Abstract
Objective:
to identify predictors of long-term aortic diameter change and disease progression in a population cohort of patients with newly diagnosed aortic dissection (AD), intramural hematoma (IMH), or penetrating aortic ulcer (PAU).
Methods:
We used the Rochester Epidemiology Project record linkage system to identify all Olmsted County, MN-USA, residents diagnosed with AD, IMH, and PAU (1995–2015). The endpoints were aortic diameter change, freedom from clinical disease progression (any related intervention, aortic aneurysm, new aortic syndrome, rupture or death) and disease resolution (complete spontaneous radiological disappear). Linear regression was used to assess aortic growth rate; predictors of disease progression were identified with Cox proportional hazards.
Results:
Of 133 incident cases, 46 ADs, 12 IMHs, and 28 PAUs with sufficient imaging data were included. Overall median follow-up was 8.1 years. Aortic diameter increase occurred in 40 ADs (87%, median 1.0 mm/year), 5 IMHs (42%, median 0.2 mm/year) and 14 PAUs (50%, median 0.4 mm/year). Symptomatic presentation (P=.045), connective tissue disorders (P=.005), and initial aortic diameter >42 mm (P=.013) were associated with AD growth rate. PAU depth >9 mm (P=.047) and female sex (P=.013) were associated with aortic growth rate in PAUs and IMHs.
At 10 years, freedom from disease progression was 22% (95%CI 12–41) for ADs, 44% (95%CI 22–92) for IMHs, and 46% (95%CI 27–78) for PAUs. DeBakey I/IIIB AD (HR 3.09; P=.038), initial IMH aortic diameter (HR 1.4; P=.037) and PAU depth >10 mm (HR 3.92; P=.018) were associated with disease progression. No AD spontaneously resolved; resolution rate at 10 years was 22% (95%CI 0–45) for IMHs and 11% (95%CI 0–23) for PAUs.
Conclusions:
Aortic growth and clinical disease progression are observed in most patients with aortic syndromes, while spontaneous resolution is uncommon. Predictors of aortic growth and disease progression may be used to tailor appropriate follow-up and eventual early intervention.
INTRODUCTION
Aortic dissection (AD), intramural hematoma (IMH) and penetrating aortic ulcer (PAU) are considered different manifestations of aortic syndromes (AS), a spectrum of related aortic pathologies characterized by involvement of the media layer.
Although significant acute and long-term morbidity and mortality are described1–8, the natural history of aortic syndromes is still not completely clear, since follow-up is usually short-term and inconsistent, and their long-term clinical behavior is extremely variable. Some patients may experience aortic diameter increase, progression to other AS, recurrent AS, or aortic rupture, that in turn are responsible of not only aortic-related mortality, but also readmissions and recurrence of symptoms8. In contrast, a benign stable disease or also a complete pathology resolution are described in up to 40–50% of ADs and 40–70% of IMHs/PAUs7–12. In this scenario, the identification of baseline predictors of late adverse events could be clinically useful to recognize patients at risk of disease progression, and therefore to target a specific follow-up protocol and eventual prophylactic treatment.
The present study aimed to validate clinical and anatomical predictors of aortic diameter changes and clinical disease progression, in a cohort of patients with newly diagnosed AD, IMH, or PAU, using a population-based approach with a long-term follow-up.
METHODS
Patients.
We used the Rochester Epidemiology Project (REP) record linkage system to identify all Olmsted County, MN, residents diagnosed with AD, IMH, or PAU from 1995 to 2015. The detailed methods for the identification of the original cohort are described elsewhere3,14. In brief, the REP represents a unique collaboration of healthcare providers linking together medical records of virtually all residents of Olmsted County, MN. This allows the identification of incident diagnoses at a population level and allows follow-up of patients across providers. Within the REP, adult residents (≥18 years of age) with a new diagnosis of AS from 1995–2015 were identified using the International Classification of Diseases (ICD), Ninth Revision diagnosis code (441.0–441.9), equivalent ICD-10 codes (I71.00–I71.03, I71.1–I71.6, I71.8, and I71.9, for October–December 2015) or Hospital Adaptation of the International Classification of Diseases, Second Edition (a modification of the ICD-8) from inpatient and outpatient encounters. For diagnosis, patients were required to have imaging confirmation of AD, IMH, or PAU (computed tomography with arterial contrast, magnetic resonance imaging, ultrasound, or conventional angiography), primary diagnosis of AS on their death certificate, or autopsy confirmation of AS. All patients were confirmed residents of Olmsted County at time of diagnosis to allow a true population-based assessment. The study was approved by the Institutional Review Boards of the two major health care providers in the REP, Mayo Clinic and Olmsted Medical Center. All individuals included in the study had already provided informed consent for the use of their medical records in research as part of the REP.
To assess aortic morphological changes, only patients with at least two available imaging studies were included in the present analysis. Imaging analysis was censored at the time of eventual related aortic intervention, and patients were excluded from the analysis in the case they received a resolutive intervention during the acute phase. Only computed tomography (CT, with or without contrast) or magnetic resonance (MR) were considered for the image analysis. In order to use all the available information and accurately estimate aortic growth rate, all the imaging studies with any useful information were used, and these included aortic diameter measurement also at non-contrast CTs. Patients were excluded if the diagnosis was based on autopsy or in case of death during the index hospitalization.
Categorization of AS was based on standard criteria; AD was defined as an intimal tear with the presence of a false lumen. IMH was defined as crescent or circular thickening of the aortic wall without an intimal tear or dissection. PAU was defined as a focal lesion of the aorta with erosion of the intima and the absence of IMH or dissection15,16.
AD was classified using the Stanford and the De Bakey (to assess also for distal extension of the AD) classifications; the Stanford classification was used to classify also IMH and PAU. Acuity of the disease was classified as acute (<14 days from initial symptoms), subacute (2 weeks to 3 months) or chronic (>3 months)15,17.
Image analysis.
For AD, size and site of the entry tear, presence of re-entries, aortic diameter, false lumen (FL) status, and presence of any other associated AS were evaluated. The maximum aortic diameter, as well as the false lumen and true lumen diameters were measured as the outer to outer wall distance perpendicular to the centerline. The levels of interest were the ascending aorta (zone 0), aortic arch (zones 1 to 3), descending thoracic aorta (zones 4 and 5), paravisceral aorta (zones 6 to 8), abdominal aorta (zone 9), and iliac arteries (zones 10–11) at the baseline imaging study19,20. In case of residual dissection after repair of the ascending aorta, only the native aorta affected by the residual dissection was considered for the morphological measurements. The FL was classified as patent or as partially or completely thrombosed according to current reporting standards15.
The maximum aortic diameter was measured in a similar way also for IMH and PAU, at the aortic level affected by the disease. In IMHs, also location, hematoma thickness (maximum intima to adventitia distance perpendicular to the centerline)18 and longitudinal extension were assessed (Figure 1A). In PAUs, location, PAU depth, longitudinal extension, PAU width, and neck width were evaluated as reported by Nathan et al19 (Figure 1B and 1C).
