Abstract
Objectives
EUCAST changed the definition of the ‘intermediate’ (I) category in 2019, now defined as ‘susceptible, increased exposure’. This new definition could lead to an increased prescription of antibiotics still reported as ‘S’, compared with those now reported as ‘I’. The objective of this study was to evaluate the influence of this definition on the use of overly broad-spectrum antibiotics for the treatment of infections caused by WT Pseudomonas aeruginosa.
Methods
A retrospective observational multicentre study was conducted, involving five hospitals. Two 15 month study periods were defined, before and after the implementation of the new definition. All patients with an infection caused by WT P. aeruginosa treated by β-lactams were included. The main endpoint was the proportion of patients treated by an overly broad-spectrum antibiotic treatment by meropenem or ceftolozane/tazobactam.
Results
Two hundred and ninety-one patients were included. No difference between groups was found, in terms of infection, microbiology or demographic characteristics. Two overly broad-spectrum antibiotic treatments by meropenem or ceftolozane/tazobactam were observed in Period 1 (1.2%), versus 13 in Period 2 (10.8%; P < 0.001). No overly broad-spectrum treatment was observed when the antimicrobial stewardship team had given advice.
Conclusions
This new definition can cause a negative impact on the use of overly broad-spectrum antibiotic treatment due to misunderstanding by clinicians. Its successful implementation requires adaptation of software for reporting antibiotic susceptibility, a sustained strong information campaign by microbiologists and support by an antimicrobial stewardship team.
Introduction
A new definition of the ‘intermediate’ (I) category on antibiograms became effective in the v. 9.0 update of the EUCAST breakpoint tables that was applicable on 1 January 2019.1,2 It was implemented the same year in the recommendations of the French antimicrobial susceptibility testing committee3 (CA-SFM). As a result, the category ‘susceptible, increased exposure’ has been applied to the microorganism/antibiotic pairs for which the breakpoints have been defined for high dosages. EUCAST decided to propose these new definitions while retaining the acronyms ‘S’, ‘I’ and ‘R’ in the electronic medical record (EMR). The objective was to eliminate the uncertainty of the old ‘I’ meaning and to emphasise that all breakpoints are dose/exposure dependent and to encourage the use of high dosages when the bacterium to treat is intrinsically less susceptible to the agent.
Pseudomonas aeruginosa is a microorganism that presents all the factors that could lead to a misunderstanding of these new susceptibility test results. With the new EUCAST definitions, isolates with a WT phenotype for β-lactams remained susceptible at standard dose (reported as ‘S’) to meropenem and ceftolozane/tazobactam, while first-line anti-pseudomonal β-lactams such as piperacillin or ceftazidime were reported as ‘I’ because they required only high-dose use. Since P. aeruginosa is responsible for opportunistic infections that result in high mortality, non-infectious disease (ID) specialists could be encouraged to favour a molecule reported as ‘S’ over a high-dose molecule reported as ‘I’.4
The aim of this study was to assess the influence of the new EUCAST definition of the I category on the choice of β-lactam antibiotics for the treatment of infections caused by WT P. aeruginosa.
Methods
Study design and setting
A retrospective observational multicentre study was conducted, involving five French hospitals with a unique centralized bacteriology laboratory. An antimicrobial stewardship (AMS) team, composed of three ID specialists, was located in the biggest hospital, giving advice 5 days a week for all the five hospitals.
Clinical samples were inoculated on media recommended by ESCMID and the Société Française de Microbiologie (SFM).5 Bacterial identification was determined using MALDITOF MS (Microflex LT, Bruker Daltonics, Bremen, Germany). Antibiotic susceptibility was performed by the disc diffusion method on Mueller–Hinton media (Bio-Rad, Marnes-la-Coquette, France) for all antimicrobial agents for which breakpoint diameter zones were available in EUCAST tables v. 9.0.
The new EUCAST definition of susceptibility categories (v. 9.0) was implemented in these five hospitals by the bacteriology laboratory on 4 February 2019. The susceptibility results were returned to EMR systems in the form of ‘S’, ‘I’ or ‘R’ letters, with a definition of these terms in a footnote. In accordance with CA-SFM recommendations, for a P. aeruginosa strain without acquired resistance, meropenem and ceftolozane/tazobactam were reported as ‘S’ and all other β-lactams were reported as ‘I’.
