Abstract
The objective of the present study was to investigate the usefulness of a recombinant flagellar protein, FlaB, of Leptospira interrogans serovar pomona in the serodiagnosis of leptospirosis by the fluorescence polarization assay (FPA). The recombinant protein FlaB was purified to homogeneity by a combination of nickel-nitriloacetic acid agarose chromatography, electrophoresis, and electroelution. Purified FlaB was labeled with fluorescein isothiocyanate (FITC). Western blotting was performed by using bovine sera with microscopic agglutination test (MAT) titers of antibodies against L. interrogans serovar pomona and L. bergpetersenii serovars hardjo and sejroe to confirm the antigenicity of FlaB. Western blot analysis demonstrated that labeled as well as unlabeled FlaB was recognized by the positive sera tested, indicating the broad serovar cross-reactivity of this protein. It also indicated that labeling with FITC did not affect the antigenicity. By using FITC-labeled FlaB as a tracer antigen, a homogeneous FPA was developed to detect antileptospiral antibodies in bovine sera. A population of 208 MAT-positive and 208 MAT-negative serum samples was tested by FPA. The FPA cutoff was determined by receiver operating characteristic analysis. By FPA, 83.7% of the MAT-positive serum samples were positive and 81.2% of the MAT-negative serum samples were negative. Compared to the results of MAT, the positive predictive value of FPA was 81.7% and the negative predictive value of FPA was 83.3%. The FPA is a simple and rapid technique for the detection of anti-Leptospira antibodies.
Leptospirosis is a zoonotic infection which exhibits a broad spectrum of clinical manifestations, ranging in severity from acute to chronic with multiorgan syndrome to fatal (16). Leptospirosis affects wild rodents and domestic animals such as cattle, swine, horses, sheep, goats, and dogs (17). Animals with infections with leptospires also represent occupational hazards to farmers, butchers, and veterinarians (15, 16). The pathogenic leptospires were formerly classified as Leptospira interrogans, but the genus has recently been reorganized and pathogenic leptospires are now identified in seven species of Leptospira (59). These bacteria are divided into 23 serogroups and are subdivided into approximately 212 serovars on the basis of common cross-reacting agglutinins (16, 18).
The diagnosis of leptospirosis depends either on the detection of antibodies in the sera or the presence of the organisms in tissues or body fluids (6, 16, 58). Since the isolation of leptospires is difficult and laborious, serological diagnosis is extensively used. The microscopic agglutination test (MAT) is the most commonly used diagnostic test (11). Several other techniques, such as the passive hemagglutination test (40), the immunofluorescence test (4), and enzyme immunoassays (55, 58), have also been investigated. However, these assays have significant drawbacks, such as the use of a battery of live leptospires, with an associated risk of a laboratory-acquired infection, and the involvement of multiple reagents and steps (16, 23, 44, 48, 49, 54, 55). There is a need for a diagnostic laboratory test which can detect anti-Leptospira antibodies in biological fluids with high sensitivity and specificity and which can be performed within a short period of time. Although the enzyme-linked immunosorbent assay (ELISA) (44, 48) meets most of the desired requirements, improved simplicity and rapidity are still needed.
Dandliker et al. (13) introduced a homogeneous fluorescence polarization assay (FPA) that is sensitive, specific, and rapid and that requires few reagents. Fluorescence polarization is a measure of the time-averaged rotational motion of fluorescent molecules. When a fluorescently labeled antigen binds to the antibody, its fluorescence polarization will increase due to the larger hydrodynamic volume of the antigen-antibody complex (17, 35, 47).
Nielsen et al. (41) reported on the development of an FPA with fluorescein-labeled Brucella abortus O polysaccharide as a tracer antigen for the detection of Brucella antibodies in bovine sera. Those investigators demonstrated that FPA is a highly accurate assay with a sensitivity and a specificity of 99.02 and 99.96%, respectively. In a similar study, Lin et al. (33) reported on the use of fluorescein-labeled Mycobacterium bovis secretory protein MPB70 as a tracer antigen in an FPA. Those investigators indicated that FPA is able to detect anti-MPB70 antibodies in the sera of infected animals but not in the sera of uninfected animals.
Information on the nature and identity of leptospiral antigens is important for elucidation of their significance in the immunity to leptospirosis and the pathogenesis and diagnosis of leptospirosis. Leptospires have a characteristic corkscrew-like movement which is mediated by two periplasmic flagella inserted subterminally into the protoplasmic cylinder (16). Sequence analyses of flagellar genes and proteins from several spirochetes show that there are two distinct classes of proteins, the FlaA and FlaB proteins, in the filament. FlaA proteins are associated with the sheath surrounding a core which is composed of FlaB proteins (38, 42, 45). Electrophoresis has revealed that the periplasmic flagella are composed of three prominent proteins: proteins with molecular masses of 31 and 37 kDa and a 33- and 34-kDa doublet (8, 31). Nunes-Edwards et al. (43) identified several proteins with molecular masses of between 30 and 67 kDa from L. interrogans serovar hardjo. However, the significance of these proteins in the diagnosis or pathogenesis of leptospirosis or immunity to infection is not yet clear.
Recently, a 35-kDa protein from L. interrogans serovar pomona which is recognized by mono- and polyclonal anti-Leptospira antibodies has been identified. This protein was identified as the flagellin protein FlaB, and a gene encoding L. interrogans serovar pomona FlaB protein was cloned (34) and expressed in Escherichia coli (36).
The objectives of the present study were (i) to investigate the application of the recombinant protein FlaB in the diagnosis of leptospirosis and (ii) develop a sensitive and specific, yet simple and rapid assay for the serological screening of large numbers of leprospiral sera. This paper reports on the purification of recombinant protein FlaB and development and preliminary analysis of a homogeneous FPA for the diagnosis of leptospirosis. The performance of FPA in terms of its diagnostic sensitivity and specificity relative to the results of MAT was determined by receiver operating characteristic (ROC) analysis.
MATERIALS AND METHODS
Production of recombinant FlaB protein.
