from PMN of women in their third trimester of pregnancy (n = 6) and nonpregnant age-matched women (n = 6). The results represent the extracellular release of [3H]AA from radioisotope-loaded, fMLP-stimulated PMN under primed (TNF-α, IL-1β, IL-8, and cytochalasin B [cyto b]) and unprimed conditions. The data are expressed as mean ± SEM and are significantly different (P values) at the following levels: ∗, <0.05; ∗∗, <0.02. Significance was determined with the Mann-Whitney U test.
LTB4 production.
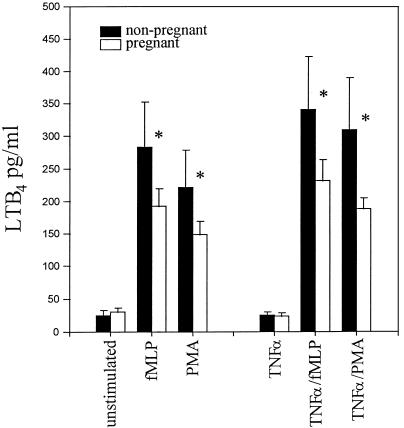
Once AA has been liberated from membrane phospholipids, it can be further metabolized by 5-lipoxygenase to yield LTB4. LTB4 is recognized as a potent inflammatory mediator and is capable of priming PMN (38) and further activating the NADPH oxidase system (14). Figure 2 illustrates LTB4 production over a 15-min period from fMLP-stimulated, TNF-α-primed and unprimed cells. No differences were evident in resting-cell responses between the two study groups. However, under both primed and unprimed conditions, pregnancy PMN did show a marked reduction in LTB4 production following stimulation.
FIG. 2.
Comparison of LTB4 release by PMN of pregnant women (30 to 34 weeks of gestation) and nonpregnant women following fMLP and PMA activation under TNF-α-primed and unprimed conditions. The data are expressed as mean ± SEM and are significantly different at a P value of <0.05 (Mann-Whitney U test) (asterisks).
Serum and PMN fatty acids.
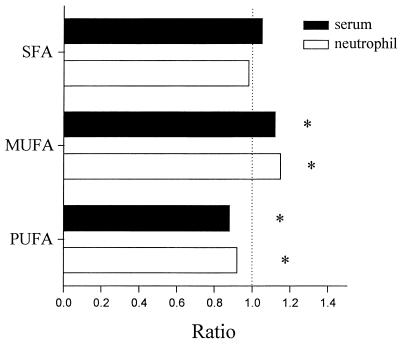
The fatty acid compositions of sera and PMN from pregnant and nonpregnant subjects were determined by gas-liquid chromatography. The mean fatty acid compositions of PMN from both study groups, expressed as relative molar percentages, are given in Table 1. Almost all major fatty acid classes, including saturated, monounsaturated, and polyunsaturated species, were altered in pregnancy. Of these differences in PMN, oleic acid and α-linolenic acid showed significant increases of 13 and 26%, respectively, while stearic acid and AA showed significant decreases of 8 and 30%, respectively. Serum variations in total fatty acids included significant increases for palmitic acid (13%) and oleic acid (11%) and significant decreases for stearic acid (27%), linoleic acid (9.9%), and AA (18%) in pregnancy. Interestingly, the stearic acid, oleic acid, and AA compositions of all the PMN analyzed correlated with corresponding changes in the serum levels of these fatty acids. Figure 3 shows the ratios of unsaturated fatty acids, monounsaturated fatty acids (MUFA), and polyunsaturated fatty acids (PUFA) for the study groups. In pregnancy, a more general pattern of fatty acid abnormalities emerges. For serum and PMN, an increase in PUFA (11.4 and 11.2%, respectively) and a decrease in MUFA (10.5 and 12.7%, respectively) would indicate an overall shift in fatty acid species in pregnancy.
TABLE 1.
