Abstract
Current proposed method allows for the determination of manganese in serum sample using aqueous standard calibration technique on Graphite Furnace atomic absorption spectrophotometer (GFAAS) using deuterium background correction. This method involves determination of manganese from digested serum samples without the use of matrix modifier. Pyrolysis and atomization temperatures are 1200 °C and 2200 °C respectively. The limit of detection (LoD) and limit of quantitation (LoQ) of the method are 0.0097 ng/ml (0.18 nmol/l) and 0.032 ng/ml (0.58 nmol/l) respectively. Validation of the method was carried out using seronorm trace element level-1 serum standard with excellent agreement between measured value and certified value. Accuracy was demonstrated by the spike and recovery study with analytical recovery between 98.8 and 100.6% in serum. The serum reference value for manganese in adolescent girls of rural Konkan region of India range from 4.7 to 215 nmol/l.
Keywords: GFAAS, Manganese, Trace elements, Manganese reference range, Adolescents
Introduction
Transition metals play a vital role in the biochemical processes in the body; they are structural motifs of proteins and co-factors in enzymes. Transition metals exist in different oxidation states which, is important for their function as co-factor in enzymes catalyzing redox reactions. However, in high doses these elements could cause acute and chronic toxicity in humans [1–3].
Manganese is an essential micronutrient naturally present in food and water. Foods sources such as nuts, beans and legumes, oat meal, brown rice, oysters etc. are rich in manganese. It is an essential component of enzymes superoxide dismutase and glucotransferase. In humans, mitochondrial manganese superoxide dismutase (MnSOD) protects the organelle from damage due to oxidative stress [4]. Manganese is a preferred cofactor of glucotransferase; an enzyme catalyzing the synthesis of mucopolysaccharide required for the formation of cartilage and bone. It also helps to activate other enzymes with regulatory functions such as decarboxylase and hydrolase [5–7]. Manganese deficiency may cause poor reproductive performance, growth retardation, congenital malformations in off springs, abnormal function of bone and cartilage and impaired glucose tolerance [8–10]. Acute toxicity of manganese due to inhalation of manganese dust leads to inflammatory response in the lungs. Accumulation of manganese in the body over several years due to high level of occupational or environmental exposure or can lead to impaired cognitive performance and permanent neurological syndrome known as manganism [11].
Development of reliable methods to quantitate manganese in biospecimens is necessary to access nutritional deficiency and for the biomonitoring of environmental exposure. Several techniques have been reported for the determination of manganese in biological sample and other matrices such as ICP-MS [12], HPLC–MS [13], ICP-OES [14], neutron activation analysis [15], cathodic stripping voltammetry [16]. Atomic Absorption spectrophotometry offers sensitivity, specificity and reduced sample handling requirements hence is widely used for the determination of heavy metals in water, pharmaceutical, chemical, and biological samples [17, 18]. Biological samples are complex; in addition to the analyte, they have interfering substances that can impact the sensitivity of the method. Therefore, additional sample preparation techniques may be required to minimize the matrix effect special procedures are required to follow the analysis.
Considering the significance of manganese for health it is imperative to have an accurate method of estimation that is cost effective for laboratories that cannot invest in expensive techniques like ICP-MS. With this background we have designed a method to estimate manganese in serum using GFAAS without the use of matrix modifier. The proposed method was applied for the estimation of manganese in serum samples obtained from healthy adolescent girls of the Konkan region of India.
Experimental
Instrumentation
The measurements were carried out using Shimadzu graphite furnace atomic absorption spectrometer (GFA-6880) equipped with deuterium background correction and a Shimadzu model (ASC-6880) graphite tube atomizer with auto sampler. The manganese hollow cathode lamp was operated at a current of 10 mA. The most sensitive wavelength of manganese at 279.5 nm was used with a bandwidth of 0.2 nm. Pyrolytically coated graphite tubes from SCP Science, Canada was used for the analysis. High purity (99.99%) argon (Ar) was used as a carrier gas and gas flow was interrupted during atomization.
