Abstract
We proposed a specially designed sequential injection (SI) amperometric system coupling with a bioreactor for in-line glucose monitoring in cell culture. The system is composed of three main parts which are the bioreactor, SI system, and electrochemical detection unit. The bioreactor accommodates six individual cell culture units which can be operated separately under different conditions. The SI system enables automatic in-line sampling and in-line sample dilution, with a specially designed mixing unit; therefore, it has the benefits of fast analysis time and less contamination risk. The use of 3D-printed microfluidic components, a mixing channel, and a flow cell helped to reduce operational time and sample volume. A disposable screen-printed electrode (SPE), modified with glucose oxidase (GOD), carbon nanotube, and gold nanoparticle, was used for detection. The developed system provided a linear range up to 3.8 mM glucose in cell culture media. In order to work with cell culture in higher glucose media, the in-line sample dilution can be applied. The developed SI system was demonstrated with mouse fibroblast (L929) cell culture. The results show that glucose concentration obtained from the SI system is comparable with that obtained from the conventional colorimetric method. This work can be further developed and applied for in vitro cell-based experiments in biomedical research.
Keywords: flow analysis, sequential injection, in-line sampling, in-line dilution, bioreactor, amperometry, screen-printed electrode, glucose, glucose oxidase
1. Introduction
Sequential injection (SI) analysis is a flow-based analysis technique that allows multiple samples to be manipulated separately in a segment controlled by a multi-position selective valve [1]. In past decades, a lot of SI systems have been developed and widely applied in various areas. Bioprocess monitoring is one of the SI applications that benefits from the in-line monitoring system [2]. The SI system allows a complex, multi-reagent, and multi-step chemical analysis, which is suitable for bioprocess including biopharmaceutical studies, fermentation bioreactor, and cell culture. Applications of the SI system to the biopharmaceutical process involving in vitro interaction with various types of cells have been previously reported; for example, monitoring of drug liberation [3,4], release test [5,6], and product quality [7].
SI has also been employed in fermentation bioreactors for in-line monitoring metabolites and products such as lactic acid, ammonia, glycerol, glucose, and formaldehyde [8,9]. In recent years, it has been applied to the mammalian cell culture in order to monitor conditions in a bioreactor and control product qualities. For cell culture application, the SI system has been combined with various analytical methods such as capillary electrophoresis [10], chromatography [11,12], and electrochemical analysis [13]. The SI systems have been beneficial for in-line determination in bioprocesses by allowing a short time process, automation, and multiple assays in one system [14].
Glucose is one of the major nutrients in cell culture media. Determination of glucose uptake rate in cell culture under experimental condition is necessary for cellular metabolism study. Therefore, an in-line monitoring system in a bioreactor can be beneficial for cell culture application. In a cell production, a change in glucose level is crucial for cell growth. The monitoring system can also be further developed into feedback control system to refresh culture media automatically.
Glucose sensors can be divided into several types depending on its transducer part of the sensor: electrochemical, optical, thermometric, piezoelectric, and magnetic. The major glucose sensor type is the electrochemical type because of its better sensitivity and reproducibility, easy maintenance, and low cost [15]. There have been numerous attempts to enhance the efficiency of glucose sensors by using nanomaterials, such as carbon nanotubes (CNTs), gold nanoparticles (AuNPs), platinum nanoparticles (PtNPs), etc., combined with glucose oxidase enzyme (GOD) coated on the electrode [16,17,18,19,20]. Some sensors have been applied to cell culture as shown in Table 1. Moreover, a couple of studies have reported the modification of glucose sensors advanced from a commercially available screen-printed electrode (SPE) [21,22].
Table 1.
Specification of available different enzymatic glucose sensors used in cell culture application.
| Detection Technique |
Sensor Components | Linear Range (mM) | Cell Type | In-Line Measurement | Ref |
|---|---|---|---|---|---|
| Optical | Oxygen sensor covered with glucose oxidase enzyme, bovine serum albumin (BSA), glycerol, glutaraldehyde | 0–20 | Chinese hamster ovarian (CHO) cells | Yes | [23] |
| Electrochemical | Multi-walled carbon nanotubes, chitosan, glucose oxidase enzyme | 5–25 | Human myeloid leukemia (U937) cells | Yes | [21,24] |
| Electrochemical (Amperometric) |
Water-based carbon ink formulations containing cobalt phthalocyanine (CoPC), glucose oxidase enzyme | up to 5 | Human choriocarcinoma (BeWo) cells | Yes | [25] |
| Electrochemical (Amperometric) |
pHEMA enzyme membrane containing glucose oxidase |
up to 10 | Human glioblastoma multiforme (T98G) brain cancer cells | Yes | [26] |
| Electrochemical (Amperometric) |
Carbon black–Prussian blue screen-printed electrode modified with Glucose oxidase and cellulose nanocrystals | 0.1–2 | NIH 3T3 fibroblast cells | No | [27] |
In this work, a SI system coupled with bioreactor and amperometric detection was designed for in-line glucose monitoring in cell culture media. The bioreactor was modified from a previous report [28]; it was able to handle six individual cell culture units with different cell types or conditions up to six types/conditions at the same time. The SI system allowed programable sampling and adjustable in-line sample dilution for glucose analysis.
The amperometric detection unit utilized a commercial SPE modified with single-walled carbon nanotubes (SWCNTs), AuNPs, chitosan, and GOD. The application of the SI-bioreactor system with GOD–SWCNTs–AuNPs-modified SPE was demonstrated for the determination of glucose concentration in cell-cultured media from L929 cell line cultivation.
