Abstract
Reactions of 5,5,5-trichloropent-3-en-2-one Cl3CCH=CHC(=O)Me with arenes in Brønsted superacid CF3SO3H at room temperature for 2 h–5 days afford 3-methyl-1-trichloromethylindenes, a novel class of indene derivatives. The key reactive intermediate, O-protonated form of starting compound Cl3CCH=CHC(=OH+)Me, has been studied experimentally by NMR in CF3SO3H and theoretically by DFT calculations. The reaction proceeds through initial hydroarylation of the carbon-carbon double bond of starting CCl3-enone, followed by cyclization onto the O-protonated carbonyl group, leading to target indenes. In general, 5,5,5-trichloropent-3-en-2-one in CF3SO3H acts as a 1,3-bi-centered electrophile.
Keywords: enones, indenes, Friedel-Crafts reaction, carbocations, triflic acid
1. Introduction
Superelectrophilic activation under the action of strong Brønsted and Lewis acids is a useful tool in organic synthesis, giving access to a variety of compounds [1,2,3,4,5,6,7,8]. Protonation (or coordination) of basic centers of organic molecules in Brønsted (or Lewis) acids affords intermediate highly reactive cationic species. In particular, superelectrophilic activation of conjugated enones consequently gives rise to O-protonated and O,C-diprotonated species. The latter takes part in electrophilic aromatic substitution reactions with arenes (Scheme 1a) [9,10,11,12,13,14,15,16,17,18]. The formation of O,C-diprotonated species from various conjugated enone structures, such as butenones [9,18], indenones [12], cinnamic acids, and their esters and amides [13,14,15,16], was proved experimentally by NMR and theoretically by DFT calculations. It has been shown that these dications are key reactive intermediates in various Friedel–Crafts processes [9,10,11,12,13,14,15,16,17,18].
Scheme 1.
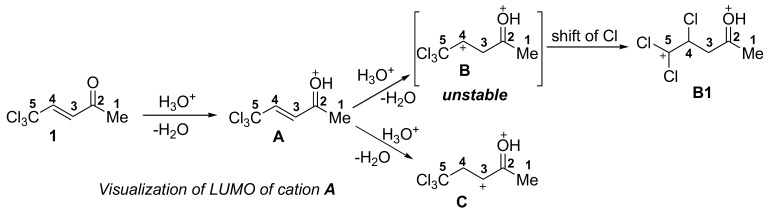
Generating of O-protonated and O,C-diprotonated species from conjugated enones and 5,5,5-trichloropent-3-en-2-one 1 under superelectrophilic activation conditions [9,10,11,12,13,14,15,16,17,18].
Based on our recent studies on superelectrophilic activation of electron deficient alkenes [19,20,21], we undertook this study on electrophilic activation of E-5,5,5-trichloropent-3-en-2-one 1 (CCl3-enone). The presence of two electron withdrawing groups, COMe and CCl3, at the carbon-carbon double bond increases its electrophilicity, especially under protonation of the carbonyl oxygen-resulting O-protonated species A (Scheme 1b). The second protonation of C=C bond in cation A may be hampered due to the strong acceptor characteristics of substituents C(OH+)Me and CCl3. However, species A possesses enough electrophilicity to react with aromatic nucleophiles.
The main goals of this study were to investigate the protonation of E-5,5,5-trichloropent-3-en-2-one 1 by NMR and DFT calculations and study its reactions with arenes under the action of strong Brønsted and Lewis acids.
2. Results and Discussion
Protonation of CCl3-enone 1 in various Brønsted acids (CH3COOH, CF3COOH, H2SO4, CF3SO3H) was initially investigated by means of NMR. According to 1H and 13C NMR data, CCl3-enone 1 gives stable O-protonated form A in these acids at room temperature (Table 1, see original spectra in Supplementary Materials). Upon increasing the acidity in the row CH3COOH→CF3COOH→H2SO4→CF3SO3H [1], signals of protons H3, H4 and carbons C2, C4 are shifted more and more downfield. The corresponding differences in chemical shifts (∆δ = δacid– δCDCl3) for atoms H3, H4 and C2, C4 are gradually increased (Table 1). These data reveal that the positive charge is mainly localized on carbons C2 and C4 in cation A, and both these atoms may act as reactive electrophilic centers in consequent interactions with aromatic nucleophiles.
Table 1.