Total fatty acid content of serum and PMN for pregnant women (30 to 34 weeks of gestation) and nonpregnant women
| Fatty acid (C) | Common name | Relative molar % fatty acid content (mean ±
SEM)a in:
|
PMN/serum Spearman’s correlation probability (n = 17) | |||
|---|---|---|---|---|---|---|
| PMN
|
Serum
|
|||||
| Nonpregnant women (n = 10) | Pregnant women (n = 10) | Nonpregnant women (n = 12) | Pregnant women (n = 12) | |||
| 22:4 (cis-7,10,13,16) | 8.76 ± 0.47 | 8.81 ± 0.37 | ||||
| 16:0 | Palmitic | 20.63 ± 0.57 | 21.08 ± 0.53 | 26.78 ± 0.74 | 30.75 ± 0.99b | 0.070 |
| 18:0 | Stearic | 18.37 ± 0.24 | 16.95 ± 0.16c | 8.53 ± 0.23 | 6.21 ± 0.20b | 0.004 |
| 18:1 (cis-9) | Oleic | 26.29 ± 0.59 | 30.10 ± 0.38c | 24.68 ± 0.61 | 27.56 ± 0.62b | 0.001 |
| 18:2 (cis-9,12) | Linoleic | 9.52 ± 0.43 | 10.73 ± 0.42 | 32.65 ± 0.91 | 29.41 ± 0.81c | 0.199 |
| 18:3 (cis-9,12,15) | α-Linolenic | 1.14 ± 0.12 | 1.55 ± 0.11b | |||
| 20:4 | Arachidonic | 14.06 ± 0.46 | 9.88 ± 0.23b | 7.38 ± 0.37 | 6.05 ± 0.70c | 0.050 |
| 22:6 (cis-4,7,10,13,16,19) | Docasahexaenoic | 1.28 ± 0.15 | 0.92 ± 0.07 | |||
Based on peak areas. Only data for the major fatty acids are included.
P, <0.01 (Mann-Whitney U test).
P, <0.05 (Mann-Whitney U test).
FIG. 3.
Ratios of mean fatty acids in PMN and serum for pregnant women (30 to 34 weeks of gestation) and nonpregnant women. SFA, saturated fatty acids. The degree of statistical significance is indicated by asterisks (P, <0.05) (Mann-Whitney U test).
Respiratory burst activity.
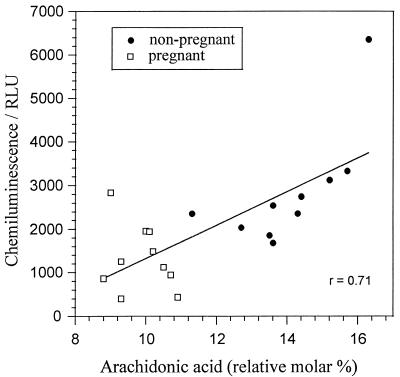
Lucigenin is a cell-impermeable probe that amplifies photoemissions from oxygenation events. It is considered to be highly selective for the extracellular generation of superoxide anions and thus provides a convenient measure of PMN NADPH oxidase activity (19). In response to fMLP, PMN isolated from pregnant subjects generated fewer superoxide anions than those from age-matched controls (2,825 ± 384 versus 1,379 ± 225; P, <0.01, Mann-Whitney U test). Taken together, the cellular concentrations of AA from both groups correlated positively with the NADPH oxidase activity (Fig. 4). In nonpregnancy cells, a more intimate relationship was recorded. This observation may suggest the influence of other cellular fatty acids or even additional regulatory factors in pregnancy, where AA concentrations are limited.
FIG. 4.
Linear regression plot of AA content and fMLP-stimulated superoxide anion release for PMN of pregnant women (30 to 34 weeks of gestation) and nonpregnant age-matched women. The relationship between parameters was significant at a P value of <0.05 (r, Spearman’s rho correlation coefficient). RLU, relative light units.
Fatty acid incorporation.
