Abstract
Heterophile antibodies are a well-recognized cause of erroneous results in immunoassays. We describe here a 22-month-old child with heterophile antibodies reactive with bovine serum albumin and caprine proteins causing false-positive results to human immunodeficiency virus type 1 and other infectious serology testing.
Heterophile antibodies are a well-recognized cause of interference in immunoassays, potentially giving rise to false-positive results (3, 5, 8). Heterophile antibodies may have some specificity, as with human anti-mouse antibodies (HAMA), but by definition they react with a number of different epitopes. Two-site immunoassays are susceptible to interference when heterophile antibodies bridge the capture and detection antibodies, as can occur with HAMA (2). HAMA and other heterophile antibodies may be present in as many as 40% of individuals, especially in patients treated with monoclonal antibody immunotherapy (6, 7). Heterophile antibodies reactive with other molecules used in immunoassays have not been well characterized but can also cause false assay results (4).
We describe here a case of heterophile antibodies that are cross-reactive with bovine and caprine proteins occurring in a 22-month-old child, causing false-positive immunoassay results to human immunodeficiency virus type 1 (HIV-1) and a number of other infectious serology tests. A 22-month-old boy with a history of Down syndrome and endocardial cushion defect repair was admitted for fevers of up to 103°F of several days’ duration and for respiratory distress. A chest radiograph showed bilateral upper-lobe pneumonias. The patient failed to respond to antibiotic therapy and a chest computerized tomogram (CT) showed a left upper-lobe abscess. The abscess was surgically drained and cultures grew Candida, Enterobacter, and gram-positive cocci.
Table 1 shows results of enzyme-linked immunosorbent assay (ELISA) laboratory testing for a number of infectious agents. Notably, an HIV-1 enzyme immunoassay (EIA) was positive (Table 1), with an inconclusive pattern on confirmatory Western blot (Fig. 1). Also, the pattern of Epstein-Barr virus (EBV) serologies was atypical, since the immunoglobulin G (IgG) antibody to early antigen appeared to be present, but neither IgM nor IgG antibodies for the viral capsid antigen were found. In addition, an unusual pattern was seen on immunodiffusion for Histoplasma antibodies. An abnormal line of precipitation was seen between the patient’s serum and the goat anti-histoplasma control antibodies, suggesting the presence of human anti-goat antibodies in the patient’s serum.
TABLE 1.
Results of infectious serology testinga
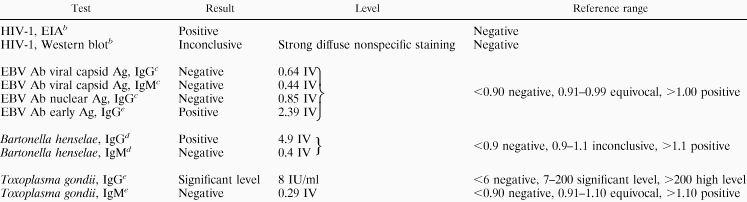 |
Abbreviations: Ab, antibody; Ag, antigen; IV, index value.
Organon Teknika, Durham, N.C.
DiaSorin, Stillwater, Minn.
ARUP Laboratories, Salt Lake City, Utah.
Beckman Coulter, Fullerton, Calif.
FIG. 1.
HIV Western blot results. (A) Negative control; (B) patient showing strong nonspecific staining; (C) positive control.
Further immunodiffusion tests were performed with the patient’s serum to confirm the presence of human anti-goat antibodies. These immunodiffusion tests showed immunoprecipitation bands between the patient’s serum and goat serum, bovine serum albumin (BSA), fetal bovine serum (FBS), and powdered-milk proteins. These results indicated the presence of heterophile antibodies that react with components of goat serum, BSA, FBS, and powdered milk.
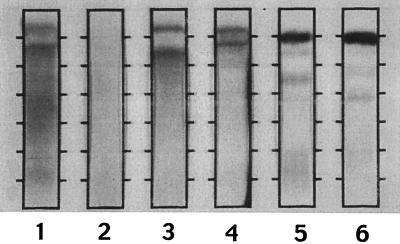
We also performed immunofixation electrophoresis of goat serum, BSA, FBS, and powdered milk by using the patient’s serum as the overlaying antibody (Fig. 2). This showed reactivity of patient antibodies to goat and bovine albumin, some reactivity in the gamma region (immunoglobulins) of the goat serum, and mild diffuse staining of the powdered-milk proteins.