Chemicals and Materials
Palladium Nitrate trace element grade was obtained from Sigma Aldrich, USA; Magnesium Nitrate trace element grade from Sigma Aldrich, USA; Nickel Nitrate from Thermo fisher Scientific India Pvt. Ltd.; (NH4)H2PO4 from Merck life sciences Pvt. Ltd, Mumbai; triton X-100 was from Sigma Aldrich, USA and concentrated Nitric Acid Trace element grade from Honeywell, USA. Polypropylene microcentrifuge tubes (1.5 ml) and 5.0 ml polypropylene centrifuge tubes were obtained from Tarsons Products Pvt. Ltd, India. Stock manganese standard solution (1000 ppm) from Merck Germany and standard reference material (SRM) Seronorm trace element serum (Level-I; Lot-1801802) was obtained from LGC standards Ltd. Solutions were prepared in deionized water (DI) with a specific resistivity of 18.2 MΩ/cm (at 25 °C) procured from Loba Chemie Pvt. Ltd., India.
Sample Collection and Storage
The fasting blood samples of healthy adolescent girls of 16–18 years of age with no history of illness, physical or intellectual disability or genetic disorders, from Konkan region of India were collected as a part of the longitudinal cohort study. The subjects were recruited from the villages of the Ratnagiri district of Maharashtra in India. Subjects were screened based on inclusion criteria as described in the published study protocol [19]. The study was approved by the institutional ethics committee of BKL Walawalkar Hospital and informed consent/assent was taken from the subjects prior to the sample collection.
Fasting blood samples were collected in blue capped 10.0 ml trace element grade BD vacutainer tubes spray dried with K2EDTA (Becton Dickinson and Company, USA). The tubes were centrifuged at 3000 rpm for 15 min. After the centrifugation, 500 μl aliquots of serum samples were collected in cryovials (Corning life sciences, China) and stored in a deep freezer (Thermo Fisher Scientific) at -80 °C.
Serum internal control (IC) was prepared by pooling 30 ml serum collected from healthy laboratory volunteers. Collection of blood samples from volunteers and preparation of serum was performed according to the protocol mentioned above. Three hundred microliter aliquots of pooled serum were kept in 1.5 ml polypropylene microtubes at − 80 °C until further use. One aliquot of internal control was analyzed along with the SRM with each batch of samples.
Sample Preparation
In order to minimize contamination and leaching, polypropylene tubes and beakers were soaked in 1% HNO3 for 24 h, washed with DI water several times and dried. Several dilutions and concentration of reagents were tested before we adopted a sample processing procedure. Optimized parameters are given in Table 1. The serum/SRM/IC sample (200 µl) was taken in 1.5 ml polypropylene microcentrifuge tube and diluted with 200 µl of 10% HNO3. The tube was vortexed for 1 min to break the protein lumps and then digested at 90–95 °C for 30 min. After digestion, the tube was cooled to room temperature and again vortexed for 1 min. It was centrifuged at 3000 rpm for 10 min to separate the supernatant and solid residue. After centrifugation, 100 µl of the supernatant was collected in a microcentrifuge tube and diluted five-fold with matrix modifier or 1% (v/v) HNO3 solution. The final dilution of sample was tenfold and 20 µl of this sample was injected in the graphite tube for analysis. The working standard and standard dilutions were prepared daily by stepwise dilution of the manganese stock solution using 1.0% (v/v) HNO3.
Table 1.