2. Results and Discussion
2.1. Performance of the Modified SPE
For glucose determination in cell culture media, a carbon SPE was modified with a mixture of GOD, SWCNTs, and AuNPs. The GOD was immobilized on the working electrode surface together with the negatively charged SWCNTs and AuNPs to increase surface area and conductivity, while a chitosan provided a cationic 3D network covered on the electrode surface as illustrated in Figure 1a. Chitosan is a polycation natural polymer derived from chitin and a entangle network of chitosan can form hydrogel to immobilize enzyme and nanoparticles on the electrode surface. Amperometric determination of glucose was based on the activity of GOD as shown in Equations (1)–(3):
| Glucose + GOD − FAD+ → Glucolactone + GOD − FADH2 | (1) |
| GOD − FADH2 + O2 → GOD − FAD + H2O2 | (2) |
| H2O2 → 2H+ + O2 + 2e− | (3) |
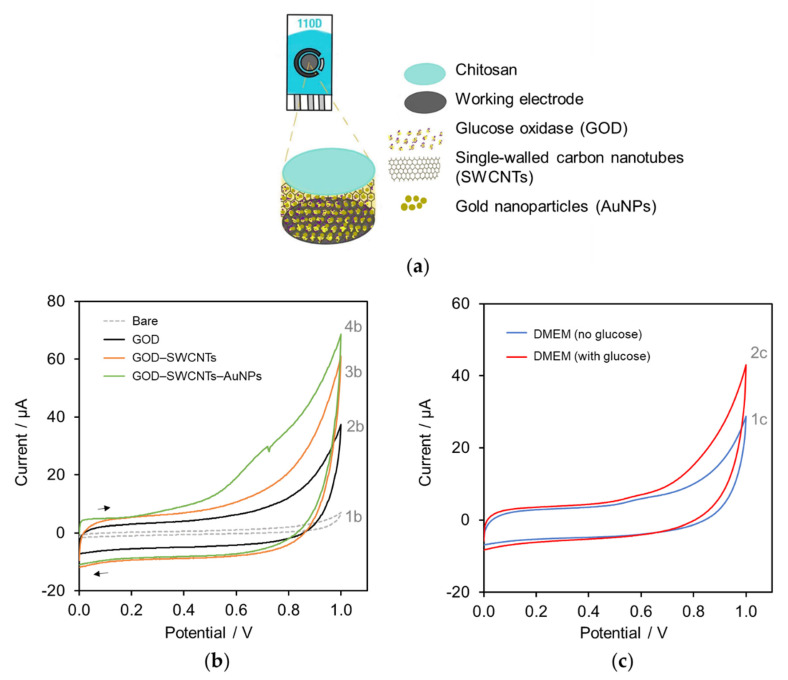
Figure 1.
Modification of a SPE: (a) a schematic diagram showing GOD-AuNPs-SWCNTs-chitosan layer on the carbon working electrode surface (not to scale); (b) cyclic voltammograms of different modified electrodes; a bare commercial SPE (CV 1b), GOD-coated SPE (CV 2b), the mixing of GOD–SWCNTs-coated SPE (CV 3b), and the mixing of GOD–SWCNTs–AuNPs-coated SPE (CV 4b) in PBS; (c) cyclic voltammograms of GOD–SWCNTs–AuNPs-modified electrodes in DMEM in the presence of 10 mM glucose (CV 2c) and in the absence of glucose (CV 1c). Scan rate at 50 mV s−1.
The modified carbon SPE was characterized by cyclic voltammetry (CV). Figure 1b shows the comparison between a bare commercial SPE (CV 1b) and three different modified glucose sensors which are a GOD-coated SPE (CV 2b), the mixing of GOD–SWCNTs-coated SPE (CV 3b), and the mixing of GOD–SWCNTs–AuNPs-coated SPE (CV 4b). The result from CV of four different sensors in PBS shows an increase in charging current due to the electric double layer formed on the electrode interface increased when nanoparticles were incorporated onto the electrode surface, suggesting a change in electrical properties on the surface. It was found that GOD–SWCNTs–AuNPs-modified SPE exhibited higher current response compared with other three glucose sensors.
The GOD–SWCNTs–AuNPs-modified SPE was further examined in, instead of PBS, Dulbecco’s Modified Eagle Medium (DMEM) which is used in mammalian cell cultures for this work containing various nutrients, amino acids, vitamins, inorganic salts (e.g., CaCl2, KCl, and MgSO4), and carbonate buffer. It was found that the current obtained from DMEM (see Figure 1c, CV 1c) was lower than that obtained from the PBS medium (see Figure 1b, CV 4b). The observed phenomena were similar to the previous report [27]. It can be seen in Figure 1c that, for DMEM, the oxidative current due to glucose (CV 2c) was higher than that without glucose (CV 1c).
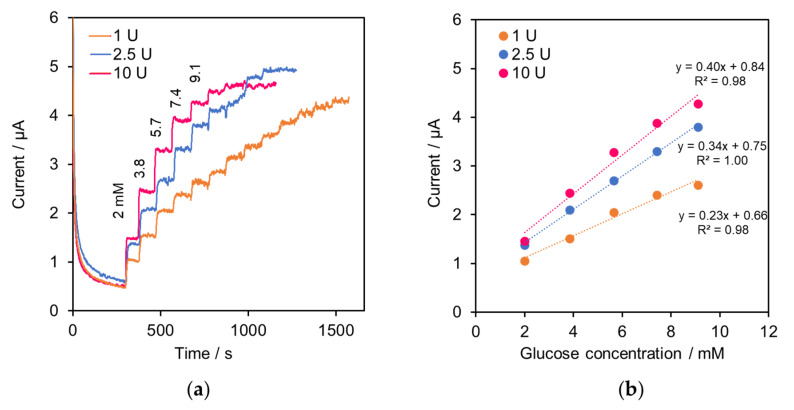
The GOD–SWCNTs–AuNPs modification of SPE was optimized by performing chronoamperometry with glucose in a bulk solution of PBS. The SPE were fabricated with various glucose oxidase enzyme amounts; 1, 2.5, and 10 U. Then, the amperometric measurement was conducted at applied potential +0.8 V. Figure 2a shows the amperometric response of three different sensors when the concentration of glucose increased in bulk solution. The relationship between the oxidative current and glucose concentration was plotted as the calibration curve shown in Figure 2b. The result shows that the current response increased linearly with glucose concentration in the working range up to 9.1 mM in all sensors. From this result, the sensor with 2.5 U GOD has compromised characteristics of sensitivity and proper working range to detect glucose compared with the others; hence, it was chosen to be employed as the detection unit for the SI system.
Figure 2.
Optimization of GOD–SWCNTs–AuNPs-modified SPE: (a) a series of chronoamperograms of GOD–SWCNTs–AuNPs-modified SPE with various amount of GOD (1, 2.5 and 10 U) at applied potential +0.8 V showing increasing current response as glucose was added in PBS bulk solution; (b) plots between current response and added glucose concentration for each SPEs.
