Abstract
Objective
To test the feasibility of a telephone delivered intervention, informed by cognitive behavioural principles, for post-stroke fatigue, and estimated its effect on fatigue and other outcomes.
Design
Randomised controlled parallel group trial.
Setting
Three Scottish stroke services.
Subjects
Stroke survivors with fatigue three months to two years post-stroke onset.
Interventions
Seven telephone calls (fortnightly then a ‘booster session’ at 16 weeks) of a manualised intervention, plus information about fatigue, versus information only.
Main measures
Feasibility of trial methods, and collected outcome measures (fatigue, mood, anxiety, social participation, quality of life, return to work) just before randomisation, at the end of treatment (four months after randomisation) and at six months after randomisation.
Results
Between October 2018 and January 2020, we invited 886 stroke survivors to participate in postal screening: 188/886 (21%) returned questionnaires and consented, of whom 76/188 (40%) were eligible and returned baseline forms; 64/76 (84%) returned six month follow-up questionnaires. Of the 39 allocated the intervention, 23 (59%) attended at least four sessions. At six months, there were no significant differences between the groups (adjusted mean differences in Fatigue Assessment Scale −0.619 (95% CI −4.9631, 3.694; p = 0.768), the Generalised Anxiety Disorder 7 −0.178 (95% CI −3.823, 3.467, p = 0.92), and the Patient Health Questionnaire −0.247 (95% CI −2.935, 2.442, p = 0.851). There were no between-group differences in quality of life, social participation or return to work.
Conclusion
Patients can be recruited to a trial of this design. These data will inform the design of further trials in post-stroke fatigue.
Keywords: Fatigue, stroke, randomized controlled trial
Background
Fatigue after stroke is common-estimates of prevalence range from 29% to 68%.1 It adversely affects quality of life, there is a paucity of data about treatments2 and it is a research priority.3 Post-stroke fatigue is associated with anxiety and depression, and although these may not be causative factors, targeting anxiety and depression, if it is present, might in theory reduce fatigue.4 Although not extensively studied, evidence is emerging that fatigue is also associated with physical inactivity and in one study inactivity preceded fatigue, suggesting that inactivity might be causal.5 Thus, addressing mood, anxiety and physical inactivity might improve fatigue. Targeting these factors can be helpful in cancer fatigue and chronic fatigue syndrome,6–9 but there is a lack of randomised controlled trials in post-stroke fatigue. A 2020 network meta-analysis of non-pharmacological treatments for post-stroke fatigue identified only 10 randomised controlled trials (777 patients).10 The review included two trials of cognitive behavioural therapy (CBT) recruiting 75 patients in total; both trials found that the intervention improved fatigue.11,12 However, all 10 trials were at high or unclear risk of bias in at least one methodological domain.
In our model of post-stroke fatigue, we proposed that pre-stroke fatigue, depressive symptoms, anxiety, low self-efficacy, passive coping, reduced physical activity, sleep problems, and inadequate social support might all contribute to the development and/or maintenance of post-stroke fatigue.13 We then developed an intervention, targeting those factors that were potentially reversible. Our intervention was informed by CBT principles.14 It was then adapted for delivery by non-psychology health care professionals15 because in stroke services clinical psychologists and cognitive behavioural therapists are in short supply.16 Here we report the feasibility of its delivery by telephone, and the clinical outcome measures in a parallel group randomised controlled trial.
Methods
The study was a United Kingdom based, multi-site, parallel, 1:1 allocation, feasibility randomised controlled trial of participants with post-stroke fatigue. Follow-up was six months after randomisation.15 The trial protocol was published previously and the trial materials are available on request.15 We aimed to recruit at least 75 participants. Recruitment was from 19th November 2018 to 20th January 2020.
Inclusion criteria were: age ≥ 18 years, stroke (ischaemic or haemorrhagic, first or recurrent, including subarachnoid haemorrhage) between three months and two years previously, mental capacity to consent, not living in a nursing home, medically stable, answered ‘Yes’ to both the Greater Manchester Stroke Assessment Tool fatigue questions17: ‘Do you feel tired all the time or get tired very quickly since your stroke’? and ‘Would you like additional help and support for this’?
Exclusion criteria were: unlikely to be available for follow-up (as judged by the trial team); life-threatening illness (e.g. advanced cancer or advanced heart failure); aphasia or cognitive impairment severe enough to prevent participation (‘very difficult’ or ‘could not do at all’ to either relevant item in the Short Stroke Impact Scale);18 actively suicidal, requiring in-patient treatment for depression, depression-related cognitive impairment; known diagnosis of post-traumatic stress disorder syndrome or panic disorder along with a score of at least 15 on the Generalised Anxiety Disorder scale,19 previously enrolled in the post stroke intervention trial in fatigue, enrolled in another trial of psychological therapy or physical activity; inability to understand spoken and/or written English.
