Abstract
Background
Current evidence on obstetric patients requiring advanced ventilatory support and impact of delivery on ventilatory parameters is retrospective, scarce, and controversial.
Research Question
What are the ventilatory parameters for obstetric patients with COVID-19 and how does delivery impact them? What are the risk factors for invasive mechanical ventilation (IMV) and for maternal, fetal, and neonatal mortality?
Study Design and Methods
Prospective, multicenter, cohort study including pregnant and postpartum patients with COVID-19 requiring advanced ventilatory support in the ICU.
Results
Ninety-one patients were admitted to 21 ICUs at 29.2 ± 4.9 weeks; 63 patients (69%) delivered in ICU. Maximal ventilatory support was as follows: IMV, 69 patients (76%); high-flow nasal cannula, 20 patients (22%); and noninvasive mechanical ventilation, 2 patients (2%). Sequential Organ Failure Assessment during the first 24 h (SOFA24) score was the only risk factor for IMV (OR, 1.97; 95% CI, 1.29-2.99; P = .001). Respiratory parameters at IMV onset for pregnant patients were: mean ± SD plateau pressure (PP), 24.3 ± 4.5 cm H2O; mean ± SD driving pressure (DP), 12.5 ± 3.3 cm H2O; median static compliance (SC), 31 mL/cm H2O (interquartile range [IQR], 26-40 mL/cm H2O); and median Pao2 to Fio2 ratio, 142 (IQR, 110-176). Respiratory parameters before (< 2 h) and after (≤ 2 h and 24 h) delivery were, respectively: mean ± SD PP, 25.6 ± 6.6 cm H2O, 24 ± 6.7 cm H2O, and 24.6 ± 5.2 cm H2O (P = .59); mean ± SD DP, 13.6 ± 4.2 cm H2O, 12.9 ± 3.9 cm H2O, and 13 ± 4.4 cm H2O (P = .69); median SC, 28 mL/cm H2O (IQR, 22.5-39 mL/cm H2O), 30 mL/cm H2O (IQR, 24.5-44 mL/cm H2O), and 30 mL/cm H2O (IQR, 24.5-44 mL/cm H2O; P = .058); and Pao2 to Fio2 ratio, 134 (IQR, 100-230), 168 (IQR, 136-185), and 192 (IQR, 132-232.5; P = .022). Reasons for induced delivery were as follows: maternal, 43 of 71 patients (60.5%); maternal and fetal, 21 of 71 patients (29.5%); and fetal, 7 of 71 patients (9.9%). Fourteen patients (22.2%) continued pregnancy after ICU discharge. Risk factors for maternal mortality were BMI (OR, 1.10; 95% CI, 1.006-1.204; P = .037) and comorbidities (OR, 4.15; 95% CI, 1.212-14.20; P = .023). Risk factors for fetal or neonatal mortality were gestational age at delivery (OR, 0.67; 95% CI, 0.52-0.86; P = .002) and SOFA24 score (OR, 1.53; 95% CI, 1.13-2.08; P = .006).
Interpretation
Contrary to expectations, pregnant patient lung mechanics were similar to those of the general population with COVID-19 in the ICU. Delivery was induced mainly for maternal reasons, but did not change ventilatory parameters other than Pao2 to Fio2 ratio. SOFA24 score was the only risk factor for IMV. Maternal mortality was associated independently with BMI and comorbidities. Risk factors for fetal and neonatal mortality were SOFA24 score and gestational age at delivery.
Key Words: critical care, delivery, mechanical ventilation, mortality, pregnancy
Graphical Abstract
FOR EDITORIAL COMMENT, SEE PAGE 473
Take-home Points.
Study Question: What are ventilatory parameters for obstetric patients with COVID-19 and how does delivery impact them?
Results: Lung mechanics of pregnant patients were similar to those of the general ICU population with COVID-19, and induced delivery did not improve ventilatory parameters associated with mortality in patients with ARDS.
Interpretation: Recommended ventilatory settings for general patients with ARDS also should be used for pregnant patients with ARDS, and delivery should not be indicated to improve maternal lung mechanics.
Evidence on obstetric patients requiring invasive mechanical ventilation (IMV) and the impact of delivery on maternal respiratory mechanics and oxygenation is scarce, controversial, and limited to retrospective studies.1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11 Before the COVID-19 pandemic, most studies included myriad causes of respiratory failure leading to IMV, either obstetric (preeclampsia, hemorrhage, and so forth) or nonobstetric (pneumonia, sepsis, and so forth), with only 10 to 71 patients included.5, 6, 7 , 10 Effects on respiratory parameters after delivery proved different in these two categories, preventing us from generalizing conclusions for specific diseases such as pneumonia. Evidence supporting use of other respiratory support, for example, high-flow nasal cannula (HFNC) or noninvasive mechanical ventilation (NIMV), in obstetric patients was even poorer and was based on case reports or series of no more than four patients.12, 13, 14, 15, 16
During the second wave of the pandemic, pregnant patients were particularly affected, presenting an increased risk of ICU admission and IMV compared with nonpregnant reproductive-age women.17 To date, few case reports and retrospective case series—primarily from high-income countries—have described obstetric patients with COVID-19 requiring IMV.1, 2, 3, 4 , 8 , 9 , 11 , 18 Evidence-based decisions supporting terminating or continuing pregnancy in patients with pneumonia requiring any kind of ventilatory support remains up for debate. Given this scenario, we launched a prospective multicenter binational (Argentina and Colombia) study including pregnant and postpartum patients with COVID-19 requiring any type of advanced ventilatory support in the ICU. Our primary objective was to describe maternal ventilatory parameters and the impact of delivery on them. Our secondary objective was to describe risk factors for IMV and maternal, fetal, and neonatal mortality.
Study Design and Methods
We followed the Strengthening the Reporting of Observational Studies in Epidemiology statement checklist for this study.19 This was a multicenter, binational (Argentina and Colombia), prospective, cohort study conducted in 20 ICUs in seven provinces in Argentina and one ICU in Colombia (16 medical-surgical ICUs and five obstetric ICUs). Pregnant or postpartum (< 42 days) patients admitted to the ICU between March 20 and December 31, 2021, because of COVID-19, confirmed by polymerase chain reaction testing of nasopharyngeal specimens requiring any type of advanced respiratory support—HFNC, NIMV, or IMV—were included.
