Abstract
Replacing rockwool with more sustainable materials, such as coir, is an effective measure to improve the sustainability of soilless cultivation in the greenhouse. To comprehensively assess the feasibility of coir before using it widely, coir was compared to rockwool as a cucumber cultivation substrate to evaluate its performance on mineral elements in the substrates, drainage, and in the plants. Plant growth, amino acids, and flavor substances of cucumber fruits were also compared between the two substrates. Compared to rockwool, coir significantly increased the LAI and yield of cucumber crops as well as contents of Ca, Mg, S, Cl and Zn in leaves and fruits. Contents of P, K, Ca, Mg, Cl, Zn, and B in the substrate were higher for coir while those of Fe, Cu, and Mn in the drainage lower. Moreover, coir also significantly increased contents of amino acids (His, Leu, Ile, Phe, Lys, Asp, Glu and Pro) and flavor substance (TC, PS, TP, CLL, CuB, and LA) in cucumber fruits. Our results demonstrated the potential of coir as a replacement of rockwool to improve sustainability of soilless cultivation in the greenhouse.
Keywords: Amino acids, Coir, Cucumber, Flavor substance, Mineral elements, Rock wool
Amino acids; Coir; Cucumber; Flavor substance; Mineral elements; Rock wool.
1. Introduction
As a modern cultivation system, soilless cultivation could significantly improve the yield and the quality of horticultural plants through controlling the quantity, composition of the nutrient solution and the growing medium, compared to conventional soil culture (Nerlich and Dannehl, 2021). Presently, rockwool and coir are two cultured substrates commonly used for greenhouse production. Rockwool offers optimum chemical properties and physical and it has been widely used in greenhouse cultivation for decades. However, rockwool is a non-biodegradable product whose production process from non-renewable resources is energy-intensive and poses great environmental impacts. Furthermore, rockwool is associated with negative health effect (Kudo et al., 2009). Considering the growth of greenhouse production and the difficulty to treat and to dispose of used rockwool products, we should make more effort to recycle rockwool or reduce the high energy demand dyring the manufacturing processes (Antón et al., 2012).
Cost and environmental concerns are pushing growers to find alternative sustainable and recyclable materials such as bark, compost, and especially, coir to replace rockwool (Fornes et al., 2015). In many tropical and subtropical countries, coir is one of the most abundant organic waste of plant origin, and offers high porosity, good aeration, and high water-holding capacity. Often used either as a pure substrate or as a constituent, coir is a stable material as a growing medium in horticulture production (van Gerrewey et al., 2020). It may also can be reused, for example, with strawberries in growing modules (Carlile et al., 2019). In hydroponic systems, some growers even prefer organic substrates to inorganic substrates because of the potential for good plant production with organic substrates (Mattson and Lieth, 2019). Previous research showed that coir added with pumice or stabilized wood fiber can improve plant growth and nutrition compared with the peat cultured L. vulgare Lam. (di Lonardo et al., 2021). Scagel (2003) reported that the growth of many ericaceous species could be promoted when plants were cultured in media mixed with coir; but the volume of coir in media never contained more than 20%. If coir could be used as a mono-substrate without other unsustainable substrates, then the environmental impact of greenhouse production could be reduced and the sustainable production could be achieved. Recent years, pure coir substrate has been increasingly used in some soft fruit plants such as strawberries and blueberries, as well as lilies (Feng et al., 2010), tomatoes (Jia et al., 2020), and cucumbers (He et al., 2021). However, physical, chemical and biological properties are quite different between rockwool and coir, which may affect both plant growth and fruit quality. Previous reports in tomatoes had shown that the coir significantly increased K and S uptake by crops, photosynthesis, individual fruit weight, total fruit yield and organic acid of fruit in first truss compared to rockwool (Xiong et al., 2017). However, there are fewer relevant studies on cucumbers, thus a deeper understanding of the characteristics of coir cultivation is required in order to improve coir substrate use and management, especially for high-quality production of greenhouse cucumbers.
The main purpose of this work was to investigate and compare the effects of commercial rockwool and coir slab on growth, chemical elements, fruit quality and flavor substance of cucumbers. Furthermore, we also analyzed the chemical elements of rockwool and coir, as well as those of their irrigation drain. The finding of this study greatly facilitated the development of novel and improved coir-based cultivation in modern greenhouses highlighting the cost-efficiency and sustainability.
2. Materials and methods
2.1. Plant material and growing conditions
The experiment was conducted during the autumn of 2020 in a greenhouse in Shanghai (31.4°N, 121.5°E, P. R. China). Cucumber seeds (Cucumis sativus L. cv. Deltastar, Rijk Zwaan Distribution B.V., De Lier, the Netherlands) were sown on 15-09-2021 and germinated in rockwool cubes (10 cm × 10 cm×7 cm, Grodan, Roermond, the Netherlands) or coir cube (10 cm × 10 cm × 6.5 cm, 100% 0–6 mm coir, EC < 1, pH 5.8–6.8, Remmy, Qingdao Remmy Commerce and Trade Co., LTD). Cucumber seedlings grown under natural light condition, maximum photosynthesis photon flux density (PPFD) about 1200 μmol m−2 s−1. The day and night temperature were maintained at 25 ± 2 °C (day) and 17 ± 2 °C (night), respectively. The modified Hoagland nutrient solution (pH = 5.5, EC = 2.0 dS m−1) was used to irrigate the cucumber seedlings regularly. When plants reached 7–8 leaves, cucumber seedlings were transferred to rockwool slab (100 cm × 20 cm × 7.5 cm) and coir slab (100 cm × 20 cm × 8 cm, 50 % 0–6 mm coir, 50 % 10–20 mm coir, EC < 1, pH 5.8–6.8), respectively. Plants were irrigated with nutrient solution ((pH = 5.5–6.5, EC = 2.8–3.2 dS m−1) through an automatic irrigation system (Nutrjet 300 inline, Priva B. V., De Lier, the Netherlands) with one drip of 100 mL every 80–100 J cm−2 of radiation sum per plant per day. The irrigation frequency and volume were the same for all rockwool slabs or coir slabs and the drainage ratio was maintained at around 20 % every day. The A Tank (500 L) of the irrigation system contains Ca(NO3)2 87.5 kg, EDTA-Fe 750 g, and the B Tank (500 L) of the irrigation system contains KNO3 25 kg, MgSO4 30 kg, KH2PO4 12 kg, K2SO4 3 kg, Na2B4O7 90 g, MnSO4 120 g, ZnSO4 62 g, CuSO4 13 g, Na2MoO4 8 g.
2.2. Measurements of plant height, leaf area index, gas exchange parameters, yield, and the quality index of cucumber fruits
Plant height, length, and width of every leave were determined using a ruler, every 4–8 d after transplanting. The leaf area was calculated according to Cho et al. (2007), and the leaf area index (LAI) was calculated according to the equation:
| LAI = total leaf area per plant × planting density |
where the planting density was 2.8 plants m−2, and the same numbers of old leaves were removed every 3–5 d from the bottom.
A portable photosynthesis system (CIRAS-3, PP Systems, Amesbury, MA, USA) equipped with the leaf chamber fluorometer (PLC3 Universal Leaf Cuvette, 18 ∗ 25 mm window, CFM-3) were used to monitor the net photosynthetic rate (Pn), stomatal conductance (gs), and intercellular CO2 concentration (Ci) of the fully expanded leaves. During measurements, the photosynthetic photon flux density (PPFD) of cuvette conditions were maintained at 1000 μmol photons·m−2 s−1 (90 % red, 635 nm, and 10 % blue, 465 nm). The relative humidity, leaf temperature and external CO2 concentration were approximately at 70 %, 25 °C and 390 μmol mol−1, respectively.
