Introduction
Infantile bullous pemphigoid (IBP) is an extremely rare autoimmune bullous disease that, in certain cases, emerges following various vaccinations.1, 2, 3, 4 The treatment of IBP primarily relies on corticosteroids, but dapsone and intravenous immunoglobulins (IVIG) are also used in refractory cases.5, 6 In the following report, we present the first case of IBP treated successfully with cyclosporine as a monotherapy.
Case report
A 3-month-old healthy baby boy presented to the pediatric dermatology department with a widespread pruritic rash that appeared four days after he received vaccinations against Neisseria meningitidis group C, diphtheria, tetanus, pertussis, poliomyelitis (inactivated), and Haemophilus influenzae type b. Three days prior to the commencement of the rash, he received metamizole for postvaccination fever. He did not suffer any additional symptoms, and his personal and family medical history was unremarkable.
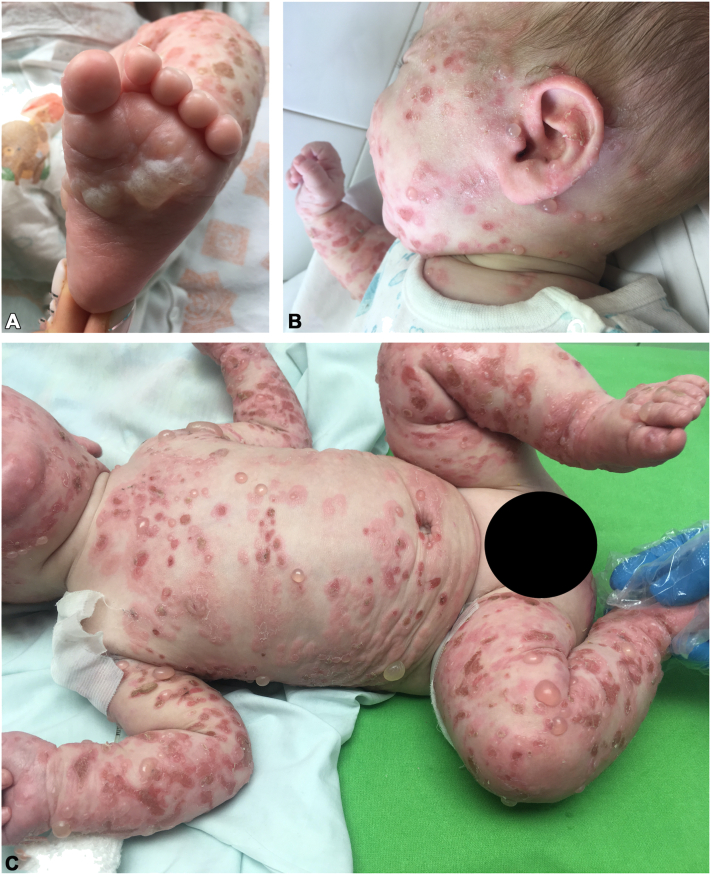
Physical examination revealed widespread erythematous urticarial plaques, numerous tense bullae, and erosions, some of which were seen in an annular distribution. Initially, the skin lesions were located on the palms and soles; subsequently, they spread to involve substantial portions of the infant’s body, including the face, scalp, ears, trunk, extremities, and anogenital region (Fig 1).
Fig 1.
Infantile bullous pemphigoid. Clinical photographs at the time of admission. Widespread erythematous plaques, blisters, and erosions were seen on (A), left sole (B), left side of the face and(C) a generalized view.
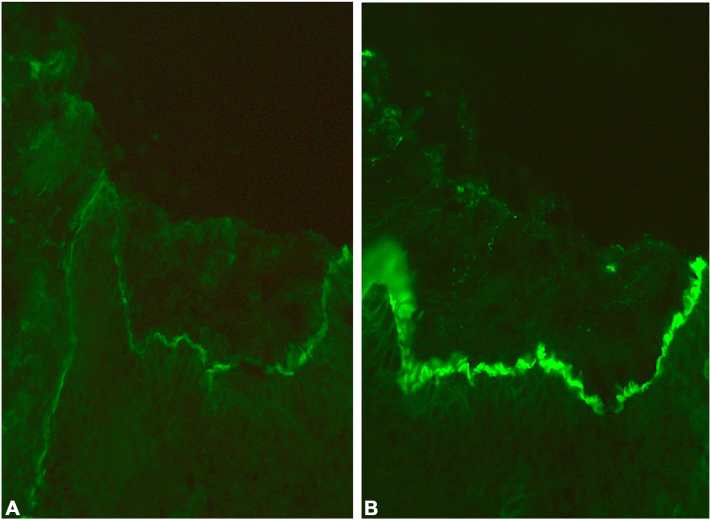
Laboratory investigations at the time of his admission revealed extremely high eosinophilia (41%; normal range, <4%); however, no other significant abnormalities were present. Skin biopsy demonstrated several loci of spongiosis with eosinophils accompanied by mild inflammation in the upper dermis. Direct immunofluorescence revealed subepidermal separation with continuous linear depositions of IgG and C3 along the basement membrane zone (Fig 2), while IgA deposition was not identified. Indirect immunofluorescence on monkey esophagus detected circulating IgG antibasement membrane autoantibodies. The clinical picture in combination with the histologic and immunologic findings confirmed the diagnosis of IBP.
