Abstract
Coronavirus disease 19 (COVID-19) is an infectious disease caused by severe acute respiratory syndrome 2 (SARS-CoV-2). Throughout the pandemic, evidence on the effects of COVID-19 during pregnancy has been inadequate due to the limited number of studies published. Therefore, the objective of this systematic review was to evaluate current literature regarding the effects of COVID-19 during pregnancy and establish pregnancy outcomes and vertical and perinatal transmission during pregnancy. Multiple databases were searched, including Embase, Medline, Web of Science, Scopus, and Cochrane Central Register of Control Clinical Trials, using the following keywords: [Pregnancy] AND [COVID-19 OR SARS-CoV-2 OR nCoV-19] OR [Perinatal transmission, Vertical transmission (VT), Pregnancy complications], [Pregnancy] AND [Hyperinflammation OR Cytokine storm]. We excluded in vitro and experimental studies, but also ex-vivo and animal study methods. To exclude the risk of bias during data collection and interpretation, all included studies were peer-reviewed publications. This review is estimated to tabulate the study intervention characteristics and compare them against the planned groups for each synthesis. Our findings showed that pregnant women are commonly susceptible to respiratory viral infections and severe pneumonia due to physiological immune suppression and pregnancy-induced changes. VT of SARS-CoV-2 infection during pregnancy is associated with a great deal of controversy and conflict. However, there is still no robust clinical evidence of VT. Furthermore, the clinical presentation and management of COVID-19 during pregnancy are nearly identical to those of non-pregnant women. Finally, chloroquine and remdesivir are the only two drugs evaluated as adequate for the management of COVID-19 during pregnancy.
Keywords: Vertical transmission, Pregnancy, COVID-19, SARS-CoV-2, Neonates
Introduction
Coronavirus disease 19 (COVID-19) is an infectious disease caused by severe acute respiratory syndrome 2 (SARS-CoV-2), which is a positive-sense single-strand RNA virus sharing genomic correspondence with other Betacoronaviruses, such as the Middle East Respiratory Syndrome (MERS-CoV) and SARS-CoV [1]. More specifically, SARS-CoV-2 binds to angiotensin-converting enzyme 2 (ACE2), an enzyme that is highly expressed in lung epithelial cells, proximal renal tubules, the heart, and the brain, and which is the receptor mediating viral entry into the epithelial cells [2, 90]. Consequently, SARS-CoV-2 infection triggers acute host immune responses, inflammatory reactions, and cytokine storm, causing acute lung injury (ALI) and acute respiratory syndrome [2, 91, 92]. The World Health Organization (WHO) declared the COVID-19 outbreak as a pandemic disease in December 2019 after emergence of several unidentified pneumonia cases in Wuhan, China[3].Chinese researchers recognized that the genetic sequence of SARS-CoV-2 was similar to that of SARS-CoV (approximately 79%)[4, 93].
In January 2020, WHO defined this outbreak as a global pandemic disease due to its rapid spread worldwide. As of early April 2022, more than 489 million affected individuals and approximately six million deaths have been documented globally. Patients with COVID-19 present symptoms of dry cough, headache, fatigue, sweating, and fever [5], and laboratory findings include leukocytosis, lymphopenia, high lactate dehydrogenase, and ferritin with a bilateral pulmonary ground-glass appearance on computed chest tomography (CT) scan [6, 94]. Primarily, data regarding the effect of COVID-19 on pregnancy were insufficient due to the limited number of studies performed during the early stages of this pandemic. Consequently, researchers and medical experts faced a significant challenge in managing COVID-19 during pregnancy, even though a significant number of pregnant women with COVID-19 had already been reported [7]. At the commencement of this outbreak, the American College of Obstetricians and Gynecologists guideline was published to safeguard a scientific approach to COVID-19 during pregnancy [8]. Furthermore, the fatality rate of COVID-19 during pregnancy varied according to individual countries because early assessment could overestimate the percentage of case fatalities [9]. In late December 2020, the European Journal of obstetrics, gynecology, and reproductive biology published a review study entitled "Is pregnancy a risk factor for COVID-19" [48], in which Phoswa et al. found that pregnant women were at higher risk of developing COVID-19 due to lymphopenia and deregulation of inflammatory cytokines with over-expression of ACE2 [48, 95]. Introductory documents had initially suggested that pregnant women were not strictly affected by COVID-19 compared to the general population [48, 49]. The total number of affected pregnant women was low, and it was not found to be associated with matched non-pregnant females. Similarly, different studies confirmed that pregnant women with COVID-19 were at increased risk of severe illness with COVID-19 compared to non-pregnant women [48]. Also, primary data regarding perinatal and intrauterine infection with SARS-CoV-2 were lacking as there was no confirmation or strong evidence pertaining to neonatal infection from infected mothers. The purpose behind this phenomenon was that nearly all infected pregnant women were in their third trimester, in which the effect of SARS-CoV-2 infection was practically unknown [10, 11, 95].
Amidst large body of literature, findings regarding the potential effect of SARS-CoV-2 infection on pregnancy outcomes and vertical and perinatal transmission are diverse. Therefore, the rationale for the review was to investigate existing literature and assess whether COVID-19 could be mild during pregnancy. Thus, the objectives of the present study were to identify COVID-19 outcomes during pregnancy and evaluate the risk of vertical and perinatal transmission during pregnancy. The novelty of this review was the illustration of the high or low risk of SARS-CoV-2 during pregnancy. This review also revealed that the DPP4 and CD137 receptors that are upregulated during pregnancy might increase the risk of vertical transmission (VT), of SARS-CoV-2 during pregnancy. In addition, this review highlighted that gestational diabetes mellitus (DM) might augment the risk of VT of SARS-CoV-2 during pregnancy due to ACE2 upregulation and associated inflammatory changes.
Search strategy
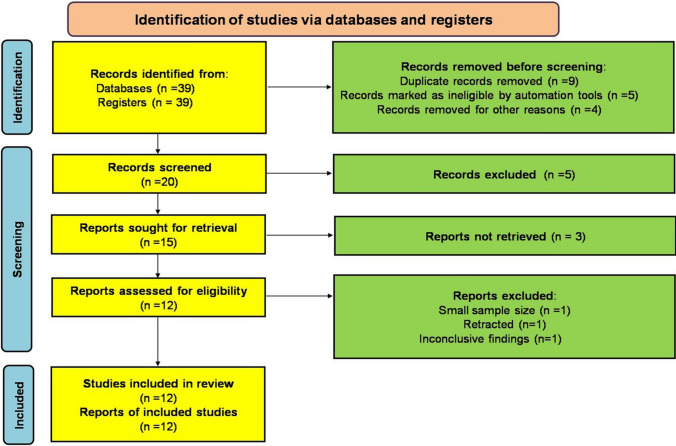
We applied different search strategies in various databases, including Embase, Medline, Web of Science, Scopus, and Cochrane Central Register of Control Clinical Trials using specific keywords such as [Pregnancy] AND [COVID-19 OR SARS-CoV-2 OR nCoV-19] OR [Perinatal transmission, VT, Pregnancy complications], [Pregnancy] AND [Hyperinflammation OR Cytokine storm]. In addition, the reference lists of relevant research studies were examined, and we considered articles published in languages other than English because it was our purpose to also include case-reported studies in this mini-review study. Exclusion criteria were as follows: in vitro and experimental studies; ex-vivo studies; and animal studies methods (Fig. 1). According to the PRISMA 2020 checklist [12], this review met the inclusion criteria according to the retrieved reports. To exclude the risk of bias during data collection and interpretation, all included studies were peer-reviewed publications. The mean difference and risk ratios regarding COVID-19 in pregnancy were also evaluated. This review is estimated to tabulate study intervention characteristics and compare them against the planned groups for each synthesis. In addition, heterogeneity among study results was assessed.
