Abstract
A new series of 3-acetyl-1,3,4-oxadiazoline hybrid molecules was designed and synthesized using a condensation between acyclonucleosides and substituted phenylhydrazone. All intermediates and final products were screened against Leishmania donovani, a Protozoan parasite and against three viruses SARS-CoV-2, HCMV and VZV. While no significant activity was observed against the viruses, the intermediate with 6-azatymine as thymine and 5-azathymine-3-acetyl-1,3,4-oxadiazoline hybrid exhibited a significant antileishmanial activity. The later compound was the most promising, exhibiting an IC50 value at 8.98 µM on L. donovani intramacrophage amastigotes and a moderate selectivity index value at 2.4.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s11030-022-10548-9.
Keywords: 3-Acetyl-1,3,4-oxadiazoline; Pyrimidines analogs; Antileishmanial activity; Antiviral activity
Introduction
Neglected tropical diseases (NTDs) are widespread in several countries in Africa, Europe, America, and Asia. Every year, NTDs contaminate millions of people in 149 countries, which cause wasting billions of dollars and claiming thousands of lives [1]. According to the World Health Organization (WHO), leishmaniases are a class of illnesses prompted by the Protozoan parasite Leishmania, showing four crucial clinical syndromes: post kala azar dermal leishmaniasis (PKDL), cutaneous leishmaniasis (CL), visceral leishmaniasis/kala azar (VL), and mucocutaneous leishmaniasis (MCL) [2].
On the other hand, the immune system response to the illnesses is altered by co-infection with the human immunodeficiency virus, HIV [3]. Furthermore, leishmaniases have an increased medical value in infected people with HIV-1 (human immunodeficiency virus type 1) because of the spread of both pathogens in several regions of the world (e.g., South America, India, the Mediterranean Basin, and many African countries). There is absolutely no doubt that the exact number of reported cases of co-infection is undercounted due to various issues with HIV-1 infection, leishmaniasis, or both in the setting of developing countries [4]. Ellen Heirwegh et al., reported that Leishmania major with phleboviral infection was more infectious than L. major alone. A better understanding of the possible role of viral co-infection might lead to more effective treatment regimens [5]. One of the key challenges in the management of leishmaniasis and viral disease co-infection is the invention of a clinically effective treatment that not only treats leishmaniasis but also prevents viral disease. However, there is a lack of knowledge on the correlation between leishmaniasis and viral disease co-infection. Moreover, Antonis Pikoulas et al., presented a case of co-infection with COVID-19 and visceral leishmaniasis, and discuss recent reports on co-existence of leishmania and SARS-CoV2 spp. to date [6].
Current treatments for visceral leishmaniasis including antimonials, liposomal amphotericin B, miltefosine, and paromomycin, have some issues related to toxicity, emerging resistance, high cost and relatively long treatment regimens. Therefore, there is an urgent need for the development of new and better medicines [7, 8].
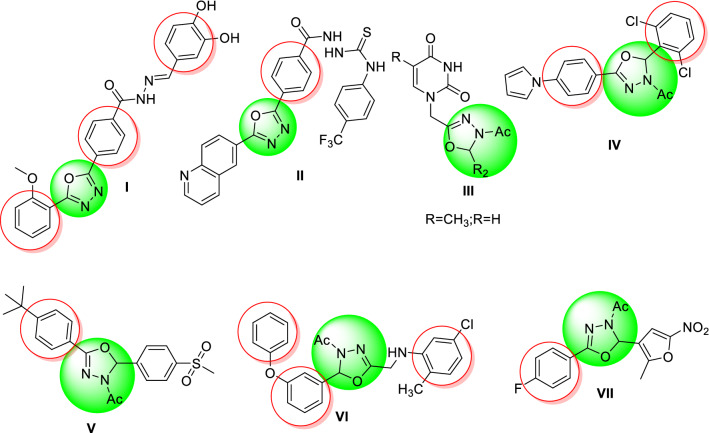
1,3,4-Oxadiazoles are a very important series in chemistry as they have several biological activities. The 1,3,4-oxadiazole scaffold is present in numerous drugs: Nesapidil® (anti-hypertensive) [9], Furamizol® (antibiotic) [10], Raltegravir® (anti-retroviral) [11], and Zibotentan® (anticancer) [12, 13]. We also notice that the N-acetyl-1,3,4-oxadiazoline is among the derivatives of 1,3,4-oxadiazoles that showed different antileishmanial activities. For instance, Taha et al. have synthesized phenyl-linked oxadiazoline–phenylhydrazone hybrids I as the most potential antileishmanial candidate, with an IC50 value of 0.95 ± 0.01 µM [14]. Moreover, another series of quinolinyl–oxadiazole hybrids had promising antileishmanial activities, and compound II emerged as the most potent agent with an IC50 value of 0.10 ± 0.001 µM [15]. Meanwhile, some N-acetyl-1,3,4-oxadiazoline derivatives have been reported as antiviral agents. For instance, Ali et al. prepared a series of monosaccharide 1-acetylhydrazinouracils that was evaluated for antiviral activity against hepatitis B virus and showed moderate activities III [16]. Moreover, they showed activities as antibacterial [17, 18], antimicrobial [19, 20], antifungal [21], and anti-inflammatory [22] (Fig. 1).
Fig. 1.
Structures of some bioactive 1,3,4-oxadiazoline based hybrids
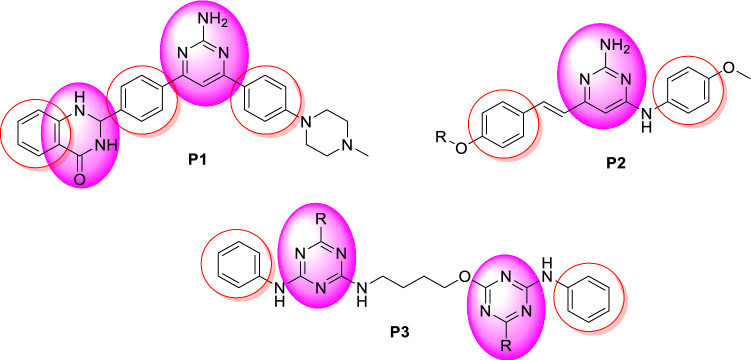
Substituted pyrimidines are widely available in living organisms and are one of the most important compounds studied by chemists. Pyrimidines, including thymine, uracil, and derivatives, are among the most abundant heterocyclic diazines. According to the literature, pyrimidine derivatives exhibit a wide range of pharmacological properties, including antifungal activity [23, 24], antibacterial [25, 26], antileishmanial [27], antiviral [28, 29], and anticancer [30, 31]. A rational discovery of novel antiparasitic drugs should be based on parasite-specific metabolisms [32]. The biosynthesis of pyrimidine is a biologically important process that occurs via both de novo production and pyrimidine salvage routes. The enzymes of this pathway are considered potential drug targets. Meanwhile, several studies have shown that pyrimidines have widely antileishmanial activities. For instance, the synthesis of pyrimidine derivatives led to the discovery of the potent compound P1 (Fig. 2) with an EC50 value of 0.65 μM against intracellular Leishmania donovani amastigotes [33]. Moreover, pyrimidines P2 have been demonstrated to be efficacious in vivo, with each compound leading to 78% parasite inhibition when dosed in L. donovani infected hamsters for five days (50 mg kg−1, i.p.) [34]. Finally, Chauhan and coworkers have described that aza-pyrimidine–pentamidine hybrids P3 have promising activity on intracellular L. donovani amastigotes (EC50 values of < 1 μM) [35].
Fig. 2.
Structures of some bioactive pyrimidine-based hybrids
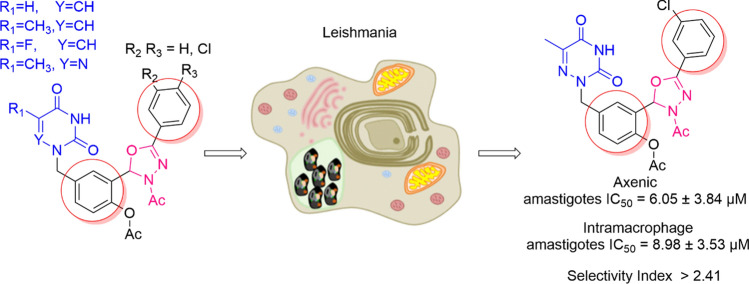
Prompted with these literature reports, we designed some novel hybrid molecules which combined the pyrimidine and 3-acetyl-1,3,4-oxadiazoline scaffolds in order to investigate firstly their in vitro antileishmanial activity against the axenic amastigotes and intramacrophage amastigotes forms of L. donovani, and second, their antiviral activity against SARS-CoV-2, HCMV, and VZV (Fig. 3).
Fig. 3.