Invitations (patient information leaflet, consent form, screening questionnaires) were sent to patients discharged from three stroke services in Scotland, United Kingdom. Those with aphasia were sent easy access patient information leaflets and consent forms. Site one invited all patients discharged without obvious exclusion criteria, whilst sites two and three contacted only patients they thought might be eligible and interested. This was because sites two and three were aware through their clinical services of which patients had fatigue and might be interested in participation and did not want to use additional resource to screen all of their data on discharges from their stroke service, whereas site one did not systematically record this information. As this was a pragmatic trial, we wanted to use a range of approaches to recruitment to reflect the services from which were recruiting; but the drawback of this was that sites two and three probably approached particular patients who they ‘knew’ had fatigue. Those who were sent an invitation, and who were interested in participating returned a consent form and screening questionnaires; they were then sent baseline questionnaires if they answered ‘yes’ to both of the fatigue questions. From August 2019, we advertised our trial throughout the United Kingdom, using an advertisement on the trial website to invite stroke survivors to get in touch, but did not recruit any patients in this way.
After the baseline questionnaire was returned, those who were eligible were then randomised to either the intervention group plus a Stroke Association leaflet about fatigue, or to the control group (Stroke Association leaflet only). There could sometimes be a week or more between screening and randomisation, because we had to wait for questionnaires to be returned. When baseline questionnaires suggested severe depression or anxiety, or when patients responded ‘several days’ (or more frequently) on the patient health questionnaire question 9 suicide question,20 clinicians at the sites contacted the patient to assess suicidal intent, and if needed, recommended the patient contacted their general practitioner. If there were serious concerns, the clinician contacted the general practitioner directly.
The intervention was six telephone calls (each up to 1 h) over 12 weeks, then a ‘booster’ call two to four weeks later, delivered by one of seven stroke health care professionals (six nurses and one physiotherapist) who had received very brief training in CBT principles by a clinical psychologist with expertise in stroke (David Gillespie) and a cognitive behavioural psychotherapist with expertise in fatigue (Trudie Chalder).15 We decided to use individual therapy rather than group therapy, to ensure that there was the necessary focus in each session to tailor goals (e.g. increasing physical activity, improving sleep) to the individual participant, rather than trying to provide advice and support to a group of survivors, with different experiences of fatigue. Cognitive behaviour therapy is a form of psychological therapy which is based on the concept that thoughts, symptoms, feelings and behaviours are interconnected and that identifying unhelpful thoughts, and challenging them, for example through the use of behavioural experiments, can lead to changes. The intervention we provided in this trial focused on the potentially reversible nature of fatigue, encouraged participants to (a) overcome fears about physical activity, (b) increase physical activity using diary monitoring and activity scheduling, (c) achieve a balance between activities, rest and sleep and (d) address unhelpful thoughts related to fatigue and low mood if present. The content of each session, and the ‘homework’ between sessions were detailed in a participant manual and a health care professional manual (available on request) and has been described previously in two papers.14,15 Briefly, session 1: introduction and psychoeducation about fatigue, session 2: goal setting and activity planning; session 3: progress assessment and goal modification; session 4: cognitive restructuring; session 5: dealing with blocks and setbacks, session 6: overview and future plans, booster session: review progress and make further plans. Immediately after each intervention session, the health care professional recorded on a checklist which intervention items had been addressed. The checklist was devised by the principal investigator, based on the content of each session as described in the intervention handbooks. Telephone calls were audio recorded on encrypted voice dictation devices. The health care professionals received supervision, every two weeks, in person or by telephone (30 min sessions) from the study clinical psychologist David Gillespie during the intervention.
Our feasibility outcomes were recruitment rates, intervention adherence, trial retention, return of follow-up questionnaires and intervention fidelity. For progression to a main trial, we pre-specified (a) recruiting 75 patients in one year, (b) all randomised to the intervention would attend at least four intervention sessions and (c) at least 90% would return the six-month questionnaire. Also a main trial would have needed further funding. We decided on a sample size of 75 based on our prior experience of feasibility trials of this size giving us sufficiently precise data to plan a main study, and because this was a practical target given the funding that was available.
After protocol publication, but before database lock, two independent external CBT therapists rated nine aspects of intervention delivery (7 = extensive, 1 = not at all) of a random sample of recordings (generated using a random number table) from either session 3 or session 5,21 in order to explore fidelity of intervention delivery. We also estimated staff time needed for intervention delivery.
