Abstract
Background
Many studies have explored the association between diabetes and nocturia, but it remains unclear. This article systematically analyses existing evidence of the relationship between diabetes and nocturia, including subgroup analysis based on the number of voids, gender, and continent, in the hope of reaching more reliable clinical conclusions relating to diabetes and nocturia.
Methods
PubMed, Web of Science, and Cochrane Library were searched for identifying studies relating to diabetes and nocturia prior to July 2021. Literature quality evaluation was performed using the Newcastle Ottawa Scale. A random effect meta-analysis was used for pooled odds ratios (ORs) and confidence intervals (CIs) as a means of evaluating the relationship between diabetes and nocturia.
Results
In total, 29 of 781 potentially relevant studies were proven to be eligible. The overall pooled OR demonstrated that diabetes increases the risk of nocturia (OR: 1.49; 95% CI: 1.38, 1.61; P < 0.00001). The association was found to be more robust among subjects ≥ 1 void than ≥ 2 void (OR: 1.74; 95% CI: 1.41, 2.14; P < 0.00001 vs. OR: 1.45; 95% CI: 1.33, 1.59; P < 0.00001), in males than females (OR: 1.59; 95% CI: 1.41, 1.79; P < 0.00001 vs. OR: 1.41; 95% CI: 1.20, 1.66; P < 0.0001) and in Asia than Europe or North America (OR: 1.54; 95% CI: 1.36, 1.75; P < 0.00001 vs. OR: 1.43; 95% CI: 1.19, 1.72; P = 0.0001 vs. OR: 1.45; 95% CI: 1.22, 1.73; P < 0.0001).
Conclusions
Diabetes has an association with a 1.49-fold higher risk of nocturia. This association is more robust for Asian and male subjects or those at a lower nocturia cut-off.
Keywords: nocturia, diabetes, risk, systematic review, meta-analysis
Introduction
Nocturia is an incredibly common and bothersome lower urinary tract symptom (1). The incidence of nocturia increases with age. Large-scale investigations have found the incidence of nocturia of ≥ 2 times per night in 60-year-old to be approximately 25% (2). In addition to sleep disruption and impaired quality of life, nocturia can also result in falls, fractures, and increased mortality among the elderly. High-quality meta-analysis has proven that nocturia increases the risk of falls by approximately 20% and that of fractures by 32% (3). In addition, another meta-analysis has demonstrated that nocturia has an association with a 1.27-fold risk of mortality (4). Therefore, identifying the risk factors of nocturia is of great importance.
Nocturia is closely related to age, but it has many influencing factors, namely, hypertension and diabetes (4). Recent studies have shown diabetes to be related to nocturia with a limited level of evidence. However, with the interference of age, gender, race, and other confounding factors, further research is required regarding whether diabetes is an independent risk factor for nocturia. Therefore, the aim of this article is to comprehensively analyze the relationship between diabetes and nocturia and reach a reliable conclusion for further guiding the clinical management of nocturia.
Materials and methods
Search strategy
Standard preferred reporting items for systematic reviews and meta-analysis (PRISMA) guidelines were adhered to when conducting this review. PubMed, Web of Science, and Cochrane Library were searched in order to identify studies relating to diabetes and nocturia that were published before July 2021. Search terms included: “nocturia and (diabetes or hyperglycemia).” Only articles that were published in English were included in the meta-analysis.
Inclusion and exclusion criteria
Inclusion and exclusion criteria were utilized based on the PICOS (patient/population, intervention, control, outcome, systematic) methodology.
Inclusion criteria: Studies that investigated diabetes and nocturia; Articles that included odds ratios (ORs) and confidence intervals (CIs); All the included articles provided the definition of diabetes and nocturia.
Exclusion criteria: System reviews or case reports were excluded; Incomplete data or no OR and 95% CI were excluded; Data from repeatedly published articles was only included once.