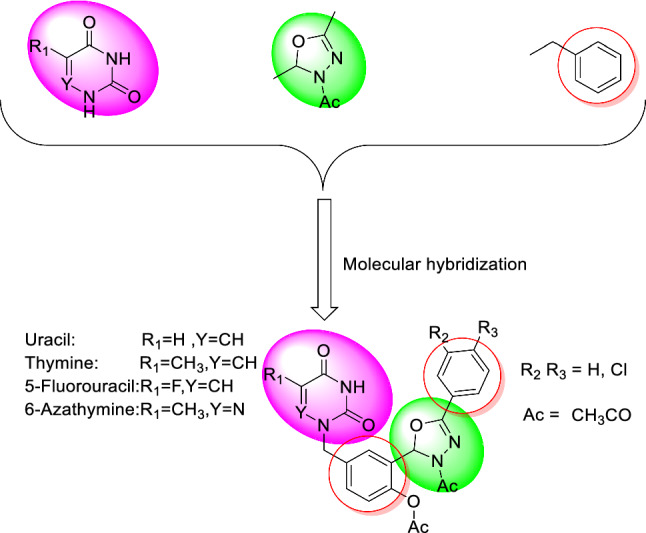
Design of new N-acetyl 1,3,4-oxadiazoline–pyrimidine hybrids
Results and discussion
Chemistry
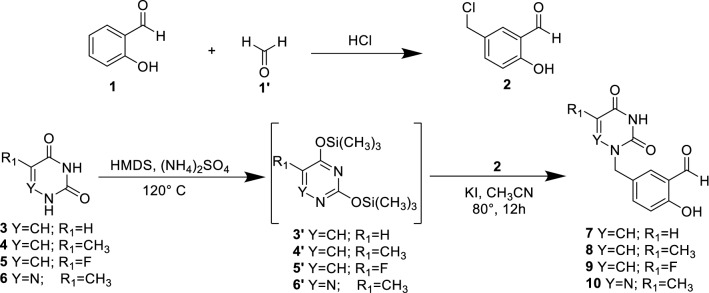
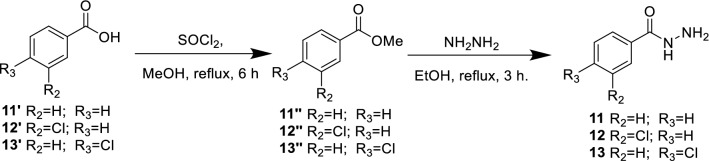
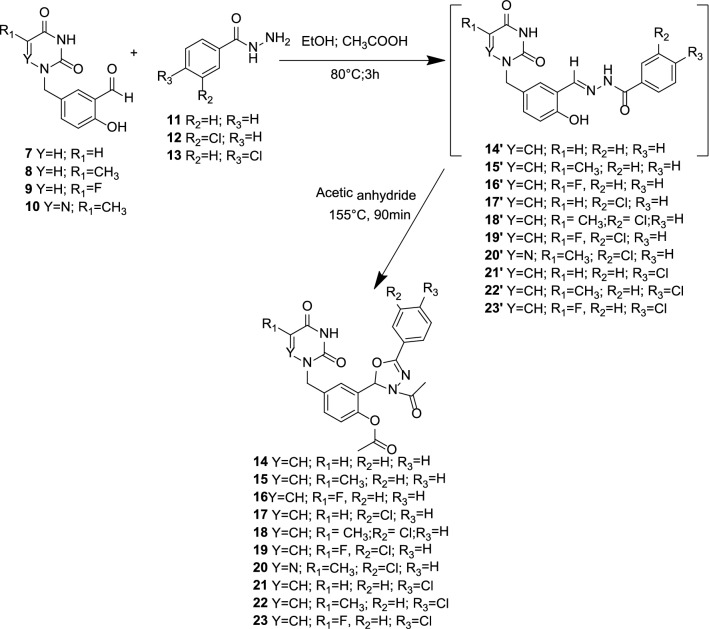
The synthetic strategy to achieve compounds (14–23) is shown in Schemes 1, 2, and 3. The crucial step for synthesizing acyclonucleosides derivatives (7–10) (Scheme 1) known as modified Hilbert-Johnson reaction [36] corresponded to the condensation between silylated nucleobases (3′–6′) and alkylating agent 2 [37]. The desired products were obtained in synthetically good yields between 53 and 84%. Thereafter, the methyl benzoate derivatives (11″–13″) were subjected to a hydrazinolysis reaction after treatment with 80% aqueous hydrazine hydrate in ethanol under reflux to afford the corresponding hydrazides (11–13) in good yield (Scheme 2). The latter were reacted with the aldehyde derivatives (7–10) to give the imine derivatives (14′–23′) which were treated with acetic anhydride under heating (155 °C) to promote cyclization and furnished the N3-acetyl-1,3,4-oxadiazoline derivatives (14–23) in yield ranging from 40 to 71%. All the oxadiazoles were completely characterized using NMR (13C and 1H), IR, and high-resolution mass spectrometry. In the 1H NMR spectra, the signal for the hydrogen present in the oxadiazole ring (H-2) was observed within the 7.20–7.67 ppm range. The hydrogen atoms of methylene group attached to nitrogen (nucleobases) were noticed as singlet in the 1H NMR spectra. The carbon chemical shifts are compatible with the structures of the compounds. In the IR spectra, expected bands for functional groups were noticed. The exact mass of the hybrid molecules (14–23) was confirmed by high-resolution mass spectrometry analyses.
Scheme 1.
Synthesis of compound 2 and N1-alkylated pyrimidines 7–10
Scheme 2.
Synthesis of hydrazide derivatives 11–13
Scheme 3.
Preparation of 3-acetyl-1,3,4-oxadiazoline–pyrimidine hybrids 14–23
Biological evaluation
Antileishmanial activity
The novel series of nucleosides analogs of 3-acetyl-1,3,4-oxadiazoline 14–23 and alkylated pyrimidines (uracil, thymine, 5-fluorouracil, and 5-azathymine) 7–10 were tested for their biological activity. This series was not highly cytotoxic on the RAW 264.7 macrophage model since eleven compounds exhibited CC50 values superior to 100 µM. However, the most cytotoxic (compounds 10, 20, 23) with CC50 values in a range from 19 to 56 µM, were the single ones having a significant antileishmanial activity against the axenic amastigotes and intramacrophage amastigotes forms of L. donovani.
The IC50 and CC50 values of the compounds were compared to those of miltefosine as the reference drug. The results, presented in Table 1, clearly showed that the starting material compound 10 presented a moderate activity (CC50 = 56.15 µM, IC50 = 29.02 µM (Axenic amastigotes), IC50 = 29.96 µM (intramacrophage amastigotes), and SI = 1.87). It is worth to note that when thymine as a nucleobase 8 was changed to 6-azathymine 10, the IC50 decrease from 100 to 29.02 µM. Furthermore, when the oxadiazole group is introduced in the hybrid molecule 20, the IC50 decrease from 29.02 to 6.05 µM. The most active derivatives 20 showed a significant activity: IC50 = 6.05 µM (Axenic amastigotes) and IC50 = 8.98 µM (Intramacrophage amastigotes) CC50 = 21.66 µM and very moderate selectivity index value of up to 2.41 comparatively to miltefosine (Table 1).
Table 1.
Cytotoxicity, antileishmanial activities of compounds 7–10 and 14–23 on axenic and intramacrophage forms of Leishmania donovani and selectivity index
| Product | Cytotoxicity CC50 | Axenic amastigotes IC50 | Intramacrophage amastigotes IC50 | SI (CC50/IC50) | ClogP |
|---|---|---|---|---|---|
| 7 | > 100 | > 100 | > 100 | – | 0.239 |
| 8 | > 100 | > 100 | > 100 | – | 0.734 |
| 9 | > 100 | > 100 | > 100 | – | 0.719 |
| 10 | 56.15 ± 16.75 | 29.02 ± 2.84 | 29.96 ± 2.18 | 1.87 | 1.791 |
| 14 | > 100 | > 100 | > 100 | – | 1.015 |
| 15 | > 100 | > 100 | > 100 | – | 1.514 |
| 16 | > 100 | > 100 | > 100 | – | 1.494 |
| 17 | > 100 | > 100 | > 100 | – | 1.728 |
| 18 | > 100 | > 100 | > 100 | – | 2.227 |
| 19 | > 100 | > 100 | > 100 | – | 2.207 |
| 20 | 21.66 ± 3.11 | 6.05 ± 3.84 | 8.98 ± 3.53 | 2.41 | 3.28 |
| 21 | > 100 | > 100 | > 100 | – | 1.728 |
| 22 | > 100 | > 100 | > 100 | – | 2.227 |
| 23 | 19.16 ± 1.39 | 56.29 ± 3.20 | > 19.16 | < 1 | 2.207 |
| Miltefosine | 52.24 ± 11.08 | 0.51 ± 0.29 | 11.82 ± 2.43 | > 4.41 | – |
The structure–activity relationship showed that oxadiazole derivatives 14–23 had better activity than the acyclonucleosides 7–10. As a result, it was concluded that the creation of a stereoisomer center C2 on the oxadiazole ring may influence the antileishmanial activity, this was supported by the fact that the oxadiazole derivative 20 was 5 times more potent than the acyclonucleoside derivative 10. From the biological data collected with derivatives 19 and 23 having 5-fluorouracil as a nucleobase, SARs showed that, at the para position of the phenyl moiety, the chlorine substituent was favorable while the meta-chlorine was not suitable for providing antileishmanial activity.
Moreover, a comparison of the activity profiles of compounds 10 and 20 draws speculation that the oxadiazole group may be serving as a chromophore group. Thus, additional modifications will be pursued in subsequent studies, as this approach has been shown to greatly enhance antileishmanial activity of oxadiazole analogs.