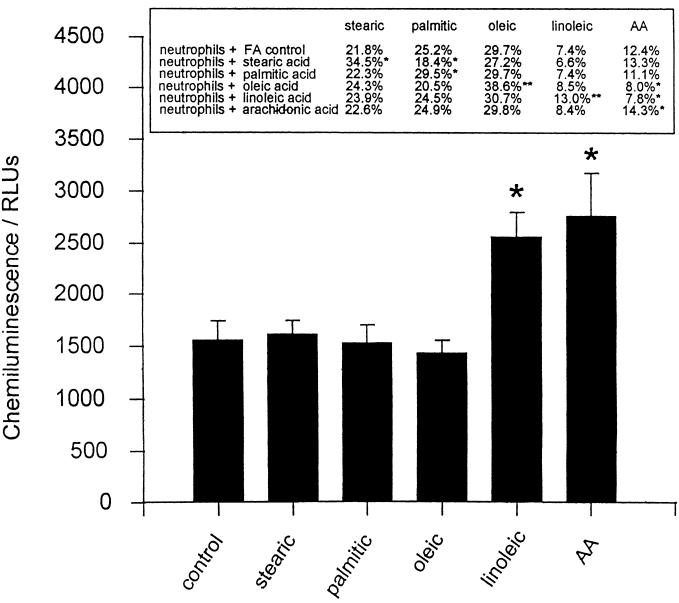
Individual fatty acids, incorporated as complexes with BSA, were successfully taken up by isolated PMN over a 5-h incubation period. This procedure produced a range of cells enriched in saturated fatty acids, MUFA, and PUFA (Fig. 5). PMN with enhanced saturated fatty acids and MUFA levels showed no difference in their respiratory burst response to fMLP; however, cells with increased PUFA levels all showed exaggerated responses (Fig. 5). Adhesion molecule markers of PMN, including CD18, CD11b, and CD62L, indicated that prior activation or priming of the cells did not occur as a result of the incubation procedure (data not shown). Incubations were limited to 5 h, as longer times were associated with a loss of cell viability and responsiveness.
FIG. 5.
Incubation of PMN in environments enriched for saturated fatty acids (FA), MUFA, and PUFA. Isolated PMN were incubated for 5 h at 37°C in a 1:1 molar ratio of individual fatty acids complexed with BSA. The final concentrations of fatty acids was 33 μM, and control samples were incubated in BSA alone. Cellular fatty acid profiles and chemiluminescence responses to fMLP were recorded for the washed cells. The data are expressed as mean ± SEM for six different PMN donors. The results were significantly different (P values) at the following levels: ∗, <0.05; ∗∗, <0.01. Significance was determined with the Wilcoxon rank sum test.
Serum incubations.
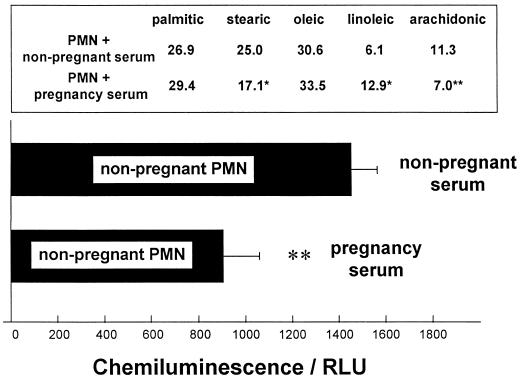
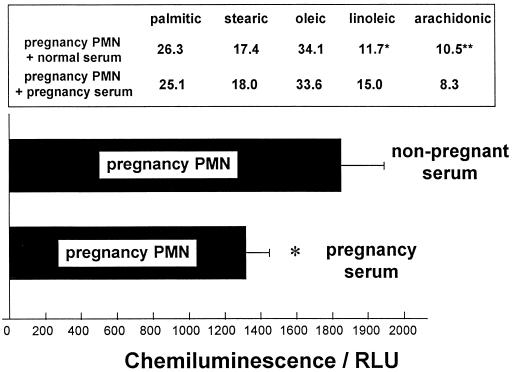
To further investigate the relationship between both cellular and circulating fatty acid levels and PMN NADPH oxidase activity, PMN isolated from pregnant or nonpregnant subjects were incubated (5 h, 37°C) in either heat-inactivated (56°C, 30 min) autologous serum or heat-inactivated pooled heterologous serum. Figure 6 demonstrates how incubation with 50% (vol/vol) pregnancy serum modifies the cellular fatty acid profile of normal nonpregnancy PMN to one comparable to that of pregnancy PMN. Similarly, the fMLP-stimulated NADPH oxidase activity of these cells is reduced to a level comparable to that of pregnancy PMN. Figure 7 shows the reverse experiment. Incubation with 50% (vol/vol) nonpregnancy serum appears to redress the changes to the cellular fatty acids of pregnancy PMN as well as to increase the total respiratory burst output.