Figure 1.

A) Example of intramural hematoma. The hematoma thickness is measured as the maximum intima to adventitia distance on a plane perpendicular to the centerline (red arrow). B) Penetrating aortic ulcer. The measurements of the PAU depth (yellow dotted arrow), width (blue dashed arrow), and neck width (red solid arrow) are shown. C) Example of the measurement of a PAU longitudinal extension (red arrow).
To ensure reproducibility, images were reviewed by a radiologist and confirmed by a vascular surgeon. The diameter measurements were repeated twice, and the Intraclass Correlation Coefficient (ICC) for intra-observer variability was 0.92.
Endpoints.
The primary outcome was aortic growth rate. For AD, aortic diameter change was assessed for each aortic segment; for IMH and PAU, only the maximum aortic diameter of the aortic segment affected by the IMH or PAU was considered.
Secondary outcome were freedom from clinical disease progression and disease resolution. Disease progression was defined considering only clinically significant events: any related intervention, aortic aneurysm formation (defined by a total aortic diameter sufficiently large to be considered for repair: >60 mm for the thoracic aorta and >55 mm for the abdominal aorta), evolution into a different AS, recurrent AS, rupture or related death. Aortic-related mortality was defined as any death from aortic rupture, malperfusion, aortic dissection or related intervention. Disease resolution was defined by complete spontaneous radiological disappear of the primary aortic disease.
Statistical analysis.
Results were reported as a number and percentage for categorical variables, mean±standard deviation or median and range for continuous variables. Aortic growth rate was determined by linear regression. This was calculated as the β coefficient resulting from the regression of the aortic diameter measured at every available time point for each patient. Only imaging studies performed before any aortic intervention were considered for the calculation of the growth rate. Univariable linear regression was used to assess the impact of baseline clinical and morphological factors on the aortic growth rate. Time-dependent outcomes were reported using Kaplan-Meier estimates. Univariable Cox proportional hazards models were used to assess the impact of baseline characteristics on disease progression and disease resolution. Firth’s penalized maximum likelihood bias reduction method was applied in case of complete or quasi-complete data separation. A P-value of less than .05 was used to determine statistical significance.
RESULTS
Study population.
Of 133 total incident cases in the initial cohort3, 46 ADs, 12 IMHs, and 28 PAUs with sufficient imaging data were included in the present analysis. Of the 45 patients excluded from this series, 6 were diagnosed on autopsy reports, 15 died during the acute phase with no available imaging follow-up, and 24 had no sufficient CT or MR available for imaging analysis. Eighty percent of patients included in the final cohort were initially admitted at the major institution (Mayo Clinic). Overall baseline demographics and clinical characteristics are reported in Table I. Presentation was acute in the majority of patients (65% AD, 67% IMH, 32% PAU); initial management was surgical for 16 type A AD during the acute phase (among the 21 type A ADs, surgery was not performed due to high comorbidities in 1 patient, due to patient’s refusal in 1 case; there were 3 cases of non-A non-B ADs that were conservatively managed); surgery consisted in surgical grafting of the ascending aorta in 10 cases, hemiarch repair in 6 cases, and associated aortic valve replacement in 6 cases. After repair, a patent FL persisted in 12 cases; partial thrombosis was observed in 3 cases and complete FL thrombosis in 1 case. No type B patients of our cohort received surgical treatment during the acute phase. Anatomical characteristics are showed in Table II. Median radiological follow-up was 8.1 years (IQR 5–13 years). Fifty-six patients (63%) died during follow-up; the estimated survival was 48.1% (95%CI 38–61) at 10 years and 20.3% (95%CI 11–37) at 15 years. Mortality was aortic-related in 8 cases, resulting in a freedom from aortic-related mortality of 75.2% (95%CI 61–93) at 10 years and 75.2% (95%CI 61–93) at 15 years.
Table I.
Baseline demographics and clinical data.
| AD (n=46) |
IMH (n=12) |
PAU (n=28) |
P | |
|---|---|---|---|---|
| Age, years | 67.1±14.0 | 69.8±13.3 | 76.1±9.2 | |
| Male sex | 32 (69.6) | 6 (50.0) | 15 (53.6) | .299 |
| Race | .592 | |||
| White | 41 (89.1) | 11 (91.7) | 27 (100.0) | |
| Black | 1 (2.2) | 1 (8.3) | 0 (0.0) | |
| Hawaiian/Pacific islands | 1 (2.2) | 0 (0.0) | 0 (0.0) | |
| Unknown | 3 (6.5) | 0 (0.0) | 1 (3.5) | |
| BMI, kg/m 2 | 28.5±7.1 | 29.6±7.2 | 27.0±6.7 | .629 |
| Risk factors | ||||
| Previous aortic surgery | 6 (13.0) | 1 (8.3) | 2 (7.1) | .689 |
| Aortic atherosclerosis | 35 (76.1) | 11 (91.7) | 28 (100.0) | .080 |
| Bicuspid aortic valve | 0 (0.0) | 0 (0.0) | 1 (3.6) | .732 |
| Connective tissue disorder | 5 (10.9) | 0 (0.0) | 0 (0) | .207 |
| Acuity of diagnosis | .014a | |||
| Acute | 30 (65.2) | 8 (66.7) | 9 (32.1) | |
| Subacute | 0 (0.0) | 1 (8.3) | 1 (3.6) | |
| Chronic | 2 (4.3) | 0 (0.0) | 1 (3.6) | |
| Indeterminate | 14 (30.4) | 3 (25.0) | 17 (60.7) | |
| Medications at discharge | ||||
| B-blocker | 39 (84.8) | 12 (100.0) | 18 (64.3) | .052 |
| ACEi/ARB | 18 (39.1) | 11 (91.7) | 12 (42.9) | .059 |
| Calcium channel blocker | 17 (37.0) | 5 (41.7) | 9 (32.1) | .589 |
ACEi, Angiotensin-converting enzyme inhibitor; ARB, Angiotensin II Receptor Blockers.
Statistically significant.
Table II.