1H and 13C NMR data of CCl3-enone 1 (in CDCl3) and its O-protonated form A (in Brønsted acids).
| Compound 1 and Cation A | Solvent | 1H NMR, δ, ppm | 13C NMR, δ, ppm | ||||||
|---|---|---|---|---|---|---|---|---|---|
| H1 | H3 | H4 | C1 | C2 | C3 | C4 | 5CCl3 | ||
|
CDCl3 | 2.40 | 6.61 | 7.05 | 28.9 | 196.7 | 128.0 | 144.4 | 92.6 |

|
CH3CO2H a ∆δ b |
2.29 | 6.56 | 7.06 | 27.6 | 198.4 | 128.1 | 144.3 | 92.4 |
| −0.11 | −0.05 | 0.01 | −1.3 | 1.7 | 0.1 | −0.1 | −0.2 | ||
| CF3CO2H a ∆δ b |
2.62 | 6.85 | 7.34 | 31.7 | 210.8 | 131.9 | 153.4 | 96.2 | |
| 0.22 | 0.24 | 0.29 | 2.8 | 14.1 | 3.9 | 9.0 | 3.6 | ||
| H2SO4 a ∆δ b |
3.03 | 7.11 | 7.86 | 26.4 | 221.8 | 123.9 | 158.5 | 89.3 | |
| 0.63 | 0.5 | 0.81 | −2.5 | 25.1 | −4.1 | 14.1 | −3.3 | ||
| CF3SO3H a ∆δ b |
3.22 | 7.31 | 8.14 | 27.5 | 226.7 | 124.3 | 163.2 | 89.9 | |
| 0.82 | 0.7 | 1.09 | −1.4 | 30.0 | −3.7 | 18.8 | −2.7 | ||
Notes.a CH2Cl2 was used as internal standard. b ∆δ = δacid − δCDCl3.
Then, DFT calculations of cations A–C derived from the protonation of CCl3-enone 1 have been carried out. The thermodynamics of their formation, such as Gibbs energies ΔG298 of protonation reactions, energies of HOMO/LUMO, electrophilicity indices ω [22,23], charge distribution, and contribution of atomic orbital into LUMO of species A–C have been estimated (Table 2, see full data in Supplementary Materials).
Table 2.
Selected calculated (DFT) electronic characteristics of the protonated forms A, B, C of CCl3-enone 1, and values of Gibbs energies of protonation reactions (ΔG, kJ/mol).

| Entry | Species | EHOMO, eV | ELUMO, eV | ω, a eV | q(C2), b e | q(C3), b e | q(C4), b e | k(C2)LUMO, c % | k(C3)LUMO, c % | k(C4)LUMO, c % | ΔG, d kJ/mol |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |

|
−7.62 | −2.61 | 2.6 | 0.57 | −0.26 | −0.18 | 9 | 10 | 14 | - |
| 2 |

|
−8.87 | −4.21 | 4.6 | 0.66 | −0.31 | −0.06 | 28 | 4 | 21 | 1→A −35 |
| 3 |

|
−9.73 | −5.51 | 6.9 | 0.75 | −0.53 | −0.29 | 5.4 | 8.9 | 15.3 | A→B1 196 |
| 4 |

|
−9.25 | −6.98 | 14.5 | 0.65 | 0.26 | −0.56 | 15 | 41 | 7 | A→C 268 |
Notes. a Global electrophilicity index ω = (EHOMO + ELUMO)2 /8(ELUMO − EHOMO). b Natural charges. c Contribution of atomic orbital into the molecular orbital. d Gibbs energy of protonation reactions.
The formation of O-protonated species A is very favorable, as the ΔG298 value of the protonation is negative (−35 kJ/mol). Secondly, the protonation of the C=C bond, both onto carbons C3 and C4, which leads to dications B and C, is, correspondingly, extremely unfavorable, due to the very high positive values of protonation Gibbs energies (Table 2). Thus, the generation of O,C-diprotonated species B and C from CCl3-enone 1 is very unlikely; that is, in accordance with NMR data (Table 1). Apart from that, it has been found that dication B is extremely unstable. It is spontaneously rearranged into species B1 via a shift of a chlorine atom.
Calculations show that the largest part of positive charge in species A is localized on atom C2 (0.66 e). Apart from that, this carbon atom contributes significantly to LUMO by 28%. There are similarities between the charge and orbital factors of the electrophilic properties of carbon C2. Contrary to that, carbon C4 bears no positive charge (−0.06 e), but it contributes significantly into LUMO by 21% (see LUMO visualization of cation A in Table 2). Electrophilic properties of atom C4 can be mainly explained by orbital factors.
Reactions of CCl3-enone 1 with benzene under the action of various Brønsted and Lewis acids have also been conducted (Table 3). The use of strong Lewis acids AlCl3 or AlBr3 yields complex mixtures of oligomeric materials (entries 1–3). Reaction in H2SO4 results in the formation of alcohol 3 as a product of hydration of the carbon-carbon double bond; no reaction with benzene occurs (entry 4). Reaction in Brønsted superacid CF3SO3H (triflic acid, TfOH) at room temperature for 5 days affords indene 2a in yields 29% (entry 7). Under other conditions (temperature and time) in CF3SO3H, the formation of 2a is unsatisfactory (entries 5, 6, 8, 9), as is the reaction in stronger acid FSO3H at a low temperature of −78 °C (entry 10). In weaker acids, CH3CO2H and CH3CO2H, the reaction does not take place (entries 11–14). These data reveal that the formation of indene 2a in CF3SO3H is accompanied by cationic oligomerization processes, which leads to a decrease in the yield of the target compound. The formation of indene 2a points out that the starting compound 1 in CF3SO3H behaves as a precursor of the bi-centered electrophile, with reactive cationic centers on carbons C2 and C4.