Determination of compound lipophilicity
There are physical differences between the test systems on promastigote and amastigote forms of L. donovani, which could explain the obtained results. The promastigote test employs an extracellular axenic parasite, which is more accessible than the intracellular amastigotes. Indeed, the parasite starts the creation of a membrane (parasitophorous vacuole membrane, which surrounds the intracellular parasite) during the invasion process. Permeation of the two membranes (plasmatic and vacuole) is a critical criterion for activity and varies depending on the hydrophobicity of the compound [38, 39].
The octanol/water partition coefficients (ClogP) reflect the hydrophobicity, and products that have higher ClogP are more lipophilic. Chemdraw was used to predict partition coefficients in this study. As shown in Table 1, we notice that the incorporation of 1,3,4-oxadiazoline increases the hydrophobic character. For instance, when we compare compound 10 and 20, ClogP is enhanced by about two times, which explain that oxadiazoline 20 is more active than 10. Similarly, by comparing all nucleobases, 6-azathymine is the most hydrophobic, which follows the results of biological activities.
Antiviral activity
All the synthesized compounds (7–10, 14–23) were evaluated for their antiviral activities against human cytomegalovirus (HCMV) and human varicella-zoster virus (VZV), both thymidine kinase-deficient TK and wild type. The pyrimidine–1,3,4-oxadiazole hybrids were unable to substantially inhibit the replication of these two DNA viruses at non-toxic concentrations, in contrast to the compounds used as reference (Ganciclovir, and Cidofovir against for HCMV, and Acyclovir for VZV) (Tables 2, 3).
Table 2.
Anti-VZV activities of compounds 7–10 and 14–23
| Product | Antiviral activity EC50 (µM)a | Cytotoxicity (µM) | ||
|---|---|---|---|---|
| TK+ VZV strain | TK− VZV strain | Cell morphology | Cell growth | |
| OKA | 07-1 | (MCC)b | (CC50)c | |
| 7 | > 100 | > 100 | > 100 | ND |
| 8 | > 100 | > 100 | > 100 | ND |
| 9 | > 100 | > 100 | > 100 | ND |
| 10 | 46.95 | > 20 | ≥ 20 | ND |
| 14 | > 100 | > 100 | ≥ 100 | ND |
| 15 | > 100 | > 100 | ≥ 20 | ND |
| 16 | > 100 | > 100 | > 100 | ND |
| 17 | 43.02 | > 20 | 100 | ND |
| 18 | > 20 | > 20 | 100 | ND |
| 19 | 34.38 | > 20 | ≥ 100 | ND |
| 20 | > 20 | > 20 | 100 | ND |
| 21 | > 20 | > 20 | 100 | ND |
| 22 | > 20 | > 20 | 100 | ND |
| 23 | > 20 | > 20 | 100 | ND |
| Acyclovir (ACV) | 6.26 | 46.80 | > 444 | > 444 |
aEffective concentration required to reduce virus plaque formation by 50%. Virus input was 20 plaque forming units (PFU)
bMinimum cytotoxic concentration that causes a microscopically detectable alteration of cell morphology
cCytostatic concentration required to reduce cell growth by 50%
Table 3.
Anti-HCMV activities of compounds 7–10 and 14–23
| Product | Antiviral activity EC50 (µM)a | Cytotoxicity (µM) | ||
|---|---|---|---|---|
| AD-169 strain | Davis strain | Cell morphology (MCC)b | Cell growth (CC50)c | |
| 7 | > 100 | > 100 | ≥ 100 | ND |
| 8 | > 20 | > 100 | ≥ 100 | ND |
| 9 | > 100 | > 100 | 100 | ND |
| 10 | 63.14 | 44.72 | ≥ 100 | ND |
| 14 | > 100 | > 100 | 100 | ND |
| 15 | > 100 | > 100 | ≥ 100 | ND |
| 16 | > 100 | > 100 | 100 | ND |
| 17 | > 100 | > 100 | 100 | ND |
| 18 | > 20 | > 20 | 100 | ND |
| 19 | > 20 | > 20 | 100 | ND |
| 20 | > 20 | > 20 | ≥ 20 | ND |
| 21 | > 20 | > 20 | 100 | ND |
| 22 | > 20 | > 20 | ≥ 20 | ND |
| 23 | > 20 | > 20 | 100 | ND |
| Ganciclovir (GCV) | 6.5 ± 1.77 | 1.64 ± 0.22 | > 394 | > 394 |
| Cidofovir (CDV) | 0.84 ± 0.21 | 0.12 ± 0.03 | 317 | > 317 |
aEffective concentration required to reduce virus-induced cytopathic effect by 50%. Virus input was 100 plaque forming units (PFU)
bMinimum cytotoxic concentration that causes a microscopically detectable alteration of cell morphology
cCytostatic concentration required to reduce cell growth by 50%
Only three compounds 10, 17, and 19 showed moderate activity against VZV with EC50 = 46.95 µM, 43.02 µM, and 34.38 µM, respectively. We notice that when the fluorine atom is present in the acyclonucleoside, the value of EC50 decreases from 43.02 µM for uracil acyclonucleoside 17 to 34.38 µM for 5-fluorouracil acyclonucleoside 19 (Table 2). Whereas only compound 10 is moderately active against HCMV with EC50 = 63.14 µM (AD-169 strain) and 44.72 µM (Davis strain) (Table 3).
The rapidly developing pandemic, known as coronavirus disease 2019 (COVID-19), is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). The rapid global emergence of SARS-CoV-2 has advanced research efforts toward the development of therapeutic intervention and finding viral drug to control the pandemic. The functional importance of RNA-dependent RNA polymerase (RdRp) in the viral life cycle, makes it an attractive target for designing antiviral drugs. Moreover, SARS-CoV-2 is an RNA virus like HCV, HIV, and other flaviviruses that share a similar replication mechanism requiring an RNA-dependent RNA polymerase (RdRp). In addition, the most promising broad-spectrum class of viral RdRp inhibitors are nucleos(t)ide analogs [40, 41]. Elfiky used a molecular docking study to predict that Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir may have inhibitory activity against SARS-CoV-2 RdRp [42]. Significant efforts have been made to discover novel or repurposed therapeutics to select the best candidates for the management of COVID-19 disease. To date, the US Food and Drug Administration (US FDA) has proved eleven agents emergency for the treatment of COVID patients, including two nucleosides analogs: remdesivir and Beta-d-N4-hydroxycytidine (NHC, molnupiravir) [43]. In this context, all synthesized hybrid molecule analogs (7–10, 14–23) were evaluated for their anti-SARS-CoV-2 activity against Beta-Cov/Belgium/GHB-03021/2020. All tested compounds showed no anti-SARS-CoV-2 activity, with EC50 > 100 µM.
Experimental section
Chemistry
General
Melting points have been measured without correction using a Büchi B-545 electronic capillary melting temperature apparatus. TLC was employed to check reactions utilizing aluminum sheets coated with Merck's silica gel 60 F254. FTIR spectra were taken on a Perkin-Elmer VERTEX 70 FTIR spectroscopy covering the frequency 400–4000 cm−1. The spectrums of 1 H and 13C NMR were recorded in DMSO-d6 or CDCl3 solution on a Bruker Advance 300 spectrometer at 300 and 75 MHz, respectively. Using DMSO-d6 as an internal reference, the chemical shifts are displayed in parts per million (ppm). The next abbreviations represent the signal multiplicities: s = singlet, d = doublet, m = multiplet, t = triplet, q = quadruplet, and as well as the coupling constants J, are documented in Hertz. The used chemicals in the synthesis were purchased from Fluka and Sigma Aldrich.
HPLC/MS conditions
HPLC/MS was performed on a Thermo Scientific Dionex Ultimate 3000 consisted of a quaternary (HPG-3400RS) pump, a WPS-3000 (TSL) analytical auto-sampler, a TCC-3000 column oven, and a TSQ Endura (Thermo Fisher Scientific) triple quadrupole equipped with heated-electrospray ionization (H-ESI). The separation was performed on a Eurospher II 100-5 C18 vertex plus column (250 4.6 mm, 5m) at 25 °C. The mobile phases were composed of acetonitrile (A) and water/0.1% formic acid (B) with an elution program as follows: 20% of (A) for 8.6 min and 85% of (A) for 5.4 min. The solvent system flow rate was set to 1.0 mL min−1, and the sample injection volume was 20 L. UV–Vis detection was monitored at 260 nm, while DAD acquisition was done between 200 and 600 nm.
General procedure for the preparation of the 2-hydroxybenzaldehyde homonucleosides
The mixture of the pyrimidine base (1 mmol) (3–6) and ammonium sulfate (0.10 mmol, 10 mg) in hexamethyldisilazane (1 mL) was refluxed (3 h) at 120 °C to give a clear solution (silylation step). Then the benzyl derivative (2.5 mmol, 425 mg), KI (0.50 mmol, 84 mg) and the solvent (acetonitrile) (5 mL) were added. The mixture was heated at 85 ◦C for 12 h, diluted with dichloromethane/methanol, and evaporated to dryness. The residue was purified by flash chromatography (solvent CH2Cl2/MeOH 99/1).