Anatomical characteristics.
| AD (n=46) |
IMH (n=12) |
PAU (n=28) |
P | |
|---|---|---|---|---|
| Stanford classification | <.001a | |||
| Type A | 21 (45.7) | 1 (8.3) | 0 (0) | |
| Type B | 25 (54.3) | 11 (91.7) | 28 (100.0) | |
| De Bakey classification | - | |||
| I | 15 (32.6) | - | - | |
| II | 6 (13.0) | - | - | |
| IIIa | 4 (8.7) | - | - | |
| IIIb | 21 (45.7) | - | - | |
| Any other associated AS | 1 (2.3) | 3 (25.0) | 4 (14.3) | .028a |
| Max aortic diameter 1 , mm | .001a | |||
| Mean±SD | 39.0±8.2 | 39.4±5.0 | 30.5±10.1 | |
| Range | 21.0–60.0 | 33.0–52.0 | 13.0–55.0 | |
| AD entry tear location | - | |||
| Ascending aorta | 16 (34.7) | - | - | |
| Aortic arch | 13 (28.2) | - | - | |
| Descending thoracic | 10 (21.7) | - | - | |
| Other | 7 (15.2) | |||
| Entry tear size, mm | - | |||
| Mean±SD | 10.6±7.7 | - | - | |
| Range | 1.0–30.0 | - | - | |
| Presence of visible re-entry | ||||
| Yes | 14 (41.2) | - | - | - |
| False lumen status | - | |||
| Patent | 33 (71.7) | - | - | |
| Partial thrombosis | 11 (23.9) | - | - | |
| Complete thrombosis | 2 (5.6) | - | - | |
| PAU/IMH depth, mm | - | |||
| Mean±SD | - | 9.5±3.7 | 9.2±6.5 | |
| Range | - | 5.0–16.0 | 3.0–37.0 | |
| PAU/IMH longitudinal extension, mm | - | |||
| Median | - | 222 | 21 | |
| Range | - | 5.0–336.0 | 5.0–104.0 | |
| PAU neck width, mm | - | |||
| Mean±SD | - | - | 10.4±3.7 | |
| Range | - | - | 3.0–19.0 | |
| PAU width, mm | - | |||
| Mean±SD | - | - | 13.4±6.1 | |
| Range | - | - | 4.0–30.0 |
Maximum diameter considering only segments of aorta involved by AD, IMH or PAU.
Statistically significant.
AD morphological change.
Mean initial aortic diameter was 39±8 mm. During a median follow-up of 8.5 years (IQR, 4–14 years), aortic diameter was stable in 6 (13%) cases and increased in 40 (87%) cases; aortic growth >5mm occurred in 16 cases after a mean period of 6.7 years. Freedom from aortic growth >5 mm was 77.6% (95%CI 65–92) at 5 years, 64.8% (95%CI 50–85) at 10 years, and 52.8% (95%CI 36–78) at 15 years. No patients had aortic diameter decrease. The median growth rate calculated by linear regression was 1.0 mm/year (IQR 0.4–1.7 mm/year) in the overall cohort and 1.2 mm/year (IQR 0.6–3. mm/year) in the subset of AD with any diameter increase. Growth rate stratified by aortic segments was 0.2 mm/year (IQR 0.1–0.2 mm/year) for the ascending aorta, 0.8 mm/year (IQR 0.3–4.0 mm/year) for the aortic arch, 0.9 mm/year (IQR 0.5–2.7) for the descending thoracic aorta, 0.5 mm/year (IQR 0.2–1.1 mm/year) for the visceral aorta, 0.7 mm/year (IQR 0.3–0.9 mm/year) for the infrarenal aorta, and 0.4 mm/year (IQR 0.1–0.6 mm/year) for the iliac arteries.
Connective tissue disorders (P=.005), presence of symptoms at onset (P=.045), initial maximum aortic diameter (P=.013), and location of the entry tear (P=.043) were significantly associated with increased aortic growth rate (Table III). In particular, a higher growth rate was registered in case of aortic diameter >42 mm (2.4 mm/year, IQR 0.9–5.4 mm/year) and intimal tear located in the descending thoracic aorta (5.7 mm/year, IQR 1.7–9.6 mm/year). Stanford classification was not significantly associated (P=.726).
Table III.
Aortic growth rate (cm/year) determined by linear regression.
| AD | IMH | PAU | ||||
|---|---|---|---|---|---|---|
| Age, years | .812 | .832 | .565 | |||
| <65 | .094 (.027, .314) | 0 (−.007, .043) | .111 (.041, .187) | |||
| 66–79 | .088 (.039, .128) | .017 (−.482, .373) | .029 (0, .047) | |||
| >80 | .206 (.072, .333) | .061 (.061, .061) | .084 (0, .196) | |||
| Gender | .264 | .013a | ||||
| Male | .097 (.052, .217) | .043 (0, .280) | 0 (0, .040) | |||
| Female | .130 (.016, .381) | .005 (−.245, .039) | .174 (.043, .200) | |||
| BMI, kg/m 2 * | .251 | .056 | .907 | |||
| 17.2–24.9 | .171 (.016, .487) | .061 (−.482, .373) | .179 (.040, .200) | |||
| 25–29.9 | .106 (.049, .352) | .022 (0, .043) | 0 (0, .149) | |||
| >30 | .096 (.049, .192) | .005 (−.079, .148) | .016 (0, .039) | |||
| Connective Tissue Disorder (CTD) | .005a | -- | -- | |||
| No | .089 (.039, .242) | .017 (−.007, .061) | .040 (0, .179) | |||
| Yes | .381 (.171, .732) | -- | -- | |||
| Stanford Classification | .726 | -- | .507 | |||
| A | .093 (.049, .269) | −.151 | -- | |||
| B | .102 (.043, .242) | .030 (−.003, .170) | .040 (0, .179) | |||
| De Bakey Classification | .732 | .268 | .614 | |||
| I | .093 (.049, .248) | -- | -- | |||
| II | .197 (.039, .352) | −.151 | -- | |||
| IIIa | .110 (.036, .206) | .030 (−.233, .162) | .047 (0, .200) | |||
| IIIb | .102 (.043, .361) | .030 (−.003, .217) | .036 (0, .159) | |||
| Acuity of Diagnosis | .171 | .313 | .828 | |||
| Acute | .107 (.049, .381) | .017 (−.007, .043) | .115 (.029, .204) | |||
| Subacute | -- | 0 | -- | |||
| Chronic | .070 (.016, .123) | -- | 0 | |||
| Indeterminate | .084 (.024, .171) | .061 (−.151, .373) | .036 (0, .169) | |||
| Symptoms at onset | .045a | .674 | .528 | |||
| No | .072 (.020, .149) | .061 (−.151, .373) | .043 (0, .169) | |||
| Yes | .116 (.056, .381) | .008 (−.007, .043) | .015 (0, .196) | |||