Fig. 1.
Flowchart of the present study
Pregnancy and COVID-19
Increased female sex hormones during pregnancy induce an immune suppression status which renders pregnant women more susceptible to viral infections. Accordingly, SARS-CoV-2 in pregnant women leads to severe complications with significant mortality rate, due to comorbidities [13, 96]. The Center for Disease Control (CDC) has published distinct guidelines for the effective management of COVID-19 during pregnancy, depending on the previous coronavirus outbreaks during pregnancy. Furthermore, CDC has issued diverse recommendations regarding the pathogenesis, epidemiology, medical sequence, and disease progression of COVID-19 during pregnancy [14, 97]. Though clinical experience in the management of COVID-19 during pregnancy was rather restricted, various complications were reported, including preterm delivery, fetal distress, premature rupture of membrane, and abortion in pregnant women with COVID-19 [15, 81].
It has been reported that most pregnant women are frequently predisposed to the development of respiratory viral infections and severe pneumonia due to physiological immune suppression alterations and pregnancy-induced changes [16]. Previously, approximately 50% of pregnant women with SARS met the prerequisites for admission to intensive care units and mechanical ventilation with a 25% case-fatality rate [17]. Pregnant women during the 2009 swine influenza (H1N1) were at higher risk of hospitalization compared to non-pregnant women [18]. Nonetheless, COVID-19 in pregnant women is not significantly different from that in non-pregnant women; however, COVID-19 pulmonary consolidations and a ground-glass appearance are more evident at both lower lobes on low-dose CT scan imaging [19, 90, 98].
A previous study on 11 patients with COVID-19 pneumonia revealed that the primary symptoms were mild fever and cough. Among these patients, one pregnant woman delivered vaginally, whereas the remaining ten delivered via cesarean section [20]. The minor clinical presentation of COVID-19 during pregnancy might be related to the higher expression of ACE2 [21]. Lai et al. [22] confirmed that advanced expression of ACE2 during pregnancy, mainly in the feto-maternal interface, may increase the risk of COVID-19 severity. However, the renin–angiotensin–aldosterone system (RAAS) is highly activated during pregnancy and plays a possible role in pulmonary vasoconstriction and the progress of ALI. Consequently, a higher expression of ACE2 during pregnancy overwhelms the harmful effect of activated RAAS [23].
The connotation between over-expression of ACE2 and COVID-19 severity is weak since the ACE2 protein is located on the X chromosome, which is more abundant in females. Despite the low expression of ACE2, the COVID-19 fatality rate is higher in males, and the severity of COVID-19 in elderly patients is high [99, 100]. Also, ACE2 has a protective role against ALI in influenza [22]. Therefore, the ultimate link between COVID-19 severity during pregnancy and ACE2 over-expression should be reviewed and appropriately explained. Moreover, pregnancy and fetal delivery did not worsen the clinical outcomes and lung radiological imaging findings of COVID-19. In addition, most pregnant women with COVID-19 presented with mild symptoms and did not require antiviral therapy [24, 101].
Pregnancy and risk of vertical transmission in COVID-19
Low risk of vertical transmission
Viral infections during pregnancy are typically alarming due to likelihood of VT to the fetus. Viral infections during pregnancy are associated with intrauterine growth retardation, preterm birth, abortion, and poor perinatal outcomes [25].It has been proposed that the typical VT pathways include intrauterine transmission (IUT), standard vaginal delivery, and breast milk [25]. IUT requires the least amount of management and control, yet poses the greatest risk to both the mother and the fetus [26].
Karimi-Zarchi et al. [27] did not established any proof that COVID-19 during pregnancy may affect the fetus through IUT due to the negative results obtained by RT-PCR of cord blood, placental blood, amniotic fluid, and vaginal secretions. Nevertheless, neonatal infection has been established and reveals that direct contact between mothers and their neonates is the main route of infection. Al-kuraishy et al. [7] found no evidence for VT of SARS-CoV-2 during pregnancy, a finding that is also supported by the Li et al.’s study [25]. However, VT was confirmed in a single case report study by Dong et al. [28] as a result of positive RT-PCR tests, which nonetheless, did not estimate amniotic, cord, and placental blood. Furthermore, the majority of published studies on VT of SARS-CoV-2 infections during pregnancy based on RT-PCR tests and nasal swabs reveal sensitivities of 63 and 29%, respectively. Subsequently, immunoglobulin (IgG, IgM) appears within two weeks of SARS-CoV2 infection. It is evident that IgG but not IgM crosses the placenta; thus, anti-SARS-CoV-2 IgG in fetal blood does not indicate an intrauterine infection, as described by a recent Chinese study [37]. Also, SARS-CoV-2 viremia is present transiently only in 1% of patients with symptomatic COVID-19 pneumonia, suggesting the low possibility of this virus to be transmitted across the placenta. In addition, pathological studies of placental samples have not shown any histopathological changes associated with SARS-CoV-2 infections [38, 102, 103]. Dissimilar experimental studies exemplify that low expression of placental ACE2 receptors, principally at 6–12 weeks of gestation, limits SARS-CoV-2 transmission from mother to fetus[39]. A cross-sectional study demonstrated that all samples examined (vaginal secretions, amniotic fluid, and breast milk) from (90% of 71) pregnant women with COVID-19 were negative, suggesting a negative indication for VT [40]. Further, positive RT-PCR tests for SARS-CoV-2 were obtained in approximately 9% of delivered neonates from COVID-19 mothers. This value, however, is not very reliable because IgM does not cross the placenta and is subjected to different cross-reactivity; hence, this positive result reflects perinatal infection but not congenital infection [41].
High risk of vertical transmission
Analysis of SARS-CoV-2 during pregnancy confirmed the detection of SARS-CoV-2 genomes in umbilical cord blood, vaginal mucosa, and milk specimens [50]. A systematic review including 69 studies found that VT of SARS-CoV-2 infection could occur during pregnancy [51]. Moreover, a meta-analysis and systematic review comprised of 179 studies on the maternal outcomes in SARS-CoV-2 infection revealed that 70 and 30% of infected neonates with SARS-CoV-2 were due to environmental exposure and VT, respectively [52]. These observations suggest the possible VT of SARS-CoV-2 infection during pregnancy. However, there is a need for more adequate and appropriate data to direct clinical recommendations with the certainty of the evidence.
Discussion
Despite the clinical evidence presented, VT of SARS-CoV-2 infection during pregnancy is still debatable. Of interest, a low risk of VT might be elucidated by selecting cesarean sections to reduce the impact of SARS-CoV2 infection on pregnancy outcomes. However, most published studies have not assessed risks in the first and second trimesters. The risk of viral infections during pregnancy is trimester-dependent [53, 104, 105]. Viral infection during the first and second trimesters leads to numerous intrauterine and fetal effects. For instance, rubella viral infection causes congenital malformations in 90% of cases in the first trimester; however, this risk is reduced to 50% in the second trimester, while it is eliminated in the third trimester [30].