We obtained clinical outcome measures at baseline, four months (i.e. immediately after the intervention end) and six months including the Fatigue Assessment Scale;22 Patient Health Questionnaire question 9;20 Generalised Anxiety Disorder 7;21 prescription of new antidepressants or anxiolytics; Short Form of Stroke Impact Scale;18 Euroqol 5D-5L;23 return to work and whether hours worked had changed. Self-reported fearful beliefs about physical activity (subscale of Cognitive and Behavioural Responses Questionnaire)24 were added after trial registration but before recruitment started. We did not measure physical activity directly, as this would have added complexity to the trial, nor did we add measures of self-efficacy or sleep, as these would have increased the length of the follow-up questionnaires and would have been too burdensome to participants and reduced the likelihood of them returning the questionnaires. Had we obtained main phase funding, the Fatigue Assessment Scale would have been the primary outcome.
Screening and baseline data were entered into a computerised central randomisation service through a secure 24/7 web interface. The computer program checked data for completeness and consistency, allocated participants a unique study identification number and assigned them to either the intervention or control arm. A minimisation program achieved balance for time since stroke (<1 year versus ≥1 year), sex and baseline and Patient Heath Questionnaire 9 (0–9 versus 10–27).
Following randomisation, an automatically generated letter was sent to participants and their general practitioner. The intervention commenced as soon as possible following randomisation.
Participants were told that we wanted to compare two approaches to fatigue management. The trial statistician (Steff Lewis) was blinded. The trial manager, the statistician performing the analyses (Hannah Ensor) and a validating statistician had full unblinded access to the database at the point of analysis.
Categorical variables (counts, percentages) and continuous variables (mean, standard deviation (SD)) are presented overall and by allocated treatment. Analysis was by intention to treat. The Fatigue Assessment Score, Generalised Anxiety Disorder 7 and Patient Health Questionnaire 9 at four and six months were regressed on treatment group using linear mixed models adjusting for minimisation factors as binary fixed effects. To account for clustering within health care professionals in the intervention arm, the health care professional was modelled as a random effect. Clustering does not occur in the control arm, so each control participant was treated as a single cluster.25 Mean differences (and 95% confidence intervals) in treatment groups are presented. Significance tests are two-sided and presented at 5% significance. Residual plots were visually inspected for distributional assumptions, and no issues were identified. The final report was validated by a second statistician. The analysis of the cognitive and behavioural responses questionnaire subscale was post-hoc.
Results
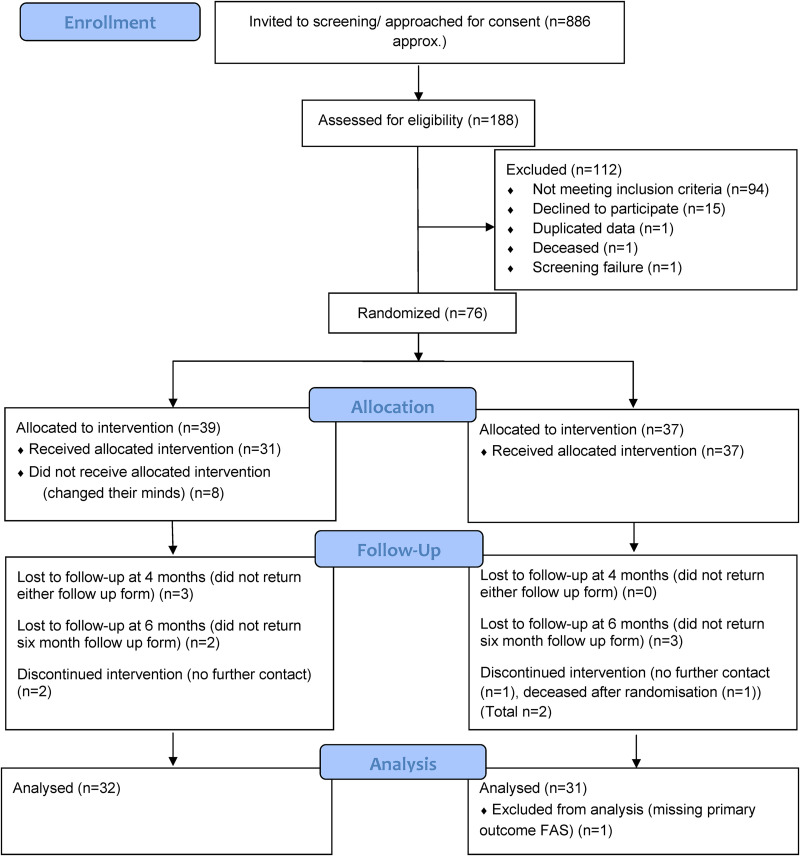
Invitation leaflets were sent to 886 patients from three services. Overall, 188 people (21%) consented to screening and returned questionnaires (126/650 (19%) in site one, 46/200 (23%) in site two and 16/36 (44%) in site three); 94/188 (50%) were eligible (Figure 1), though 5/76 were recruited more than two years since their last stroke, due to the lag between sending out invitations and randomisation. 76/94 (81%) of eligible participants were randomised between 18th December 2018 and 4th February 2020. Recruitment ended on 21st January 2020 (Figure 1). None were recruited through the online advertisement.
Figure 1.