Optimized program for manganese determination in serum samples
| S.N | Temperature (°C) | Time (s) | Heat mode | Sensitivity | Gas type | Gas flow rate (L/min) | Stage |
|---|---|---|---|---|---|---|---|
| 1 | 60 | 3 | RAMP | – | Ar | 0.10 | Drying |
| 2 | 120 | 20 | RAMP | – | Ar | 0.10 | |
| 3 | 250 | 10 | RAMP | – | Ar | 0.10 | |
| 4 | 1000 | 7 | RAMP | – | Ar | 1.0 | Pyrolysis |
| 5 | 1000 | 10 | STEP | – | Ar | 1.0 | |
| 6 | 1200 | 1 | STEP | – | Ar | 1.0 | |
| 7 | 1200 | 2 | STEP | READ | Ar | 0.0 | |
| 8 | 2200 | 3 | STEP | READ | Ar | 0.0 | Atomization |
| 9 | 2500 | 2 | STEP | – | Ar | 1.0 | Cleaning |
Data Analysis
Data analysis was performed by IBM® SPSS® Statistics (ver. 25) software. A value of p < 0.05 was considered statistically significant.
Results and Discussion
Temperature Program
We started our study with manufacturer recommended temperature program. However, we observed higher background noise at lower pyrolysis temperature (Fig. 1a, b). In this work pyrolysis and atomization temperatures were optimized for Mn analysis. Significant reduction in background noise was observed as the pyrolysis temperature was increased from 800 to 1200 °C (Fig. 1c). However, at pyrolysis temperatures higher than 1200 °C we observed loss of analyte in addition to lower background noise (Fig. 2a). The optimal pyrolysis and atomization temperatures were 1200 °C and 2200 °C respectively (Fig. 2a, b). We adopted this optimized temperature program for further analysis (Table 1).
Fig. 1.
Signal profile obtained for Mn in IC at an atomization temperature of 2200 °C. a Sample and background peak obtained for undigested IC at a pyrolysis temperature of 800 °C, b sample and background peak for digested IC by keeping the pyrolysis temperature at 800 °C; c sample peak obtained for digested IC by keeping pyrolysis temperature 1200 °C
Fig. 2.
a Impact of pyrolysis temperature on the background and sample (IC) signal intensity at atomization temperature of 2200 °C, b impact of atomization temperature on sample (0.125 ppb standard) absorbance
Effect of Matrix Modifier
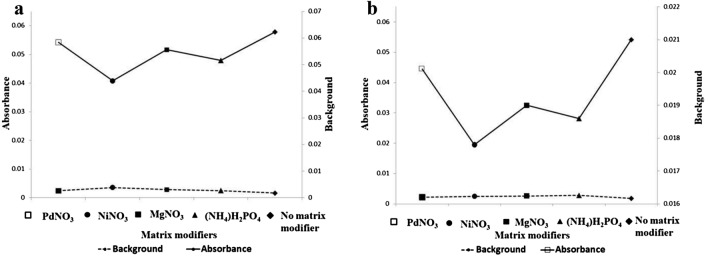
Matrix modifiers are frequently used in GFAAS analysis to improve the thermal stability of analyte in the presence of matrix components. In case of complex matrix such as serum, higher pyrolysis temperature and use of suitable matrix modifiers enables complete volatilization of matrix components. PdNO3, MgNO3, (NH4)H2HPO4, NiNO3 are frequently used as matrix modifiers during the analysis of biological samples by GF-AAS. A method of measuring manganese in serum by GF-AAS was developed with tungsten as a permanent modifier. In this study several experiments were carried out to select a suitable modifier [20]. Diaz et al. determined serum manganese in Canarian population by GF-AAS using Mg(NO3)2 as a matrix modifier [21].
In this study, digested/undigested samples were diluted with the solution containing 0.1% (w/v) of matrix modifier in 1% HNO3. Significant signal suppression was observed in the presence of MgNO3 and (NH4)2HPO4 as compared to sample without any matrix modifier (Fig. 3a, b). The RSD value without addition of matrix modifier was 0.6 (n = 10). Hence, we decided to determine manganese in serum without addition of any matrix modifiers.