FIG. 6.
Serum incubation study for nonpregnancy PMN. The effect of pregnancy serum (50% [vol/vol]) on the fatty acid content and fMLP-stimulated superoxide anion release of normal nonpregnancy PMN was examined. Isolated peripheral blood PMN from nonpregnant subjects were incubated (5 h, 37°C) in either heat-inactivated (56°C, 30 min) autologous serum or heat-inactivated pooled pregnancy sera (n = 6). The data are expressed as mean ± SEM for eight different PMN donors. The results were significantly different (P values) at the following levels: ∗, <0.05; ∗∗, <0.01. Significance was determined with the Wilcoxon rank sum test. RLU, relative light units.
FIG. 7.
Serum incubation study for pregnancy PMN. The effect of nonpregnancy serum (50% [vol/vol]) on the fatty acid content and fMLP-stimulated superoxide anion release of pregnancy PMN was examined. Isolated peripheral blood PMN from pregnant subjects were incubated (5 h, 37°C) in either heat-inactivated (56°C, 30 min) autologous serum or heat-inactivated pooled nonpregnancy sera (n = 6). The data are expressed as mean ± SEM for seven different PMN donors. The results were significantly different (P values) at the following levels: ∗, <0.05; ∗∗, <0.03. Significance was determined with the Wilcoxon rank sum test. RLU, relative light units.
DISCUSSION
PMN play an important role in host defense against infectious agents; paradoxically, however, their “unrestricted” activation has also been implicated in the pathology of a variety of inflammatory conditions, such as RA. Activated PMN generate superoxide anions, an initial step leading to the formation of a variety of reactive oxygen species that have both microbicidal and proinflammatory properties. The formation of reactive oxygen species requires the correct assembly of a membrane-associated, multicomponent NADPH oxidase system. We have previously investigated the activity of this enzyme complex in PMN isolated from pregnant subjects and have shown a reduction in its activity compared to that in nonpregnant controls; furthermore, we have shown that this reduction parallels the symptomatic relief seen in pregnant RA patients (11). To further investigate the biochemical mechanisms underlying this observation, we have examined the effect of pregnancy upon the activation of PLA2 in human PMN.
PLA2 catalyzes the hydrolysis of the ester bond between a fatty acid and the hydroxyl group at the sn-2 position of the glycerol backbone of a phospholipid. Although other pathways have been suggested to cause the release of AA from intact cells, PLA2 is still considered to be the dominant, rate-limiting step in the formation of AA from membrane phospholipids. In human PMN, exogenously added AA has been shown to induce Ca2+ influx (23), degranulation (2), leukotriene synthesis (29), and NADPH oxidase activation (12). AA formed as a result of PLA2 activation also serves as the substrate for both lipoxygenase and cyclooxygenase enzymes, whose action leads to the generation of a group of proinflammatory lipids termed the eicosanoids. In PMN, the main products of this “AA cascade” are LTB4, the 5-lipoxygenase product of AA, and platelet-activating factor (PAF), resulting from the liberation of AA from phosphotidylcholine. Both PAF and LTB4 can act in an autocrine manner to activate or prime the PMN NADPH oxidase system (14).