Consort flow diagram.
Table 1 shows baseline data. Baseline medications were typical of a stroke population (as judged by the research team) and about half had major comorbidity (data on request).
Table 1.
Baseline characteristics.
| Intervention N = 39 | Control N = 37 | All N = 76 | |
|---|---|---|---|
| Age (Years) | |||
| N | 39 | 37 | 76 |
| Mean (SD) | 67.3 (12.5) | 66.1 (14.3) | 66.7 (13.3) |
| Gender | |||
| Male | 22 (56%) | 21 (57%) | 43 (57%) |
| Female | 17 (44%) | 16 (43%) | 33 (43%) |
| Months since most recent stroke | |||
| N | 39 | 37 | 76 |
| Mean (SD) | 10.3 (6.5) | 10.2 (7.1) | 10.3 (6.7) |
| Number of strokes | |||
| 1 | 31 (79%) | 28 (76%) | 59 (78%) |
| 2or more | 8 (21%) | 9 (24%) | 17 (22%) |
| Stroke subtype-self-reported | |||
| Bleed into brain | 9 (24%) | 7 (20%) | 16 (22%) |
| Clot (also known as ischaemic stroke) | 17 (46%) | 18 (51%) | 35 (49%) |
| Not sure | 11 (30%) | 10 (29%) | 21 (29%) |
| Missing | 2 | 2 | 4 |
| Modified Rankin score | |||
| 0–2 | 30 (77%) | 27 (73%) | 57 (75%) |
| 3–5 | 9 (23%) | 9 (26%) | 18 (24%) |
| Missing | 1 | 1 | |
| Short stroke impact scale (total score) | |||
| N | 35 | 34 | 69 |
| Mean (SD) | 31.1 (4.9) | 31.1 (5.2) | 31.1 (5.0) |
| Euroquol-5D-5L stroke recovery | |||
| N | 39 | 37 | 76 |
| Mean (SD) | 72.1 (18.4) | 67.4 (21.3) | 69.8 (19.9) |
Our feasibility outcomes were recruitment, attendance at sessions, follow-up, adherence and resources. We extended the recruitment period from 12 to 14 months to achieve ≥ 75 participants. Of the 39 randomised to the intervention, 31 (79%) attended at least one session and 23 (59%) attended at least four sessions. Seven health care professionals delivered a total of 151 intervention sessions. Six-month follow-up was obtained for 64/76 (84%) participants.
The health care professionals reported high adherence to all intervention topics (Appendix). Rater A rated 15 recordings and Rater B rated 14 recordings; of which 10 recordings were rated by both raters for training, calibration, inter-rater reliability and rater drift (Table 2). The health care professionals worked well with the participant to plan, or to practise alternative behaviours between sessions, but less frequently addressed unhelpful thoughts, sleep or how to maintain any gains.
Table 2.
Fidelity assessment of two external raters.
| Overall, how would you rate the therapeutic alliance? | Did the health professional or client develop one or more specific assignments for the client to engage in between sessions? | Did the health professional provide a rationale which emphasised the importance for the client of undertaking specific activities in order to alleviate the client’s symptoms? | Did the health care professional discuss ways of addressing any sleep problems? | Did the health professional work with the client to plan, or to practice alternative overt behaviours for the client to utilise outside of therapy? | Did the health professional provide a rationale for evaluating the accuracy of the client’s beliefs and evaluating beliefs in order to manage fatigue more effectively? | Did the health professional help the client to identify specific types of cognitive distortions or errors (e.g. all-or-nothing thinking, over-generalisation) that were present in the client’s thinking? | Did the health professional help the client to consider alternative explanations for events besides the client’s initial explanations for those events? | Did the health professional encourage the continued use after the end of therapy, of the skills the client had acquired during therapy? | |
|---|---|---|---|---|---|---|---|---|---|
| Rater A N = 15) Median/IQR | 5 (4,6) | 5 (5,6) | 5 (4,5) | 3 (1,4) | 5 (5,5) | 3 (2,4) | 2 (1,2) | 3 (2,4) | 3 (2,3) |
| Rater B N = 14. Median/IQR | 6 (5,7) | 5 (4.75,7) | 4.5 (3,5) | 3 (2.75, 4.5) | 5 (5,7) | 3 (1,3) | 3 (1,3) | 3 (2.75, 5) | 2.5 (1,5.25) |
Total of 29 tapes assessed: 10 by both raters and 9 by either rater A or rater B.
Each item is scored on a scale 1 to 7 (1 = not at all, 7 = extensive).
Seven health care professionals received 12.5 h training in total over three days from the trial manager, David Gillespie, Trudie Chalder and Gillian Mead. Health care professionals delivered at least one session to between 1 and 17 participants each. Each session lasted between 30 and 60 min; an additional 30 min per session was needed to prepare, arrange appointments and record each session. David Gillespie provided 54.5 h of supervision, that is about 9 h per health care professional. Stroke services can use these data to estimate the cost of implementation delivery if they wish to develop their post-stroke follow-up services. We have not provided a detailed breakdown of costs in this paper because salaries vary substantially according to the grade and experience of staff.