Table 3.
Reactions of CCl3-enone 1 with benzene under the action of excess of various acids.
| Entry | Acid | Temperature | Time | Reaction Product, Yield, % |
|---|---|---|---|---|
| 1 | AlCl3 | room temperature | 1 h | oligomeric material a |
| 2 | AlBr3 | room temperature | 1 h | oligomeric material b |
| 3 | AlBr3 | room temperature | 2 h | oligomeric material a |
| 4 | H2SO4 | room temperature | 6 days | 3, 75% |
| 5 | CF3SO3H | room temperature | 0.5 h | quantitative isolation of starting compound 1 |
| 6 | CF3SO3H | room temperature | 3 days | 2a, 20% b |
| 7 | CF3SO3H | room temperature | 5 days | 2a, 29% a |
| 8 | CF3SO3H | 60 °C | 0.5 h | oligomeric material a |
| 9 | CF3SO3H | 80 °C | 1 h | oligomeric material a |
| 10 | FSO3H | −78 °C | 2 h | quantitative isolation of starting compound 1 |
| 11 | CH3CO2H | room temperature | 2 days | quantitative isolation of starting compound 1 |
| 12 | CH3CO2H | 80 °C | 1 h | quantitative isolation of starting compound 1 |
| 13 | CF3CO2H | room temperature | 2 days | quantitative isolation of starting compound 1 |
| 14 | CF3CO2H | 80 °C | 1 h | quantitative isolation of starting compound 1 |
Notes. a Full conversion of starting compound 1. b Incomplete conversion of starting compound 1.
Reactions of CCl3-enone 1 with other arenes (o-, m-, p-xylenes, pseudocumene, and veratrole) in CF3SO3H, leading to indenes 2b–f, are presented in Scheme 2. These reactions with electron donating arenes take much less time (2 h only) at room temperature compared to the reaction with benzene (5 days, Table 1, entry 7). The yields of target indenes 2b–f are moderate (20–47%) due to secondary cationic oligomerization processes.
Scheme 2.
Reactions of CCl3-enone 1 with arenes in CF3SO3H leading to indenes 2b–f.
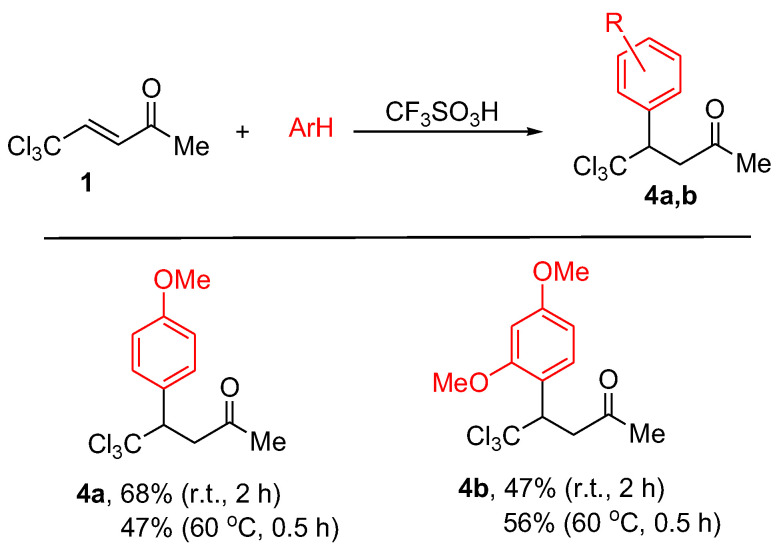
However, the same reactions with anisole (methoxybenzene) and 1,3-dimethoxybenzene at room temperature for 2 h furnish compounds 4a,b as products of hydroarylation of the carbon-carbon double bond of starting CCl3-enone 1 (Scheme 3). Running these reactions at the higher temperature of 60 °C does not lead to the consequent cyclization of compounds 4a,b into the corresponding indenes 2.
Scheme 3.
Reactions of CCl3-enone 1 with arenes in CF3SO3H under different conditions leading to compounds 4a,b.