In this study, the expression construct pHTFlaB, which was generated as described previously (36), was used to produce the recombinant FlaB protein in E. coli DH5α. Briefly, Luria-Bertani broth (500 ml) supplemented with ampicillin (100 μg/ml) was inoculated with 5 ml of an overnight culture of the E. coli harboring pHTFlaB. Cultures were grown at 37°C in a incubator (Environ Shaker) until an optical density at 590 nm of 0.8 to 1.0 (approximate time, 4 to 5 h) was achieved. Expression of FlaB was initiated by the addition of isopropyl-β-d-thiogalactopyranoside (IPTG) at a final concentration of 0.6 mM. Cultures were incubated for an additional 3 h to allow maximal protein expression. The cells were harvested by centrifugation at 10,000 × g for 20 min at 4°C. The cell pellet was stored at −80°C until further analysis. In order to confirm protein expression, a 1.0-ml aliquot of the E. coli culture was taken before and after the addition of IPTG and was analyzed by electrophoresis.
SDS-PAGE and Western blotting.
Sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) was performed by the method of Laemmli (32) in 12% (wt/vol) resolving and 4% (wt/vol) stacking polyacrylamide gels with a mini-PROTEAN II gel apparatus (Bio-Rad). After electrophoresis, the proteins were either stained with Coomassie brilliant blue or electrophoretically transblotted (57) onto a nitrocellulose membrane (NC) (Bio-Rad) with a Trans-Blot Cell (Hoeffer Scientific) at 100 V for 2.5 h with cooling (7). The NC was blocked overnight with Tris-buffered saline (TBS) supplemented with Tween 20 (10 mM Tris-HCl, 140 mM NaCl [pH 7.4], 0.5% Tween) (TBS-T) plus 5% heat-inactivated horse serum (Armour, Kankakee, Ill.). After blocking, the NC was reacted with diluted bovine antiserum (1:100) for 3 h at room temperature and then with anti-bovine immunoglobulin G (IgG) conjugated with horseradish peroxidase (1:5,000). The NC was washed (five times) between each reaction step with TBS-T. Bound conjugate was visualized with a horseradish peroxidase substrate kit (Bio-Rad). The molecular masses of the separated proteins were determined by comparison with those of prestained protein standards (Bio-Rad).
Nickel-chelate affinity chromatography.
The recombinant protein FlaB was partially purified by affinity chromatography on a nickel-nitriloacetic acid (Ni-NTA)-agarose column (27, 28, 36) according to the manufacturer’s instructions (Qiagen). Briefly, IPTG-induced E. coli cells harboring pHTFlaB were resuspended in 10 ml of buffer A (8 M urea, 0.1 M Na2HPO4, 0.01 M Tris-HCl [pH 8.0]), lysed for 1 h at room temperature with continuous stirring, and then kept overnight at 4°C. Insoluble material was removed by centrifugation at 27,000 × g for 40 min at 4°C. The supernatant was applied to an Ni-NTA column (1 by 7.5 cm) preequilibrated with buffer A. The E. coli proteins were eluted by sequential washing with 50 ml each of buffer B (8 M urea, 0.1 M Na2HPO4, 0.01 M Tris-HCl, 1 M NaCl [pH 6.3]) and buffer C (which had the same composition as buffer B except that it was at pH 5.9). Selective elution of the FlaB protein was achieved with buffer D (which had the same composition as buffer C except that it contained 80 mM imidazole). The fractions were collected, and the absorbance was measured at 280 nm in a spectrophotometer (Pharmacia LKB Ultrospec plus). All eluted fractions were subjected to SDS-PAGE to confirm the presence of the FlaB protein. The peak fractions containing the FlaB protein were pooled and stored at −20°C.
Electroelution.
Following Ni-NTA affinity chromatography, the partially purified FlaB preparation was subjected to SDS-PAGE as described above. Individual protein bands were visualized by staining with Tris-Coomassie buffer (0.125 M Tris-HCl [pH 6.8] containing 0.2% Coomassie brilliant blue) and destaining with 0.125 M Tris-HCl (pH 6.8) containing 0.1% SDS and 1 mM EDTA. The FlaB protein band was excised and electroeluted (1, 20) with a Bio-Rad model 422 Electroelutor (25) at a constant current of 10 mA/tube for 4 to 6 h at room temperature with elution buffer (25 mM Tris-HCl, 192 mM glycine, 0.1% SDS [pH 8.5]). The purified FlaB was concentrated in an Ultrafiltration Stirred Cell (Amicon) fitted with a PM-10 (25 mm) ultrafiltration membrane (Amicon). Purified FlaB was dialyzed overnight against 0.02 M phosphate-buffered saline (PBS; pH 7.2) containing 0.01% SDS. This sample was used as an antigen for fluorescein labeling.
FITC labeling.
Purified FlaB (650 μg in 1 ml) mixed with 0.3 ml of fluorescein isothiocyanate (FITC; 1 mg/ml) in 0.15 M sodium phosphate buffer (pH 9.5) was routinely used. The reaction mixture was incubated for 3 h at 37°C. In order to separate free dye from the labeled FlaB protein, the reaction mixture was applied to a Sephadex G-25 column (1 by 23 cm; Pharmacia) that had been preequilibrated with 0.1 M sodium phosphate buffer (pH 7.0). The absorbance of the fractions was monitored at 492 nm (Pharmacia LKB Ultrospec plus). The eluted fractions were pooled and analyzed by SDS-PAGE. The protein bands were visualized with UV light (Transilluminator; VWR Scientific) followed by staining with Coomassie brilliant blue. The FITC-labeled recombinant protein FlaB was used as a tracer antigen in an FPA.
Serum samples.
A total of 416 field bovine serum samples were examined by FPA for the detection of antileptospiral antibodies. All serum samples were supplied by the Animal Diseases Research Institute, Lethbridge, Alberta, Canada. All of the positive and negative serum samples were initially tested once by MAT (53). All serum samples that had been stored at −20°C prior to processing were equilibrated to room temperature before testing by FPA.
MAT.
