Dear Editor,
Vaginal candidiasis (VC) in pregnancy is a distressing infection and has emerged as an important cause of neonatal infections. The clinical symptoms and manifestations of VC include cottage cheese-like vaginal discharge, swelling, pruritus, pain, irritation, burning sensation, dyspareunia, and dysuria [1]. The hormonal milieu of the vagina during pregnancy can enhance candidal colonization and serves as risk factor. Progesterone has suppressive effect on the anti-candidal activity of neutrophils, while oestrogen has been found to reduce the ability of vaginal epithelial cells to inhibit the growth of Candida albicans. Moreover, a large proportion of women with chronic recurrent candidiasis first experienced the infection during pregnancy. In pregnancy, VC has been related to emotional stress and suppression of immune system [2].
Candida albicans is both a commensal and a pathogen that can exhibit yeast, pseudohyphae and hyphae morphology. These morphological transitions promote colonization and invasion at different anatomical sites, which also occur in other Candida species. C. albicans possesses several virulence factors that are involved in hyphae formation, phenotype switching, cell adhesion and extracellular production of hydrolytic enzymes. Secreted aspartyl proteases (SAPs) are enzymes that are secreted by Candida species and are coded for by the SAP gene family (SAP1-SAP10). The SAP superfamily members have been demonstrated as virulence factors in opportunistic pathogens of the genus Candida, and SAP1 and SAP6 have been known to be associated with vaginal candidiasis [2,3].
The study was carried out to describe the Candida species in vagina of pregnant women, their susceptibility to antifungal agents and the carriage of virulence genes. Pregnant women (390), attending antenatal clinic of Ekiti State University Teaching Hospital, Ado-Ekiti, Nigeria, between 2016 and 2017, were enlisted in the study. Isolation of Candida species from high vaginal swab of the subjects was carried out using Brilliance Candida Agar® (Oxoid, England). Antifungal susceptibility testing, on nystatin, fluconazole and variconazole, was performed on the Candida isolates by disk diffusion method. Quick-DNATM Universal Kit (Zymoresearch, USA) was used for DNA extraction from pure cultures of the Candida isolates. The DNA of isolates with green colour on Candida CHROMO agar were subjected to polymerase chain reactions (PCR) using the C. albicans specific primers INT1-F:5′-AAGTATTTGGGAGAAGGGAAAGGG-3′ and INT2-R:5′AAAATGGGCATTAAGGAAAAGAGC-3′, to distinguish C. albicans from C. dublinensis [4]. PCR amplification of virulence genes SAP 1 and SAP 6 of Candida albicans were carried out using the primers: SAP1 (F:5′-TCAATCAATTTACTCTTCCATTTCTAACA-3'; R:5′-CCAGTAGCATTAACAGGAGTTTTAATGACA-3′) and SAP 6 (F:5′-CCCGTTTTGAAATTAAATATGCTGATGG-3'; R:5′-GTCGTAAGGAGTTCTGGTAGC TTC G-3′) as described by Lima et al. [5]. Molecular identification of Candida species by PCR analysis and sequencing using the universal primer ITS4/ITS5 (5′-GGAAGTAAAAGTCGTAACAAGG-3’; 5′-TCCTCCGCTTATTGATATGC-3′) as described by El-Naggar et.al. [4].
The 390 healthy pregnant women enlisted in this study were 19–46 (30.93 ± 4.60) years old, with median and modal age of 26-32 years. Candida species were isolated from HVS of 260 (66.7 %) of the subjects. Based on phenotypical characteristics, the Candida species isolated were identified as C. albicans (46.8%), C. dubliniensis (3.2%), C. tropicalis (0.8%), C. krusei (29.8%), C. glabrata (12.1%) and C. parapsilosis (7.3%). The Candida isolates showed high level of resistance to the three antifungal agents used; nystatin (50.4 %), fluconazole (79.7 %) and variconazole (81.3 %). Candidal isolation increased with parity, with women after forth delivery recording 75 % compared to 59.6 % of women expecting their first delivery. HIV positive women recorded higher occurrence of Candida (75.0 %) than HIV negative individuals (66.4 %). Candidal isolation from HVS of the women was found to be significantly associated with vaginal itching (p < 0.001), vaginal discharge (p < 0.001), previous abortion (p = 0.022) and antibiotics usage (p = 0.044).