The mechanism of VT in SARS-CoV-2 is complex. Wastnedge et al. [31] found that placental expression of ACE2 and transmembrane-protease serine (TMPRSS2), including their co-localization, which is essential for viral entry, in the trophoblast is low during pregnancy. It has been suggested that the low rate of VT is linked to the low expression of ACE2 at the feto-maternal tissue interface, as marked by a lack of placental pathological changes during SARS-CoV2 infections during pregnancy [29]. In contrast, Turco and co-workers found that the expression of ACE2 was higher in the syncytiotrophoblast and villous cytotrophoblast cells in the decidua during the first trimester of pregnancy [54]. Syncytiotrophoblast cells regulate feto-maternal nutrient supply and gas exchange [55]. Therefore, a higher expression of placental ACE2 during pregnancy increases the risk of SARS-CoV2 infections during pregnancy and increases the risk of VT [39, 56, 105, 106].
These findings suggest that another pathway for SARS-CoV-2 VT, involving dipeptidyl peptidase 4 (DPP4) and CD147, which are highly expressed in the placental trophoblastic cells, might be responsible for SARS-CoV-2 entry [32, 87, 107]. Therefore, DPP4 and CD147 have been proposed to be additional receptors for SARS-CoV-2 entry [44, 57, 89, 90, 108]. In addition, different proteolytic enzymes, such as trypsin, furin, cathepsin B, and plasmin, which are involved in cleaving and activating SARS-CoV-2 spike protein, are highly expressed in the placental cells during pregnancy [33].
Notably, hyperinflammation caused by high circulating pro-inflammatory cytokines and chemokines in severe COVID-19 infections may disrupt placental barriers and favor VT of SARS-CoV2 infections [50, 109, 110].
Consequently, the direct cytopathic effect of SARS-CoV-2 infection and associated hyperinflammation during pregnancy may induce placental dysfunction and increase the risk of VT.
Breastfeeding and the risk of vertical transmission
Concerning breastfeeding, there have not been any positive RT-PCR findings reported in the breast milk of infected mothers [34]. Nonetheless, infected mothers are advised to stop breastfeeding until the results of RT-PCR are negative [35]. Obstetricians are responsible for deciding the type and timing of delivery depending on gestational age and fetal and maternal health. Each pregnant woman should be individually consulted and advised in accordance with her specific health conditions [36]. Till now, there has been no satisfactory proof in literature to support the occurrence of VT through breastfeeding, but the prevalence of prematurity was high among pregnant women infected by SARS-Cov-2. Likewise, only a single study has identified this virus in breast milk so far [58]. A review consisting of eight studies that analyzed the occurrence of SARS-CoV-2 RNA in the breast milk of 24 pregnant women infected with SARS-CoV-2 during the third trimester of pregnancy established that all breast milk samples were negative for SARS-CoV-2 [59]. The WHO endorses that women with suspected or confirmed COVID-19 can continue breastfeeding because neonates acquire antibodies and anti-infective factors through breast milk that will in turn help protect them from infections [60]. Therefore, it seems reasonable to conclude that there is no restriction for pregnant women with COVID-19 to breastfeed provided that they respect the commendations for prevention of SARS-CoV-2 infection.
Gestational diabetes and the risk of vertical transmission
Gestational diabetes and other types of diabetes during pregnancy may affect maternal, fetal, and neonatal outcomes during the COVID-19 pandemic [61]. A systematic scoping review found that higher expression of ACE2 with underlying chronic inflammatory reactions and exaggerated platelet activation in pregnant women with DM might cause placental dysfunction, which may increase the risk of VT SARS-CoV-2 infection during pregnancy [61–64]. According to a meta-analysis study performed by Yang et al., DM was the most common comorbidity observed during the COVID-19 pandemic [65]. Al-kuraishy et al. illustrated that DM was linked with severe COVID-19 complications due to changes in blood glucose and hyperinflammation, which enhance the pathogenesis of SARS-CoV-2 infection [66, 67]. Notably, diabetes increases the risk of SARS-CoV-2 infection due to T cell function and immune response impairment caused by high advanced glycation end-product diet [65–69]. Likewise, insulin resistance and endothelial dysfunction augment the risk of prothrombotic events, a hallmark of severe COVID-19 [70]. Preexistent DM during pregnancy increases the risk of developing severe COVID-19 and the requirement for intensive care support [71].
Higher expression of ACE2 and TMPRSS2 in the placenta, concurrent with high rates of pro-inflammatory cytokines during pregnancy with DM, may favor SARS-CoV-2 infection and the dysregulation of the placenta environment with subsequent VT of SARS-CoV-2 infection [61, 111]. Besides, placental hypoxic-ischemic injury induced by high circulating AngII may cause vasoconstriction and disruption of the placental function [72]. Interestingly, SARS-CoV-2 infection during pregnancy may increase the risk of new-onset gestational DM development through injury of pancreatic β-cells [61, 112].
These findings suggest that gestational DM may augment the risk of COVID-19 and VT of SARS-CoV-2 infection during pregnancy.
Management of COVID-19 during pregnancy
The common principles in the management of COVID-19 during pregnancy comprise early diagnosis, isolation, oxygen therapy, experiential antibiotics for secondary bacterial infections, monitoring uterine contraction and fetal distress, and preparation and planning for delivery [42]. Regular monitoring and consultations for the prompt recognition of early and late difficulties and complications, such as preterm delivery and premature rupture of the membrane, should be performed [42, 113].
Corticosteroids must be avoided during pregnancy because they delay clearance of SARS-CoV-2 and may increase the risk of secondary bacterial infections [43]. Nevertheless, corticosteroid use for induction of fetal lung maturity should be contingent on guidelines and medical consultations [43].
At present, there are no FDA-approved antiviral medications during pregnancy, though broad-spectrum antiviral drugs against MERS are used against SARS-CoV-2 [44]. However, during the pandemic, the proper use of non-licensed medications during pregnancy should adhere to the Monitored Use of Unregistered Interventions (MEURI). Both chloroquine and remdesivir are effective and safe in managing COVID-19 during pregnancy. However, chloroquine has a large volume of distribution, thus a large dose is required during pregnancy [45]. Chloroquine and remdesivir reduce radiological opacities and accelerate clinical and serological resolutions in COVID-19 pregnant women [46]. Chloroquine is safe during pregnancy and is recommended for use as malaria prophylaxis [73]. A small amount of chloroquine can pass through lactating women’s breast milk, which does not cause any serious adverse effects on the neonates [74]. However, an experimental study showed that chloroquine could cross the placenta and accumulate in the fetal eyes, and remained for up to five months after clearance from the body [75]. Similarly, a very high dose of ≥ 250 mg/kg of chloroquine during pregnancy was found to increase the risk of developing anophthalmia and microphthalmia in rats [75–77]. According to the current literature, the effectiveness of chloroquine and hydroxychloroquine is of limited value in COVID-19 [78, 79].
In contrast, remdesivir is a prodrug that needs to be activated by host intracellular enzymes and converted to an active drug that inhibits viral RNA polymerase. It was originally developed to treat Marburg and Ebola virus infections before being repurposed to treat SARS-CoV-2 infections [80–82]. The most common adverse effects of remdesivir involve elevated liver enzymes, nausea, sweating, and hypotension, and remdesivir metabolism is affected by enzyme inducers or inhibitors [83]. It has also been shown that hydroxychloroquine and chloroquine reduce the antiviral activity of remdesivir by inhibiting its metabolic activation [84]. Remdesivir induces minor side effects during pregnancy that are well tolerated in most cases. Remdesivir increases the recovery of pregnant women with COVID-19 [85]. A cohort study involving 95 pregnant women with COVID-19, of which 39 women were treated by remdesivir, revealed no severe or allergic adverse effects [86].