5-((2,4-Dioxo-3,4-dihydropyrimidin-1(2H)-yl) methyl)-2-hydroxybenzaldehyde (7)
Rf = 0.26 [CH2Cl2/MeOH (97/3)]. Yield = 84%, m = 207 mg. Mp = (218–220) °C. UV (methanol) λmax = 259 nm. IR υ (cm−1): 3020 (= CH); 1689 (C=O aldehyde); 1350 (CH2). 1H NMR (300 MHz, DMSO d-6) δ (ppm): 4.80 (s, 2H, CH2); 5.57 (d, 3JH–H = 7.8 Hz, 1H, H-5); 6.97 (d, 3JH–H = 8.4 Hz, 1H, HPh); 7.47 (d, 3JH–H = 8.4 Hz, 1H, HPh); 7.60 (s, 1H, HPh); 7.76 (d, 3JH–H = 7.8 Hz, 1H, H-6); 10.25 (s, 1H, H-aldehyde); 10.77 (s, 1H, OH); 11.30 (s, 1H, H-3). 13C NMR (75 MHz, DMSO-d6) δ (ppm): 49.63 (CH2); 101.22 (C-5); 117.63 (CPh); 122.20 (CPh); 127.84 (CPh); 127.93 (CPh); 135.94 (CPh); 145.40 (C-6); 150.95 (C-2); 160.40 (CPh); 163.59 (C-4); 190.80 (C-aldehyde). HPLC/MS (m/z) 247.07 (M+H)+, HRMS for C12H11N2O4: Calc 247.0713, Found 247.0716.
2-Hydroxy-5-((5-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl) methyl) benzaldehyde (8)
Rf = 0.38 [CH2Cl2/MeOH (97/3)]. Yield = 76%, m = 198 mg. Mp = (218–220) °C. UV (methanol) λmax = 259 nm. IR υ (cm−1): 3053 (=CH); 1696 (C=O aldehyde); 1364 (CH2). 1H NMR (300 MHz, DMSO d-6) δ (ppm): 1.74 (s, 3H, CH3); 4.76 (s, 2H, CH2); 6.97 (d, 3JH–H = 8.4 Hz, 1H, HPh); 7.47 (d, 3JH–H = 8.4 Hz, 1H, HPh); 7.59 (s, 1H, H-6); 7.63 (s, 1H, HPh); 10.24 (s, 1H, H-aldehyde); 10.76 (s, 1H, OH); 11.29 (s, 1H, H-3). 13C NMR (75 MHz, DMSO d-6) δ (ppm): 11.88 (CH3); 48.90 (CH2); 109.00 (C-5); 117.62 (CPh); 122.09 (CPh); 127.95 (CPh); 128.02 (CPh); 135.92 (CPh); 141.07 (C-6); 150.95 (C-2); 160.30 (CPh); 164.17 (C-4); 190.99 (C-aldehyde). HPLC/MS (m/z) 261.08 (M+H)+, HRMS for C13H13N2O4: Calc 261.0870, Found 261.0877.
5-((5-Fluoro-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl) methyl)-2-hydroxybenzaldehyde (9)
Rf = 0.37 [CH2Cl2/MeOH (97/3)]. Yield = 72%, m = 190 mg. Mp = (236–238) °C. UV (methanol) λmax = 258 nm. IR υ (cm−1): 3026 (=CH); 1691 (C=O aldehyde); 1348 (CH2). 1H NMR (300 MHz, DMSO d-6) δ (ppm): 4.75 (s, 2H, CH2); 6.97 (d, 3JH–H = 8.4 Hz, 1H, HPh); 7.49 (d, 3JH–H = 8.4 Hz, 1H, HPh); 7.63 (s, 1H, HPh); 8.21 (d, 3JH–F = 6.9 Hz, 1H, H-6); 10.24 (s, 1H, H-aldehyde); 10.78 (s, 1H, OH); 11.82 (s, 1H, H-3). 13C NMR (75 MHz, DMSO d-6) δ (ppm): 48.61 (CH2); 117.59 (CPh); 122.10 (CPh); 127.37 (CPh); 128.27 (CPh); 129.60 (d, 2JC–F = 32.7 Hz, C-6); 136.03 (CPh); 138.21 (d, 1JC–F = 230.85, C-5); 149.58 (C-2); 160.30 (CPh); 157.16 (d, 2JC–F = 25.75 Hz, C-4); 190.82 (C-aldehyde). HPLC/MS (m/z) 265.06 (M+H)+, HRMS for C12H10FN2O4: Calc 265.0619, Found 265.0623.
2-Hydroxy-5-((6-methyl-3,5-dioxo-4,5-dihydro-1,2,4-triazin-2(3H)-yl) methyl) benzaldehyde (10)
Rf = 0.47 [CH2Cl2/MeOH (97/3)]. Yield = 53%, m = 138 mg. Mp = (198–200) °C. UV (methanol) λmax = 258 nm. IR υ (cm−1): 3018(=CH); 1664 (C=O aldehyde); 1379 (CH2). 1H NMR (300 MHz, DMSO d-6) δ (ppm): 2.05 (s, 3H, CH3); 4.92 (s, 2H, CH2); 6.95 (d, 3JH–H = 8.7 Hz, 1H, HPh); 7.45 (d, 3JH–H = 8.4 Hz, 1H, HPh); 7.59 (s, 1H, HPh); 10.23 (s, 1H, H-aldehyde); 10.75 (s, 1H, OH); 12.11 (s, 1H, H-3). 13C NMR (75 MHz, DMSO d-6) δ (ppm): 15.85 (CH3); 51.84 (CH2); 117.46 (CPh); 122.00 (CPh); 127.77 (CPh); 128.27 (CPh); 135.94 (CPh); 143.44 (C-5); 148.69 (C-2); 157.02 (C-4); 160.21 (Cph); 191.12 (C-aldehyde). HPLC/MS (m/z) 262.08 (M+H)+, HRMS for C12H12N3O4: Calc 262.0822, Found 262.0823.
General procedure for the preparation of the N-acetyl 1,3,4 oxadiazol homonucleosides
A mixture of 2-hydroxy benzaldehyde homonucleosides (0.3 mmol) (7–10) and benzoic hydrazide acid (0.36 mmol) (11–13) was refluxed in ethanol (4 mL) for 3 h in the presence of catalytic amount of acetic acid (3 drops). The reaction mixture was evaporated to dryness, 3 mL of acetic anhydride added, and agitated at 155 °C for 90 min. The solution was poured into ice and neutralized with NaHCO3. The obtained solid was filtered and purified by flash chromatography (CH2Cl2/MeOH 98/2).
2-(3-Acetyl-5-phenyl-2,3-dihydro-1,3,4-oxadiazol-2-yl)-4-((2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl) methyl) phenyl acetate (14)
Rf = 0.26 [CH2Cl2/MeOH (97/3)]. Yield = 50%, m = 67 mg. Mp = (214–216) °C. UV (methanol) λmax = 271 nm. IR υ (cm−1): 3459 (NH); 1765 (C=O); 1690 (C=C); 691 (OH phenol). 1H NMR (300 MHz, DMSO d-6) δ (ppm): 2.11 (s, 3H, CH3); 2.19 (s, 3H, CH3); 4.90 (s, 2H, CH2); 5.62 (d, 3JH–H = 7.8 Hz, 1H, H-5); 7.17 (s, 1H, H-oxadiazoline); 7.38 (d, 1H, 3JH–H = 7.8 Hz, H-6); 7.49–7.80 (m, 8H, H-aromatic); 11.36 (s, 1H, H-3). 13C NMR (75 MHz, DMSO d-6) δ (ppm): 20.44 (CH3); 20.96 (CH3); 49.56 (CH2); 90.07 (C-oxadiazoline); 101.37 (C-5); 123.81 (Cph); 124.35 (Cph); 126.42 (Cph); 128.19 (Cph); 128.65 (Cph); 129.10 (Cph); 129.93 (Cph); 131.90 (Cph); 134.72 (Cph); 145.44 (C-6); 148.22 (Cph); 151.03 (C-2); 154.58 (C-oxadiazoline); 163.56 (C-4); 166.19 (CO acetyl); 168.55 (CO acetyl). HPLC/MS (m/z) 449.15 (M+H)+, HRMS for C23H21N4O6: Calc 449.1456, Found 449.1453.
2-(3-Acetyl-5-phenyl-2,3-dihydro-1,3,4-oxadiazol-2-yl)-4-((5-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl) methyl) phenyl acetate (15)
Rf = 0.31 [CH2Cl2/MeOH (97/3)]. Yield = 71%, m = 98 mg. Mp = (231–233) °C. UV (methanol) λmax = 277 nm. IR υ (cm−1): 3463 (NH); 1767 (C=O); 1662 (C=C); 1196 (C–N); 693 (OH phenol). 1H NMR (300 MHz, DMSO d-6) δ (ppm): 1.75 (CH3); 2.11 (s, 3H, CH3); 2.19 (s, 3H, CH3); 4.87 (s, 2H, CH2); 7.18 (s, 1H, H-oxadiazoline); 7.48 (s, 1H, H-6); 7.38–7.80 (m, 6H, H-aromatic); 11.35 (s, 1H, H-3). 13C NMR (75 MHz, DMSO d-6) δ (ppm): 11.89 (CH3); 20.55 (CH3); 20.96 (CH3); 49.31 (CH2); 90.04 (C-oxadiazoline); 109.13 (C-5); 123.82 (Cph); 124.32 (Cph); 126.41 (Cph); 128.12 (Cph);128.59 (Cph); 129.02 (Cph); 129.91 (Cph); 131.89 (Cph); 134.89 (Cph); 141.17 (C-6); 148.23 (Cph); 150.97 (C-2); 154.64 (C-oxadiazoline); 164.18 (C-4); 166.30 (CO acetyl); 168.55 (CO acetyl). HPLC/MS (m/z) 463.16 (M+H)+, HRMS for C24H23N4O6: Calc 463.1612, Found 463.1611.