We have demonstrated a reduction in the activity of PLA2 in PMN isolated from pregnant subjects. This attenuated response is seen following direct stimulation of cells with fMLP and in PMN that have first been primed with TNF-α or IL-8. To support the concept of reduced AA liberation by these cells, we have also measured their ability to generate LTB4 and have found that the production of this eicosanoid in pregnant subjects is likewise reduced. These two observations, which coincided with a previously reported reduction in PMN PAF production (3), may partially explain the parallel reduction in NADPH oxidase activity in pregnancy cells. However, whether this decrease in oxidase activity results from a reduction in LTB4 generation or more directly from an attenuation of AA release is not known.
Several lines of investigation have suggested that changes in the phospholipid and fatty acid compositions of PMN plasma membranes can modulate the function of NADPH oxidase (10, 25). With this in mind, we have examined the relative amounts of major fatty acids in the sera and PMN of both nonpregnant and pregnant subjects. The variations in the amounts of all forms of fatty acids between the groups would suggest that profound changes in cellular phospholipid metabolism occur during pregnancy. Of the observed differences, the most important with respect to NADPH oxidase activity appears to be a decrease in the levels of PUFA, more specifically, AA (4). Flesch and Ferber (18) have shown that the incorporation of PUFA into the phospholipids of macrophages is accompanied by an increase in PLA2 activity and superoxide anion generation. Conversely, it can be envisaged that a reduction in PUFA would reduce NADPH oxidase activity in PMN. Indeed, the PMN in this study showed a strong relationship between modified PUFA levels and NADPH oxidase activity both through individual fatty acid incorporations and through crossover serum incubations.
Although there is little doubt as to the importance of PUFA in both the activation and the maintenance of NADPH oxidase in human PMN, the possibility that changes in the fatty acid content may have more far-reaching effects upon PMN function cannot be disregarded. It has recently been established that compounds that readily affect membrane fluidity in PMN also affect oxidase activity (16, 25). Furthermore, since the fluidity of membranes is known to be influenced by the degree of saturation or unsaturation and/or hydrocarbon chain length of fatty acids, it seems reasonable to assume that the pregnancy-induced fatty acid changes that we have reported will alter PMN membrane fluidity. The fluidity of the plasma membrane may affect oxidase activity in a number of ways: (i) by the manner in which the multicomponent NADPH oxidase is arranged within the lipid bilayer or (ii) by the extent of expression and affinity of membrane-bound receptors. This latter explanation seems unlikely, since LTB4 generation was reduced in PMN from pregnant subjects not only in response to fMLP but also in response to PMA, an agent that bypasses receptor-mediated signalling events by directly activating protein kinase C.
The results of this study indicate a strong relationship between serum fatty acid levels and cellular fatty acid content. To put these observations into perspective and to establish a rationale for changes in pregnancy, a closer examination of the biosynthetic pathways of fatty acids is necessary. In human PMN, a deficiency exists in the stepwise elongation of short-chain fatty acids. In vivo and in vitro studies suggest that although elongase activity in PMN is normal, the cells specifically lack the Δ5-desaturase enzyme necessary for long-chain PUFA biosynthesis (8). Given this information, serum levels of fatty acids may have a direct influence on cellular fatty acids which lie upstream of those involved in this reaction. The findings of this study, showing an association between serum and cellular AA, would support this proposal, while further changes in pregnancy would cause an exaggeration of this effect. For AA, a deficiency in Δ5-desaturase would promote the cellular accumulation of its precursor, linoleic acid. This suggestion directly corresponds with our observations for cellular fatty acid content in pregnancy and accounts for the discrepancy between PMN increases and serum decreases in linoleic acid.
The correlation between the relative amounts of the individual essential fatty acids (EFA) in maternal and umbilical plasma phospholipids highlights a dependence of the growing fetus on maternal sources of EFA (22). This relationship is particularly true with respect to AA and docasahexanoic acid, the major structural and functional fatty acids involved in the development of the human brain and vascular and central nervous systems. In this study we have demonstrated a decline in serum maternal EFA (linoleic acid, AA) levels in the latter stages of pregnancy. Others have shown a progressive loss of these particular fatty acids throughout pregnancy, with a more pronounced reduction in the third trimester, when fetal brain development is maximal (37). This pattern of increased burden on the essential PUFA from maternal sources would fit with our progressive reduction in PMN activity in pregnancy and would also parallel the progressive improvement in the symptoms of pregnant RA patients.