Furthermore, antiviral drugs, such as ritonavir and lopinavir, are effective against SARS-CoV-2 in COVID-19 pregnant women. However, a large population-based study did not confirm their safety. Contrariwise, favipiravir, arbidol, ribavirin, and baricitinib cause teratogenic effects on the fetus and are thus contraindicated during pregnancy [47, 87, 88].
The present study has several limitations, including a paucity of clinical studies that focus on COVID-19 infection in different trimesters of pregnancy, and the risk of VT was mainly speculative. However, this review produced evidence that VT of SARS-CoV-2 during pregnancy is rare yet possible, and the clinical course of COVID-19 during pregnancy was not different from that followed by non-pregnant women. The novelty of this review was that it confirmed the high or low risk of SARS-CoV-2 during pregnancy. This review also revealed that the DPP4 and CD137 receptors, which are upregulated during pregnancy, might increase the risk of VT of SARS-CoV-2 during pregnancy. In addition, this review highlighted that gestational DM might augment the risk of VT of SARS-CoV-2 during pregnancy due to upregulation of ACE2 and associated inflammatory changes.
Conclusion
The clinical presentation and management of COVID-19 during pregnancy are nearly identical to the ones in non-pregnant women. VT of SARS-CoV-2 infection during pregnancy is associated with a great deal of controversy and conflict. However, there is still no robust clinical evidence of VT. Only chloroquine and remdesivir are evaluated as adequate for managing COVID-19 during pregnancy. Large-prospective clinical studies are required to evaluate SARS-CoV-2 transmission during pregnancy and determine the true effects of current antiviral agents on pregnancy outcomes.
Acknowledgements
Not applicable.
Authors contributions
Hayder M. Al-kuraishy and Ali I. Al-Gareeb contributed to project development, data collection, data analysis, and manuscript writing; Nisreen Khalid Aref Albezrah and Haitham Ahmed Bahaa performed data collection, data analysis, and manuscript writing; Maisra M. El-Bouseary was involved in project development, data analysis, manuscript writing/editing, and visualization; Athanasios Alexiou, Shatha Hallal Al-Ziyadi, and Gaber El-Saber Batiha performed project development and manuscript writing/editing. All authors read and approved the final manuscript.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Declarations
Conflict of interests
The authors have no relevant financial or non-financial interests to disclose.
Consent for publication
This article does not contain any studies with human or animal subjects.
Human or animal right
This article does not contain any studies with human or animal subjects.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hayder M. Al-kuraishy, Email: hayderm36@yahoo.com
Ali I. Al-Gareeb, Email: dr.alialgareeb78@yahoo.com
Nisreen Khalid Aref Albezrah, Email: dr.nisreen@tu.edu.sa.
Haitham Ahmed Bahaa, Email: haitham_bahaa@yahoo.com.
Maisra M. El-Bouseary, Email: maysra_mohamed@pharm.tanta.edu.eg
Athanasios Alexiou, Email: alextha@yahoo.gr.
Shatha Hallal Al-Ziyadi, Email: Shatha.h@tu.edu.sa.
Gaber El-Saber Batiha, Email: gaberbatiha@gmail.com.
References
- 1.Al-Kuraishy HM, Al-Gareeb AI, Alzahrani KJ, Cruz-Martins N, Batiha GE. The potential role of neopterin in Covid-19: a new perspective. Mol Cell Biochem. 2021;476(11):4161–4166. doi: 10.1007/s11010-021-04232-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Kuraishy HM, Al-Gareeb AI, Alzahrani KJ, Alexiou A, Batiha GE. Niclosamide for Covid-19: bridging the gap. Mol Biol Rep. 2021;48(12):8195–8202. doi: 10.1007/s11033-021-06770-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Kuraishy HM, Al-Gareeb AI. From SARS-CoV to nCoV-2019: ruction and argument. Arch Clin Infect Dis. 2020;15(COVID19):e102624. doi: 10.5812/archcid.102624. [DOI] [Google Scholar]
- 4.Al-Kuraishy HM, Al-Niemi MS, Hussain NR, Al-Gareeb AI, Al-Harchan NA, Al-Kurashi AH. The potential role of Renin Angiotensin System (RAS) and dipeptidyl peptidase-4 (DPP-4) COVID-19: navigating the uncharted. In: Kibel A, editor. Selected chapters from the reninangiotensin system. London: IntechOpen; 2020. pp. 151–165. [Google Scholar]
- 5.Onohuean H, Al-Kuraishy HM, Al-Gareeb AI, Qusti S, Alshammari EM, Batiha GE. Covid-19 and development of heart failure: mystery and truth. Naunyn Schmiedebergs Arch Pharmacol. 2021;394(10):2013–2021. doi: 10.1007/s00210-021-02147-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Kuraishy HM, Al-Gareeb AI, Abdullah SM, Cruz-Martins N, Batiha GE. Case report: hyperbilirubinemia in Gilbert syndrome attenuates Covid-19-induced metabolic disturbances. Front Cardiovasc Med. 2021;8:642181. doi: 10.3389/fcvm.2021.642181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Gareeb AI, Musa RA, Ali ZH, Al-kuraishy HM, Al-Maiahy TJ. COVID-19 pneumonia in an Iraqi pregnant woman with preterm delivery. Asian Pac J Reprod. 2020;9(3):156. doi: 10.4103/2305-0500.282984. [DOI] [Google Scholar]
- 8.Poon LC, Yang H, Lee JCS, Copel JA, Leung TY, Zhang Y, et al. ISUOG interim guidance on 2019 novel coronavirus infection during pregnancy and puerperium: information for healthcare professionals. Ultrasound Obstet Gynecol. 2020;55(5):700–708. doi: 10.1002/uog.22013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Kuraishy HM, Hussien NR, Al-Naimi MS, Al-Buhadily AK, Al-Gareeb AI, Lungnier C. Is ivermectin–azithromycin combination the next step for COVID-19? Biomed Biotechnol Res J. 2020;4(5):101. doi: 10.4103/bbrj.bbrj_109_20. [DOI] [Google Scholar]
- 10.Zhang L, Jiang Y, Wei M, Cheng BH, Zhou XC, Li J, et al. Analysis of the pregnancy outcomes in pregnant women with COVID-19 in Hubei Province. Zhonghua Fu Chan Ke Za Zhi. 2020;55(3):166–171. doi: 10.3760/cma.j.cn112141-20200218-00111. [DOI] [PubMed] [Google Scholar]
- 11.Bashyam AM, Feldman SR. Should patients stop their biologic treatment during the COVID-19 pandemic. J Dermatolog Treat. 2020;31(4):317–318. doi: 10.1080/09546634.2020.1742438. [DOI] [PubMed] [Google Scholar]
- 12.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021 doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barbero P, Mugüerza L, Herraiz I, García Burguillo A, San Juan R, Forcén L, Mejía I, Batllori E, Montañez MD, Vallejo P, Villar O. SARS-CoV-2 in pregnancy: characteristics and outcomes of hospitalized and non-hospitalized women due to COVID-19. J Matern Fetal Neonatal Med. 2022;35(14):2648–2654. doi: 10.1080/14767058.2020.1793320. [DOI] [PubMed] [Google Scholar]
