Abstract
Context
Abdominal pain after surgery can occur for numerous reasons. Postoperative radiographs may be indicated to evaluate for ileus or other reasons for the pain. Whether outcomes are significantly different based on whether patients get radiographs following lateral lumbar interbody fusion (LLIF) are unclear.
Aims:
To investigate the postoperative outcomes of patients experiencing abdominal pain after LLIF.
Settings and Design:
This retrospective cohort study included patients at a tertiary academic medical center and surrounding affiliated hospitals.
Materials and Methods:
Patients >18 years of age who underwent elective LLIF at a single institution were retrospectively identified. Patients were stratified into two groups depending on whether they received a postoperative abdominal radiograph or computed tomography (CT) scan for postoperative abdominal pain.
Statistical Analysis:
Patient demographics, surgical characteristics, and surgical outcomes were compared between groups utilizing independent t-tests or Mann–Whitney U-tests for continuous variables or Pearson's Chi-square tests for categorical variables.
Results:
A total of 153 patients (18 with abdominal scans, 135 without) were included. Patients who received a postoperative abdominal radiograph or CT scan were more likely to undergo exploratory laparotomy (11.1% vs. 0.00%, P = 0.013). Ultimately, patients with abdominal scans had a longer hospital length of stay (6.67 vs. 3.79 days, P = 0.002) and were discharged home less frequently (71.4% vs. 83.7%, P = 0.002).
Conclusions:
Patients who received abdominal imaging after LLIF were more likely to undergo exploratory laparotomy, experience longer hospital length of stay, and were discharged home less frequently. Intra-abdominal air on postoperative imaging without corresponding physical exam findings consistent with bowel injury is not an appropriate indication for surgical intervention.
Keywords: Exploratory laparotomy, intra-abdominal air, lateral lumbar interbody fusion, length of stay, postoperative imaging
INTRODUCTION
The minimally invasive aspect of the lateral lumbar interbody fusion (LLIF) imparts distinct advantages to access the anterior column of the spine when compared to open techniques including reduced blood loss, lower complication rates, decreased costs, and a shorter hospital length of stay.[1] Access to the anterior column via the lateral approach allows for a wider interbody cage footprint when compared to transforaminal interbody fusions, which may minimize subsidence rates, while still allowing for indirect neural decompression.[2,3] Further, LLIFs avoid the need to retract the major vessels anteriorly, while also avoiding potential transection of the facet capsules and ligamentous structures posteriorly, which may result in increased adjacent segment motion and instability.[2,4,5] For these reasons, LLIFs have become a popular option for lumbar fusions.[6]
Although postoperative ileus is the most common abdominal complication after LLIF, it is important for clinicians to recognize more insidious peritoneal signs and symptoms of bowel perforation (i.e., abdominal pain, hyperemesis, hypotension, peritonitis, and sepsis).[7,8] Visceral bowel perforations are associated with high risk of morbidity and warrant further appropriate workup and timely treatment.[9] Common diagnostic findings for bowel perforations on computed tomography (CT) scans include fluid in the abdomen, pneumoperitoneum, extraluminal trapped air, loss of bowel continuity, and increased bowel wall thickness.[10]
Only a few cases of bowel perforation from LLIF have been reported in the literature.[11,12,13] These studies are largely limited to case series or reports, with variability in patient demographics, postoperative presentation, and possible etiologies of injury. Therefore, the purpose of this study was to investigate the surgical outcomes after LLIF procedures which required additional in-hospital radiographic imaging.
MATERIALS AND METHODS
Patient selection and data collection
After approval from the Institutional Review Board, a retrospective analysis was conducted on patients who received surgery at a single-academic center and six affiliated sites. Patients >18 years of age who underwent an LLIF between 2010 and 2021 were retrospectively identified and included in our analysis using Current Procedural Terminology code 22,558 and a manual review of operative notes to confirm if the patient underwent a LLIF. Patients were excluded from the study if they received surgical intervention for any tumors, infections, trauma, or revision procedures at the index level.
Patient demographic data, including age, sex, body-mass index (BMI), smoking status (never, former, current smoker), Charlson Comorbidity Index (CCI), primary preoperative diagnosis, number of levels fused, and follow-up duration were all collected via manual chart review. Chart review was also performed to identify patients who had a postoperative abdominal radiograph or CT scan. Patients were then stratified into groups depending on whether they received any postoperative abdominal scan.