Fig. 3.
a Shows the absorbance of 0.4 ppb Mn std. using PdNO3, MgNO3, (NH4)H2PO4 and without matrix modifier at an atomization temperature of 2200 °C, b shows the absorbance of IC using PdNO3, MgNO3, (NH4)H2PO4 and without Matrix Modifier
Effect of Digestion of Serum Sample
Direct analysis of serum sample diluted 1:10 with 0.1% HNO3 resulted in problems with sample injection, loss of manganese during the pyrolysis step and high background noise and signal overcorrection. Surfactant Triton X-100 in concentration ranging from 0.001 to 0.5% was used for the analysis of undigested samples. Surfactant helped with the sample injection however, we still observed high background. The analysis of digested sample containing Triton X-100 in the diluent indicated slight loss of signal intensity during the atomization stage. Digested samples diluted fivefold without the addition of triton X-100 did not present any problem with sample injection.
Linearity and Selectivity
The calibration curve was prepared by using aqueous standard solutions from 1000 ppm Mn stock solution from 0 to 4.0 ppb. The coefficient correlation factor (r) obtained corresponding to linear regression equation was 0.9998. Coefficient correlation factor greater than 0.995 for Mn indicate an excellent and precise linear relationship between concentration and peak height in our study [22].
Selectivity is the ability of a method to accurately quantify the analyte in presence of interference, under standard conditions of assay for the sample matrix under study. To access the selectivity of this method, matrix matching calibration curve (0 – 1.0 ppb) was prepared within the linearity range obtained for aqueous standard by spiking lyophilized serum in the aqueous standard solutions (Table 2). There was no statistical difference (p > 0.09) between the two slopes by two tailed students t-distribution test indicating no matrix interference. Therefore, aqueous calibration standards (0 – 1.0 ppb) were used for analysis of serum samples.
Table 2.
Agreement between aqueous linearity and lyophilized serum spiked linearity
| Analyte | Aqueous calibration range (ppb) | Slope | Correlation coefficient (r) | Lyophilized serum spiked calibration range (ppb) | Slope | Correlation coefficient (r) |
|---|---|---|---|---|---|---|
| Mn | 0–1.0 | 0.1642 | 0.9998 | 0–1.0 | 0.189 | 0.997 |
Determination of LoD and LoQ
The method and instrument performance were assessed by calculating the limit of detection (LoD) and limit of quantitation (LoQ). Instrument LoD was calculated as the three times the standard deviation of the mean of 21 blank readings while instrument LoQ was measured as six times of the standard deviation of the mean of 21 blank readings [23]. The LoD and LoQ for serum samples (Table 3) were calculated by multiplying each instrument LoD and LoQ with a factor of 10.
Table 3.
Agreement between certified SRM value and mean of measured SRM value and mean value of measured IC
| Seronorm External Reference Material: Mean value for Mn obtained on ICP-SFMS (Lot No. 1801802) | 9.4 ppb |
| Laboratory Mean of Measured Seronorm External Reference material on GF-AAS (n = 10) |
9.4968 ppb SD = 0.163638 |
| Mean of Measured IC (n = 32) |
2.208728 ppb SD = 0.624536 |
| Method LoD | 0.0097 ng/ml (0.18 nmol/l) |
| Method LoQ | 0.032 ng/ml (0.59 nmol/l) |
Accuracy and Precision
Accuracy is be defined as the closeness of agreement between true value and measured value. For determination of accuracy of the method we used serum reference standard material from seronorm (Level-1, Lot-1801802). The mean laboratory measured value of SRM was in agreement with the certified value assigned by seronorm. The agreement between true value and measured value is given in the Table 3.
Spiked Percentage Recovery Study
To study the spiked percentage recovery, 200 µl samples and 200 μl working standards (0.4, 1.2, 4.0 ppb) were diluted to 1000 µl using 1% HNO3 (n = 6). Percentage recoveries (%R) obtained for the spiked samples as shown in the Table 4.
Table 4.
