Abstract
Ethiopian barley germplasm is a potential source of useful traits to fight the production challenges of barley farming and to enhance yield productivity in favorable and marginal environments. A study was carried out to assess the distribution and patterns of 17 qualitative trait variations among 85 Ethiopian barley accessions using an alpha lattice design with two replications. The Shannon-Weaver diversity (H′) index was used to estimate morphological diversity. Fifteen morphological traits of barley accessions originating from various regions of origins and altitude ranges were polymorphic. However, two traits including stem branching and lemma awn were monomorphic. The highest (0.94) overall mean of H′ was obtained for glume colour, kernel row and kernel shape. The estimated H′ ranged from 0.41 to 0.99 across regions, and 0.52 to 0.99 across altitude ranges with an overall mean of 0.76. The analysis of variance of H′ showed significant variation for most studied traits. Principal components analysis revealed that eight traits were the major loading on the first two principal components that describe 38.3% of the total morphological variance. Heat map analysis based on morphological traits of barley accessions was also grouped into three distinct clusters. Thus, the present finding confirmed that the Ethiopian barley accessions showed vast morphological variations across the region of origins and altitude ranges. Based on the result, further evaluation is ongoing to exploit specific gene variations through phenotyping and genotyping trait association.
Keywords: Accessions, Altitude range, Diversity index, Ex-situ, Hordeum vulgare, Region of origin, Qualitative trait
Accessions; Altitude range; Diversity index; Ex-situ; Hordeum vulgare; Region of origin; Qualitative trait.
1. Introduction
Barley (Hordeum vulgare L. 2n = 2x = 14) is widely cultivated on a global basis, it ranks fourth in terms of production, as a cool-season food cereal crop for humans (Purugganan and Fuller, 2009; FAOSTAT, 2019), feeds and malting for alcohol industries (Shaaf et al., 2019). It is believed to be among the oldest crop species in the world and it was domesticated from the large-seeded wild barley (Hordeum vulgare ssp. spontaneum) (Pourkheirandish and Komatsuda, 2007). It is grown worldwide and in a wide range of agro-ecological zones (Leino and Jenny, 2010; Russell et al., 2016).
Barley is one of the earliest cultivated and the most essential staple food crop in Ethiopia (MoARD, 2018; Bekele et al., 2020). It is used in numerous traditional foods such as injera, kinche, dabo, kolo, basso, porridge and in making local drinks including tela, borde, areki (Hailemichael and Sopade, 2011; Mohammed et al., 2016). Barley is also used as feed for animals, as a thatching of roots and as a source of income generation for many smallholder farmers in Ethiopia (Bekele et al., 2020). It constitutes one of the major crops (Taffesse et al., 2013) for food and industrial crops used as raw material for malting and brewing industries in Ethiopia (CSA, 2018).
Barley is grown in a broad range of agro-climatic regions under several production systems. It can be cultivated in the highland of Ethiopia (Oromia, Amhara, Tigray and part of SNNP regions) at altitude ranges of 1800 and 3500 meter above sea level, but the crop is most widely grown at altitudes between 2000 and 3000 masl (Lakew et al., 1997; Mohammed et al., 2016). It ranks fifth, after teff (Eragrostis tef), wheat (Triticum aestivum), maize (Zea mays) and sorghum (Sorghum bicolor) both in area coverage and quantity of production in the country (CSA, 2018). The main producing regions are Oromia, Amhara, Tigray and Southern Nations Nationalities and Peoples (SNNP), which account for about 99.5% of the total annual barley production (Bakana et al., 2018; MoARD, 2018). Ethiopia is the second-largest barley producer in Africa after Morocco (FAOSTAT, 2019), accounting for about 25% of the total barley production in Africa. However, the average yield of barley in the country is 2.18 tons ha−1 (CSA, 2018), which is slightly less than the world average of 2.89 tons ha−1 (FAOSTAT, 2019). Conversely, it is much less than the average yield in Belgium (8.6 t/ha), France (7.0 t/ha), and Germany (6.8 t/ha) (FAOSTAT, 2019). This low productivity of barley in Ethiopia is due to various production challenges such as drought and frost (Gardi et al., 2022), disease (Mulatu and Grando, 2011) and shootfly (Amsal et al., 1997; Tafa et al., 2004). Hence, the use of genetic diversity is one of the main approaches to enhancing crop productivity and achieving food security (Tilman et al., 2001; Hernandez et al., 2020; Temesgen, 2021).
Barley germplasm is a potential source of useful traits to fight the production challenges of barley farming and to enhance yield productivity. To cite the case, Ethiopian barley accessions are the source of valuable traits for Ethiopia and world agriculture, such as resistance and tolerance to disease (barley yellow dwarf virus, net blotch, powdery mildew, scald and loose smut) and insect pests (Yitbarek et al., 1998; Bonman et al., 2005), high lysine and protein content (Munck et al., 1970), and malting and brewing quality (Lance and Nilan, 1980). Thus, barley germplasm preserved in the Ethiopian Biodiversity Institute (EBI), with more than 16,000 accessions, is a useful resource of genetic diversity and can play an important role in developing new crop varieties having desirable traits that help to support resilience and enhance barley yield potential (www.ebi.gov.et).
Morphological diversity study is a very useful step prior to advanced tools such as molecular and genomic studies. Thus, understanding the existing morphological diversity in barley germplasm is an important step in enhancing the selection of breeding materials with varied genetic backgrounds and efficient management of crop genetic resources (Demissie and Bjørnstad, 1996; Nyiraguhirwa et al., 2021). Previous research reports showed that high morphological variation of barley germplasm in Ethiopia. Twenty two accessions for nine qualitative traits (Kebebew et al., 2001), forty four landraces for four qualitative traits (Assefa and Labuschagne, 2004), one hundred six landraces for eight qualitative traits (Tanto Hadado et al., 2009), forty three landraces for eight qualitative traits (Shumet and Tesema, 2014), one hundred two accessions for six qualitative traits (Mekonnon et al., 2015), thirty-six landraces for eleven qualitative traits (Addisu et al., 2018), and one hundred twenty landraces for seven qualitative traits (Gadissa et al., 2021) were reported. These reports provide primary information to distinguish genetic resources from diverse geographic sites in relation to efficient germplasm collection and valorization in barley breeding (Mekonnon et al., 2015; Kaur et al., 2022). Although these researchers realized the role of morphological diversity in past decades, in general, there is still a gap in the characterization and evaluation of the preserved germplasm and their valorization in barley breeding. These diversity studies on barley germplasm have evaluated a limited number of qualitative traits, some accessions from specific collection periods, and specific regions of origins. In addition, there is limited information about the diversity and practically useful morphological traits in barley breeding in Ethiopia (Abdi, 2011). Therefore, continuous characterization and evaluation of barley germplasm for qualitative traits can be an alternative approach to generate directly adaptable new varieties for smallholder farmers under both favorable and marginal production environments. Therefore, this study was conducted to assess the extent and patterns of morphological variation in Ethiopia's barley accessions.
2. Materials and methods
2.1. Experimental sites and plant materials
The experiment was conducted at Kulumsa Agricultural Research Center, located at 8°1′7″N latitude and 39°9′35″E longitude with an altitude of 2200 masl. It is found 160 km southeast of Addis Ababa and is characterized by an annual rainfall of 832 mm and with the means maximum and minimum temperature of 22.8 and 10.4 °C, respectively. The soil is classified as clay loam with a pH of 6 (Abebele et al., 2020).
Eighty five barley accessions having wide variation for various agro-morphological traits were evaluated at Kulumsa Agricultural Research Center during the two consecutive main cropping seasons in 2020 and 2021 under rain-fed conditions. The accessions were obtained from the ex-situ collection of the Ethiopian Biodiversity Institute along with their passport data (Table S1). The random sampling procedure was modified to allow the equal representation of barley accessions from 1976 to 2018 collection periods from four regions of the country (Oromia, Amhara, Tigray, and SNNPR) as well as at four major altitude ranges (less or equal to 2000, between 2001 to 2500, from 2501 to 3000, and above 3001 masl). The geographical distribution of the barley accessions for morphological characterization of the collection and testing sites is indicated in Figure 1.
Figure 1.
Map of Ethiopia showing the experimental sites, collection region of origins and their altitudes. Where: org = origins, Agr. Res. Center = Agricultural Research Center.
2.2. Experimental design and field management
The experiment was laid out in an alpha lattice design with two replications. The trial was arranged in 5 blocks, with 17 entries in each block. The gross plot area was 0.4 m2 which accommodates two rows having a length of 1m. The spacing between plots and rows was 0.5 m and 0.2 m, respectively. Ten grams of seed for each genotype were sowed manually at the rate of 125 kg ha−1, on 11 July 2020 and 19 July 2021. Nitrogen and phosphorous fertilizers were used at the recommended rate for the area, 50 kg ha−1 Urea and 120 kg ha−1 DAP at planting, respectively. Maximum care was taken during the experiment to minimize the possible confounding factors which could affect the expected result of the study. All other management practices such as hand weeding (three times weeding at seedling and vegetative stages) were uniformly applied.
2.3. Data collection
Each barley accession was morphologically characterized and observations on different qualitative traits were recorded on ten randomly selected plants from the most frequent variant in each accession following morphological and taxonomical descriptors based on the barley descriptors (IPGRI, 1994) (Table 1). Colour traits were also recorded based on the Munsell colour Company manual (1957).
Table 1.