The data obtained allow proposing plausible reaction mechanisms for transformations of CCl3-enone 1 in Brønsted acids (Scheme 4). The formation of compounds 4 reveals that the first interaction of arenes with cation A occurs at carbon C4 of the latter, leading to species D. Hydrolysis of these cations affords compounds 4 (Scheme 3). In the case of electron donating aryl groups, cations D undergo intramolecular cyclization into species E. At this stage of the reaction, carbon C2 acts as an electrophilic center. Finally, dehydration of E gives rise to indenes 2. Another reaction pathway takes place in H2SO4. The interaction of cation A with hydrosulfate anion HSO4− affords species F, which is hydrolyzed into alcohol 3. We additionally examined the reaction of alcohol 3 with benzene in TfOH to obtain indene 2a. However, only a mixture of oligomeric materials was obtained, with no target indene 2a. In general, upon the formation of indenes 2, starting CCl3-enone 1 in CF3SO3H behaves as a precursor of 1,3-bi-centered electrophilic synthon.
Scheme 4.
Plausible reaction mechanisms for transformations of CCl3-enone 1 in Brønsted acids.
It should be especially emphasized that the development of routes for the synthesis of novel indene derivatives such as compounds 2 is a highly important goal for organic chemistry. Indenes are valuable molecules for medicinal uses [24,25,26]. They are widely exploited as ligands in organometallic chemistry [27,28,29,30,31], as structural units in molecular machines [32] and organic photovoltaics [33].
3. Experimental Section
3.1. General Information
The NMR spectra of solutions of compounds in CDCl3 and in acids (CH3COOH, CF3COOH, H2SO4, CF3SO3H) were recorded on a Bruker 400 spectrometer (Billerica, MA, USA) at 25 °C at 400 and 101 MHz for 1H and 13C NMR spectra, respectively. The residual proton-solvent peaks CDCl3 (δ 7.26 ppm) for 1H NMR spectra, and the carbon signals of CDCl3 (δ 77.0 ppm) for 13C NMR spectra were used as references. NMR spectra in acids were referenced to the signal of CH2Cl2 added as internal standard: δ 5.30 ppm for 1H NMR spectra, and δ 53.52 ppm for 13C NMR spectra. HRMS-APCI was carried out using the instruments Bruker maXis HRMS-ESI-QTOF (Billerica, MA, USA). Preparative TLC was performed on silica gel 5−40 μm (Merck Co., Kenilworth, NJ, USA) with petroleum ether or petroleum ether-ethyl acetate mixture elution.
3.2. DFT Calculations
All computations were carried out at the DFT/HF hybrid level of theory using hybrid exchange functional B3LYP, by using GAUSSIAN 2009 program packages [34]. The geometries optimization was performed using the 6-311+G(2d,2p) basis set (standard 6-311G basis set added with polarization (d,p) and diffuse functions). Optimizations were performed on all degrees of freedom and solvent-phase optimized structures were verified as true minima with no imaginary frequencies. The Hessian matrix was calculated analytically for the optimized structures in order to prove the location of correct minima and to estimate the thermodynamic parameters. For solvent-phase calculations, the Polarizable Continuum Model (PCM, solvent=water) was used.
3.3. Preparation and Characterization of Compounds 1–4
First, E-5,5,5-trichloropent-3-en-2-one 1 was obtained in a yield of 83% according to the procedure shown in the literature [35]. Yellow oil. 1H NMR (CDCl3, 400 MHz) δ, ppm: 7.06 d (J 15 Hz, 1H), 6.62 d (J 15 Hz, 1H), 2.41 s (3H). 13C NMR (CDCl3, 101 MHz) δ, ppm: 196.58, 144.35, 127.95, 92.59, 28.85.
The general procedure for the synthesis of indenes 2, compounds 3 and 4 from E-5,5,5-trichloropent-3-en-2-one 1 and arenes in CF3SO3H. Solution of compound 1 (50 mg, 0.27 mmol) and arene (1.2 equiv., 0.320 mmol) in 2 mL of CF3SO3H involved stirring at room temperature for 2 h (or other temperature and time, see Table 3 and Scheme 3). Then, the reaction mixture was poured into water (25 mL) and extracted with CH2Cl2 (3 × 20 mL). Combined extract was washed with water (20 mL), saturated aqueous solution of NaHCO3 (10 mL), water again (20 mL), and dried over Na2SO4. The solvent was distilled off under a reduced pressure. The residue was subjected to preparative TLC using petroleum ether or petroleum ether-ethyl acetate mixtures (20:1, vol.) as eluent.
Reactions under the action of other Brønsted (H2SO4, FSO3H) and Lewis (AlCl3 and AlBr3, 5 equiv. in 5 mL of benzene) acids were carried out in the same way (Table 1).
3-Methyl-1-trichloromethylinden (2a), yield of 29% (Table 3). Yellow oil. 1H NMR (CDCl3, 400 MHz) δ, ppm: 7.96 d (J 7.8 Hz, 1Harom), 7.44 d (J 7.4 Hz, 1Harom), 7.35–7.27 m (2Harom), 6.32 br. s. (1H, =CH), 4.49 br. s. (1H, CR3H), 2.22 t (J 1.8 Hz, 3H, CH3). 13C NMR (CDCl3, 101 MHz) δ, ppm: 146.5, 144.1, 141.0, 128.6, 128.4, 125.8, 125.1, 119.4, 100.0 (CCl3), 67.5 (CR3H), 13.0 (Me). HRMS-APCI: m/z calc. C11H9Cl3 [M + H]+ 246.9848, found 246.9843.