Data extraction and quality assessment
Two authors independently searched and screened the literature based on the established inclusion and exclusion criteria. The following data was extracted: First name of author, publication year, patient country, study design, sample size, gender, the definition of diabetes, the minimum number of voids per night, and the number of patients with nocturia. The Newcastle Ottawa Scale (NOS) was used for evaluating the quality of the included studies (5). All the aforementioned work was independently performed by two authors and any differing opinions were resolved through a discussion with a third author.
Statistical analysis
The data was analyzed using RevMan 5.3. We pooled the OR and 95% CI to evaluate the effect of diabetes on nocturia, and z-test was used to assess for statistical significance. Computed values for Cochran's Q test were used to evaluate heterogeneity. Random effects model was performed for high heterogeneity among studies (P < 0.05 or I2 > 50%). Otherwise, the fixed effects model was used. A funnel plot across all studies was made for the evaluation of publication bias. Sensitivity analysis was performed through the removal of individual studies.
For accurately investigating the relationship between diabetes and nocturia, multiple subgroup analyses were conducted. Weighted ORs were pooled in different subgroups according to 1-void and 2-void, male and female, patient continent, single-factor and multi-factor analysis.
Results
Literature screening and quality assessment
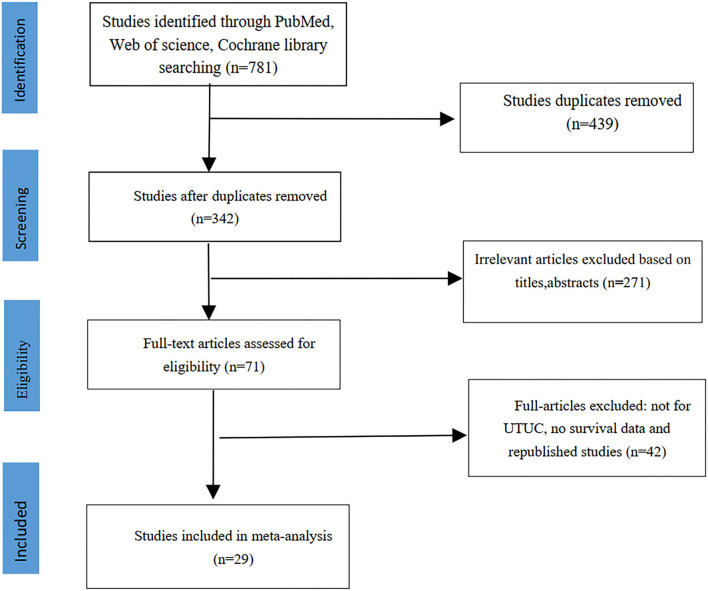
We initially screened 781 abstracts, and 439 articles were deleted due to duplication. After reading the full text of 71 articles, 29 articles met the inclusion criteria and were included in this meta-analysis (6–34). The screening flowchart was shown in Figure 1.
Figure 1.
The flowchart showing study search and selection process.
In total, 29 articles were included in the analysis performed in this article and the quality scores of the included literature are shown in Table 1. A total of 197,809 subjects were incorporated into the meta-analysis. The basic characteristics of the literature, namely, gender, the definition of diabetes, the minimum number of voids per night, and the number of patients with nocturia, can be seen in Table 1.
Table 1.
Basic characteristics and data of included articles.