2.2. Characterization of the System for Determination of Glucose in Cell Culture Media
The performance characteristics of the system (see Section 3.1) for determination of glucose in culture media sample were investigated. The optimized sensor was assembled with the flow cell connected with the SI system. A series of glucose standard solutions with various concentrations were added to cell culture media (glucose-free DMEM), and each solution was sequentially transferred to the detection flow cell by an in-house syringe pump with a controlling program for the system calibration. The amperometric measurement was performed at applied potential +0.8 V.
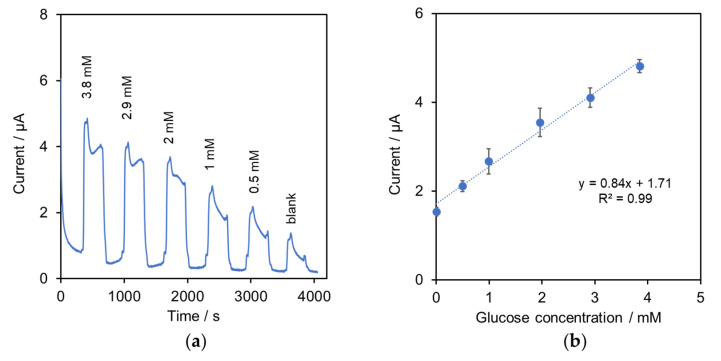
In Figure 3a, the chronoamperogram shows the current response when DMEM with various glucose concentrations sequentially flowed through the sensor. It was found that peak current increased with glucose concentration. A calibration of peak current (I) and glucose concentration in DMEM was obtained up to 3.8 mM glucose as shown in Figure 3b: I = 0.84[glucose] + 1.71, R2 = 0.99, n = 3. The limit of detection (3σ, [29]) of glucose in DMEM was 0.3 mM. The sensitivity per electrode area was 66.8 nA mM−1 mm−2.
Figure 3.
The system with the GOD–SWCNTs–AuNPs-modified SPE: (a) amperometric responses obtained from the system with various concentrations of glucose in DMEM from 0.5 to 3.8 mM with flow rate 0.4 mL min−1; (b) calibration plot of peak current and glucose concentration, n = 3.
Table 2 summarizes the performance of the reported enzymatic glucose electrodes. The developed system provided proper sensitivity for glucose in media, by which the working range was considered to be suitable for cell culture in biomedical work, e.g., proliferation and cytotoxicity assays. In order to work with cell culture in higher glucose media, in-line sample dilution could then be applied. This also helped to minimize matrix interferences such as lactate and serum protein.
Table 2.
Performance of reported different enzymatic glucose electrodes.
| Electrode | Sensitivity | Linear Range | Limit of Detection | Ref. |
|---|---|---|---|---|
| Multiwalled carbon nanotubes, chitosan, platinum nanoparticles, glucose oxidase enzyme, methyltrimethoxysilane (MTOS) | 2.8 µA mM−1 | 1.2 µM−6 mM | 0.3 µM | [17] |
| Silver nanoparticles, carbon nanotubes, chitosan, glucose oxidase enzyme | 135.9 µA mM−1 | 0.5–50 µM | 0.1 µM | [18] |
| Carbon nanotubes, gold nanoparticles, chitosan, glucose oxidase enzyme | n/a | 6 µM–5 mM | 3 µM | [19] |
| Carbon-coated tin sulfide (C-SnS) nanoparticles, glucose oxidase enzyme | 439 nA mM−1 mm−2 | 0.03–0.7 mM | n/a | [20] |
| Multi-walled carbon nanotubes, chitosan, glucose oxidase enzyme | 4.7 ± 1.3 nA mM−1 mm−2 | 5–25 mM * | 1.4 mM | [21] |
| Water-based carbon ink formulations containing cobalt phthalocyanine (CoPC), glucose oxidase enzyme | 6 nA mM−1 | Up to 5 mM * | n/a | [25] |
| pHEMA enzyme membrane containing glucose oxidase |
3.3 nA mM−1 mm−2 | Up to 10 mM * | 75 µM | [26] |
| Carbon black–Prussian blue screen-printed electrode modified with glucose oxidase and cellulose nanocrystals | 57 ± 3 nA mM−1mm−2 | 0.1–2 mM | 4 µM | [27] |
| This work | 66.8 nA mM−1 mm−2 | Up to 3.8 mM * | 0.3 mM |
* Indicating glucose concentration in culture media.
2.3. Application
The application of the developed system was demonstrated using L929 cell line as a model. The L929 cell line is commonly used as a tool in biomedical research, such as the cytotoxicity test. In order to apply the developed SI system and sensor in L929 cell line cultivation, the amperometric measurement of the GOD–SWCNTs–AuNPs-modified SPE in 0.5–3.8 mM glucose in DMEM was repeated for 3 successive days to determine the stability of the sensor. It was observed that the glucose sensor exhibited 81–94% of current responses compared with initial values. This indicated that the sensor can be used for usual cell culture application (72 h incubation) [28].
The cell culture of L929 cell was incubated in the bioreactor for 72 h without media refresh. At 24 and 72 h after cell seeding, glucose contents were determined by two methods; the SI system (in-line analysis) and the conventional colorimetric assay based on GOD enzymatic reaction (off-line analysis).