3,4,7-Trimethyl-1-trichloromethylinden (2b), yield of 20% (Scheme 2). Yellow oil. 1H NMR (CDCl3, 400 MHz) δ, ppm: 7.04 d (J 7.8 Hz, 1Harom), 6.97 d (J 7.8 Hz, 1Harom), 6.25 br. s. (1H, =CH), 4.50 br. s. (1H, CR3H), 2.56 s (3H, Carom-CH3), 2.54 s (3H, Carom-CH3), 2.35 t (J 1.6 Hz, 3H, Caliph-CH3). 13C NMR (101 MHz, CDCl3) δ, ppm: 146.3, 144.3, 132.8, 132.0, 131.2, 130.5, 129.1, 128.8, 101.8 (CCl3), 65.7 (CR3H), 22.6 (Capom-CH3), 19.5 (Capom-CH3), 17.8 (CH3). HRMS-ESI: m/z calc. C13H13Cl3 [M + Ag + CH3CN]+ 421.9399, found 421.9394.
3,5,6-Trimethyl-1-trichloromethylinden (2c), yield of 35% (Scheme 2). Yellow oil. 1H NMR (CDCl3, 400 MHz) δ, ppm: 7.72 s (1Harom), 7.11s (1Harom), 6.22 br. s. (1H, =CH), 4.43 br. s. (1H, CR3H), 2.35 s (6H, Carom-CH3), 2.19 t (J 1.7 Hz, 3H, Caliph-CH3). 13C NMR (CDCl3, 101 MHz) δ, ppm: 144.47, 143.88, 138.72, 136.85, 134.05, 127.53, 126.50, 120.70, 100.40 (CCl3), 67.28 (CR3H), 20.21 (Carom-CH3), 20.09 (Carom-CH3), 13.03 (CH3). HRMS-APCI: m/z calc. C13H13Cl3 [M + H]+ 275.0161, found 275.0156.
3,5,7-Trimethyl-1-trichloromethylinden (2d), yield of 23% (Scheme 2). Yellow oil. 1H NMR (CDCl3, 400 MHz) δ, ppm: 6.94 s (1H), 6.92 s (1Harom), 6.29 br. s. (1H, =CH), 4.54 br. s. (1H, CR3H), 2.58 s (3H, Carom-CH3), 2.39 s (3H, Carom-CH3), 2.15 t (J 1.6 Hz, 3H). 13C NMR (CDCl3, 101 MHz) δ, ppm: 147.8, 144.4, 138.7, 136.56, 134.8, 129.9, 129.8, 118.0, 101.6 (CCl3), 66.6 (CR3H), 22.8 (Carom-CH3), 21.3 (Carom-CH3), 13.0 (CH3). HRMS-ESI: m/z calc. C13H13Cl3 [M + Ag + CH3CN]+ 421.9399, found 421.9394.
3,4,5,7-Tetramethyl-1-trichloromethylinden (2e), yield of 47% (Scheme 2). Yellow oil. 1H NMR (CDCl3, 400 MHz) δ, ppm: 6.90 s (1Harom), 6.25 br. s. (1H, =CH), 4.44 br. s. (1H, CR3H), 2.52 s (3H, Carom-CH3), 2.44 s (3H, Carom-CH3), 2.37 s (J 1.6 Hz, 3H, Caliph-CH3), 2.30 s (3H, Carom-CH3). 13C NMR (CDCl3, 101 MHz) δ, ppm: 146.4, 144.4, 138.2, 138.1, 132.1, 131.2, 130.8, 127.9, 102.0 (CCl3), 65.1 (CR3H), 22.4 (CH3), 20.2 (CH3), 18.8 (CH3), 14.8 (CH3). HRMS-APCI: m/z calc. C14H15Cl3 [M + H]+ 289.0318, found 289.0312.
5,6-Dimethoxy-3-methyl-1-trichloromethylinden (2f), yield of 28% (Scheme 2). Yellow oil. 1H NMR (CDCl3, 400 MHz) δ, ppm: 7.54 s (1Harom), 6.85 s (1Harom), 6.21 br. s. (1H, =CH), 4.39 br. s. (1H, CR3H), 3.97 s (3H, OCH3), 3.94 s (3H, OCH3), 2.19 t (J 1.8 Hz, 3H, CH3). 13C NMR (CDCl3, 101 MHz) δ, ppm: 149.7, 147.4, 143.6, 139.7, 133.4, 127.2, 109.6, 102.9, 100.3 (CCl3), 67.2 (CR3H), 56.4 (OCH3), 56.1 (OCH3), 13.2 (CH3). HRMS-APCI: m/z calc. C13H13Cl3O2 [M + H]+ 307.0059, found 307.0054.
