Abstract
Bioconjugation techniques for biomolecule-polymer conjugation are numerous, however, slow kinetics and steric challenges generally necessitate excess reagents or long reactions times. Organometallic transformations are known to circumvent these issues; however, harsh reaction conditions, incompatibility in aqueous media and substrate promiscuity often limit their use in a biological context. This work reported herein, demonstrates a facile and benign organometallic Au(III) S-arylation approach that enables the synthesis of poly(ethylene glycol) monomethyl ether (mPEG)-protein conjugates with high efficiency. Isolable and bench-stable 2, 5, and 10 kDa mPEG-Au(III) reagents were synthesized via oxidative addition into terminal aryl iodide substituents installed on mPEG substrates with a (Me-DalPhos)Au(I)Cl precursor. Reaction of the isolable mPEG-Au(III) oxidative addition complexes with a cysteine thiol on a biomolecule resulted in facile and selective cysteine arylation chemistry forging covalent S-aryl linkages and affording the stable mPEG-biomolecule conjugates. Notably, low polymer reagent loadings were used to achieve near quantitative conversion at room temperature in one minute due to the rapid kinetics and high chemoselectivity of this Au-based bioconjugation approach. Therefore, this work represents an important addition to the protein-polymer conjugation chemical tool box.
Graphical Abstract
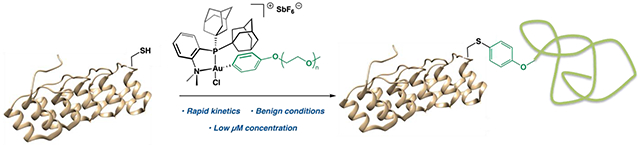
Introduction
Protein and peptide therapeutics represent a large class of treatments for a plethora of diseases, with more than 100 therapeutics garnering approval by the Food and Drug Administration (FDA) for clinical use.1–3 Conjugation of synthetic polymers to these biomacromolecule therapeutics significantly increases both the stability and in vivo lifetime of the drug, making the application of proteins and peptides as disease treatments more feasible and effective.4 Currently, polyethylene glycol (PEG) comprises the polymer component of all of the 27 protein-polymer conjugates that are approved by the FDA. Therefore, the pharmacokinetics and immunogenicity of PEG conjugates are well established, and the ability of the polymer to stabilize therapeutics has been shown extensively.5–7
Bioconjugation techniques for the efficient synthesis of PEGylated-biomacromolecular conjugates generally necessitate a coupling strategy with extremely rapid kinetics and high chemoselectivity to overcome the steric hindrance and low target functional group concentration inherent to these systems. Examples of these types of bioconjugation strategies are numerous; however, most require large excess of polymer reagents, often 10 equivalents or more.8–14 This large excess of reagent not only exhibits poor atom economy, but it also necessitates complicated purification strategies to separate multiple macromolecules from one another. Additionally, elevated reaction temperatures are occasionally employed to achieve near quantitative conversion, which can be detrimental to the structure and function of more delicate biomolecules.9,15,16 In an attempt to overcome some of these challenges, bioconjugation strategies have been developed utilizing non-canonical amino acids with more traditional “click” reactivity, notably alkynes with azides, to achieve faster rates.10,17 However, these non-natural modifications require challenging protein expression techniques, and can also perturb the natural structure and activity of the biomolecule.
In contrast, classical canonical bioconjugation techniques often utilize nucleophilic amino acid residues, particularly lysine and cysteine, to achieve efficient conjugation.18–24 However, modification of lysine residues often provides poor residue selectvity and can affect the solubility, structure and stability of the resulting biomolecule conjugate. To circumvent these challenges, free cysteine is often employed as a nucleophilic amino acid residue for bioconjugation, due to its low abundance and ease of engineering into proteins. Further benefits of the thiol functionality of cysteine include its unique chemical characteristics, such as its ionizability and soft nucleophilicity. However, traditional cysteine conjugations, such as maleimide conjugations and disulfide exchanges, forge bonds that are generally considered reversible in biologically relevant media (Figure 1a).25–29 While this can be advantageous in some cases where release of the protein is desired, in others, more stable bonds are preferred. Certain pH or redox conditions found in the body can undesirably cleave labile bonds, causing decomposition, clearance, toxic effects or an otherwise negative impact on pharmacokinetic properties.26,30–33
Figure 1:
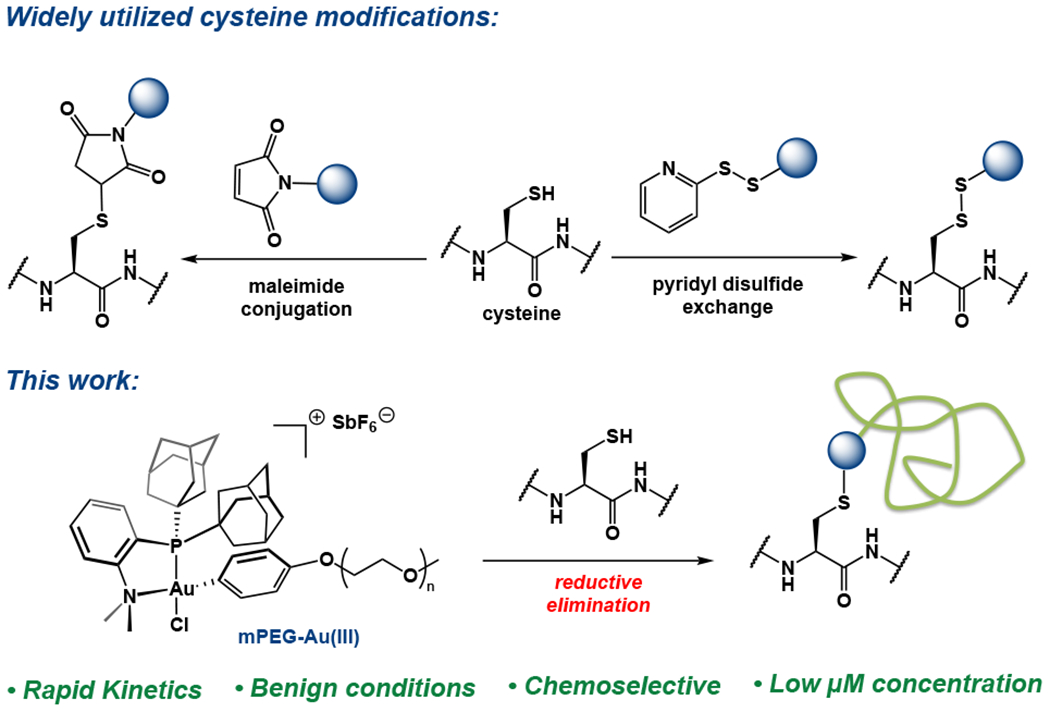
Comparison of widely utilized cysteine bioconjugation strategies with this Au(III) mediated S-arylation approach for biomolecule PEGylation.