The weight of cucumber fruits (plant number = 144 for rockwool and coir cultured, respectively) was recorded after harvest every time over two months and then calculated for the yield per plant.
The fruit soluble protein (SP) of cucumber was measured according to Bradford (1976). The extraction and activities determination of polyphenol oxidase (PPO) were performed according to the method of Tang and Newton (2004). The extraction buffer that contained 100 mm NaPO4, pH 7.2, 0.1 % [w v−1] SDS and 3 mM ascorbate was used to extract and centrifuge the cucumber fruit tissues. The supernatant obtained was used for PPO activity determination using a spectrophotometer at 490 nm and 28 °C. The change in absorbance per milligram of protein per minute was used to define the activity of enzyme, and every 0.05 change in absorbance was defined as one unit (U).
The soluble sugar (SS) contents of cucumber fruit were determined using the previous method (Bai et al., 2013). Fruit tissues (0.05 g) was boiled in 6 mL of deionized water for 30 min and then centrifuged at 12 000 × g for 10 min. The precipitate was obtained and dissolved in 50 mL deionized water. Anthrone reagent was prepared from 0.15 g anthrone, 84 ml sulphuric acid and 16 ml H2O. 0.1 mL extract was added to 3 mL anthrone reagent and the absorbance was recorded at 620 nm by a spectrophotometer.
To measure the total polysaccharide (TP) of cucumber fruits, 2 g samples of cucumber and 20 g distilled water were mixed and extracted 3 times at 100 °C for 3 h. The filtrate was obtained by filtering the suspension and then mixed with 4 times the volume of cooled ethanol (8 °C). After freezing for 48 h (4 °C) the samples were centrifuged at 5000 × g for 10 min. Finally, the phenol-sulfuric acid method was used to determined the polysaccharides content (Wang et al., 2013).
For ascorbate (AsA) content, 10 μl ascorbate oxidase (0.01 units ml−1), 10 μL of extracted AsA and 80 μl of 0.1 M potassium phosphate buffer (pH 7.0) were mixed and form a mixture. The increase in extinction of dehydroascorbate was determined by adding 10 μl of 4 mM dithiothreitol (DTT) and 80 μl of 0.1 M potassium phosphate buffer (pH 7.8) to 10 μl of mixture prepared above. The sum of decreased ascorbate absorbance at 265 nm was calculated as total AsA concentrations (Ali et al., 2019).
The chlorophyll content of cucumber peel, as one of the characteristics of cucumber fruits, can be affected by various environmental factors and may be related to the content of other flavor substances. The total chlorophyll contents of the cucumber peel were measured following the method of Chung et al. (2016). The absorbance of the extracts at 663, 645, and 470 nm was assayed by a spectrophotometer (UV-Vis 2550, Shimadzu, Kyoto, Japan). Chlorophyll and carotenoid contents were expressed in mg·g−1 fresh weight (FW) and calculated through following equations:
| Chlorophyll a = 0.01 × (12.7 × A663 − 2.69 × A645) FW−1 |
| Chlorophyll b = 0.01 × (22.9 × A645 − 4.68 × A663) FW−1 |
| Total chlorophylls = 0.01 × (8.02 × A663 + 20.21 × A645) FW−1 |
Total phenols (TP), were determined by adding 180 μl of distilled water to 5 μl of fruit extract solution. Then, 1200 μl Folin (10 %) was added to the mixture and let it stand for 5 min before adding sodium carbonate (7.5 %). Finally, absorption was measured at 760 nm wavelength using a spectrophotometer (Dynamica HALO DB-20, UK). Distilled water was used as blank and gallic acid as standard. The calibration curve was plotted based on the method of Alizadeh and Fattahi (2021).
The nitrate (NO3–N) and nitrite (NO2–N) contents of cucumber fruits were spectrophotometrically according to Qiao et al. (2018) and the cellulose (CLL) contents of cucumber fruits were determined using a previously described method (Tong et al., 2015).
2.3. Elements analysis by inductively coupled plasma mass spectrometry
The ultra-high purity water (Milli-Q) was used to wash cucumber leaves and fruits, before the chemical element analysis. The fresh cucumber samples were then dried in an oven at 105 °C for 2 h and then at 65 °C until the weight was constant. The dry samples were milled to powder and stored to measure chemical elements.
The multi-element composition was evaluated and inproved according to the procedures established by Mi et al. (2022). The digested cucumber samples were analysed by inductively coupled plasma mass spectrometry (ICP-MS 7700X, Agilent Technologies Inc., Palo Alto, California, USA). The chemical element of drain water from rockwool and coir was analyzed by using a Inductively Coupled Plasma Optical Emission Spectrometer (ICP-OES) at the following wavelengths: S (181.9 nm), Mo (202.0 nm), Zn (206.2 nm), P (213.6 nm), Fe (238.2 nm), B (249.7 nm), Mn (257.6 nm), Mg (285.2 nm), Ca (317.9 nm), Cu (327.4 nm) and K (766.5 nm), respectively. The nitrate (NO3-) content was determined by Flow Injection Analysis (FIA).
2.4. Amino acids analysis by high-performance liquid chromatography (HPLC)
The free amino acid contents of cucumber fruits were carried out following a method described previously (Riga et al., 2019) and with some modifications. Briefly, cucumber fruits (0.5 g) of two treatments were homogenized in 100 ml ultrapure water and prepared for the derivatization. A 1.5ml propylene vial was added 200 μl extracted sample before and 200 μl amino acids standard solution. Thereafter, the vial was added in 200 μl triethylamine acetonitrile, 100 μl phenyl isothiocyanate acetonitrile and 20 μl norleucine standard solution. The vial was vortexed for a few seconds and then left stand at room temperature for 1 h. Then the vial was added in 400 μl n-hexane and stand for 10 min after vortexed. A high-performance liquid chromatography (Rigol L3000, Rigol Technologies Co., Ltd. Suzhou, China) equipped with a reverse-phase column (Sepax C18, 25 0 mm × 4.6 mm, 5 μm) was used to separate the derivatized free amino acids. The column were injected with 10 μl of derivatized amino acid standards or samples and the flow rate was set at 0.5 mL min−1. The column oven temperature was kept at 40 °C and the acquisition time was 45 min for each sample. The peak time of each amino acid is as follows: Asp, 4.043 min; Glu, 4.527 min; Ser, 9.823 min; Gly, 10.907 min; His, 11.670 min; Arg, 15.297 min; Thr, 16.630 min; Ala, 17.353 min; Pro, 18.603 min; Tyr, 24.600 min; Val, 26.070 min; Met, 26.940 min; Cys, 27.427 min; Ile, 29.037 min; Leu, 29.397 min; Phe, 30.913 min; Lys, 32.627 min. The results were expressed as μg amino acid g−1 of fresh weight.
2.5. Flavor substance analysis by HPLC and GS
Some important flavor compounds of cucumber fruits, including gallic acid, caffeic acid, apigenin, rutin, and cucurbitacin B were quantitatively determined by HPLC according to the method described by Canas et al. (2015) and Mi et al. (2022), using the same equipment as described for measuring free amino acid. The chromatographic peaks of the compounds was identified as described.
Linoleic acid of cucumber fruits was produced by acid catalysed transmethylation and analyzed by gas chromatography (GS) according to Moretti et al. (2019).