Extensive studies in the late 1970s and 1980s have strongly suggested the presence in the circulation of pregnant women of an immunomodulating factor(s) which is capable of modifying PMN function. Pregnancy PMN have been shown to exhibit reduced chemotaxis (24), microbial killing (15), and adherence to nylon wool (5), while pregnancy serum has been demonstrated to suppress PMN phagocytosis and killing of Staphylococcus aureus (34) and Escherichia coli (33). A number of factors produced by both the placenta and maternal tissues, including cortisol (32), progesterone, and pregnancy-associated α2-glycoprotein (30), have been suggested to explain these effects. However, to date no single factor can satisfactorily explain the pattern of improvement and relapse in inflammatory diseases during pregnancy. We believe that while efforts in the past have concentrated on the identification of a particular factor during pregnancy, one important mechanism of cell modulation has been neglected. This study has fostered the idea that an alteration in the fatty acid metabolism of PMN and perhaps other cells of the immune system allows pregnancy-induced changes in the circulating levels of fatty acids to have a direct bearing on their inflammatory responsiveness.
REFERENCES
- 1.Andresen R H, Monroe G W. Experimental study of the behavior of adult human skin homografts during pregnancy. Am J Obstet Gynecol. 1962;84:1096–1103. doi: 10.1016/0002-9378(62)90560-4. [DOI] [PubMed] [Google Scholar]
- 2.Barnette M S, Rush J, Marshall L A, Foley J J, Schmidt D B, Sarau H M. Effects of Scalaradial, a novel inhibitor of 14KDa phospholipase A2 on human neutrophil function. Biochem Pharmacol. 1994;47:1661–1668. doi: 10.1016/0006-2952(94)90545-2. [DOI] [PubMed] [Google Scholar]
- 3.Beilin L J, Croft K D, Michael C A, Ritchie J, Schmidt L, Vandongen R, Walter B N J. Neutrophil platelet activating factor in normal and hypertensive pregnancy and in pregnancy-induced hypertension. Clin Sci. 1993;85:63–70. doi: 10.1042/cs0850063. [DOI] [PubMed] [Google Scholar]
- 4.Bellavite P, Guarini P, Biasi D, Carletto A, Trevisan M T, Caramaschi P, Bambara L M, Corrocher R. Correlations between the intensity of fMLP-dependent respiratory burst and cellular fatty acid composition in human neutrophils. Br J Haematol. 1995;89:271–276. doi: 10.1111/j.1365-2141.1995.tb03300.x. [DOI] [PubMed] [Google Scholar]
- 5.Bjorksten B, Soderstrom T, Damber M-G, Von Schoultz B, Stigbrand T. Polymorphonuclear leucocyte function during pregnancy. Scand J Immunol. 1978;8:257–262. doi: 10.1111/j.1365-3083.1978.tb00518.x. [DOI] [PubMed] [Google Scholar]
- 6.Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Scand J Clin Lab Investig. 1968;21:77–89. [PubMed] [Google Scholar]
- 7.Brabin B J. Epidemiology of infection in pregnancy. Rev Infect Dis. 1985;7:579–603. doi: 10.1093/clinids/7.5.579. [DOI] [PubMed] [Google Scholar]
- 8.Chilton-Lopez T, Surette M E, Swan D D, Fonteh A N, Johnson M M, Chilton F H. Metabolism of gammalinolenic acid in human neutrophils. J Immunol. 1996;156:2941–2947. [PubMed] [Google Scholar]
- 9.Cockcroft S, Stutchfield J. The receptors for ATP and f-MetLeuPhe are independently coupled to phospholipase C and A2 via G-proteins. Biochem J. 1989;263:715–723. doi: 10.1042/bj2630715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corey S J, Rossoff P M. Unsaturated fatty acids and lipoxygenase products regulate phagocytic NADPH oxidase activity by a non-detergent mechanism. J Lab Clin Med. 1991;118:343–351. [PubMed] [Google Scholar]