Demographic data collected included level of education (years), comorbidities (Charlson comorbidity index score,20 Acute Physiology and Chronic Health Evaluation [APACHE] II score,21 and Sequential Organ Failure Assessment during the first 24 h [SOFA24] score22), ICU length of stay (LOS), hospital LOS, maternal mortality, and fetal or neonatal mortality (spontaneous or induced abortion, fetal death, and 28-d mortality), hypertension, and obesity.23 Obstetric history included antepartum or postpartum admission; gestational age on admission to the ICU, at delivery (weeks), or both; preterm birth (< 37 weeks) classified as late or early preterm (32-36.6 weeks and < 32 weeks, respectively)24; minimal vs standard prenatal care (minimum one vs five visits for term pregnancies)25; parity; frequency and type of fetal monitoring; route of delivery (vaginal or C-section); and reason for induced delivery, if occurred (maternal, fetal, or both). Also collected were respiratory support on admission (nasal prongs, nonrebreathing masks [NRMs], HFNC, NIMV, and IMV), type of maximum ventilatory support (HFNC, NIMV, and IMV), tracheostomy status, and prone positioning status. Pao 2 to Fio 2 ratio was registered on admission, before IMV (< 2 h), after intubation (days 0, 2, and 7) and before (< 2 h) and after (< 2 h and at 24 h) delivery. For those requiring IMV, ventilatory support before IMV, peripheral oxygen saturation before intubation, and mechanical ventilation parameters (positive-end expiratory pressure, plateau pressure [PP], driving pressure [DP], and static compliance) after intubation (days 0, 2, and 7) and before (< 2 h) and after (< 2 h and at 24 h) delivery were recorded. Neuromuscular blocker and vasopressor treatments were recorded. Treatments received included steroid type and dose (per the Randomized Evaluation of COVID-19 Therapy- RECOVERY-trial: for pregnant patients, prednisolone 40 mg or hydrocortisone 80 mg bid, and for postpartum patients, dexamethasone 6 mg/d for 10 days26), convalescent plasma, tocilizumab, and anticoagulation. ICU complications were detailed as follows: ARDS,27 preeclampsia,28 septic shock,29 acute renal failure requiring dialysis, pulmonary embolism or DVT,30 and acquired infections.
Statistical Analysis
Categorical variables are presented as number (percentage) and continuous variables are presented as mean ± SD or median (interquartile range [IQR]). Variables were compared with the Student t test, Wilcoxon test, χ 2 test, or Fisher exact test. Multiple comparisons of parametric and nonparametric variables were analyzed with repeated-measures analyses of variance and Friedman tests, respectively, whereas both post hoc analyses were carried out with Bonferroni correction. A P value of ≤ .05 was considered significant. A binary logistic regression was built to find independent predictors of IMV and maternal, fetal, and neonatal mortality. Variables with P value of ≤ .20 were included in univariate analysis. The multivariate model included variables with a P value of ≤ .05 on Wald test, confounding effects (variation coefficient, ≥ 20%), or both. The model was calibrated with the Hosmer-Lemeshow goodness-of-fit test to evaluate discrepancy between observed and expected ICU mortality and IMV. SPSS version 15 software (SPSS, Inc.) was used for analysis.
Ethical Considerations
This study was approved by the institutional review board of the Argentinian Society of Critical Care Medicine (Identifier: PRIISABA-5012). It was performed in accordance with ethical standards laid down in the 1964 Declaration of Helsinki and later amendments and with provincial laws regarding research on human beings. Patient information was de-identified before analysis. Informed written consent was waived; instead, written information regarding the study was given to patients and relatives.
Results
Population Characteristics and Outcomes
Forty-three ICUs were enrolled, but 22 were excluded for not recruiting patients. Twenty-one ICUs recruited 91 pregnant or postpartum patients, 73 patients (80%) from the public health sector and 18 patients (20%) from the private health sector. General and obstetric characteristics and differences between survivors and nonsurvivors are presented in Table 1 . Organ dysfunction was frequent and was associated with differential mortality (Fig 1 ).
Table 1.
Characteristics and Outcomes of 91 Pregnant or Postpartum Patients With COVID-19 Requiring Advanced Respiratory Support in 21 ICUs in Argentina and Colombia During 2021
| Variable | All | ICU Status |
P Value | |
|---|---|---|---|---|
| Survivors | Nonsurvivors | |||
| No. of patients | 91 | 75 (82.4) | 16 (17.6) | . . . |
| Age, y | 31 ± 6 | 31.5 ± 6 | 31.7 ± 7 | .90 |
| Level of education, y | 11 ± 3.5 | 11 ± 3.5 | 10 ± 3.6 | .43 |
| Public health sector | 73 (80.2) | 57 (76) | 16 (100) | .04 |
| No comorbiditya | 74 (81.3) | 65 (86.6) | 9 (56.25) | .005 |
| BMI | 30 ± 6 | 28.9 ± 5.7 | 33.2 ± 7.6 | .013 |
| Obesity | 36 (39.5) | 26 (34.6) | 10 (62.5) | .05 |
| Obesity class | .021b | |||
| I (30-34.9 kg/m2) | 17 (18.7) | 16 (21.3)b | 1 (6.25) | |
| II (35-39.9 kg/m2) | 15 (16.5) | 8 (10.6) | 7 (43.75) | |
| III (≥ 40 kg/m2) | 4 (4.4) | 2 (2.7) | 2 (12.5) | |
| Vaccination (one dose)c | 8/75 (10.6) | 5/62 (8) | 3/13 (23) | .28 |
| APACHE II score | 13 (7-16) | 12 (6-18) | 14 (9.5-14.5) | .31 |
| APACHE II risk, % | 14 (5.2-21) | 12.6 (5.1-23) | 15 (7.9-19.3) | .41 |
| SOFA24 scored | 4 (3-6) | 4 (3-6) | 5 (4-7) | .077 |
| LOS, d | ||||
| ICU | 15 (8-28) | 16 (8-31) | 11 (9-15) | .12 |
| Hospital | 22 (11-36) | 23 (13-37) | 12 (10-17) | .01 |
| Obstetric characteristics | ||||
| Antepartum admission | 63/91 (69.2) | 54/75 (72) | 9/16 (56.25) | .21 |
| Parity | 2 (1-3) | 2 (1-3) | 2 (1-3) | .65 |
| Minimal prenatal caree | 84 (92) | 70 (93) | 14 (87.5) | .43 |
| Standard prenatal caref | 43 (47) | 38 (55) | 5 (31) | .36 |
| Gestational age on admission, wk | 31 ± 5 | 31 ± 4 | 29.5 ± 7 | .28 |
| Gestational age at delivery, wk | 32.5 ± 3 | 32.3 ± 3 | 30.9 ± 6.5 | .24 |
| Births, wk | 74 | 61 | 13 | .79 |
| Term, ≥ 37 | 7 (9.5) | 6 (9.8) | 1 (7.7) | . . . |
| Late preterm, 32-36.6 | 40 (54) | 33 (54.1) | 7 (53.8) | . . . |
| Early preterm, < 32 | 27 (36.5) | 22 (36.1) | 5 (38.5) | . . . |
| Route of delivery | .68 | |||
| Cesarean section | 70 (96) | 56 | 13 | |
| Vaginal | 3 (4) | 0 | ||
Data are presented as No. (%), mean ± SD, or median (interquartile range), unless otherwise indicated. APACHE = Physiology and Chronic Health Evaluation; LOS = length of stay; SOFA24 = Sequential Organ Failure Assessment during the first 24 h.