Fatigue Assessment Scale scores fell in both groups by around 4 points between baseline and six-month follow-up (Table 3). Between groups, there was no statistically significant differences in six-month Fatigue Assessment Scale (mean difference −0.619 (95% CI−4.931, 3.694 p = 0.7684), six-month Generalised Anxiety Disorder 7 (−0.178 (95% CI −3.823, 3.467), p = 0.9201) or in six-month Patient Health Questionnaire 9 (−0.247 (95% CI −2.935, 2.442), p = 0.8505) (Tables 4 and 5). The small negative mean differences indicate that the intervention was slightly better than the control.
Table 3.
Fatigue assessment scale; baseline, four months, six months.
| Intervention N = 39 | Control N = 37 | All N = 76 | ||
|---|---|---|---|---|
| Fatigue Assessment Scale (baseline) | ||||
| N | 39 | 36 | 75 | |
| Mean (SD) | 28.9 (6.2) | 30.1 (6.7) | 29.5(6.5) | |
| Fatigue Assessment Scale (four months) | ||||
| N | 30 | 30 | 60 | |
| Mean (SD) | 24.0 (7.2) | 25.8 (6.9) | 24.9 (7.1) | |
| Fatigue Assessment Scale (six months) | ||||
| N | 32 | 31 | 63 | |
| Mean (SD) | 24.2 (7.9) | 25.0 (6.8) | 24.6 (7.3) | |
| Change in Fatigue Assessment Scale (four months–baseline) | ||||
| N | 30 | 29 | 59 | |
| Mean (SD) | −4.6 (6.0) | −4.1(5.9) | −4.4 (5.9) | |
| Change in Fatigue Assessment Scale (six months–baseline) | ||||
| N | 32 | 30 | 62 | |
| Mean (SD) | −5.0 (7.1) | −5.3 (5.9) | −5.1 (6.5) | |
Numbers are n(%) or n, mean (SD).
There is one missing baseline fatigue assessment scale score.
Table 4.
Patient Health Questionnaire 9 scores at baseline, four months and six months.
| Treatment | ||||
|---|---|---|---|---|
| Intervention N = 39 | Control N = 37 | All N = 76 | ||
| Patient Health Questionnaire 9 (baseline) | ||||
| N | 39 | 37 | 76 | |
| Mean (SD) | 9.4 (5.6) | 10.6 (6.0) | 10.0 (5.8) | |
| Patient Health Questionnaire 9 (four months) | ||||
| N | 30 | 30 | 60 | |
| Mean (SD) | 5.1 (5.3) | 8.1 (5.4) | 6.6 (5.5) | |
| Patient Health Questionnaire 9 (six months) | ||||
| N | 32 | 32 | 64 | |
| Mean (SD) | 6.6 (6.1) | 7.1 (5.3) | 6.8 (5.7) | |
| Change in Patient Health Questionnaire 9 (four months–baseline) | ||||
| N | 30 | 30 | 60 | |
| Mean (SD) | −3.4 (4.9) | −1.5 (3.9) | −2.5 (4.5) | |
| Change in Patient Health Questionnaire 9 (six months–baseline) | ||||
| N | 32 | 32 | 64 | |
| Mean (SD) | −2.2 (4.6) | −3.1 (4.8) | −2.7 (4.7) | |
Table 5.
Generalised Anxiety Disorder 7 at baseline, four and six months.
| Intervention N = 39 | Control N = 37 | All N = 76 | |||||
|---|---|---|---|---|---|---|---|
| Generalised Anxiety Disorder 7 (baseline) | |||||||
| N | 39 | 37 | 76 | ||||
| Mean (SD) | 6.4 (4.9) | 6.8 (5.5) | 6.6 (5.2) | ||||
| Generalised Anxiety Disorder 7 (four months) | |||||||
| N | 29 | 30 | 59 | ||||
| Mean (SD) | 2.8 (3.9) | 5.3 (5.7) | 4.1 (5.0) | ||||
| Generalised Anxiety Disorder 7 (six months) | |||||||
| N | 32 | 32 | 64 | ||||
| Mean (SD) | 4.4 (4.1) | 5.4 (5.7) | 4.9 (5.0) | ||||
| Change in Generalised Anxiety Disorder 7 (four months–baseline) | |||||||
| N | 29 | 30 | 59 | ||||
| Mean (SD) | −3.2 (3.6) | −0.9 (3.4) | −2.1 (3.6) | ||||
| Change in Generalised Anxiety Disorder 7 (six months–baseline) | |||||||
| N | 32 | 32 | 64 | ||||
| Mean (SD) | −1.6 (3.9) | −1.1 (3.1) | −1.4 (3.5) | ||||
For the outcomes without formal statistical testing (Appendix), there was no indication of differences in descriptive statistics, including the social participation item of short stroke impact scale (change between baseline and six months: 0.1 (SD 1.1, n = 30) in intervention and −0.1 (SD 1.2, n = 31) in control), Euroquol 5D 5L (mean patient reported stroke recovery at six months was 72.7% (SD 21.1, n = 32) in the intervention and 68.1% (SD 26.9, n = 32) in control, no difference in return to work, or in self-reported prescriptions of antidepressants or anxiolytics (one in control and one in the intervention group) or in the fear avoidance subscale of cognitive and behavioural responses questionnaire.