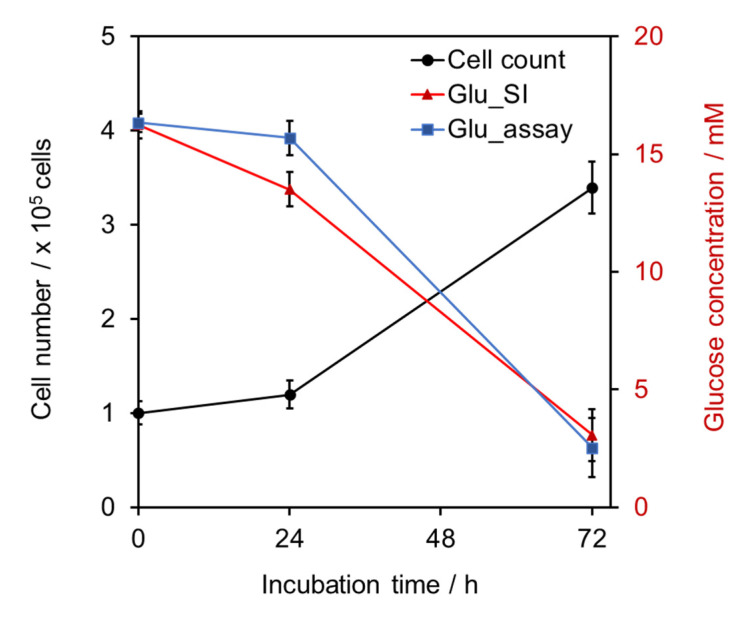
Results achieved with the proposed system and the conventional colorimetric assay were in agreement (paired t-test, p > 0.05) as shown in Figure 4. The developed SI system with amperometric detection demonstrated fast, simple, and high performance compared with the conventional assay. The system could process and prepare samples, carry detection, and display a result within approximately 5 min per sample, while the conventional method required an enzyme incubation time of at least 30 min. Another advantage of the electrochemical method is that there is no interference from the color and turbidity of the culture media; for example, phenol red which is a pH indicator commonly used in many commercial media formulations can interfere with the colorimetric method. Moreover, there is no need for sample and reagent preparation for each measurement. Therefore, the SI system can reduce working time and waste generation.
Figure 4.
The relationship between glucose concentration in cell media determined by the developed system (red) and conventional enzymatic assay (blue) and the number of L929 cell (black) after incubated in the bioreactor for 72 h, n = 3.
Moreover, the measured glucose contents were related to the number of cells, i.e., the amount of glucose decreased as the number of cells increased. This result also suggested that the nutrient in the culture media was depleted; therefore, refreshing of the media or cell subculture was critically needed before the cell started dying. It can be developed further for an automatic culture system or a feedback control system. Therefore, this SI-bioreactor system with GOD–SWCNTs–AuNPs SPE can be employed for advanced cell culture in tissue engineering applications.
This work demonstrated the feasibility of using the developed system for monitoring metabolites in cell proliferation of L929 cell culture. It would be beneficial as a system model for applying in the study of other cell proliferation, and multiple cell monitoring in drug screening to observe the efficacy of drugs such as cancer treatment medicines. Moreover, it can be employed in multiple cell monitoring under various conditions to understand the cell behavior and its related effects in different conditions such as cell hyperglycemia and cell hyperinsulinemia that can possibly cause some diseases [30,31,32].
3. Materials and Methods
3.1. System Design and Fabrication
3.1.1. The Design Concept
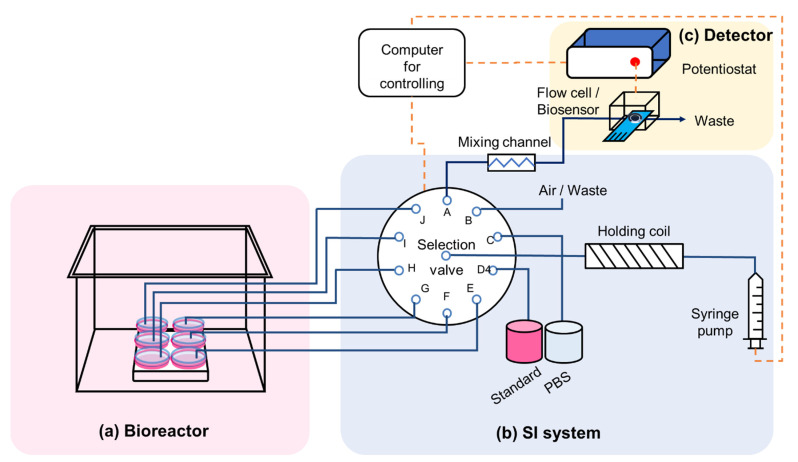
A specially designed system for in-line glucose monitoring in cell culture is composed of three parts: (a) bioreactor, (b) SI system, and (c) detector; as illustrated in Figure 5.
Figure 5.
A specially designed new system for in-line glucose monitoring in cell culture application is composed of three parts: (a) bioreactor, (b) SI system, and (c) detector.
3.1.2. The Bioreactor
An SI-bioreactor system was designed for in-line glucose analysis in mammalian cell culture for biomedical research in a general biological laboratory. The bioreactor is where cells are accommodated under controlled conditions. We previously reported the development of a programable automatic bioreactor for both adherent and suspension cell culture [28].
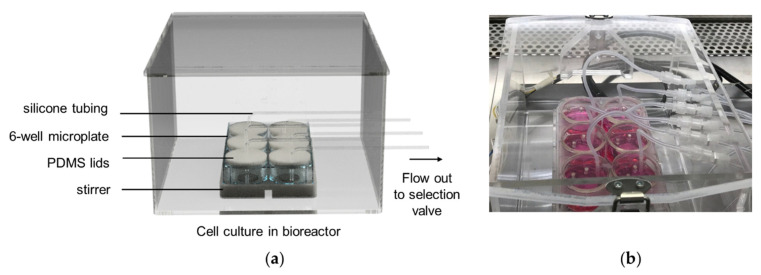
In this work, the bioreactor model was modified from the previous work. The bioreactor was designed to accommodate cell culture in a standard 6-well microplate that is generally used in biological laboratory, as illustrated in Figure 6. It served as a cluster of6 units under the same incubation condition (such as temperature and CO2 atmosphere), but individual cell culture may be differently treated. This setup was able to monitor each bioreactor unit separately. The system was designed to be a closed system with minimal modification to a cell container in order to avoid cell stress and reduce contamination risks. The bioreactor was coupled with the SI system via a PTFE connector to create a closed system for in-line glucose analysis. Most components were sterilizable and disposable. All components that were in contact with cell or culture media were sterilized by either an autoclave, ethanol, or UV radiation, to minimize contamination.
Figure 6.
The bioreactor: (a) a computer-aided design (CAD) file showing components of a bioreactor; (b) a photograph of bioreactor with cell culture in a 6-well microplate.