Seventeen qualitative traits and their classes were used for the study.
| No | Traits | Classes |
|---|---|---|
| 1 | Stem pigmentation (Immature) | Green-1, Purple (basal only)-2, Purple (half or more)-3 |
| 2 | Leaf colour (LfC) | Light green-1, Medium green-2, Dark green-3 |
| 3 | Stem branching (StBr) | Opposite-1, Alternate-2, Ternate-3, Mixed-4 |
| 4 | Glume colour at hard dough stage (GlC) | White-1, Yellow-2, Brown-3, Black-4, Purple-5 |
| 5 | Glume colour at physiological maturity (GlCM) | White-1, Yellow-2, Brown-3, Black-4, Purple-5, Grey-6 |
| 6 | Lemma colour at the hard dough stage (LmC) | Amber/normal-1, Red-2, Purple-3, Black-4, Grey-5, Yellow-6, Brown-7 |
| 7 | Lemma colour at physiological maturity (LmCM) | |
| 8 | Glume hairiness (GlH) | Glabrous-1, Hairy-2 |
| 9 | Spike density (SpDs) | Lax-3, Intermediate-5, Dense-7 |
| 10 | Kernel row number (KrRw) | Two row-1, Irregular-3, Six row-5 |
| 11 | Awn colour (AwC) | Amber/White-1, Yellow-2, Brown-3, Reddish-4, Black-5 |
| 12 | Lemma awn (LmA) | Awnless-1, Awnleted-2, Awned-3, Sessile-4, Elevated-5 |
| 13 | Lemma awn barbs (LmAB) | Smooth-3, intermediate-5, Rough-7 |
| 14 | Beak length (BkLn) | Very short-1, Short-2, Medium-3, Long-4, Very long-5 |
| 15 | Beak shape (BkSh) | Straight-1, slightly curved-2, Medium curved-3, Strongly Curved-4, Geniculated-5 |
| 16 | Kernel shape (KrSh) | Spherical-1,Spherical-Flattened-2,Elongated-3, Elongated-Flattened-4 |
| 17 | Kernel colour (KrC) | White-1, Red-2, Purple-3, Black-4, Grey-5, Amber-6, Yellow-7, Brown-8 |
2.4. Data analysis
The percentage of frequency of the morphological traits for classes of each trait, region, and altitudinal ranges were calculated using GenStat (2015) for the seventeen qualitative traits. The chi-square (X2) was analyzed to test deviation from the pool mean of qualitative traits using the PROC FREQ procedure in SAS software for the region of origins and altitude ranges (SAS, 2008). The Shannon-Weiner evenness index (H′) was estimated for the region of origins and altitude ranges using GenStat (2015) with bootstrapping. A principal component analysis (PCA) was conducted using the autoplot.prcomp () function to summarize the variation in morphological traits. An ANOVA was performed on morphological data collected including region of origins and altitude ranges in R using the aov () function. The R program was also used to make a plot using heatmap () function using R.4.2 statistical software (R development core Team, 2022).
3. Results and discussion
3.1. Distribution of qualitative traits of barley across regions
The frequency distribution of each qualitative trait of barley accessions across regions is presented in Table 2 and showed high morphological diversity (Table 2). Chi-square (X2) values are also displayed to understand the degree of deviation of the observed frequency distribution of qualitative traits from expected values. The result indicated that the highest percentage frequency distribution of green and purple stems (basal only) was recorded in Oromia and Tigray regions with values of 90.9 and 53.3%, respectively. A comparable result was reported by Gadissa et al. (2021) showed that most collections of barley revealed green stem pigmentation. Light green leaf (66.7%) in the Tigray region followed by medium green leaf (51.9%) in the Amhara region as a dark-green leaf was the least abundant (9.5%) in the SNNP region. The high frequency of green stem and light green leaf colours in barley accessions can be valorized in drought resistance breeding. Indeed, the stay-green trait, retaining green leaf area, which is related to the retention of chlorophyll content has been identified as a key target trait for improving light interception and utilization and can contribute to enhancing cereal yield potential under moisture deficit growing conditions (Cossani and Reynolds, 2012; Tshikunde et al., 2019).
Table 2.
Frequency distribution of 17 qualitative traits (in percent of the total) of barley accessions by region of origins and altitude ranges. The chi-square (X2) statistic is given deviations of observed frequencies from the expectations.
| Traits | Classes |
Regions |
Chi-square (X2) |
Altitudes |
Chi-square (X2) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Oromia | Amhara | Tigray | SNNP | ≤2000 | 2001–2500 | 2501–3000 | ≥3001 | ||||
| StP | 1 | 90.9 | 74.1 | 20.0 | 85.7 | 149∗∗ | 63.6 | 65.5 | 73.3 | 86.7 | 63.5∗∗ |
| 2 | 4.5 | 22.2 | 53.3 | 9.5 | 9.1 | 31.0 | 16.7 | 13.3 | |||
| 3 | 4.5 | 3.7 | 26.7 | 4.8 | 27.3 | 3.4 | 10.0 | 0.0 | |||
| LfC | 1 | 45.5 | 48.1 | 66.7 | 52.4 | 28.4∗∗ | 63.6 | 58.6 | 50.0 | 33.3 | 40.6∗∗ |
| 2 | 45.5 | 51.9 | 33.3 | 38.1 | 27.3 | 34.5 | 46.7 | 66.7 | |||
| 3 | 9.1 | 0.0 | 0.0 | 9.5 | 9.1 | 6.9 | 3.3 | 0.0 | |||
| GlH | 1 | 86.4 | 81.5 | 73.3 | 71.4 | 8.6∗ | 54.5 | 75.9 | 80.0 | 100.0 | 60∗∗ |
| 2 | 13.6 | 18.5 | 26.7 | 28.6 | 45.5 | 24.1 | 20.0 | 0.0 | |||
| StBr | 1 | 0.0 | 0.0 | 0.0 | 0.0 | 0 | 0.0 | 0.0 | 0.0 | 0.0 | 0 |
| 2 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | |||
| AwC | 1 | 95.5 | 85.2 | 66.7 | 66.7 | 45∗∗ | 90.9 | 75.9 | 76.7 | 86.7 | 50∗∗ |
| 2 | 0.0 | 3.7 | 13.3 | 9.5 | 9.1 | 6.9 | 6.7 | 0.0 | |||
| 3 | 0.0 | 0.0 | 0.0 | 4.8 | 0.0 | 3.4 | 0.0 | 0.0 | |||
| 4 | 4.5 | 7.4 | 20.0 | 14.3 | 0.0 | 10.3 | 16.7 | 6.7 | |||
| 5 | 0.0 | 3.7 | 0.0 | 4.8 | 0.0 | 3.4 | 0.0 | 6.7 | |||
| SpDs | 3 | 9.1 | 22.2 | 40.0 | 33.3 | 40.4∗∗ | 54.5 | 27.6 | 13.3 | 20.0 | 48.7∗ |
| 5 | 63.3 | 66.7 | 53.33 | 47.6 | 36.4 | 58.6 | 66.7 | 60.0 | |||
| 7 | 27.3 | 11.1 | 6.7 | 19.0 | 9.1 | 13.8 | 20.0 | 20.0 | |||
| GlC | 1 | 50.0 | 44.4 | 60.0 | 57.1 | 23.8∗∗ | 63.6 | 44.8 | 43.3 | 73.3 | 45∗∗ |
| 2 | 9.1 | 0.0 | 0.0 | 4.8 | 9.1 | 0.0 | 6.7 | 0.0 | |||
| 3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |||
| 4 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |||
| 5 | 40.9 | 55.6 | 40.0 | 38.1 | 27.3 | 55.2 | 50.0 | 26.7 | |||
| GlCM | 1 | 77.3 | 59.3 | 86.7 | 66.7 | 57∗∗ | 72.7 | 62.1 | 76.7 | 73.3 | 74.4∗∗ |
| 2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |||
| 3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |||
| 4 | 4.5 | 14.8 | 6.7 | 28.6 | 0.0 | 13.8 | 13.3 | 26.7 | |||
| 5 | 13.6 | 25.9 | 6.7 | 4.8 | 27.3 | 24.1 | 6.7 | 0.0 | |||
| 6 | 4.5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 3.3 | 0.0 | |||
| LmC | 1 | 54.5 | 37.0 | 66.7 | 52.4 | 70.1∗∗ | 54.5 | 44.8 | 46.7 | 66.7 | 87.6∗∗ |
| 2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |||
| 3 | 36.4 | 59.3 | 33.3 | 42.9 | 27.3 | 55.2 | 50.0 | 26.7 | |||
| 4 | 0.0 | 3.7 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 6.7 | |||
| 5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |||
| 6 | 9.1 | 0.0 | 0.0 | 0.0 | 9.1 | 0.0 | 3.3 | 0.0 | |||
| 7 | 0.0 | 0.0 | 0.0 | 4.8 | 9.1 | 0.0 | 0.0 | 0.0 | |||
| LmCM | 1 | 50.0 | 48.4 | 38.0 | 52.4 | 117.2∗∗ | 27.3 | 37.9 | 60.0 | 60.0 | 118∗∗ |
| 2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |||
| 3 | 4.5 | 21.0 | 22.0 | 4.8 | 27.3 | 17.2 | 10.0 | 0.0 | |||
| 4 | 4.5 | 15.8 | 6.7 | 28.6 | 0.0 | 13.8 | 13.3 | 26.7 | |||
| 5 | 22.7 | 14.8 | 33.3 | 9.5 | 27.3 | 27.2 | 13.3 | 6.7 | |||
| 6 | 13.6 | 0.0 | 0.0 | 0.0 | 9.1 | 3.4 | 3.3 | 0.0 | |||
| 7 | 4.5 | 0.0 | 0.0 | 4.8 | 9.1 | 0.0 | 0.0 | 6.7 | |||
| KrRw | 1 | 18.2 | 37.0 | 80.0 | 47.0 | 97.8∗∗ | 63.6 | 58.6 | 26.7 | 26.7 | 79.1∗∗ |
| 3 | 18.2 | 7.4 | 6.7 | 0.0 | 0.0 | 6.9 | 16.7 | 0.0 | |||
| 5 | 63.6 | 55.6 | 13.3 | 52.4 | 36.4 | 34.5 | 56.7 | 73.3 | |||
| LmAB | 3 | 81.8 | 81.5 | 80.0 | 57.1 | 23.1∗∗ | 72.7 | 72.4 | 73.3 | 86.7 | 8.1∗∗ |
| 5 | 18.2 | 18.5 | 20.0 | 42.9 | 27.3 | 27.6 | 26.7 | 13.3 | |||
| 7 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |||
| LmA | 1 | 0.0 | 0.0 | 0.0 | 0.0 | 0 | 0.0 | 0.0 | 0.0 | 0.0 | 0 |
| 2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |||
| 3 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | |||
| 4 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |||
| 5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |||
| BkLn | 1 | 0.0 | 7.4 | 6.7 | 4.8 | 46.3∗∗ | 0.0 | 6.9 | 3.3 | 6.7 | 60.1∗∗ |
| 2 | 4.5 | 3.7 | 13.3 | 0.0 | 9.1 | 6.9 | 3.3 | 0.0 | |||
| 3 | 27.3 | 33.3 | 46.7 | 42.9 | 27.3 | 41.4 | 26.7 | 53.3 | |||
| 4 | 59.1 | 51.9 | 26.7 | 47.6 | 45.5 | 44.8 | 60.0 | 33.3 | |||
| 5 | 9.1 | 3.7 | 6.7 | 4.8 | 18.2 | 0.0 | 6.7 | 6.7 | |||
| BkSh | 1 | 18.2 | 40.7 | 40.0 | 28.6 | 19.4∗ | 36.4 | 37.9 | 23.3 | 33.3 | 22.8∗∗ |
| 2 | 63.6 | 44.4 | 40.0 | 47.6 | 45.5 | 41.4 | 56.7 | 53.3 | |||
| 3 | 18.2 | 14.8 | 20.0 | 23.8 | 18.2 | 20.7 | 20.0 | 13.3 | |||
| 4 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |||
| 5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |||
| KrSh | 1 | 0.0 | 0.0 | 0.0 | 0.0 | 45∗∗ | 0.0 | 0.0 | 0.0 | 0.0 | 30.8∗∗ |
| 2 | 4.5 | 3.7 | 20.0 | 0.0 | 0.0 | 10.3 | 3.3 | 6.7 | |||