Statistical analysis
Descriptive statistics including mean and standard deviation were used to report patient demographics, surgical characteristics, and clinical outcomes. A Shapiro–Wilk test was used to analyze the normality of each continuous variable, and parametric data was analyzed with independent t-tests while nonparametric data was analyzed with Mann–Whitney U-tests. All categorical variables were compared using a Pearson's Chi-square test.
RESULTS
A total of 153 patients (18 with abdominal scans, 135 without) were included. There were no differences in age (63.8 ± 9.48 vs. 63.4 ± 10.5, P = 0.937), sex (female: 66.7% vs. 54.1% female, P = 0.449), smoking status (current smokers: 8.33% vs. 20.7%, P = 0.308), BMI (28.7 ± 5.84 vs. 31.1 ± 7.11, P = 0.315), diabetic status (28.6% vs. 16.8%, P = 0.480), or CCI (0.87 ± 1.92 vs. 0.74 ± 1.09, P = 0.425) between groups [Table 1]. Patients who received a postoperative abdominal scan were more likely to undergo exploratory laparotomy (2 vs. 0 patients, P = 0.013) [Table 1]. Each exploratory laparotomy case was ultimately negative for bowel injury. Representative CT images of intrabdominal air can be seen in [Figures 1 and 2].
Table 1.
Demographics of cohort when stratified into patients with and without abdominal imaging
| Variable | No abdominal imaging (n=135), n (%) | Received abdominal imaging (n=18), n (%) | P a, b |
|---|---|---|---|
| Age | 63.4 (10.5) | 63.8 (9.48) | 0.937 |
| Sex | |||
| Female | 73 (54.1) | 12 (66.7) | 0.449 |
| Male | 62 (45.9) | 6 (33.3) | |
| BMI | 31.1 (7.11) | 28.7 (5.84) | 0.315 |
| Diabetes | |||
| No | 84 (83.2) | 10 (71.4) | 0.283 |
| Yes | 17 (16.8) | 4 (28.6) | |
| CCI | 0.74 (1.09) | 0.87 (1.92) | 0.425 |
| Smoking | |||
| Current smoker | 18 (20.7) | 1 (8.33) | 0.473 |
| Former smoker | 30 (34.5) | 6 (50.0) | |
| Nonsmoker | 39 (44.8) | 5 (41.7) | |
| Hospital length of stay | 3.79 (1.71) | 6.67 (3.97) | 0.002 |
| Discharge | |||
| Home | 72 (83.7) | 10 (71.4) | 0.002 |
| Inpatient rehab facility | 2 (2.33) | 4 (28.6) | |
| Skilled nursing facility | 12 (14.0) | 0 | |
| Levels operated on | |||
| L1–L2 | |||
| No | 129 (95.6) | 15 (83.3) | 0.073 |
| Yes | 6 (4.44) | 3 (16.7) | |
| L2–L3 | |||
| No | 84 (62.2) | 8 (44.4) | 0.234 |
| Yes | 51 (37.8) | 10 (55.6) | |
| L3–L4 | |||
| No | 51 (37.8) | 10 (55.6) | 0.234 |
| Yes | 84 (62.2) | 8 (44.4) | |
| L4–L5 | |||
| No | 87 (64.4) | 13 (72.2) | 0.698 |
| Yes | 48 (35.6) | 5 (27.8) | |
| Underwent exploratory laparotomy | |||
| No | 135 (100) | 16 (88.9) | 0.013 |
| Yes | 0 | 2 (11.1) |
aIndependent-samples t-test or Mann–Whitney U-test for age, BMI, CCI, hospital length of stay, bPearson Chi-square test or Fisher's exact test for sex, diabetes, smoking status, disposition, levels operated on, exploratory laparotomy status. BMI - Body mass index, CCI - Charlson comorbidity index
Figure 1.
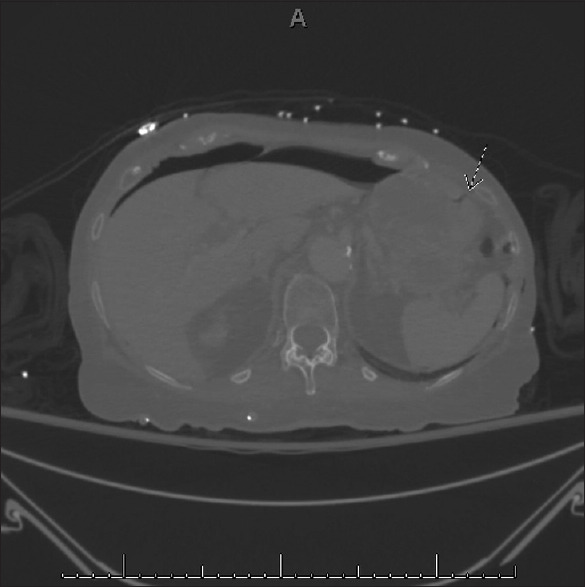
Axial cut of a computed tomography scan. A small quantity of air in the gastric fundus can be seen extending to the gastric wall
Figure 2.