Fig 2.
Direct immunofluorescence examination of a perilesional skin biopsy. Subepidermal separation with a continuous linear deposition of (A) IgG and (B) C3 was detected in the basement membrane zone.
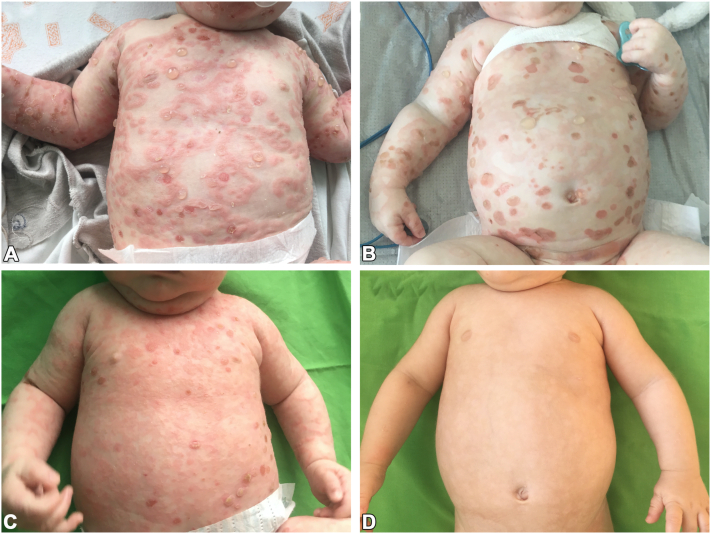
At the beginning of his hospitalization, the infant was treated with intravenous methylprednisolone at a dose of 1 to 2 mg/kg/day. However, the rash continued to spread with the emergence of multiple new bullae. Consequently, IVIG (two 5-g infusions with a one-week interval) was added to the methylprednisolone, but this paring did not lead to any significant improvement either (Fig 3, A). Afterwards, IVIG was discontinued and replaced by dapsone (0.8-1.6 mg/kg/day). The combination of methylprednisolone and dapsone achieved only a partial response (Fig 3, B), and, unfortunately, dapsone triggered severe hemolytic anemia and dapsone hypersensitivity syndrome. Symptoms of dapsone hypersensitivity syndrome included maculopapular exanthema, fever, extremely high eosinophilia (78.7% with an absolute count of 29.4 × 109/L), and pulmonary involvement and required immediate treatment termination, which followed by a rapid recovery.
Fig 3.
Patient with infantile bullous pemphigoid following administration of different treatments. A, Extensive skin involvement with tense blisters following the administration of intravenous methylprednisolone in combination with IVIG. B, Partial response after a combination therapy of methylprednisolone and dapsone at the baseline of initiation of cyclosporine treatment. C, Four weeks after the start of cyclosporine as a monotherapy, blister formation ceased, and the majority of the lesions showed resolution. D, Complete response after three months of cyclosporine therapy, with only residual postinflammatory hypopigmentation.
Subsequently, methylprednisolone was also stopped because of life threatening Staphylococcus aureus sepsis that necessitated admission to the intensive care unit. This occurred three days after discontinuing dapsone therapy, while the child was recovering from the hypersensitivity syndrome. Between the sepsis and the dapsone hypersensitivity syndrome, the patient did not suffer from fever. Ten days afterwards, the patient returned to the Pediatric Dermatology Department. Then, because of the recalcitrant and refractory course of the disease and the inefficiency of the steroid therapy, modified cyclosporine syrup was administered at five months of age.
The standard therapeutic drug level for cyclosporine in the blood is 100 to 350 ng/mL.7 The cyclosporine dosage was increased gradually from 1.5 to 9 mg/kg/day until reaching a therapeutic drug level of 115 ng/mL. At the beginning, the drug level in the blood was controlled every 3 to 4 days, then weekly, and the last two measurements were done with one-month interval. During this whole period, the dose did not exceed the therapeutic drug level, and the maximal cyclosporine level in the blood was 146 ng/mL.
With the cyclosporine treatment, the infant experienced remarkable improvement within a few weeks (Fig 3, C), and complete resolution occurred after 1.5 months after reaching therapeutic drug level. At the age of eight months, after 1.5 months of remission and a total of three months of cyclosporine therapy (Fig 3, D), we began to decrease the cyclosporine dosage. Cyclosporine was tapered gradually, during a period of one month, until complete cessation of therapy was achieved at nine months of age. After we began tapering the cyclosporine dosage, measurements of cyclosporine levels were not preformed.