Information to explain the new meaning of the intermediate category was distributed 1 week before the implementation by sending several e-mails to all doctors prescribing antibiotics. After implementation, continuous training was provided to all the new residents of the hospital group welcomed during Period 2 and the AMS team informed the other doctors of this change during their daily visits when additional training was required.
Two study periods were defined: from 4 September 2017 to 2 February 2019 (Period 1) and from 11 February 2019 to 29 August 2020 (Period 2). A washout period of 1 week between the two periods was considered, corresponding to the period of communication by the bacteriology laboratory.
Population and data collection
Patients were eligible if they were aged 18 years or more, presented a monomicrobial infection due to P. aeruginosa with a WT phenotype for β-lactams,6 regardless of the site of infection, and were treated by IV β-lactams for this infection during more than 48 h after the antibiotic susceptibility results had been delivered. Data were collected retrospectively from the medical record. This study was approved by Institutional Review Board (IRB) Mondor (IRB #00011558).
Endpoints and statistical analysis
The main endpoint was the proportion of patients treated by an overused antimicrobial therapy, defined as treatment by meropenem or ceftolozane/tazobactam for a monomicrobial infection due to WT P. aeruginosa for β-lactams, at the time of assessment (TOA), i.e. 48 h after the antibiotic susceptibility results had been delivered. Secondary endpoints were: (i) the proportion of patients treated by an overly broad-spectrum antimicrobial therapy in absence of AMS team advice in the EMR; (ii) the proportion of patients with AMS advice; and (iii) the number of days of treatment in excess with an overly broad-spectrum antibiotic.
Results
Inclusions
During the study periods, 1268 patients had a bacteriological specimen culture positive for P. aeruginosa with a WT phenotype for β-lactams. Of these, 977 patients were not included for the following reasons: the sample was polymicrobial for 563; no antimicrobial treatment had been started by TOA for 393; and treatment data were missing for 21. Finally, 291 patients were included in the study: 171 in Period 1 and 120 in Period 2 (Table 1). The lower number of inclusions in Period 2 is linked to a lower incidence of positive samples with WT P. aeruginosa.
Table 1.
Demographic data of included patients (n = 291)
| Total (n = 291) | Period 1 (N = 171) | Period 2 (N = 120) | P value | |
|---|---|---|---|---|
| Sex (male), n (%) | 195 (67) | 113 (66) | 82 (68) | 0.69 |
| Age (years), mean ± SD | 70.2 ± 15.5 | 70.2 ± 15.8 | 70.1 ± 15.0 | 0.96 |
| Ward type, n (%) | ||||
| Medical unit | 111 (39) | 66 (40) | 45 (38) | 0.89 |
| Surgical unit | 37 (13) | 23 (14) | 14 (12) | |
| ICU | 92 (32) | 51 (31) | 41 (34) | |
| Follow-up care unit | 44 (15) | 25(15) | 19 (16) | |
| Biological sample | ||||
| Blood culture | 73 (25) | 49 (29) | 24 (20) | 0.13 |
| Pulmonary sample | 93 (32) | 48 (28) | 45 (38) | |
| Urine culture | 87 (30) | 48 (28) | 39 (33) | |
| Abscess or collection | 27 (9) | 20 (12) | 7 (6) | |
| Superficial sample | 11 (4) | 6 (4) | 5 (4) |
Categorical variables were expressed as number (%), continuous variables as mean (± SD). Chi-squared tests or Fisher’s exact tests were used in the case of categorical variables while t-tests or Mann–Whitney tests were used in the case of continuous variables. P < 0.05 in two-tailed tests was considered as statistically significant.
The mean age of patients was 70.2 years (± 15.5 years) and 195 (67%) were men. The majority of patients were hospitalized in the acute care hospital (89%), mostly in medical and ICU wards (39% and 32%, respectively). Bacteriological samples were mainly pulmonary, urinary or blood culture (32%, 30% and 25%, respectively). Patient characteristics are summarized in Table 1. No difference between groups was found.