Charlson comorbidity index score of 0.
P = .01 obesity class I vs class II.
No patient received a full vaccination schedule; only eight patients in total received one dose.
Within the first 24 h of ICU admission.
Minimal prenatal care: at least one visit for term pregnancies.
Standard prenatal care: at least five visits for term pregnancies.
Figure 1.
Bar graph showing organ dysfunction during the first 24 h in the ICU and its corresponding ICU mortality among 91 pregnant or postpartum patients with COVID-19.
Sixteen maternal deaths (17.5%) occurred and 14 fetal or neonatal losses (15.4%) occurred; however, only five of these deaths or losses were both maternal and fetal. BMI and presence of at least one comorbidity (Charlson comorbidity index score, ≥ 1) were the only risk factors for maternal mortality (OR, 1.10 [95% CI, 1.006-1.204; P = .037] and OR, 4.15 [95% CI, 1.212-14.20; P = .023], respectively). Of the 14 fetal or neonatal losses, two were spontaneous abortions, four fetal deaths, and eight were neonatal deaths. Of the four fetal deaths, three were nonviable fetuses (two at 23 weeks and one at 26.1 weeks), and one at 35 weeks was the fetus of a severely ill patient (APACHE II score, 22; Pao 2 to Fio 2 ratio, 87; SOFA24 score, 5). The threshold for fetal viability in Argentina is considered to be approximately 26 weeks.31 Risk factors for fetal or neonatal mortality were gestational age at delivery and SOFA24 score (Table 2 ).
Table 2.
Multivariate Analysis of Variables Associated With Fetal or Neonatal Mortality Among 91 Pregnant or Postpartum Patients Admitted to the ICU Because of COVID-19 in 21 ICUs in Argentina and Colombia
| Variable | OR (95% CI)a | P Value |
|---|---|---|
| Sequential Organ Failure Assessment during the first 24 h | 1.53 (1.13-2.08) | .006 |
| Gestational age at delivery (wks) | 0.67 (0.52-0.86) | .002 |
Hosmer and Lemeshow test significance, 0.82.
Respiratory Support, Ventilatory Parameters, and Impact of Delivery
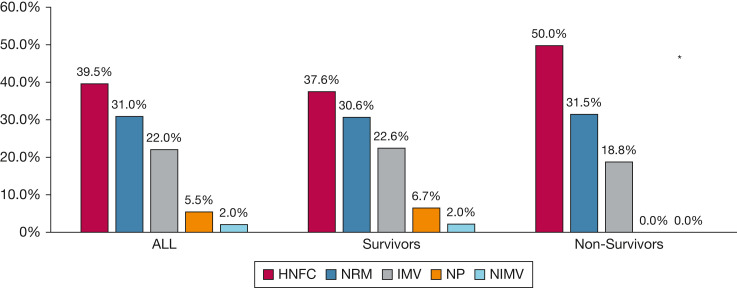
Respiratory support on admission and differences between survivors and nonsurvivors is illustrated in Figure 2 . Of the 36 patients admitted receiving HFNC treatment, 24 patients (66.6%) required IMV, and of the 28 patients admitted receiving NRM, 21 patients (75 %) eventually required IMV (P = .48). Respiratory support on admission did not affect days receiving IMV or ICU LOS (Table 3 ). Among patients receiving noninvasive oxygen support on admission (n = 49), the only independent risk factor for IMV was SOFA24 score (OR, 1.97; 95% CI, 1.29-2.99; P = .001), even after adjusting for APACHE II score and BMI.
Figure 2.
Bar graph showing type of respiratory support on admission for 91 pregnant or postpartum patients with COVID-19 and differences between survivors and nonsurvivors. aP = .71 for comparison of type of respiratory support on admission between SV and NonSV groups. HFNC = high-flow nasal cannula; IMV = invasive mechanical ventilation; NIMV = noninvasive mechanical ventilation; NonSV = nonsurvivors; NP = nasal prong; NRM = nonrebreathing mask; SV = survivor.
Table 3.
No. of Days of IMV and ICU LOS According to Ventilatory Support on Admission Among Obstetric Patients With COVID-19 Requiring IMV as Maximum Ventilatory Support in 21 ICUs in Argentina and Colombia During 2021
| Variable |
Type of respiratory support on admission |
PValue | ||||
|---|---|---|---|---|---|---|
| HFNC | NRM | NP | NIMV | IMV | ||
| No. of patients | 24 | 21 | 2 | 2 | 20 | . . . |
| IMV duration, d | 10 (10-20) | 14 (5-23) | 18 (11-32) | 18 (7-28) | 21 (11-35) | .22 |
| ICULOS, d | 14 (11-27) | 18 (11-32) | 21 (18-24) | 26 (15-36) | 30 (18-43) | .07 |
Data are presented as median (interquartile range), unless otherwise indicated. HFNC = high-flow nasal cannula; IMV = invasive mechanical ventilation; LOS = length of stay; NIMV = noninvasive mechanical ventilation; NP = nasal prong; NRM = nonrebreathing mask.
Sixty-nine patients required IMV as maximum ventilatory support, 16 of whom died (23.2%). Forty-seven of these patients were pregnant at IMV onset (day 0); mechanical ventilation characteristics and differences between survivors and nonsurvivors over the first week are presented in Table 4 and Figure 3 . Delivery did not improve ventilatory parameters other than oxygenation (Table 5 , Fig 4 ).
Table 4.