2-(3-Acetyl-5-phenyl-2,3-dihydro-1,3,4-oxadiazol-2-yl)-4-((5-fluoro-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl) methyl) phenyl acetate (16)
Rf = 0.31 [CH2Cl2/MeOH (97/3)]. Yield = 47%, m = 66 mg. Mp = (153–155) °C. UV (methanol) λmax = 278 nm. IR υ (cm−1): 3480 (NH); 1760 (C=O); 1707 (C=C); 1196 (C–N); 699 (OH phenol). 1H NMR (300 MHz, DMSO d-6) δ (ppm): 2.10 (s, 3H, CH3); 2.19 (s, 3H, CH3); 4.86 (s, 2H, CH2); 5.62 (s, 1H, H-aromatic); 7.18 (s, 1H, H-oxadiazoline); 7.42–7.81 (m, 8H, H-aromatic); 8.24 (d, 1H, 3JH–F = 6.9 Hz, H-6); 11.87 (s, 1H, H-3). 13C NMR (75 MHz, DMSO d-6) δ (ppm): 20.47 (CH3); 20.96 (CH3); 49.82 (CH2); 90.11 (C-oxadiazoline); 123.81(Cph); 124.32 (Cph); 126.41 (Cph); 128.11 (Cph); 128.74 (Cph); 129.10 (Cph); 129.71 (Cph); 130.01 (d, 2JC–F = 10.95 Hz, C-6); 131.90 (Cph); 134.31 (Cph); 148.33 (C-2); 151.03 (Cph); 154.63 (C-oxadiazoline); 141.36 (d, 1JC–F = 228.45 Hz, C-5); 157.02 (d, 2JC–F = 28.72 Hz, C-4); 166.31 (CO acetyl); 168.55 (CO acetyl). HPLC/MS (m/z) 467.14 (M+H)+, HRMS for C23H20FN4O6: Calc 467.1361, Found 467.1364.
2-(3-Acetyl-5-(3-chlorophenyl)-2,3-dihydro-1,3,4-oxadiazol-2-yl)-4-((2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl) methyl) phenyl acetate (17)
Rf = 0.23 [CH2Cl2/MeOH (97/3)]. Yield = 60%, m = 89 mg. Mp = (229–231) °C. UV (methanol) λmax = 271 nm. IR υ (cm−1): 3436 (NH); 1763 (C=O); 1689 (C=C); 689 (OH phenol). 1H NMR (300 MHz, DMSO d-6) δ (ppm): 2.12 (s, 3H, CH3); 2.19 (s, 3H, CH3); 4.90 (s, 2H, CH2); 5.60 (d, 3JH–H = 9 Hz, 1H, H-5); 7.19 (s, 1H, H-oxadiazoline); 7.39–7.80 (m, 8H, H-aromatic, H-6); 11.36 (s, 1H, H-3). 13C NMR (75 MHz, DMSO d-6) δ (ppm): 20.57 (CH3); 20.94 (CH3); 49.49 (CH2); 90.44 (C-oxadiazoline); 101.49 (C-5); 124.35 (Cph); 125.02 (Cph); 125.75 (Cph); 125.84 (Cph); 127.99 (Cph);128.68 (Cph); 130.05 (Cph); 131.22 (Cph); 131.75 (Cph); 133.80 (Cph); 134.77 (Cph); 145.51 (C-6); 148.28 (Cph); 150.98 (C-2); 153.36 (C-oxadiazoline); 163.54 (C-4); 166.49 (CO acetyl); 168.57 (CO acetyl). HPLC/MS (m/z) 483.11(M+H)+, HRMS for C23H20ClN4O6: Calc 483.1066, Found 483.1064.
2-(3-Acetyl-5-(3-chlorophenyl)-2,3-dihydro-1,3,4-oxadiazol-2-yl)-4-((5-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl) methyl) phenyl acetate (18)
Rf = 0.33 [CH2Cl2/MeOH (97/3)]. Yield = 71%, m = 106 mg. Mp = (231–233) °C. UV (methanol) λmax = 277 nm. IR υ (cm−1): 3486 (NH); 1769 (C=O); 1678 (C=C); 1204 (C–N); 694 (OH phenol). 1H NMR (300 MHz, DMSO d-6) δ (ppm): 1.74 (s, 3H, CH3); 2.12 (s, 3H, CH3); 2.19 (s, 3H, CH3); 4.87 (s, 2H, CH2); 7.19 (s, 1H, H-oxadiazoline); 7.66 (s, 1H, H-6); 7.39–7.76 (m, 7H, H-aromatic); 11.34 (s, 1H, H-3). 13C NMR (75 MHz, DMSO d-6) δ (ppm): 11.97 (CH3); 20.57 (CH3); 20.94 (CH3); 49.30 (CH2); 90.41 (C-oxadiazoline); 109.07 (C-5); 124.33 (Cph); 125.06 (Cph); 125.74 (Cph); 125.85 (Cph); 127.99 (Cph); 128.60 (Cph); 130.03 (Cph); 131.22 (Cph); 131.75 (Cph); 133.80 (Cph); 134.95 (Cph); 141.17 (C-6); 148.23 (Cph); 150.97 (C-2); 153.42 (C-oxadiazoline aromatic); 164.18 (C-4); 166.41 (CO acetyl); 168.51 (CO acetyl). HPLC/MS (m/z) 497.12 (M+H)+, HRMS for C24H22ClN4O6: Calc 497.1222, Found 497.1221.
2-(3-Acetyl-5-(3-chlorophenyl)-2,3-dihydro-1,3,4-oxadiazol-2-yl)-4-((5-fluoro-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl) methyl) phenyl acetate (19)
Rf = 0.33 [CH2Cl2/MeOH (97/3)]. Yield = 54%, m = 81 mg. Mp = (198–200) °C. UV (methanol) λmax = 279 nm. IR υ (cm−1): 3450 (NH); 1769 (C=O); 1705 (C=C); 1191 (C–N); 695 (OH phenol). 1H NMR (300 MHz, DMSO d-6) δ (ppm): 2.12 (s, 3H, CH3); 2.19 (s, 3H, CH3); 4.86 (s, 2H, CH2); 7.19 (s, 1H, H-oxadiazoline); 7.43–7.76 (m, 7H, H-aromatic); 8.24 (d, 1H, 3JH–F = 6 Hz, H-6); 11.88 (s, 1H, H-3). NMR 13C (75 MHz, DMSO d-6) δ (ppm): 20.48 (CH3); 20.95 (CH3); 49.89 (CH2); 90.49 (C-oxadiazoline); 124.33 (Cph); 125.06 (Cph); 125.86 (Cph); 128.00 (Cph); 128.76 (Cph); 129.70 (Cph); 130.13 (d, 2JC–F = 1.2 Hz, C-6); 131.23 (Cph); 131.75 (Cph); 133.80 (Cph); 134.37 (Cph); 148.33 (C-2); 149.63 (Cph); 153.38 (C-oxadiazoline); 141.37(d, 1JC–F = 228.37 Hz, C-5); 157.52 (d, 2JC–F = 21.82 Hz, C-4); 166.47 (CO acetyl); 168.56 (CO acetyl). HPLC/MS (m/z) 501.10 (M+H)+, HRMS for C23H19ClFN4O6: Calc 501.0972, Found 501.0970.
2-(3-Acetyl-5-(3-chlorophenyl)-2,3-dihydro-1,3,4-oxadiazol-2-yl)-4-((6-methyl-3,5-dioxo-4,5-dihydro-1,2,4-triazin-2(3H)-yl) methyl) phenyl acetate (20)
Rf = 0.38 [CH2Cl2/MeOH (97/3)]. Yield = 60%, m = 89 mg. Mp = (157–158) °C. UV (methanol) λmax = 284 nm. IR υ (cm−1): 3449 (NH); 1769 (C=O); 1705 (C=C); 1191 (C–N); 695 (OH phenol). 1H NMR (300 MHz, DMSO d-6) δ (ppm): 2.05 (s, 3H, CH3); 2.12 (s, 3H, CH3); 2.20 (s, 3H, CH3); 5.02 (s, 2H, CH2); 7.20 (s, 1H, H-oxadiazoline); 7.41–7.76 (m, 7H, H-aromatic); 12.16 (s, 1H, H-3). 13C NMR (75 MHz, DMSO d-6) δ (ppm): 15.97 (CH3); 20.45 (CH3); 20.88 (CH3); 51.79 (CH2); 90.43 (C-oxadiazoline); 124.20 (Cph); 125.06 (Cph); 125.62 (Cph); 125.94 (Cph); 127.89 (Cph); 128.80 (Cph); 130.20 (Cph); 131.23 (Cph); 131.65 (Cph); 133.80 (Cph); 134.72 (Cph); 143.66 (C-5); 148.17 (Cph); 148.88 (C-2); 153.43 (C-oxadiazoline); 157.05 (C-4); 166.47 (CO acetyl); 168.60 (CO acetyl). HPLC/MS (m/z) 498.12 (M+H)+, HRMS for C23H21ClN5O6: Calc 498.1175, Found 498.1176.