2.6. Data processing and statistical analyses
Experimental data were processed with SAS (version 9.2, SAS Institute, Cary, NC, USA) using t-test to identify differences between means. For the data of plant height, LAI and yield, there were five replicates (n = 5) while for that of elements of plants and substrates; the quality, amino acids, and flavor substance of fruits, there were three replicates (n = 3). All statistical tests were conducted at a probability level of α = 0.05.
Principal component analysis (PCA) were also applied for data exploration and samples classification.
3. Results
3.1. The effect of different substrates on growth, photosynthesis and crop productivity of cucumber plants
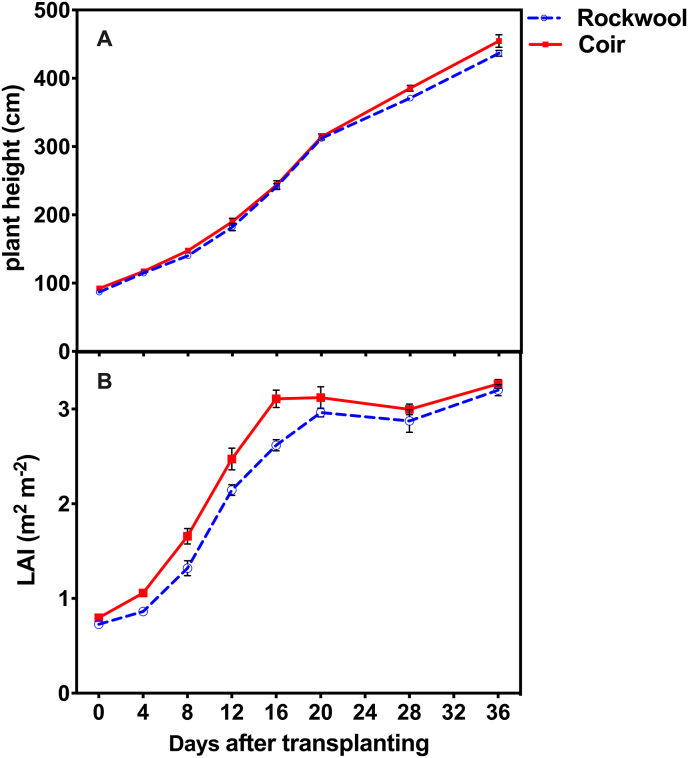
No significant differences in plant height were found between crops grown on rockwool and coir substrates during the beginning 20 days after transplanting. The plant height of cucumbers grown in coir slabs was around 3.9 % and 4.1 % higher than that grown in rockwool slabs at 28 days and 36 days after transplanting, respectively (Figure 1A). LAI of plants grown in coir was significantly higher than those grown in rockwool and reached the value of 3 on 16 days after transplant (Figure 1B). Regular removal of full-grown leaves from below is a common practice in the greenhouse when LAI reached 3, and LAI was maintained from 2.8 to 3.3 after 16 days of transplanting (Figure 1B). There was no difference in gas exchange parameters between the two substrates, but the yield of single plants grown in coir was significantly higher (7.7 % higher) than that grown in rockwool, during the whole harvesting season (Figure 2D).
Figure 1.
Dynamics of plant height and leaf area index (LAI) value of cucumber crops grown on rockwool (R) and coir (C). Error bars show ± standard deviation (n = 5).
Figure 2.
The gas exchange parameters of leaves and yield of the single cucumber plant grown in rockwool and coir during a rotation (80 d). Error bars show ± standard deviation (n = 5). Different letters indicate significant different at P < 0.05.
3.2. The effect of different substrates on elements, amino acids, and flavor substance of cucumber
To investigate the effects of different substrates on elements accumulation in cucumber plants, we analyzed macro and micronutrients concentrations in the leaves and fruits of cucumber plants grown in rockwool and coir (Table 1). The results showed that the contents of Ca, Mg, S, Cl, and Zn in leaves and fruits of cucumber grown in coir were significantly higher than that grown in rockwool. The cucumbers that grown in rockwool were rich in Fe, Mn, Cu, and Mo. The contents of P, K, Na, and Cl were higher in fruits than in leaves significantly, but these elements had no significance between the two substrates.
Table 1.
The content of macro and micronutrients of cucumber leaves and fruits.
| Chemical elements | Leaves |
Fruits |
||
|---|---|---|---|---|
| Rockwool | Coir | Rockwool | Coir | |
| N | 62.00±0.21 a | 64.00±1.73 a | 28.60±2.50 b | 30.47±2.35 b |
| P | 4.97±0.12 b | 5.17±0.21 b | 10.07±1.21 a | 11.30±0.06 a |
| K | 34.97±4.37 b | 36.20±4.68 b | 56.28±3.05 a | 57.33±0.57 a |
| Ca | 40.40±4.94 b | 54.67±0.58 a | 9.60±0.89 d | 11.27±0.15 c |
| Mg | 6.80±0.70 b | 9.43±0.65 a | 3.50±0.30 d | 3.93±0.06 c |
| Na | 0.13±0.06 b | 0.17±0.06 b | 0.25±0.05 a | 0.20±0.01 a |
| Cl | 0.67±0.32 c | 1.00±0.10 b | 1.20±0.14 b | 1.93±0.39 a |
| S | 8.20±0.53 b | 10.53±1.07 a | 4.62±0.41 d | 5.63±0.06 c |
| Fe | 160.00±17.32 a | 125.33±5.74 b | 80.67±8.07 c | 73.33±2.88 d |
| Mn | 116.00±5.57 a | 71.00±6.93 b | 56.32±3.51 c | 40.12±1.73 d |
| Zn | 41.00±1.73 c | 50.67±2.89 b | 53.63±3.21 b | 61.33±0.57 a |
| Cu | 9.67±0.25 b | 7.43±0.68 c | 14.03±1.05 a | 8.93±1.38 b |
| B | 31.47±0.93 a | 29.87±1.80 a | 24.08±1.08 b | 24.83±0.55 b |
| Mo | 2.53±0.25 a | 0.50±0.10 c | 1.40±0.15 b | 0.37±0.12 d |
Note: The levels of N, P, K, Ca, Mg Na, Cl and S are expressed in gram per kilogram of cucumber leaves and fruits on dry weight (g kg−1 DW); Fe, Mn, Zn, Cu, B and Mo are express in milligram per kilogram of cucumber leaves and fruits on dry weight (mg kg−1 DW). All values are expressed as the mean (n = 3) ± standard deviation. Different letters in the same row represent significant difference at P < 0.05.
We detected 17 free amino acids in cucumber fruits, including arginine (Arg), alanine (Ala), aspartic acid (Asp), cysteine (Cys), glycine (Gly), glutamic acid (Glu), histidine (His), isoleucine (Ile), leucine (Leu), lysine (Lys), methionine (Met), proline (Pro), phenylalanine (Phe), serine (Ser), threonine (Thr), tyrosine (Tyr) and valine (Val), were identified and the results were shown in Table 2. Cucumber fruits grown in coir contained significantly more His (24.7 % increase), Leu (10.8 % increase), Ile (15.1 % increase), Phe (20.2 % increase), Lys (25.5 % increase), Asp (8.5 % increase), Glu (26.5 % increase) and Pro (18.2 % increase) than cucumber fruits grown under rockwool. Compared to coir, cucumber fruits grown in rockwool presented significantly more Ser (9.6 % increase), Arg (7.1 % increase), Ala (3.4 % increase) and Tyr (2.5 % increase). Other free amino acids (Thr, Val, Met, Gly and Cys) showed no difference cucumber fruits grown in rockwool and coir.
Table 2.