Recently, cysteine S-arylation has emerged as a strategy toward more stable heteroatom-C(sp2) linkages that can help overcome the existing limitations in cysteine bioconjugation.34,35 Transition metal complexes for arylation of biomolecules have been shown to exhibit high functional group tolerance, chemoselectivity and rapid reaction kinetics to achieve efficient synthesis of bioconjugates.34,36,37 We previously showcased a Au(III) organometallic cysteine S-arylation approach in which small molecule Au(III) aryl reagents forge stable, S-C(sp2) bonds rapidly and selectively with cysteine thiols via reductive elimination.36,38–42 The relative thiophilicity of Au(III), as well as the reluctance of the Au(I) byproduct to undergo oxidative addition provided the benefit of minimal background reactivity with the many reactive substrates present in a biological setting.
While we previously showed the utility of this approach for the synthesis of small molecule S-aryl bioconjugates, we sought to explore this transformation to achieve fast and efficient synthesis of biomolecule-polymer conjugates. To this end, poly(ethylene glycol) monomethyl ether (mPEG)-Au(III)(Me-DalPhos) reagents were developed for the arylation of proteins and peptides containing cysteine (Figure 1b). Notably, the conservation of rapid reaction rates associated with our Au(III) mediated S-arylation approach when employing large coupling partners enables the use of near equimolar amounts of polymer reagent and extremely mild reaction conditions to achieve near quantitative conversion in a minute.
Results and Discussion
Synthesis of mPEG-Au(III) Reagents
Commercial mPEG reagents of 2, 5 and 10 kDa molecular weights were first subjected to tosylation conditions, followed by reaction with base and iodophenol to generate mPEG-aryl iodide reagents of each size in 74%, 82% and 78% isolated yield, respectively. Treatment of these mPEG-aryl iodide reagents with (Me-DalPhos)Au(I)Cl in the presence of the halide scavenger silver hexafluoroantimonate(V) (AgSbF6) results in oxidative addition across the terminal mPEG aryl-iodide bonds thereby generating 2, 5 and 10 kDa mPEG-Au(III) reagents in quantitative conversions and high isolated yields (Figure 2a). In our previous small molecule work, excess aryl iodide was utilized to promote the traditionally slow oxidative addition step thereby allowing us to achieve quantitative conversion of the Au(III) complexes.. However, because it is challenging to purify away the excess aryl iodide polymer from the Au(III) polymer product, further reaction optimization was needed. Extending the reaction time and applying heat enabled the quantitative conversion of mPEG-Au(III) reagents using sub-stoichiometric amounts of the aryl iodide polymer starting material.
Figure 2:
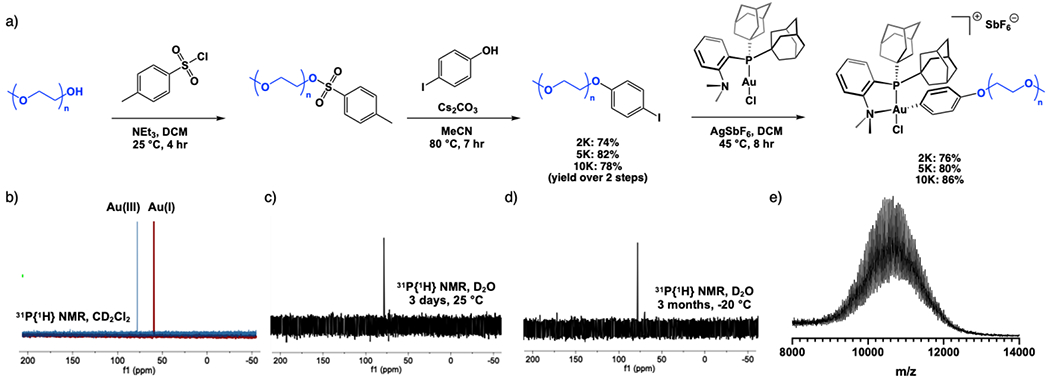
a) Synthesis of mPEG-Au(III) reagents. b) 31P{1H} NMR of 10 kDa mPEG-Au(III) reagent indicating quantitative conversion to mPEG-Au(III) product (blue) from Me-DalPhosAu(I)Cl starting material (red). c) 31P{1H} NMR of 5 kDa mPEG-Au(III) reagent after 3 days dissolved in water and stored at room temperature in air. d) 31P{1H} NMR of 5 kDa mPEG-Au(III) reagent after 3 months dissolved in water and stored at −20 °C. e) MALDI-TOF spectrum of 10 kDa mPEG-Au(III) reagent.