- 14.Marinelli KA, Lawrence RM. Response to letters to the editor about the safe handling of containers of expressed human Milk in all settings during the SARS-CoV-2 (COVID-19) Pandemic. J Hum Lact. 2020;36(3):543–547. doi: 10.1177/0890334420924351. [DOI] [PubMed] [Google Scholar]
- 15.Poon LC, Yang H, Kapur A, Melamed N, Dao B, Divakar H, et al. Global interim guidance on coronavirus disease 2019 (COVID-19) during pregnancy and puerperium from FIGO and allied partners: information for healthcare professionals. Int J Gynaecol Obstet. 2020;149(3):273–286. doi: 10.1002/ijgo.13156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Maiahy TJ, Al-Gareeb AI, Al-kuraishy H. Testosterone is a surrogate and proxy biomarker for severity of late-onset preeclampsia: a cross-sectional study. Asian Pac J Reprod. 2020;9(1):1. doi: 10.4103/2305-0500.275522. [DOI] [Google Scholar]
- 17.Schneider E, Duncan D, Reiken M, Perry R, Messick J, Sheedy C, et al. SARS in pregnancy. AWHONN Lifelines. 2004;8(2):122–128. doi: 10.1177/1091592304265557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong SF, Chow KM, Leung TN, Ng WF, Ng TK, Shek CC, et al. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol. 2004;191(1):292–297. doi: 10.1016/j.ajog.2003.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu D, Li L, Wu X, Zheng D, Wang J, Yang L, Zheng C. Pregnancy and perinatal outcomes of women with coronavirus disease (COVID-19) pneumonia: a preliminary analysis. AJR Am J Roentgenol. 2020;215(1):127–132. doi: 10.2214/AJR.20.23072. [DOI] [PubMed] [Google Scholar]
- 21.Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai YJ, Chang CM, Lin CK, Yang YP, Chien CS, Wang PH, Chang CC. Severe acute respiratory syndrome coronavirus-2 and the deduction effect of angiotensin-converting enzyme 2 in pregnancy. J Chin Med Assoc. 2020;83(9):812–816. doi: 10.1097/JCMA.0000000000000362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu H, Liu F, Li J, Zhang T, Wang D, Lan W. Clinical and CT imaging features of the COVID-19 pneumonia: focus on pregnant women and children. J Infect. 2020;80(5):e7–13. doi: 10.1016/j.jinf.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al-Kuraishy HM, Hussien NR, Al-Naimi MS, Al-Buhadily AK, Al-Gareeb AI, Lungnier C. Renin–angiotensin system and fibrinolytic pathway in COVID-19: one-way skepticism. Biomed Biotechnol Res J. 2020;4(5):33. doi: 10.4103/bbrj.bbrj_105_20. [DOI] [Google Scholar]
- 25.Arora N, Sadovsky Y, Dermody TS, Coyne CB. Microbial vertical transmission during human pregnancy. Cell Host Microbe. 2017;21(5):561–567. doi: 10.1016/j.chom.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karimi-Zarchi M, Neamatzadeh H, Dastgheib SA, Abbasi H, Mirjalili SR, Behforouz A, et al. Vertical transmission of coronavirus disease 19 (COVID-19) from infected pregnant mothers to neonates: a review. Fetal Pediatr Pathol. 2020;39(3):246–250. doi: 10.1080/15513815.2020.1747120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Zhao R, Zheng S, Chen X, Wang J, Sheng X, et al. Lack of vertical transmission of severe acute respiratory syndrome coronavirus 2. China Emerg Infect Dis. 2020;26(6):1335–1336. doi: 10.3201/eid2606.200287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dong L, Tian J, He S, Zhu C, Wang J, Liu C, Yang J. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA. 2020;323(18):1846–1848. doi: 10.1001/jama.2020.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Z, Liu Y. Vertical transmission of severe acute respiratory syndrome coronavirus 2: a systematic review. Am J Perinatol. 2020;37(10):1055–1060. doi: 10.1055/s-0040-1712161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bukasa A, Campbell H, Brown K, Bedford H, Ramsay M, Amirthalingam G, Tookey P. Rubella infection in pregnancy and congenital rubella in United Kingdom, 2003 to 2016. Euro Surveill. 2018;23(19):17–00381. doi: 10.2807/1560-7917.ES.2018.23.19.17-00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wastnedge EAN, Reynolds RM, van Boeckel SR, Stock SJ, Denison FC, Maybin JA, Critchley HOD. Pregnancy and COVID-19. Physiol Rev. 2021;101(1):303–318. doi: 10.1152/physrev.00024.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kreis NN, Ritter A, Louwen F, Yuan J. A message from the human placenta: structural and immunomodulatory defense against SARS-CoV-2. Cells. 2020;9(8):1777. doi: 10.3390/cells9081777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al-Kuraishy HM, Al-Gareeb AI, Al-Hussaniy HA, Al-Harcan NAH, Alexiou A, Batiha GE. Neutrophil extracellular traps (NETs) and Covid-19: a new frontiers for therapeutic modality. Int Immunopharmacol. 2022;104:108516. doi: 10.1016/j.intimp.2021.108516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Al-Kuraishy HM, Al-Gareeb AI, Atanu FO, El-Zamkan MA, Diab HM, Ahmed AS, et al. Maternal transmission of SARS-CoV-2: safety of breastfeeding in infants born to infected mothers. Front Pediatr. 2021;9:738263. doi: 10.3389/fped.2021.738263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Z, Wang M, Zhu Z, Liu Y. Coronavirus disease 2019 (COVID-19) and pregnancy: a systematic review. J Matern Fetal Neonatal Med. 2022;35(8):1619–1622. doi: 10.1080/14767058.2020.1759541. [DOI] [PubMed] [Google Scholar]
- 36.Qi H, Luo X, Zheng Y, Zhang H, Li J, Zou L, et al. Safe delivery for pregnancies affected by COVID-19. BJOG. 2020;127(8):927–929. doi: 10.1111/1471-0528.16231. [DOI] [PubMed] [Google Scholar]
- 37.Kimberlin DW, Stagno S. Can SARS-CoV-2 infection be acquired in utero?: More definitive evidence is needed. JAMA. 2020;323(18):1788. doi: 10.1001/jama.2020.4868. [DOI] [PubMed] [Google Scholar]
- 38.Chen S, Huang B, Luo DJ, Li X, Yang F, Zhao Y, et al. Pregnant women with new coronavirus infection: a clinical characteristics and placental pathological analysis of three cases. Zhonghua Bing Li Xue Za Zhi. 2020;49:E005. doi: 10.3760/cma.j.cn112151-20200225-00138. [DOI] [PubMed] [Google Scholar]