5,5,5-Trichloro-4-hydroxypentane-2-one (3) [36], yield of 75% (Table 3). Yellow oil. 1H NMR (CDCl3, 400 MHz) δ, ppm: 5.03 d (J 9.3 Hz, 1H), 3.44 d (J 17.4 Hz, 1H), 3.24 dd (J 17.4, 9.3 Hz, 1H), 2.29 c (3H). 13C NMR (CDCl3, 101 MHz) δ, ppm: 201.9, 100.4, 67.2, 48.4, 30.6.
5,5,5-Trichloro-4-(4-methoxyphenyl)pent-2-one (4a), yield of 68% (at room temperature for 2 h), 47% (at 60 °C for 0.5 h) (Scheme 3). 1H NMR (CDCl3, 400 MHz) δ, ppm:7.40 d (J 8.8 Hz, 2Harom), 6.88 d (J 8.8 Hz, 2Harom), 4.32 dd (J 9.2, 3.5 Hz, 1H), 3.80 s (3H, OCH3), 3.41 dd (J 17.4, 3.5 Hz, 1H), 3.32 dd (J 17.4, 9.2 Hz, 1H), 2.11 s (3H, CH3). 13C NMR (CDCl3, 101 MHz) δ, ppm: 204.2 (C=O), 159.7 (Carom-OCH3), 131.3 (Carom), 128.7 (Carom), 113.6 (Carom), 103.6 (CCl3), 59.7, 55.2, 46.5, 30.6 (CH3). HRMS-APCI: m/z calc. C12H13Cl3O2 [M + H]+ 295.0054, found 295.0054.
5,5,5-Trichloro-4-(2,4-dimethoxyphenyl)pent-2-one (4b), yield of 47% (at room temperature for 2 h), 56% (at 60 °C for 0.5 h) (Scheme 3). 1H NMR (CDCl3, 400 MHz) δ, ppm: 7.39 d (J 9.3 Hz, 1Harom), 6.55–6.44 m (2Harom), 5.02 d (J 7.9 Hz, 1H), 3.89 s (3H, OCH3), 3.81 s (3H, OCH3), 3.38 dd (J 16.7, 3.6 Hz, 1H), 3.26 dd (J 16.7, 10.2 Hz, 1H), 2.09 s (3H, CH3). 13C NMR (CDCl3, 101 MHz) δ, ppm: 204.6 (C=O), 160.8 (Carom-OCH3), 159.4 (Carom-OCH3), 128.8 (Carom), 117.81 (Carom), 104.4 (Carom), 103.9 (Carom), 98.8 (CCl3), 55.1 (OCH3), 55.3 (OCH3), 50.7, 46.6, 30.2 (CH3). HRMS-APCI: m/z calc. C13H15Cl3O3 [M + H]+ 325.0165, found 325.0160.
4. Conclusions
A novel method for the synthesis of 3-methyl-1-trichloromethylindenes has been developed based on the reaction of 5,5,5-trichloropent-3-en-2-one with arenes in Brønsted superacid CF3SO3H. In this transformation, the initial 5,5,5-trichloropent-3-en-2-one in CF3SO3H behaves as a 1,3-bi-centered electrophile.
Acknowledgments
The spectral studies were performed at Center for Magnetic Resonance, Center for Chemical Analysis and Materials Research of Saint Petersburg State University, Saint Petersburg, Russia.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/molecules27196675/s1, NMR spectra of compounds and cations, data of DFT calculations.