Quantitative conversion was determined via 31P NMR wherein the resonance corresponding to the phosphine ligand underwent a shift from 55 ppm to 75 ppm indicating consumption of the (Me-DalPhos)Au(I)Cl and formation of the mPEG-Au(III)(Me-DalPhos)Cl complex (Figure 2b). These 2, 5 and 10 kDa mPEG Au(III) reagents were isolated and characterized as yellow powders in 76%, 80% and 86% isolated yield, respectively. Notably, these reagents proved to be bench stable for several months, including at least 3 days dissolved in water at room temperature on the bench in open air, as well as 3 months when dissolved in water and stored at −20 °C as observed by a lone resonance at 75 ppm in the 31P NMR spectra. (Figure 2c–d). Additionally, MALDI-TOF was used to confirm the appropriate molar masses of each reagent, observed as a broad mass envelope, characteristic of a polymer, including the mass corresponding to the addition of gold and its ligands (Figure 2e).
Biomolecule PEGylation with mPEG-Au(III) Reagents
We next sought to challenge the kinetics of the rapid organometallic bioconjugation by PEGylation of a cysteine-containing model protein. For this purpose, we chose a designed ankyrin repeat protein (DARPin) which belongs to a class of genetically engineered proteins that bind tightly to a target protein and are often implicated as antibody mimics with moderate molecular weight and high levels of stability due to their repeating alpha helical motifs. Biomolecules are often insoluble and unstable, at room temperature in high μM concentrations, generally necessitating relatively dilute concentrations of coupling partners in these types of conjugations. We hypothesized that the inherent thiophilicity of Au(III) and the observed rapid kinetics would yield quantitative conversion to the desired conjugate at low μM concentrations and at room temperature.
When conjugating polymers to proteins using the grafting to approach, it is helpful to avoid employing large excess of polymer reagent, as the purification of excess polymer from the conjugate is challenging due to the large size of each component. Additionally, the instability of proteins at room temperature is a common problem when considering reaction times and temperatures for these transformations. As such, we sought to investigate optimize this bioconjugation strategy to achieve full conjugate conversion at or below room temperature while minimizing the amount of polymer reagent loading and reaction time. Our previous report utilized 20 equivalents of Au(III) small molecule oligoethylene glycol reagent to achieve complete conversion in 30 minutes. We sought to optimize the reaction conditions by minimizing both reaction time and mPEG Au(III) reagent loading while retaining high levels of conversion.
First, DARPin was pre-treated with 4 equivalents of TCEP•HCl to reduce any disulfide bonds that result in protein dimer formation during storage. Then, the sample was subjected to treatment with a range of equivalents of 5 kDa mPEG Au(III) reagents in Tris buffer, pH 7.5 for 1 minute at 25 °C (Figure S20). It was found that incubation withbetween 1.2 and 1.5 equivalents of the Au(III) reagent resulted in gave high yields of producthigh product yields. The same concentration screens wereConcentration screening with SDS-PAGE analysis performed at 15 min, 4 h, and 14 h with similar resultsdemonstrated similar results, indicating that the reaction was complete within the 1 minutes (data not shown) and that longer time periodsreaction times to do not increase yields when employing 1.0 equivalent of the Au(III) reagent. We next proceeded using near equimolar amounts of mPEG-Au(III) reagents (1.3 equivalents) in one minute at a 70 μM reaction concentration (Figure 3a). High conversion was observed in reactions of 2, 5 and 10 kDa mPEG-Au(III) reagents with DARPin in Tris buffer at pH 7.5 and 25 °C (Figure 3b) demonstrating that within this polymer molecular weight range, no differences in conversion were observed.
Figure 3:
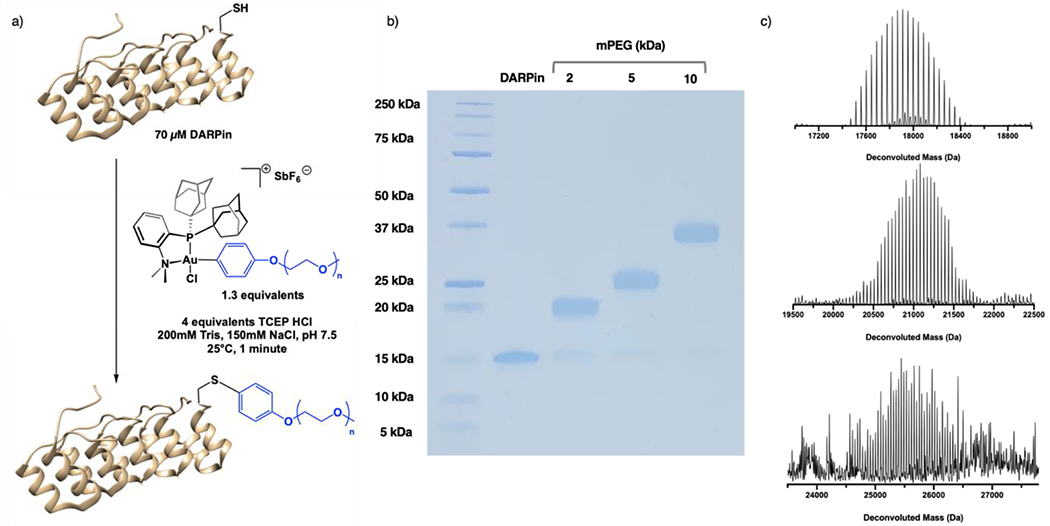
a) Synthetic scheme demonstrating reaction between DARPin protein and mPEG-Au(III) reagent leading to the formation of a cysteine-functionalized conjugate within one minute. b) SDS-PAGE gel displaying conversion to the 2, 5 and 10 kDa mPEG-DARPin conjugates (left to right). c) Deconvoluted mass spectra of the 2, 5 and 10 kDa (top to bottom) conjugates obtained by QTOF-LCMS.