Effect of rockwool and coir on amino acid contents of cucumber fruits.
| Essential amino acid (μg g−1 F.W.) |
Non-essential amino acid (μg g−1 F.W.) |
||||
|---|---|---|---|---|---|
| Name | Rockwool | Coir | Name | Rockwool | Coir |
| Histidine | 143.1±2.66 b | 182.35±6.89 a | Alanine | 55.3±0.28 a | 53.5±1.14 b |
| Isoleucine | 59.8±0.89 b | 68.8±1.34 a | Arginine | 88.0±0.63 a | 82.2±3.68 b |
| Leucine | 69.5±0.47 b | 77.0±1.01 a | Aspartic acid | 54.3±0.11 b | 58.9±1.38 a |
| Lysine | 19.2±0.21 b | 24.1±0.15 a | Cystine | 241.8±1.24 a | 236.6±7.18 a |
| Methionine | 32.3±0.46 a | 28.2±3.79 a | Glutamic acid | 22.3±0.33 b | 28.2±1.10 a |
| Phenylalanine | 53.9±0.35 b | 64.8±0.73 a | Glycine | 58.3±2.82 a | 59.4±1.33 a |
| Threonine | 77.9±1.86 a | 80.1±2.69 a | Proline | 29.3±0.37 b | 35.8±0.49 a |
| Valine | 53.7±0.29 a | 54.6±0.66 a | Serine | 111.7±1.52 a | 101.9±0.77 b |
| Tyrosine | 50.1±0.54 a | 48.9±0.63 b | |||
All values are expressed as the mean (n = 3) ± standard deviation. Different letters in the same row represent significant difference at P < 0.05.
A total of 16 flavor substances, including soluble protein (SP), polyphenol oxidase (PPO), soluble sugar (SS), polysaccharide (PS), ascorbate (AsA), total chlorophyll (TC), total phenol (TP), nitrate (NO3–N), nitrite (NO2–N), cellulose (CLL), gallic acid (GA), caffeic acid (CA), apigenin (AP), rutin (RT), cucurbitacin B (CuB), linoleic acid (LA), were qualitatively and quantitatively determined and the results were presented in Table 3. Cucumber fruits grown in coir had higher contents in TC (12.5 % increase), PS (23.9 % increase), TP (12.3 % increase), CLL (24.1 % increase), CuB (38.9 % increase) and LA (78. 1% increase), significantly (p < 0.05). Rockwool significantly increased the contents of NO3–N (12.5 %), NO2–N (80.0 %), GA (75.0 %), CA (33.3 %), AP (20.7 %), and RT (31.9 %) in cucumber fruits, compared to coir cultured. The concentration of SP, PPO, SS, and AsA showed no different in cucumber fruits under the two substrates treatment.
Table 3.
Effect of rockwool and coir on fruit quality and flavor substance of cucumber fruits.
| Name | Rockwool | Coir |
|---|---|---|
| NO3–N (mg kg−1 FW) | 115.5 ± 0.10 a | 102.7 ± 0.22 b |
| SP (mg g−1 FW) | 27.5 ± 1.53 a | 25.8 ± 0.93 a |
| SS (mg g−1 FW) | 13.0 ± 0.45 a | 13.5 ± 0.11 a |
| TC (mg g−1 FW) | 0.48 ± 0.01 b | 0.54 ± 0.03 a |
| PS (mg g−1 DW) | 30.6 ± 0.17 b | 37.9 ± 0.32 a |
| TP (mg g−1 DW) | 7.3 ± 0.28 b | 8.2 ± 0.42 a |
| CLL (mg g−1 DW) | 209.8 ± 13.09 b | 260.3 ± 19.4 a |
| LA (mg g−1 DW) | 0.64 ± 0.01 b | 1.14 ± 0.01 a |
| NO2–N (μg g−1 FW) | 0.09 ± 0.006 a | 0.05 ± 0.004 b |
| ASA (μg g−1 FW) | 49.2 ± 1.38 a | 48.6 ± 1.06 a |
| GA (μg g−1 FW) | 0.35 ± 0.02 a | 0.20 ± 0.01 b |
| CA (μg g−1 FW) | 0.16 ± 0.01 a | 0.12 ± 0.01 b |
| AP (μg g−1 FW) | 0.35 ± 0.03 a | 0.29 ± 0.01 b |
| RT (μg g−1 FW) | 1.20 ± 0.03 a | 0.91 ± 0.04 b |
| Cub (μg g−1 FW) | 1.90 ± 0.08 b | 2.64 ± 0.11 a |
| PPO (U g−1 FW) | 69.2 ± 1.73 a | 67.8 ± 1.43 a |
Abbreviations used: soluble protein (SP), polyphenol oxidase (PPO), soluble sugar (SS), polysaccharide (PS), ascorbate (AsA), total chlorophyll (TC), total phenols (TP), nitrate (NO3-N), nitrite (NO2-N), gallic acid (GA), caffeic acid (CA), apigenin (AP), rutin (RT), cucurbitacin B (CuB), linoleic acid (LA), cellulose (CLL), the dry weight of CLL is cell wall dry mass. All values are expressed as the mean (n = 3) ± standard deviation. Different letters in the same row represent significant difference at P < 0.05.
Principal component analysis (PCA) was used to visualize the data set of amino acids and flavor substances of cucumber fruit grown in rockwool and coir. As shown in Figure 3, PC1 and PC2 accounted for 95.92 % of total variance under two substrates. Coir treatments had a higher score and were distributed in high quadrant. Figure 3 also indicated the correlations between the metabolic substance mentioned above and the cucumber samples. Rockwool and coir cultured cucumber groups were separated from each other clearly. There were more (18) metabolic substances closely groupted to coir treatments, including His, Leu, Ile, Phe, Lys, Asp, Glu, Pro, Thr, Gly, Val, SS, TC, PS, TP, CLL, CuB and LA.
Figure 3.
Principal component analysis (PCA) of amino acid and flavor substance of cucumber fruits under two substrates. Green circles indicate rockwool, red circles indicate coir.
3.3. The difference in element composition in new and used substrates
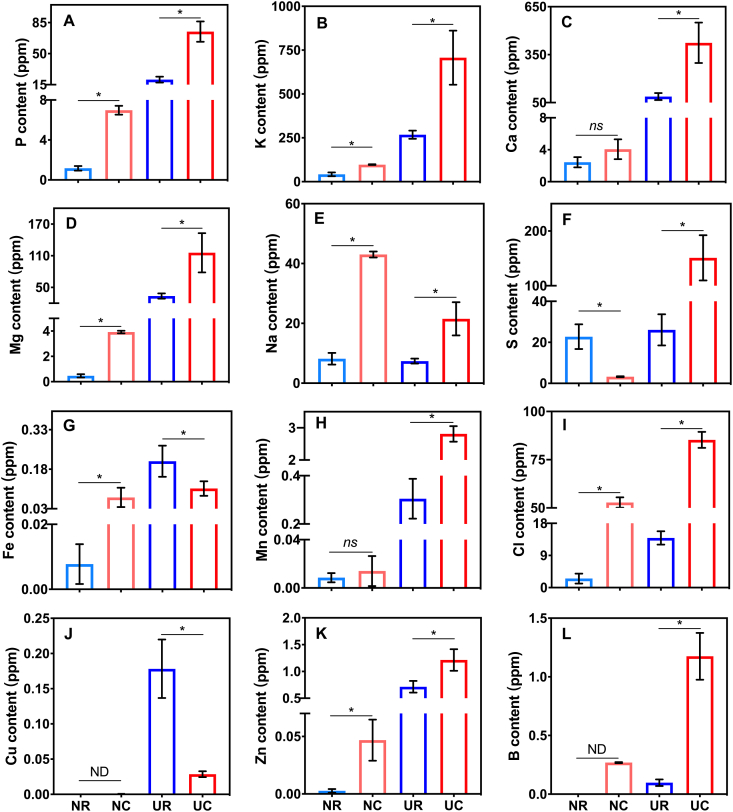
Most elements in new substrates were lower than in used substrates. The Ca, Mg, Mn, Cu, and Zn elements were low in new rockwool and new coir (Figure 4C, D, H, J, and K). However, these elements increased significantly in used substrates, and the concentrations in used coir were significantly higher than in used rockwool, except Cu element (Figure 4C, D, H, and K). The content of Cu in used rockwool was 6.2 times as many as in used coir (Figure 4J). S was detected in new rockwool (22.75 mg kg−1) and was significantly higher than that in new coir (3.22 mg kg−1), but was significantly increased in used coir (151.00 mg kg−1) compared to used rockwool (26.12 mg kg−1) (Figure 4F). New and used coir had a higher content in Na, P, K, Cl and B compared to new and used rockwool, respectively (Figure 4A, B, E, I, and L). The content of Fe in new coir (0.07 mg kg−1) was higher than in new rockwool (0.05 mg kg−1), but in used coir (0.11 mg kg−1) was lower than in used rockwool (0.21 mg kg−1) (Figure 4G).