| Study | Year | Participants country | Study design | Sample size |
Gender (Female/ Male) |
Diabetes definition | Nocturia (minimum episodes) | Number of nocturia patients | NOS |
|---|---|---|---|---|---|---|---|---|---|
| Tikkinen et al. (28) | 2009 | Finnish | Questionnaires sent to subjects in the Population Register Center | 3,307 | N | History of diabetes | 2 | N | 6 |
| Liao et al. (18) | 2011 | Taiwan | Participants in health examinations at a Taiwan hospital | 509 | 0/509 | History of diabetes | 2 | N | 7 |
| Yoshimura et al. (32) | 2004 | Taiwan | Multistage health screening program in Taiwan | 6,517 | 1,949/4,568 | History of diabetes or fasting plasma glucose ≥ 126 mg/dL, or random glucose ≥200 mg/ dL. | 2 | 1,856 | 8 |
| Wen et al. (31) | 2015 | China | Multi-staged, stratified, random sampling of participants over 40 years in Zhengzhou City, China | 9,637 | 6,621/3,016 | History of diabetes | 2 | 3,053 | 8 |
| Gourova et al. (11) | 2006 | Netherlands | Questionnaires sent to elderly men in 21 general practices in Maastricht | 2,934 | 0/2,934 | History of diabetes | 2 | 965 | 8 |
| Hsieh et al. (12) | 2008 | Taiwan | Multistage selection of female participants over 60 older in Taiwan and neighboring islands | 1,523 | 1,523/0 | History of diabetes | 1 | 1,120 | 8 |
| Johnson II et al. (15) | 2005 | USA | Data from the Medical, Epidemiologic, and Social aspects of Aging (MESA) Study in Michigan | 1,652 | 987/665 | History of diabetes | 2 | 520 | 8 |
| Liew et al. (19) | 2006 | Singapore | A population-based cross-sectional survey was conducted in Singapore | 2,273 | 1,134/1,139 | History of diabetes | 1 | 1,250 | 7 |
| Obayashi et al. (24) | 2015 | Japan | A cross-sectional study of community-based elderly individuals | 862 | 435/427 | History of diabetes or fasting plasma glucose levels ≥ 7.0 mmol/L | 2 | 262 | 8 |
| Nakagawa et al. (23) | 2010 | Japan | Community sample ≥ 70 years in Japan | 784 | 427/357 | History of diabetes | 2 | 359 | 7 |
| Stone | 2016 | USA | Participants in USA health screening of men | 30,500 | 0/30,500 | History of diabetes | 2 | 9,440 | 7 |
| Kim et al. (16) | 2018 | USA | Participants aged ≥ 65 years were included from the NHANES dataset | 4,698 | 2,323/2,375 | History of diabetes or fasting plasma glucose ≥126 mg/dL, or random glucose ≥200 mg/ dL. | 2 | 2,333 | 8 |
| Azuero et al. (6) | 2021 | Colombia | A cross-sectional study conducted in five major cities in Colombia. | 1,060 | 530/530 | History of diabetes | 1 | 593 | 7 |
| Rembratt et al. (26) | 2003 | Sweden | Questionnaires sent to all inhabitants aged ≥ 65 years in Tierp, Sweden. | 2,081 | 1,061/1,020 | History of diabetes | 2 | 603 | 7 |
| Lightner et al. (20) | 2012 | USA | A random sample of men aged 40 – 79 years from Olmsted County, MN, USA | 2,447 | 0/2,447 | History of diabetes | 2 | 440 | 7 |
| Wang | 2014 | China | A cross-sectional survey for adults aged ≥18 in five geographical regions of China. | 3,023 | 1,472/1,551 | History of diabetes | 2 | 747 | 8 |
| Chow et al. (8) | 2018 | Taiwan | An internet-based study for subjects aged ≥ 40 years in China, South Korea, and Taiwan | 8,284 | 4,208/4,076 | History of diabetes | 2 | 2,976 | 7 |