Ventilatory and Oxygenation Parameters Among 47 Pregnant Patients Requiring IMV Because of COVID-19 and Differences Between Survivors and Nonsurvivors in 21 ICUs in Argentina and Colombia During 2021
| Variable | All (n = 47) | Survivors (n = 38) | Nonsurvivors (n = 9) | P Value |
|---|---|---|---|---|
| Pao2 to Fio2 ratio before IMV (< 2 h) | 121 (86.5-162.2) | 123.5 (87.7-174) | 102.5 (90-129) | .20 |
| SpO2 before IMV, % | 90 (88-94) | 91 (90-95) | 89 (88-90) | .055 |
| Pao2 to Fio2 ratio on IMV day 0 (< 2 h) | 142 (110-176) | 147.5 (120-176) | 120 (100-142) | .17 |
| Pao2 to Fio2 ratio | ||||
| Day 2 | 200 (139-232.2) | 203 (157-230) | 144 (105-237) | .31 |
| Day 7 | 183 (141.75-235.5) | 186 (168-236) | 100 (80-188) | .08 |
| PEEP, cm H2O | ||||
| Day 0 | 11.1 ± 2.7 | 11 ± 2.4 | 11.8 ± 3.8 | .45 |
| Day 2 | 11 ± 3 | 10.7 ± 2.6 | 11.8 ± 4 | .31 |
| Day 7 | 10.2 ± 2.7 | 9.9 ± 2.4 | 11.8 ± 3.5 | .10 |
| PP, cm H2O | ||||
| Day 0 | 24.3 ± 4.5 | 23.7 ± 4.5 | 26.8 ±3.9 | .062 |
| Day 2 | 23.8 ± 6.4 | 22.7 ± 5.7 | 29.2 ± 7.1 | .011 |
| Day 7 | 25.1 ± 5.9 | 23.7 ± 4.7 | 31.8 ± 7.2 | .001 |
| SC, mL/cm H2O | ||||
| Day 0 | 31 (26-40) | 32.5 (26-41) | 27 (23-38) | .48 |
| Day 2 | 33 (23.2-42) | 33 (24-42) | 27 (21-40) | .75 |
| Day 7 | 29 (23.7-38) | 30 (24-38) | 29 (21-34.5) | .83 |
| DP, cm H2O | ||||
| Day 0 | 12.5±3.3 | 12.1 ± 3.5 | 14.1 ±2.1 | .10 |
| Day 2 | 12.6 ± 4.3 | 12.1 ± 4 | 15.3 ± 4.8 | .069 |
| Day 7 | 13.8 ± 4.8 | 12.7 ± 3.7 | 19.3 ± 4.1 | .000 |
Data are presented as No. (%), mean ± SD, or median (interquartile range), unless otherwise indicated. DP = driving pressure; IMV = invasive mechanical ventilation; PEEP = positive-end expiratory pressure; PP = plateau pressure; SC = static compliance; SpO2 = peripheral oxygen saturation.
Figure 3.
A-D, Box-and-whisker plots showing mechanical ventilation and oxygenation parameters over the first week after intubation for 47 patients admitted before delivery because of COVID-19 and differences between survivors and nonsurvivors. A, Plateau pressure. B, Driving pressure. C, Static compliance. D, Pao2 to Fio2 ratio. aDifference between survivors (blue) and nonsurvivors (red) (P < .05) that were significant for plateau pressure at days 2 and 7, and driving pressure at day 7. bDifferences in Pao2 to Fio2 ratio over the first week for the entire group (gray) (P = .000): post hoc Bonferroni significant differences between day 0 vs day 2 and day 0 vs day 7, P = 000.
Table 5.
Ventilatory and Oxygenation Parameters Before (< 2 h) and After (< 2 h and 24 h) Delivery Among 47 Pregnant Patients With COVID-19 Requiring Invasive Mechanical Ventilation in 21 ICUs in Argentina and Colombia During 2021
| Variable | Before Delivery (< 2 h) | After Delivery (< 2 h) | After Delivery (24 h) | P Value |
|---|---|---|---|---|
| PP, cm H2O | 25.6 ± 6.6 | 24 ± 6.7 | 24.6 ± 5.2 | .59 |
| DP, cm H2O | 13.6 ± 4.2 | 12.9 ± 3.9 | 13 ± 4.4 | .69 |
| SC, mL/cm H2O | 28 (22.5-39) | 30 (24.5-44) | 30 (24.5-44) | .058 |
| Pao2 to Fio2 ratio | 134 (100-230) | 168 (136-185) | 192 (132-232.5) | .022a |
| PEEP, cm H2O | 11 ± 3 | 12 ± 4 | 11 ± 2 | .8 |
Data are presented as No. (%), mean ± SD, or median (interquartile range), unless otherwise indicated. DP = driving pressure; PEEP = positive-end expiratory pressure; PP = plateau pressure; SC = static compliance.
In the post hoc Bonferroni analysis, differences were observed between the subgroup before delivery and the subgroup at 24 h after delivery (P = .025).
Figure 4.
A-D, Box-and-whisker plots showing mechanical ventilation and oxygenation parameters before (< 2 h) and after (< 2 h and 24 h) delivery among 47 patients with COVID-19 admitted before delivery. A, Plateau pressure. B, Driving pressure. C, Static compliance. D, Pao2 to Fio2 ratio. Comparisons of variables before (< 2 h) and after (< 2 h and 24 h) delivery: aD, Pao2 to Fio2 ratio = P = .022 for the entire group (P = .025 between before [< 2 h] and at 24 h of delivery in the post hoc Bonferroni analysis).
Twenty patients required HFNC as maximum respiratory support, 12 of whom were admitted and continued to receive it until discharge, whereas three and five patients, respectively, required HFNC after trials of nasal prongs and NRM. Patients continuing to receive HFNC vs those requiring IMV differed in, respectively, BMI (mean ± SD, 27.2 ± 3.5 kg/m2 vs 30.2 ± 6 kg/m2; P = .010), APACHE II score (median, 7 [IQR, 5.5-12.5] vs 14 [IQR, 9-19]; P = .001), and SOFA24 score (median, 2.5 [IQR, 2-4] vs 5 [IQR, 4-7]; P = .000). No maternal, fetal, or neonatal deaths occurred among the HFNC group.
Four patients required NIMV during ICU stay: two were receiving NIMV on admission and treatment failed in both, which required IMV; two patients were admitted receiving NRM, but required NIMV as maximum respiratory support. The latter two patients received NIMV for 5 and 9 days, respectively, were admitted before delivery (at 28 and 35.3 weeks), and successfully continued their pregnancies after ICU discharge. Both showed obesity class II (BMI, 38.7 kg/m2 and 39.1 kg/m2), but no other comorbidities according to Charlson comorbidity index score. Pao 2 to Fio 2 ratio on admission was 150 and 250, and SOFA24 score was 3 for both patients.
