Introduction
Granuloma annulare (GA) most commonly presents as annular plaques on the hands or feet. On histology, this disease is defined by the presence of a palisaded or interstitial lymphohistiocytic infiltrate, mucin deposition, and collagen alteration.1 Localized, generalized, and subcutaneous GA is frequently encountered, but rarer GA variants also exist.1 Acute-onset, painful, acral GA (AOPAGA) is a rarely reported localized GA variant.2 Here, we present a 52-year-old AOPAGA patient.
Case report
A 52-year-old female with a past medical history significant for migraines, transient ischemic attack, iron deficiency anemia, gastroesophageal reflux disease, and multinodular goiter presented in April 2021 with a multi-year history of intermittent, painful, bilateral, finger rash. The rash consisted of tender papules that limited her normal daily activities. Lesions progressed proximally along her fingers but stayed distal to the metacarpophalangeal joints. Lesions appeared without a clear trigger and resolved without intervention. During this time, the patient also developed distal interphalangeal and ankle arthralgias.
Prior to being seen in our clinic, the patient underwent an autoimmune, infectious, and malignancy workup (Supplementary Table I, available via Mendeley at https://doi.org/10.17632/yn3r6bd7bf.2) that did not identify a cause for her rash and arthralgias. She was given betamethasone valerate 0.1% cream and clobetasol propionate 0.05% ointment for her rash. After 6 weeks of use, there was no improvement, which led the patient to seek evaluation at our institution.
In our clinic, the patient reported no personal or family history of similar dermatologic findings or rheumatologic conditions. While experiencing her hand rash in June 2020, she did report a subjective increase in the size of her thyroid nodules, though her thyroid-stimulating hormone and T4 were normal at that time (Supplementary Table I, available via Mendeley at https://doi.org/10.17632/yn3r6bd7bf.2). Her medications included omeprazole, ferrous sulfate, cardioprotective aspirin, cholecalciferol, naproxen, rizatriptan, promethazine, and metaxalone. She had no history of tobacco use.
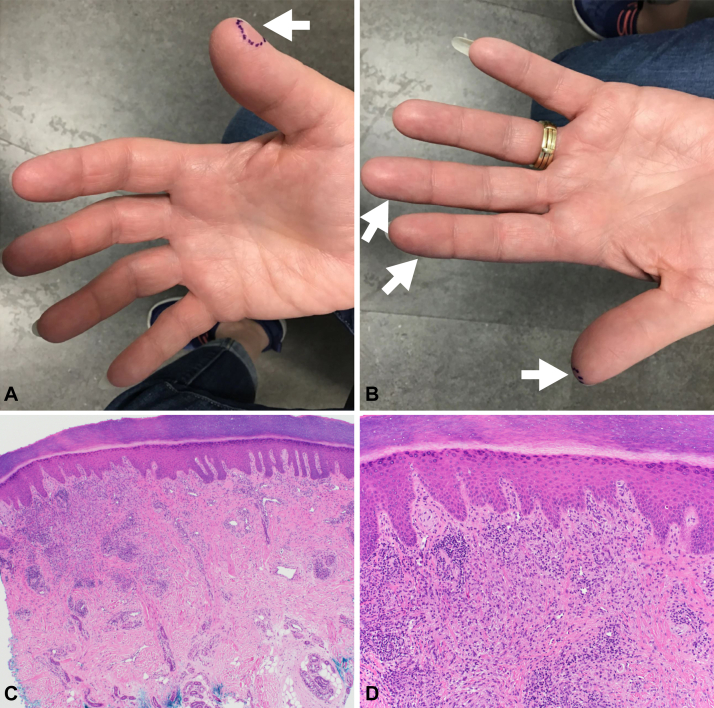
Physical examination revealed slightly violaceous, mildly indurated, thin papules on the palmar/glabrous surfaces of the right and left fingers (Fig 1, A and B). Punch biopsies of the newest and oldest lesions demonstrated lymphocytes and histiocytes around and between collagen bundles, an associated perivascular lymphocytic infiltrate, and no notable neutrophilic infiltrate (Fig 1, C and D). The dermatopathologist’s diagnosis based on these biopsies was granulomatous dermatitis with findings most suggestive of GA. These histology findings were similar to those of a right distal thumb biopsy of the patient’s hand rash performed by an outside provider in November 2020. The histopathology observed in our patient’s multiple finger biopsies mirrors that described in published reports of palmar GA.2, 3, 4, 5
Fig 1.
Acute-onset, painful, acral granuloma annulare clinical images and histology. A and B, Painful, slightly violaceous, thin papules on the palmar/glabrous surfaces of the fingers. The (A—right thumb) newest and (B—left thumb) oldest lesions were marked in purple and biopsied. AOPAGA lesions are indicated by white arrows (A—right hand, tip of digit 1; B—left hand, tips of digits 1-3). C and D, Granulomatous, lymphohistiocytic inflammation in the dermis of the patient’s biopsy specimen is shown at (C) 40× and (D) 100× magnification. Lymphocytes and histiocytes are arranged around bundles of collagen. AOPAGA, Acute-onset, painful, acral granuloma annulare.