Percentage recovery obtained using IC spiked with known standards
| Spike + sample | %R | %RSD |
|---|---|---|
| 0.4 ppb + IC | 98.8 | 2.0 |
| 1.2 ppb + IC | 100.6 | 3.0 |
| 4.0 ppb + IC | 98.0 | 2.1 |
Analytical Application of the Method: Reference Interval for Adolescent Girls
The method was applied for the determination of manganese in serum samples obtained from 584 healthy adolescent girls in the age group of 16–18 years residing in three distinct administrative regions (Taluka) of the Ratnagiri district of Maharashtra, India. These three regions of the Ratnagiri district differ in their dietary diversity and socioeconomic conditions; Sangameshwar is tribal, Chiplun is semi-urban and Guhagar is a coastal region. Table 5 shows the distribution of serum manganese concentration. The serum manganese concentration demonstrated a wide range (4.7–215 nmol/l) in adolescent girls. Table 6 shows the serum manganese results grouped into talukas. Manganese results between Chiplun-Sangameshwar and Chiplun-Guhagar differed significantly (p < 0.05). These differences could be attributed to dietary diversity and manganese concentration of the soil of the respective region. A detailed analysis of the dietary diversity and trace element levels in biological fluids will be presented in subsequent publications. To our knowledge very few plasma or serum reference ranges for manganese in adolescents are available in the literature. Our mean manganese serum value (51.97 nmol/l) is higher than the mean value reported for healthy adolescents (21.84 nmol/l) from Spain [21]. Kruse-Jarres, JD et al. reported manganese plasma reference value of 17.8 ± 5.0 nmol/l for adolescents obtained from a smaller subset (n = 17) of 205 cases [24]. In a data analysis report of National Health and Nutrition Examination Survey (NHANES) 2011–2012; a cross-sectional survey of US population, Yao et al. reported a mean blood manganese concentration of 195.6 nmol/l in female adolescents [25].
Table 5.
Serum manganese levels of adolescent girls in three talukas of Ratnagiri district
| Taluka | Samples (N) | Range (nmol/l) | Mean (nmol/l) |
Median (nmol/l) |
|---|---|---|---|---|
| Chiplun | 235 | 4.66–178.7 | 45.32 | 37.6 |
| Sangameshwar | 113 | 14.4–215.0 | 55.15 | 51.2 |
| Guhagar | 236 | 6.6–175.2 | 55.87 | 47.7 |
| Reference value for the entire group | 584 | 4.66–215 | 51.97 ± 1.87 | 44.30 |
Table 6.
Correlations obtained between Sangameshwar, Chiplun, Guhagar talukas
| Taluka | P value |
|---|---|
| Chiplun-Sangameshwar | 0.024 |
| Chiplun-Guhagar | 0.001 |
| Sangameshwar-Guhagar | 1.00 |
Conclusion
A method of total serum manganese estimation by GF-AAS was optimized. This method works without the use of matrix modifier with optimal pyrolysis temperature of 1200 °C and atomization temperature of 2200 °C using deuterium background correction. The method is simple and robust with good accuracy, precision and recovery over a wide range. The method LOQ was low enough for accurate analysis of serum manganese and comparable to ICP-DRC-MS and ICP-MS [12, 26]. This method was applied for the estimation of serum manganese in 584 serum samples obtained from healthy adolescent girls residing in three distinct geographical regions of the Ratnagiri district of India. To the best of our knowledge this is the first time that normal reference range for serum manganese has been presented for adolescent girls in India. We hope that present analytical method and the established reference range for manganese in adolescents will be useful to clinicians and researchers alike.
Acknowledgements
Authors would like to thank Mr. Charudatta Joglekar, Mr. Dnyaneshwar Jadhav and Mr. Omkar Dervankar for their assistance in statistical analysis.
Authors' Contribution
SC did the experimental work, data analysis and preparation of manuscript. RB supervised the experimental work, data analysis, manuscript preparation and submission. AN performed initial experimental work. PB, SRR, RRM performed sample collection and processing. SP the principal investigator of the Dervan Cohort project under which this work was done, developed the concept, manuscript preparation, and submission.