- 11.Crouch S P M, Crocker I P, Fletcher J. The effect of pregnancy on polymorphonuclear leukocyte function. J Immunol. 1995;155:5436–5443. [PubMed] [Google Scholar]
- 12.Dana R, Malech H L, Levy R. The requirement for phospholipase A2 for activation of the assembled NADPH oxidase in human neutrophils. Biochem J. 1994;297:217–233. doi: 10.1042/bj2970217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Da Silva J A P, Spector T D. The role of pregnancy in the course and etiology of rheumatoid arthritis. Clin Rheumatol. 1992;11:189–194. doi: 10.1007/BF02207955. [DOI] [PubMed] [Google Scholar]
- 14.Dewald B, Baggiolini M. Activation of NADPH oxidase in human neutrophils. Synergism between fMLP and the neutrophil products PAF and LTB4. Biochem Biophys Res Commun. 1985;128:297–304. doi: 10.1016/0006-291x(85)91678-x. [DOI] [PubMed] [Google Scholar]
- 15.El-Maallem H, Fletcher J. Impaired neutrophil function and myeloperoxidase deficiency in pregnancy. Br J Haematol. 1980;44:375–381. doi: 10.1111/j.1365-2141.1980.tb05906.x. [DOI] [PubMed] [Google Scholar]
- 16.Ferrante A, Carman K, Nandoskar M, McPhee A, Poulos A. Cord-blood neutrophil responses to polyunsaturated fatty-acids—effects on degranulation and oxidative respiratory burst. Biol Neonate. 1996;69:368–375. doi: 10.1159/000244333. [DOI] [PubMed] [Google Scholar]
- 17.Finn R, St. Hill C A, Govan A J. Immunologic response in pregnancy and survival of the fetal homograft. Br Med J. 1972;3:150–152. doi: 10.1136/bmj.3.5819.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flesch I, Ferber E. Effect of cellular fatty acid composition on the phospholipase A2 activity of bone-marrow derived macrophages and their ability to induce lucigenin-dependent chemiluminescence. Biochim Biophys Acta. 1986;889:6–14. doi: 10.1016/0167-4889(86)90003-0. [DOI] [PubMed] [Google Scholar]
- 19.Gyllenhammer H. Lucigenin chemiluminescence in the assessment of neutrophil superoxide anion production. J Immunol Methods. 1987;97:209–213. doi: 10.1016/0022-1759(87)90461-3. [DOI] [PubMed] [Google Scholar]
- 20.Hempel K H, Fernandez L A, Persellin R H. Effect of pregnancy sera on isolated lysosomes. Nature. 1970;225:955–957. doi: 10.1038/225955a0. [DOI] [PubMed] [Google Scholar]
- 21.Hench P S. The ameliorating effect of pregnancy on chronic atrophic (infectious rheumatoid) arthritis, fibrositis, and intermittent hydrarthrosis. Proc Staff Meet Mayo Clin. 1938;13:161–167. [Google Scholar]
- 22.Hornstra G. Essential fatty acids in pregnancy and early human development. Eur J Obstet Gynecol Reprod Biol. 1995;61:57–62. doi: 10.1016/0028-2243(95)02153-j. [DOI] [PubMed] [Google Scholar]
- 23.Jacobson P B, Schier D J. Regulation of CD11b/CD18 expression in human neutrophils by PLA2. J Immunol. 1993;151:5639–5652. [PubMed] [Google Scholar]
- 24.Krause P J, Ingardia C J, Pontius L T, Malech H L, Lobello T M, Maderazo E G. Host defense during pregnancy: neutrophil chemotaxis and adherence. Am J Obstet Gynecol. 1987;157:274–280. doi: 10.1016/s0002-9378(87)80150-3. [DOI] [PubMed] [Google Scholar]