Obstetric and Delivery Characteristics
Twenty-eight patients (31%) delivered at a mean ± SD of 34 ± 2.7 weeks and were admitted to ICU after delivery, whereas 63 patients (69%) were admitted to the ICU before delivery (at a mean ± SD of 29.2 ± 4.9 weeks) and delivered there at a mean ± SD of 31 ± 4.4 weeks (P = .002 for gestational age at delivery for patients admitted after delivery vs before delivery). Fetal monitoring in ICU consisted mainly of intermittent fetal heart rate monitoring (18/21 ICUs [86%]) every 8 to 12 h and Doppler ultrasound (15/21 [71.4%]) every 2 to 3 days; only one ICU performed continuous cardiotocography. Reasons for induced delivery were: maternal (43/71 [60.5%]), maternal and fetal (21/71 [29.5%]), and fetal (7/71 [9.9%]). Among the 63 patients admitted before delivery, 43 patients (68.3%) required delivery during ICU stay, 14 patients (22.2%) were discharged pregnant, two patients (3.2%) experienced spontaneous abortion, and fetal death occurred in four patients (6.3%). Most patients admitted before delivery who required delivery gave birth during the first 24 to 48 h of ICU admission; population median was 3 days (IQR, 1-6.75 days). For the 14 patients who continued pregnancy after ICU discharge, maximum respiratory support in the ICU was HFNC in eight patients (57%), IMV in four patients (29%), and NIMV in two patients (14%); median Pao 2 to Fio 2 ratio on admission was 157.5 (IQR, 120-240) vs 180 (IQR, 100-240) for those requiring ICU delivery (P = .9).
ICU Management and Complications
Of 69 patients requiring IMV, 64 patients (92.7%) received neuromuscular blockers, 25 patients (36.2%) required tracheostomy, and 49 patients (71%) required prone positioning, 33 patients (67%) of whom were admitted before delivery with a median of 3 proning sessions (IQR, 1-3 sessions) for a median duration of 30 h (IQR, 21-48 h) each. Twenty-six of the 49 patients (53%) requiring prone positioning received pressure-point protection. Fifteen of the 49 patients (30.6%) who underwent prone positioning demonstrated skin lesions.
In the entire population, 46 patients (50.5%) experienced some type of hospital-acquired infection, 35 patients (38.5%) showed septic shock, 13 patients (14.3%) showed acute renal failure requiring dialysis, and 15 patients (16.4%) demonstrated preeclampsia. DVT or pulmonary embolism were documented in two patients (2.2%).
All patients (n = 91) received steroids, 61 patients (67%) per RECOVERY trial types and dosages.25 However, 30 patients (33%) received nonstandardized steroids, dosages, or both. No patient received convalescent plasma or tocilizumab. Sixty-two patients (68%) received vasopressors, and 35 patients (38.5%) were treated with anticoagulants.
Discussion
To our knowledge, this is the first prospective study evaluating pregnant and postpartum patients admitted to the ICU with respiratory failure requiring advanced ventilatory support that sequentially analyzed the impact of delivery on lung mechanics and oxygenation. The use of HFNC did not prevent intubation. The only risk factor for IMV was SOFA24 score. Ventilatory parameters in pregnant patients were similar to those in general ICU populations with COVID-19. Delivery was induced mainly for maternal reasons, but did not change ventilatory parameters other than Pao 2 to Fio 2 ratio. Twenty-two percent of patients were discharged pregnant with good outcomes. Risk factors for maternal mortality were BMI and presence of comorbidities. Risk factors for fetal or neonatal mortality were SOFA24 score and gestational age at delivery.
Population Characteristics and Outcomes
Ninety-one pregnant or postpartum patients with COVID-19 requiring advanced ventilatory support in the ICU were included in this multicenter, binational, prospective cohort study. Most patients were treated in the public health sector and none were vaccinated fully; this correlates with the time frame of the study, which was performed when vaccination was not recommended for pregnant patients.18 Although in Argentina and Colombia the health-care system comprises public and private sectors, the quality of care is comparable in both sectors.32 , 33 Although most patients demonstrated no comorbidities, the presence of at least one comorbidity was associated independently with maternal mortality, as was described for the general population with COVID-19.34 , 35 Forty percent of patients presented obesity, also associated independently with increased maternal mortality. As noted in previous studies, pregnant patients with COVID-19 experience higher maternal, fetal, and neonatal mortality compared with pregnant patients without COVID-19.36 They also have higher ICU and IMV requirements compared with nonpregnant patients with COVID-19, even those with no comorbidities or obesity.37
In this study, mortality in patients requiring IMV (23.2%) was considerably lower than in the general ICU population with COVID-19 (37%-57%),34 , 35 which may be explained by younger age and recovery capacity of these patients. However in this cohort, maternal mortality numbers were between those described for obstetric patients in high-income countries (0%-8.4%)1 , 4 , 8 , 9 , 11 , 18 , 37 , 38 and upper and lower middle-income countries (78%-100%),2 , 3 which may be related to health budgets, patient characteristics such us underlying diseases, and health-care variables such as quality of care, accessibility, or system overload, among others.
Patients were severely ill on admission according to the SOFA24 score, similar to critically ill obstetric patients with other community-acquired pneumonias.39 Respiratory and cardiovascular dysfunctions were the most frequent, and their associated mortality coincided with previous reports (18% and 16%, respectively).39 However, renal dysfunction, although less frequent, was associated with the highest mortality in this group (23%) and exhibited higher mortality compared with the general critically ill obstetric population without COVID-19 (15%).39 The proportion of obstetric patients with COVID-19 requiring dialysis was 14.3%, compared with 3.3% among critically ill obstetric patients in general,33 which may explain why this particular group showed worse outcomes associated with renal dysfunction.
Respiratory Support, Ventilatory Parameters, and Impact of Delivery
The most frequently used types of ventilatory support on admission were HFNC and NRM, followed by IMV; however, neither type of respiratory support nor oxygenation parameters on admission had an impact on maternal mortality. Furthermore, use of HFNC did not prevent IMV requirement compared to use of NRM, contrary to what was reported in the last meta-analysis.40 Discrepancies between our results and those from the meta-analysis may be related to sample size issues or different populations evaluated. In our study, 20 patients (22%) were able to continue to receive HFNC as maximum respiratory support, with excellent maternal, fetal, and neonatal outcomes. These patients were less severely ill on admission and showed lower BMI than those requiring IMV. SOFA24 score was the only risk factor for IMV among patients admitted with noninvasive respiratory support. This may provide additional information to consider before intubating these patients.