Notably, this reaction operates in the presence of tris(2-carboxyethyl)phosphine hydrochloride TCEP•HCl, a phosphine reducing agent commonly employed to prevent disulfide formation. TCEP does not negatively affect the reaction and therefore does not necessitate removal from the reaction mixture. Additionally, a His6 tag was incorporated into DARPin during expression to enable Ni-NTA purification. The cysteine arylation process proceeds without necessitating the removal of the TCEP•HCl or the His6 tag, both of which are considered generally coordinating to traditional transition metal complexes. This is advantageous because the His6 tag could potentially be used as a handle for purification from remaining excess mPEG Au(III) reagent or Au(I) byproducts.
Reaction compatibility with various pH and buffer conditions was studied next. Near quantitative conversion to conjugate was observed in citrate buffer at pH 4, also within one minute at 25 °C. This compatibility allows for PEGylation of biomolecules that are only stable and/or soluble in acidic conditions. Further experiments demonstrated similarly high levels of conversion at concentrations as low as 7 μM in one minute, at both 25 °C and at 4 °C (Table 1). These results collectively demonstrate that the fast kinetics of the Au(III) mediated bioconjugation process can efficiently couple large, potentially thermally unstable partners quickly under mild reaction conditions.
Table 1.
Screening of reaction conditions for the PEGylation of DARPin with mPEG-Au(III) reagents
| entry | polymer MW (kDa) | Au(III) equivalents | buffer | PH | [protein] (μM) | temperature (°C) | time (min) | % conversion* |
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 1.3 | Tris | 7.5 | 70 | 25 | 1 | 90 |
| 2 | 5 | 1.3 | Tris | 7.5 | 70 | 25 | 1 | 92 |
| 3 | 10 | 1.3 | Tris | 7.5 | 70 | 25 | 1 | 91 |
| 4 | 5 | 1.3 | Tris | 7.5 | 7 | 25 | 1 | 93 |
| 5 | 2 | 1.3 | Tris | 7.5 | 70 | 4 | 1 | 94 |
| 6 | 5 | 1.3 | Tris | 7.5 | 7 | 4 | 1 | 92 |
| 7 | 10 | 1.3 | Citrate | 5.5 | 70 | 25 | 1 | 92 |
% conversion calculated by optical densitometry of SDS-PAGE gels (SI)
Competition with Maleimide mPEG Reagents
Maleimide conjugation remains the most widely utilized strategy for cysteine bioconjugation to date. While maleimide-based polymer reagents react at room temperature, excess polymer loadings are required and the reaction solution often needs to incubate for several hours to achieve high conjugation conversion11,43,44. To compare the strategies, a solution of DARPin in Tris buffer was treated with 1.3 equivalents of 5 kDa mPEG-maleimide and the reaction mixture was allowed to sit at room temperature for one minute at which point approximately 5% conversion to the conjugate was observed via SDS-PAGE. In contrast, treatment of DARPin with 2 kDa mPEG-Au(III) under the same conjugation conditions resulted in nearly complete conversion to the conjugate. Additionally, when incubating the 5 kDa mPEG-maleimide and 2kDa mPEG-Au(III) reagents in the same reaction, the mPEG-Au(III) exclusively converted to product, as observed by formation of only the 2 kDa conjugate by SDS-PAGE (Figure S17).
Characterization of mPEG-DARPin Conjugate
Mild reaction conditions for the synthesis of protein-polymer conjugates are crucial for the preservation of the structure and function of the biomolecule. To investigate this, circular dichroism (CD) was performed on the 5 kDa mPEG-DARPin conjugate to ensure that the presence of any remaining Au(I) byproduct or the reaction itself, did not affect the secondary structure. The CD spectra for the DARPin and mPEG-DARPin conjugates were nearly identical, showing a set of peaks with intensities between −15 to −20 mdeg at 200-230 nm, indicating highly alpha helical character consistent with that of DARPin. Additionally, CD spectra collected throughout a temperature ramp from 22 °C to 100 °C provided thermal denaturation curves which show similar melting temperatures (Tm) for both DARPin and the conjugate, suggesting no significant change in secondary structure (Figure 4).