Discussion
We recruited 76 patients in 14 months and delivered 151 interventions sessions to 31/39 participants randomised to the intervention, and achieved 84% follow-up at six months. Participants were younger than the general stroke population, men outnumbered women, and most had mild disability. There was no statistically significant difference between groups for the Fatigue Assessment Scale, Patient Health Questionnaire 9 and Generalised Anxiety Disorder 7, though effect sizes were in favour of the intervention. We did not recruit anyone from our online advertisement.
To the best of our knowledge, we have more than doubled the number of people recruited into randomised controlled trials of CBT approaches for post-stroke fatigue. Although we fell just short of our pre-specified progression criteria, randomised controlled trials often proceed to a main phase despite just missing one criterion.26 We might have achieved 90% follow-up rates had the team been able to access the trials unit during Covid-19 lockdown.
Of those randomised to the intervention, adherence was 59% for at least four sessions; with 8/39 not attending any sessions. This could have been due to the length of the manual or because participants had not understood the need for active participation and ‘homework’ between sessions. In future trials, we would need to make it clearer from the outset that active involvement and ‘homework’ between sessions is expected of participants, and we would need to consider reducing the length of the handbook. Ideally we would have performed a process evaluation including qualitative interviews to explore in-depth reasons for low participation, but we did not have the resource to do this. The health care professionals formed good therapeutic relationships with participants and the sessions were guided by the manual, but addressing sleep and identifying unhelpful beliefs appeared more difficult to implement. Note though, that this was introduced later in the manual and it might have been better to introduce the concept much earlier. Thus, our invention may not have been potent enough. Health care professionals may have needed more extensive training in cognitive behaviour therapy or more supervision. However, in most health services there would be insufficient cognitive behaviour therapists to treat all patients with fatigue; a reason for choosing a ‘light touch’ approach to training in the principles of cognitive behaviour therapy, to health care professionals who already had extensive knowledge of stroke. This approach has preliminary evidence of efficacy in cancer-related fatigue.27 We used telephone rather than video calls; this might have contributed to small effect sizes.
There was a fall in Fatigue Assessment Scale scores in the control group as well as in the intervention group. This might be due to natural recovery of fatigue over time, though previous cohort studies have found mixed patterns of either improvement or deterioration of fatigue over time.28 Another possible explanation is that being involved in a trial of post-stroke fatigue may have validated the participant's symptoms, and this external validation may have been helpful. The patient information leaflet provided by the Stroke Association, in the context of a clinical trial of fatigue, may have brought about the improvement in fatigue; though there is evidence that information alone is not helpful after stroke.29 In clinical practice, it would be reasonable to provide the Stroke Association leaflet to stroke survivors with post-stroke fatigue. Our data also suggest that the Fatigue Assessment Scale scores is responsive to change—an important consideration when deciding which fatigue scale is suitable for use in future clinical research.
Post-stroke fatigue shares similarities with fatigue in other conditions; yet there are important differences too. For example, in breast cancer,30 fatigue is associated with depression and anxiety, but breast cancer treatments (including surgery, radiotherapy, hormonal therapy and chemotherapy) are probably more likely to cause fatigue directly than are stroke treatments (statins, antiplatelets and antihypertensives). In multiple sclerosis, which is primarily an inflammatory disease of the central nervous system, fatigue is common; it is associated with depression and sleep disorders, and it may be triggered by the disease process. Although the causes of cancer fatigue and fatigue in multiple sclerosis are likely to be different, both can be improved by cognitive behavioural approaches or exercise.31,32
In summary, we have shown that it is possible to recruit people with post-stroke fatigue to a randomised trial of a complex intervention for fatigue. Stroke survivors were willing to be randomised, though the adherence to our intervention was lower than hoped. Further work needs to be done to establish how to increase adherence. Also, the intervention might be more effective if delivered by staff who have already been trained in cognitive behaviour therapy. Post-stroke fatigue is still a priority for research,3 and further work, particularly around intervention design and testing, is needed in this neglected area.
Clinical messages.
It is feasible to recruit stroke survivors with fatigue into a randomised controlled clinical trial of a CBT informed intervention.