Most patients required IMV as maximum respiratory support and most were pregnant when intubated. To our knowledge, this is the first prospective study describing obstetric patients with community acquired pneumonia requiring IMV and the impact of delivery on ventilatory parameters. Current evidence available is from retrospective case series of patients with respiratory failure resulting from myriad obstetric and nonobstetric causes,5, 6, 7 , 10 and limited retrospective case series of patients with COVID-19.1, 2, 3, 4 , 8 , 9 , 11 , 18 , 38 Pregnancy is characterized by decreased chest wall compliance because of a gravid uterus, commonly believed to impact PP or DP as well as positive-end expiratory pressure settings, leading some to suggest permitting, for example, higher PP (35 cm H2O).41 However, one relevant finding of our study is that pregnant patient lung mechanics were similar to those from the general ICU population with COVID-19.34 , 35 Therefore, because potential differences were not evident, we need to dig deeper for the explanation. In patients with acute lung injury, the impact of the chest wall on respiratory mechanics was described insufficiently and, excluding those with intraabdominal hypertension,42 it might have been overestimated.43 Changes in respiratory mechanics seem to be determined by abnormalities in lung vs chest wall mechanics.44 Finally, although in pregnant patients intraabdominal pressure can be higher than in nonpregnant critically ill patients, presence of intraabdominal hypertension is rare.45 These findings have clear clinical implications because they may guide health-care professionals to adjust ventilator settings for pregnant patients with nonobstetric causes of respiratory failure to those proven to reduce mortality in general ICU population, such as PP of < 30 cm H2O.46
One of the most controversial issues regarding pregnant patients with respiratory failure is the impact of delivery on maternal lung mechanics and oxygenation. To date, the evidence available comes from four retrospective case series that indicate some improvement in oxygenation after delivery, with only one describing improvement in DP.5 , 7 , 10 , 18 Tomlinson et al10 found decreased oxygen requirements after delivery in 10 patients receiving IMV (nine with pneumonia). Lapinsky et al7 described improvement in oxygenation index after delivery in three of 10 patients and static compliance in five of 10 patients, most of whom were ventilated for nonobstetric causes. Hung et al5 recorded improvement in oxygenation and peak pressure after delivery, but only in the subgroup of patients with obstetric causes of respiratory failure. Finally, Péju et al18 described improvement in oxygenation and inconsistent improvement in DP after delivery. This is the first prospective study evaluating impact of delivery on maternal lung mechanics and oxygenation. In pregnant patients with COVID-19, delivery did not change PP or DP, which are respiratory parameters strongly associated with mortality in patients with ARDS34 , 47; instead, it improved only Pao 2 to Fio 2 ratio, which is not associated independently with mortality. Our results for DP are not consistent with those of the COVIDPREG study18; however, their own limitations noted large interindividual variability. Furthermore, its retrospective nature could have increased missing data and reporting bias. In most of these patients, delivery was chosen for maternal reasons, as described by Eman et al,2 highlighting another key finding of this study: unsupported beliefs lead to the removal of the fetus under the assumption that this will automatically improve maternal parameters.5 , 6
Obstetric and Delivery Characteristics
After maternal stabilization, interrupting pregnancy for fetal reasons when fetal alterations persist is completely warranted because continuing pregnancy would not benefit the fetus. Notably, in this population, nonviable fetuses died, with only one death occurring at 35 weeks, but this was because of an extremely adverse maternal condition. Fetal and neonatal mortality was associated independently with gestational age and SOFA24 score. Risking the fetus without significantly improving maternal conditions could be eliminated with these results. In this study, most deliveries were performed within 48 h after admission, meaning that SOFA24 score was readily available and potentially might have saved fetuses. Physicians should strive for equipoise to obtain maximum benefit for both mother and child.
ICU Interventions and Complications
Evidence about prone positioning during pregnancy is scarce.11 , 18 , 48 In this study, 33 patients required prone positioning for a median of 30 h during pregnancy. Prone positioning was deemed feasible, resulting in only skin complications, which might have been related to inadequate use of pressure point protection. This information may encourage physicians to use prone positioning—so beneficial in treating patients with ARDS—in this particular group of patients as well.49
In terms of treatment, although all patients received steroids, one-third did not receive an evidenced-based formula or dose, per the RECOVERY trial.26 None received tocilizumab. In most patients receiving anticoagulants, thrombosis was not documented, underscoring common, unsatisfactory medical treatment for pregnant patients.2 , 18
Strengths and Limitations
Strengths of this study are in the number of obstetric patients included and its prospective design with consecutive enrollment. Community-acquired pneumonia requiring advanced respiratory support during pregnancy is uncommon, but the pandemic affected obstetric patients in particular, increasing the number of cases to evaluate. The prospective design allowed us to minimize missing data, to improve data quality, and to measure variables before outcomes occur, contributing to causal pathways. It also afforded us the opportunity to gather sequential data related to impact of delivery on maternal respiratory parameters, the first study ever to do so.
Limitations of this study are its observational nature, which could have introduced some bias and confounding, although the latter was controlled by regression analysis. Selection bias also might have occurred because of overrepresentation of patients treated in the public health sector. Response bias is possible because of inclusion of qualified ICUs more prone to participate in studies. Generalizability could be uncertain. Although evaluating the impact of maternal hypercapnia on fetal outcomes would have provided relevant information, we opted not to request daily Pco 2 given pandemic workload considerations. Finally, neonatal morbidity was not measured; instead, we focused entirely on fetal and neonatal mortality, because it is a nonbiased outcome.
Interpretation
Maternal mortality in patients with COVID-19 requiring advanced ventilatory support was associated independently with BMI and presence of comorbidities, which are outside the control of critical care practitioners. Pregnant patient lung mechanics and ventilator settings were similar to those in general ICU populations with COVID-19, supporting the use of evidenced-based ventilator settings proven to reduce mortality in nonobstetric patients with ARDS. Ventilatory parameters associated with mortality in ARDS patients were not improved by inducing delivery. Fetal and neonatal mortality was associated independently with gestational age at delivery and SOFA24 score. Given what we know now, the clinical practice of inducing delivery in pregnant patients with pneumonia to improve maternal conditions should be abandoned. Gestational age and SOFA24 score should be considered in the decision-making process, thereby improving both maternal and fetal outcomes, as well as considering the psychological impact of losing a child.