Figure 4.
The content of macro and micronutrients of new rockwool (NR), new coir (NC), used rockwool (UR) and used coir (UC). Graphs A, B, C, D, E, F, G, H, I, J, K and L represent P, K, Ca, Mg, Na, S, Fe, Mn, Cl, Cu, Zn and B content, respectively. Asterisks indicate level of significance difference between NR and NC, or UR and UC at P < 0.05.
4. Discussion
Rockwool, as a substrate traditionally used in greenhouse crop production, has its limitations due to negative environmental and ecological impacts (Steiner and Harttung, 2014). Although coir gained popularity as an alternative to rockwool, thorough investigation of its performance in greenhouse production is still scarce (Xiong et al., 2017).
LAI, defined as the total leaf area per unit ground area, is a useful index to evaluate crop growth and it contributes not only to estimation of current dry matter production but also to prediction of subsequent growth and yields (Fukuda et al., 2021). Increasing LAI from 2 to 3 increases light interception from 80 % to 90 %, while further increasing LAI from 3 to 4 only leads to a 4% increase (Heuvelink et al., 2005). In the present study, cucumber plants grown in coir had a larger leaf area and higher LAI than that in rockwool at the beginning of the transplanting (0–16 d) and had a higher plant height 20 days after transplanting (Figure 1). The higher plant height and LAI indicated that cucumber plants grown in coir had a higher growth rate due to increased light interception for photosynthesis. We found that cucumber plants are grown in coir also had more yield, although the gas exchange parameters of cucumber leaves were no different in rockwool or coir (Figure 2). Previous research also found that when coir was used as the cultivation substrate could significantly increase the fruit yields of bell pepper, compared with soil-based cultivation, and this result was due to the pepper plants grown in coir having a greater plant height, leaf length, leaf width, and stem thickness (Camposeco-Montejo et al., 2018). Compared to rockwool and peat, coir also showed a higher individual fruit weight and total fruit weight in tomatoes (Xiong et al., 2017).
Chemical elements, EC and pH in substrates are critical factors for plant growth, and growers also need to detect these parameters of drainage, in order to adjust the fertilizer and irrigation strategy. It is well known that coir usually have a high pH and the capacity of cation-exchange, as well as a high content in K, Na and Cl, but has a low concentration of P (Ross et al., 2012). Due to the high salt content, coir needs to be cleaned and buffered (treated with calcium nitrate) before it can be ready for horticultural growing (van Gerrewey et al., 2020). The composition of elements and their configuration under coir or rockwool condition may be used to explain their good properties as a substrate for plant growth. In tomatoes, coir culture could significantly increase the concentrations of NO3−, SO42− and Mg2+ than those in water culture (Xing et al., 2019). The K concentration in the root-zone and K, Ca, and Mg concentration in crops were also increased by coir, compared to rockwool (Xiong et al., 2017). In our study, we also found that P, K, Ca, and Mg content was higher in new and used coir than those in rockwool (Figure 4). Although the accumulation of K in cucumber leaves and fruits was not influenced by two substrates, the P, Ca, and Mg concentrations in crops were increased by coir (Table 1). Because of the accumulation of P, Ca, and Mg, a organic substrates (coir) could prevent Ca deficiency more effectively than an inorganic substrate (rockwool), such as blossom-end rot of tomato (Xiong et al., 2017), and then enhanced fruit quality.
Organic substrates, such as coir, could release certain kinds of ions into the solution while absorbing other ions (Xing et al., 2019). It is necessary to evaluate the microelements, except the P, K, Ca, and Mg, to better and more comprehensively understand the difference between ions’ release and absorption in rockwool and coir. Kingston et al. (2017) reported that, compared to bark, peat and coir had a better nutrients uptake efficiency of N, P, K, S, Ca, Mg, Mn, B, Cu, and Zn. Eleven mineral ion binding and transport-related proteins were identified through functional annotation analysis of the root proteome, suggesting that coir cultivation induced complex proteomic changes involving mineral ion binding and transport, compared to hydroponics (Xing et al., 2019). In this research, we found that S, Mn, Cl, Zn and B were significantly accumulated in used coir (Figure 4), but only S, Cl, and Zn were found to increase in leaves and fruits of cucumber under coir cultivations simultaneously (Table 1). The content of Mn not only decreased in crops under coir cultivations but also had a lower concentration in drainage from coir (Supplementary Figure 1). In contrast, the contents of B in crops and drainage were not influenced by substrates (Table 1 and Supplementary Figure 1). All concentrations of Fe and Cu in coir, drainage from coir, and crops under coir cultivations were lower than that treated with rockwool. These results indicated that the coir could accumulate most ions and have a better nutrient availability, due to its cation exchange capacity (Barrett et al., 2016). But, coir might not accumulate Fe and Cu, and absorb a lot but release very little of Mn, resulting in the contents of these elements being decreased in crops compared to rockwool cultivations. Previous research also showed that the substrates that contained coir had higher levels of many macro and micronutrients, namely N–NH4, K, Ca, Mg, Mn, and Zn compared to other peat-containing substrates (di Lonardo et al., 2021). The higher C/N ratio in coir and N content in used coir is due to its a high content of lignin and cellulose that can cause immobilization of soluble N (Atzori et al., 2021) (Supplementary Figure 2). These consequences could guide growers to adjust the recipes of fertilizer and improve the fruit quality when using coir as a soilless substrate.
As organic compounds, amino acids are the compositions of proteins, involved in the peptide hormone synthesis in plants (Hirakawa et al., 2017). With the increasing focus on fruit quality, the contents of amino acids and flavor substances in cucumber fruits were measured, which are considered to have important nutritional value because of their health-promoting functions (Nicolle et al., 2004). In lettuces, it has already been reported that sphagnum moss and wood could be used as an alternative to rockwool for lettuce cultivation (Nerlich and Dannehl, 2021). The arbuscular mycorrhizal fungi (AMF) also could increase total and specific amino acid concentrations in strawberry and cucumber fruits (Guo et al., 2021; Matsubara et al., 2009). In our study, the production of amino acids components in cucumber fruits was strongly influenced by different substrates (p < 0.05). Such as, the content of His, Leu, Ile, Phe, Lys, Asp, Glu, and Pro were increased in cucumber fruits grown under coir (Table 2). It suggested that coir could enhance most essential amino acids contents in cucumber fruits. The His, Leu, Ile, Phe, and Lys are five out of nine essential amino acids that must be absorbed from the diet, so far the largest share is coming from plants (Hou and Wu, 2018). For example, plants often contain low levels in Lys and thus limit their nutritional value (Trovato et al., 2021). In addition, Lys has relationship with epigenome and stress biology in plants due to involve in histone modifications (Yuan et al., 2013). Kavi Kishor et al. (2020) also reported the Lys and Ser were involved in plant development and stress tolerance related regulation through transcriptional and post-transcriptional regulation mechanisms and highlighted the importance of proteins enrichment.