Author Contributions
Conceptualization, A.V.V.; methodology, A.V.V.; investigation, I.A.S. and I.A.B.; writing—original draft preparation, A.V.V.; writing—review and editing, A.V.V.; supervision, A.V.V.; funding acquisition, A.V.V. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the Russian Scientific Foundation grant number 21-13-00006.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Olah G.A., Prakash G.K.S., Molnár Á., Sommer J. Superacid Chemistry. 2nd ed. Wiley-Interscience; Hoboken, NJ, USA: 2008. [Google Scholar]
- 2.Olah G.A., Klumpp D.A. Superelectrophiles and Their Chemistry. Wiley; New York, NY, USA: 2008. [Google Scholar]
- 3.Naredla R.R., Klumpp D.A. Contemporary carbocation chemistry: Applications in organic synthesis. Chem. Rev. 2013;113:6905–6948. doi: 10.1021/cr4001385. [DOI] [PubMed] [Google Scholar]
- 4.Kazakova A.N., Vasilyev A.V. Trifluoromethanesulfonic acid in organic synthesis. Russ. J. Org. Chem. 2017;53:485–509. doi: 10.1134/S1070428017040017. [DOI] [Google Scholar]
- 5.Klumpp D.A., Kennedy S. Superelectrophiles in ring-forming reactions. Arkivoc. 2018;87:215–232. doi: 10.24820/ark.5550190.p010.270. [DOI] [Google Scholar]
- 6.Vasilyev A.V. Superelectrophilic activation of alkynes, alkenes, and allenes. Adv. Org. Synth. 2018;8:81–120. [Google Scholar]
- 7.Zhao W., Sun J. Triflimide (HNTf2) in Organic Synthesis. Chem. Rev. 2018;118:10349–10392. doi: 10.1021/acs.chemrev.8b00279. [DOI] [PubMed] [Google Scholar]
- 8.Fernandes A.J., Panossian A., Michelet B., Martin-Mingot A., Leroux F.R., Thibaudeau S. CF3-substituted carbocations: Underexploited intermediates with great potential in modern synthetic chemistry. Beilst. J. Org. Chem. 2021;17:343–378. doi: 10.3762/bjoc.17.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koltunov K.Y., Repinskaya I. Interaction of phenols and their derivatives with aromatic compounds in the presence of acidic agents XIV. Protonation of α,β-enones in the HF-SbF5-SO2FCl superacid system. Russ. J. Org. Chem. 1994;30:82–85. [Google Scholar]
- 10.Allen J.M., Johnston K.M., Jones J.F., Shotter R.G. Friedel-crafts cyclisation—VI: Polyphosphoric acid-catalysed reactions of crotonophenomes and chalcones. Tetrahedron. 1977;33:2083–2087. doi: 10.1016/0040-4020(77)80317-7. [DOI] [Google Scholar]
- 11.Suzuki T., Ohwada T., Shudo K. Superacid-Catalyzed Electrocyclization of Diphenylmethyl Cations to Fluorenes. Kinetic and Theoretical Revisit Supporting the Involvement of Ethylene Dications. J. Am. Chem. Soc. 1997;119:6774–6780. doi: 10.1021/ja971100g. [DOI] [Google Scholar]
- 12.Walspurger S., Vasilyev A.V., Sommer J., Pale P. Chemistry of 3-arylindenones: Behavior in superacids and photodimerization. Tetrahedron. 2005;61:3559–3564. doi: 10.1016/j.tet.2005.01.110. [DOI] [Google Scholar]
- 13.Koltunov K.Y., Walspurger S., Sommer J. Superacidic Activation of α,β-Unsaturated Amides and Their Electrophilic Reactions. Eur. J. Org. Chem. 2004:4039–4047. doi: 10.1002/ejoc.200400313. [DOI] [Google Scholar]
- 14.Koltunov K.Y., Walspurger S., Sommer J. Friedel–Crafts alkylation of benzene with α,β-unsaturated amides. Tetrahedron Lett. 2004;45:3547–3549. doi: 10.1016/j.tetlet.2004.03.067. [DOI] [Google Scholar]
- 15.Rendy R., Zhang Y., McElrea A., Gomez A., Klumpp D.A. Superacid-Catalyzed Reactions of Cinnamic Acids and the Role of Superelectrophiles. J. Org. Chem. 2004;69:2340–2347. doi: 10.1021/jo030327t. [DOI] [PubMed] [Google Scholar]
- 16.Stadler D., Goeppert A., Rasul G., Olah G.A., Prakash G.K.S., Bach T.J. Chiral Benzylic Carbocations: Low-Temperature NMR Studies and Theoretical Calculations. Org. Chem. 2009;74:312–318. doi: 10.1021/jo802296e. [DOI] [PubMed] [Google Scholar]
- 17.Prakash G.K.S., Yan P., Török B., Olah G.A. Superacidic Trifluoromethanesulfonic Acid-Induced Cycli-Acyalkylation of Aromatics. Cat. Lett. 2003;87:109–112. doi: 10.1023/A:1023482904174. [DOI] [Google Scholar]
- 18.Genaev A.M., Salnikov G.E., Koltunov K.Y. DFT insight into superelectrophilic activation of α,β-unsaturated nitriles and ketones in superacids. Org. Biomol. Chem. 2022;20:6799–6808. doi: 10.1039/D2OB01141G. [DOI] [PubMed] [Google Scholar]