3.1.3. The SI System
The SI system was responsible for solution handling between the bioreactor and the detector unit. For in-line glucose assay in cell culture, the SI system first performed a sampling of each cell culture unit in the cluster independently. Second, the sample was in-line diluted to match the calibration graph. Before detection, a special design zigzag mixing channel was exploited instead of flow reversal operation for mixing efficiency. The sample zone ends with an air segment to minimize dispersion. The mixed solution was introduced to the electrochemical detection part without removing the air segment. These operational steps offer efficiency of the SI system and shorten operation time.
The SI system consisted of a 1-mL syringe pump (in-house assembly), a holding coil, a 10-port selection valve, a newly designed mixing channel (in-house 3D-printed), and a flow cell (in-house 3D-printed) as illustrated in Figure 5. It was connected to a laptop computer and controlled by in-house software. The software allowed both manual and pre-programed controlling. The SI sequence and volume are pre-programmable, including the in-line sample dilution step.
A mixing channel was designed in-house using a computer-aided design (CAD) program (Fusion 360, Autodesk Incorporation, San Francisco, CA, USA) and printed using an LCD-based 3D printer (Anycubic photon mono x, Hongkong anycubic technology, Hongkong, China). The acrylic resin-based 3D printing allowed precise small-scale fabrication suitable for the microfluidic platform. The design was also customizable to be able to fit with any available devices in the laboratory.
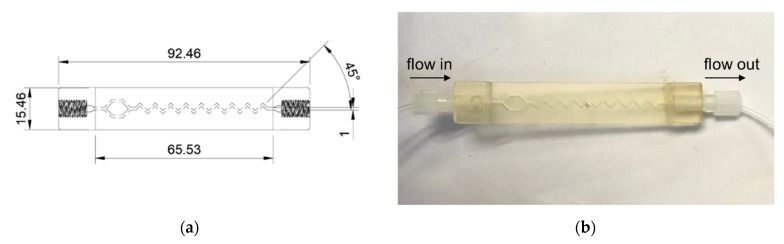
In Figure 7a,b, a mixing channel was designed as a 45° zigzag channel modified from previous reports [33,34]. In addition, for this system with one input channel, a Y-split part was added before the mixing channel to help mixing performance. The Y-split was designed in one to two ways with 45° and rejoined before the mixing channel. The Reynolds number was estimated and found to be 0.402 (at flow rate 0.4 mL min−1). The Reynolds numbers between 0.01 and 100 indicate the mixing flows are expected to be laminar, and mixing system would be highly dependent on diffusion.
Figure 7.
3D-printed mixing channel: (a) CAD design; (b) photograph of a mixing channel.
3.1.4. The Electrochemical Detection Unit
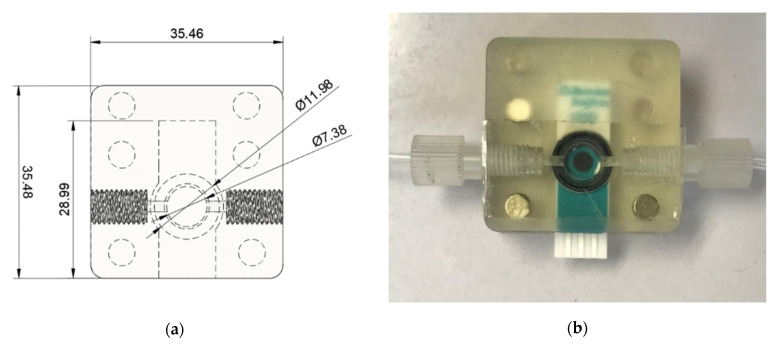
For the detection unit, a flow cell was specially designed to accommodate the SPE with a flow-through chamber, as shown in Figure 8a,b. It was printed using LCD-based 3D printing, similar to the mixing channel. It allowed flow-through electrochemical measurement for a solution volume less than 100 μL. The sample was aspirated to the flow chamber at 0.4 mL min−1. The flow-through analysis also helped avoid biofouling on electrode by proteins.
Figure 8.
Detection unit: (a) CAD file of 3D-printed flow cell; (b) photograph of the flow cell accommodating a SPE.
An SPE was employed for amperometric measurement in the detection unit. It has advantages of small size, ease of use, and cost-effectiveness compared with standard three-electrode system. This makes the SPE a suitable sensor for the glucose monitoring in the SI-bioreactor system. A simple modification of commercially available, disposable SPE with GOD enzyme can be performed in a laboratory beforehand.
3.2. Reagents and Chemicals
All reagents and chemicals used were of analytical reagent grade. A glucose standard solution was prepared by dissolving D-(+)-glucose (C6H12O6, Sigma-Aldrich, Saint Louis, MO, USA) in a phosphate buffered saline (PBS) pH 7.4 (Amresco Inc, Solon, OH, USA). A glucose-free Dulbecco’s Modified Eagle Medium (DMEM) (Gibco, Thermo Fisher Scientific, Waltham, MA, USA), DMEM powder (Gibco, Thermo Fisher Scientific, USA), fetal bovine serum (FBS) (Thermo Fisher Scientific, USA), and antibiotic-antimycotic (Thermo Fisher Scientific, USA) were used without further purification.
For electrode modification, a 1000 U mL−1 glucose oxidase (GOD) stock solution was prepared by dissolving glucose oxidase (E.C.1.1.3.4) from Aspergillus niger, recombinant (269 U mg−1, Merck, Darmstadt, Germany) in PBS and then diluted to final concentration. A single-walled carbon nanotube (SWCNT, dispersion in H2O, Sigma-Aldrich, Saint Louis, MO, USA) and colloidal gold (20 nm, Kestrel Bio Science, Bangkok, Thailand) were sonicated for one hour before use. A 1% w/v chitosan solution was prepared by dissolving 1 g of chitosan oligomer (100 mesh, Taming Enterprise, Beijing, China) in 100 mL of 1% acetic acid solution and then purified by dialysis.