- 39.Li M, Chen L, Zhang J, Xiong C, Li X. The SARS-CoV-2 receptor ACE2 expression of maternal-fetal interface and fetal organs by single-cell transcriptome study. PLoS ONE. 2020;15(4):e0230295. doi: 10.1371/journal.pone.0230295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lamouroux A, Attie-Bitach T, Martinovic J, Leruez-Ville M, Ville Y. Evidence for and against vertical transmission for severe acute respiratory syndrome coronavirus 2. Am J Obstet Gynecol. 2020;223(1):91.e1–4. doi: 10.1016/j.ajog.2020.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu Q, Shi Y. Coronavirus disease (COVID-19) and neonate: whatneonatologist need to know. J Med Virol. 2020;92(6):564–567. doi: 10.1002/jmv.25740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luo Y, Yin K. Management of pregnant women infected with COVID-19. Lancet Infect Dis. 2020;20(5):513–514. doi: 10.1016/S1473-3099(20)30191-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McIntosh JJ. Corticosteroid guidance for pregnancy during COVID-19 pandemic. Am J Perinatol. 2020;37(8):809–812. doi: 10.1055/s-0040-1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Al-Kuraishy HM, Al-Naimi MS, Lungnier CM, Al-Gareeb AI. Macrolides and COVID-19: an optimum premise. Biomed Biotechnol Res J. 2020;4(3):189. doi: 10.4103/bbrj.bbrj_103_20. [DOI] [Google Scholar]
- 45.Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;323(18):1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 46.Maleki Dana PM, Kolahdooz F, Sadoughi F, Moazzami B, Chaichian S, Asemi Z. COVID-19 and pregnancy: a review of current knowledge. Infez Med. 2020;28(suppl 1):46–51. [PubMed] [Google Scholar]
- 47.Li L, Wang X, Wang R, Hu Y, Jiang S, Lu X. Antiviral agent therapy optimization in special populations of COVID-19 patients. Drug Des Devel Ther. 2020;14:3001–3013. doi: 10.2147/DDDT.S259058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Phoswa WN, Khaliq OP. Is pregnancy a risk factor of COVID-19? Eur J Obstet Gynecol Reprod Biol. 2020;252:605–609. doi: 10.1016/j.ejogrb.2020.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whitaker KM, Hung P, Alberg AJ, Hair NL, Liu J. Variations in health behaviors among pregnant women during the COVID-19 pandemic. Midwifery. 2021;95:102929. doi: 10.1016/j.midw.2021.102929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fenizia C, Biasin M, Cetin I, Vergani P, Mileto D, Spinillo A, et al. Analysis of SARS-CoV-2 vertical transmission during pregnancy. Nat Commun. 2020;11(1):5128. doi: 10.1038/s41467-020-18933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Musa SS, Bello UM, Zhao S, Abdullahi ZU, Lawan MA, He D. Vertical transmission of SARS-CoV-2: a systematic review of systematic reviews. Viruses. 2021;13(9):1877. doi: 10.3390/v13091877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raschetti R, Vivanti AJ, Vauloup-Fellous C, Loi B, Benachi A, De Luca D. Synthesis and systematic review of reported neonatal SARS-CoV-2 infections. Nat Commun. 2020;11(1):5164. doi: 10.1038/s41467-020-18982-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Groulx T, Bagshawe M, Giesbrecht G, Tomfohr-Madsen L, Hetherington E, Lebel CA. Prenatal care disruptions and associations with maternal mental health during the COVID-19 Pandemic. Front Glob Womens Health. 2021;2:648428. doi: 10.3389/fgwh.2021.648428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Turco MY, Moffett A. Development of the human placenta. Development. 2019;146(22):dev163428. doi: 10.1242/dev.163428. [DOI] [PubMed] [Google Scholar]
- 55.Tashev SA, Parsons D, Hillman C, Harris S, Lofthouse EM, Goggin P, et al. Folding of the syncytiotrophoblast basal plasma membrane increases the surface area available for exchange in human placenta. Placenta. 2022;117:57–63. doi: 10.1016/j.placenta.2021.11.002. [DOI] [PubMed] [Google Scholar]
- 56.Terefe EM, Okalebo FA, Derese S, Batiha GE, Youssef A, Alorabi M, Muriuki J. Cytotoxicity and anti-HIV activities of extracts of the twigs of Croton dichogamus Pax. BMC Complement Med Ther. 2022;22(1):49. doi: 10.1186/s12906-022-03532-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Al-Kuraishy HM, Al-Gareeb AI, Qusty N, Alexiou A, Batiha GE. Impact of sitagliptin in nondiabetic Covid-19 patients. Curr Mol Pharmacol. 2022;15(4):683–692. doi: 10.2174/1874467214666210902115650. [DOI] [PubMed] [Google Scholar]
- 58.Trapani Júnior A, Vanhoni LR, Silveira SK, Marcolin AC. Childbirth, puerperium and abortion care protocol during the COVID-19 pandemic. Rev Bras Ginecol Obstet. 2020;42(6):349–355. doi: 10.1055/s-0040-1713587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martins-Filho PR, Santos VS, Santos HP. To breastfeed or not to breastfeed?Lack of evidence on the presence of SARS-CoV-2 in breastmilk of pregnant women with COVID-19. Rev Panam Salud Publica. 2020;44:e59. doi: 10.26633/RPSP.2020.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pereira A, Cruz-Melguizo S, Adrien M, Fuentes L, Marin E, Forti A, Perez-Medina T. Breastfeeding mothers with COVID-19 infection: a case series. Int Breastfeed J. 2020;15(1):69. doi: 10.1186/s13006-020-00314-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eberle C, James-Todd T, Stichling S. SARS-CoV-2 in diabetic pregnancies: a systematic scoping review. BMC Pregnancy Childbirth. 2021;21(1):573. doi: 10.1186/s12884-021-03975-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Elnagar A, El-Dawy K, El-Belbasi HI, Rehan IF, Embark H, Al-Amgad Z, et al. Ameliorative effect of oxytocin on FBN1 and PEPCK gene expression, and behavioral patterns in rats’ obesity-induced diabetes. Front Public Health. 2022;10:777129. doi: 10.3389/fpubh.2022.777129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Onikanni AS, Lawal B, Oyinloye BE, Mostafa-Hedeab G, Alorabi M, Cavalu S, et al. Therapeutic efficacy of Clompanus pubescens leaves fractions via downregulation of neuronal cholinesterases/Na+-K+ ATPase/IL-1β, and improving the neurocognitive and antioxidants status of streptozotocin-induced diabetic rats. Biomed Pharmacother. 2022;148:112730. doi: 10.1016/j.biopha.2022.112730. [DOI] [PubMed] [Google Scholar]
- 64.Momoh TB, Oniwon WO, Elazab ST, Sharkawi SMZ, Waheed RM, Youssef A, Batiha GE-S, Dakwoji PA, Atanu FO, Nweje-Anyalowu PC. Pharmacological studies of anti-inflammatory, anti-nociceptive and anti-pyretic compounds found in chromatographic fractions of Anogeissus leiocarpa (DC). Guill. & Perr. leaves. J Pharm Pharmacogn Res. 2022;10(3):459–468. doi: 10.56499/jppres21.1265_10.3.459. [DOI] [Google Scholar]
- 65.Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Al-Gareeb AI, Guerreiro SG, Cruz-Martins N, Batiha GE, Al-kuraishy H. COVID-19 in relation to hyperglycemia and diabetes mellitus. Front Cardiovasc Med. 2021;8:335. doi: 10.3389/fcvm.2021.644095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ogunyemi OM, Gyebi GA, Ibrahim IM, Esan AM, Olaiya CO, Soliman MM, Batiha GES. Identification of promising multi-targeting inhibitors of obesity from Vernonia amygdalina through computational analysis. Mol Divers. 2022 doi: 10.1007/s11030-022-10397-6. [DOI] [PubMed] [Google Scholar]