FIG. 2.
Immunofixation electrophoresis with patient’s serum as the overlaying antibody. Lane 1, goat serum; lane 2, powdered milk; lane 3, BSA; lane 4, FBS. Strong reactivity with goat and bovine albumin is seen with prozone effect, as well as reactivity in the gamma region of the goat serum. Lane five shows the patient’s serum, and lane six contains pooled human plasma, both immunofixed with polyclonal anti-human serum.
We wanted to test the possible role of the patient’s heterophile antibodies in causing false-positive ELISA test results. Therefore, we preabsorbed the patient’s serum with BSA and goat serum to remove the heterophile antibodies. After preabsorption, testing for Bartonella henselae was negative, and the HIV-1 EIA was less reactive. Preabsorption with powdered milk did not change any results.
Based on the electrophoresis and preabsorption studies, we believe the positive test results observed in this patient were due to heterophile antibodies reactive with BSA and caprine proteins. All of the positive tests observed used BSA as a blocking agent for the preparation of the microELISA reaction wells. BSA is commonly used to cover other epitopes that may be present in the wells. In this case the patient’s heterophile antibody reacted with the BSA in the reaction well and is then detected by the labeled anti-human detection antibody, thus giving a false-positive reaction. A false-positive result was not seen when BSA was used in the specimen diluent, resulting in the heterophile antibodies being preabsorbed. Review of the specific test components used in the different test kits in this case showed that the heterophile antibody caused a false-positive result only when BSA was used to block the microtiter wells but was not in the specimen diluent. Anti-BSA antibodies have previously been investigated in the pathogenesis of diabetes mellitus (1), but their prevalence and interference in immunoassays are not known. Conceivably, anti-BSA antibodies could be quite common, since most immunoassays use BSA in the specimen diluent, so that in most instances these antibodies would be preabsorbed and not detected. Heterophile antibodies should be considered in instances of multiple presumed false-positive test results that are not consistent with the clinical situation.
REFERENCES
- 1.Atkinson M A, Bowman M A, Kao K J, Campbell L, Dush P J, Shah S C, Simell O, Maclaren N K. Lack of immune responsiveness to bovine serum albumin in insulin-dependent diabetes. N Engl J Med. 1993;329:1853–1858. doi: 10.1056/NEJM199312163292505. [DOI] [PubMed] [Google Scholar]
- 2.Hansen H J, Sullivan C L, Sharkey R M. HAMA interference with murine monoclonal antibody-based immunoassays. J Clin Immunoassay. 1993;16:294–299. [Google Scholar]
- 3.Kricka L J, Schmerfeld-Pruss D, Senior M, Goodman D B, Kaladas P. Interference by human anti-mouse antibody in two-site immunoassays. Clin Chem. 1990;36:892–894. [PubMed] [Google Scholar]
- 4.Levinson S S. Antibody multispecificity in immunoassay interference. Clin Biochem. 1992;25:77–87. doi: 10.1016/0009-9120(92)80048-l. [DOI] [PubMed] [Google Scholar]
- 5.Nahm M H, Hoffmann J W. Heteroantibody: phantom of the immunoassay. Clin Chem. 1990;36:829. [PubMed] [Google Scholar]
- 6.Reynolds J C, Del Vecchio S, Sakahara H, Lora M E, Carrasquillo J A, Neumann R D, Larson S M. Anti-murine antibody response to mouse monoclonal antibodies: clinical findings and implications. Int J Radiat Appl Instr. 1989;16:121–125. doi: 10.1016/0883-2897(89)90182-7. [DOI] [PubMed] [Google Scholar]
- 7.Schroff R W, Foon K A, Beatty S M, Oldham R K, Morgan A C., Jr Human anti-murine immunoglobulin responses in patients receiving monoclonal antibody therapy. Cancer Res. 1985;45:879–885. [PubMed] [Google Scholar]
- 8.Sosolik R C, Hitchcock C L, Becker W J. Heterophilic antibodies produce spuriously elevated concentrations of the MB isoenzyme of creatine kinase in a selected patient population. Am J Clin Pathol. 1997;107:506–510. doi: 10.1093/ajcp/107.5.506. [DOI] [PubMed] [Google Scholar]