3.3. Modification of SPE
A carbon SPE (working electrode: carbon; counter electrode: carbon; reference electrode: silver) (Metrohm Dropsens, Madrid, Spain) was modified with SWCNTs, AuNPs, chitosan, and GOD. First, the SPE was activated by performing cyclic voltammetry in 0.5 M H2SO4 solution. A 10 μL aliquot of glucose oxidase solution with optimal concentration, 1 μL of 0.1 mg mL−1 SWCNT suspension and 1 μL of colloidal gold suspension were mixed. Later, a mixture was drop-casted on a working electrode surface and left to semi-dry. A 2 μL aliquot of chitosan solution was then drop-casted and the electrode was kept at 4 °C overnight. The SPE was rinsed with PBS thoroughly before use.
3.4. Electrochemical Measurement
All electrochemical measurements, cyclic voltammetry, and chronoamperometry were performed using a potentiostat (µStat 300, Dropsens, Spain) at 25 °C. The potential was reported as V vs. Ag pseudo-reference electrode.
3.5. Glucose Monitoring Using the System
The system was controlled by an in-house program modified from the previous study [28]. The controlling program allowed preset command script (.csv) as listed in Table 3.
Table 3.
List of command in the SI system controlling program.
| Command | Value | Description |
|---|---|---|
| Loop | Number | Set number of scripts to repeat |
| Pump | Number | Set volume of syringe pump to aspirate or dispense (mL) |
| Goto | Number | Jump to a line number that set of the script |
| Wait | hh:mm:ss | Set delay time |
| Dir | F or B | Set syringe pump direction (forward or backward) |
| Ch | Selection valve (A or B or C or else) |
Select selection valve position |
| Start | Starting syringe pump | |
| Msgbox | Text | Displays the specified text in the message box |
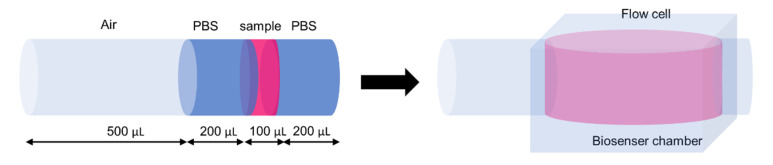
A glucose standard solution or sample was sequentially aspirated into a holding coil to create mono-segmentation and then introduced to the detection unit where electrochemical measurement occurred. In the case of the real sample, 100 μL of culture media was sampled from the bioreactor and in-line diluted with two portions of 200 μL of PBS before detection as shown in the script in Table 4 and illustrated in Figure 9. A ratio between sample and diluent can be adjusted to obtain desired dilution factor. This also can help reduce interference from serum protein in the culture media.
Table 4.
A script for glucose analysis using the SI system.
| Line Number | Command | Value | Action |
|---|---|---|---|
| 1 | Loop | 3 | Repeat all actions for 3 times |
| 2 | Pump | 0.5 | Aspirate air 500 μL into holding coil |
| 3 | Dir | B | |
| 4 | Ch | B | |
| 5 | Start | ||
| 6 | Wait | 00:00:10 | |
| 7 | Pump | 0.2 | Aspirate PBS 200 μL into holding coil |
| 8 | Dir | B | |
| 9 | Ch | C | |
| 10 | Start | ||
| 11 | Wait | 00:00:10 | |
| 12 | Pump | 0.1 | Aspirate sample 100 μL into holding coil |
| 13 | Dir | B | |
| 14 | Ch | E | |
| 15 | Start | ||
| 16 | Wait | 00:00:10 | |
| 17 | Pump | 0.2 | Aspirate PBS 200 μL into holding coil |
| 18 | Dir | B | |
| 19 | Ch | C | |
| 20 | Start | ||
| 21 | Wait | 00:00:10 | |
| 22 | Pump | 1 | Dispense all segment 1000 μL from holding coil to sensor |
| 23 | Dir | F | |
| 24 | Ch | A | |
| 25 | Start | ||
| 26 | Wait | 00:00:10 | |
| 27 | Pump | 0.5 | Aspirate air 500 μL into holding coil (cleaning) |
| 28 | Dir | B | |
| 29 | Ch | B | |
| 30 | Start | ||
| 31 | Wait | 00:00:10 | |
| 32 | Pump | 0.5 | Aspirate PBS 500 μL into holding coil (cleaning) |
| 33 | Dir | B | |
| 34 | Ch | C | |
| 35 | Start | ||
| 36 | Wait | 00:00:10 | |
| 37 | Pump | 1 | Dispense all segment 1000 μL from holding coil to sensor (cleaning) |
| 38 | Dir | F | |
| 39 | Ch | A | |
| 40 | Start | ||
| 41 | Wait | 00:00:10 | |
| 42 | Goto | 2 | Repeat all actions for 3 times |
Figure 9.
A SI sequence profile for amperometric determination of glucose in culture media.
3.6. Cell Culture of L929 Cell Line
A glucose analysis using the developed SI system was demonstrated using a cell culture of a mouse fibroblast cell line (L929: NCTC clone 929, JCRB cell bank, Osaka, Japan). The cells were maintained in a complete DMEM with 10% FBS and 1% antibiotic-antimycotic. The L929 cells were seeded in a 6-well plate at 1 × 105 cells per well and incubated in the bioreactor at 37 °C with 5% CO2 for 72 h.
3.7. Glucose Enzymatic Assay
Glucose enzymatic colorimetric assay is a conventional method for the determination of glucose in a biological sample. The analysis is based on the reaction between glucose and glucose oxidase enzyme, then the released hydrogen peroxide reacts with peroxidase reagent and induces oxidative condensation of 4-aminoantipyrine and phenol to form a quinoneimine dye [35]. A total of 4 μL of GOD solution was added to 100 μL of glucose standard or sample and then the mixture was incubated at 37 °C for 30 min. A 2 μL aliquot of the incubated product was added to a 200 μL of peroxidase reagent. The absorbance of the quinoneimine dye product was measured by spectrophotometry (HiPo MPP-96, Biosan, Riga, Latvia).
4. Conclusions
A system consisted of bioreactor, SI system, and amperometric detector was developed for in-line glucose monitoring. The system was designed for operating six units of bioreactor for individual cell culture under different treatments. The SI system allowed automation for handling of various solutions, i.e., in-line sampling, in-line sample dilution, and mixing. The GOD–SWCNTs–AuNPs-modified SPE combined with a 3D-printed flow cell was used as a detection unit. Application on cell culture was demonstrated on fibroblast cell L929 culture.