Larger trials in post-stroke fatigue could use the methodology we developed.
Supplemental Material
Supplemental material, sj-docx-1-cre-10.1177_02692155221113908 for Post stroke intervention trial in fatigue (POSITIF): Randomised multicentre feasibility trial by Gillian Mead, David Gillespie, Mark Barber, Allan House, Steff Lewis, Hannah Ensor, Simiao Wu, and Trudie Chalder in Clinical Rehabilitation
Acknowledgements
We thank the participants and their families, the grant holders not on the writing committee (Marian Brady, Yvonne Chun, Maree Hackett, Martin Dennis, Vera Cvoro, Alan Carson), Data monitoring committee (Frederike Van Wijck, Sally Kerry, Helen Rodgers), the patient advisor (Euan Haig), Health Care Professionals (Pat Taylor, Allan Macraild, Brenda Bain, Seona Burgess, Michelle Coakley, Jessica Teasdale, Mandy Couser), the Scottish Stroke Research Network, independent raters (Brenda Barrass and Debbie Brewin), Edinburgh Clinical Trials Unit (particularly Fiona Wee, Alix Macdonald and Richard Parker). Jill Pearl helped develop easy access trial materials. Aryelly Carbonell Rodriguez was the validating statistician.
Footnotes
Disclosures: TC has authored self-help books on fatigue. GM has received honoraria (used to support further research) and expenses to lecture on post-stroke fatigue.
Availability of data and materials: ECTUdatashare@ed.ac.uk
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Chest Heart & Stroke Scotland (Grant reference: Res16/A168). The funder had no influence on trial design or analysis. TC is part funded by National Institute for Health Research (NIHR) Specialist Biomedical Research Centre for Mental Health award to the South London and Maudsley NHS Foundation Trust and Institute of Psychiatry at King’s College London.
ORCID iDs: Gillian Mead https://orcid.org/0000-0001-7494-2023
David Gillespie https://orcid.org/0000-0003-1325-9727
Study registration: U.S. National Institutes of Health on ClinicalTrials.gov (11 June 2018) (NCT03551327). Ethics approval: East of Scotland Research Ethics Committee (Reference: 17/ES/0159).
Supplemental material: Supplemental material for this article is available online.
References
- 1.Annoni J-M, Staub F, Bogousslavsky J, et al. Frequency, characterisation and therapies of fatigue after stroke. Neurol Sci 2008; 29: S244–S246. [DOI] [PubMed] [Google Scholar]
- 2.Hinkle JL, Becker KJ, Kim J, et al. On behalf of the American heart association council on cardiovascular and stroke nursing and stroke council poststroke fatigue: emerging evidence and approaches to management: a scientific statement for healthcare professionals from the American heart association. Stroke 2017; 48: e159–e170. [DOI] [PubMed] [Google Scholar]
- 3.Stroke Association. Shaping stroke research to rebuild lives. The Stroke Priority Setting partnership results for investment. June 2021. Item code: A08C19 © Stroke Association 2021.
- 4.Wu S, Barugh A, MacLeod M, et al. Psychological association of post-stroke fatigue: a systematic review and meta-analysis. Stroke 2014; 45: 1778–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duncan F, Greig CA, Dennis MS, et al. An exploratory longitudinal cohort study of associations of fatigue after stroke. Stroke 2015; 46: 1052–1058. [DOI] [PubMed] [Google Scholar]
- 6.Berger AM, Abernethy AP, Atkinson Aet al. et al. Cancer-related fatigue. Practice Guidelines Oncol Version 1. 2009. Retrieved September 2016.