Funding
Financial support from Rajiv Gandhi Science and Technology Commission (RGSTc), Mumbai is greatly acknowledged. Award No. RGSTC/File-2018/DPP-195/CR-38.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research Involving Human Participants—Ethical Approval
The study was approved by the institutional ethics committee of BKL Walawalkar Hospital Diagnostic and Research Center Sawarde, Maharashtra. (Registration code: EC/755/INST/MH/2015/RR-18). All procedures performed in this study involving human participants were under the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Written informed consent was obtained from those who were 18 years old. For those below 18 years of age, written informed consent was obtained from parents of the adolescent girl and written informed assent was obtained from the adolescent girl.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Avila DS, Puntel RL, Aschner M. Manganese in health and disease. Met Ions Life Sci. 2013;13:199–227. doi: 10.1007/978-94-007-7500-8_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aschner M, Guilarte TR, Schneider JS, Zheng W. Manganese: recent advances in understanding its transport and neurotoxicity. Toxicol Appl Pharmacol. 2007;221:131–147. doi: 10.1016/j.taap.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erikson KM, Aschner M. Manganese neurotoxicity and glutamate-GABA interaction. Neurochem Int. 2003;43:475–480. doi: 10.1016/S0197-0186(03)00037-8. [DOI] [PubMed] [Google Scholar]
- 4.Perry JJP, Hearn AS, Cabelli DE, Nick HS, Tainer JA, Silverman DN. Human Manganese Superoxide Dismutase Tyrosine 34 Contribution to Structure and Catalysis. Biochemistry. 2009;48:3417–3424. doi: 10.1021/bi8023288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wedler FC. Biochemical and nutritional role of manganese: an overview. In: Klimis-Tavantzis DJ, editor. Manganese in health and disease. CRC Press: CRC Press; 1994. pp. 1–38. [Google Scholar]
- 6.Prohaska JR. Functions of trace elements in brain metabolism. Physiol Rev. 1987;67(3):858–901. doi: 10.1152/physrev.1987.67.3.858. [DOI] [PubMed] [Google Scholar]
- 7.Hazell AS, Butterworth RF. Hepatic encephalopathy: An update of pathophysiologic mechanisms. Proc Soc Exp Biol Med. 1999;222:99–112. doi: 10.1046/j.1525-1373.1999.d01-120.x. [DOI] [PubMed] [Google Scholar]
- 8.Goldhaber SB. Trace element risk assessment: essentiality vs. toxicity. Regul Toxicol Pharmacol. 2003;38:232–242. doi: 10.1016/S0273-2300(02)00020-X. [DOI] [PubMed] [Google Scholar]
- 9.Yang TJ, Perry PJ, Ciani S, Pandian S, Schmidt W. Manganese deficiency alters the patterning and development of root hairs in Arabidopsis. J Exp Bot. 2008;59(12):3453–3464. doi: 10.1093/jxb/ern195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li L, Yang X. The essential element manganese, oxidative stress, and metabolic diseases: links and interactions. Oxid Med Cell Longev. 2018 doi: 10.1155/2018/7580707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.ATSDR. Toxicological profile for Manganese. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service. 2012. https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=102&tid=23. Accessed 16 May 2021.