The system can be further developed for simultaneous monitoring of a change in metabolite of cell culture with multiple cell types or simulated conditions up to six types/conditions. In addition, it can be utilized in automatic cell culture systems, such as automatic feeding of media or other solutions. The system can be applied to a wide area of biomedical research such as cellular metabolism studies for drug and cancer therapy, and in vitro cytotoxicity tests for biomaterial and tissue engineering applications.
Acknowledgments
The authors would like to acknowledge Chiang Mai University through Biomedical Engineering Institute and Center of Excellence for Innovation in Analytical Science and Technology for Biodiversity-Based Economic and Society (I-ANALY-S-T_B.BES-CMU), CMU Junior Research Fellowship Program (P.P. and C.W.). We would like to thank Chawan Manaspon for providing cell culture sample. We are thankful to Sarawut Kumphune for advice on cell culture.
Author Contributions
Conceptualization, P.P. and K.G.; methodology, P.P. and S.U.; software, A.B.; validation, P.P., S.U. and K.K.; formal analysis, C.W. and P.P.; investigation, C.W., A.B., S.U. and P.P.; resources, K.K., P.P. and K.G.; data curation, C.W.; writing—original draft preparation, C.W., A.B., S.U. and P.P.; writing—review and editing, P.P., K.K. and K.G.; visualization, P.P. and S.U.; supervision, K.G.; project administration, P.P.; funding acquisition, P.P. and K.G. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was supported by CMU Junior Research Fellowship Program, Chiang Mai University.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ruzicka J.M., Graham D. Sequential injection: A new concept for chemical sensors, process analysis and laboratory assays. Anal. Chim. Acta. 1990;237:329–343. doi: 10.1016/S0003-2670(00)83937-9. [DOI] [Google Scholar]
- 2.Baxter P.J., Christian G.D. Sequential Injection Analysis: A Versatile Technique for Bioprocess Monitoring. Acc. Chem. Res. 1996;29:515–521. doi: 10.1021/ar950214z. [DOI] [Google Scholar]
- 3.Solich P., Sklenářová H., Huclová J., Šatínský D., Schaefer U.F. Fully automated drug liberation apparatus for semisolid preparations based on sequential injection analysis. Anal. Chim. Acta. 2003;499:9–16. doi: 10.1016/j.aca.2003.09.002. [DOI] [Google Scholar]
- 4.Sklenarova H., Beran M., Novosvetska L., Smejkalova D., Solich P. Sequential Injection Analysis for Automation and Evaluation of Drug Liberation Profiles: Clotrimazole Liberation Monitoring. Molecules. 2021;26:5538. doi: 10.3390/molecules26185538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klimundova J., Satinsky D., Sklenarova H., Solich P. Automation of simultaneous release tests of two substances by sequential injection chromatography coupled with Franz cell. Talanta. 2006;69:730–735. doi: 10.1016/j.talanta.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 6.Aguinaga Martinez M.V., Jozicova N., Dusek J., Horstkotte B., Pavek P., Miro M., Sklenarova H. Real-time monitoring of Metridia luciferase release from cells upon interaction with model toxic substances by a fully automatic flow setup—A proof of concept. Talanta. 2022;245:123465. doi: 10.1016/j.talanta.2022.123465. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y., Zhang C., Chen J., Fernandez J., Vellala P., Kulkarni T.A., Aguilar I., Ritz D., Lan K., Patel P., et al. A Fully Integrated Online Platform for Real Time Monitoring of Multiple Product Quality Attributes in Biopharmaceutical Processes for Monoclonal Antibody Therapeutics. J. Pharm. Sci. 2022;111:358–367. doi: 10.1016/j.xphs.2021.09.011. [DOI] [PubMed] [Google Scholar]
- 8.Hun-Chi S.H.H., Mattiasson B. On-line monitoring of D-lactic acid during a fermentation process using immobilized D-lactate dehydrogenase in a sequential injection analysis system. Anal. Chim. Acta. 1995;300:277–285. doi: 10.1016/0003-2670(94)00371-R. [DOI] [Google Scholar]
- 9.Mauser H., Busch M., Höbel W., Polster J. Bioprocess monitoring by sequential injection analysis. Lat. Am. Appl. Res. 2001;31:463–468. [Google Scholar]
- 10.Alhusban A.A., Gaudry A.J., Breadmore M.C., Gueven N., Guijt R.M. On-line sequential injection-capillary electrophoresis for near-real-time monitoring of extracellular lactate in cell culture flasks. J. Chromatogr. A. 2014;1323:157–162. doi: 10.1016/j.chroma.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Tharmalingam T., Wu C., Callahan S., Goudar C.T. A framework for real-time glycosylation monitoring (RT-GM) in mammalian cell culture. Biotechnol. Bioeng. 2015;112:1146–1154. doi: 10.1002/bit.25520. [DOI] [PubMed] [Google Scholar]
- 12.Wu C.H., Wee S. Micro sequential injection system as the interfacing device for process analytical applications. Biotechnol. Prog. 2015;31:607–613. doi: 10.1002/btpr.2055. [DOI] [PubMed] [Google Scholar]
- 13.Zabadaj M., Szuplewska A., Kalinowska D., Chudy M., Ciosek-Skibińska P. Studying pharmacodynamic effects in cell cultures by chemical fingerprinting − SIA electronic tongue versus 2D fluorescence soft sensor. Sens. Actuators B Chem. 2018;272:264–273. doi: 10.1016/j.snb.2018.05.137. [DOI] [Google Scholar]
- 14.Kiwfo K., Wongwilai W., Sakai T., Teshima N., Grudpan K. Determination of Albumin, Glucose, and Creatinine Employing a Single Sequential Injection Lab-at-Valve with Mono-Segmented Flow System Enabling In-Line Dilution, In-Line Single-Standard Calibration, and In-Line Standard Addition. Molecules. 2020;25:1666. doi: 10.3390/molecules25071666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoo E.H., Lee S.Y. Glucose biosensors: An overview of use in clinical practice. Sensors. 2010;10:4558–4576. doi: 10.3390/s100504558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai C., Chen J. Direct electron transfer of glucose oxidase promoted by carbon nanotubes. Anal. Biochem. 2004;332:75–83. doi: 10.1016/j.ab.2004.05.057. [DOI] [PubMed] [Google Scholar]