2-(3-Acetyl-5-(4-chlorophenyl)-2,3-dihydro-1,3,4-oxadiazol-2-yl)-4-((2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl) methyl) phenyl acetate (21)
Rf = 0.28 [CH2Cl2/MeOH (97/3)]. Yield = 59%, m = 85 g. Mp = (215–217) °C. UV (methanol) λmax = 273 nm. IR υ (cm−1): 3456 (NH); 1765 (C=O); 1667 (C=C); 1208 (C–N); 726 (OH phenol). 1H NMR (300 MHz, DMSO d-6) δ (ppm): 2.11 (s, 3H, CH3); 2.18 (s, 3H, CH3); 4.90 (s, 2H, CH2); 5.60 (d, 3JH–H = 7.8 Hz, 1H, H-5); 7.18 (s, 1H, H-oxadiazoline); 7.38–7.81 (m, 7H, H-aromatic, H-6); 11.36 (s, 1H, H-3). NMR 13C (75 MHz, DMSO d-6) δ (ppm): 20.57 (CH3); 20.94 (CH3); 49.66 (CH2); 90.18 (C-oxadiazoline); 101.48 (C-5); 122.70 (Cph); 124.34 (Cph); 128.06 (Cph); 128.19 (Cph); 128.65 (Cph); 129.32 (Cph); 130.00 (Cph); 134.76 (Cph); 136.57 (Cph); 145.51 (C-6); 148.19 (Cph); 150.97 (C-2); 153.85 (C-oxadiazoline); 163.51 (C-4); 166.30 (CO acetyl); 168.54 (CO acetyl). HPLC/MS (m/z) 483.11 (M+H)+, HRMS for C23H20ClN4O6: Calc 483.1066, Found 483.1063.
2-(3-Acetyl-5-(4-chlorophenyl)-2,3-dihydro-1,3,4-oxadiazol-2-yl)-4-((5-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl) methyl) phenyl acetate (22)
Rf = 0.34 [CH2Cl2/MeOH (97/3)]. Yield = 49%, m = 73 mg. Mp = (219–221) °C. UV (methanol) λmax = 278 nm. IR υ (cm−1): 3446 (NH); 1762 (C=O); 1702 (C=C); 1203 (C–N); 698 (OH phenol). 1H NMR (300 MHz, DMSO d-6) δ (ppm): 1.74 (s, 3H, CH3); 2.12 (s, 3H, CH3); 2.18 (s, 3H, CH3); 4.87 (s, 2H, CH2); 7.19 (s, 1H, H-oxadiazoline); 7.38–7.81 (m, 7H, H-aromatic, H-6); 11.34 (s, 1H, H-3). 13C NMR (75 MHz, DMSO d-6) δ (ppm): 11.80 (CH3); 20.49 (CH3); 20.94 (CH3); 49.31 (CH2); 90.31 (C-oxadiazoline); 109.13 (C-5); 122.70 (Cph); 124.32 (Cph); 127.99 (Cph); 128.18 (Cph); 128.59 (Cph); 129.33 (Cph); 129.99 (Cph); 134.93 (Cph); 136.58 (Cph); 145.17 (Cph); 148.22 (C-6); 150.97 (C-2); 153.85 (C-oxadiazoline); 164.18 (C-4); 166.36 (CO acetyl); 168.55 (CO acetyl). HPLC/MS (m/z) 497.12 (M+H)+, HRMS for C24H22ClN4O6: Calc 497.1222, Found 497.1215.
2-(3-Acetyl-5-(4-chlorophenyl)-2,3-dihydro-1,3,4-oxadiazol-2-yl)-4-((5-fluoro-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl) methyl) phenyl acetate (23)
Rf = 0.31 [CH2Cl2/MeOH (97/3)]. Yield = 40%, m = 60 mg. Mp = (218–220) °C. UV (methanol) λmax = 281 nm. IR υ (cm−1): 3434 (NH); 1769 (C=O); 1709 (C=C); 1195 (C–N); 658 (OH phenol). 1H NMR (300 MHz, DMSO d-6) δ (ppm): 2.11 (s, 3H, CH3); 2.18 (s, 3H, CH3); 4.85 (s, 2H, CH2); 7.18 (s, 1H, H-oxadiazoline); 7.42–7.81 (m, 7H, H-aromatic); 8.23 (d, 1H, 3JH–H = 6 Hz, H-6); 11.88 (s, 1H, H-3). 13C NMR (75 MHz, DMSO d-6) δ (ppm): 20.51 (CH3); 20.94 (CH3); 49.96 (CH2); 90.38 (C-oxadiazoline); 122.70 (Cph); 124.32 (Cph); 128.06 (Cph); 128.18 (Cph); 128.75 (Cph); 129.33 (Cph); 129.71 (Cph); 134.35 (Cph); 136.58 (Cph); 130.15 (d, 2JC–F = 4.57 Hz, C-6); 145.51 (Cph); 148.32 (C-2); 153.85 (C-oxadiazoline); 141.23 (d, 1JC–F = 222.07 Hz, C-5); 157.58 (d, 2JC–F = 22.8 Hz, C-4); 166.31 (CO acetyl); 168.46 (CO acetyl). HPLC/MS (m/z) 501.10 (M+H)+, HRMS for C23H19ClFN4O6: Calc 501.0972, Found 501.0970.
Materials and methods in biology
SARS-CoV-2
The used SARS-CoV-2 was derived from the Beta-Cov/Belgium/GHB-03021/2020 (EPI ISL407976|2020-02-03), which has been isolated in February 2020 from a Belgian patient having returned from Wuhan. The isolate was passed through VeroE6 cells seven times, producing two series of amino acid deletions in the spike protein [44]. Titration on Vero E6 cells was used to determine the infectious material of the viral stock.
The antiviral assay for SARS-CoV-2 is established on the previous used SARS-CoV assay [45]. After infection, the fluorescence of VeroE6-GFP cell cultures declines because of the cytopathogenic influence of the replicating virus. The cytopathogenicity is inhibited, and the fluorescent signal is maintained in the presence of an antiviral agent. To this end, VeroE6-GFP cells (kindly provided by Marnix Van Loock, Janssen Pharmaceutica, Beerse, Belgium) have been used as described previously [46, 47]. Because VeroE6 cells exhibit high chemotype efflux, the antiviral assays were carried out in the presence of the P-glycoprotein (Pgp) efflux inhibitor CP-100356 (0.5 M) [48].
VZV and HCMV
The synthetized compounds were tested against two human herpesviruses {cytomegalovirus (HCMV) strains AD-169 and Davis varicella-zoster virus strains [Oka (wild type) and 07-1 (thymidine kinase-deficient strain)]} in human embryonic lung (HEL) fibroblasts as reported previously [49].
Antileishmanial evaluation
Cell lines
The mouse monocyte/macrophage cell lines RAW 264.7 and L. donovani (MHOM/ET/67/HU3, also called LV9) promastigotes and axenic amastigotes were kept in accordance with the procedures presented by Pomel et al. [50]
Cytotoxicity evaluation of the compounds on RAW 264.7 macrophages
The resazurin technique, described in Pomel et al., was used to assess cytotoxicity in RAW 264.7 macrophages [50].
In vitro antileishmanial evaluation on L. donovani axenic amastigotes
This evaluation was performed using the SYBR Green method as previously described [50]. IC50 values were calculated using the IC Estimator version 1.2 software [50]. Miltefosine was used as the reference drug.
Evaluation of in vitro antileishmanial on intramacrophage amastigotes
Cytotoxicity determination, as described above, was employed to choose the highest concentrations of drug that could be investigated on the L. donovani intramacrophage amastigote model using RAW 264.7 cells. Macrophages were contaminated with L. donovani axenic amastigotes at a 10 parasites per macrophage ratio. The rate of infected macrophages was about 80% in these conditions, as well as the mean number of amastigotes for each infected macrophage was 4 to 5 in the untreated controls. The in vitro treatment was applied 24 h post-infection during 48 h. The results of the product’s effect are given as rate reduction of parasite growth, measured using the SYBR Green incorporation method. The activity of the compounds is expressed as IC50, calculated using the IC Estimator version 1.2 software [50]. Miltefosine was used as the reference drug.
Conclusion
In conclusion, a novel series of homonucleosides analogs of N-acetyl-1,3,4-oxadiazoline was synthesized and evaluated for their antileishmanial and antiviral activities against HCM, VZV, and SARS-CoV-2. Furthermore, the synthetic strategy to synthetize hybrid compounds was proved to be simple and efficient. Compound 20 was the most active with IC50 value less than 10 µM against the axenic and intramacrophage amastigotes forms of L. donovani. The absence of significant antiviral activity of this series prompts us to focus further studies on its antileishmanial potential but trying to enhance its antiviral effect. Consequently, additional chemistry work is under progress to synthesize new derivatives, to strengthen their development on a rational base, and to optimize the antileishmanial activity and antiviral activity of the 6-azathymine as a pharmacophore.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors are extremely grateful to M. Brecht Dirix for excellent technical assistance and dedication to evaluate the anti-herpes virus activity of the derivatives. The authors would also like to thank the technical staff of the CAC (Centre of Analysis and Characterization), University Cadi Ayyad for running the spectroscopic analysis.