| B-blockers at discharge | .555 | .490 | -- | |||
| No | .079 (.049, .128) | -- | .043 (0, .195) | |||
| Yes | .102 (.039, .315) | .017 (−.007, .061) | .036 (0, .179) | |||
| ACE-i/ARB at discharge | .534 | .894 | .017a | |||
| No | .114 (.027, .292) | −.482 | .043 (0, .187) | |||
| Yes | .086 (.049, .242) | .030 (−.003, .170) | .031 (0, .179) | |||
| CCB at discharge | .608 | .457 | .981 | |||
| No | .093 (.043, .248) | .030 (−.151, .280) | .040 (0, .169) | |||
| Yes | .105 (.039, .361) | 0 (−.007, .061) | .039 (.015, .196) | |||
| Depth of PAU, mm * | -- | -- | .047a | |||
| 3.5–4 | -- | -- | .015 (0, .037) | |||
| 5–9 | -- | -- | .095 (0, .187) | |||
| 10–37 | -- | -- | .108 (0, .195) | |||
| PAU neck width, mm * | -- | .065 | ||||
| 5–10 | -- | -- | .043 (0, .179) | |||
| 10–12 | -- | -- | .031 (0, .159) | |||
| 12–19 | -- | -- | .040 (0, .195) | |||
| PAU width, mm * | -- | -- | .138 | |||
| 4–12 | -- | -- | .037 (0, .149) | |||
| 12–16 | -- | -- | .015 (0, .040) | |||
| 16–23 | -- | -- | .108 (0, .198) | |||
| Max aortic diameter, mm * | .013a | -- | -- | |||
| 21–35 | .086 (.043, .171) | -- | -- | |||
| 35–42 | .052 (0, .192) | -- | -- | |||
| 42–60 | .242 (.093, .541) | -- | -- | |||
| Max diameter at PAU, mm * | -- | -- | .051 | |||
| 13–24 | -- | -- | .015 (0, .106) | |||
| 24–32 | -- | -- | .040 (0, .149) | |||
| 32–55 | -- | -- | .198 (.098, .280) | |||
| Max diameter at IMH, mm * | -- | .165 | -- | |||
| 33–35 | -- | .017 (0, .373) | -- | |||
| 35–39 | -- | .043 (−.007, .061) | -- | |||
| 39–43 | -- | −.151 | -- | |||
| IMH: Longitudinal extent, mm * 1 | .948 | -- | ||||
| 5–32 | −.045 (−.151, .061) | -- | ||||
| 32–240 | −.017 (−.007, .280) | -- | ||||
| 240–330 | −.241 (−.482, 0) | -- | ||||
| Depth of IMH, mm * | .552 | -- | ||||
| 6–7 | −.151 | -- | ||||
| 7–10 | .061 (.017, .280) | -- | ||||
| 10–16 | 0 (−.482, .043) | -- | ||||
| AD entry tear location | .043a | |||||
| Ascending | .084 (.035, .292) | -- | -- | |||
| Arch | .126 (.086, .381) | -- | -- | |||
| Descending thoracic | .565 (.171, .959) | -- | -- | |||
| Abdominal | .082 (.033, .115) | -- | -- | |||
| Entry tear size, mm * | .616 | |||||
| ≤10 | .078 (0, .381) | |||||
| >10 | .123 (.030, .352) | |||||
| Overall status FL | .329 | -- | -- | |||
| Patent | .107 (.027, .258) | -- | -- | |||
| Partial/complete thrombosis | .093 (.056, .352) | -- | -- | |||
| Initial AD management | .726 | -- | -- | |||
| Conservative | .102 (.043, .242) | -- | -- | |||
| Surgical | .093 (.049, .269) | -- | -- | |||
| Presence of visible re-entry | .730 | -- | -- | |||
| No | .115 (0.31, .333) | -- | -- | |||
| Yes | .107 (.030, .192) | -- | -- |
AD, aortic dissection; ACEi, Angiotensin-converting enzyme inhibitor; ARB, Angiotensin II Receptor Blockers BMI, body mass index; CCB, calcium channel blocker; FL, false lumen.
Log transformed.
Statistically significant.
Growth rate was 1.0 mm/year (IQR 0.4–2.4 mm/year) in patients undergoing initial conservative treatment, 0.9 mm/year (IQR 0.5–2.7 mm/year; P=.726) for patients with residual dissection after type A repair. Considering only the 30 patients treated conservatively, De Bakey IIIb AD had a higher growth rate (5.2 mm/year, IQR 0.4–10.0 mm/year; P=.034), as well as initial aortic maximum diameter >42 mm (7.4 mm/year, IQR 2.6–12.1 mm/year; P=.003). Other considered clinical and anatomical factors were not significantly associated with the growth rate.
AD clinical progression.
Thirty-four patients had disease progression during follow-up. The estimated freedom from AD progression was 65.2% (95%CI 52.8–80.5) at 1 year, 38.6% (95%CI 26.4–56.5) at 5 years, and 21.9% (95%CI 11.6–41.5) at 10 years (Figure 2). Aortic-related mortality was 9.3% (95%CI 0–19.7) and aortic rupture rate was 7.3% (95%CI 0–18.2) at 10 years. Nine patients (6 with type B AD and 3 with type A residual dissection) underwent related intervention after an average time of 4 years (range 3 months-14 years), due to 8 aneurysm formation and 1 type A dissection. No patients underwent “prophylactic” TEVAR during the subacute phase for uncomplicated type B AD. Freedom from intervention was 80% (95%CI 68–94) at 10 years. Patients with De Bakey I or IIIa AD had an increased risk of disease clinical progression (HR 3.09, 95%CI 1.07–8.97; P=.038) compared to more limited De Bakey II/IIIb AD (Table IV). Stanford classification was not significantly associated to disease progression (HR 0.73, 95%CI 0.36–1.46; P=.368). No patients with AD had a spontaneous resolution.
Figure 2.

A) Kaplan-Meier estimate of freedom from disease progression in patients with aortic dissection. * indicates a standard error >10%. B) Kaplan-Meier estimate of freedom from disease progression in patients with intramural hematoma. * indicates a standard error >10%. C) Kaplan-Meier estimate of freedom from disease progression in patients with penetrating aortic ulcer. * indicates a standard error >10%.
Table IV.
Disease Progression univariate analysis with Cox proportional hazards regression.