Figure 4:
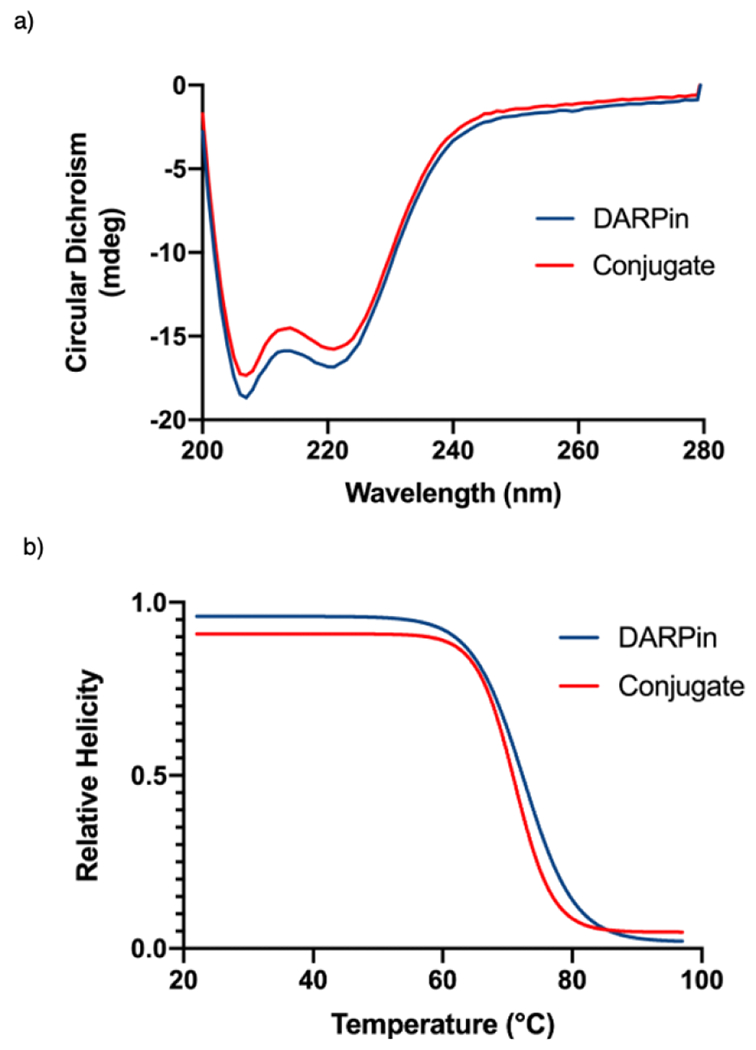
a) Circular dichroism (CD) spectra of native DARPin and DARPin conjugate showing no significant difference in helicity. b) CD thermal denaturation curves of native DARPin and DARPin conjugate showing no significant difference in melting temperature between 22 – 98°C. This data indicates that the Au(III) cysteine arylation does not alter DARPin’s secondary structure.
Liquid chromatography–mass spectrometry (LC-MS) experiments were also performed to confirm the absence of degradation or aggregation of the conjugate. The UV and total ion count (TIC) traces showed a peak corresponding to the mPEG-DARPin conjugate with deconvoluted masses corresponding to the mass of mPEG-DARPin for the 2, 5 and 10 kDa conjugates. (Figure 3c, S24). A peak at a later retention time was also observed corresponding to the Au(I) byproducts produced by this reaction.
Purification of mPEG-DARPin Conjugates
Utilizing near equimolar amounts of coupling reagent is beneficial when considering purification of these conjugates from excess polymer coupling partners that are similar in size. To remove the Au(I) byproduct and the limited excess of mPEG Au(III) reagent remaining, the conjugates were purified by size exclusion—fast protein liquid chromatography (SEC-FPLC). The conjugates were again analyzed by LC-MS after purification, and complete disappearance of the peak corresponding to the Au(I) byproducts was observed, providing evidence that this purification method is sufficient to obtain pure conjugate. Importantly, it was further confirmed by ICP-OES that 98.77 + 0.17% of gold was removed from this material (SI).
Conclusions
Herein is reported an organometallic Au(III) cysteine S-arylation bioconjugation strategy for the efficient coupling of large reaction partners, specifically mPEG with a cysteine-containing model protein, DARPin. This strategy utilizes readily accessible mPEG-aryl iodide reagents, which are synthesized in two steps from commercial mPEGs of varying molecular weights. These mPEG-aryl iodide reagents readily undergo oxidative addition when treated with (Me-DalPhos)Au(I)Cl in the presence of AgSbF6 to generate the Au(III)-mPEG reagents in quantitative yield. Notably, this process proceeds efficiently in air without the exclusion of water or oxygen, and these complexes are bench stable and can be stored at room temperature for up to three months permitting the long-term storage of these reagents. Quantitative conversion to the mPEG Au(III) product is observed, precluding the need for laborious purification techniques, and 31P NMR serves as a useful characterization tool to determine reagent purity.
Moreover, this strategy utilizes near equimolar amounts of these 2, 5 and 10 kDa mPEG-Au(III) reagents to achieve high levels of conversion to the mPEG-DARPin conjugates at various pHs and temperatures in one minute. The reaction proceeds efficiently at low micro-molar reaction concentrations (7 μM), without large excess of remaining polymer reagents or undesired byproducts. The preformation and isolation of these mPEG-Au(III) oxidative addition complexes decouples the traditionally slow oxidative addition step, common in organometallic cross coupling reactions, from the fast transmetalation and reductive elimination steps. This allows for extremely rapid kinetics under mild reaction conditions amenable to delicate biomolecules. The resulting conjugates are purified efficiently using size exclusion chromatography with protein secondary structure remaining intact.
This strategy could allow for conjugation of polymers to proteins with limited stability at room temperature, or which are only stable or soluble at non-neutral pH, for the exploration of new biologics for therapeutic applications. Overall, this work represents an expansion for the growing organometallic reagent toolkit available for bioconjugation chemistry.16,21,45–56
Supplementary Material
Acknowledgments
This work was funded by the National Science Foundation grant number CHE-2003946. A. M. S. thanks NIGMS (R35GM124746) for support. M.S.M thanks the NSF for the Bridge-to-Doctorate (HRD-1400789) and the Predoctoral (GRFP) (DGE-0707424) Fellowships and UCLA for the Christopher S. Foote and John Stauffer Fellowships. NIH award S10OD028491 is acknowledged for providing funding for the circular dichroism spectrometer. Mr. Austin Ready (UCLA) is thanked for assistance with ICP-OES measurements.