During the cyclosporine therapy, we regularly monitored complete blood cell count, electrolytes, magnesium, kidney and liver function, lipid profile, and blood pressure. All the measurements were unremarkable, except for a slight elevation in cholesterol and triglycerides, which were normalized after treatment cessation.
Following the resolution of the rash, small milia and residual hyperpigmentation developed. Subsequently, the milia resolved, and the hyperpigmentation continues to improve and is presently barely noticeable. Cyclosporine syrup therapy was well tolerated and did not result in any significant adverse events. The patient is currently under close follow-up without signs of recurrence.
Discussion
IBP is usually a benign disease that typically responds to corticosteroids and has a favorable prognosis.1 Recalcitrant cases of IBP were described to benefit from dapsone and IVIG, and the use of rituximab was also reported.5,6,8
Cyclosporine was shown to be effective in steroid-resistant BP in adults.9 However, there is no available information about cyclosporine as a monotherapy for IBP. To date, cyclosporine was reported only once in the literature for the treatment of IBP, but as a combination therapy with IVIG.10
Cyclosporine is used for dermatologic disorders, typically with a dosage of 3 to 6 mg/kg/day. In our case, the patient responded partially to 6 mg/kg/day, as the medication did not reach the therapeutic drug level. Thus, we consulted the hematology and the nephrology departments, both of which concurred to increase the dosage to 9 mg/kg/day. With this dose, the therapeutic drug level was reached, and a significant improvement in the condition of the patient was observed. We recommend dose selection of cyclosporine based on careful continuous monitoring of the drug level in the blood. In addition, we suggest regular measurements of complete blood cell counts, electrolytes, magnesium, kidney and liver function, lipid profile, and blood pressure, until cyclosporine tapering.
In conclusion, our case demonstrated that cyclosporine is an additional treatment option for recalcitrant IBP. We believe this low-cost, relatively accessible, easily administered medication with a favorable safety profile should be considered in therapy-resistant IBP cases.
Conflicts of interest
None disclosed.
Footnotes
Funding sources: None.
IRB approval status: Not applicable.
References
- 1.Pérez-Feal P., Pita da Veiga G., Sánchez-Aguilar D., Aliste C., Vázquez-Veiga H., Vázquez-Osorio I. Infantile bullous pemphigoid after meningococcal B vaccine. Int J Dermatol. 2021;60(9):1172–1173. doi: 10.1111/ijd.15440. [DOI] [PubMed] [Google Scholar]
- 2.de la Fuente S., Hernández-Martín Á., de Lucas R., et al. Postvaccination bullous pemphigoid in infancy: report of three new cases and literature review. Pediatr Dermatol. 2013;30(6):741–744. doi: 10.1111/pde.12231. [DOI] [PubMed] [Google Scholar]
- 3.Baykal C., Okan G., Sarica R. Childhood bullous pemphigoid developed after the first vaccination. J Am Acad Dermatol. 2001;44(2 Suppl):348–350. doi: 10.1067/mjd.2001.103034. [DOI] [PubMed] [Google Scholar]
- 4.Baroero L., Coppo P., Bertolino L., Maccario S., Savino F. Three case reports of post immunization and post viral bullous pemphigoid: looking for the right trigger. BMC Pediatr. 2017;17(1):60. doi: 10.1186/s12887-017-0813-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomsen K., Deleuran M., Vestergaard C., Holm M., Riber-Hansen R., Bech R. Severe infantile bullous pemphigoid treated with dapsone after bridging with systemic glucocorticoid. Case Rep Dermatol. 2019;11(2):187–193. doi: 10.1159/000501359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tekin B., Yücelten A.D. Infantile bullous pemphigoid treated using intravenous immunoglobulin: case report and review of the literature. Pediatr Dermatol. 2015;32(5):723–726. doi: 10.1111/pde.12635. [DOI] [PubMed] [Google Scholar]
- 7.Karaalp A., Demir D., Gören M.Z., et al. Therapeutic drug monitoring of immunosuppressant drugs in Marmara University Hospital. Ther Drug Monit. 2004;26(3):263–266. doi: 10.1097/00007691-200406000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Schulze J., Bader P., Henke U., Rose M.A., Zielen S. Severe bullous pemphigoid in an infant--successful treatment with rituximab. Pediatr Dermatol. 2008;25(4):462–465. doi: 10.1111/j.1525-1470.2008.00751.x. [DOI] [PubMed] [Google Scholar]
- 9.Curley R.K., Holden C.A. Steroid-resistant bullous pemphigoid treated with cyclosporin A. Clin Exp Dermatol. 1991;16(1):68–69. doi: 10.1111/j.1365-2230.1991.tb00304.x. [DOI] [PubMed] [Google Scholar]
- 10.Okada K., Kakeda M., Yamamoto S., et al. Infantile bullous pemphigoid successfully treated with i.v. immunoglobulin and cyclosporin. J Dermatol. 2019;46(6):e213–e214. doi: 10.1111/1346-8138.14726. [DOI] [PubMed] [Google Scholar]