- 68.Ilesanmi OB, Odewale TT, Avwioroko OJ, Ahmed EI, Alaneme C, Atanu FO, et al. Trévo abrogates lead acetate neurotoxicity in male Wistar rats viz antiamyloidogenesis, antiglutaminergic, and anticholinesterase activities. Ann Neurosci. 2022 doi: 10.1177/09727531221077642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fawzy MA, Maher SA, El-Rehany MA, Welson NN, Albezrah NKA, Batiha GE-S, Fathy M. Vincamine modulates the effect of pantoprazole in renal ischemia/reperfusion injury by attenuating MAPK and apoptosis signaling pathways. Molecules. 2022;27(4):1383. doi: 10.3390/molecules27041383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Al-Kuraishy HM, Al-Gareeb AI, Alblihed M, Cruz-Martins N, Batiha GE. COVID-19 and risk of acute ischemic stroke and acute lung injury in patients with type II diabetes mellitus: theanti-inflammatory role of metformin. Front Med (Lausanne) 2021;8:644295. doi: 10.3389/fmed.2021.644295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Allotey J, Stallings E, Bonet M, Yap M, Chatterjee S, Kew T, et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ (Clin Res Ed) 2020;370:3320. doi: 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Abbas AM, Ahmed OA, Shaltout AS. COVID-19 and maternal pre-eclampsia: a synopsis. Scand J Immunol. 2020;92(3):e12918. doi: 10.1111/sji.12918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Plantone D, Koudriavtseva T. Current and future use of chloroquine and hydroxychloroquine in infectious, immune, neoplastic, and neurological diseases: a mini-review. Clin Drug Investig. 2018;38(8):653–671. doi: 10.1007/s40261-018-0656-y. [DOI] [PubMed] [Google Scholar]
- 74.Hassan SA, Ibrahim N, Elzanfaly ES, El Gendy AE. Analytical quality by design approach for the control of potentially counterfeit chloroquine with some NSAIDs using HPLC with fluorescence detection in pharmaceutical preparation and breast milk. Acta Chromatogr. 2021;33(3):234–244. doi: 10.1556/1326.2020.00793. [DOI] [Google Scholar]
- 75.Lacroix I, Bénévent J, Damase-Michel C. Chloroquine and hydroxychloroquine during pregnancy: what do we know? Therapies. 2020;75(4):384–385. doi: 10.1016/j.therap.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Olatubosun MO, Abubakar MB, Batiha GE, Malami I, Ibrahim KG, Abubakar B, et al. LncRNA SNHG15: a potential therapeutic target in the treatment of colorectal cancer. Chem Biol Drug Des. 2022 doi: 10.1111/cbdd.14036. [DOI] [PubMed] [Google Scholar]
- 77.Ojo OA, Oni AI, Grant S, Amanze J, Ojo AB, Taiwo OA, et al. Antidiabetic activity of Elephant grass (Cenchrus purpureus (Schumach.) Morrone) via activation of PI3K/AkT signaling pathway, oxidative stress inhibition, and apoptosis in Wistar rats. Front Pharmacol. 2022;13:651. doi: 10.3389/fphar.2022.845196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Eze P, Mezue KN, Nduka CU, Obianyo I, Egbuche O. Efficacy and safety of chloroquine and hydroxychloroquine for treatment of COVID-19 patients-a systematic review and meta-analysis of randomized controlled trials. Am J Cardiovasc Dis. 2021;11(1):93–107. [PMC free article] [PubMed] [Google Scholar]
- 79.Batiha GE, Shaheen HM, Al-Kuraishy HM, Teibo JO, Akinfe OA, Al-Garbee AI, et al. Possible mechanistic insights into iron homeostasis role of the action of 4-aminoquinolines (chloroquine/hydroxychloroquine) on COVID-19 (SARS-CoV-2) infection. Eur Rev Med Pharmacol Sci. 2021;25(23):7565–7584. doi: 10.26355/eurrev_202112_27456. [DOI] [PubMed] [Google Scholar]
- 80.Burwick RM, Yawetz S, Stephenson KE, CollierAY SP, Blackburn BG, et al. Compassionate use of remdesivir in pregnant women with severe coronavirus disease 2019. Clin Infect Dis. 2021;73(11):e3996–4004. doi: 10.1093/cid/ciaa1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Al-Kuraishy HM, Al-Gareeb AI, Butnariu M, Batiha GE. The crucial role of prolactin-lactogenic hormone in Covid-19. Mol Cell Biochem. 2022;477(5):1381–1392. doi: 10.1007/s11010-022-04381-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Abdelzaher WY, Nassan MA, Ahmed SM, Welson NN, El-Saber Batiha G, Khalaf HM. Xanthine oxidase inhibitor, febuxostat is effective against 5-fluorouracil-induced parotid salivary gland injury in rats via inhibition of oxidative stress, inflammation and targeting TRPC1/CHOP signalling pathway. Pharmaceuticals (Basel) 2022;15(2):232. doi: 10.3390/ph15020232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Igbinosa I, Miller S, Bianco K, Nelson J, Kappagoda S, Blackburn BG, et al. Use of remdesivir for pregnant patients with severe novel coronavirus disease 2019. Am J Obstet Gynecol. 2020;223(5):768–770. doi: 10.1016/j.ajog.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McCoy JA, Short WR, Srinivas SK, Levine LD, Hirshberg A. Compassionate use of remdesivir for treatment of severe coronavirus disease 2019 in pregnant women at a United States academic center. Am J Obstet Gynecol MFM. 2020;2(3):100164. doi: 10.1016/j.ajogmf.2020.100164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dande R, Qureshi A, Persaud K, Puri C, Zulfiqar S, Awasthi S. Remdesivir in a pregnant patient with COVID-19 pneumonia. J Community Hosp Intern Med Perspect. 2021;11(1):103–106. doi: 10.1080/20009666.2020.1857510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gutierrez R, Mendez-Figueroa H, Biebighauser JG, Bhalwal A, Pineles BL, Chauhan SP. Remdesivir use in pregnancy during the SARS-CoV-2 pandemic. J Matern Fetal Neonatal Med. 2022 doi: 10.1080/14767058.2022.2041595. [DOI] [PubMed] [Google Scholar]
- 87.Apalowo OA, Adediji AO, Balogun OS, Fakolujo TI, Izuogu NB, Archibong JM et al. Genetic structure of Cucumber mosaic virus (Bromoviridae: Cucumovirus) from natural hosts in Nigeria reveals high diversity and occurrence of putative novel recombinants strains. Front Microbiol. 97 [DOI] [PMC free article] [PubMed]
- 88.Cheng SY, Wu ATH, Batiha GE-S, Ho CL, Lee JC, Lukman HY, et al. Identification of DPP4/CTNNB1/MET as a theranostic signature of thyroid cancer and evaluation of the therapeutic potential of sitagliptin. Biology. 2022;11(2):324. doi: 10.3390/biology11020324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Saber S, Nasr M, Kaddah MMY, Mostafa-Hedeab G, Cavalu S, Mourad AAE, et al. Nifuroxazide-loaded cubosomes exhibit an advancement in pulmonary delivery and attenuate bleomycin-induced lung fibrosis by regulating the STAT3 and NF-κB signaling: a new challenge for unmet therapeutic needs. Biomed Pharmacother. 2022;148:112731. doi: 10.1016/j.biopha.2022.112731. [DOI] [PubMed] [Google Scholar]