| 3 | 59.1 | 44.4 | 53.3 | 52.4 | 36.4 | 44.8 | 63.7 | 53.3 | |||
| 4 | 36.4 | 51.9 | 26.7 | 47.6 | 63.5 | 44.8 | 33.1 | 40.0 | |||
| KrC | 1 | 54.5 | 40.7 | 26.7 | 57.1 | 132.5∗∗ | 36.4 | 34.5 | 56.7 | 53.3 | 114.8∗∗ |
| 2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |||
| 3 | 4.5 | 22.2 | 20.0 | 4.8 | 27.3 | 17.2 | 10.0 | 0.0 | |||
| 4 | 4.5 | 14.8 | 6.7 | 28.6 | 0.0 | 13.8 | 13.3 | 26.7 | |||
| 5 | 18.2 | 14.8 | 33.3 | 4.8 | 18.2 | 24.1 | 13.3 | 6.7 | |||
| 6 | 0.0 | 7.4 | 6.7 | 0.0 | 0.0 | 6.9 | 0.0 | 6.7 | |||
| 7 | 13.6 | 0.0 | 6.7 | 0.0 | 9.1 | 3.4 | 6.7 | 0.0 | |||
| 8 | 4.5 | 0.0 | 0.0 | 4.8 | 9.1 | 0.0 | 0.0 | 6.7 | |||
Abbreviations: StP = Stem pigmentation, LfC = Leaf colour, GlH = Glume hairiness, StBr = Stem branching, AwC = Awn colour, SpDs = Spike density, GlC = Glume colour at hard dough stage, GlCM = Glume colour at physiological maturity stage, LmC = Lemma colour at hard dough stage and LmCM = Lemma colour at physiological maturity stage. KrRw = Kernel rows, LmAB = Lemma awn barbs, LmA = Lemma awn, BkLn = Beak length, BkSh = Beak shape, KrSh = Kernel shape and KrC = Kernel colour. ∗∗, significant at the 1% level; ∗ significant at the 5 % level; n.s, not significant.
Awn colours of barley accessions showed different distribution among the region of origins (Table 2). Among the awn colours, amber or white was the most frequent (95.5%) colour in the Oromia region, however, reddish (20.0%), yellow (13.3%), brown (4.8%) and black (4.8%) colours were with low frequencies. Similarly, in Derbew et al. (2013), most of the barley landraces were amber or white in awn colour followed by red and yellow. On the contrary, Bouhaouel et al. (2019) showed yellow awn colour (99%) was dominant in Tunisian barley accessions. White GlC was highly variable with a percentage frequency distribution of 60.0% in the Tigray region followed by purple colour (55.6%) in the Amhara region (Table 2, Figure S1A and B). In contrast, Tanto Hadado et al. (2009) found that the most frequent glumes colour was white followed by brown with black and yellow colours. Besides, white (86.7%) and black (28.6%) glume colours were commonly obtained in Tigray and SNNP regions at the maturity stage (Table 2, Figure S2A). The amber or normal (66.7%) in the Tigray and purple (59.3%) in the Amhara regions were the most prevalent LmCs (Table 2). On the other hand, both amber or normal (52.2%) and black (28.6%) colours were predominantly obtained from the SNNP regions whereas grey (33.3%) colour was mainly recorded in the Tigray region at maturity time (Table 2, Figure S2A). This study explained by the dominance of the traits may be due to farmers’ selections for desirable traits being key forces in determining the variations of the barley accessions (Kebebew et al., 2001).
Awned LmA was monomorphic in all regions, while awnless, awnleted, sessile and elevated traits were absent (Table 2). This result is comparatively consistent with the awned spikes that were predominant (96% and 89%) of barley materials from India, Syria, Canada, the USA and Mexico (Kaur et al., 2022) and from the world collection (Tolbert et al., 1979), respectively. The presence of awn is an advantageous trait in cereals (Ahmadi et al., 2018; Ntakirutimana and Xie, 2020) which may be associated with tolerance to drought stress climatic conditions as well as an adaptive structure against leaf diseases (Paperson and Ohm, 1975; Negassa, 1986), yet further evaluations are required to understand the relationship between awn length, and grain yield potential in moisture deficit area as well in leaf disease affected environments. Research report showed that the presence of awns helps the spikes to maintain higher rates of photosynthesis and WUE throughout the grain filling period as well as significantly enhance kernel dry weight in barley (Bort et al., 1994). Besides, awns could improve yield potential by improving the photosynthetic rate and WUE in wheat (Motzo and Giunta, 2002).
Spike related traits of barley accessions showed different distribution among regions (Table 2), which are useful traits of smallholder farmers in Ethiopia (Mancini et al., 2017) as well as a selection tool that provides key traits for yield enhancement in breeding (Gao et al., 2015; Allel et al., 2017). Intermediate SpDs (66.7%) was commonly found in the Amhara region followed by lax type (40.0%) in the Tigray region and dense type (27.3%) in the Oromia region. Two-rowed (80.0%) is the predominant type of KrRw in the Tigray region while six-rowed (63.6% and 55.6%) was frequently found in the Oromia and Amhara regions, respectively. The prevalence of these traits may be related to the presence of better precipitations in the highlands of Oromia and Amhara than in the lowlands of the Tigray regions. These results are in agreement with Bouhaouel et al. (2019) and Tolbert et al. (1979) who reported the dominance of the six-rowed number in the Tunisian barley collection and the world barley collection, respectively. Six-rowed barley is associated with a higher number of seeds per spike and enhanced productivity at higher elevations and high rainfall areas (Pourkheirandish and Komatsuda, 2007), while the two-rowed barley is frequently produced in marginal areas (García del Moral et al., 2003).
The high percentage distribution of long (59.1%) and medium (46.7%) types of BkLn was recorded in Oromia and Tigray regions, respectively (Table 2). The BkSh of slightly curved (63.6%) in Oromia and straight (40.7%) types in Amhara regions showed the highest frequency (Table 2, Figure S2B). Correspondingly, the elongated (59.1%) and elongated-flattened (51.9%) kernel shapes were primarily originated in Oromia and Amhara regions, respectively, and this material can be valorized in barley quality improvement in Ethiopia and beyond. Indeed, the landraces of barley from Ethiopia are a source of genes that control useful nutrient traits, such as high lysine and protein quality and content (Munck et al., 1970). The kernel size and shape related traits showed different distribution among regions (Table 2), this may be associated with local requirements to cultural preferences such as cooking and taste qualities, and local adaptation. Earlier research reports showed that kernel shape is one of the most determinant traits for the preparation of food and taste qualities (Sun et al., 2013), and malting quality (Nielsen, 2003).
Kernel colours (KrC) of barley accessions showed different distribution among regions (Table 2, Figure 2A–E). KrC was variable with 1–8 classes (Table 2). The existence of different KrC could be linked to the local requirement of farmers’ traits for making cultural purposes (Asfaw, 1988), which could ensure the in-situ conservation of indigenous knowledge by the farming community in Ethiopia (Abebe et al., 2010). White colour was predominantly observed in the SNNP region (57.1%) followed by Oromia (54.5%), Amhara (40.7%) and Tigray (26.7 %) regions (Table 2, Figure 2A), which may be associated with Ethiopian smallholder farmer selection pressures operating for white phenotypic class (Demissie and Bjørnstad, 1996). A similar finding was reported by (Assefa and Labuschagne, 2004; Tanto Hadado et al., 2009) indicating that the majority of Ethiopian barley landraces have white kernel colour. Unlikely, the barley accessions were largely characterized by the grey kernel colour (91%) in Tunisia (Bouhaouel et al., 2019). The highest frequency of black kernel accessions was observed from the region of SNNP and Amhara regions (Table 2, Figure 2C). The brown kernel was only sourced from Oromia and SNNP regions while the red kernel was absent in all regions. The purple kernel was frequent in Amhara and Tigray regions (Table 2, Figure 2D). Grey was dominant in the Tigray region (Table 2, Figure 2B).
Figure 2.
Diversity of kernel colour of barley accessions (A) White (64255, 17672, 242096, 27507, 17190, 236254, 15397, 29689, 18319, 244774, 235059, 29524, 15366, 208857, 244890, 25915, 29523, 29682, 235057, 239077, 29705, 15277, 1625, 16857, 208843, 208845, 16853, 64265, 237839, 242092, 30228, 1661, 15291, 16739, 16861, 236811,30230,243574, 3248), (B) Grey (242583, 242584, 234311, 4496, 235252, 242067, 15255, 17220, 239538, 18871, 208815, 3509, 17231, 16862) (C) Black (16804, 1637, 26514, 244771, 244772, 15260, 243605, 16737, 1652, 16863, 235530, 24126) (D) Purple (24237, 3470, 244944, 3489, 3473, 240795, 242094, 26519, 17255, 243586, 238349) and (E) Amber colours (25919, 9950, 243598). Numbers in the bracket are accession numbers at various classes of kernel colour. The kernel colours of barley accessions are measured with a scale of 1–8 classes (white-1, red-2, purple-3, black-4, grey-5, amber-6, yellow-7 and brown-8) following the barley descriptors.