MAT was performed by a modification of a previously described method (11). Live 4-day-old Leptospira cultures (Leptospira serovars hardjo, pomona, sejroe, canicola, icterohaemorrhagiae, and grippotyphosa) were used as antigens. The endpoint titer was the highest dilution of serum that agglutinated at least 50% of the leptospires. Agglutination at a serum dilution of 1:100 was considered positive.
FPA.
FPA was carried out on an FPM-1 fluorescence polarization analyzer (Jolley Consulting and Research, Inc., Grayslake, Ill.). The baseline for the tracer antigen alone was established with 0.1 M PBS (pH 7.2). Serum samples were diluted (1:50) in 0.01 M PBS containing 0.1% sodium azide and 0.05% lithium dodecyl sulfate. Two milliliters of diluent was dispensed into a glass tube (12 by 75 cm). Test serum (0.04 ml) was then added to the diluent tube and the contents were mixed thoroughly. Each sample was blanked in the fluorescence polarization analyzer. The predetermined amount of the tracer antigen (0.02 ml) was then added to the serum sample and the contents were mixed thoroughly. The mixture was allowed to equilibrate for 10 to 30 min at room temperature. Following the equilibration period, the fluorescence polarization was measured as millipolarization units (mP) in a fluorescence polarization analyzer. In this study, the same lot of labeled FlaB tracer antigen was used for all FPAs.
Protein determination.
The concentration of FlaB was determined with a bicinchoninic acid protein assay kit (Pierce) by using bovine serum albumin as the standard, as described previously (50).
Data analysis.
The Excel 7.0 program (Microsoft) and ROC analysis (37) were used to evaluate the performance of FPA for the detection of antileptospiral antibodies. The ROC curve plots the relative sensitivity (true-positive ratio) versus relative specificity (false-positive ratio) while the cutoff value for a positive or negative result is varied and is completely independent of disease prevalence. The area under the ROC curve provides a quantitative assessment of a test’s diagnostic performance. The 95% confidence intervals are calculated as indicators of the precision of the relative sensitivity and relative specificity estimates. ROC analysis analyzes the diagnostic performance for the full range of cutoff points and thus eliminates the bias resulting from selection of a single value (24).
RESULTS
Recombinant protein overproduction and purification.
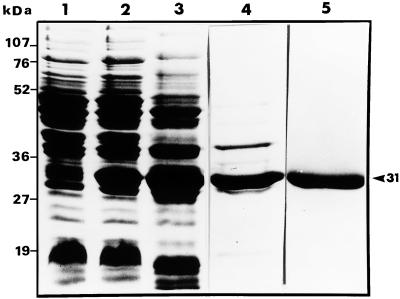
SDS-PAGE analysis of the total lysate of E. coli harboring pHTFlaB demonstrated that the recombinant FlaB protein showed up as a strong band at the expected 31-kDa position (Fig. 1, lane 2) in the cells induced with IPTG. However, FlaB was not detected in the E. coli extracts grown in the absence of IPTG (Fig. 1, lane 1). It was observed that FlaB was contaminated with several high- and low-molecular-mass proteins of E. coli (Fig. 1, lanes 2 and 3).
FIG. 1.
Expression and purification of recombinant FlaB protein. Samples from each step in the purification process were subjected to SDS-PAGE. Lane 1, soluble lysate of uninduced E. coli; lane 2, soluble lysate of induced E. coli; lane 3, crude extract of induced cells prior to Ni-NTA affinity chromatography; lane 4, protein eluted from the Ni-NTA column with 80 mM imidazole; lane 5, purified FlaB following electroelution. The positions of the molecular mass markers are indicated. Lanes 1 and 2 contain total cell proteins from 1 ml of culture with an A590 of 0.2. The recombinant protein FlaB is indicated with an arrowhead.
The expression of the L. interrogans serovar pomona flaB gene in E. coli (36) resulted in the FlaB fusion protein with a polyhistidine tag at the N terminus which facilitates purification. Ni-NTA affinity chromatography was used as the first step in the purification of FlaB from the bacterial cell lysate. The proteins were eluted in progressively acidic steps. The elution profile at 280 nm showed two well-separated peaks. SDS-PAGE analysis demonstrated the presence of E. coli proteins in the fractions of the first peak and the presence of a strong band in the 31-kDa region (which corresponds to the molecular mass of FlaB) in the second peak. Affinity-purified FlaB was found to be contaminated with one higher-molecular-mass band and one lower-molecular-mass band (Fig. 1, lane 4).
FlaB was further purified by electroelution. The elution time for maximal recovery of FlaB was 6 h. A purified product represented by a strong single band at the 31-kDa position was obtained, with no detectable contaminating protein bands (Fig. 1, lane 5). Purified FlaB was concentrated, dialyzed, and labeled with fluorescein dye.
FITC labeling.
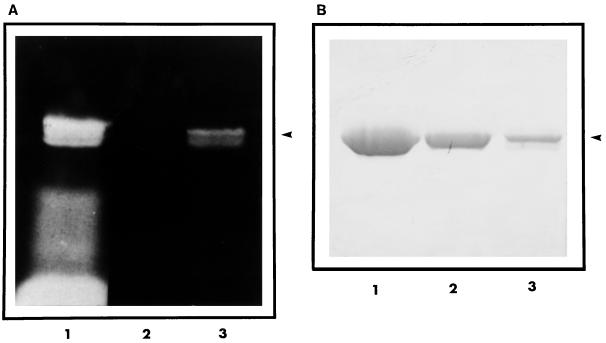
Analysis of Sephadex G-25 gel filtration chromatography fractions at 492 nm revealed the presence of two distinct peaks. The fractions of the first peak contained a bluish green fluorescent eluate. The second peak contained free FITC. The covalent attachment of FITC to FlaB was confirmed by SDS-PAGE. The gels exposed to UV light prior to staining revealed the presence of at least two fluorescently labeled FlaB protein bands at the 31-kDa site (Fig. 2A, lanes 1 and 3), which corresponds to the position of unlabeled FlaB (Fig. 2B, lane 2). However, it was observed that unlabeled FlaB migrated as a single band with an apparent molecular mass of 31 kDa (Fig. 2B, lane 2). The fractions of the first peak containing FITC-labeled FlaB were pooled and used as the tracer antigen.