Funding
None.
Data availability
Spectroscopic data are available in the Supporting Material.
Consent for publication
Not applicable.
Declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Statement involving human and animal rights
No animals/humans were used for studies that are the basis of this research.
Ethical approval
Not applicable.
Informed consent
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hotez PJ, Nathan CL (2020) Neglected tropical diseases: public health control programs and mass drug administration. In: Hunter's tropical medicine and emerging infectious diseases. Elsevier, Inc., Amsterdam, pp 209–213
- 2.Kapil S, Singh PK, Silakari O. An update on small molecule strategies targeting leishmaniasis. Eur J Med Chem. 2018;157:339–367. doi: 10.1016/j.ejmech.2018.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Croft SL, Olliaro P. Leishmaniasis chemotherapy—challenges and opportunities. Clin Microbiol Infect. 2011;17:1478–1483. doi: 10.1111/j.1469-0691.2011.03630.x. [DOI] [PubMed] [Google Scholar]
- 4.Ezra N, Ochoa MT, Craft N. Human immunodeficiency virus and leishmaniasis. J Glob Infect Dis. 2010;2:248–257. doi: 10.4103/0974-777X.68528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heirwegh E, MacLean E, He J, Kamhawi S, Sagan SM, Olivier M. Sandfly Fever Sicilian Virus-Leishmania major co-infection modulates innate inflammatory response favoring myeloid cell infections and skin hyperinflammation. PLoS Negl Trop Dis. 2021;15:e0009638. doi: 10.1371/journal.pntd.0009638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pikoulas A, Piperaki ET, Spanakos G, Kallianos A, Mparmparousi D, Rentziou G, Trakada GV. Leishmaniasis and COVID-19 coinfection—a case report. IDCases. 2022;27:e01358. doi: 10.1016/j.idcr.2021.e01358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sundar S, Sinha PK, Rai M, Verma DK, Alam KS, Chakravarty J, Vaillant M, Verma N, Pandey K, Kumari P, Lal CS, Arora R, Sharma B, Ellis S, Strub Wourgaft N, Balasegaram M, Olliaro P, Das P, Modabber F. Comparison of short-course multidrug treatment with standard therapy for visceral leishmaniasis in India: an open-label, non-inferiority, randomised controlled trial. Lancet. 2011;377:477–486. doi: 10.1016/S0140-6736(10)62050-8. [DOI] [PubMed] [Google Scholar]
- 8.Torres-Guerrero E, Quintanilla-Cedillo MR, Ruiz-Esmenjaud J, Arenas R. Leishmaniasis: a review. F1000 Res. 2017;6:750. doi: 10.12688/f1000research.11120.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schlecker R, Thieme PC. The synthesis of antihypertensive 3-(1, 3, 4-oxadiazol-2-yl) phenoxypropanolahines. Tetrahedron. 1988;44:3289–3294. doi: 10.1016/S0040-4020(01)85962-7. [DOI] [Google Scholar]
- 10.Bala S, Kamboj S, Kumar A. Heterocyclic 1, 3, 4-oxadiazoline compounds with diverse biological activities: a comprehensive review. J Pharm Res. 2010;3:2993–2997. [Google Scholar]
- 11.Cocohoba J, Dong BJ. Raltegravir: the first HIV integrase inhibitor. Clin Ther. 2008;30:1747–1765. doi: 10.1016/j.clinthera.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 12.James N, Growcott JW. Zibotentan endothelin ETA receptor antagonist oncolytic. Drugs Future. 2009;34:624–633. [Google Scholar]
- 13.Fizazi K, Higano CS, Nelson JB, Gleave M, Miller K, Morris T, Nathan FE, McIntosh S, Pemberton K, Moul JW. Phase III, randomized, placebo-controlled study of docetaxel in combination with zibotentan in patients with metastatic castration-resistant prostate cancer. J Clin Oncol. 2013;31:1740–1747. doi: 10.1200/JCO.2012.46.4149. [DOI] [PubMed] [Google Scholar]
- 14.Taha M, Ismail NH, Imran S, Anouar EH, Selvaraj M, Jamil W, Ali M, Kashif SM, Rahim F, Khan KM, Adenan MI. Synthesis and molecular modeling studies of phenyl linked oxadiazoline–phenylhydrazone hybrids as potent antileishmanial agents. Eur J Med Chem. 2017;126:1021–1033. doi: 10.1016/j.ejmech.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 15.Taha M, Ismail NH, Ali M, Rashid U, Imran S, Uddin N, Khan KM. Molecular hybridization conceded exceptionally potent quinolinyl–oxadiazoline hybrids through phenyl linked thiosemicarbazide antileishmanial scaffolds: in silico validation and SAR studies. Bioorg Chem. 2017;71:192–200. doi: 10.1016/j.bioorg.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Ali OM, Amer HH, Abdel Rahman AH. Synthesis and antiviral evaluation of sugar uracil-1-ylmethylhydrazones and their oxadiazoline derivatives. Synthesis. 2007;2007:2823–2828. doi: 10.1055/s-2007-983878. [DOI] [Google Scholar]
- 17.Mallikarjuna BP, Sastry BS, Suresh Kumar GV, Rajendraprasad Y, Chandrashekar SM, Sathisha K. Synthesis of new 4-isopropylthiazole hydrazide analogs and some derived clubbed triazole, oxadiazoline ring systems—a novel class of potential antibacterial, antifungal and antitubercular agents. Eur J Med Chem. 2009;44:4739–4746. doi: 10.1016/j.ejmech.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 18.Joshi SD, Vagdevi HM, Vaidya VP, Gadaginamath GS. Synthesis of new 4-pyrrol-1-yl benzoic acid hydrazide analogs and some derived oxadiazoline, triazole and pyrrole ring systems: a novel class of potential antibacterial and antitubercular agents. Eur J Med Chem. 2008;4:1989–1996. doi: 10.1016/j.ejmech.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 19.Rollas S, Nehir G, Erdeniz H. Synthesis and antimicrobial activity of some new hydrazones of 4-fluorobenzoic acid hydrazide and 3-acetyl-2, 5-disubstituted-1, 3, 4-oxadiazolines. Il Farmaco. 2002;57:171–174. doi: 10.1016/S0014-827X(01)01192-2. [DOI] [PubMed] [Google Scholar]
- 20.Popiolek L, Biernasiuk A, Paruch K, Malm A, Wujec M. Synthesis and in vitro antimicrobial activity screening of new 3-acetyl-2, 5-disubstituted-1, 3, 4-oxadiazoline derivatives. Chem Biodivers. 2019;16:e1900082. doi: 10.1002/cbdv.201900082. [DOI] [PubMed] [Google Scholar]
- 21.Kaymakçioglu BK, Emre EEO, Unsalan SU, Tabanca N, Iqrar Khan S, Wedge ED, Iscan G, Demirci F, Rollas S. Synthesis and biological activity of hydrazide–hydrazones and their corresponding 3-acetyl-2, 5-disubstituted-2, 3-dihydro-1, 3, 4-oxadiazolines. Med Chem Res. 2012;21:3499–3508. doi: 10.1007/s00044-011-9882-z. [DOI] [Google Scholar]
- 22.Grover J, Bhatt N, Kumar V, Patel NK, Gondaliya BJ, Elizabeth Sobhia M, Bhutani KK, Jachak SM. 2,5-Diaryl-1,3,4-oxadiazoles as selective COX-2 inhibitors and anti-inflammatory agents. RSC Adv. 2015;5:45535–45544. doi: 10.1039/C5RA01428J. [DOI] [Google Scholar]
- 23.Sharma V, Chitranshi N, Agarwal AK. Significance and biological importance of pyrimidine in the microbial world. Int J Med Chem. 2014;2014:1–31. doi: 10.1155/2014/202784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakagawa Y, Bobrov S, Semer CR, Kucharek TA, Harmoto M (2004) Fungicidal pyrimidine derivatives. United States Patent
- 25.Cieplik J, Stolarczyk M, Pluta J, Gubrynowicz O, Bryndal I, Lis T, Mikulewicz M. Synthesis and antibacterial properties of pyrimidine derivatives. Acta Pol Pharm. 2015;72:53–64. [PubMed] [Google Scholar]
- 26.Thomson JM, Lamont IL. Nucleoside analogues as antibacterial agents. Front Microbiol. 2019;10:952. doi: 10.3389/fmicb.2019.00952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vega S, Alonso J, Diaz JA, Junquera F. Synthesis of 3-substituted-4-phenyl-2-thioxo-1, 2, 3, 4, 5, 6, 7, 8-octahydrobenzo [4, 5] thieno [2, 3-á] pyrimidines. J Heterocycl Chem. 1990;27:269–273. doi: 10.1002/jhet.5570270229. [DOI] [Google Scholar]
- 28.Luo MZ, Liu MC, Mozdziesz DE, Lin TS, Dutschman GE, Gullen EA, Cheng YC, Sartorelli AC. Synthesis and biological evaluation of l- and d-configuration 1, 3-dioxolane 5-azacytosine and 6-azathymine nucleosides. Bioorg Med Chem Lett. 2000;10:2145–2148. doi: 10.1016/S0960-894X(00)00418-2. [DOI] [PubMed] [Google Scholar]