3.2. Distribution of qualitative traits in the four altitude ranges
The frequency distribution of the 17 qualitative traits of barley accessions was observed in relation with four altitude ranges (Table 2). Chi-square (X2) values are also indicated to understand the degree of deviation of the observed frequency distribution of qualitative traits from expected values. Research report showed that variations of barley traits at different altitudes may have resulted from the random or selective accumulation of certain genes at high or low altitudes and partly from farmers’ selection of morphological types (Asfaw, 1989). High percentage frequency distribution was obtained in the green stem (86.7%) at higher altitudes followed by the purple stem (31.0%) (basal only) at medium altitudes. Consecutively, medium (66.7%) and light green (63.6%) leaves were observed at higher and lower altitudinal ranges, respectively. Leaf traits are important for breeding of enhanced grain yield in cereals (Mathan et al., 2016: Shaaf et al., 2019) and determining yield potential (Tesso et al., 2011), traits related to photosynthetic efficiency as well as to water balance are less exploited in barley breeding programs in Ethiopia. Leaf traits could be useful for enhancing the performance of triticale under drought stress growing conditions (Lonbani and Arzani, 2011). Therefore, it is good to consider strategic breeding combines leaf traits with measured traits to enhance the grain yield of barley under both favourable and marginal production conditions.
The distribution of the two classes of GlH, glabrous and hairy, showed a strong trend in the four altitude ranges (Table 2). Glabrous increased its frequency of distribution from 54.5% in lowland (≤2000) to 100% in highland (≥3001), while hairy type decreased its distribution from 45.5% in low altitude (≤2000) and absent at greater than 3001 masl. Higher altitude in Ethiopia is characterized by high rainfall, low temperature and frost (Etana et al., 2020). The more abundance of glabrous in highland and hairy in lowland may be associated with adaptive significance in drought and frost-affected areas in Ethiopia, respectively. Glume hairiness is usually common in Ethiopian wheat (Negassa, 1986), it can be used by breeders engaged in resistance breeding to insects. A similar finding was reported in this study by Belay et al. (1997) indicating that the greater parts of Ethiopian wheat landraces have glabrous glume with preset at the highest altitudes.
The most abundant GlC at the hard dough stage was white colour (73.6%) at higher altitudes (≥3001 masl) followed by purple colour (55.2%) at medium altitudes (2001–2500 masl) (Table 2, Figure S1A and B). At the GlCM stage, white (76.7%) and purple (27.3%) colours were also mainly occurred at altitudes 2501–3000 and ≤2000 masl, respectively (Table 2). The variations of glume colours from the medium altitudes towards the lower and higher altitudes were decreased; this is possible due to the direct influence of both human and natural selection (Shumet and Tesema, 2014). The LmC of amber or normal (66.7%) in higher altitudes and purple (55.2%) in medium altitudes had abundantly occurred while yellow (9.1%), brown (9.1%) and black (6.7%) colours were the least abundances. This predominance of LmC in this study is in line with Demissie and Bjørnstad (1996), the amber or white or normal colour was the prevalent lemma colour in Ethiopian barley collections. Additionally, white or normal colour was predominately found at higher altitudes (60%) followed by purple (27.3%) and grey (27.3%) colours recorded at lower altitudes during maturity.
The distribution of spike density (SpDs) in barley accessions showed different distributions in the four altitude ranges (Table 2). Intermediate SpDs (66.7%) was the most abundant class for all altitude ranges except in lower altitude (≤2000). Lax SpDs was found in higher frequency at lower altitude while the dense type had a relatively higher frequency at higher altitude (Table 2). This result is in agreement with the finding of Kebebew et al. (2001), who reported that intermediate SpDs was largely found at higher altitudes whereas the lax type concentrated below 2650 masl. Research report showed a significant association between altitude and spike related traits in Ethiopian barley (Asfaw, 1989). Indeed, variation in altitude ranges affects barley cultivation in Ethiopia by influencing the amount of rainfall and temperature (Engels, 1994). Further, moisture deficit is decreased from lower altitudes towards higher altitude ranges in central Ethiopia (Etana et al., 2020).
The distribution of KrRw types varied in the four altitude ranges (Table 2). Six-rowed type in barley accessions (73.3%) was the most dominant in higher altitude range (≥3001 masl) while two-rowed barley (63.6%) was more dominant in lower altitude (≤2000 masl), this could be related to local adaptation/related to the amount and distribution of rainfall and temperature (Demissie and Bjørnstad, 1996; Kebebew et al., 2001). Two-rowed barley over six-rowed barley is earlier to mature, which is a useful trait for breeding to escape high temperature and low precipitations during grain filling periods (Kandic et al., 2018), while six-rowed barley is a high yielder type at higher altitudes and high rainfall areas (Pourkheirandish and Komatsuda, 2007).
Kernel related traits of barley accessions including beak length (BkLns), beak shape (BkSh) and kernel shape (KrSh) showed different distribution in the four altitude ranges (Table 2). High frequency of long (60%) and medium (53%) BkLns was common in higher altitudes. The slightly curved (56.7%) BkSh was mainly observed at 2501–3000 masl, followed by the straight (37.9%) BkSh at medium altitude (Figure S2B). However, KrSh of the elongated-flattened and elongated were mainly found at altitudes ≤2000 and 2501–3000 masl, respectively. The dominant traits of BkLn and BkSh were largely originated from higher altitudes except for KrSh which was concentrated at lower and higher altitudes. This showed that most of them are high variations may due to they are particularly influenced by altitude, climate and soil conditions and human selections. Previously described by the human being dependent on the yields for it is the existence or a variation of it is desirable traits (Fuller, 2007).
The high frequency of long, slightly curved and elongated traits of kernels in this study (Table 2) indicates that these barley accessions may be valorized in quality and yield enhancement breeding programs. Indeed, kernel related traits are important yield related traits in barley breeding (Xin et al., 2018). Yield has been highly related with enhanced kernel size in cereals (Kesavan et al., 2013; Tshikunde et al., 2019). Kernel size is characterized by the grain width, grain length and thousand grain weights (Li et al., 2019). Variation of kernel size related traits in Ethiopian barley germplasm has received little research attention. Therefore, future barley research should focus on understanding the variation and relationship among kernel related traits and grain yield potential, which will enhance the efficiency of genetic selection in barley breeding and improvement programs.
The barley accessions showed a wide range of kernel colour (KrC) distribution in the four altitude ranges (Table 2, Figure 2A–E). Among the KrC, the high frequency was observed in white colour (56.7%) followed by purple (27.3%), black (26.7%) and grey (24.1%) colours, and the white colour was the most abundant for all altitude ranges. The possible factors explained by responsible for the highest frequency of the white kernel colours could be the human selection for barley against the pigmented strains (Demissie and Bjørnstad, 1996). The white colour showed an increasing trend from low to high altitude, while the brown colour showed decreasing trend from low to high altitude ranges this may be related to the local requirement of smallholder farmers for making various traditional foods and local adaptive evolution. This result agreed with the findings of, Amsalu and Endashaw (1998) for sorghum and Woldeyohannes et al. (2020) for teff crops, who reported that kernel colours showed altitude variations in Ethiopian accessions. Though the adaptive implication of kernel colour has not been well-known, a previous study by Abate et al. (2013) revealed that the brown seeded Dima variety (nationally released teff variety for production) showed better Al- tolerance indices than the white seeded ones.
3.3. Diversity index of qualitative traits
The level of barley diversity was analyzed using the Shannon-Weaver diversity index (H′) in relation to region of origins and altitude ranges (Table 3). Across the region of origins, the overall mean values for H′ in Oromia, Amhara, SNNP and Tigray regions were 0.71, 0.76, 0.77 and 0.79, respectively (Table 3), indicating high H′ in the barley accessions for the current and future barley breeding purpose. High H′ values were estimated across the regions with the highest (0.99) values for LfC and GlC in Amhara, GlC in Tigray and KrRw, KrSh and LmAB in SNNP regions. However, very low to low H′ values were estimated for StBr (H′ = 0.00) and LmA (H′ = 0.00) in all regions, AwC (H′ = 0.41) and StP (H′ = 0.46) in the Oromia region, and StP(H′ = 0.58) in SNNP regions. Relatively overall lower estimates of H′ in the Oromia region are probably related to the cultivation of commercial varieties of barley by smallholder farmers in the highlands of the region (Dawit and Zewdie, 2019). Therefore, future collection campaigns should focus on remote areas of the Oromia region to capture the barley diversity for the breeding program.
Table 3.
Estimates of morphological diversity index (H′) for 17 qualitative traits of barley accessions within region of origins and altitude ranges, the value of H′ ranges from 0 to 1, with greater values corresponding to greater diversity in the corresponding class. Mean bootstrap estimates are given with the corresponding standard error.