FIG. 2.
SDS-PAGE analysis of FITC-labeled and unlabeled recombinant FlaB protein. (A) A photograph of the gel taken under UV illumination. (B) The same gel after Coomassie blue staining. Lane 1, reaction mixture (FlaB plus FITC) following 3 h of incubation at 37°C; lane 2, unlabeled FlaB; lane 3, FITC-labeled FlaB after gel filtration chromatography. The recombinant protein FlaB is indicated with an arrowhead.
Western blotting.
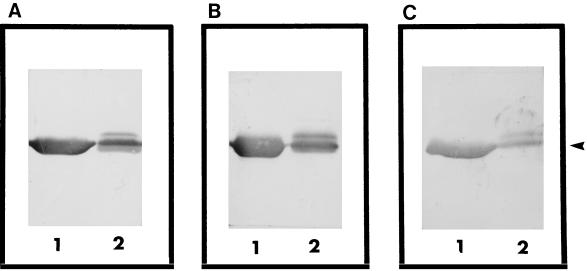
The antigenicities of labeled and unlabeled FlaB were examined by Western blotting with bovine sera containing antibodies against L. interrogans serovar pomona and Leptospira borgpetersenii serovars hardjo and sejroe. Sera from uninfected animals were used as negative controls. Western blot analysis revealed that both labeled and unlabeled FlaB proteins were recognized by antibodies against various Leptospira serovars (Fig. 3A to C). In the case of unlabeled FlaB, a serum sample with a high titer of antibodies against L. interrogans serovar pomona, as determined by MAT, recognized a single band at the 31-kDa site (Fig. 3A, lane 1). However, in the case of labeled FlaB, the same serum sample reacted with at least two FlaB protein bands at the 31-kDa position (Fig. 3, lane 2) with the same intensity. Similar patterns were obtained with serum samples with high titers of antibodies against L. borgpetersenii serovars hardjo and sejroe (Fig. 3B and C, respectively). This suggests that the purified unlabeled and labeled FlaB proteins were immunologically active and that the immunogenicity of the epitope was not altered by modification with fluorescein. The MAT-negative serum samples did not react with labeled or unlabeled FlaB.
FIG. 3.
Western blot analysis of unlabeled FlaB (lanes 1) and FITC-labeled FlaB (lanes 2). Recombinant protein was electrophoresed on an SDS–12% polyacrylamide gel, transblotted onto an NC, and immunostained with bovine sera containing antibodies against L. interrogans serovar pomona (A), L. borgpetersenii serovar hardjo (B), and L. borgpetersenii serovar sejroe (C). Ten microliters of labeled and unlabeled protein was loaded into each lane. The recombinant protein FlaB is indicated with an arrowhead.
FPA.
FPA was performed with fluorescein-labeled FlaB as a tracer antigen. A mean fluorescence millipolarization value (mP) value of 147 mP (n = 4) was obtained when the fluorescence polarization of the tracer antigen diluted in PBS was measured. Initially, five MAT-positive and four MAT-negative bovine serum samples (diluted 1:50) were analyzed by FPA. In the presence of the tracer antigen, the MAT-positive sera gave mean mP values ranging from 201 to 214 (n = 5). In the presence of the tracer antigen, the MAT-negative sera gave mean mP values of between 151 and 155 (n = 4), which was very close to the mP value obtained with the tracer antigen alone. When the same serum samples were tested by Western blotting with the tracer antigen, the presence or absence of antileptospiral antibodies was confirmed. It was demonstrated that Western blotting results were in agreement with FPA results. These results suggested the feasibility of using FITC-labeled recombinant FlaB as a tracer antigen for the detection of antileptospiral antibodies by FPA.
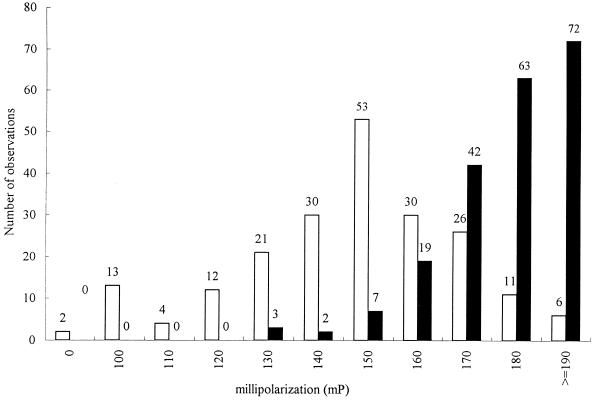
A total of 416 field serum samples were then tested by FPA. A total of 208 serum samples which were MAT positive for at least one of the Leptospira serovars, pomona, sejroe, canicola, icterohaemorrhagiae, grippotyphosa, or hardjo, were used to estimate the relative sensitivity of FPA, and 208 serum samples which were MAT negative for the serovars listed above were used to estimate the relative specificity of FPA. The relative sensitivity and relative specificity were calculated by ROC analysis at different cutoff points. In this study, a large area (0.90; 95% confidence interval = 0.856 to 0.918) was observed under the ROC curve for FPA (data not shown), suggesting that FPA could be a useful test for the serodiagnosis of leptospirosis. It was demonstrated that at low cutoff points, FPA has a high relative sensitivity and high negative predictive values; however, the relative specificity and positive predictive values were low. At higher cutoff points, although the relative specificity and positive predictive values were high, the relative sensitivity and negative predictive values were low. The optimum cutoff point for FPA from the ROC curve was 161 mP, which yielded a relative sensitivity value of 83.7 ± 0.01% and a relative specificity value of 81.2% ± 0.01% (Table 1). The positive predictive value was 81.7% and the negative predictive value was 83.3% (Table 1). The distribution of data obtained with the 416 serum samples that were positive or negative for antileptospiral antibodies is shown in Fig. 4. The sample numbers (y axis) were plotted against the mP value (x axis). An area of overlap between MAT-positive and MAT-negative samples was observed, in that some MAT-negative serum samples had high mP values by FPA.