Cucumber fruits also contained other nutritional contents and some specific flavor substances. Our results showed that coir cultured could significantly increase the contents of total chlorophyll (TC), polysaccharide (PS), total phenols (TP), cellulose (CLL), cucurbitacin B (CuB), and linoleic acid (LA) in cucumber fruits (Table 3). Phenolics are kinds of antioxidants, could act as reducing and hydrogen donors donating agents, mainly due to their inhibitory effects (Alizadeh and Fattahi, 2021). The biological functions of phenolic compounds are included protecting against biotic and abiotic stresses, and phenolic compounds also could be health benefits to humans act as potential dietary antioxidants (Braidot et al., 2008). It has been reported that hetero-grafted could significantly increase the proanthocyanidins (PAs) and flavonoid in grape berry skin compared to the auto-grafted control (Zhang et al., 2022). Cucurbitacin, a kind of triterpenoids, can produce the bitter taste in cucumber, melon, watermelon, squash, and pumpkin. The bitterness in some cultivars can be increased by temperature stress (Che and Zhang, 2019), these bitter compounds could pretect plants against most pests and have also been proved to have anti-tumor characteristics and hepatoprotective activities (Yi et al., 2014) and may have some beneficial effects to human health and plants themselves. Recent research identified and characterized several gene clusters to participate in CuB biosynthesis in cucurbitaceae plants, such as cucumber, watermelon and melon (Luo et al., 2020), and a cluster harboring several bHLHs was reported to regulate the synthesis of cucurbitacins (Zhou et al., 2016). Through the analysis of evolutionary history and expression profiles of two tandem bHLH genes, and one gene (Brp) had been identified to regulate the cucurbitacin biosynthesis in roots (Xu et al., 2022). These results indicated roots and roots’ genes could involve in the regulation of the biosynthesis of phenols and CuB in cucumber plants. Due to the difference in root-room environment between the rockwool and coir, the coir may stimulate the cucumber roots and regulate the related genes to increase the total phenols and CuB in cucumber fruits.
Polysaccharides (PS), including cellulose (CLL), are important biomacromolecules presenting in all plants, most of which are integrated into a fibrous structure called the cell wall (Cai et al., 2021). The biological activities of polysaccharides are mainly existing in antioxidant, antibacterial, anticancer, healing, antiviral, immunomodulatory, antidiabetic and radioprotective effects. Due to the antiviral activity of polysaccharides, the Coronavirus pandemic has stimulated significantly the number of publications about related research (Albuquerque et al., 2022). When non-enzymatic cleavage of the cell wall polysaccharides is prevented, the tomatoer resistance against pathogenic infections were enhanced and the deterioration of postharvest quality during storage was suppressed (Li et al., 2021). Linoleic acid (LA) is an essential fatty acid and can be obtained through the daily diet. It is well known that LA have cardiovascular protective, anti-cancer, neuroprotective, anti-osteoporotic, anti-inflammatory, and antioxidative effects (Kim et al., 2014). LA also could decrease the population of nematode in soil and reduce M. incognita infection of tomato roots through downregulating the expressions of Mi-flp-18 and Mi-mpk-1 (Dong et al., 2018). Nitrogen application and 2,4-epibrassinolide (EBR) treatment can also increase the concentrations of linoleic acid in purslane and loquat fruit (Chen et al., 2022; Montoya-García et al., 2018). In this study, we found that coir could increase the contents of linoleic acid (LA), polysaccharides (PS), and other a series of flavor substances in cucumber fruits (Tables 2 and 3). It indicated that coir could be wide-spread adopted as an organic substrate in cucumber production to improve the fruit quality.
As a rockwool alternative, physical properties of coir are generally suitable for soilless culture. Coir usually has higher wettability, hydrophilic character, structural stability, total porosity and air content, lower bulk density and total water-holding capacity, compared with sphagnum peat (Atzori et al., 2021). In our study, we also found that the coir had a higher aeration porosity and a lower water-holding porosity than rockwool, whether used or not used (Supplementary Table 2). Also, the limited change in aeration porosity and water-holding porosity of coir after being used also indicated that coir had higher structural stability and could ensure an optimal air and water content for crops.
5. Conclusion
In this study, we measured the chemical element of coir and rockwool and their drainage, the effect of coir and rockwool used as a substrate on the growth, fruit quality, flavor substance, and multi-element of cucumbers was also evaluated. We found coir was a potential substrate that could be widely used in greenhouse cucumber production. Compared with rockwool, coir showed higher P, K, Ca, and Mg content in the substrate and lower Fe, Cu and Mn content in the drainage. Coir not only increased the LAI and yield but also increased the concentration of essential amino acids (His, Leu, Ile, Phe, Lys, Asp, Glu and Pro) and flavor substance (TC, PS, TP, CLL, CuB and LA). Little research studied the effect of coir on the amino acids profile and some special flavor substances of cucumber fruits and further in-depth research is needed to elucidate the physiological and biochemical mechanisms, but our research is instrumental for developing and improving the application of coir as a soilless substrate to move towards a low rockwool sustainable horticulture.
Declarations
Author contribution statement
Jizhu Yu, Qiang Zhou: Conceived and designed the experiments.
Lizhong He: Performed the experiments; Wrote the paper.
Xiaotao Ding: Performed the experiments.
Haijun Jin, Hongmei Zhang: Analyzed and interpreted the data.
Jiawei Cui, Jianfeng Chu, Rongguang Li: Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by the National Key Research and Development Program of China, grant number: 2019YFD1001900.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
supplementary figure_V2.tiff.