In conjunction with the patient’s biopsies, a repeat inflammatory workup (erythrocyte sedimentation rate, C-reactive protein, antinuclear antibodies) was obtained to determine if the patient’s arthralgias represented an evolving autoimmune process. These labs were normal. Based on this patient’s clinical, laboratory, and biopsy findings, alternate diagnoses for this patient’s presentation were ruled out (Supplementary Table II, available via Mendeley at https://doi.org/10.17632/yn3r6bd7bf.2).
A diagnosis of AOPAGA was made, and the patient was treated with a 7-week prednisone taper starting at 50 mg daily. The patient’s AOPAGA resolved within 2 weeks but recurred at prednisone doses below 40 to 45 mg/day. An extended prednisone course (40 mg/day for 2 months followed by a 4-week prednisone taper) was started to overlap with the initiation of methotrexate as a steroid-sparing therapy for GA.
The patient then decided to not pursue methotrexate therapy or any other steroid-sparing treatment. She was lost to follow-up for 1 year. After contact was re-established, the patient reported that her AOPAGA had recurred off prednisone, but that she did not wish to pursue further treatment.
Discussion
AOPAGA was first described by Brey et al2 in 2006. This rare variant of localized GA is characterized by the abrupt formation of tender papules and annular plaques primarily on the hands and feet. In our patient, a diagnosis of AOPAGA was made via clinicopathological correlation.
While GA commonly involves the dorsal hands/feet, the volar/glabrous surfaces are typically spared. Formation of GA lesions on palmar skin, as seen in our patient, is exceptionally rare.1,3 Among the 4 cases included in the original description of AOPAGA, Brey et al reported 2 patients with lesions on the palms.2 Including this case report’s patient, 18 AOPAGA cases have now been reported, with 14 cases noting AOPAGA involvement of volar/glabrous hand skin (eg, palms, fingertips) and 9 cases reporting AOPAGA exclusively on volar/glabrous hand skin (Table I).2, 3, 4, 5, 6, 7, 8, 9
Table I.
Summary of AOPAGA cases
| First author (year) | Patient (age, sex) | GA location(s)∗ | Systemic symptoms | Successful treatment | Significant Comorbidities |
|---|---|---|---|---|---|
| Barksdale SK (1994)6 | 75, male | Fingers† | Not reported | Not reported | Lymphoma |
| Barksdale SK (1994)6 | 66, female | Fingertips‡ | Not reported | Not reported | Lymphoma |
| Barksdale SK (1994)6 | 60, female | Dorsal hands | Not reported | Not reported | Lymphoma |
| Brey NV (2006)2 | 42, female | Hands§, legs, feet | Arthralgias of knees and ankles | Hydroxychloroquine sulfate | None |
| Brey NV (2006)2 | 50, female | Lateral hands, dorsal hands | None | Intralesional triamcinolone, fluticasone propionate cream, nicotinamide-folic acid-zinc oxide combination | Not reported |
| Brey NV (2006)2 | 48, female | Dorsal hands, marginal hands, wrists, upper and lower extremities, trunk, occipital scalp | Diffuse arthralgias | Hydroxychloroquine sulfate, hydrocortisone acetate/pramoxine, hydrochloride lotion | Not reported |
| Brey NV (2006)2 | 65, female | Upper and lower palms | None | Prednisone, betamethasone dipropionate ointment | Not reported |
| Haushalter K (2007)7 | 36, female | Palms, fingertips | None | Not reported | None |
| Spencer B (2007)8 | 72, female | Palms | None | Dapsone | Not reported |
| Gutte R (2012)3 | Patient 1‖ | Palms | None | Not reported | Not reported |
| Gutte R (2012)3 | Patient 2‖ | Palms | None | Not reported | Not reported |
| Gutte R (2012)3 | Patient 3‖ | Palms | None | Not reported | Not reported |
| Gutte R (2012)3 | Patient 4‖ | Palms | None | Not reported | Not reported |
| Huh JW (2016)4 | 57, female | Fingers§, legs | Febrile sensations, myalgia, back pain | None identified¶ | None |
| Sonthalia S (2014)5 | 44, male | Palms, soles | None | Clobetasol ointment | None |
| Rai T (2017)9 | 55, female | Palms, soles | None | Prednisone, clobetasol, levocetirizine | Not reported |
| Hsing MT (2018)10 | 37, female | Dorsal foot | Migrating joint pain, myalgia | Prednisone, fluocinonide cream | IDA, Meniere's disease |
Ahmad FS (2022)
|
52, female | Palms, fingertips | Arthralgias | Prednisone | IDA, multinodular goiter, GERD, migraines, TIA, osteopenia |
AOPAGA, Acute-onset, painful, acral granuloma annulare; GA, granuloma annulare; GERD, gastroesophageal reflux disease; IDA, iron-deficiency anemia; TIA, transient ischemic attack.
Glabrous/volar skin is highlighted in Bold.
Fingers were assumed to be acral but not necessarily glabrous/volar (eg, palms/soles).