TABLE 1.
Characteristics of FPA for diagnosis of leptospirosisa
| ROC cutoff (mP) | Relative sensitivity (%) | Relative specificity (%) | Positive predictive value (%) | Negative predictive value (%) |
|---|---|---|---|---|
| 121 | 99.5 | 15.9 | 54.2 | 97.0 |
| 141 | 97.1 | 40.4 | 62.0 | 93.3 |
| 151 | 93.3 | 66.3 | 73.5 | 90.8 |
| 161b | 83.7 | 81.2 | 81.7 | 83.3 |
| 171 | 62.0 | 93.3 | 90.2 | 71.1 |
| 182 | 28.4 | 97.1 | 90.8 | 57.5 |
| 192 | 7.7 | 99.0 | 88.9 | 51.8 |
The area under the ROC curve was 0.9, the standard error was 0.01, and the 95% confidence interval was 0.856 to 0.918.
Cutoff point giving the most efficient screening characteristics of FPA.
FIG. 4.
Distribution of 416 MAT-positive (■) and -negative (□) bovine serum samples tested by FPA for the presence of antileptospiral antibodies. Total serum sample numbers (y axis) were plotted against mP values (x axis), which is a measure of the fluorescence polarization.
DISCUSSION
In the present study, we report on the development and preliminary evaluation of a novel immunoassay, FPA, for the detection of antileptospiral antibodies in a panel of bovine sera with a fluorescein-labeled recombinant protein, FlaB, as the tracer antigen. The FPA has been applied to the quantitation of analytes, investigations of protein-protein interactions, ligand binding, and enzymatic activity, and monitoring of the interaction between antibody and its epitope (5, 12, 14, 20, 21, 26, 29, 30, 35, 41, 46, 51, 52).
The first aim of this study was to produce highly purified recombinant protein FlaB. By using a combination of Ni-NTA affinity chromatography, electrophoretic separation by SDS-PAGE, and electroelution, FlaB protein was recovered in a homogeneous and antigenically active form. The role of protein FlaB in the immune response to Leptospira is not yet known. Chang and Faine (9) demonstrated that leptospiral flagella are highly immunogenic. Several studies have reported that leptospiral flagella are recognized by antibodies induced by natural and experimental leptospirosis in animals and humans, and antiflagellar sera have been shown to agglutinate the whole leptospires (2, 10, 19, 22).
The second goal of this study was to label purified FlaB with fluorescein and examine if it could be used as a tracer antigen for the detection of antileptospiral antibodies by an FPA. Some discrepancies in the migration of labeled and unlabeled FlaB on SDS-polyacrylamide gels were observed. The labeled FlaB seem to comprise at least two bands; however, unlabeled FlaB appeared as a single band. The reason for this difference is unclear. It is possible that the modification of FlaB with FITC may have resulted in at least two populations of labeled proteins with different degrees of substitution.
Western blots of FITC-labeled and unlabeled FlaB were performed with bovine serum samples with titers against Leptospira serovars pomona, hardjo, and sejroe, as determined by MAT, to confirm the antigenicity of FlaB. It was observed that the labeled as well as the unlabeled FlaB proteins demonstrated similar reactivities to the MAT-positive sera, indicating the broad antigenic cross-reactivity of this recombinant protein. It also indicated that labeling with fluorescein did not affect the antigenicity.
To evaluate the relative sensitivity and relative specificity of FPA, a total of 416 field bovine serum samples were used. All serum samples were initially tested once by MAT against Leptospira serovars pomona, sejroe, canicola, icterohaemorrhagiae, grippotyphosa, and hardjo. The majority of positive serum samples reacted to Leptospira serovar pomona, with other commonly reacting serovars being Leptospira serovars sejroe, hardjo, and canicola. The relative sensitivity and relative specificity were calculated at different cutoff points by ROC analysis. The area under the ROC curve indicates the ability of FPA to discriminate the sera with and without antibodies. The larger the area under the ROC curve the better the test discriminates. A perfectly informative test has an area of 1.0, whereas a noninformative test has an area of 0.5 or less (24, 41). In this study, a large area (0.90; 95% confidence interval = 0.856 to 0.918) was observed under the ROC curve for FPA, suggesting that FPA could be a useful test for the serodiagnosis of leptospirosis. A cutoff point of 161 mP yielded the best combination of relative sensitivity (83.7%) and relative specificity (81.2%). However, the overall relative sensitivity may be increased up to 99.5% (with a corresponding relative specificity of 15.9%) if the cutoff is established at a lower point (121 mP). The overall relative specificity may be increased to up to 99.0% (with a corresponding relative sensitivity of 7.7%) if the cutoff is established at a higher point (192 mP). The frequency distribution histogram for 416 MAT-positive and MAT-negative bovine serum samples demonstrated an area of overlap between MAT-positive and MAT-negative samples, in that some MAT-negative serum samples had high mP values by FPA. One explanation for this overlap could be that MAT-negative sera may contain nonagglutinating antibodies which were detected by FPA but not by MAT since MAT detects agglutinating antibodies only.
It must be stressed that these results were obtained from measurements performed with serum samples kept at −20°C for a period of 2 to 3 years. It must also be noted that the antisera used in this study are defined as positive or negative for particular Leptospira serovars on the basis of MAT results alone. Due to the difficulty and expense associated with the culture of Leptospira, culture-positive or culture-negative serum samples are not available.
In our study, some MAT-positive bovine serum samples were found to be negative by FPA and some MAT-negative serum samples were found to be positive by FPA. The results could be inconsistent for a number of reasons, including the presence of nonspecific components in the serum samples or the presence of antibodies of an isotype(s) not recognized by MAT. Several studies (2, 3, 22, 53) have demonstrated that the agglutinins produced are mainly IgM. Some other studies (22, 56) have reported that very few animals and humans with leptospirosis produce IgG agglutinins that can be measured by MAT; however, the presence of IgG antibodies was detectable by ELISA. Presumably, they were directed against nonagglutinating antigens (39).