Funding/Support
The authors have reported to CHEST that no funding was received for this study.
Financial/Nonfinancial Disclosures
None declared.
Acknowledgments
Author contributions: D. N. V. takes responsibility for the content of the data and analysis. D. N. V. conceived and designed the study. D. N. V., A. V. D. N., M. K., M. F. V., G. A. P., and A. D. I. made substantial contributions to analysis and interpretation of data and drafting the manuscript. R. G., A. S., K. C., L. F., D. L., F. E., M. M., P. J., L. V., V. M., E. B., M. C. C., I. R., P. M., A. K. M., E. R., C. P., L. R., J. M. A. S., R. N., S. T., M. A. G., D. N., and P. O. acquired data. All authors revised the draft for intellectual content and approved the final version.
Other contributions: The authors thank all the patients who participated in this study, all the health-care workers who struggled against this disease, and Maria-Teresa Pérez, who coedited the manuscript and revised the English.
References
- 1.Douedi S., Albayati A., Alfraji N., Mazahir U., Costanzo E. Successful maternal and fetal outcomes in COVID-19 pregnant women: an institutional approach. Am J Case Rep. 2020;21 doi: 10.12659/AJCR.925513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eman A., Balaban O., Kocayigit H., Suner K.O., Cirdi Y., Erdem A.F. Maternal and neonatal outcomes of critically ill pregnant and puerperal patients diagnosed with COVID-19 disease: retrospective comparative study. J Korean Med Sci. 2021;36(44):e309. doi: 10.3346/jkms.2021.36.e309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hantoushzadeh S., Shamshirsaz A.A., Aleyasin A., et al. Maternal death due to COVID-19. Am J Obstet Gynecol. 2020;223(1):109.e1–109.e16. doi: 10.1016/j.ajog.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirshberg A., Kern-Goldberger A.R., Levine L.D., et al. Care of critically ill pregnant patients with coronavirus disease 2019: a case series. Am J Obstet Gynecol. 2020;223(2):286–290. doi: 10.1016/j.ajog.2020.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hung C.Y., Hu H.C., Chiu L.C., et al. Maternal and neonatal outcomes of respiratory failure during pregnancy. J Formos Med Assoc. 2018;117(5):413–420. doi: 10.1016/j.jfma.2017.04.023. [DOI] [PubMed] [Google Scholar]
- 6.Jenkins T.M., Troiano N.H., Graves C.R., Baird S.M., Boehm F.H. Mechanical ventilation in an obstetric population: characteristics and delivery rates. Am J Obstet Gynecol. 2003;188(2):549–552. doi: 10.1067/mob.2003.68. [DOI] [PubMed] [Google Scholar]
- 7.Lapinsky S.E., Rojas-Suarez J.A., Crozier T.M., et al. Mechanical ventilation in critically-ill pregnant women: a case series. Int J Obstet Anesth. 2015;24(4):323–328. doi: 10.1016/j.ijoa.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 8.Lucarelli E., Behn C., Lashley S., Smok D., Benito C., Oyelese Y. Mechanical ventilation in pregnancy due to COVID-19: a cohort of three cases. Am J Perinatol. 2020;37(10):1066–1069. doi: 10.1055/s-0040-1713664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polcer R.E., Jones E., Pettersson K. A case series on critically ill pregnant or newly delivered patients with Covid-19, treated at Karolinska University Hospital, Stockholm. Case Rep Obstet Gynecol. 2021;2021:8868822. doi: 10.1155/2021/8868822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomlinson M.W., Caruthers T.J., Whitty J.E., Gonik B. Does delivery improve maternal condition in the respiratory-compromised gravida? Obstet Gynecol. 1998;91(1):108–111. doi: 10.1016/s0029-7844(97)00585-1. [DOI] [PubMed] [Google Scholar]
- 11.Wong M.J., Bharadwaj S., Lankford A.S., Galey J.L., Kodali B.S. Mechanical ventilation and prone positioning in pregnant patients with severe COVID-19 pneumonia: experience at a quaternary referral center. Int J Obstet Anesth. 2022;49:103236. doi: 10.1016/j.ijoa.2021.103236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Ansari M.A., Hameed A.A., Al-jawder S.E., Saeed H.M. Use of noninvasive positive pressure ventilation during pregnancy: case series. Ann Thorac Med. 2007;2(1):23–25. doi: 10.4103/1817-1737.30358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bach J.R. Successful pregnancies for ventilator users. Am J Phys Med Rehabil. 2003;82(3):226–229. doi: 10.1097/01.PHM.0000053395.41165.73. [DOI] [PubMed] [Google Scholar]
- 14.DeBolt C.A., Bianco A., Limaye M.A., et al. Pregnant women with severe or critical coronavirus disease 2019 have increased composite morbidity compared with nonpregnant matched controls. Am J Obstet Gynecol. 2021;224(5):510.e1–510.e12. doi: 10.1016/j.ajog.2020.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mazlan M.Z., Ali S., Zainal Abidin H., et al. Non-invasive ventilation in a pregnancy with severe pneumonia. Respir Med Case Rep. 2017;21:161–163. doi: 10.1016/j.rmcr.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plotnikow G.A., Vasquez D., Pratto R., Carreras L. High-flow nasal cannula in the treatment of acute hypoxemic respiratory failure in a pregnant patient: case report. Rev Bras Ter Intensiva. 2018;30(4):508–511. doi: 10.5935/0103-507X.20180072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan D.S.A., Pirzada A.N., Ali A., Salam R.A., Das J.K., Lassi Z.S. The differences in clinical presentation, management, and prognosis of laboratory-confirmed COVID-19 between pregnant and non-pregnant women: a systematic review and meta-analysis. Int J Environ Res Public Health. 2021;18(11):5613. doi: 10.3390/ijerph18115613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Péju E., Belicard F., Silva S., et al. Management and outcomes of pregnant women admitted to intensive care unit for severe pneumonia related to SARS-CoV-2 infection: the multicenter and international COVIDPREG Study. Intensive Care Med. 2022;48(9):1185–1196. doi: 10.1007/s00134-022-06833-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P., STROBE Initiative The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 20.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 21.Knaus W.A., Draper E.A., Wagner D.P., Zimmerman J.E. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 22.Vincent J.L., de Mendonca A., Cantraine F., et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26(11):1793–1800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- 23.National Institutes of Health Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—the evidence report. Obes Res. 1998;6(suppl 2):51S–209S. [PubMed] [Google Scholar]