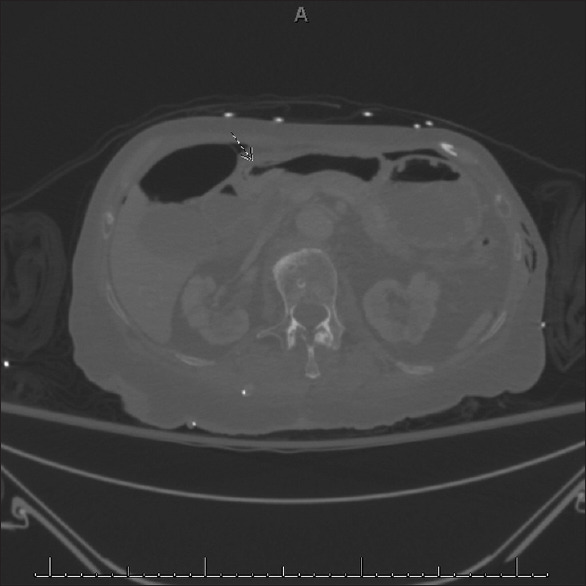
Axial cut of a computed tomography scan. Curvilinear air posterior to the gastric wall can be seen adjacent to air-fluid levels in the duodenum
Of the 18 patients who received an abdominal scan, 15 patients were found to have an ileus, 2 had suspected free air in the abdomen, and 1 had a postoperative fluid collection in the pelvis. Patients with abdominal scans had a longer hospital length of stay (6.67 ± 3.97 vs. 3.79 ± 1.71 days, P = 0.002) and were discharged home less frequently compared to patients without abdominal imaging (71.4% vs. 83.7%, P = 0.002) [Table 1].
There were no differences in complications (33.3% vs. 15.6%, P = 0.093), 90-day hospital readmissions (0.00% vs. 5.93%, P = 0.597), or revision surgeries (5.56% vs. 9.63%, P = 1.000) between groups [Table 2].
Table 2.
90 days readmissions, complications, and revisions
| Variable | No abdominal imaging (n=135), n (%) | Received abdominal imaging (n=18), n (%) | P a |
|---|---|---|---|
| 90-day readmissions | |||
| No | 127 (94.1) | 18 (100) | 0.597 |
| Yes | 8 (5.93) | 0 | |
| Surgical complications | |||
| No | 114 (84.4) | 12 (66.7) | 0.093 |
| Yes | 21 (15.6) | 6 (33.3) | |
| Revisions | |||
| No | 122 (90.4) | 17 (94.4) | 1.000 |
| Yes | 13 (9.63) | 1 (5.56) |
aPearson Chi-square test or Fisher's exact test comparing groups
DISCUSSION
LLIFs are a popular lumbar fusion technique due to its ability to optimize sagittal alignment, while avoiding manipulation of the great vessels anteriorly.[14,15,16,17] The minimally invasive nature of the surgery allows for shorter overall hospital length of stay when compared to open surgeries.[18] When compared to similar minimally invasive fusions such as oblique lateral interbody fusion, LLIF has been shown to be safer for new surgeons due to a lower learning curve.[19] However, LLIFs do pose a threat for intraperitoneal violation and postoperative ileus due to manipulation of the abdominal contents.[20] Our study identified 18 of 136 patient's required postoperative abdominal imaging due to prolonged abdominal pain with an additional two patients having intraperitoneal air identified on CT imaging. These patients ultimately underwent an exploratory laparotomy without the identification of violation of the peritoneal space during the LLIF procedure.