- 17.Kang X., Mai Z., Zou X., Cai P., Mo J. Glucose biosensors based on platinum nanoparticles-deposited carbon nanotubes in sol-gel chitosan/silica hybrid. Talanta. 2008;74:879–886. doi: 10.1016/j.talanta.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 18.Lin J., He C., Zhao Y., Zhang S. One-step synthesis of silver nanoparticles/carbon nanotubes/chitosan film and its application in glucose biosensor. Sens. Actuators B Chem. 2009;137:768–773. doi: 10.1016/j.snb.2009.01.033. [DOI] [Google Scholar]
- 19.Wang Y., Wei W., Liu X., Zeng X. Carbon nanotube/chitosan/gold nanoparticles-based glucose biosensor prepared by a layer-by-layer technique. Mater. Sci. Eng. C. 2009;29:50–54. doi: 10.1016/j.msec.2008.05.005. [DOI] [Google Scholar]
- 20.Chung R.-J., Wang A.-N., Peng S.-Y. An Enzymatic Glucose Sensor Composed of Carbon-Coated Nano Tin Sulfide. Nanomaterials. 2017;7:39. doi: 10.3390/nano7020039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boero C., Casulli M.A., Olivo J., Foglia L., Orso E., Mazza M., Carrara S., De Micheli G. Design, development, and validation of an in-situ biosensor array for metabolite monitoring of cell cultures. Biosens. Bioelectron. 2014;61:251–259. doi: 10.1016/j.bios.2014.05.030. [DOI] [PubMed] [Google Scholar]
- 22.Gonzalez-Fuentes F.J., Molina G.A., Silva R., Lopez-Miranda J.L., Esparza R., Hernandez-Martinez A.R., Estevez M. Developing a CNT-SPE Sensing Platform Based on Green Synthesized AuNPs, Using Sargassum sp. Sensors. 2020;20:6108. doi: 10.3390/s20216108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tric M., Lederle M., Neuner L., Dolgowjasow I., Wiedemann P., Wolfl S., Werner T. Optical biosensor optimized for continuous in-line glucose monitoring in animal cell culture. Anal. Bioanal. Chem. 2017;409:5711–5721. doi: 10.1007/s00216-017-0511-7. [DOI] [PubMed] [Google Scholar]
- 24.Jacopo Olivo L.F. Maria Antonietta Casulli, Cristina Boero, Sandro Carrara, and Giovanni De Micheli, Glucose and Lactate Monitoring in Cell Cultures with a Wireless Android Interface; Proceedings of the IEEE Biomedical Circuits and Systems Conference (BioCAS) Proceedings; Lausanne, Switzerland. 22–24 October 2014. [Google Scholar]
- 25.Pemberton R.M., Cox T., Tuffin R., Drago G.A., Griffiths J., Pittson R., Johnson G., Xu J., Sage I.C., Davies R., et al. Fabrication and evaluation of a micro(bio)sensor array chip for multiple parallel measurements of important cell biomarkers. Sensors. 2014;14:20519–20532. doi: 10.3390/s141120519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weltin A., Slotwinski K., Kieninger J., Moser I., Jobst G., Wego M., Ehret R., Urban G.A. Cell culture monitoring for drug screening and cancer research: A transparent, microfluidic, multi-sensor microsystem. Lab Chip. 2014;14:138–146. doi: 10.1039/C3LC50759A. [DOI] [PubMed] [Google Scholar]
- 27.Tang Y., Petropoulos K., Kurth F., Gao H., Migliorelli D., Guenat O., Generelli S. Screen-Printed Glucose Sensors Modified with Cellulose Nanocrystals (CNCs) for Cell Culture Monitoring. Biosensors. 2020;10:125. doi: 10.3390/bios10090125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Udomsom S., Budwong A., Wongsa C., Sangngam P., Baipaywad P., Manaspon C., Auephanwiriyakul S., Theera-Umpon N., Paengnakorn P. Automatic Programmable Bioreactor with pH Monitoring System for Tissue Engineering Application. Bioengineering. 2022;9:187. doi: 10.3390/bioengineering9050187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller J., Miller J. Statistics and Chemometrics for Analytical Chemistry. 5th ed. Pearson/Prentice Hall; Upper Saddle River, NJ, USA: 2005. [Google Scholar]
- 30.Ito M., Makino N., Matsuda A., Ikeda Y., Kakizaki Y., Saito Y., Ueno Y., Kawata S. High Glucose Accelerates Cell Proliferation and Increases the Secretion and mRNA Expression of Osteopontin in Human Pancreatic Duct Epithelial Cells. Int. J. Mol. Sci. 2017;18:807. doi: 10.3390/ijms18040807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karbach S., Jansen T., Horke S., Heeren T., Scholz A., Coldewey M., Karpi A., Hausding M., Kröller-Schön S., Oelze M., et al. Hyperglycemia and oxidative stress in cultured endothelial cells—A comparison of primary endothelial cells with an immortalized endothelial cell line. J. Diabetes Its Complicat. 2012;26:155–162. doi: 10.1016/j.jdiacomp.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 32.Fiorello M.L., Treweeke A.T., Macfarlane D.P., Megson I.L. The impact of glucose exposure on bioenergetics and function in a cultured endothelial cell model and the implications for cardiovascular health in diabetes. Sci. Rep. 2020;10:19547. doi: 10.1038/s41598-020-76505-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Squires T.M., Quake S.R. Microfluidics: Fluid physics at the nanoliter scale. Rev. Mod. Phys. 2005;77:977–1026. doi: 10.1103/RevModPhys.77.977. [DOI] [Google Scholar]
- 34.Tsai C.-H.D., Lin X.-Y. Experimental Study on Microfluidic Mixing with Different Zigzag Angles. Micromachines. 2019;10:583. doi: 10.3390/mi10090583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Menezes F., Neves A., Lima D., Lourenço S., Silva L., Lima K. Bioorganic Concepts Involved in the Determination of Glucose, Cholesterol and Triglycerides in Plasma via Enzymatic Colorimetric Method. Química Nova. 2015;38:588–594. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.