| AD | IMH | PAU | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | HR | 95% CI | P | |
| Age at baseline, years | 1.00 | (0.98, 1.03) | .733 | 1.01 | (0.95, 1.07) | .739 | 1.00 | (0.95, 1.06) | .863 |
| Gender, female | 1.22 | (0.58, 2.58) | .601 | 2.16 | (0.47, 9.94) | .324 | 1.66 | (0.59, 4.68) | .336 |
| Acute presentation | 1.57 | (0.76, 3.24) | .223 | 0.46 | (0.09, 2.30) | .345 | 1.45 | (0.51, 4.10) | .485 |
| Connective Tissue Disorder (CTD) | 1.65 | (0.57, 4.79) | .353 | -- | -- | -- | -- | -- | -- |
| Symptoms at onset | 1.13 | (0.55, 2.29) | .744 | 0.26 | (0.05, 1.29) | .100 | 2.81 | (0.93, 8.48) | .066 |
| Beta blockers at discharge | 0.70 | (0.29, 1.72) | .442 | 0.13 | (0.01, 2.14) | .155 | 0.68 | (0.24, 1.88) | .454 |
| ACE-i/ARB med at discharge | 1.08 | (0.55, 2.15) | .816 | 2.06 | (0.34, 12.63) | .433 | 1.94 | (0.69, 5.49) | .210 |
| CCB medication at discharge | 0.66 | (0.63, 1.30) | .165 | 0.73 | (0.13, 4.02) | .772 | 0.77 | (0.24, 2.44) | .659 |
| Type B | 0.73 | (0.36, 1.46) | .368 | 0.47 | (0.05, 4.57) | .517 | -- | -- | -- |
| Residual AD after surgery | 1.37 | (0.69–2.75) | .577 | - | - | - | - | ||
| Disease extension | .038a | - | - | ||||||
| Distal (De Bakey I/IIIb) | 3.09 | (1.07, 8.97) | - | - | - | - | |||
| Proximal (De Bakey II/IIIa) | Ref. | - | - | - | - | - | - | ||
| AD entry tear location (grouped) | 0.848 | -- | -- | ||||||
| Ascending | 1.38 | (0.61, 3.13) | -- | -- | -- | -- | |||
| Arch | 1.25 | (0.55, 2.86) | -- | -- | -- | -- | |||
| Other | Ref. | -- | -- | -- | -- | ||||
| Aortic atherosclerosis | 2.41 | (0.98, 5.89) | .054 | -- | -- | -- | -- | -- | -- |
| PAU location | |||||||||
| Thoracic | -- | -- | -- | -- | -- | -- | 2.14 | (0.72, 6.39) | .171 |
| Abdominal | -- | -- | -- | -- | -- | -- | Ref. | -- | -- |
| IMH location | |||||||||
| Aortic arch | -- | -- | -- | 6.84 | (1.10, 43) | .040a | -- | -- | -- |
| Descending thoracic | -- | -- | -- | Ref. | -- | -- | -- | -- | -- |
| Max aortic diameter, mm | 0.98 | (0.94, 1.02) | .244 | 1.18 | (1.02, 1.32) | .037a | 1.03 | (0.98, 1.07) | .246 |
| PAU/IMH Longitudinal extent, mm | -- | -- | -- | 0.62 | (0.37, 1.03) | .062 | 1.73 | (0.85, 3.51) | .128 |
| Depth of PAU/IMH, mm | -- | -- | -- | 1.06 | (0.85, 1.32) | .600 | 1.03 | (0.98, 1.09) | .247 |
| Depth of PAU/IMH >10 mm | -- | -- | -- | -- | -- | -- | 3.92 | (1.26, 12.2) | .018a |
| PAU neck width, mm | -- | -- | -- | -- | -- | -- | 1.04 | (0.91, 1.19) | .577 |
| PAU width, mm | -- | -- | -- | -- | -- | -- | 1.08 | (0.99, 1.18) | .072 |
AD, aortic dissection; ACEi, Angiotensin-converting enzyme inhibitor; ARB, Angiotensin II Receptor Blockers.
Statistically significant.
No clinical or anatomical factor was associated with AD progression in the subset of patients receiving medical treatment alone as initial treatment.
IMH morphological change.
Initial maximum aortic diameter in patients with IMH was 39±5 mm. After a median follow-up of 8 years (IQR 6–17 years), total aortic diameter increased in 6 (50%) patients, decreased in 4 (33.3%), and remained unchanged in 2 (16.7%). Maximum aortic diameter increased >5 mm in 5 cases; freedom from aortic growth >5 mm was 78.5% (95%CI 59–100) at 5 years and 62.8% (95%CI 37–100) at 10 years. Overall aortic growth rate was 0.2 mm/year (IQR −0.1–0.6 mm/year); 0.6 mm/year (IQR 0.4–2.8) for those with increasing diameter and −1.5 mm/year (IQR −4.8; −0.1 mm/year) for those with decreasing diameter. IMH depth increased in 3 (25%) patients with a median rate of 0.61 mm/year (IQR 0.4–3.5 mm/year); it decreased in 4 (33.3%) with a median rate of −0.4 mm/year (IQR −0.8;−0.2). Longitudinal extension increased in 1 (8.3%) patient and had no change in all other 11 (81.7%) cases. Men had a significantly higher aortic growth rate compared to women (0.4 mm/year vs 0.1 mm/year; P=.013) (Table III).
IMH clinical progression and resolution.
Clinical progression occurred in 7 cases. Kaplan-Meier freedom from disease progression was 44.4% (95%CI 21.4–92.3) at 1 year, 44.4% (95%CI 21.4–92.3) at 5 year, and 44.4% (95%CI 21.4–92.3) at 10 years (Figure 2). Specific rates of aortic mortality and rupture at 10 years were 28.9% (95%CI 0–56.7) and 20% (95%CI 0–48.4). An aortic intervention was necessary in 2 cases during follow-up: transition to complicated AD with malperfusion occurred in one case 9 months from the initial diagnosis, and a pseudoaneurysm formation occurred in one case 11 years from the diagnosis. Initial maximum aortic diameter (HR 1.18, 95%CI 10.02–1.32; P=.037) and IMH location in the aortic arch (HR 6.84, 95%CI 1.10–43; P=.040) were significant predictors of IMH progression (Table IV). A transition to an aortic aneurysm occurred in in 3 (25%) patients.
One case remained stable during follow-up while IMH resolution occurred in 3 (25%) cases. The estimated rate of radiological disappear was 0% at 1 year, 22.2% (95%CI 0–45.1) at 5 years (Figure 3). No clinical or anatomical predictors of IMH resolution were identified (Table V).
Figure 3.