Previous literature has found that postoperative ileus is the most common abdominal complication after LLIF, occurring in 7% of cases.[7] Prior independent risk factors for developing ileus after LLIF include gastroesophageal reflux disease, posterior instrumentation, and LLIF at L1–L2.[7] Interestingly, our study found that no preoperative demographic factor had a significant impact on the likelihood of postoperative abdominal pain and the subsequent need for abdominal imaging. In addition, there were no significant differences with regard to postoperative outcomes, complications, readmissions, or revisions. In our study, patients who underwent postoperative abdominal imaging had a significantly longer hospital length of stay and were less likely to discharge home. The prolonged length of stay following postoperative ileus is consistent with a previous study which found that elderly patients who underwent ALIF complicated by a postoperative ileus had a significantly greater length of hospital stay, which was associated with an additional healthcare cost of more than $2000.[21] However, our study did identify a nonsignificant increase in the frequency of LLIFs performed at L1-L2, possibly contributing to the number of patients who received abdominal scans since postoperative ileus is more commonly encountered after performing LLIFs at that level.[7] This data, in conjunction with previous literature, suggests that preoperative counseling of patients regarding the elevated risk of postoperative ileus for LLIFs performed at the L1-2 level is warranted.
One feared complication with anterior lumbar spine surgery, including lateral fusion, is visceral bowel injury. Anatomic studies have previously found there is a risk for bowel injury, especially with lateral interbody fusion at L2-L3 and L3-L4.[22] However, the incidence of these injuries is quite low, typically around 0.1%.[13,16,22,23] However, our study did not find an association between abdominal scans and LLIFs at L2-L3 or L3-L4.
A retrospective analysis of twelve patients undergoing combined LLIF with open posterior pedicle instrumentation for the correction of adult degenerative scoliosis reported on one case of intraoperative bowel injury that necessitated open laparotomy with subsequent segmental bowel resection.[13] This case illustrates the learning curve inherent to LLIF procedures, especially when performed on patients with severe degenerative anatomy. However, in a large nationwide survey of 2998 LLIFs performed in Japan, the rate of bowel injury was found to be as low as 0.03%.[23] The range in intraperitoneal complications may be in part due to surgical technique, preoperative degenerative disease severity, and surgeon experience.[19]
In general, it is reasonable to expect bowel injuries should be recognized either intraoperatively or on postoperative examination. Patients with bowel injury typically present with increased abdominal pain, guarding, vital sign changes, and a pneumoperitoneum visualized on CT scan.[4,11] Bowel perforations are associated with a high risk of patient morbidity and must be urgently evaluated and treated by general surgery. Described treatments include exploratory laparotomy followed by bowel resection or colostomy.[4,11] Bowel injuries have previously been classified as sentinel events in spine surgery. In a large national database study including 543,146 lumbar spine surgeries, there were a total of 30 bowel injuries which had a relative risk of mortality 200.9 times greater than patients without these complications.[24]
Our study suggests that patients who underwent further workup for abdominal pain with radiographic imaging were significantly more likely to undergo exploratory laparotomy (P = 0.014). Exploratory laparotomy was recommended by our general surgery team for two patients due to vague abdominal pain and the finding of free air in the abdomen, as interpreted by our radiologist on CT imaging, which was ordered due to vague abdominal pain. Neither patient had an obvious intraoperative complication during the LLIF procedure nor did the patients have documented physical examination findings or vital signs suggestive of acute abdomen. Exploratory laparotomy was performed by general surgery in both cases with both negative for bowel injury. The phenomenon of incidental pneumoperitoneum was described in a recent retrospective study of 90 patients who underwent LLIF and were subsequently taken for abdominal CT scans within 48 h of surgery.[25] There was a positive pneumoperitoneum on CT in 5.5% of patients who underwent CT scan with no patients having evidence of bowel perforation and each of these patients only had mild vague abdominal symptoms.[25] None of the patients required additional postoperative treatment. This study is suggestive of the relatively high rate of postoperative pneumoperitoneum in the absence of bowel injury. In addition, prior trauma surgery literature has demonstrated a false-positive rate of 2%–4.5% for the detection of pneumoperitoneum on CT scans.[26,27]
Overall, this study highlights important factors regarding postoperative complications and care of patients who undergo LLIF. Abdominal pain postoperatively is a common complaint necessitating further radiographic imaging and work up. However, the most common etiology of abdominal pain is ileus, and although this complication is associated with increased length of stay and increased rate of discharge to rehabilitation, there is no indication for an exploratory laparoscopy unless there was an intraoperative complication or physical exam finding concerning for acute abdomen.[21] A departmental focus on these complications could possibly help with new protocol implementation designed to anticipate and address clinically insignificant pneumoperitoneum before it results in unnecessary surgical interventions.