Footnotes
Supporting information available free of charge at:
Detailed summary of the chemistry and conjugation conditions, NMR spectra, SDS-PAGE, protein sequences, ICP-OES and supporting MS data. (PDF)
References
- (1).Alconcel SNS; Baas AS; Maynard HD FDA-Approved Poly(Ethylene Glycol)–Protein Conjugate Drugs. Polym. Chem 2011, 2 (7), 1442–1448. 10.1039/C1PY00034A. [DOI] [Google Scholar]
- (2).FDA Approved PEGylated Drugs Up To 2022 | Biopharma PEG. https://www.biochempeg.com/article/58.html (accessed 2022-05-20). [Google Scholar]
- (3).Gupta V; Bhavanasi S; Quadir M; Singh K; Ghosh G; Vasamreddy K; Ghosh A; Siahaan TJ; Banerjee S; Banerjee SK Protein PEGylation for Cancer Therapy: Bench to Bedside. J. Cell Commun. Signal 2019, 13 (3), 319–330. 10.1007/s12079-018-0492-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Harris JM; Chess RB Effect of Pegylation on Pharmaceuticals. Nat. Rev. Drug Discov 2003, 2 (3), 214–221. 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- (5).Lawrence PB; Price JL How PEGylation Influences Protein Conformational Stability. Curr. Opin. Chem. Biol 2016, 34, 88–94. 10.1016/j.cbpa.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Yang C; Lu D; Liu Z How PEGylation Enhances the Stability and Potency of Insulin: A Molecular Dynamics Simulation. Biochemistry 2011, 50 (13), 2585–2593. 10.1021/bi101926u. [DOI] [PubMed] [Google Scholar]
- (7).Diwan M; Park TG Pegylation Enhances Protein Stability during Encapsulation in PLGA Microspheres. J. Controlled Release 2001, 73 (2), 233–244. 10.1016/S0168-3659(01)00292-9. [DOI] [PubMed] [Google Scholar]
- (8).White CJ; Bode JW PEGylation and Dimerization of Expressed Proteins under Near Equimolar Conditions with Potassium 2-Pyridyl Acyltrifluoroborates. ACS Cent. Sci 2018, 4 (2), 197–206. 10.1021/acscentsci.7b00432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Swierczynski MJ; Ball ZT One-Step Protein–Polymer Conjugates from Boronic-Acid-Functionalized Polymers. Bioconjug. Chem 2020, 31 (11), 2494–2498. 10.1021/acs.bioconjchem.0c00516. [DOI] [PubMed] [Google Scholar]
- (10).Deiters A; Cropp TA; Summerer D; Mukherji M; Schultz PG Site-Specific PEGylation of Proteins Containing Unnatural Amino Acids. Bioorg. Med. Chem. Lett 2004, 14 (23), 5743–5745. 10.1016/j.bmcl.2004.09.059. [DOI] [PubMed] [Google Scholar]
- (11).Mantovani G; Lecolley F; Tao L; Haddleton DM; Clerx J; Cornelissen JJLM; Velonia K Design and Synthesis of N-Maleimido-Functionalized Hydrophilic Polymers via Copper-Mediated Living Radical Polymerization: A Suitable Alternative to PEGylation Chemistry. J. Am. Chem. Soc 2005, 127 (9), 2966–2973. 10.1021/ja0430999. [DOI] [PubMed] [Google Scholar]
- (12).Stenzel MH Bioconjugation Using Thiols: Old Chemistry Rediscovered to Connect Polymers with Nature’s Building Blocks. ACS Macro Lett. 2013, 2 (1), 14–18. 10.1021/mz3005814. [DOI] [PubMed] [Google Scholar]
- (13).Turecek PL; Bossard MJ; Schoetens F; Ivens IA PEGylation of Biopharmaceuticals: A Review of Chemistry and Nonclinical Safety Information of Approved Drugs. J. Pharm. Sci 2016, 105 (2), 460–475. 10.1016/j.xphs.2015.11.015. [DOI] [PubMed] [Google Scholar]
- (14).Belén LH; Rangel-Yagui C. de O.; Beltrán Lissabet JF; Effer B; Lee-Estevez M; Pessoa A; Castillo RL; Farías JG From Synthesis to Characterization of Site-Selective PEGylated Proteins. Front. Pharmacol 2019, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Dumas A; Spicer CD; Gao Z; Takehana T; Lin YA; Yasukohchi T; Davis BG Self-Liganded Suzuki–Miyaura Coupling for Site-Selective Protein PEGylation. Angew. Chem. Int. Ed 2013, 52 (14), 3916–3921. 10.1002/anie.201208626. [DOI] [PubMed] [Google Scholar]
- (16).Ko H-M; Deng J-R; Cui J-F; Kung KK-Y; Leung Y-C; Wong M-K Selective Modification of Alkyne-Linked Peptides and Proteins by Cyclometalated Gold(III) (C^N) Complex-Mediated Alkynylation. Bioorg. Med. Chem 2020, 28 (7), 115375. 10.1016/j.bmc.2020.115375. [DOI] [PubMed] [Google Scholar]