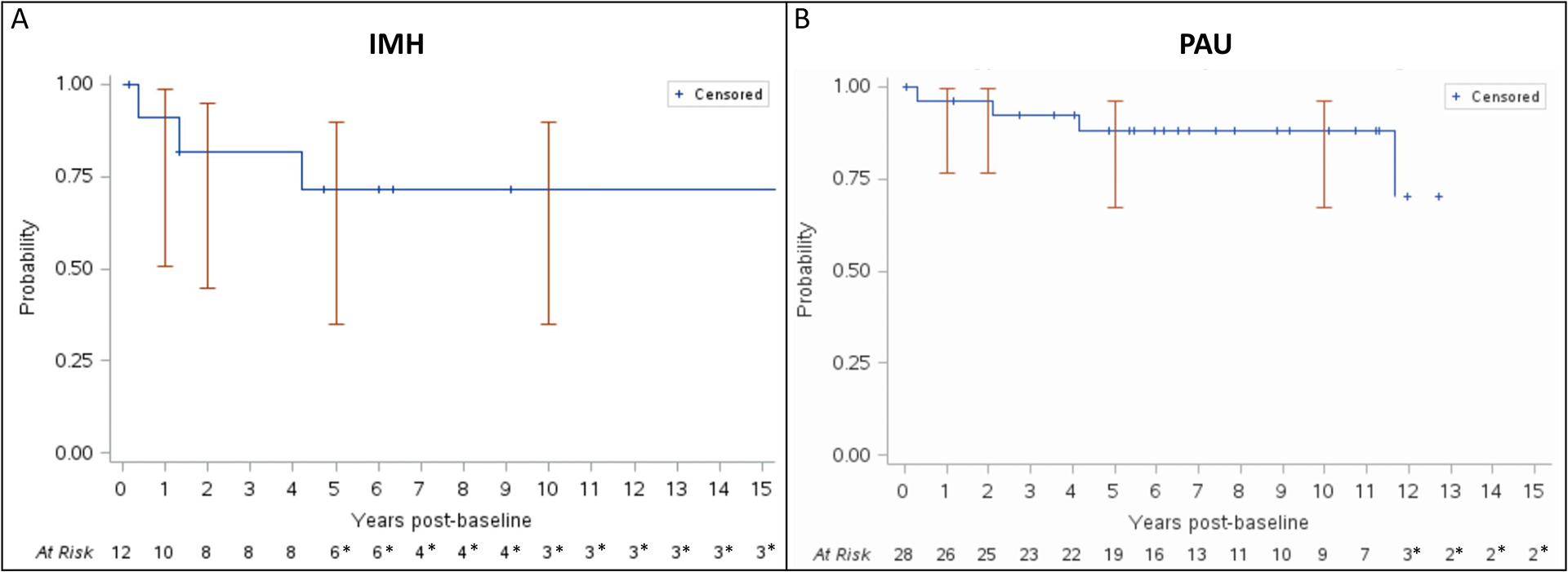
A) Kaplan-Meier estimate of freedom from disease resolution in patients with intramural hematoma. * indicates a standard error >10%. B) Kaplan-Meier estimate of freedom from disease resolution in patients with penetrating aortic ulcer. * indicates a standard error >10%.
Table V.
Disease spontaneous resolution univariate analysis with Cox proportional hazards regression.
| PAU | IMH | |||||
|---|---|---|---|---|---|---|
| HR | 95 CI | P | HR | 95 CI | P | |
| Age, years | 0.96 | (0.89, 1.05) | .403 | 1.06 | (0.97, 1.19) | .192 |
| Female gender | 2.27 | (0.37, 23.6) | .454 | 1.41 | (0.20, 10.2) | .731 |
| BMI, kg/m 2 | 0.97 | (0.79, 1.18) | .746 | 0.81 | (0.60, 1.09) | .162 |
| Acute presentation | 0.80 | (0.08, 4.88) | .834 | .342 | ||
| Symptoms at onset | 0.13 | (0, 1.27) | .230 | 0.95 | (0.16, 9.84) | .963 |
| Beta blockers at discharge | 0.26 | (0.02, 1.67) | .226 | -- | -- | -- |
| ACE-i/ARB med at discharge | 0.68 | (0.07, 4.17) | .726 | -- | -- | -- |
| CCB at discharge | 0.26 | (0, 2.45) | .418 | -- | -- | -- |
| Any other associated aortic pathology | 0.71 | (0.01, 7.40) | .847 | -- | -- | -- |
| PAU location | ||||||
| Thoracic | 3.98 | (0.41, 5.30) | .412 | -- | -- | -- |
| Abdominal | Ref. | - | -- | -- | -- | |
| Depth of PAU/IMH, mm | 0.27 | (0.07, 1.08) | .064 | 0.87 | (0.59, 1.30) | .502 |
| PAU width, mm | 0.40 | (0.08, 0.76) | .043a | -- | -- | -- |
| PAU neck width, mm | 0.41 | (0.07, 0.74) | .041a | -- | -- | -- |
| Max diameter at IMH/PAU, mm | 0.85 | (0.72, 1.00) | .055 | 0.87 | (0.63, 1.18) | .366 |
| PAU/IMH Longitudinal extent, mm | 0.04 | (0, 0.32) | .021a | 1.22 | (0.58, 2.56) | .596 |
Statistically significant.
PAU morphological change.
Initial total aortic diameter was 31±10 mm. After a median observation period of 7.9 years (IQR 5–11 years), aortic diameter increased in 14 (50%) cases, decreased in 3 (11%) and was unchanged in 11 (39%). Overall aortic growth rate was 0.4 mm/year (IQR 0–1.8 mm/year); 1.6 mm/year (IQR 0.4–2.0 mm/year) in cases with increasing size and −0.3 mm/year (IQR −0.6;0 mm/year) in cases with decreasing diameter. Also PAU depth (0.5 mm/year, IQR 0.1–1.4 mm/year), PAU width (1.1 mm/year, IQR 0.8–1.7 mm/year) and neck width (1.1 mm/year, IQR 0.6–4.6 mm/year) tended to increase during follow-up. PAU initial diameter was >2 cm in 24/28 (85.7%) cases, and PAU growth to >2 cm occurred in 1 additional case after 2 years of follow-up. The use of ACEi/ARBs (P=.017) was protective from aortic growth, while initial PAU depth (P=.047) was significantly associated to an increased aortic growth rate; in particular a depth >10 mm (1.08 mm/year, IQR 0–1.95 mm/year) was a predictor of increased growth rate. A similar trend was recognized also for PAU neck width (P=.065) and total aortic diameter (P=.051), but without reaching statistical significance (Table III).
PAU clinical progression and resolution.
Twelve (43%) PAUs had clinical progression, 3 (11%) had complete resolution, and 13 (46%) remained stable. Freedom from clinical PAU progression was 90.5% (95%CI 78.8–100) at 1 year, 70.6% (95%CI 53.3–93.5) at 5 years, and 45.8% (95%CI 26.8–78.4) at 10 years (Figure 2). PAU mortality and rupture rates at 10 years were 8.4% (95%CI 0–19.2) and 7.2% (95%CI 0–16.4) respectively. A PAU intervention was performed in three cases after a mean period of 5.7 years; freedom from intervention was 71.6% (95%CI 47–100) at 10 years. PAU depth >10 mm was the only identified factor associated with disease progression (HR 3.92, 95%CI 1.26–12.2; P=.018) (Table IV).
PAU resolution rate was 0% at 1 year, 10.6% (95%CI 0–23.5) at 5 years, and 10.6% (95%CI 0–23.5) at 10 years (Figure 3). A smaller PAU width (HR 0.40, 95%CI 0.08–0.76; P=0.43), PAU neck width (HR 0.41, 95%CI 0.07–0.74; P=.041) and longitudinal extent (HR 0.04, 95%CI 0–0.32; P=0.21) were associated to disease resolution (Table V).
DISCUSSION
Our study analyzed the morphological changes and clinical outcomes of ASs at a population level, showing that aortic growth is observed in most patients with AD, IMH or PAU, and that clinical disease progression is frequent. Prior studies focused on the early identification of clinical or anatomical predictors of aortic diameter enlargement and/or clinical course in patients with AS. However, the results are often inconsistent for patients with AD20, and only scarce data are available for IMH and PAU. The limited follow-up, the variable definition of outcomes, and the lack of a standardized measurement methods may justify the conflicting results between reports. Our patient cohort is not subject to the referral bias that usually characterizes registries or single center reports. A reliable long-term follow-up was available (median 8 years); differently most imaging studies on both AD and IMH/PAU have a 2–5 years follow-up period7,9–11,13,18,19,21–25, that may be sufficient to detect initial morphological changes, but may fail in showing the clinical impact of the aortic growth rate, and do not allow to generalize the results to longer follow-up.