This study is limited by its retrospective nature and the low number of patients who met inclusion criteria. The low power of the study may underestimate potentially significant differences in surgical complications between patients who require abdominal radiographs or CT scans compared to those who do not. In addition, we were unable to obtain institutional cost data to determine the relative increase in healthcare utilization caused by the additional surgeries, greater length of stay, and greater rate of inpatient rehabilitation admissions between the two groups. Future prospective studies may improve our knowledge of the cost effectiveness of LLIFs and their association with abdominal injuries.
CONCLUSIONS
Patients who receive abdominal radiographic imaging due to abdominal pain after LLIFs were more likely to undergo exploratory laparotomy, experience longer hospital length of stay, and were discharged home less frequently. No bowel injury was identified during the exploratory laparotomies. Intraabdominal air without physical exam findings of peritonitis is not an appropriate indication for further surgical intervention. New protocols designed to anticipate and address abdominal complications and increased recognition of the insidious pneumoperitoneum phenomenon after LLIF is essential to providing better patient care and avoiding unnecessary surgeries.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Lucio JC, Vanconia RB, Deluzio KJ, Lehmen JA, Rodgers JA, Rodgers W. Economics of less invasive spinal surgery: An analysis of hospital cost differences between open and minimally invasive instrumented spinal fusion procedures during the perioperative period. Risk Manag Healthc Policy. 2012;5:65–74. doi: 10.2147/RMHP.S30974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma AK, Kepler CK, Girardi FP, Cammisa FP, Huang RC, Sama AA. Lateral lumbar interbody fusion. J Spinal Disord Tech. 2011;24:242–50. doi: 10.1097/BSD.0b013e3181ecf995. [DOI] [PubMed] [Google Scholar]
- 3.Sembrano JN, Tohmeh A, Isaacs R Group SDS. Two-year comparative outcomes of MIS lateral and MIS transforaminal interbody fusion in the treatment of degenerative spondylolisthesis. Spine. 2016;41:S123–32. doi: 10.1097/BRS.0000000000001471. [DOI] [PubMed] [Google Scholar]
- 4.Malham GM, Ellis NJ, Parker RM, Seex KA. Clinical outcome and fusion rates after the first 30 extreme lateral interbody fusions. Sci World J. 2012;2012:246989. doi: 10.1100/2012/246989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wangsawatwong P, Sawa AG, Pereira BA, Lehrman JN, Turner JD, Uribe JS, et al. Does the choice of spinal interbody fusion approach significantly affect adjacent segment mobility? Spine. 2021;46:E1119–24. doi: 10.1097/BRS.0000000000004058. [DOI] [PubMed] [Google Scholar]
- 6.Souslian FG, Patel PD. Review and analysis of modern lumbar spinal fusion techniques. Brit J Neurosurg. 2021. Epub ahead of print:1-7. [doi: 10.1080/02688697.2021.1881041] [DOI] [PubMed]
- 7.Maaieh MA, Du JY, Aichmair A, Huang RC, Hughes AP, Cammisa FP, et al. Multivariate analysis on risk factors for postoperative ileus after lateral lumbar interbody fusion. Spine. 2014;39:688–94. doi: 10.1097/BRS.0000000000000238. [DOI] [PubMed] [Google Scholar]
- 8.Siasios I, Vakharia K, Khan A, Meyers JE, Yavorek S, Pollina J, et al. Bowel injury in lumbar spine surgery: A review of the literature. J Spine Surg. 2018;4:130–7. doi: 10.21037/jss.2018.03.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bielecki K, Kamiński P, Klukowski M. Large bowel perforation: Morbidity and mortality. Tech Coloproctol. 2002;6:177–82. doi: 10.1007/s101510200039. [DOI] [PubMed] [Google Scholar]
- 10.Rustagi T, Yilmaz E, Alonso F, Schmidt C, Oskouian R, Tubbs RS, et al. Iatrogenic bowel injury following minimally invasive lateral approach to the lumbar spine: A retrospective analysis of 3 cases. Global Spine J. 2019;9:375–82. doi: 10.1177/2192568218800045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Balsano M, Carlucci S, Ose M, Boriani L. A case report of a rare complication of bowel perforation in extreme lateral interbody fusion. Eur Spine J. 2015;24:405–8. doi: 10.1007/s00586-015-3881-6. [DOI] [PubMed] [Google Scholar]