This report presents an efficient method for the purification of flagellin recombinant protein FlaB from E. coli. The purification procedures presented here are rapid and easy to perform and provide the recombinant protein at a good recovery rate and a high purity. This procedure can readily be scaled up, therefore allowing the production of sufficient antigen for immunological and biochemical studies. We are encouraged by the fact that FlaB appears to retain most of the features of its native properties even after the multiple-step purification procedure. The analysis of our FPA data reveals that this test could be a valuable addition to the existing methods for the diagnosis of leptospirosis. MAT is the standard reference method for the serodiagnosis of leptospirosis. MAT is laborious and time-consuming; requires multiple serovars of fresh, live bacteria and the maintenance of a large number of stock cultures to provide antigens, which increases the risk of laboratory-acquired infection; requires specialized laboratory facilities; and is a sensitive detector of IgM antibodies alone. In addition, the interpretation of the results is subjective, and there is no field application. ELISA is simple and easily automated, but it is time-consuming and expensive. FPA has several advantages over MAT and ELISA: (i) FPA is safe since it uses fluorescein-labeled proteins instead of live bacteria of multiple serovars as the antigen(s); (ii) it is a homogeneous assay, with no blocking, washing, or antigen immobilization steps required; (iii) it uses a single reagent; (iv) it is relatively low cost; (v) it is simple; (vi) it is fast; (vii) it is a sensitive detector of IgM, IgG, and IgA; (viii) its results can be assessed objectively; and (ix) due to its rapidity and ease, it can be adapted as a field test. These qualities indicate that FPA may be a useful technique for diagnosis and epidemiological studies of leptospirosis. We are in the process of conducting a large-scale validation study in order to further investigate the performance of FPA. Statistical analysis of the results obtained with a larger panel of sera may result in a reevaluation of the cut-off point and the relative sensitivity and relative specificity values.
ACKNOWLEDGMENTS
We are indebted to Klaus Nielsen, Donna Hutchings, and Brian Brooks for helpful comments. We also thank David Gall for excellent assistance with the statistical analysis and Pat Smith for help with FPA.
The work was supported in part by Diachemix Corporation (Grayslake, Ill.).
REFERENCES
- 1.Acil Y, Brinckmann J, Behrens P, Muller P K, Batge B. Semipreparative isolation of collagen types I, II, III and V by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electroelution. J Chromatogr. 1997;758:313–318. doi: 10.1016/s0021-9673(96)00729-7. [DOI] [PubMed] [Google Scholar]
- 2.Adler B, Faine S. Antibodies against leptospiral axial filament in human anti-Leptospira sera. FEMS Microbiol Lett. 1979;11:387–400. [Google Scholar]
- 3.Adler B, Murphy A M, Locarnini S A, Faine S. Detection of specific anti-leptospiral immunoglobulins M and G in human serum by solid-phase enzyme-linked immunosorbent assay. J Clin Microbiol. 1980;11:452–457. doi: 10.1128/jcm.11.5.452-457.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Appassakij H, Silpapojakul K, Wansit R, Woodtayakorn J. Evaluation of the immunofluorescent antibody test for the diagnosis of human leptospirosis. Am J Trop Med Hyg. 1995;52:340–343. doi: 10.4269/ajtmh.1995.52.340. [DOI] [PubMed] [Google Scholar]
- 5.Bentley K L. Fluorescence polarization: a general method for measuring ligand binding and membrane microviscosity. BioTechniques. 1985;5:356–366. [Google Scholar]
- 6.Bolin C A. Diagnosis of leptospirosis: a reemerging disease of companion animals. Semin Vet Med Surg. 1996;11:166–171. doi: 10.1016/s1096-2867(96)80029-6. [DOI] [PubMed] [Google Scholar]
- 7.Bughio N I, Faubert G M, Prichard R K. Characterization and biological activities of monoclonal antibodies against Brugia pahangi tubulin. Int J Parasitol. 1993;7:913–918. doi: 10.1016/0020-7519(93)90057-6. [DOI] [PubMed] [Google Scholar]
- 8.Champion C I, Miller J N, Lovett M A, Bianco D R. Cloning, sequencing and expression of two class B endoflagellar genes of Treponema pallidum subsp. pallidum encoding the 34.5 and 31.0 kDa proteins. Infect Immun. 1990;58:1697–1704. doi: 10.1128/iai.58.6.1697-1704.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang A, Faine S. Effect of anti-cell and anti-axial filament sera on Leptospira. Aust J Exp Biol Med Sci. 1973;52:549–568. doi: 10.1038/icb.1973.80. [DOI] [PubMed] [Google Scholar]
- 10.Chapman A J, Adler B, Faine S. Antigens recognized by the human immune response to infection with L. interrogans serovar hardjo. J Med Microbiol. 1988;25:269–278. doi: 10.1099/00222615-25-4-269. [DOI] [PubMed] [Google Scholar]
- 11.Cole J R, Sulzer C R, Pursell A R. Improved microtechnique for the leptospiral microscopic agglutination test. Appl Microbiol. 1973;25:976–980. doi: 10.1128/am.25.6.976-980.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dandliker W B, Hsu M, Vanderlaan W P. Fluorescence polarization immunoreceptor assays. Lab Res Met Biol Med. 1980;4:65–88. [Google Scholar]