In our cohort of AD patients, aortic growth rate was 1.0 mm/year (IQR 0.4–1.7 mm/year). This result is similar to the 2-years results from the International Registry on Aortic Dissection (IRAD), describing a 1.7 mm/year diameter increase22. However, growth rates of 3 mm/year or higher have been described in other multicenter analyses21,22 or single-center studies10,23. Besides the longer observation period, also the different methods to calculate the growth rate (based on linear regression) and the different study design, may justify these different results. The population-based design, collecting information on all imaging studies across different healthcare providers, might have mitigated the bias for which patients with any grade of aortic enlargement are more prone to be prescribed control CTAs, to be compliant to follow-up, and thus to be included in single- or multi-center cohorts.
Aortic diameter >42 mm (P=.013) was significantly associated with increased aortic growth rate. This is consistent with a large body of literature, including a recent literature review highlighting that aortic diameter was associated with aortic growth or aortic events in 26 studies, with only 3 studies reporting contrasting results20. The effect of location of the intimal tear is more controversial24–25. We showed that location in the descending thoracic aorta (P=.043) was associated with aortic growth in our study, similarly to Kato et al.24.
AD extension (P=.038) was associated with clinical aortic events but not with the aortic growth rate. This may be related to the fact that aortic diameter and growth rate represent a surrogate of just the risk of aneurysm formation or rupture, while other possible complications, as recurrent dissection, incidence of a new AS, or branch malperfusion, may be independent from the aortic diameter, and be more frequent in distally extended ADs. Also, the major involvement of aortic side branches in De Bakey I or IIIb AD may have a role in maintaining FL patency and AD progression26–28. Together these findings on AD support a conservative treatment for acute uncomplicated AD with aortic diameter <42 mm and absence of other risk factors for disease progression.
In case of IMH, aortic diameter (P=.037) and location in the aortic arch (P=.040) were predictors of adverse events. The effect of total aortic diameter has been previously described7,12,18, however other possible factors as the presence of concomitant PAU, longitudinal extension, or IMH depth failed to predict late outcomes. Regarding this last aspect, it is also interesting to note that depth decrease during follow-up did not necessarily indicate a disease resolution. In fact IMH depth reduction occurred in 4 cases (with complete radiological disappear of the IMH in 3), but IMH turned into an aortic aneurysm in 3 of these patients, probably as a result of the weakened aortic wall. Therefore, a lifelong follow-up may be still warranted in all cases of IMH.
Compared to AD and IMH, PAU represents a different disease, as it usually occurs in older patients, is associated with aortic atherosclerosis, and the clinical presentation may be subtle or asymptomatic, with less cases diagnosed in the acute phase. The use of ACEi/ARBs was protective from aortic enlargement, but it is difficult to understand if this was a marker of better blood pressure control or if there is any direct effect of ACEi/ARBs on aortic remodeling. A PAU depth ≥10 mm predicted both an increased aortic growth rate and disease progression. Our results also suggest that PAUs with large width, neck width, or great longitudinal extension are not significantly at risk for progression, but are less likely to spontaneously resolve. Differently from IMH, all PAUs with diameter decrease during follow-up showed also a disease resolution within 5 years from the diagnosis. Another interesting observation was that PAU neck width enlargement was associated in all cases with total aortic diameter increase. These results seem to support the current recommendation for intervention in case of PAU depth >10 mm29, while a watchful follow-up may be appropriate for those with large neck width, PAU width, or long longitudinal extent. Differently, a PAU diameter >2cm is relatively frequent at the time of diagnosis, and indications to treatment should not be based only on the total PAU diameter alone.
Our study has several limitations that are worth mentioning. This is a retrospective study where the initial code-based identification of patients with AS may have led to inherent biases. Although we have tried to identify all possible cases, there may be patients with imprecise codes classification that would have been missed. Also, the limited number of patients included in the final cohort limited the power of the statistical analysis, particularly for IMH and PAU. Due to the low number of events we did not perform any multivariable analyses to avoid the risk of overfitting. The small sample size may cause type II errors, and it is possible that only factors with large effect size had a significant impact in our analysis, while there may be false negatives in case of smaller effect size. The indication to follow-up protocol and to treatment were not standardized and were at discretion of the treating physician. No cases of uncomplicated type B AD received prophylactic TEVAR, therefore it was not possible to assess the role of TEVAR in this specific clinical setting. Type B AD and residual AD after type A repair were grouped together in the main analysis since they may have a similar behavior; however, the subanalysis on only non-operated type B ADs was limited by the low number of patients.
Our findings are strengthened by the fact that this population-based approach allowed to report a long-term follow-up results, since our patient cohort is not subject to the referral bias that is usually seen in registries or single center reports. As previously reported3, the identification of patients with AS was consistent during the study period, and the incidence has remained stable since 1995. Also, the method to assess diameter changes took into account all the available measurements for each patient. Moreover our definition of disease progression included only clinically relevant events, differently from other reports that included also minor morphological changes (ie. any increase in size of the aorta, IMH, or PAU parameters) that may carry an unclear clinical significance.
CONCLUSION
Aortic growth is observed in most patients with AD, IMH or PAU. Clinical disease progression is frequent for all AS, while a spontaneous resolution is uncommon. Follow-up imaging, particularly early after aortic events, is important in these patients. High risk predictors of aortic growth rate and disease progression should be used to additionally tailor appropriate aortic follow up.