- 19.Kalyaev M.V., Ryabukhin D.S., Borisova M.A., Ivanov A.Y., Boyarskaya I.A., Borovkova K.E., Nikiforova L.R., Salmova J.V., Ulyanovskii N.V., Kosyakov D.S., et al. Synthesis of 3-aryl-3-(furan-2-yl)propanoic acid derivatives and study of their antimicrobial activity. Molecules. 2022;27:4612. doi: 10.3390/molecules27144612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kochurin M.A., Ismagilova A.R., Zakusilo D.N., Khoroshilova O.V., Boyarskaya I.A., Vasilyev A.V. Reactions of linear conjugated dienone structures with arenes under superelectrophilic activation conditions. An experimental and theoretical study of intermediate multicentered electrophilic species. New J. Chem. 2022;46:12041–12053. doi: 10.1039/D2NJ01828D. [DOI] [Google Scholar]
- 21.Ismagilova A.R., Zakusilo D.N., Osetrova L.V., Vasilyev A.V. Reaction of 5-phenylpent-2,4-dienoic acid in trifluoromethanesulfonic acid. Russ. Chem. Bull. Int. Ed. 2020;69:1928–1932. doi: 10.1007/s11172-020-2980-7. [DOI] [Google Scholar]
- 22.Parr R.G., Szentpaly L.V., Liu S. Electrophilicity Index. J. Am. Chem. Soc. 1999;121:1922–1924. doi: 10.1021/ja983494x. [DOI] [Google Scholar]
- 23.Chattaraj P.K., Giri S., Duley S. Update 2 of: Electrophilicity index. Chem. Rev. 2011;111:PR43–PR75. doi: 10.1021/cr100149p. [DOI] [PubMed] [Google Scholar]
- 24.Koca M., Yerdelen K.O., Anil B., Kasap Z., Sevindik H., Ozyurek I., Gunesacar G., Turkaydin K. Design, synthesis and biological activity of 1H-indene-2-carboxamides as multi-targeted anti-Alzheimer agents. J. Enzyme Inhib. Med. Chem. 2016;31:13–23. doi: 10.1080/14756366.2016.1186019. [DOI] [PubMed] [Google Scholar]
- 25.Liu Z., Zhang R., Meng Q., Zhang X., Sun Y. Discovery of new protein kinase CK2 inhibitors with 1,3-dioxo-2,3-dihydro-1H-indene core. Med. Chem. Commun. 2016;7:1352–1355. doi: 10.1039/C6MD00189K. [DOI] [Google Scholar]
- 26.Huffman J.W., Padqett L.W. Recent developments in the medicinal chemistry of cannabimimetic indoles, pyrroles and indene. Current Med. Chem. 2005;12:1395–1411. doi: 10.2174/0929867054020864. [DOI] [PubMed] [Google Scholar]
- 27.Sui-Seng C., Castonguay A., Chen Y., Gareau D., Groux L.F., Zargarian D. Catalytic Reactivities of Indenyl–nickel, Indenyl–palladium, and PCsp3P–nickel Complexes. Top. Catal. 2006;37:81–90. doi: 10.1007/s11244-006-0008-7. [DOI] [Google Scholar]
- 28.Deck P.A. Perfluoroaryl-substituted cyclopentadienyl complexes of transition metals. Coord. Chem. Rev. 2006;250:1032–1055. doi: 10.1016/j.ccr.2005.11.001. [DOI] [Google Scholar]
- 29.Leino R., Lehmus P., Lehtonen A. Heteroatom-Substituted Group 4 Bis(indenyl)metallocenes. Eur. J. Inorg. Chem. 2004;2004:3201–3222. doi: 10.1002/ejic.200400214. [DOI] [Google Scholar]
- 30.Zargarian D. Group 10 metal indenyl complexes. Coord. Chem. Rev. 2002;233–234:157–176. doi: 10.1016/S0010-8545(02)00201-1. [DOI] [Google Scholar]
- 31.Stradiotto M., McGlinchey M.J. η1-indenyl derivatives of transition metal and main group elements: Synthesis, characterization and molecular dynamics. Coord. Chem. Rev. 2001;219–221:311–378. doi: 10.1016/S0010-8545(01)00339-3. [DOI] [Google Scholar]
- 32.McGlinchey M.J., Nikitin K. Palladium-catalysed coupling reactions en route to molecular machines: Sterically hindered indenyl and ferrocenyl anthracenes and triptycenes, and biindenyls. Molecules. 2020;25:1950. doi: 10.3390/molecules25081950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang G., Lin F.R., Qi F., Heumüller T., Distler A., Egelhaaf H.-J., Li N., Chow P.C.Y., Brabec C.J., Jen A.K.-Y. Renewed prospects for organic photovoltaics. Chem. Rev. 2022;122:14180–14274. doi: 10.1021/acs.chemrev.1c00955. [DOI] [PubMed] [Google Scholar]
- 34.Frisch M.J., Trucks G.W., Schlegel J., Scuseria G.E., Robb M.A., Cheeseman J.R., Schlegel H.B., Scalmani G., Barone V., Mennucci B. Gaussian 09, Revision C.01. Gaussian, Inc.; Wallingford, CT, USA: 2010. [Google Scholar]
- 35.Nakatsua S., Gubaidullin A.T., Mamedov V.A., Tsuboi S.A. convenient synthesis of olefins via deacylation reaction. Tetrahedron. 2004;60:2337–2349. doi: 10.1016/j.tet.2004.01.022. [DOI] [Google Scholar]
- 36.Dias L.C., de Marchi A.A., Ferreira M.A.B., Aguilar A.M. The influence of a β-electron withdrawing substituent in aldol reactions of methylketone boron enolates. Org. Lett. 2007;9:4869–4872. doi: 10.1021/ol702204h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.