- 29.El Mansouri A, Oubella A, Dânoun K, Ahmad M, Neyts J, Jochmans D, Snoeck R, Andrei G, Morjani H, Zahouily M, Lazrek HB. Discovery of novel furo[2,3-d] pyrimidin-2-one–1,3,4-oxadiazole hybrid derivatives as dual antiviral and anticancer agents that induce apoptosis. Arch Pharm. 2021;354:2100146. doi: 10.1002/ardp.202100146. [DOI] [PubMed] [Google Scholar]
- 30.Ae EM, Oubella A, Maatallah M, AitItto MY, Zahouily M, Morjani H, Lazrek HB. Design, synthesis, biological evaluation and molecular docking of new uracil analogs-1, 2, 4-oxadiazoline hybrids as potential anticancer agents. Bioorg Med Chem Lett. 2020;30:127438. doi: 10.1016/j.bmcl.2020.127438. [DOI] [PubMed] [Google Scholar]
- 31.El Mansouri A, Maatallah M, Ait Benhassou H, Moumen A, Mehdi A, Snoeck R, Andrei G, Zahouily M, Lazrek HB. Design, synthesis, chemical characterization, biological evaluation, and docking study of new 1,3,4-oxadiazole homonucleoside analogs. Nucleosides Nucleotides Nucleic Acids. 2020;39:1088–1107. doi: 10.1080/15257770.2020.1761982. [DOI] [PubMed] [Google Scholar]
- 32.Vincent IM, Barrett MP. Metabolomic-based strategies for anti-parasite drug discovery. J Biomol Screen. 2015;20:44–55. doi: 10.1177/1087057114551519. [DOI] [PubMed] [Google Scholar]
- 33.Sharma M, Chauhan K, Shivahare R, Vishwakarma P, Suthar MK, Sharma A, Gupta S, Saxena JK, Lal J, Chandra P, Kumar B, Chauhan PMS. Discovery of a new class of natural product-inspired quinazolinone hybrid as potent antileishmanial agents. J Med Chem. 2013;56:4374–4392. doi: 10.1021/jm400053v. [DOI] [PubMed] [Google Scholar]
- 34.Suryawanshi SN, Kumar S, Shivahare R, Pandey S, Tiwari A, Gupta S. Design, synthesis and biological evaluation of aryl pyrimidine derivatives as potential leishmanicidal agents. Bioorg Med Chem Lett. 2013;23:5235–5238. doi: 10.1016/j.bmcl.2013.06.060. [DOI] [PubMed] [Google Scholar]
- 35.Chauhan K, Sharma M, Shivahare R, Debnath U, Gupta S, Prabhakar YS, Chauhan PM. Discovery of triazine mimetics as potent antileishmanial agents. ACS Med Chem Lett. 2013;4:1108–1113. doi: 10.1021/ml400317e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El Mansouri A, Zahouily M, Lazrek HB. HMDS/KI a simple, a cheap and efficient catalyst for the one-pot synthesis of N-functionalized pyrimidines. Synth Commun. 2019;49:1802–1812. doi: 10.1080/00397911.2019.1602655. [DOI] [Google Scholar]
- 37.Guieu S, Rocha J, Silva AMS. Synthesis of unsymmetrical methylenebisphenol derivatives. Synlett. 2013;24:762–764. doi: 10.1055/s-0032-1318394. [DOI] [Google Scholar]
- 38.Marhadour S, Marchand P, Pagniez F, Bazin MA, Lozach CPO, Ruchaud S, Antoine M, Meijer L, Rachidi N, Le Pape P. Synthesis and biological evaluation of 2,3-diarylimidazo[1,2-a] pyridines as antileishmanial agents. Eur J Med Chem. 2012;58:543–556. doi: 10.1016/j.ejmech.2012.10.048. [DOI] [PubMed] [Google Scholar]
- 39.Gamal El Din MM, El Gamal MI, Abdel Maksoud MS, Ho Yoo K, Oh CH. Synthesis and broad-spectrum antiproliferative activity of diarylamides and diarylureas possessing 1,3,4-oxadiazoline derivatives. Bioorg Med Chem Lett. 2015;25:1692–1699. doi: 10.1016/j.bmcl.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 40.Zhao J, Liu Q, Yi D, Li Q, Guo S, Ma L, Zhang Y, Dong D, Guo F, Liu Z, Wei T, Li X, Cen S. 5-Iodotubercidin inhibits SARS-CoV-2 RNA synthesis. Antiviral Res. 2022;198:105254. doi: 10.1016/j.antiviral.2022.105254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dustin LB, Bartolini B, Capobianchi MR, Pistello M. Hepatitis C virus: life cycle in cells, infection and host response, and analysis of molecular markers influencing the outcome of infection and response to therapy. Clin Microbiol Infect. 2016;22:826–832. doi: 10.1016/j.cmi.2016.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elfiky AA. Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): a molecular docking study. Life Sci. 2020;253:117592. doi: 10.1016/j.lfs.2020.117592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parsons TL, Kryszak LA, Marzinke MA. Development and validation of assays for the quantification of β-d-N4-hydroxycytidine in human plasma and β-d-N4-hydroxycytidine-triphosphate in peripheral blood mononuclear cell lysates. J Chromatogr B. 2021;1182:122921. doi: 10.1016/j.jchromb.2021.122921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boudewijns R, Thibaut HJ, Kaptein SJF, Li R, Vergote V, Seldeslachts L, Weyenbergh JV, De Keyzer C, Bervoets L, Sharma S, Liesenborghs L, Ma J, Jansen S, Van Looveren D, Vercruysse T, Wang X, Jochmans D, Martens E, Roose K, De Vlieger D, Schepens B, Van Buyten T, Jacobs S, Liu Y, Martí Carreras J, Vanmechelen B, Wawina Bokalanga T, Delang L, Rocha Pereira J, Coelmont L, Chiu W, Leyssen P, Heylen E, Schols D, Wang L, Close L, Matthijnssens J, Van Ranst M, Compernolle V, Schramm G, Van Laere K, Saelens X, Callewaert N, Opdenakker G, Maes P, Weynand B, Cawthorne C, Vande Velde G, Wang Z, Neyts J, Dallmeier K. STAT2 signaling restricts viral dissemination but drives severe pneumonia in SARS-CoV-2 infected hamsters. Nat Commun. 2020;11:5838. doi: 10.1038/s41467-020-19684-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ivens T, Van den Eynde C, Van Acker K, Nijs E, Dams G, Bettens E, Ohagen A, Pauwels R, Hertogs K. Development of a homogeneous screening assay for automated detection of antiviral agents active against severe acute respiratory syndrome-associated coronavirus. J Virol Methods. 2005;129:56–63. doi: 10.1016/j.jviromet.2005.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Do TND, Donckers K, Vangeel L, Chatterjee AK, Gallay PA, Bobardt MD, Bilello JP, Cihlar T, De Jonghe S, Neyts J, Jochmans D. A robust SARS-CoV-2 replication model in primary human epithelial cells at the air liquid interface to assess antiviral agents. Antiviral Res. 2021;192:105122. doi: 10.1016/j.antiviral.2021.105122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abdelnabi R, Foo CS, Jochmans D, Vangeel L, De Jonghe S, Augustijns P, Mols R, Weynand B, Wattanakul T, Hoglund RM. The oral protease inhibitor (PF-07321332) protects Syrian hamsters against infection with SARS-CoV-2 variants of concern. Nat Commun. 2022;13:1–9. doi: 10.1038/s41467-022-28354-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoffman RL, Kania RS, Brothers MA, Davies JF, Ferre RA, Gajiwala KS, He M, Hogan RJ, Kozminski K, Li LY, Lockner JW, Lou J, Marra MT, Mitchell LJ, Jr, Murray BW, Nieman JA, Noell S, Planken SP, Rowe T, Ryan K, Smith GJ, III, Solowiej JE, Steppan CM, Taggart B. Discovery of ketone-based covalent inhibitors of coronavirus 3CL proteases for the potential therapeutic treatment of COVID-19. J Med Chem. 2020;63:12725–12747. doi: 10.1021/acs.jmedchem.0c01063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yuan W, Chen X, Liu N, Wen Y, Yang B, Andrei G, Snoeck R, Xiang Y, Wu YW, Jiang Z, Schols D, Zhang Z, Wu Q. Synthesis, anti-varicella-zoster virus and anti-cytomegalovirus activity of 4,5-disubstituted 1,2,3-(1H)-triazoles. Med Chem. 2019;15:801–812. doi: 10.2174/1573406414666181109095239. [DOI] [PubMed] [Google Scholar]
- 50.Pomel S, Cojean S, Pons V, Cintrat JC, Nguyen L, Vacus J, Pruvost A, Barbier J, Gillet D, Loiseau PM. An adamantine derivative as a drug candidate for the treatment of visceral leishmaniasis. J Antimicrob Chemother. 2021;76:2640–2650. doi: 10.1093/jac/dkab226. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Spectroscopic data are available in the Supporting Material.
Consent for publication
Not applicable.