Antimicrobial therapy
Main endpoint
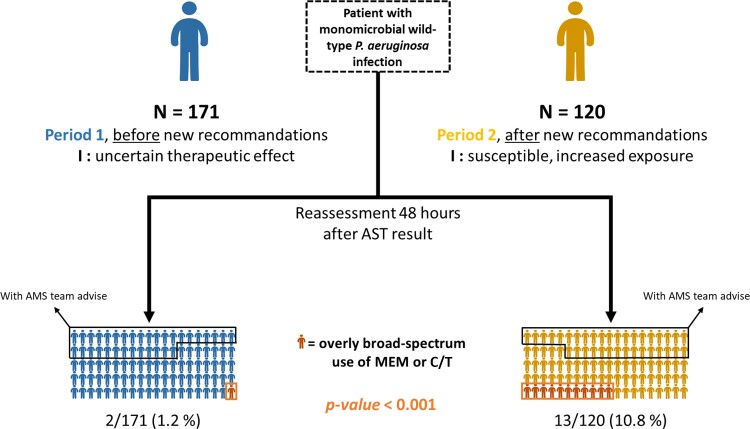
At TOA, two treatments by meropenem or ceftolozane/tazobactam were continued in Period 1 (2/171; 1.2%), whereas 13 were continued or started in Period 2 (13/120; 10.8%) (P < 0.0001, Figure 1). In Period 2, we observed overly broad-spectrum use of meropenem or ceftolozane/tazobactam in an average of one in nine patients.
Figure 1.
Overly broad-spectrum antimicrobial therapy according to period and AMS advice. MEM, meropenem; C/T, ceftolozane/tazobactam.
Secondary endpoints
AMS team written advice was found in the EMR in 57/171 patients in Period 1 and 42/120 in Period 2 (P = 0.77). No treatment by meropenem or ceftolozane/tazobactam was observed for patients with AMS advice, in both periods. For patients without AMS advice, 2 of 114 (2%) in Period 1 and 13 of 78 in Period 2 (17%) presented an overly broad-spectrum prescription. Thereby, we observed an overly broad-spectrum prescription for one in six patients after the implementation of new recommendations and in the absence of AMS advice.
AMS advice was observed in, respectively, 57 (34%) and 42 patients (33%) during Periods 1 and 2 (P = 0.77). Finally, the median duration of the overly broad-spectrum antibiotic was 7 days [IQR (6–10)] in Period 2 (7 and 10 days for the two patients in Period 1).
Discussion
To our knowledge, this is second study analysing the association between the new EUCAST definitions of susceptibility testing categories and the inappropriate use of second-line β-lactams for the treatment of infections due to WT strains of P. aeruginosa. This study confirms previous results observed in another European country,7 illustrating the significant and potentially widespread impact of this new definition in Europe. We showed that after implementation of these definitions, overly broad-spectrum antibiotic therapy represented 10.8% of the cases.
Several studies have shown that the reporting of susceptibility test results significantly influences prescribing decisions.4,8 The reception of information is particularly dependent on the way it is reported. We think that the misunderstanding of the new ‘intermediate’ category definition—corresponding, in fact, to ‘susceptible, increased exposure’—by clinicians was the reason for the use of overly broad-spectrum antibiotics in our study. We assume that the way of reporting results with complete information (including ‘susceptible, increased exposure’ rather than ‘intermediate’ or ‘I’ and a dosage table) would be more appropriate in order to better advise antimicrobial prescribers with regard to these definitions. Manufacturers of EMRs and bacteriology software should be aware of this problematic issue and propose adapted solutions. This study also showed that AMS team accompaniment could compensate the negative effect of this misunderstanding.
This study had some limitations. First, as the number of events was small, we cannot assess the other factors that can be associated with overly broad-spectrum antimicrobial therapy, such as the ward type or the type of infection. Second, our EMRs did not allow us to report the result in a way other than ‘I’, which could have affected the understanding by clinicians.
In conclusion, this new definition is an important paradigm shift aiming to promote the use of high-dose regimen therapy for some bacteria/antibiotic couples, but which can cause a negative effect of prescription of overly broad-spectrum β-lactams such as meropenem or ceftolozane/tazobactam due to misunderstanding of these recommendations by clinicians.
To improve the understanding of this new definition, susceptibility testing reports to prescribers should be presented as plain text in EMRs, i.e. ‘susceptible, standard dosing regimen’, ‘susceptible, increased exposure’ and ‘resistant’. Its successful implementation requires a sustained strong information campaign by microbiologists and support by an AMS team.