| Traits | Regions |
Altitude ranges |
Overall mean diversity | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oromia |
Amhara |
Tigray |
SNNP |
BSE | BSs.e | ≤2000 |
2001–2500 |
2501–3000 |
≥3001 |
BSE | BSs.e | ||
| H′ | H′ | H′ | H′ | H′ | H′ | H′ | H′ | ||||||
| StP | 0.46 | 0.69 | 0.96 | 0.58 | 0.83 | 0.02 | 0.88 | 0.73 | 0.79 | 0.64 | 0.80 | 0.02 | 0.72 |
| LfC | 0.87 | 0.99 | 0.95 | 0.87 | 0.77 | 0.03 | 0.84 | 0.82 | 0.75 | 0.96 | 0.77 | 0.03 | 0.89 |
| StBr | 0.00 | 0.00 | 0.00 | 0.00 | – | – | 0.00 | 0.00 | 0.00 | 0.00 | – | – | – |
| GlH | 0.65 | 0.76 | 0.89 | 0.91 | 0.8 | 0.05 | 0.00 | 0.86 | 0.79 | 0.99 | 0.81 | 0.05 | 0.88 |
| GlC | 0.94 | 0.99 | 0.99 | 0.91 | 0.9 | 0.01 | 0.93 | 0.99 | 0.92 | 0.94 | 0.90 | 0.02 | 0.94 |
| LmC | 0.89 | 0.77 | 0.96 | 0.79 | 0.68 | 0.02 | 0.91 | 0.99 | 0.75 | 0.83 | 0.68 | 0.02 | 0.86 |
| AwC | 0.41 | 0.64 | 0.90 | 0.83 | 0.67 | 0.04 | 0.52 | 0.74 | 0.81 | 0.68 | 0.66 | 0.03 | 0.68 |
| KrRw | 0.91 | 0.88 | 0.73 | 0.99 | 0.97 | 0.01 | 0.98 | 0.88 | 0.93 | 0.95 | 0.97 | 0.01 | 0.94 |
| SpDs | 0.91 | 0.83 | 0.81 | 0.95 | 0.89 | 0.02 | 0.86 | 0.89 | 0.88 | 0.92 | 0.90 | 0.02 | 0.90 |
| GlCM | 0.79 | 0.95 | 0.68 | 0.83 | 0.82 | 0.02 | 0.95 | 0.94 | 0.78 | 0.93 | 0.83 | 0.02 | 0.91 |
| LmCM | 0.91 | 0.97 | 0.94 | 0.88 | 0.88 | 0.01 | 0.95 | 0.92 | 0.88 | 0.87 | 0.88 | 0.01 | 0.89 |
| LmAB | 0.76 | 0.76 | 0.79 | 0.99 | 0.85 | 0.04 | 0.89 | 0.89 | 0.89 | 0.65 | 0.84 | 0.05 | 0.76 |
| LmA | 0.00 | 0.00 | 0.00 | 0.00 | – | – | 0.00 | 0.00 | 0.00 | 0.00 | – | – | – |
| BkSh | 0.89 | 0.93 | 0.97 | 0.97 | 0.94 | 0.02 | 0.95 | 0.97 | 0.93 | 0.90 | 0.94 | 0.02 | 0.92 |
| BkLn | 0.87 | 0.83 | 0.91 | 0.86 | 0.81 | 0.01 | 0.95 | 0.92 | 0.84 | 0.89 | 0.81 | 0.02 | 0.90 |
| KrSh | 0.91 | 0.92 | 0.96 | 0.99 | 0.93 | 0.01 | 0.98 | 0.96 | 0.90 | 0.94 | 0.92 | 0.01 | 0.94 |
| KrC | 0.91 | 0.96 | 0.92 | 0.84 | 0.89 | 0.01 | 0.96 | 0.93 | 0.93 | 0.90 | 0.89 | 0.01 | 0.92 |
| 0.71 | 0.76 | 0.79 | 0.77 | 0.74 | 0.79 | 0.75 | 0.76 | ||||||
| Overall mean in Ethiopia | 0.76 | ||||||||||||
Abbreviations: H′ = Shannon-Weiner index, BSE = Bootstrap estimate, BSs.e = Bootstrap s.e., StP = Stem pigmentation, LfC = Leaf colour, StBr = Stem branching, GlH = Glume hairiness, GlC = Glume colour at hard dough stage, LmC = Lemma colour at hard dough stage, AwC = Awn colour, KrRw = Kernel rows, SpDs = Spike density, GlCM = Glume colour at physiological maturity stage, LmCM = Lemma colour at physiological maturity stage, LmAB = Lemma awn barbs, LmA = Lemma awn, BkSh = Beak shape, BkLn = Beak length, KrSh = Kernel shape and KrC = Kernel colour.
The H′ values for each qualitative trait, over regions and altitude ranges, varied from 0.68 for AwC to 0.94 for KrRw, GlC and KrSh with an overall mean of 0.76 (Table 3), indicating the accessions harnesses many useful traits and merit further evaluation. The overall mean H′ obtained in this study was higher than that reported in earlier Ethiopian barley germplasm collection (Demissie and Bjørnstad, 1996, H′ = 0.71; Addisu et al., 2018, H′ = 0.55), Nepalese naked barley landraces (Yadav et al., 2018, H′ = 0.73), Algerian barley accessions (Taibi et al., 2019, H′ = 0.53), Tunisian barley landraces (Bouhaouel et al., 2019, H′ = 0.28) and the world barley collection (Tolbert et al., 1979, H′ = 0.50). This is likely due to variation in source and type of barley materials, and the variation of studied qualitative traits. In general, our results confirm the huge variation of Ethiopian barley genetic resources and need further evaluation for breeding purposes.
The diversity index of barley accessions was also estimated across four altitude ranges. Across the altitude ranges, the overall mean value for H′ was 0.74 for altitude ≤2000, 0.79 for altitude ranges 2001–2500, 0.75 for 2501–3000 and 0.76 for altitude ≥3001 masl, suggesting high genetic diversity and this calls for barley breeding to valorize these resources to improve new barley varieties for different growing areas. Most qualitative traits such as spike, kernel and leaf related traits showed very high diversity index values (Table 3) over the four altitude ranges, indicating that the barley accessions had high divergence for these traits. However, a very low H′ value was estimated for StBr (H′ = 0.00) and LmA (H′ = 0.00) in all altitude ranges. The barley accessions showed higher diversity at an altitude range of 2001–2500 masl, this may be associated with climatic variability and agro-ecological heterogeneity in these altitude ranges (Demissie and Bjørnstad, 1997). The research report also showed that the variation in the collection of genetic resources is related to the climatic variation existing across the adaptation zones (Woldeyohannes et al., 2020). Our result is in agreement with the highest variation of traits at medium altitude ranges in the Ethiopian barley collection (Shumet and Tesema, 2014).
The analysis of the variance of diversity index (H′) based on the regions showed highly significant (P < 0.01) variation for StP, LfC, GlC, LmC, LmCM, KrRw and KrC. There were also significant variations for GlCM and KrSh (P < 0.05) (Table 4) showing a variation for useful morphological traits of breeding importance. Previous research reports showed that high variability for agro morphological traits in Ethiopian barley collection (Demissie and Bjørnstad, 1997; Abebe et al., 2010), an ancient world barley collection (Karagöz et al., 2017), North African barley germplasm (Allel et al., 2017), Tunisia barley landraces (Bouhaouel et al., 2019), Algerian barley accessions (Taibi et al., 2019) and the world barley collection (Tolbert et al., 1979), which could be a source of a new gene for valorizing in future barley improvement program for world agriculture. A very high significant difference (P < 0.001) was observed at altitudinal ranges for StP and GlC (Table 4). The altitudes exhibited a highly significant difference (P < 0.01) in traits of GlH and GlCM. However, KrRw, LmCM, LmAB and KrC were significant variations (P < 0.05) (Table 4). In general, this huge morphological variation suggests that there may be a margin to further enhance useful traits either through a crossing of molecular breeding to support barley yield potential in both favourable and marginal environments. The regional and altitudinal level of significant difference in traits in this study is supported by Kebebew et al. (2001), the distinctive agro-ecological conditions, and differences in natural and artificial selection pressure may have resulted in the significant difference. The present result is explained by the humans selecting traits based on particular needs and utilization may have also an effect on variation (Fuller, 2007).
Table 4.
Mean square values from the analysis of variance of diversity index (H′) for the region of origins and altitude ranges based on 15 morphological traits.
| Traits | Region of origins (df = 3) |
Altitude ranges (df = 3) |
|---|---|---|
| Mean square | Mean square | |
| StP | 0.08∗∗ | 0.020∗∗∗ |
| LfC | 0.007∗∗ | 0.0070ns |
| GlH | 0.008ns | 0.363∗∗ |
| GlC | 0.020∗∗ | 0.432∗∗∗ |
| LmC | 0.003∗∗ | 0.002ns |
| AwC | 0.149ns | 0.002ns |
| KrRw | 0.022∗∗ | 0.004∗ |
| SpDs | 0.014ns | 0.017ns |
| GlCM | 0.027∗ | 0.387∗∗ |
| LmCM | 0.002∗∗ | 0.008∗ |
| LmAB | 0.025ns | 0.032∗ |
| BkSh | 0.001ns | 0.0002ns |
| BkLn | 0.003ns | 0.0002ns |
| KrSh | 0.003∗ | 0.0008ns |
| KrC | 0.001∗∗ | 0.003∗ |
Significant codes: 0 ‘∗∗∗’ 0.001, ‘∗∗’ 0.01, ‘∗’ 0.05, ‘ns’ 0.1, ‘ns’ 1, ns-non significant. Abbreviations: StP = Stem pigmentation, LfC = Leaf colour, GlH = Glume hairiness, GlC = Glume colour at hard dough stage, LmC = Lemma colour at hard dough stage, AwC = Awn colour, KrRw = Kernel rows, SpDs = Spike density, GlCM = Glume colour at physiological maturity stage, LmCM = Lemma colour at physiological maturity stage, LmAB = Lemma awn barbs, BkSh = Beak shape, BkLn = Beak length, KrSh = Kernel shape and KrC = Kernel colour.
3.4. Principal component analysis
A Principal component analysis (PCA) was also used to visualize variation among 85 accessions for 15 qualitative traits in the barley accessions (Figure 3A), which refine and reduce the number of selection criteria into a few meaningful and practically useful traits. The PCA on 15 qualitative traits described 21.91% and 16.37% of the total variation in PC1 and PC2 percent of the total variance (Figure 3A) and could be considered as the major factors that determine morphological variation in barley accessions. These percentages of variance captured in PC axes were previously reported by different researchers using morphological traits for Ethiopia barley collections (Demissie and Bjørnstad, 1996), Nepalese naked barley landraces (Yadav et al., 2018), Algerian barley accessions (Taibi et al., 2019) and Ethiopian wheat materials (Mengistu et al., 2015). Qualitative traits such as KrC (0.37), GlC (0.37), LmC (0.42), GlCM (0.43) and LmCM (0.44) were the major loadings on the PC1 (Figure 3A and B). The majority of the morphological diversity among barley accessions was brought because of these major qualitative traits and should be emphasized in barley breeding (Yadav et al., 2018). Other traits, such as KrRw (−0.46), LfC (−0.42) and SpDs (−0.36) were the major contributors to PC2 (Figure 3A and B) showing that yield traits including rowed types, spike density (kernel weight) and leaf colour (light-harvesting to maximize productivity) had also a large contribution to the total morphological diversity in the barley accessions.
Figure 3.