References
- Ali B., Pantha S., Acharya R., Ueda Y., Wu L.-B., Ashrafuzzaman M., Ishizaki T., Wissuwa M., Bulley S., Frei M. Enhanced ascorbate level improves multi-stress tolerance in a widely grown indica rice variety without compromising its agronomic characteristics. J. Plant Physiol. 2019;240 doi: 10.1016/j.jplph.2019.152998. [DOI] [PubMed] [Google Scholar]
- Albuquerque P.B.S., de Oliveira W.F., dos Santos Silva P.M., dos Santos Correia M.T., Kennedy J.F., Coelho L.C.B.B. Skincare application of medicinal plant polysaccharides — a review. Carbohydr. Polym. 2022 doi: 10.1016/j.carbpol.2021.118824. [DOI] [PubMed] [Google Scholar]
- Alizadeh Z., Fattahi M. Essential oil, total phenolic, flavonoids, anthocyanins, carotenoids and antioxidant activity of cultivated Damask Rose (Rosa damascena) from Iran: with chemotyping approach concerning morphology and composition. Sci. Hortic. 2021;288 [Google Scholar]
- Antón A., Torrellas M., Montero J.I., Ruijs M., Vermeulen P., Stanghellini C. Acta Horticulturae. International Society for Horticultural Science (ISHS); Leuven, Belgium: 2012. Environmental impact assessment OF Dutch tomato crop production IN a venlo glasshouse; pp. 781–791. [Google Scholar]
- Atzori G., Pane C., Zaccardelli M., Cacini S., Massa D. Agronomy; 2021. The Role of Peat-free Organic Substrates in the Sustainable Management of Soilless Cultivations. [Google Scholar]
- Bai J., Liu J., Zhang N., Yang J., Sa R., Wu L. Effect of alkali stress on soluble sugar, antioxidant enzymes and yield of Oat. J. Integr. Agric. 2013;12:1441–1449. [Google Scholar]
- Barrett G.E., Alexander P.D., Robinson J.S., Bragg N.C. Achieving environmentally sustainable growing media for soilless plant cultivation systems – a review. Sci. Hortic. 2016;212:220–234. [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Braidot E., Zancani M., Petrussa E., Peresson C., Bertolini A., Patui S., Macrì F., Vianello A. Transport and accumulation of flavonoids in grapevine (Vitis vinifera L.) Plant Signal. Behav. 2008;3:626–632. doi: 10.4161/psb.3.9.6686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y., Zhang B., Liang L., Wang S., Zhang L., Wang L., Cui H.-L., Zhou Y., Wang D. A solid-state nanopore-based single-molecule approach for label-free characterization of plant polysaccharides. Plant Commun. 2021;2 doi: 10.1016/j.xplc.2020.100106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camposeco-Montejo N., Robledo-Torres V., Ramírez-Godina F., Mendoza-Villarreal R., Pérez-Rodríguez M.Á., Cabrera-de la Fuente M. Response of bell pepper to rootstock and greenhouse cultivation in coconut fiber or soil. Agronomy. 2018 [Google Scholar]
- Canas S., Assunção M., Brazão J., Zanol G., Eiras-Dias J.E. Phenolic compounds involved in grafting incompatibility of vitis spp: development and validation of an analytical method for their quantification. Phytochem. Anal. 2015;26:1–7. doi: 10.1002/pca.2526. [DOI] [PubMed] [Google Scholar]
- Carlile W.R., Raviv M., Prasad M. Soilless Culture: Theory and Practice Theory and Practice. Elsevier B.V.; 2019. Organic soilless media components; pp. 303–378. [Google Scholar]
- Che G., Zhang X. Molecular basis of cucumber fruit domestication. Curr. Opin. Plant Biol. 2019;47:38–46. doi: 10.1016/j.pbi.2018.08.006. [DOI] [PubMed] [Google Scholar]
- Chen G., Hou Y., Zheng Y., Jin P. 2,4-epibrassinolide enhance chilling tolerance of loquat fruit by regulating cell wall and membrane fatty acid metabolism. Sci. Hortic. 2022;295 [Google Scholar]
- Cho Y.Y., Oh S., Oh M.M., Son J.E. Estimation of individual leaf area, fresh weight, and dry weight of hydroponically grown cucumbers (Cucumis sativus L.) using leaf length, width, and SPAD value. Sci. Hortic. 2007;111:330–334. [Google Scholar]
- Chung S.W., Yu D.J., Lee H.J. Changes in anthocyanidin and anthocyanin pigments in highbush blueberry (Vaccinium corymbosum cv. Bluecrop) fruits during ripening. Horticult., Environ., Biotechnol. 2016;57:424–430. [Google Scholar]
- di Lonardo S., Cacini S., Becucci L., Lenzi A., Orsenigo S., Zubani L., Rossi G., Zaccheo P., Massa D. Testing new peat-free substrate mixtures for the cultivation of perennial herbaceous species: a case study on Leucanthemum vulgare Lam. Sci. Hortic. 2021;289 [Google Scholar]
- Dong L., Li X., Huang C., Lu Q., Li B., Yao Y., Liu T., Zuo Y. Reduced Meloidogyne incognita infection of tomato in the presence of castor and the involvement of fatty acids. Sci. Hortic. 2018;237:169–175. [Google Scholar]
- Feng B., Ren S., Hang L., Liu C., Dong L. Research on substrates as peat substitute for cut flower production of Oriental lily. Acta Hortic. Sin. 2010;37:1637–1644. [Google Scholar]
- Fornes F., Belda R.M., Lidón A. Analysis of two biochars and one hydrochar from different feedstock: focus set on environmental, nutritional and horticultural considerations. J. Clean. Prod. 2015;86:40–48. [Google Scholar]
- Fukuda S., Koba K., Okamura M., Watanabe Y., Hosoi J., Nakagomi K., Maeda H., Kondo M., Sugiura D. Novel technique for non-destructive LAI estimation by continuous measurement of NIR and PAR in rice canopy. Field Crop. Res. 2021;263 [Google Scholar]
- Guo Fang, Wang Tiecheng, He Zhao, Xu Jin, Wang Shujuan, Zhu Ning. Effects of arbuscular mycorrhizal fungus on growth and quality of greenhouse spring cucumber. J. Shanxi Agric. Sci. 2021;49:1308–1311. [Google Scholar]
- He L., Ding X., Jin H., Zhang Hongmei, Cui J., Zhou Q., Yu J. Effects of commercial rock wool strip and coco coir strip on growth, photosynthesis, yield and fruit quality of cucumber. China Veg. 2021;10:91–96. [Google Scholar]
- Heuvelink E., Bakker M.J., Elings A., Kaarsemaker R.C., Marcelis L.F.M. Acta Horticulturae. International Society for Horticultural Science (ISHS); Leuven, Belgium: 2005. Effect of leaf area on tomato yield; pp. 43–50. [Google Scholar]
- Hirakawa Y., Torii K.U., Uchida N. Mechanisms and strategies shaping plant peptide hormones. Plant Cell Physiol. 2017;58:1313–1318. doi: 10.1093/pcp/pcx069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y., Wu G. Nutritionally essential amino acids. Adv. Nutr. 2018;9:849–851. doi: 10.1093/advances/nmy054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia L., Yanhai J., Baoju W., Zhanghui W., Liu Mingchi, Wang Liping. Study on the concentration of tomato nutrient solution in pot culture with coconut bran composite substrate. Northern Hortic. 2020;6:9–16. [Google Scholar]
- Kavi Kishor P., Suravajhala R., Guddimalli R., Marka N., Kavya Shridhar K., Divya D., Scinthia K., Divya K., Doma M., Edupuganti S., Suravajhala P., Polavarapu R. Lysine, lysine-rich, serine, and serine-rich proteins: link between metabolism, development, and abiotic stress tolerance and the role of ncRNAs in their regulation. Front. Plant Sci. 2020;11 doi: 10.3389/fpls.2020.546213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K.-B., Nam Y.A., Kim H.S., Hayes A.W., Lee B.-M. α-Linolenic acid: nutraceutical, pharmacological and toxicological evaluation. Food Chem. Toxicol. 2014;70:163–178. doi: 10.1016/j.fct.2014.05.009. [DOI] [PubMed] [Google Scholar]