- 7.Hilfiker R, Meichtry A, Eicher M, et al. Exercise and other non-pharmaceutical interventions for cancer-related fatigue in patients during or after cancer treatment: A systematic review incorporating an indirect-comparisons meta-analysis. Br J Sports Med 2018; 52: 651–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corbett TK, Groarke A, Devane Det al. et al. The effectiveness of psychological interventions for fatigue in cancer survivors: systematic review of randomised controlled trials. Syst Rev 2019; 8: 1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thong MSY, van Noorden CJF, Steindorf K, et al. Cancer-related fatigue: causes and current treatment options. Curr Treat Options Oncol 2020; 21: 17. Erratum in: Curr Treat Options Oncol. 2022 Mar;23(3):450-451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Su Y, Yuki M, Otsuki M. Non-pharmacological interventions for post-stroke fatigue: systematic review and network meta-analysis. J Clin Med 2020; 9: 621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen S, Wong D, McKay Aet al. Cognitive behavioural therapy for post-stroke fatigue and sleep disturbance: a pilot randomised controlled trial with blind assessment. Neuropsychol Rehabil 2019; 29: 723–738. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, Wang L, Dong X. Influence of cognitive behavioral intervention on health behavior and treatment compliance of patients with post stroke fatigue based on problem solving method. Chin Nurs Res 2018; 32: 327–329. [Google Scholar]
- 13.Wu S, Mead G, Macleod M, et al. Model of understanding fatigue after stroke. Stroke. 2015; 46: 893–898. [DOI] [PubMed] [Google Scholar]
- 14.Wu S, Chalder T, Anderson KEet al. et al. Development of a psychological intervention for fatigue after stroke. PLoS ONE 2017; 12: e0183286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gillespie DC, Barber M, Brady MC, et al. Study protocol for POSITIF. Pilot Feasibility Stud 2020; 6: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowen A, Knapp P, Hoffman A, et al. Psychological services for people with stroke: compliance with the United Kingdom national clinical guidelines. Clin Rehabil 2005; 19: 323–330. [DOI] [PubMed] [Google Scholar]
- 17.Rothwell K, Boaden R, Bamford D, et al. Feasibility of assessing the needs of stroke patients after six months using the GM-SAT. Clin Rehabil 2013; 27: 264–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacIsaac PMA, Peters M, English C, et al. On behalf of the VISTA collaboration. Derivation and validation of a modified short form of the stroke impact scale. J Am Heart Assoc 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spitzer RL, Kroenke K, Williams JB, et al. A brief measure for assessing generalised anxiety disorder. The GAD-7. Arch Intern Med 2006; 166: 1092 − 7. [DOI] [PubMed] [Google Scholar]
- 20.Kroenke K, Spitzer RL. The PHQ-9: a new depression diagnostic and severity measure. Psychiatr Ann 2002: 32: 1–7 [Google Scholar]
- 21.Godfrey E, Chalder T, Ridsdale L, et al. Investigating the active ingredients of cognitive behaviour therapy and counselling for patients with chronic fatigue in primary care: developing a new process measure to assess treatment fidelity and predict outcome. Br J Clin Psychol 2007; 46: 253–272. [DOI] [PubMed] [Google Scholar]
- 22.Michielsen HJ, De Vries J, Van Heck GL. Psychometric properties of a brief self-rated fatigue measure: the fatigue assessment scale. J Psychosom Res 2003; 54: 345–352. [DOI] [PubMed] [Google Scholar]
- 23.Herdman M., Gudex C, Lloyd A., et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res 2011; 20: 1727–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryan EG, Vitoratou S, Goldsmith KA, et al. Psychometric properties and factor structure of shortened version of the cognitive behavioural responses questionnaire. Psychosom Med 2018; 80: 230–237. [DOI] [PubMed] [Google Scholar]
- 25.Flight L, Allison A, Dimairo Met al. et al. Recommendations for the analysis of individually randomised controlled trials with clustering in one arm–a case of continuous outcomes. BMC Med Res Methodol 2016; 16: 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Avery KNL, Williamson PR, Gamble C, et al. Informing efficient randomised controlled trials: exploration of challenges in developing progression criteria for internal pilot studies. BMJ Open 2017; 7: e013537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Armes J, Chalder T, Addington-Hall J, et al. A randomized controlled trial to evaluate the effectiveness of a brief, behaviorally oriented intervention for cancer-related fatigue. Cancer 2007; 110: 1385–1395. [DOI] [PubMed] [Google Scholar]
- 28.Duncan F, Wu S, Mead GE. A systematic review of longitudinal studies of post-stroke fatigue. J Pyschosomatic Res 2012; 73: 18−27. [DOI] [PubMed] [Google Scholar]
- 29.Crocker TF, Brown L, Lam Net al. et al. Information provision for stroke survivors and their carers. Cochrane Database Syst Rev 2021; . Art No.:CD001919 DOI: 10.1002/14651858. pub4. Accessed 05 April 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muthanna FMS, Karuppannan M, Hassan BAR, et al. Impact of fatigue on quality of life among breast cancer patients receiving chemotherapy. Osong Public Health Res Perspect 2021; 12: 115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van den Akker LE, Beckerman H, Collette EHet al. et al. Effectiveness of cognitive behavioral therapy for the treatment of fatigue in patients with multiple sclerosis: a systematic review and meta-analysis. J Psychosom Res 2016; 90: 33–42. [DOI] [PubMed] [Google Scholar]
- 32.Heine M, van de Port I, Rietberg MBet al. et al. Exercise therapy for fatigue in multiple sclerosis. Cochrane Database Syst Rev 2015; (9). Art. No.: CD009956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.White PD, Goldsmith K, Johnson AL, et al. Recovery from chronic fatigue syndrome after treatments given in the PACE trial. Psychol Med 2013; 43(10): 2227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-cre-10.1177_02692155221113908 for Post stroke intervention trial in fatigue (POSITIF): Randomised multicentre feasibility trial by Gillian Mead, David Gillespie, Mark Barber, Allan House, Steff Lewis, Hannah Ensor, Simiao Wu, and Trudie Chalder in Clinical Rehabilitation