- 24.Kramer M.S., Papageorghiou A., Culhane J., et al. Challenges in defining and classifying the preterm birth syndrome. Am J Obstet Gynecol. 2012;206(2):108–112. doi: 10.1016/j.ajog.2011.10.864. [DOI] [PubMed] [Google Scholar]
- 25.Asprea I., García O, Nigri C, Lipchak D, Bermúdez S, Crespo H, et al. Recommendations for preconception, prenatal and puerperal care . Argentinian Ministry of Health; 2013. National Office of Maternity and Childhood; pp. 1–83. [Google Scholar]
- 26.Group R.C., Horby P., Lim W.S., et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Force A.D.T., Ranieri V.M., Rubenfeld G.D., et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 28.Tranquilli A.L., Dekker G., Magee L., et al. The classification, diagnosis and management of the hypertensive disorders of pregnancy: a revised statement from the ISSHP. Pregnancy Hypertens. 2014;4(2):97–104. doi: 10.1016/j.preghy.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Singer M., Deutschman C.S., Seymour C.W., et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greer I.A. Clinical practice. Pregnancy complicated by venous thrombosis. N Engl J Med. 2015;373(6):540–547. doi: 10.1056/NEJMcp1407434. [DOI] [PubMed] [Google Scholar]
- 31.Alda E, Apás Pérez de Nucci A, Corimayo L, Mariani G, Otaño L, Sebastiani M, Dirección Nacional de Maternidad e Infancia . Ministerio de Salud de la Nación Argentina; 2014. Recomendaciones para el manejo del embarazo y el recién nacido en los límites de la viabilidad; pp. 1–148. [Google Scholar]
- 32.Vasquez D.N., Das Neves A.V., Aphalo V.B., et al. Health insurance status and outcomes of critically ill obstetric patients: a prospective cohort study in Argentina. J Crit Care. 2014;29(2):199–203. doi: 10.1016/j.jcrc.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 33.Vasquez D.N., Das Neves A.V., Vidal L., et al. Characteristics, outcomes, and predictability of critically ill obstetric patients: a multicenter prospective cohort study. Crit Care Med. 2015;43(9):1887–1897. doi: 10.1097/CCM.0000000000001139. [DOI] [PubMed] [Google Scholar]
- 34.Estenssoro E., Loudet C.I., Rios F.G., et al. Clinical characteristics and outcomes of invasively ventilated patients with COVID-19 in Argentina (SATICOVID): a prospective, multicentre cohort study. Lancet Respir Med. 2021;9(9):989–998. doi: 10.1016/S2213-2600(21)00229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schmidt M, M, Hajage D, Demoule A, et al. COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med. 2021;47(1):60–73. doi: 10.1007/s00134-020-06294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Villar J., Ariff S., Gunier R.B., et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID multinational cohort study. JAMA Pediatr. 2021;175(8):817–826. doi: 10.1001/jamapediatrics.2021.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ackerman C.M., Nguyen J.L., Ambati S., et al. Clinical and pregnancy outcomes of coronavirus disease 2019 among hospitalized pregnant women in the United States. Open Forum Infect Dis. 2022;9(2):ofab429. doi: 10.1093/ofid/ofab429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trahan M.J., Malhame I., Mitric C., Simard C., Lipes J., Abenhaim H.A. Severe and critical COVID-19 in pregnancy: a case series from Montreal. Obstet Med. 2021;14(3):170–176. doi: 10.1177/1753495X21990213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vasquez D.N., Estenssoro E., Canales H.S., et al. Clinical characteristics and outcomes of obstetric patients requiring ICU admission. Chest. 2007;131(3):718–724. doi: 10.1378/chest.06-2388. [DOI] [PubMed] [Google Scholar]
- 40.Rochwerg B., Granton D., Wang D.X., et al. High flow nasal cannula compared with conventional oxygen therapy for acute hypoxemic respiratory failure: a systematic review and meta-analysis. Intensive Care Med. 2019;45(5):563–572. doi: 10.1007/s00134-019-05590-5. [DOI] [PubMed] [Google Scholar]
- 41.Lapinsky S.E. Management of acute respiratory failure in pregnancy. Semin Respir Crit Care Med. 2017;38(2):201–207. doi: 10.1055/s-0037-1600909. [DOI] [PubMed] [Google Scholar]
- 42.Hess D.R., Bigatello L.M. The chest wall in acute lung injury/acute respiratory distress syndrome. Curr Opin Crit Care. 2008;14(1):94–102. doi: 10.1097/MCC.0b013e3282f40952. [DOI] [PubMed] [Google Scholar]
- 43.Dorado J.H., Accoce M., Plotnikow G. Chest wall effect on the monitoring of respiratory mechanics in acute respiratory distress syndrome. Rev Bras Ter Intensiva. 2018;30(2):208–218. doi: 10.5935/0103-507X.20180038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Polese G., Rossi A., Appendini L., Brandi G., Bates J.H., Brandolese R. Partitioning of respiratory mechanics in mechanically ventilated patients. J Appl Physiol (1985) 1991;71(6):2425–2433. doi: 10.1152/jappl.1991.71.6.2425. [DOI] [PubMed] [Google Scholar]
- 45.Tyagi A., Singh S., Kumar M., Sethi A.K. Intra-abdominal pressure and intra-abdominal hypertension in critically ill obstetric patients: a prospective cohort study. Int J Obstet Anesth. 2017;32:33–40. doi: 10.1016/j.ijoa.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 46.Fan E., Del Sorbo L., Goligher E.C., et al. An Official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine clinical practice guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;195(9):1253–1263. doi: 10.1164/rccm.201703-0548ST. [DOI] [PubMed] [Google Scholar]
- 47.Bellani G., Laffey J.G., Pham T., et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315(8):788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 48.Tolcher M.C., McKinney J.R., Eppes C.S., et al. Prone positioning for pregnant women with hypoxemia due to coronavirus disease 2019 (COVID-19) Obstet Gynecol. 2020;136(2):259–261. doi: 10.1097/AOG.0000000000004012. [DOI] [PubMed] [Google Scholar]
- 49.Lee J.M., Bae W., Lee Y.J., Cho Y.J. The efficacy and safety of prone positional ventilation in acute respiratory distress syndrome: updated study-level meta-analysis of 11 randomized controlled trials. Crit Care Med. 2014;42(5):1252–1262. doi: 10.1097/CCM.0000000000000122. [DOI] [PubMed] [Google Scholar]