- 12.Richardson C, Roberts E, Nelms S, Roberts NB. Optimisation of whole blood and plasma manganese assay by ICP-MS without use of a collision cell. Clin Chem Lab Med. 2011;50:317–323. doi: 10.1515/CCLM.2011.775. [DOI] [PubMed] [Google Scholar]
- 13.Grygo-Szymanko E, Tobiasz A, Miliszkiewicz N, Dudek-Adamska D, Walas S. Evaluation of manganese (II) and manganese (VII) speciation in water samples by ion pair high-performance liquid chromatography-inductively coupled plasma mass spectrometry. Anal Lett. 2017;50:2147–2160. doi: 10.1080/00032719.2016.1267185. [DOI] [Google Scholar]
- 14.Oleszczuk N, Castro JT, da Silva MM, Korn M, Welz B, Vale MG. Method development for the determination of manganese, cobalt and copper in green coffee comparing direct solid sampling electrothermal atomic absorption spectrometry and inductively coupled plasma optical emission spectrometry. Talanta. 2007;73:862–869. doi: 10.1016/j.talanta.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Moav B. The determination of manganese in urine by neutron activation analysis. Int J Appl Radiat Isot. 1965;16:365–369. doi: 10.1016/0020-708X(65)90020-7. [DOI] [PubMed] [Google Scholar]
- 16.Kang W, Rusinek C, Bange A, Haynes E, Heineman WR, Papautsky I. Determination of manganese by cathodic stripping voltammetry on a microfabricated platinum thin-film electrode. Electroanalysis. 2017;29(3):686–695. doi: 10.1002/elan.201600679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nowka R, Marr I, Ansari T, Müller H. Direct analysis of solid samples by GFAAS—determination of trace heavy metals in barytes. Fresenius J Anal Chem. 1999;364:533–540. doi: 10.1007/s002160051381. [DOI] [Google Scholar]
- 18.Zhong WS, Ren T, Zhao LJ. Determination of Pb (Lead), Cd (Cadmium), Cr (Chromium), Cu (Copper), and Ni (Nickel) in Chinese tea with high-resolution continuum source graphite furnace atomic absorption spectrometry. J Food Drug Anal. 2016;24(1):46–55. doi: 10.1016/j.jfda.2015.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patil S, Patil N, Joglekar C, Yadav A, Nilawar A, Banavali U, et al. aDolescent and prEconception health peRspectiVe of Adult Non-communicable diseases (DERVAN): protocol for rural prospective adolescent girls cohort study in Ratnagiri district of Konkan region of India (DERVAN-1) BMJ Open. 2020;10:e035926. doi: 10.1136/bmjopen-2019-035926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fabrino HJ, Silveira JN, Neto WB, Goes AM, Beinner MA, da Silva JB. Multivariate approach in the optimization procedures for the direct determination of manganese in serum samples by graphite furnace atomic absorption spectrometry. J Anal Toxicol. 2011;35(8):571–576. doi: 10.1093/anatox/35.8.571. [DOI] [PubMed] [Google Scholar]
- 21.Díaz C, López F, Henríquez P, Rodríguez E, Serra-MaJem L. Serum manganese concentrations in a representative sample of the Canarian population. Biol Trace Elem Res. 2001;80:43–51. doi: 10.1385/BTER:80:1:43. [DOI] [PubMed] [Google Scholar]
- 22.Ellison SLR, Williams A. Eurachem/CITAC Guide CG 4 Quantifying Uncertainty in Analytical Measurement. 2012. https://www.eurachem.org/index.php/publications/guides/quam. Accessed 19 Mar 2021.
- 23.European Commission Regulation (EC) No. 333/2007 of 28 March 2007 laying down the methods of sampling and analysis for the official control of the levels of lead, cadmium, mercury, inorganic tin, 3-MCPD and benzo(a)pyrene in foodstuffs. 2007. https://eurlex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2007:088:0029:0038:EN:PDF. Accessed 19 May 2021.
- 24.Rükgauer M, Klein J, Kruse-Jarres JD. Reference values for the trace elements copper, manganese, selenium, and zinc in the serum/plasma of children, adolescents, and adults. J Trace Element Med Biol. 1997;11:92–98. doi: 10.1016/S0946-672X(97)80032-6. [DOI] [PubMed] [Google Scholar]
- 25.Yao Q, Zhou G, Xu M, Dai J, Qian Z, Cai Z, et al. Blood metal levels and serum testosterone concentrations in male and female children and adolescents: NHANES 2011–2012. PLoS ONE. 2019;14:e0224892. doi: 10.1371/journal.pone.0224892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D’Ilio S, Violante N, Caimi S, Di Gregorio M, Petrucci F, Senofonte O. Determination of trace elements in serum by dynamic reaction cell inductively coupled plasma mass spectrometry: Developing of a method with a desolvating system nebulizer. Anal Chim Acta. 2006;573–574:432–438. doi: 10.1016/j.aca.2006.05.010. [DOI] [PubMed] [Google Scholar]