- (17).Wang M; Svatunek D; Rohlfing K; Liu Y; Wang H; Giglio B; Yuan H; Wu Z; Li Z; Fox J Conformationally Strained Trans- Cyclooctene (STCO) Enables the Rapid Construction of 18 F-PET Probes via Tetrazine Ligation. Theranostics 2016, 6 (6), 887–895. 10.7150/thno.14742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Laserna V; Abegg D; Afonso CF; Martin EM; Adibekian A; Ravn P; Corzana F; Bernardes GJL Dichloro Butenediamides as Irreversible Site-Selective Protein Conjugation Reagent. Angew. Chem. Int. Ed 2021, 60 (44), 23750–23755. 10.1002/anie.202108791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Konč J; Brown L; Whiten DR; Zuo Y; Ravn P; Klenerman D; Bernardes GJL A Platform for Site-Specific DNA-Antibody Bioconjugation by Using Benzoylacrylic-Labelled Oligonucleotides. Angew. Chem. Int. Ed 2021, 60 (49), 25905–25913. 10.1002/anie.202109713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Ohata J; Krishnamoorthy L; Gonzalez MA; Xiao T; Iovan DA; Toste FD; Miller EW; Chang CJ An Activity-Based Methionine Bioconjugation Approach To Developing Proximity-Activated Imaging Reporters. ACS Cent. Sci 2020, 6 (1), 32–40. 10.1021/acscentsci.9b01038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Cheng W-M; Lu X; Shi J; Liu L Selective Modification of Natural Nucleophilic Residues in Peptides and Proteins Using Arylpalladium Complexes. Org. Chem. Front 2018, 5 (21), 3186–3193. 10.1039/C8QO00765A. [DOI] [Google Scholar]
- (22).Gunnoo SB; Madder A Chemical Protein Modification through Cysteine. ChemBioChem 2016, 17 (7), 529–553. 10.1002/cbic.201500667. [DOI] [PubMed] [Google Scholar]
- (23).Ochtrop P; Hackenberger CPR Recent Advances of Thiol-Selective Bioconjugation Reactions. Curr. Opin. Chem. Biol 2020, 58, 28–36. 10.1016/j.cbpa.2020.04.017. [DOI] [PubMed] [Google Scholar]
- (24).You J; Zhang J; Wang J; Jin M Cysteine-Based Coupling: Challenges and Solutions. Bioconjug. Chem 2021, 32 (8), 1525–1534. 10.1021/acs.bioconjchem.1c00213. [DOI] [PubMed] [Google Scholar]
- (25).Lyon RP; Setter JR; Bovee TD; Doronina SO; Hunter JH; Anderson ME; Balasubramanian CL; Duniho SM; Leiske CI; Li F; et al. Self-Hydrolyzing Maleimides Improve the Stability and Pharmacological Properties of Antibody-Drug Conjugates. Nat. Biotechnol 2014, 32 (10), 1059–1062. 10.1038/nbt.2968. [DOI] [PubMed] [Google Scholar]
- (26).Shen B-Q; Xu K; Liu L; Raab H; Bhakta S; Kenrick M; Parsons-Reponte KL; Tien J; Yu S-F; Mai E; et al. Conjugation Site Modulates the in Vivo Stability and Therapeutic Activity of Antibody-Drug Conjugates. Nat. Biotechnol 2012, 30 (2), 184–189. 10.1038/nbt.2108. [DOI] [PubMed] [Google Scholar]
- (27).Zhang C; Liu Y; Feng C; Wang Q; Shi H; Zhao D; Yu R; Su Z Loss of PEG Chain in Routine SDS-PAGE Analysis of PEG-Maleimide Modified Protein. ELECTROPHORESIS 2015, 36 (2), 371–374. 10.1002/elps.201400373. [DOI] [PubMed] [Google Scholar]
- (28).Hermanson GT, Bioconjugate Techniques, 3rd ed. Academic Press: New York, 1996. [Google Scholar]
- (29).Altinbasak I; Arslan M; Sanyal R; Sanyal A Pyridyl Disulfide-Based Thiol–Disulfide Exchange Reaction: Shaping the Design of Redox-Responsive Polymeric Materials. Polym. Chem 2020, 11 (48), 7603–7624. 10.1039/D0PY01215G. [DOI] [Google Scholar]
- (30).Shakya AK; Sami H; Srivastava A; Kumar A Stability of Responsive Polymer–Protein Bioconjugates. Prog. Polym. Sci 2010, 35 (4), 459–486. 10.1016/j.progpolymsci.2010.01.003. [DOI] [Google Scholar]
- (31).Masters JC; Nickens DJ; Xuan D; Shazer RL; Amantea M Clinical Toxicity of Antibody Drug Conjugates: A Meta-Analysis of Payloads. Invest. New Drugs 2018, 36 (1), 121–135. 10.1007/s10637-017-0520-6. [DOI] [PubMed] [Google Scholar]
- (32).Behrens CR; Ha EH; Chinn LL; Bowers S; Probst G; Fitch-Bruhns M; Monteon J; Valdiosera A; Bermudez A; Liao-Chan S; et al. Antibody–Drug Conjugates (ADCs) Derived from Interchain Cysteine Cross-Linking Demonstrate Improved Homogeneity and Other Pharmacological Properties over Conventional Heterogeneous ADCs. Mol. Pharm 2015, 12 (11), 3986–3998. 10.1021/acs.molpharmaceut.5b00432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Baldwin AD; Kiick KL Tunable Degradation of Maleimide–Thiol Adducts in Reducing Environments. Bioconjug. Chem 2011, 22 (10), 1946–1953. 10.1021/bc200148v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Vinogradova EV; Zhang C; Spokoyny AM; Pentelute BL; Buchwald SL Organometallic Palladium Reagents for Cysteine Bioconjugation. Nature 2015, 526 (7575), 687–691. 10.1038/nature15739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Lobba MJ; Fellmann C; Marmelstein AM; Maza JC; Kissman EN; Robinson SA; Staahl BT; Urnes C; Lew RJ; Mogilevsky CS; et al. Site-Specific Bioconjugation through Enzyme-Catalyzed Tyrosine–Cysteine Bond Formation. ACS Cent. Sci 2020, 6 (9), 1564–1571. 10.1021/acscentsci.0c00940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Messina MS; Stauber JM; Waddington MA; Rheingold AL; Maynard HD; Spokoyny AM Organometallic Gold(III) Reagents for Cysteine Arylation. J. Am. Chem. Soc 2018, 140 (23), 7065–7069. 10.1021/jacs.8b04115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Zhang C; Vinogradova EV; Spokoyny AM; Buchwald SL; Pentelute BL Arylation Chemistry for Bioconjugation. Angew. Chem. Int. Ed 2019, 58 (15), 4810–4839. 10.1002/anie.201806009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Stauber JM; Qian EA; Han Y; Rheingold AL; Král P; Fujita D; Spokoyny AM An Organometallic Strategy for Assembling Atomically Precise Hybrid Nanomaterials. J. Am. Chem. Soc 2020, 142 (1), 327–334. 10.1021/jacs.9b10770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Joost M; Amgoune A; Bourissou D Reactivity of Gold Complexes towards Elementary Organometallic Reactions. Angew. Chem. Int. Ed 2015, 54 (50), 15022–15045. 10.1002/anie.201506271. [DOI] [PubMed] [Google Scholar]