- 13.Dandliker W B, Kelly R J, Dandliker J, et al. Fluorescence polarization immunoassay: theory and experimental method. Immunochemistry. 1973;10:219–227. doi: 10.1016/0019-2791(73)90198-5. [DOI] [PubMed] [Google Scholar]
- 14.Eksborg S, Albertioni F, Rask C, Beck O, Palm C, Schroeder H, Peterson C. Methotrexate plasma pharmacokinetics: importance of assay method. Cancer Lett. 1996;108:163–169. doi: 10.1016/s0304-3835(96)04394-7. [DOI] [PubMed] [Google Scholar]
- 15.Faine S. Leptospirosis. In: Evans A S, Brachman P S, editors. Bacterial infections of humans: epidemiology and control. New York, N.Y: Plenum Publishing Corporation; 1991. pp. 367–393. [Google Scholar]
- 16.Faine S. Leptospira and leptospirosis. Boca Raton, Fla: CRC Press, Inc.; 1994. [Google Scholar]
- 17.Fairbrother J M. Antibody response to genus- and serovar-specific leptospiral antigen in Leptospira-infected cows. Am J Vet Res. 1985;46:1422–1426. [PubMed] [Google Scholar]
- 18.Farr R W. Leptospirosis. Clin Infect Dis. 1995;21:1–8. doi: 10.1093/clinids/21.1.1. [DOI] [PubMed] [Google Scholar]
- 19.Farrelly H E, Adler B, Faine S. Opsonic monoclonal antibodies against lipopolysaccharide antigens of Leptospira interrogans serovar hardjo. J Med Microbiol. 1987;23:1–7. doi: 10.1099/00222615-23-1-1. [DOI] [PubMed] [Google Scholar]
- 20.Ferns-Bridget R, Tuke P W, Sweenie C H. Characterization of a panel of monoclonal antibodies raised against recombinant HCV core protein. J Med Virol. 1996;50:221–229. doi: 10.1002/(SICI)1096-9071(199611)50:3<221::AID-JMV3>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 21.Goetschy J F, Letourneur O, Cerletti N, Horisberger M A. The unglycosylated extracellular domain of type-II receptor for transforming growth factor beta. Eur J Biochem. 1996;241:355–362. doi: 10.1111/j.1432-1033.1996.00355.x. [DOI] [PubMed] [Google Scholar]
- 22.Graves S R, Faine S. Antileptospiral agglutinins produced in rabbits. Bull W H O. 1970;43:579–587. [PMC free article] [PubMed] [Google Scholar]
- 23.Gussenhoven G C, Hoor M A, Goris M G A, Terpstra W J, Hartskeerl R A, Mol B W, Smits H L. Lepto-dipstick, a dipstick assay for detection of Leptospira-specific immunoglobulin M antibodies in human sera. J Clin Microbiol. 1997;35:92–97. doi: 10.1128/jcm.35.1.92-97.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han U K, Kim H Y. Determination of class II and class III skeletal patterns: ROC analysis on various cephalometric measurements. Am J Orthod Dentofacial Orthop. 1998;113:538–545. doi: 10.1016/s0889-5406(98)70265-3. [DOI] [PubMed] [Google Scholar]
- 25.Harrington M G. Elution of protein from gels. Methods Enzymol. 1990;182:488–495. doi: 10.1016/0076-6879(90)82039-5. [DOI] [PubMed] [Google Scholar]
- 26.Helene C, Brun F, Yaniv M. Fluorescent study of interactions between valyl-tRNA synthtase and valine-specific tRNAs from E. coli. Biochem Biophys Res Commun. 1969;37:393–398. doi: 10.1016/0006-291x(69)90927-9. [DOI] [PubMed] [Google Scholar]
- 27.Hochuli E. Purification of recombinant proteins with metal chelate absorbent. In: Setlow J K, editor. Genetic engineering, principle and method. New York, N.Y: Plenum Publishing Corporation; 1990. pp. 87–98. [DOI] [PubMed] [Google Scholar]
- 28.Holzinger A, Phillips KS, Weaver T E. Single step purification/solubilization of 6× His-tagged proteins on Ni-NTA agarose. News Qiagen. 1996;4:14–15. [Google Scholar]
- 29.Jolley M E, Stroupe S D, Schwenzer K S, Wang C J, Lu-Steffes M, Hill H D, Popelka S R, Holen J T, Lelso D M. Fluorescence polarization immunoassay. III. An automated system for therapeutic drug determination. Clin Chem. 1981;9:1575–1579. [PubMed] [Google Scholar]
- 30.Jolley M E. Fluorescence polarization immunoassay for the detection of therapeutic drug levels in human plasma. J Anal Toxicol. 1981;5:236–240. doi: 10.1093/jat/5.5.236. [DOI] [PubMed] [Google Scholar]
- 31.Kelson J S, Adler B, Chapman A J, Faine S. Identification of leptospiral flagellar antigens by gel electrophoresis and immunoblotting. J Med Microbiol. 1987;26:47–53. doi: 10.1099/00222615-26-1-47. [DOI] [PubMed] [Google Scholar]
- 32.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 33.Lin M, Sugden E A, Jolley M E, Stilwell K. Modification of the Mycobacterium bovis extracellular protein MPB70 with fluorescein for rapid detection of specific serum antibodies by fluorescence polarization. Clin Diagn Lab Immunol. 1996;4:438–443. doi: 10.1128/cdli.3.4.438-443.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin M, Surujballi O P, Nielsen K, Nadin-Davis S, Randell G. Identification of a 35 kilodalton serovar-cross-reactive flagellar protein, FlaB, from Leptospira interrogans by N-terminal sequencing, gene cloning and sequence analysis. Infect Immun. 1997;65:4355–4359. doi: 10.1128/iai.65.10.4355-4359.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin M, Nielsen K. Binding of the Brucella abortus lipopolysaccharide O-chain fragment to a monoclonal antibody. J Biol Chem. 1997;272:2821–2827. doi: 10.1074/jbc.272.5.2821. [DOI] [PubMed] [Google Scholar]
- 36.Lin M, Bughio N, Surujballi O. Expression in Escherichia coli of flaB, the gene coding for a periplasmic flagellin of Leptospira interrogans serovar pomona. J Med Microbiol. 1999;48:1–6. doi: 10.1099/00222615-48-11-977. [DOI] [PubMed] [Google Scholar]
- 37.Metz C E. Basic principles of ROC analysis. Semin Nucl Med. 1978;8:283–298. doi: 10.1016/s0001-2998(78)80014-2. [DOI] [PubMed] [Google Scholar]