Variation and relationship of morphological traits of 85 barley accessions evaluated at Kulumsain during the 2020 and 2021 main cropping seasons. A. PC represents 15 morphological traits of barley accessions (for accession names refer to Table S1). Dots or numbers are barley accessions at various altitude ranges (1 = ≤2000, 2 = 2001–2500, 3 = 2501–3000 and 4 = ≥3001, according to the legend). Golden vectors represent the traits in the PCA. PC1 and PC2 are shown on the X and Y axis, respectively, aside from their explained variance. B. Correlations between derived PC variables and original variables in the morphological traits. Correlation coefficients are reported in colours, according to the legend to the right. Abbreviations: StP = Stem pigmentation, LfC = Leaf colour, GlH = Glume hairiness, GlC = Glume colour at hard dough stage, LmC = Lemma colour at hard dough stage, AwC = Awn colour, KrRw = Kernel rows, SpDs = Spike density, GlCM = Glume colour at physiological maturity stage, LmCM = Lemma colour at physiological maturity stage, LmAB = Lemma awn barbs, BkSh = Beak shape, BkLn = Beak length, KrSh = Kernel shape and KrC = Kernel colour.
The yield of barley can be enhanced by combining spike related traits both qualitative and quantitative traits with other morphological traits (Tshikunde et al., 2019). Hence, breeding combines spike related traits with useful agronomic traits to boost grain yield. Further, other qualitative traits which loaded highly on the other principal components should also be considered (Figure 3A and B). For example, traits such as BkSh (−0.45) and BkLn (−0.41) and SpDs (0.5) were the major contributors to the morphological variability in PC3 (Figure 3A and B). The first three principal components (PC1, PC2 and PC3) accounted for 49.3% of the total variation (Table 5) and could be considered the major factors that determine morphological variation in barley. Our result comparatively agrees with Enyew et al. (2019) who showed the first three PCs contributed 74.20% of the total variations among the 48 Ethiopian barley accessions.
Table 5.
Total variance explained by the three principal components extracted from a morphological data set of 15 traits in 85 barley accessions grown at Kulumsa in the 2020 and 2021 main cropping seasons.
| Principal Components | Standard deviation | Proportion of Variance | Cumulative Proportion |
|---|---|---|---|
| PC1 | 1.812 | 0.219 | 0.219 |
| PC2 | 1.567 | 0.164 | 0.383 |
| PC3 | 1.30 | 0.11 | 0.496 |
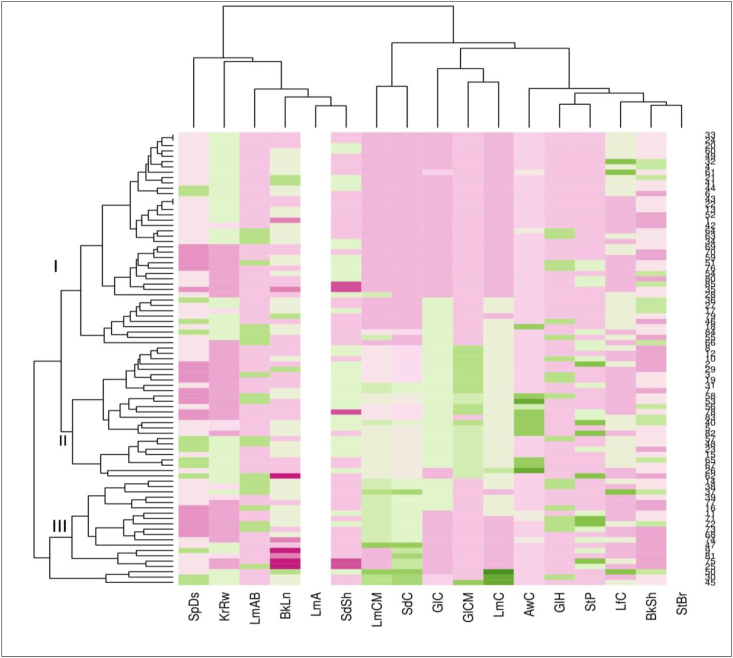
3.5. Heat map analysis
The Heat map analysis based on 17 morphological traits of 85 barley accessions was grouped into three clusters (Figure 4, Table 6). Cluster I contained the largest number of barley accessions (40) followed by Cluster II (25) and Cluster III (20). Similar to current findings, previous studies grouped North African barley germplasm into three clusters (Allel et al., 2017) and Ethiopia's sorghum accessions into five clusters using qualitative traits (Abdi et al., 2002). In addition, clustering based on regions and altitudes has also been reported in Ethiopia barely (Shumet and Tesema, 2014; Mekonnon et al., 2015; Addisu et al., 2018).
Figure 4.
Heatmap analysis shows similarity distance based on the colour distribution among 85 barley accessions of 17 qualitative traits evaluated at Kulumsa in the 2020 and 2021 main cropping seasons (for accession name and collection origins refer to Table S1). On the left of the matrix, barley accessions are grouped into 3 distinct clusters. Abbreviations: StP = Stem pigmentation, LfC = Leaf colour, StBr = Stem branching, GlH = Glume hairiness, GlC = Glume colour at hard dough stage, LmC = Lemma colour at hard dough stage, AwC = Awn colour, KrRw = Kernel rows, SpDs = Spike density, GlCM = Glume colour at physiological maturity stage, LmCM = Lemma colour at physiological maturity stage, LmAB = Lemma awn barbs, LmA = Lemma awn, BkSh = Beak shape, BkLn = Beak length, KrSh = Kernel shape and KrC = Kernel colour.
Table 6.
Clustering of 85 barley accessions into three groups using morphological traits, the distribution of accessions across region of origins and altitude ranges.
| Regions | Cluster I | Cluster II | Cluster III | Total |
|---|---|---|---|---|
| Oromia | 10 | 9 | 3 | 22 |
| Amhara | 11 | 6 | 10 | 27 |
| Tigray | 10 | 3 | 2 | 15 |
| SNNP | 9 | 7 | 5 | 21 |
| Total | 40 | 25 | 20 | 85 |
| Altitude Ranges | ||||
| ≤2000 | 3 | 6 | 2 | 11 |
| 2001–2500 | 14 | 8 | 7 | 29 |
| 2501–3000 | 18 | 8 | 4 | 30 |
| ≥3001 | 5 | 3 | 7 | 15 |
| Total | 40 | 25 | 20 | 85 |
The number of accessions per cluster varied from 20 accessions in cluster III to 40 accessions in cluster I (Figure 4, Table 6). Cluster I included accessions from four regions with the prevailing percentage in Amhara (12.94%) followed by Oromia (11.76%), Tigray (11.76%) and SNNP (10.59%) regions. The majority of the accessions were mainly found between 2001-3000 masl. Cluster II contributed 8.24% and 10.59% of accessions from the SNNP and Oromia regions, respectively. Most of the accessions included from an altitude of 2001–3000 masl. Cluster III consisted of less number of accessions from four regions with the highest percentage in the Amhara (11.76%) and the accessions encompassed both in the medium and above 3001 masl. These barley accessions may be used as a source of breeding material to enrich genetic variation in barley breeding through crossing programs. Indeed, clustering accessions into the phenotypically similar cluster of diverse collections are valuable for barley breeding such as selecting parents for crossing (Abebe et al., 2010; Allel et al., 2017) and the selection of parents should be based on specific objectives of hybridization program (Chahal and Gosal, 2002). In general, hybridization involving parents belonging to the most divergent cluster may produce maximum heterosis in the F1 population and wide variation in genetic architecture (Hosan et al., 2010).
Morphological traits did not cluster the 85 barley accessions along the collection region of origins and altitude ranges (Figure 4, Table 6). For example, Cluster-I was the largest cluster containing 40 accessions which were composed of 11 accessions from Amhara (Agew Awi, West and East Gojjam, North Gondar and shewa zone), 10 accessions from Oromia (Arssi, Bale, East Hararghe and Welega, East and North Shewa zone), and 10 accessions from Tigray (Central, East and South zone) and 9 accessions from SNNP (Gamo-Gofa, Sidama, North Omo, and Gurage zone). This shows that barley accessions from different collection origins (Amhara, Oromia, Tigray and SNNP) were clustered into different clusters with different sizes (Figure 4, Table 6). This admixture between barley accessions regardless of their collection region of origins could be associated with the continuous exchange of seeds of landraces among smallholder farmers throughout the country (Abebe et al., 2010; Yadav et al., 2018; Alemu et al., 2020; Woldeyohannes et al., 2020).
4. Conclusion
This study showed broad morphological diversity in region of origins and different altitudinal ranges based on 17 qualitative traits. The majority of the morphological traits indicated enormous variation in Amhara, SNNP and Tigray regions with medium altitude ranges. These are a sign of the potential of the regions and altitudes in contributing toward primary barley diversity areas. The first two principal components (PC1 and PC2) accounted for 38.3 % of the total variation and could be considered as the major factors that determine morphological variation in barley and should be emphasized in barley breeding program. Besides, KrC (0.37), GlC (0.37), LmC (0.42), GlCM (0.43) and LmCM (0.43) were the major loadings on the PC1 while other traits, such as KrRw (−0.46), LfC (−0.42) and SpDs (−0.36) were the major contributors to PC2. Hence, our study mainly provides useful information for further barley breeding as well as critical indication for in-situ conservation. This result shows that wide collection from various region of origins and altitude ranges is very useful than barley collection from specific agro-ecology, suggesting to capture important alleles diversity from different growing environments. Thus, this material could be manipulated for improvement either through the usual conventional breeding (direct selection) or advanced tools to study the genetic basis of phenotypic differences of these traits. Based on the result, further work is ongoing to identify a very useful gene in these barley accessions through phenotyping and genotyping.
Declarations
Author contribution statement
Mihret Yirgu: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed materials, analysis tools or data; wrote the paper.
Mulugeta Kebede, Tileye Feyissa and Berhane Lakew: Conceived and designed the experiments; Analyzed and interpreted the data.
Aemiro Bezabih Woldeyohannes: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed materials, analysis tools or data.