- (40).Joost M; Zeineddine A; Estévez L; Mallet–Ladeira S; Miqueu K; Amgoune A; Bourissou D Facile Oxidative Addition of Aryl Iodides to Gold(I) by Ligand Design: Bending Turns on Reactivity. J. Am. Chem. Soc 2014, 136 (42), 14654–14657. 10.1021/ja506978c. [DOI] [PubMed] [Google Scholar]
- (41).Zeineddine A; Estévez L; Mallet-Ladeira S; Miqueu K; Amgoune A; Bourissou D Rational Development of Catalytic Au(I)/Au(III) Arylation Involving Mild Oxidative Addition of Aryl Halides. Nat. Commun 2017, 8 (1), 565. 10.1038/s41467-017-00672-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Rodriguez J; Zeineddine A; Carrizo EDS; Miqueu K; Saffon-Merceron N; Amgoune A; Bourissou D Catalytic Au(I)/Au(III) Arylation with the Hemilabile MeDalphos Ligand: Unusual Selectivity for Electron-Rich Iodoarenes and Efficient Application to Indoles. Chem. Sci 2019, 10 (30), 7183–7192. 10.1039/C9SC01954E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).De P; Li M; Gondi SR; Sumerlin BS Temperature-Regulated Activity of Responsive Polymer–Protein Conjugates Prepared by Grafting-from via RAFT Polymerization. J. Am. Chem. Soc 2008, 130 (34), 11288–11289. 10.1021/ja804495v. [DOI] [PubMed] [Google Scholar]
- (44).Tao L; Kaddis CS; Loo RRO; Grover GN; Loo JA; Maynard HD Synthesis of Maleimide-End-Functionalized Star Polymers and Multimeric Protein–Polymer Conjugates. Macromolecules 2009, 42 (21), 8028–8033. 10.1021/ma901540p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Vinogradova EV Organometallic Chemical Biology: An Organometallic Approach to Bioconjugation. Pure Appl. Chem 2017, 89 (11), 1619–1640. 10.1515/pac-2017-0207. [DOI] [Google Scholar]
- (46).Malins LR Peptide Modification and Cyclization via Transition-Metal Catalysis. Curr. Opin. Chem. Biol 2018, 46, 25–32. 10.1016/j.cbpa.2018.03.019. [DOI] [PubMed] [Google Scholar]
- (47).Rodríguez J; Martínez-Calvo M Transition-Metal-Mediated Modification of Biomolecules. Chem. – Eur. J 2020, 26 (44), 9792–9813. 10.1002/chem.202001287. [DOI] [PubMed] [Google Scholar]
- (48).Waddington MA; Zheng X; Stauber JM; Hakim Moully E; Montgomery HR; Saleh LMA; Král P; Spokoyny AM An Organometallic Strategy for Cysteine Borylation. J. Am. Chem. Soc 2021, 143 (23), 8661–8668. 10.1021/jacs.1c02206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Jbara M Transition Metal Catalyzed Site-Selective Cysteine Diversification of Proteins. Pure Appl. Chem 2021, 93 (2), 169–186. 10.1515/pac-2020-0504. [DOI] [Google Scholar]
- (50).Ohata J; T. Ball Z. Rhodium at the Chemistry–Biology Interface. Dalton Trans. 2018, 47 (42), 14855–14860. 10.1039/C8DT03032D. [DOI] [PubMed] [Google Scholar]
- (51).Zhao Z; Shimon D; Metanis N Chemoselective Copper-Mediated Modification of Selenocysteines in Peptides and Proteins. J. Am. Chem. Soc 2021, 143 (32), 12817–12824. 10.1021/jacs.1c06101. [DOI] [PubMed] [Google Scholar]
- (52).Lin X; Haimov E; Redko B; Vigalok A Selective Stepwise Arylation of Unprotected Peptides by PtIV Complexes. Angew. Chem. Int. Ed n/a (n/a), e202205368. 10.1002/anie.202205368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Tilden JAR; Lubben AT; Reeksting SB; Kociok-Köhn G; Frost CG Pd(II)-Mediated C–H Activation for Cysteine Bioconjugation. Chem. – Eur. J 2022, 28 (11), e202104385. 10.1002/chem.202104385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Yang P; Wang X; Li B; Yang Y; Yue J; Suo Y; Tong H; He G; Lu X; Chen G Streamlined Construction of Peptide Macrocycles via Palladium-Catalyzed Intramolecular S-Arylation in Solution and on DNA. Chem. Sci 2021, 12 (16), 5804–5810. 10.1039/D1SC00789K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Gukathasan S; Parkin S; Black EP; Awuah SG Tuning Cyclometalated Gold(III) for Cysteine Arylation and Ligand-Directed Bioconjugation. Inorg. Chem 2021, 60 (19), 14582–14593. 10.1021/acs.inorgchem.1c01517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Wang W; Lorion MM; Shah J; Kapdi AR; Ackermann L Late-Stage Peptide Diversification by Position-Selective C–H Activation. Angew. Chem. Int. Ed 2018, 57 (45), 14700–14717. 10.1002/anie.201806250. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