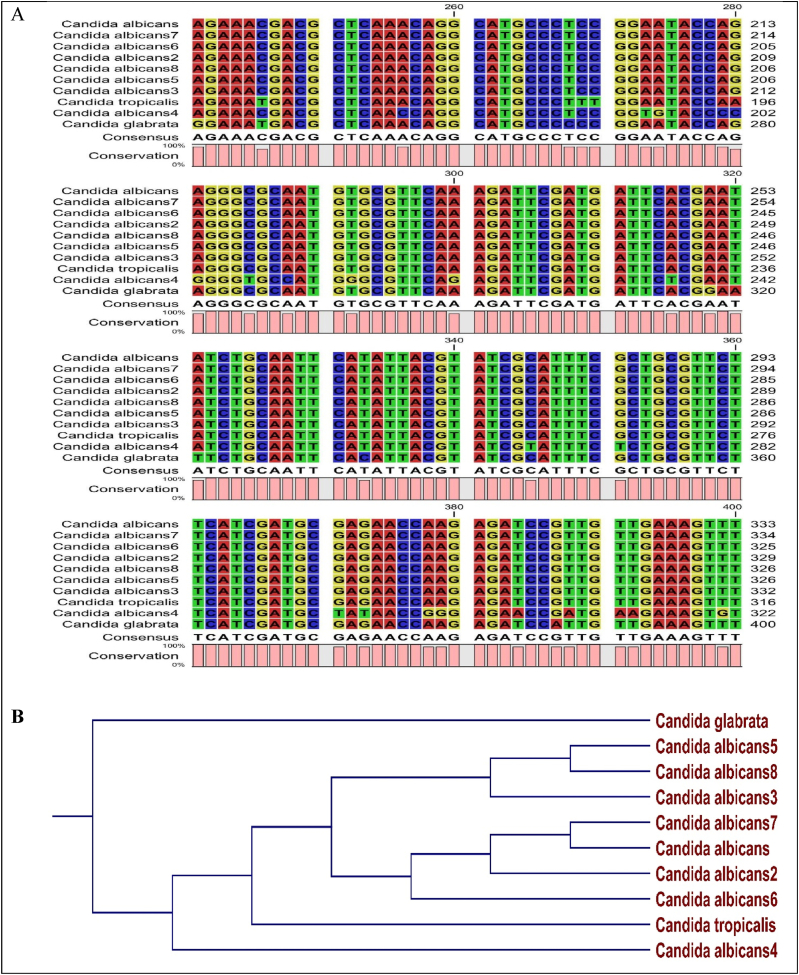
Following sequencing and BLAST, the isolates of C. albicans were found to belong to different nearest relatives, homology and accession numbers in NBCI data bank. The alignment of the nucleotides of 10 Candida isolates, as presented in Fig. 1, showed various sites of insertions, deletions, transversions and translocations. However, 7 out of the 8 C. albicans showed strong homology in their nucleotide sequence. The phylogenetic tree shows the ancestral closeness of the 10 isolates of Candida species.
Fig. 1.
Alignment (A) and phylogenetic relationship (B) among the Candida species isolated from HVS of pregnant women.
The virulence genes SAP1 and SAP6 were detected in 68.75% and 87.50%. respectively among the C. albicans isolates and were found to be associated with vaginitis among the subjects. The prevalence of virulence genes SAP1 and SAP6 (68.75 and 87.50 %, respectively), present study is in tandem with the findings in earlier work of Lima et al. [5], who reported incidence of 69.25 and 84.61% respectively of SAP1 and SAP6 genes in C. albicans isolated from vulvovaginal infection and colonization in Brazil.
In conclusion, the study showed high prevalence of vaginal candidiasis among apparently healthy pregnant women with drug resistant Candida species carrying virulent genes. Perinatal transmission of such drug resistant organisms to new-borns can be of grave consequence.
Transparency declaration
There is no conflict of interest.
References
- 1.Ghaddar N., Anastasiadis E., Halimeh R., et al. Prevalence and antifungal susceptibility of Candida albicans causing vaginal discharge among pregnant women in Lebanon. BMC Infect Dis. 2020;20(32) doi: 10.1186/s12879-019-4736-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodríguez-Cerdeira C., Martínez-Herrera E., Carnero-Gregorio M., López-Barcenas A., Fabbrocini G., Fida M., El-Samahy M., González-Cespón J.L. Pathogenesis and clinical relevance of Candida biofilms in vulvovaginal candidiasis. Front Microbiol. 2020;11:544480. doi: 10.3389/fmicb.2020.544480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hube B., Naglik J. Candida albicans proteinases: resolving the mystery of a gene family. Microbiology. 2001;147(8):1997–2005. doi: 10.1099/00221287-147-8-1997. [DOI] [PubMed] [Google Scholar]
- 4.El-Naggar M.Y., Al-Basri H.M., El-Din A.A.K. Molecular diagnosis of Candida albicans using real-time polymerase chain reaction of a CaYST1 gene. Taibah Univ Sci J. 2010;3:8–13. [Google Scholar]
- 5.Lima J.S., Braga K.R.G.S., Vieira C.A., Souza W.W.R., Chávez-Pavoni J.H., de Araújo C., Goulart L.S. Genotypic analysis of secreted aspartyl proteinases in vaginal Candida albicans isolates. J Brasileiro de Patologia e Medicina Laboratoria. 2018;54(1):28–33. [Google Scholar]