- 12.Yilmaz E, Iwanaga J, Moisi M, Blecher R, Abdul-Jabbar A, Tawfik T, et al. Risks of colon injuries in extreme lateral approaches to the lumbar spine: An anatomical study. Cureus. 2018;10:e2122. doi: 10.7759/cureus.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tormenti MJ, Maserati MB, Bonfield CM, Okonkwo DO, Kanter AS. Complications and radiographic correction in adult scoliosis following combined transpsoas extreme lateral interbody fusion and posterior pedicle screw instrumentation. Neurosurg Focus. 2010;28:E7. doi: 10.3171/2010.1.FOCUS09263. [DOI] [PubMed] [Google Scholar]
- 14.Berjano P, Balsano M, Buric J, Petruzzi M, Lamartina C. Direct lateral access lumbar and thoracolumbar fusion: Preliminary results. Eur Spine J. 2012;21:37–42. doi: 10.1007/s00586-012-2217-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pumberger M, Hughes AP, Huang RR, Sama AA, Cammisa FP, Girardi FP. Neurologic deficit following lateral lumbar interbody fusion. Eur Spine J. 2012;21:1192–9. doi: 10.1007/s00586-011-2087-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Epstein NE. Perspective on the true incidence of bowel perforations occurring with extreme lateral lumbar interbody fusions. How should they be treated? Surg Neurol Int. 2021;12:576. doi: 10.25259/SNI_1003_2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakashima H, Kanemura T, Satake K, Ishikawa Y, Ouchida J, Segi N, et al. Changes in sagittal alignment following short-level lumbar interbody fusion: Comparison between posterior and lateral lumbar interbody fusions. Asian Spine J. 2019;13:904–12. doi: 10.31616/asj.2019.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodgers WB, Gerber EJ, Patterson J. Intraoperative and early postoperative complications in extreme lateral interbody fusion. Spine. 2011;36:26–32. doi: 10.1097/BRS.0b013e3181e1040a. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Wang X, Sun Y, Zhang F, Gao Y, Li Z, et al. Safety analysis of two anterior lateral lumbar interbody fusions at the initial stage of learning curve. World Neurosurg. 2019;127:e901–9. doi: 10.1016/j.wneu.2019.03.294. [DOI] [PubMed] [Google Scholar]
- 20.Epstein NE. Review of risks and complications of extreme lateral interbody fusion (XLIF) Surg Neurology Int. 2019;10:237. doi: 10.25259/SNI_559_2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horowitz JA, Jain A, Puvanesarajah V, Qureshi R, Hassanzadeh H. Risk factors, additional length of stay, and cost associated with postoperative ileus following anterior lumbar interbody fusion in elderly patients. World Neurosurg. 2018;115:e185–9. doi: 10.1016/j.wneu.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Uribe JS, Deukmedjian AR. Visceral vascular, and wound complications following over 13,000 lateral interbody fusions: A survey study and literature review. Eur Spine J. 2015;24:386–96. doi: 10.1007/s00586-015-3806-4. [DOI] [PubMed] [Google Scholar]
- 23.Fujibayashi S, Kawakami N, Asazuma T, Ito M, Mizutani J, Nagashima H, et al. Complications associated with lateral interbody fusion: Nationwide survey of 2998 cases during the first 2 years of its use in Japan. Spine (Phila Pa 1976) 2017;42:1478–84. doi: 10.1097/BRS.0000000000002139. [DOI] [PubMed] [Google Scholar]
- 24.Marquez-Lara A, Nandyala SV, Hassanzadeh H, Sundberg E, Jorgensen A, Singh K. Sentinel events in lumbar spine surgery. Spine (Phila Pa 1976) 2014;39:900–5. doi: 10.1097/BRS.0000000000000247. [DOI] [PubMed] [Google Scholar]
- 25.Hwang ES, Kim KJ, Lee CS, Lee MY, Yoon SJ, Park JW, et al. Bowel injury and insidious pneumoperitoneum after lateral lumbar interbody fusion. Asian Spine J. 2021. Epub ahead of print. [doi: 10.31616/asj. 2021.0132] [DOI] [PMC free article] [PubMed]
- 26.Malhotra AK, Fabian TC, Katsis SB, Gavant ML, Croce MA. Blunt bowel and mesenteric injuries: The role of screening computed tomography. J Trauma Inj Infect Crit Care. 2000;48:991–1000. doi: 10.1097/00005373-200006000-00001. [DOI] [PubMed] [Google Scholar]
- 27.Hefny AF, Kunhivalappil FT, Matev N, Avila NA, Bashir MO, Abu-Zidan FM. Usefulness of free intraperitoneal air detected by CT scan in diagnosing bowel perforation in blunt trauma: Experience from a community-based hospital. Injury. 2015;46:100–4. doi: 10.1016/j.injury.2014.09.002. [DOI] [PubMed] [Google Scholar]