Fundings
This study was supported by the American Heart Association (16SDG2750043) and made possible using the resources of the Rochester Epidemiology Project (supported by the NIH National Institute on Aging under award no. R01AG34676). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
REFERENCES
- 1.Tsai TT, Nienaber CA, Eagle KA. Acute Aortic Syndromes. Circulation. 2005. Dec 13;112(24):3802–13. [DOI] [PubMed] [Google Scholar]
- 2.Weiss S, Sen I, Huang Y, Killian JM, Harmsen WS, Mandrekar J, et al. Cardiovascular morbidity and mortality after aortic dissection, intramural hematoma, and penetrating aortic ulcer. J Vasc Surg. 2019;70(3):724–731.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeMartino RR, Sen I, Huang Y, Bower TC, et al. Population-Based Assessment of the Incidence of Aortic Dissection, Intramural Hematoma, and Penetrating Ulcer, and Its Associated Mortality From 1995 to 2015. Circ Cardiovasc Qual Outcomes. 2018;11(8):e004689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsai TT, Fattori R, Trimarchi S, et al. Long-Term Survival in Patients Presenting With Type B Acute Aortic Dissection: Insights From the International Registry of Acute Aortic Dissection. Circulation. 2006. Nov 21;114(21):2226–31. [DOI] [PubMed] [Google Scholar]
- 5.Olsson C, Thelin S, Ståhle E, et al. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. Circulation. 2006. Dec 12;114(24):2611–8. [DOI] [PubMed] [Google Scholar]
- 6.Chou AS, Ziganshin BA, Charilaou P, et al. Long-term behavior of aortic intramural hematomas and penetrating ulcers. J Thorac Cardiovasc Surg. 2016. Feb;151(2):361–372, 373.e1. [DOI] [PubMed] [Google Scholar]
- 7.von Kodolitsch Y, Csösz SK, Koschyk DH, et al. Intramural Hematoma of the Aorta: Predictors of Progression to Dissection and Rupture. Circulation. 2003. Mar 4;107(8):1158–63. [DOI] [PubMed] [Google Scholar]
- 8.D’Oria M, Sen I, Day CN, Mandrekar J, Weiss S, Bower TC, Oderich GS, Goodney PP, DeMartino RR. Burden and causes of readmissions following initial discharge after aortic syndromes. J Vasc Surg. 2021. Mar;73(3):836–843.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jonker FHW, Trimarchi S, Rampoldi V, et al. Aortic Expansion After Acute Type B Aortic Dissection. Ann Thorac Surg. 2012. Oct;94(4):1223–9. [DOI] [PubMed] [Google Scholar]
- 10.Durham CA, Aranson NJ, Ergul EA, et al. Aneurysmal degeneration of the thoracoabdominal aorta after medical management of type B aortic dissections. J Vasc Surg. 2015. Oct;62(4):900–6. [DOI] [PubMed] [Google Scholar]
- 11.Kamman AV, Jonker FHW, Sechtem U, et al. Predictors of Stable Aortic Dimensions in Medically Managed Acute Aortic Syndromes. Ann Vasc Surg. 2017. Jul;42:143–9. [DOI] [PubMed] [Google Scholar]
- 12.Ganaha F, Miller DC, Sugimoto K, et al. Prognosis of Aortic Intramural Hematoma With and Without Penetrating Atherosclerotic Ulcer: A Clinical and Radiological Analysis. Circulation. 2002. Jul 16;106(3):342–8. [DOI] [PubMed] [Google Scholar]
- 13.Kaji S Long-Term Prognosis of Patients With Type B Aortic Intramural Hematoma. Circulation. 2003. Sep 9;108(90101):307II−−311. [DOI] [PubMed] [Google Scholar]
- 14.St Sauver JL, Grossardt BR, Yawn BP, et al. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol. 2011. May 1;173(9):1059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lombardi JV, Hughes GC, Appoo JJ, et al. Society for Vascular Surgery (SVS) and Society of Thoracic Surgeons (STS) reporting standards for type B aortic dissections. J Vasc Surg. 2020. Mar;71(3):723–47. [DOI] [PubMed] [Google Scholar]
- 16.Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with Thoracic Aortic Disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Circulation. 2010. Apr 6;121(13):e266–369. [DOI] [PubMed] [Google Scholar]
- 17.Dake MD, Thompson M, van Sambeek M, et al. DEFINE Investigators. DISSECT: a new mnemonic-based approach to the categorization of aortic dissection. Eur J Vasc Endovasc Surg Off J Eur Soc Vasc Surg. 2013. Aug;46(2):175–90. [DOI] [PubMed] [Google Scholar]
- 18.Sueyoshi E, Imada T, Sakamoto I, et al. Analysis of predictive factors for progression of type B aortic intramural hematoma with computed tomography. J Vasc Surg. 2002. Jun;35(6):1179–83. [DOI] [PubMed] [Google Scholar]
- 19.Nathan DP, Boonn W, Lai E, et al. Presentation, complications, and natural history of penetrating atherosclerotic ulcer disease. J Vasc Surg. 2012. Jan;55(1):10–5. [DOI] [PubMed] [Google Scholar]
- 20.Spinelli D, Benedetto F, Donato R, et al. Current evidence in predictors of aortic growth and events in acute type B aortic dissection. J Vasc Surg. 2018. Dec;68(6):1925–1935.e8. [DOI] [PubMed] [Google Scholar]
- 21.Trimarchi S, Tolenaar JL, Jonker FHW, et al. Importance of false lumen thrombosis in type B aortic dissection prognosis. J Thorac Cardiovasc Surg. 2013. Mar;145(3 Suppl):S208–212. [DOI] [PubMed] [Google Scholar]
- 22.Tolenaar JL, van Keulen JW, Jonker FHW, et al. Morphologic predictors of aortic dilatation in type B aortic dissection. J Vasc Surg. 2013. Nov;58(5):1220–5. [DOI] [PubMed] [Google Scholar]
- 23.Song J-M, Kim S-D, Kim J-H, et al. Long-term predictors of descending aorta aneurysmal change in patients with aortic dissection. J Am Coll Cardiol. 2007. Aug 21;50(8):799–804. [DOI] [PubMed] [Google Scholar]
- 24.Kato M, Bai H, Sato K, Kawamoto S, et al. Determining surgical indications for acute type B dissection based on enlargement of aortic diameter during the chronic phase. Circulation. 1995. Nov 1;92(9 Suppl):II107–112. [DOI] [PubMed] [Google Scholar]
- 25.Sueyoshi E, Sakamoto I, Uetani M. Growth Rate of Affected Aorta in Patients With Type B Partially Closed Aortic Dissection. Ann Thorac Surg. 2009. Oct;88(4):1251–7. [DOI] [PubMed] [Google Scholar]
- 26.Kitamura T, Torii S, Oka N, Horai T, et al. Impact of the entry site on late outcome in acute Stanford type B aortic dissection. Eur J Cardiothorac Surg. 2015. Nov;48(5):655–61. [DOI] [PubMed] [Google Scholar]
- 27.Kamman AV, Brunkwall J, Verhoeven EL, ADSORB trialists. Predictors of aortic growth in uncomplicated type B aortic dissection from the Acute Dissection Stent Grafting or Best Medical Treatment (ADSORB) database. J Vasc Surg. 2017. Apr;65(4):964–971.e3. [DOI] [PubMed] [Google Scholar]
- 28.Squizzato F, Oderich GS, Bower TC, Mendes BC, Kalra M, Shuja F, Colglazier J, DeMartino RR. Long-term fate of aortic branches in patients with aortic dissection. J Vasc Surg. 2021. Feb 14:S0741-5214(21)00200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riambau V, Böckler D, Brunkwall J, Cao P, et al. Editor’s Choice – Management of Descending Thoracic Aorta Diseases. Eur J Vasc Endovasc Surg. 2017. Jan;53(1):4–52. [DOI] [PubMed] [Google Scholar]