- 25.Kusner D J, Aucott J N, Franceshi D, Sarasua M M, Spagnuolo P J, King C H. Protease priming of neutrophil superoxide production. J Biol Chem. 1991;266:16465–16471. [PubMed] [Google Scholar]
- 26.Lepage G, Roy C C. Direct transesterification of all classes of lipids in a one-step reaction. Lipid Res. 1986;27:114–120. [PubMed] [Google Scholar]
- 27.Luft B J, Remington J S. Effect of pregnancy on resistance to Listeria monocytogenes and Toxoplasma gondii infections in mice. Infect Immun. 1982;38:1164–1171. doi: 10.1128/iai.38.3.1164-1171.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahoney E M, Hamill A L, Scott W A, Cohn Z A. Response of endocytosis to altered fatty acyl composition of macrophage phospholipids. Proc Natl Acad Sci USA. 1977;74:4895–4899. doi: 10.1073/pnas.74.11.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marshal L A, Winkler J D, Grinswold D E, Bolognese B, Rishar A, Sung C-M, Webb E F, Jacobs R. Effects of Scalaradial, a type II phospholipase A2 inhibitor on human neutrophil AA mobilisation and lipid mediator formation. J Pharmacol Exp Ther. 1994;268:709–717. [PubMed] [Google Scholar]
- 30.Nolten W E, Rueckert P A. Elevated free cortisol index in pregnancy; possible regulatory mechanisms. Am J Obstet Gynecol. 1981;139:492–498. doi: 10.1016/0002-9378(81)90331-8. [DOI] [PubMed] [Google Scholar]
- 31.Persellin R H. The effect of pregnancy on rheumatoid arthritis. Bull Rheum Dis. 1977;27:922–927. [PubMed] [Google Scholar]
- 32.Persellin R H. Inhibitors of inflammatory and immune responses in pregnancy serum. Clin Rheum Dis. 1981;7:769–780. [Google Scholar]
- 33.Persellin R H, Leibfarth J K. Studies of the effects of pregnancy serum on polymorphonuclear leukocyte functions. Arthritis Rheum. 1978;21:316–325. doi: 10.1002/art.1780210305. [DOI] [PubMed] [Google Scholar]
- 34.Persellin R H, Thoi L L. Human polymorphonuclear leukocyte phagocytosis in pregnancy: development of inhibition during gestation and recovery in the post partum period. Am J Obstet Gynecol. 1979;134:250–255. doi: 10.1016/s0002-9378(16)33028-9. [DOI] [PubMed] [Google Scholar]
- 35.Qian M W, Eaton J W. Free fatty-acids enhance hypochlorous acid production by activated neutrophils. J Lab Clin Med. 1994;124:86–95. [PubMed] [Google Scholar]
- 36.Reinhardt M C. Effects of parasitic infections in pregnant women. Ciba Found Symp. 1980;77:149–159. doi: 10.1002/9780470720608.ch10. [DOI] [PubMed] [Google Scholar]
- 37.Sanjurjo P, Matorras R, Ingunza N, Alonso M, Rodriguez-Alarcon J, Perteagudo L. Cross sectional study of percentual changes in total plasmatic fatty acids during pregnancy. Horm Metab Res. 1993;25:590–592. doi: 10.1055/s-2007-1002183. [DOI] [PubMed] [Google Scholar]
- 38.Serhan C N, Radin A, Smolen J E, Korchak H, Samuelson B, Weissmann G. LTB4 is a complete secretagogue in human neutrophils: a kinetic analysis. Biochem Biophys Res Commun. 1982;107:1006–1012. doi: 10.1016/0006-291x(82)90622-2. [DOI] [PubMed] [Google Scholar]
- 39.Spector T D, Da Silva J A P. Pregnancy and rheumatoid arthritis: an overview. Am J Reprod Immunol. 1992;28:222–225. doi: 10.1111/j.1600-0897.1992.tb00797.x. [DOI] [PubMed] [Google Scholar]
- 40.Taylor A J, Pandov H, Lawson N. Determination of erythrocyte fatty acids by capillary gas liquid chromatography. Ann Clin Biochem. 1998;24:293–296. doi: 10.1177/000456328702400309. [DOI] [PubMed] [Google Scholar]