- 90.Al-Kuraishy HM, Al-Gareeb AI, Al-Niemi MS, Aljowaie RM, Almutairi SM, Alexiou A, Batiha GE. The prospective effect of allopurinol on the oxidative stress index and endothelial dysfunction in Covid-19. Inflammation. 2022;45(4):1651–1667. doi: 10.1007/s10753-022-01648-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Al-Kuraishy HM, Al-Gareeb AI, Kaushik A, Kujawska M, Batiha GE. Hemolytic anemia in COVID-19. Ann Hematol. 2022;101(9):1887–1895. doi: 10.1007/s00277-022-04907-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Alkhayyat SS, Al-Kuraishy HM, Al-Gareeb AI, El-Bouseary MM, AboKamer AM, Batiha GE, Simal-Gandara J. Fenofibrate for COVID-19 and related complications as an approach to improve treatment outcomes: the missed key for Holy Grail. Inflamm Res. 2022 doi: 10.1007/s00011-022-01615-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Al-Kuraishy HM, Batiha GE, Faidah H, Al-Gareeb AI, Saad HM, Simal-Gandara J. Pirfenidone and post-Covid-19 pulmonary fibrosis: invoked again for realistic goals. Inflammopharmacology. 2022 doi: 10.1007/s10787-022-01027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Al-Kuraishy HM, Al-Gareeb AI, Alkazmi L, Habotta OA, Batiha GE. High-mobility group box 1 (HMGB1) in COVID-19: extrapolation of dangerous liaisons. Inflammopharmacology. 2022;30(3):811–820. doi: 10.1007/s10787-022-00988-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Al-Kuraishy HM, Al-Gareeb AI, El-Saber Batiha GE. The possible role of ursolic acid in Covid-19: a real game changer. Clin Nutr ESPEN. 2022;47:414–417. doi: 10.1016/j.clnesp.2021.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mostafa-Hedeab G, Al-Kuraishy HM, Al-Gareeb AI, Welson NN, El-Saber Batiha GE, Conte-Junior CA. Selinexor and COVID-19: the neglected warden. Front Pharmacol. 2022;13:884228. doi: 10.3389/fphar.2022.884228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Babalghith AO, Al-kuraishy HM, Al-Gareeb AI, De Waard M, Sabatier JM, Saad HM, Batiha GE. The potential role of growth differentiation factor 15 in COVID-19: a corollary subjective effect or not? Diagnostics. 2022;12(9):2051. doi: 10.3390/diagnostics12092051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Al-Kuraishy HM, Al-Gareeb AI, Welson NN, Batiha GE. Trimetazidine and COVID-19-induced acute cardiac injury: a missed key. Int J Clin Pharm. 2022;44(3):832–833. doi: 10.1007/s11096-022-01408-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Al-Kuraishy HM, Al-Gareeb AI, Onohuean H, El-Saber Batiha G. COVID-19 and erythrocrine function: the roller coaster and danger. Int J Immunopathol Pharmacol. 2022;36:03946320221103151–3946320221103151. doi: 10.1177/03946320221103151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Al-Kuraishy HM, Al-Gareeb AI, El-Bouseary MM, Sonbol FI, Batiha GE. Hyperviscosity syndrome in COVID-19 and related vaccines: exploring of uncertainties. Clin Exp Med. 2022 doi: 10.1007/s10238-022-00836-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Al-Kuraishy HM, Al-Gareeb AI, Fageyinbo MS, Batiha GE. Vinpocetine is the forthcoming adjuvant agent in the management of COVID-19. Future Sci OA. 2022;8(5):FSO797. doi: 10.2144/fsoa-2021-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mostafa-Hedeab G, Al-Kuraishy HM, Al-Gareeb AI, Jeandet P, Saad HM, Batiha GE. A raising dawn of pentoxifylline in management of inflammatory disorders in Covid-19. Inflammopharmacology. 2022;30(3):799–809. doi: 10.1007/s10787-022-00993-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Al-kuraishy HM, Al-Gareeb AI, Kaushik A, Kujawska M, Batiha GE. Ginkgo biloba in the management of the COVID-19 severity. Arch der Pharm. 2022 doi: 10.1002/ardp.202200188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Al-Kuraishy HM, Al-Gareeb AI, Alexiou A, Mukerjee N, Al-Hamash SMJ, Al-Maiahy TJ, Batiha GE. 5-HT/CGRP pathway and sumatriptan role in Covid-19. Biotechnol Genet Eng Rev. 2022 doi: 10.1080/02648725.2022.2108996. [DOI] [PubMed] [Google Scholar]
- 105.Al-Kuraishy HM, Al-Gareeb AI, Alexiou A, Batiha GE. Central effects of ivermectin in alleviation of Covid-19-induced dysautonomia. Curr Drug Targets. 2022 doi: 10.2174/1389450123666220810102406. [DOI] [PubMed] [Google Scholar]
- 106.Batiha GE, Al-Gareeb AI, Qusti S, Alshammari EM, Kaushik D, Verma R, Al-Kuraishy HM. Deciphering the immunoboosting potential of macro and micronutrients in COVID support therapy. Environ Sci Pollut Res Int. 2022;29:43516–43531. doi: 10.1007/s11356-022-20075-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Al-Kuraishy HM, Al-Gareeb AI, Alexiou A, Batiha GE. Covid-19 and L-arginine supplementations: yet to find the missed key. Curr Protein Pept Sci. 2022;23(3):166–169. doi: 10.2174/1389203723666220512104039. [DOI] [PubMed] [Google Scholar]
- 108.Al-Kuraishy HM, Al-Gareeb AI, Al-Niemi MS, Alexiou A, Batiha GE. Calprotectin: the link between acute lung injury and gastrointestinal injury in Covid-19: ban or boon. Curr Protein Pept Sci. 2022 doi: 10.2174/1389203723666220610124303. [DOI] [PubMed] [Google Scholar]
- 109.Al-Kuraishy HM, Al-Gareeb AI, Negm WA, Alexiou A, Batiha GE. Ursolic acid and SARS-CoV-2 infection: a new horizon and perspective. Inflammopharmacology. 2022 doi: 10.1007/s10787-022-01038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Alkazmi L, Al-Kuraishy HM, Batiha GE, Mostafa-Hedeab G, De Waard M, Sabatier JM, et al. Roxadustat for SARS-CoV-2 infection: old signaling raised new hopes. Drugs RD. 2022;22(3):183–186. doi: 10.1007/s40268-022-00397-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Al-Kuraishy HM, Al-Gareeb AI, Jalal NA, Kabrah SM, Alexiou A, Batiha GE. SARS-CoV-2 infection and C1-esterase inhibitor: camouflage pattern and new perspective. Curr Protein Pept Sci. 2022 doi: 10.2174/1389203723666220811121803. [DOI] [PubMed] [Google Scholar]
- 112.Batiha GE, Moubarak M, Shaheen HM, Zakariya AM, Usman IM, Rauf A, et al. Favipiravir in SARS-CoV-2 infection: is it Worthwhile? Comb Chem High Throughput Screen. 2022 doi: 10.2174/1386207325666220414111840. [DOI] [PubMed] [Google Scholar]
- 113.Al-Kuraishy HM, Al-Rubiey HF, Al-Buhadily AK, Al-Gareeb AI. Anti-histamines and Covid-19: hype or hope. JPMA J Pak Med Assoc. 2021;71(12):144–148. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.