Fingertip and lateral hand lesions are presumed to be on glabrous/volar hand (ie, equivalent to palms/soles).
Images included lesions on palmar/volar/glabrous skin.
Individual patient information (eg, age, sex) was not available in this case series.
Intermittent treatment with oral prednisolone did not effectively manage this patient’s AOPAGA as new cutaneous lesions persistently developed.
We hypothesize that the propensity of AOPAGA to exclusively appear on volar/glabrous hand skin (50% of AOPAGA cases) suggests an environmental trigger may initiate AOPAGA. Further, we hypothesize that the high level of sensory innervation in volar/glabrous skin underlies the unique painful presentation of AOPAGA. Other subtypes of GA are typically asymptomatic or only mildly painful/pruritic.1 Systemic symptoms (eg, arthralgias, myalgias) have been observed in 5 of the 18 AOPAGA patients (Table I), which has previously led to the hypothesis that this condition represented an early manifestation of autoimmune disease.2,4,10 Our patient’s progressive ankle and distal interphalangeal joint pain temporally coincided with the development of her palmar lesions, but no other evidence of autoimmune disease was identified. Most AOPAGA cases have not reported systemic symptoms or autoimmune disease, though 11% of AOPAGA patients had a history of iron-deficiency anemia and 17% of AOPAGA patients had lymphoma.
Associations with systemic conditions (eg, diabetes, thyroid disease, HIV, dyslipidemia, and malignancy) have been described for GA.1 Our patient’s normal laboratory workup did not support an association between a systemic condition (eg, iron-deficiency anemia, lymphoma, thyroid disease) and ongoing AOPAGA. As our case lacked laboratory data (1) immediately prior to, and (2) near the start of our patient’s AOPAGA, we cannot say whether a systemic disease (eg, iron-deficiency anemia) might initiate but then not be required to sustain AOPAGA. Based on our assessment of all published AOPAGA cases, we recommend that an AOPAGA patient be evaluated for lymphoma and iron-deficiency anemia. Further, we suggest that providers consider (1) a diagnosis of AOPAGA in patients who present with painful, volar/glabrous lesions, and (2) treating AOPAGA with a systemic therapy to alleviate the impact of this disease’s painful presentation on activities of daily living.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: NIH, United States grants: KL2TR002346, UL1TR002345, 5K08AR076464-03.
IRB approval status: Not applicable.
Consent: Written consent was given to the authors for the publication of patient photographs and medical information in print and online with the understanding that this information will be publicly available.
References
- 1.Piette E.W., Rosenbach M. Granuloma annulare: clinical and histologic variants, epidemiology, and genetics. J Am Acad Dermatol. 2016;75(3):457–465. doi: 10.1016/j.jaad.2015.03.054. [DOI] [PubMed] [Google Scholar]
- 2.Brey N.V., Malone J., Callen J.P. Acute-onset, painful acral granuloma annulare: a report of 4 cases and a discussion of the clinical and histologic spectrum of the disease. Arch Dermatol. 2006;142(1):49–54. doi: 10.1001/archderm.142.1.49. [DOI] [PubMed] [Google Scholar]
- 3.Gutte R., Kothari D., Khopkar U. Granuloma annulare on the palms: a clinicopathological study of seven cases. Indian J Dermatol Venereol Leprol. 2012;78(4):468–474. doi: 10.4103/0378-6323.98078. [DOI] [PubMed] [Google Scholar]
- 4.Huh J.W., Jeong Y.I., Choi K.H., Park H.J., Jue M.S. A case of acute-onset, painful acral granuloma annulare. Ann Dermatol. 2016;28(1):119–120. doi: 10.5021/ad.2016.28.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sonthalia S., Arora R., Sarkar R., Khopkar U. Papular granuloma annulare of palms and soles: case report of a rare presentation. F1000Res. 2014;3:2–7. doi: 10.12688/f1000research.3-32.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barksdale S.K., Perniciaro C., Halling K.C., Stickler J.G. Granuloma annulare in patients with malignant lymphoma: clinicopathologic study of thirteen new cases. J Am Acad Dermatol. 1994;31(1):42–48. doi: 10.1016/S0190-9622(94)70133-4. [DOI] [PubMed] [Google Scholar]
- 7.Haushalter K., Hughey L., Foster W., Croitrou A. Painful acral nodules as a manifestation of interstitial granuloma annulare. J Am Acad Dermatol. 2007;56(2):AB57. doi: 10.1016/j.jaad.2006.10.300. [DOI] [Google Scholar]
- 8.Spencer B., Butler D. Painful palmar granuloma annulare responsive to dapsone. J Am Acad Dermatol. 2007;56:AB48. [Google Scholar]
- 9.Rai T. Papular granuloma annulare of palms and soles. Indian Dermatol Online J. 2017;8(6):511–513. doi: 10.4103/idoj.IDOJ_338_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsing M.T., Tsai Y.Y., Hsu H.T. Acute-onset, painful acral granuloma annulare with unusual microcalcification. Australas J Dermatol. 2018;59(4):e291–e294. doi: 10.1111/ajd.12821. [DOI] [PubMed] [Google Scholar]