Funding
This work was supported by the Ethiopian Ministry of Science and Higher Education.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors would like to acknowledge the Ethiopian Ministry Education for hosting the study and EBI for the provision of barley accessions.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Abate E., Hussein S., Laing M., Mengistu F. Quantitative responses of tef [Eragrostis tef (Zucc.) Trotter] and weeping love grass [Eragrostis curvula (Schrad.) Nees] varieties to acid soil. Aust. J. Crop. Sci. 2013;7(12):1854–1860. [Google Scholar]
- Abdi A. Proceedings of the 2nd National Barley Research and Development Review Workshop: HARC, Holetta, Ethiopia. 2011. Barley genetic resources collection and conservation in Ethiopia; pp. 19–30.https://www.researchgate.net/publication/234939470 [Google Scholar]
- Abdi A., Endashaw B., Zemede A., Awgechew T. Patterns of morphological variation of sorghum (Sorghum bicolor (L.) Moench) landraces in qualitative characters in North Shewa and South Welo, Ethiopia. Hereditas. 2002;137:161–172. [Google Scholar]
- Abebe T.D., Bauer A.M., On J.L. Morphological diversity of Ethiopian barley (Hordeum vulgare L.) in relation to geographic regions and altitudes. Hereditas. 2010;147:154–164. doi: 10.1111/j.1601-5223.2010.02173.x. [DOI] [PubMed] [Google Scholar]
- Abebele G.M., Admasu M.A., Agdu B.H. Field evaluation of bread wheat (Triticum aestivum L.) genotypes for stripe rust (Puccinia striiformis W.) resistance in arsi highlands of Oromia region, South -eastern-Ethiopia. J. Plant Pathol. Microbiol. 2020;11:521. [Google Scholar]
- Addisu F., Fantahun W., Shumet T. Qualitative traits variation in barley (Hordeum vulgare L.) landraces from the Southern highlands of Ethiopia. Int. J. Biodivers. Conserv. 2018;10(5):258–264. [Google Scholar]
- Ahmadi J., Pour-Aboughadareh A., Fabriki Ourang S., Mehrabi A.A., Siddique K.H.M. Wild relatives of wheat: aegilops-Triticum accessions disclose differential antioxidative and physiological responses to water stress. Acta Physiol. Plant. 2018;40:90. [Google Scholar]
- Alemu A., Feyissa T., Letta T., Abeyo B. Genetic diversity and population structure analysis based on the high-density SNP markers in Ethiopian durum wheat (Triticum turgidum ssp. durum) BMC Genet. 2020;21(18):1–12. doi: 10.1186/s12863-020-0825-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allel D., Ben-Amar A., Lamine M., Abdelly C. Relationships and genetic structure of North African barley (Hordeum vulgare L.) germplasm revealed by morphological and molecular markers: biogeographical considerations. South Afr. J. Bot. 2017;112:1–10. [Google Scholar]
- Amsal T., Hailu G., Charles A.F. Yield limiting factors to food barley production in Ethiopia. J. Sustain. Agric. 1997;10(2–3):97–113. [Google Scholar]
- Amsalu A., Endashaw B. Geographical patterns of morphological variation in sorghum (Sorghum bicolor (L.) Moench) germplasm from Ethiopia and Eritrea: qualitative characters. Hereditas. 1998;129:195–205. [Google Scholar]
- Asfaw Z. Variation in the morphology of the spike within Ethiopian barley, [Hordeum vulgare L. (Poaceae)] Acta Agric. Scand. 1988;38(3):277–288. [Google Scholar]
- Asfaw Z. Relationships between spike morphology, hordeins and altitude within Ethiopian barley [Hordeum vulgare L. (Poaceae)] Hereditas. 1989;110:203–209. [Google Scholar]
- Assefa A., Labuschagne M.T. Phenotypic variation in barley (Hordeum vulgare L.) landraces from North Shewa in Ethiopia. Biodivers. Conserv. 2004;13(8):1441–1451. [Google Scholar]
- Bakana Z.L., Abduselam F., Biri A. Performance evaluation and adaptability of food barely (Hordeum vulgare L.) in the highlands of eastern Hararghe. Agric. Res. Technol. Open Access J. 2018;18(2):106–109. [Google Scholar]
- Bekele S., Yoseph T., Ayalew T. Growth, protein content, yield and yield components of malt barley (Hordeum vulgare L.) varieties in response to seeding rate at Sinana District, Southeast Ethiopia. Int. J. Appl. Agric. Sci. 2020;6(4):61–71. [Google Scholar]
- Belay G., Bechere E., Mitiku D., Merker A., Tsegaye S. Patterns of morphological diversity in tetraploid wheat (Triticum turgidum L.) landraces from Ethiopia. Acta Agric. Scand. B Plant Soil Sci. 1997;47(4):221–228. [Google Scholar]
- Bonman J.M., Bockelman H.E., Jackson L.F., Steffenson B.J. Disease and insect resistance in cultivated barley accession from the USDA National Small Grains Collection. Crop Sci. 2005;45:1271–1280. [Google Scholar]
- Bort J., Febrero A., Amaro T., Araus J.l. Role of awns in-ear water-use efficiency and grain weight in barley. Agronomies. EDP Sci. 1994;14(2):133–139. [Google Scholar]
- Bouhaouel I., Medini M., Belhadj H., Ayed O.S., Jabri C., Slim H. Phenotypic diversity of barley (Hordeum vulgare L.) landraces from the Center and the South of Tunisia and identification of potential areas of on-farm conservation. New Sci. 2019;66(3):4157–4169. [Google Scholar]
- Chahal G.S., Gosal S.S. Biotechnology and Conventional Approaches. Narosa Publishing House; New Delhi: 2002. Principles and procedures of plant Breeding. [Google Scholar]
- Cossani C.M., Reynolds M.P. Physiological traits for improving heat tolerance in wheat. Plant Physiol. 2012;160:1710–1718. doi: 10.1104/pp.112.207753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CSA (Central Statistical Agency) Agricultural sample survey report on area and production of major crops (private peasant holdings ‘Meher’ season) Stat. Bull. 2018/19;585 Addis Ababa, Ethiopia. [Google Scholar]
- Dawit A., Zewdie B. International Center for Agricultural Research in the Dry Areas (ICARDA); Beirut, Lebanon: 2019. Varietal Adoption and Seed Commercial Behaviour: Barley Seed System Landscape in the Highlands of Ethiopia. https://hdl.handle.net/20.500.11766/10386. [Google Scholar]
- Demissie A., Bjørnstad Å. Phenotypic diversity of Ethiopian barley in relation to geographical regions, altitudinal range and agro-ecological zones: as an aid to germplasm collection and conservation strategy. Hereditas. 1996;124:17–29. [Google Scholar]
- Demissie A., Bjørnstad Å. Geographical, altitudinal and agro-ecological differentiation of isozymes and hordein genotypes of landraces of barleys from Ethiopia: implications for germplasm conservation. Genet. Resour. Crop Evol. 1997;44:43–55. [Google Scholar]
- Derbew S., Mohammed H., Urage E. Phenotypic diversity for qualitative characters of barley (Hordeum vulgare L.). Landrace collections from southern Ethiopia. Int. J. Sci. Res. 2013;2(9):34–40. [Google Scholar]
- Engels J.M.M. Genetic diversity in Ethiopia in relation to altitude. Genet. Resour. Crop Evol. 1994;41:67–73. [Google Scholar]
- Enyew M., Dejene T., Lakew B., Worede F. Clustering and principal component analysis of Barley (Hordeum vulgare L.) Landraces for major morphological traits from North Western Ethiopia. Int. J. Agric. Sci. Food Technol. 2019;5(1):58–63. [Google Scholar]
- Etana D., Snelder D.J.R.M., van Wesenbeeck C.F.A., Buning T. de C. Trends of climate change and variability in three agro-ecological settings in central Ethiopia: contrasts of meteorological data and farmers’ perceptions. Climate. 2020;8(11):1–27. [Google Scholar]
- FAOSTAT (Food and Agriculture Organization) Food and agriculture organization of the United Nations. 2019. http://www.fao.org/faostat/en/ Available online: Accessed on 10 January 2021.