- Kingston P.H., Scagel C.F., Bryla D.R., Strik B. Suitability of sphagnum moss, coir, and Douglas fir bark as soilless substrates for container production of highbush blueberry. HortSci. Horts. 2017;52:1692–1699. [Google Scholar]
- Kudo Y., Kotani M., Tomita M., Aizawa Y. Effects of rock wool on the lungs evaluated by magnetometry and biopersistence test. J. Occup. Med. Toxicol. 2009;4:5. doi: 10.1186/1745-6673-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Xie F., Zhao Y., Cao J. Inhibitory effect of postharvest yeast mannan treatment on Alternaria rot of tomato fruit involving the enhancement of hemicellulose polysaccharides and antioxidant metabolism. Sci. Hortic. 2021;277 [Google Scholar]
- Luo F., Li Q., Yu L., Wang C., Qi H. High concentrations of CPPU promotes cucurbitacin B accumulation in melon (Cucumis melo var. makuwa Makino) fruit by inducing transcription factor CmBt. Plant Physiol. Biochem. 2020;154:770–781. doi: 10.1016/j.plaphy.2020.05.033. [DOI] [PubMed] [Google Scholar]
- Matsubara Y., Ishigaki T., Koshikawa K. Changes in free amino acid concentrations in mycorrhizal strawberry plants. Sci. Hortic. 2009;119:392–396. [Google Scholar]
- Mattson N., Lieth J.H. In: Soilless Culture. second ed. Raviv M., Lieth J.H., Bar-Tal A., editors. Elsevier; Boston: 2019. Chapter 12 – liquid culture hydroponic system Operation; pp. 567–585. [Google Scholar]
- Mi S., Zhang X., Wang Y., Ma Y., Sang Y., Wang X. Effect of different fertilizers on the physicochemical properties, chemical element and volatile composition of cucumbers. Food Chem. 2022;367 doi: 10.1016/j.foodchem.2021.130667. [DOI] [PubMed] [Google Scholar]
- Montoya-García C.O., Volke-Haller V.H., Trinidad-Santos A., Villanueva-Verduzco C. Change in the contents of fatty acids and antioxidant capacity of purslane in relation to fertilization. Sci. Hortic. 2018;234:152–159. [Google Scholar]
- Moretti S., Francini A., Hernández M.L., Martínez-Rivas J.M., Sebastiani L. Effect of saline irrigation on physiological traits, fatty acid composition and desaturase genes expression in olive fruit mesocarp. Plant Physiol. Biochem. 2019;141:423–430. doi: 10.1016/j.plaphy.2019.06.015. [DOI] [PubMed] [Google Scholar]
- Nerlich A., Dannehl D. Soilless cultivation: Dynamically changing chemical properties and physical conditions of organic substrates influence the plant phenotype of lettuce. Front. Plant Sci. 2021;11 doi: 10.3389/fpls.2020.601455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolle C., Cardinault N., Gueux E., Jaffrelo L., Rock E., Mazur A., Amouroux P., Rémésy C. Health effect of vegetable-based diet: lettuce consumption improves cholesterol metabolism and antioxidant status in the rat. Clin. Nutr. 2004;23:605–614. doi: 10.1016/j.clnu.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Qiao F., Zhang X.-M., Liu X., Chen J., Hu W.-J., Liu T.-W., Liu J.-Y., Zhu C.-Q., Ghoto K., Zhu X.-Y., Zheng H.-L. Elevated nitrogen metabolism and nitric oxide production are involved in Arabidopsis resistance to acid rain. Plant Physiol. Biochem. 2018;127:238–247. doi: 10.1016/j.plaphy.2018.03.025. [DOI] [PubMed] [Google Scholar]
- Ross P., Ronald J., Paramanandham P., Thenmozhi K.S., Abbiramy, Muthulingam M. Determination of physico-chemical properties of coir pith in relation to particle size suitable for potting medium. Int. J. Res. Environ. Sci. Technol. 2012;2:45–47. [Google Scholar]
- Scagel C.F. Growth and nutrient use of ericaceous plants grown in media amended with sphagnum moss peat or coir Dust. HortScience. 2003;38:46–54. [Google Scholar]
- Steiner C., Harttung T. Biochar as a growing media additive and peat substitute. Solid Earth. 2014;5:995–999. [Google Scholar]
- Tang W., Newton R.J. Increase of polyphenol oxidase and decrease of polyamines correlate with tissue browning in Virginia pine (Pinus virginiana Mill.) Plant Sci. 2004;167:621–628. [Google Scholar]
- Tong Z., Li H., Zhang R., Ma L., Dong J., Wang T. Co-downregulation of the hydroxycinnamoyl-CoA:shikimate hydroxycinnamoyl transferase and coumarate 3-hydroxylase significantly increases cellulose content in transgenic alfalfa (Medicago sativa L.) Plant Sci. 2015;239:230–237. doi: 10.1016/j.plantsci.2015.08.005. [DOI] [PubMed] [Google Scholar]
- Trovato M., Funck D., Forlani G., Okumoto S., Amir R. Editorial: amino acids in plants: regulation and functions in development and stress Defense. Front. Plant Sci. 2021 doi: 10.3389/fpls.2021.772810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gerrewey T., Ameloot N., Navarrete O., Vandecruys M., Perneel M., Boon N., Geelen D. Microbial activity in peat-reduced plant growing media: identifying influential growing medium constituents and physicochemical properties using fractional factorial design of experiments. J. Clean. Prod. 2020;256 [Google Scholar]
- Wang J.T., Wang Q., Han J.R. Yield, polysaccharides content and antioxidant properties of the mushroom Agaricus subrufescens produced on different substrates based on selected agricultural wastes. Sci. Hortic. 2013;157:84–89. [Google Scholar]
- Xing J., Gruda N., Xiong J., Liu W. Influence of organic substrates on nutrient accumulation and proteome changes in tomato-roots. Sci. Hortic. 2019;252:192–200. [Google Scholar]
- Xiong J., Tian Y., Wang J., Liu W., Chen Q. Comparison of coconut coir, rockwool, and peat cultivations for tomato production: nutrient balance, plant growth and fruit quality. Front. Plant Sci. 2017;8 doi: 10.3389/fpls.2017.01327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Zhang H., Zhong Y., Jiang N., Zhong X., Zhang Q., Chai S., Li H., Zhang Z. Comparative Genomics Analysis of bHLH Genes in Cucurbits Identifies a Novel Gene Regulating Cucurbitacin Biosynthesis. Hortic. Res. 2022:uhac038. doi: 10.1093/hr/uhac038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi S., Yongshuo M., Yuan Z., Huimin Z., Lixin D., Huiming C., Jianguo Z., Qian Z., Shenhao W., Wenjia G., Min L., Jinwei R., Xingfang G., Shengping Z., Ye W., Ken Y., J B.H., Xiaoquan Q., Zhonghua Z., J L.W., Sanwen H. Biosynthesis, regulation, and domestication of bitterness in cucumber. Science. 2014;346:1084–1088. doi: 10.1126/science.1259215. [DOI] [PubMed] [Google Scholar]
- Yuan L., Liu X., Luo M., Yang S., Wu K. Involvement of histone modifications in plant abiotic stress responses. J. Integr. Plant Biol. 2013 doi: 10.1111/jipb.12060. [DOI] [PubMed] [Google Scholar]
- Zhang F., Zhong H., Zhou X., Pan M., Xu J., Liu M., Wang M., Liu G., Xu T., Wang Y., Wu X., Xu Y. Grafting with Rootstocks Promotes Phenolic Compound Accumulation in Grape berry Skin during Development by Integrative Multi-Omics Analysis. Hortic. Res. 2022:uhac055. doi: 10.1093/hr/uhac055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Ma Y., Zeng J., Duan L., Xue X., Wang H., Lin T., Liu Z., Zeng K., Zhong Y., Zhang S., Hu Q., Liu M., Zhang H., Reed J., Moses T., Liu Xinyan, Huang P., Qing Z., Liu Xiubin, Tu P., Kuang H., Zhang Z., Osbourn A., Ro D.-K., Shang Y., Huang S. Convergence and divergence of bitterness biosynthesis and regulation in Cucurbitaceae. Nat. Plants. 2016;2 doi: 10.1038/nplants.2016.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.