Acknowledgements
Results of this study were presented at ‘Journées Nationales d’Infectiologie 2021’: poster BU-15.
Contributor Information
Clément Ourghanlian, AP-HP, Hôpital Henri Mondor, Unité Transversale de Traitement des Infections, F-94010 Créteil, France; AP-HP, Hôpital Henri Mondor, Pharmacie, F-94000 Créteil, France.
Vincent Fihman, Département de Microbiologie, AP-HP, Hôpital Henri Mondor, F-94010 Créteil, France.
Antoine Morel, Univ Paris Est Créteil, IMRB U955 INSERM, F-94010 Créteil, France; AP-HP, Hôpital Henri Mondor, Service de Santé Publique, F-94010 Créteil, France.
Charlotte Lafont, Univ Paris Est Créteil, IMRB U955 INSERM, F-94010 Créteil, France; AP-HP, Hôpital Henri Mondor, Service de Santé Publique, F-94010 Créteil, France.
Adrien Galy, AP-HP, Hôpital Henri Mondor, Unité Transversale de Traitement des Infections, F-94010 Créteil, France.
Eimma Calimouttoupoulle, AP-HP, Hôpital Henri Mondor, Pharmacie, F-94000 Créteil, France.
Paul-Louis Woerther, Département de Microbiologie, AP-HP, Hôpital Henri Mondor, F-94010 Créteil, France; Univ Paris Est Créteil, EA 7380 DYNAMYC, F-94010 Créteil, France.
Raphaël Lepeule, AP-HP, Hôpital Henri Mondor, Unité Transversale de Traitement des Infections, F-94010 Créteil, France.
Funding
This study was supported by internal funding.
Transparency declarations
We have no competing interests to declare.
Author contributions
C.O., A.G., P.L.W., V.F. and R.L. contributed to the conception and design of the study. E.C., V.F. and C.O. collected the data. A.M., C.L., C.O., A.G. and R.L. contributed to analysis and interpretation of data. C.O. drafted the article, and all other authors revised it critically for important intellectual content. All authors approved the final version of the manuscript.
References
- 1. Kahlmeter G. EUCAST Proposes to change the definition and usefulness of the susceptibility category ‘intermediate’. Clin Microbiol Infect 2017; 23: 894–5. 10.1016/j.cmi.2017.08.015 [DOI] [PubMed] [Google Scholar]
- 2. EUCAST . Previous versions of documents. https://www.eucast.org/ast_of_bacteria/previous_versions_of_documents/.
- 3. Société Française de Microbiologie (SFM), EUCAST . Comité de l’antibiogramme de la Société Française de Microbiologie: recommendations 2019, V.1.0 January. 2019.https://www.sfm-microbiologie.org/wp-content/uploads/2019/02/CASFM2019_V1.0.pdf.
- 4. Meylan S, Guery B. In the name of common sense: EUCAST breakpoints and potential pitfalls. Clin Microbiol Infect 2020; 26: 1593–4. 10.1016/j.cmi.2020.07.025 [DOI] [PubMed] [Google Scholar]
- 5. Cornaglia G, Courcol R, Hermann JLet al. European Manual of Clinical Microbiology. ESCMID-SFM, 2012. [Google Scholar]
- 6. Mesaros N, Nordmann P, Plésiat Pet al. Pseudomonas aeruginosa: resistance and therapeutic options at the turn of the new millennium. Clin Microbiol Infect 2007; 13: 560–78. 10.1111/j.1469-0691.2007.01681.x [DOI] [PubMed] [Google Scholar]
- 7. Munting A, Regina J, Damas Jet al. Impact of 2020 EUCAST criteria on meropenem prescription for the treatment of Pseudomonas aeruginosa infections: an observational study in a university hospital. Clin Microbiol Infect 2022; 28: 558–63. 10.1016/j.cmi.2021.03.034 [DOI] [PubMed] [Google Scholar]
- 8. Langford BJ, Daneman N, Diong Cet al. Antibiotic susceptibility reporting and association with antibiotic prescribing: a cohort study. Clin Microbiol Infect 2021; 27: 568–75. 10.1016/j.cmi.2020.10.001 [DOI] [PubMed] [Google Scholar]