- Fuller D. Contrasting patterns in crop domestication and domestication rates: recent archaeobotanical insights from the old world. Ann. Biotechnol. 2007;100(5):903–924. doi: 10.1093/aob/mcm048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadissa F., Abebe M., Bekele T. Agro-morphological traits-based genetic diversity assessment in Ethiopian barley (Hordeum vulgare L.) landrace collections from Bale highlands, Southeast Ethiopia. Agric. Food Secur. 2021;10(1):1–14. [Google Scholar]
- Gao F.M., Wen W.E., Liu J.D., Rasheed A., Yin G.H., Xia X.C., et al. Genome-wide linkage mapping of QTL for yield components, plant height and yield-related physiological traits in the Chinese wheat cross Zhou 8425B/Chinese Spring. Front. Plant Sci. 2015;6:1099. doi: 10.3389/fpls.2015.01099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García del Moral L.F., Garcia del Moral M.B., Molina-Cano L., Slafer G.A. Yield stability and development in two- and six-rowed winter barleys under Mediterranean conditions. Field Crop. Res. 2003;81(2):109–119. [Google Scholar]
- Gardi M.W., Memic E., Zewdu E., Graeff-Hönninger S. Simulating the effect of climate change on barley yield in Ethiopia with the DSSAT-CERES-Barley model. Agron. J. 2022;114(2):1128–1145. [Google Scholar]
- GenStat . VSN International; Hemel Hempstead, UK: 2015. GenStat (General Statistics) for Windows 18th Edition.http://www.vsni.co.uk/VSN Available at: [Google Scholar]
- Hailemichael S., Sopade P. Ethno botany, diverse food uses, claimed health benefits and implications on conservation of barley landraces in North Eastern Ethiopia highlands. J. Ethnobiol. Ethnomed. 2011;7:19. doi: 10.1186/1746-4269-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez J., Meints B., Hayes P. Introgression breeding in barley: perspectives and case studies. Front. Plant Sci. 2020;11:761. doi: 10.3389/fpls.2020.00761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosan S., Sultana N., Iftekharudduala K., Ahmed M., Mia S. Genetic divergence in landraces of Bangladesh rice (Oryza sativa L.) The Agriculturists. 2010;8(2):28–34. [Google Scholar]
- IPGRI (International Plant Genetic Resources Institute) International Plant Genetic Resources Institute; Rome, Italy: 1994. Descriptors for Barley (Hordeum vulgare L.) [Google Scholar]
- Kandic V., Dodig D., Zoric M., Nikolic A., Momirovic G.S., Kaitovic Z., Aleksic G., Duric N. Grain filling parameters of two and six-rowed barley genotypes in terminal drought conditions. Ital. J. Agrometeorol. 2018 [Google Scholar]
- Karagöz A., Özbek K., Akar T., Ergün N., Aydoğan S., Sayim İ. Agro-morphological variation among an ancient world barley collection. J. Agric. Sci. 2017;23(4):444–452. [Google Scholar]
- Kaur V., Aravind J., Manju, Jacob S.R., Kumari J., Panwar B.S., et al. Phenotypic characterization, genetic diversity assessment in 6,778 accessions of barley (Hordeum vulgare L. ssp. vulgare) germplasm conserved in national gene bank of India and development of a Core set. Front. Plant Sci. 2022;13:1–17. doi: 10.3389/fpls.2022.771920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebebew F., Tsehaye Y., Mcneilly T. Morphological and farmers cognitive diversity of barley [Hordeum vulgare L. (poaceae)] at Bale and North shewa of Ethiopia. Genet. Resour. Crop Evol. 2001;48:1–10. [Google Scholar]
- Kesavan M., Song J.T., Seo H.S. Seed size: a priority trait in cereal crops. Physiol. Plantarum. 2013;147(2):113–120. doi: 10.1111/j.1399-3054.2012.01664.x. [DOI] [PubMed] [Google Scholar]
- Lakew B., Semeane Y., Alemayehu F., Gebre H., Grando S., Van Leur J.A.G., Ceccarelli S. Exploiting the diversity of barley landraces in Ethiopia. Genet. Resour. Crop Evol. 1997;44(2):109–116. [Google Scholar]
- Lance R.C., Nilan R.A. Screening for low acid-soluble β -glucan barleys. Barley Genet. Newsl. 1980;10:41. [Google Scholar]
- Leino M.W., Jenny H. Nineteenth-century seeds reveal the population genetics of landrace barley (Hordeum vulgare L.) Mol. Biol. Evol. 2010;27:964–973. doi: 10.1093/molbev/msp308. [DOI] [PubMed] [Google Scholar]
- Li R., Li M., Ashraf U., Liu S., Zhang J. Exploring the relationships between yield and yield-related traits for rice varieties released in China from 1978 to 2017. Front. Plant Sci. 2019;10:543. doi: 10.3389/fpls.2019.00543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonbani M., Arzani A. Morpho-physiological traits associated with terminal drought-stress tolerance in triticale and wheat. Agron. Res. 2011;9:315–329. [Google Scholar]
- Mancini C., Yosef K., Dejene M., Workaye F.C., Pè M.E., Fadda C., Dell’Acqua M. Joining smallholder farmers’ traditional knowledge with metric traits to select better varieties of Ethiopian wheat. Nat. Sci. Rep. 2017 doi: 10.1038/s41598-017-07628-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathan J., Bhattacharya J., Ranjan A. Enhancing crop yield by optimizing plant developmental features. Development. 2016;143:3283–3294. doi: 10.1242/dev.134072. [DOI] [PubMed] [Google Scholar]
- Mekonnon B., Lakew B., Dessalegn T. Morphological diversity and association of traits in Ethiopian food barley (Hordeum vulgare L.) landraces in relation to regions of origin and altitudes. J. Plant Breed Crop Sci. 2015;7(2):44–54. [Google Scholar]
- Mengistu D.K., Kiros A.Y., Pè M.E. Phenotypic diversity in Ethiopian durum wheat (Triticum turgidum var. durum) landraces. Crop J. 2015;3(3):190–199. [Google Scholar]
- MoARD (Ministry of Agriculture and Rural Development) 2018. Ministry of Agriculture and Natural Resources and Seed Quality Control Directorate. Issue No. 21. [Google Scholar]
- Mohammed J., Seleshi S., Nega F., Lee M. Revisit to Ethiopian traditional barley-based food. J. Ethnic Foods. 2016;3(2):135–141. [Google Scholar]
- Motzo R., Giunta F. Awnedness affects grain yield and kernel weight in near-isogenic lines of durum wheat. Aust. J. Agric. Res. 2002;53:1285–1293. [Google Scholar]
- Mulatu B., Grando S. In: Proceedings of the 2nd National Barley Research Development Review Workshop. Mulatu B., Grando S., editors. HARC; ICARDA; Holetta Ethiopia; Aleppo: 2011. Barley research and development in Ethiopia: an overview in barley research and development in Ethiopia; pp. 1–15. [Google Scholar]
- Munck L., Karlsson K.E., Hagberg A., Eggum B.O. Gene for improved nutritional value in barley seed protein. Science. 1970;168:985–987. doi: 10.1126/science.168.3934.985. [DOI] [PubMed] [Google Scholar]
- Munsell Color Company . Munsell colour company InC; Baltimore: 1957. Nickerson Colour Fan. Maximum Chromas-40 Hues. [Google Scholar]
- Negassa M. Estimates of phenotypic diversity and breeding potential of Ethiopian wheat. Hereditas. 1986;104:41–48. [Google Scholar]
- Nielsen J.P. Evaluation of malting barley quality using exploratory data analysis. II. The use of kernel hardness and image analysis as screening methods. J. Cereal. Sci. 2003;38:247–255. [Google Scholar]
- Ntakirutimana F., Xie W. Unveiling the actual functions of awns in grasses: from yield potential to quality traits. Int. J. Mol. Sci. 2020;21:7593. doi: 10.3390/ijms21207593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyiraguhirwa S., Grana Z., Henkrar F., Ouabbou H., Mohammed I., Udupa S.M. Genetic diversity and structure of a barley collection predominantly from the North African region. Cereal Res. Commun. 2021 [Google Scholar]
- Paperson F.L., Ohm H.W. Compensating ability of awns in soft red winter wheat. Crop Sci. 1975;15 [Google Scholar]
- Pourkheirandish M., Komatsuda T. The importance of barley genetics and domestication in a global perspective. Ann. Bot. 2007;100(5):999–1008. doi: 10.1093/aob/mcm139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purugganan M.D., Fuller D.Q. The nature of selection during plant domestication. Nature. 2009;457:843–848. doi: 10.1038/nature07895. [DOI] [PubMed] [Google Scholar]
- R development core Team . R Foundation for Statistical Computing; Vienna, Austria: 2022. A Language and Environment for Statistical Computing. [Google Scholar]
- Russell J., Mascher M., Dawson I.K., Kyriakidis S., et al. Exome sequencing of geographically diverse barley landraces and wild relatives gives insights into environmental adaptation. Nat. Genet. 2016;48:1024–1030. doi: 10.1038/ng.3612. [DOI] [PubMed] [Google Scholar]
- SAS . SAS Institute Inc.; Cary, North Carolina, USA: 2008. Statistical Analysis Systems Institute. Version 9.1. [Google Scholar]
- Shaaf S., Bretani G., Biswas A., Fontana I.M., Rossini L. Genetics of barley tiller and leaf development. J. Integr. Plant Biol. 2019;61(3):226–256. doi: 10.1111/jipb.12757. [DOI] [PubMed] [Google Scholar]
- Shumet T.G., Tesema T.H. Genetic diversity of qualitative traits of barley (Hordeum vulgare L.) landrace populations collected from Gamo Highlands of Ethiopia. Int. J. Biodivers. Conserv. 2014;6(9):663–673. [Google Scholar]
- Sun L., Li X., Fu Y., Zhu Z., Tan L., Liu F., et al. GS6, a member of the GRAS gene family, negatively regulates grain size in rice. J. Integr. Plant Biol. 2013;55(10):938–949. doi: 10.1111/jipb.12062. [DOI] [PubMed] [Google Scholar]
- Tafa J., Tadesse G., Sakhuja P.K. Ovipositional antixenosis in some barley accessions to barley shootfly. Pest Manage. J. Ethiopia. 2004;8:51–57. [Google Scholar]
- Taffesse S.A., Dorosh P.A., Asrat S. Food and Agriculture in Ethiopia: Progress and Policy Challenges. 2013. Crop production in Ethiopia: regional patterns and trends. [Google Scholar]
- Taibi W., Belletreche A., Kharsi M., Gaouar S.B.S. Phenotypic diversity for quantitative and qualitative characters of barley (Hordeum vulgare) accession from Algeria. Biodiversitas. 2019;20(12):3794–3803. [Google Scholar]
- Tanto Hadado T., Rau D., Bitocchi E., Papa R. Genetic diversity of barley (Hordeum vulgare L.) landraces from the central highlands of Ethiopia : comparison between the Belg and Meher growing seasons using morphological traits. Genet. Resour. Crop Evol. 2009;56(8):1131–1148. [Google Scholar]
- Temesgen B. Role and economic importance of crop genetic diversity in food security. Int. J. Agric. Sci. Food Technol. 2021;7:164–169. [Google Scholar]
- Tesso T., Tirfessa A., Mohammed H. Association between morphological traits and yield components in the durra sorghums of Ethiopia. Hereditas. 2011;148:98–109. doi: 10.1111/j.1601-5223.2011.02229.x. [DOI] [PubMed] [Google Scholar]
- Tilman D., Reich P., Knops J., Wedin D., Mielke T. Diversity and productivity in a long-term grassland experiment. Science. 2001;294:843–845. doi: 10.1126/science.1060391. [DOI] [PubMed] [Google Scholar]
- Tolbert D.M., Qualset C.O., Jain S.K., Craddock J.C. A diversity analysis of the World collection of Barley. Crop Sci. 1979;19:789–794. [Google Scholar]
- Tshikunde N., Mashilo J., Shimelis H., Odindo A. Agronomic and physiological traits, and associated quantitative trait Loci (QTL) affecting yield response in wheat (Triticum aestivum L.): a review. Front. Plant Sci. 2019;10:1428. doi: 10.3389/fpls.2019.01428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldeyohannes A.B., Accotto C., Desta E.A., Kidane Y.G., Fadda C., Pè M.E., Dell’Acqua M. Current and projected eco-geographic adaptation and phenotypic diversity of Ethiopian teff (Eragrostis teff) across its cultivation range. Agric. Ecosyst. Environ. 2020;300:1–10. [Google Scholar]
- Xin X., Rajiv S., Alessandro T., Joanne R., et al. Genome-wide association analysis of grain yield-associated traits in a pan-European barley cultivar collection. Plant Genome. 2018;11(1):1–11. doi: 10.3835/plantgenome2017.08.0073. [DOI] [PubMed] [Google Scholar]
- Yadav R.K., Gautam S., Palikhey E., Joshi B.K., Ghimire K.H., Gurung R., et al. Agro-morphological diversity of Nepalese naked barley landraces. Agric. Food Secur. 2018;7(86):1–12. [Google Scholar]
- Yitbarek S., Bekele H., Getaneh W., Dereje T. Proceedings of the 1st Barley Research Review Workshop. IAR/ICARDA; Addis Ababa, Ethiopia: 1998. Disease surveys and loss assessment studies on barley. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.