- 38.Mitchison M, Rood J I, Faine S, Adler B. Molecular analysis of the Leptospira borgpetersenii gene encoding an endoflagellar subunit protein. J Gen Microbiol. 1991;137:1529–1536. doi: 10.1099/00221287-137-7-1529. [DOI] [PubMed] [Google Scholar]
- 39.Morris J A, Hussaini S N. Characterisation of the antibodies detected by the MAT for bovine leptospirosis. J Hyg Camb. 1974;73:425–432. doi: 10.1017/s0022172400042789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morris J A, Gill J E, Hussaini S N. An examination of antibodies active in the indirect hemagglutination test for bovine leptospirosis. Br Vet J. 1977;133:17–24. doi: 10.1016/s0007-1935(17)34183-0. [DOI] [PubMed] [Google Scholar]
- 41.Nielsen K, Gall D, Jolley M E, Leishman G, Balsevicus S, Smith P, Nicoletti P, Thomas F. A homogeneous fluorescence polarization antibody assay for detection of antibody to Brucella abortus. J Immunol Methods. 1996;195:161–168. doi: 10.1016/0022-1759(96)00116-0. [DOI] [PubMed] [Google Scholar]
- 42.Norris S J, Charon N W, Cook R G, Fuentes M D, Limberger R J. Antigenic relatedness and N-terminal sequence homology define two classes of major periplasmic flagellar proteins of Treponema pallidum subspecies pallidum and Treponema phagedenis. J Bacteriol. 1988;170:4072–4082. doi: 10.1128/jb.170.9.4072-4082.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nunes-Edwards P L, Thiermann A B, Bassford P J, Stamm L V. Identification and characterization of the protein antigens of Leptospira interrogans serovar hardjo. Infect Immun. 1985;48:492–497. doi: 10.1128/iai.48.2.492-497.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pappas M G, Ballou W R, Gray M R, Takafuji E T, Miller R N, Hockmeyer W T. Rapid diagnosis of leptospirosis using the IgM-specific dot-ELISA: comparison with MAT. Am J Trop Med Hyg. 1985;34:346–354. doi: 10.4269/ajtmh.1985.34.346. [DOI] [PubMed] [Google Scholar]
- 45.Parales J J, Greeberg E P. N-terminal amino acid sequences and amino acid compositions of the Spirocheta aurantia flagellar filament polypeptides. J Bacteriol. 1991;173:1357–1359. doi: 10.1128/jb.173.3.1357-1359.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perrin F. Polarization de la lumiere de fluorescence. Vie moyenne des molecules dans l’etat excite. J Phys Radium. 1921;7:390–401. [Google Scholar]
- 47.Rhys-Williams A T. Fluorescence polarization immunoassays. In: Collins W P, editor. Complementary immunoassays. Chichester, United Kingdom: John Wiley; 1988. pp. 135–147. [Google Scholar]
- 48.Silva M V, Nakamura P M, Camargo E D, Batista L, Vaz A J, Romero E C, Brandao A P. Immunodiagnosis of human leptospirosis by dot-ELISA for the detection of IgM, IgG and IgA antibodies. Am J Trop Med Hyg. 1997;56:650–655. doi: 10.4269/ajtmh.1997.56.650. [DOI] [PubMed] [Google Scholar]
- 49.Smith C R, Ketterer P J, McGowan M R, Corney B G. A review of laboratory techniques and their use in the diagnosis of Leptospira interrogans serovar hardjo infection in cattle. Aust Vet J. 1994;71:290–294. doi: 10.1111/j.1751-0813.1994.tb03447.x. [DOI] [PubMed] [Google Scholar]
- 50.Smith P, Krohn R I, Hermanson G T, Mallia A K, Gartner F, Provenzano M D, Fujimoto E K, Goeke N M, Olson B J, Klenk D C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 51.Spencer R D. Application of fluorescence polarization in clinical assays. Clin Biochem Anal. 1981;10:143–170. [Google Scholar]
- 52.Steinmann L, Thormann W. Characterization of competitive binding, fluorescent drug immunoassays based on micellar electrokinetic capillary chromatography. Electrophoresis. 1996;17:1348–1356. doi: 10.1002/elps.1150170812. [DOI] [PubMed] [Google Scholar]
- 53.Surujballi O P, Marenger R M, Eaglesome M D, Sugden E A. Development and initial evaluation of an indirect ELISA for the detection of L. interrogans serovars hardjo antibodies in bovine sera. Can J Vet Res. 1997;61:260–266. [PMC free article] [PubMed] [Google Scholar]
- 54.Terpstra W J, Lighthart G S, Schoone G J. Serodiagnosis of human leptospirosis by ELISA. Zentbl Bakteriol Parasitenkd Infektionskr Hyg Abt 1 Orig Reihe A. 1980;247:400–405. [PubMed] [Google Scholar]
- 55.Thiermann A B, Garrett L A. Enzyme-linked immunosorbent assay for the detection of antibodies to Leptospira interrogans serovars hardjo and pomona in cattle. Am J Vet Res. 1983;44:884–887. [PubMed] [Google Scholar]
- 56.Tong M J, Rosenberg E B, Voteri B A, Che C T. Immunological response in leptospirosis. Report of three cases. Am J Trop Med Hyg. 1971;20:625–630. doi: 10.4269/ajtmh.1971.20.625. [DOI] [PubMed] [Google Scholar]
- 57.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheet. Procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Watt G, Alquiza M L, Padre L P, Tuazaon M L, Laughlin L W. The rapid diagnosis of leptospirosis: a prospective comparison of the dot-ELISA and the genus-specific microscopic agglutination test at different stages of illness. J Infect Dis. 1988;157:840–842. doi: 10.1093/infdis/157.4.840. [DOI] [PubMed] [Google Scholar]
- 59.Yasuda P H, Stiegerwalt A G, Sulzer K R, Kaufmann A F, Rogers F, Brenner D J. Deoxyribonucleic acid relatedness between serogroups and serovars in the family Leptospiraceae with proposals for seven new Leptospira species. Int J Syst Bacteriol. 1987;37:407–415. doi: 10.1099/00207713-49-2-839. [DOI] [PubMed] [Google Scholar]