| Huang et al. (13) | 2012 | Taiwan | Questionnaires sent to ≥ 40 years for the lower urinary tract symptoms in Taiwan | 1,011 | N | History of diabetes | 2 | 385 | 7 |
| Kim SY et al. (17) | 2017 | Korea | Data were collected by the Korean Centers for Disease Control and Prevention | 92,626 | N | History of diabetes | 2 | 16,322 | 7 |
| Madhu et al. (21) | 2015 | UK | A cross-sectional, population-representative survey involving 30,000 men and women from the USA, UK and Sweden evaluating lower urinary tract symptoms (LUTS) | 30,000 | 15,810/14,107 | History of diabetes | 2 | 9,325 | 8 |
| Victor et al. (29) | 2019 | USA | Data from a cluster-randomized trial of BP reduction in 52 black-owned barbershops in Los Angeles County, California (Clinicaltrials.gov, NCT02321618) | 1,673 | 0/1,673 | History of diabetes | 2 | 485 | 7 |
| Bing et al. (7) | 2008 | Denmark | Questionnaire was randomized sent to 4,000 individuals living in Copenhagen County | 2,799 | 1,313/1,486 | History of diabetes | 2 | 1,022 | 8 |
| Parthasarathy | 2012 | USA | Data from the Sleep Heart Health Study (SHHS) for middle-age and older adults | 6,342 | 3,361/2,981 | History of diabetes | 1 | 3,625 | 7 |
| Chung et al. (9) | 2019 | Korea | Data were prospective collected in Hanyang University Hospital | 304 | 83/221 | History of diabetes | 2 | 83 | 7 |
| Yow et al. (33) | 2021 | Malaya | A cross-sectional was conducted among community-dwelling Malaysian adults aged≥18 years old | 4,616 | 2,634/1,982 | History of diabetes | 1 | 2,646 | 8 |
| Fitzgerald | 2006 | USA | A multistage, stratified, cluster random sample were obtained from the Boston | 5,506 | 3,205/2,301 | History of diabetes | 2 | 1,872 | 7 |
| Zhang | 2010 | China | A cross-sectional survey of nocturia in several communities in northern China | 1,198 | 592/606 | History of diabetes | 2 | 411 | 8 |
| Ito et al. (14) | 2019 | Japan | Multiphasic health screening for 18 952 women in Fukui, Japan | 18,952 | 0/18,952 | History of diabetes | 2 | 739 | 7 |
| Mekki BS | 2020 | USA | A sample of 143 patients based on outpatient cardiology clinic | 143 | 106/37 | History of diabetes patients or fasting plasma glucose ≥126 mg/dL, or a recent HbA1c ≥6.5% | 1 | 111 | 8 |
Association of diabetes and nocturia
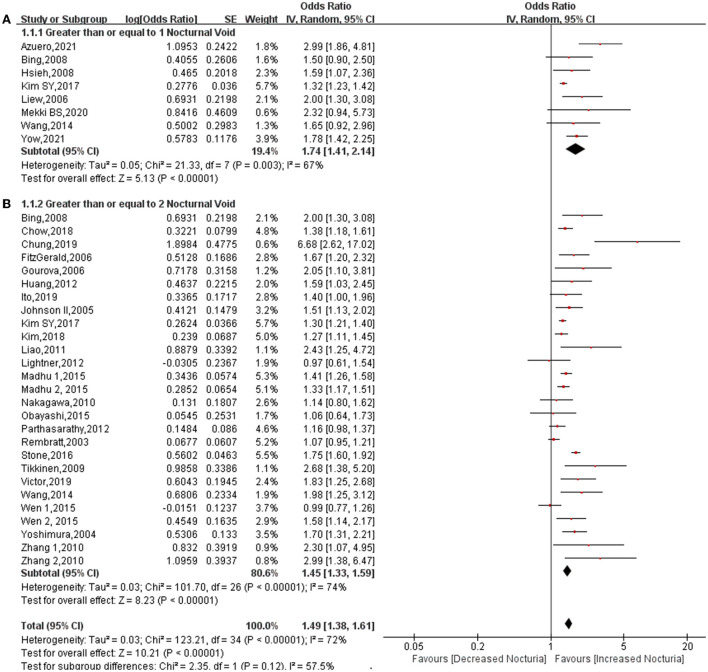
All 29 studies that were included explored the association between diabetes and nocturia (6–34). The heterogeneity among studies was found to be high and the random effect model was used (P < 0.00001, I2 = 72%). Pooled OR demonstrated that diabetes increases the risk of nocturia (OR: 1.49; 95% CI: 1.38, 1.61; P < 0.00001) (Figure 2). In subgroup analysis based on the number of voids, the association was found to be more robust in subjects ≥ 1 void than ≥ 2 void (OR: 1.74; 95% CI: 1.41, 2.14; P < 0.00001 vs. OR: 1.45; 95% CI: 1.33, 1.59; P < 0.00001).
Figure 2.
Forest plot for the association between diabetes and nocturia stratified by number of nocturia. Nocturia ≥1 void (A), nocturia ≥2 void (B).
Stratification by gender
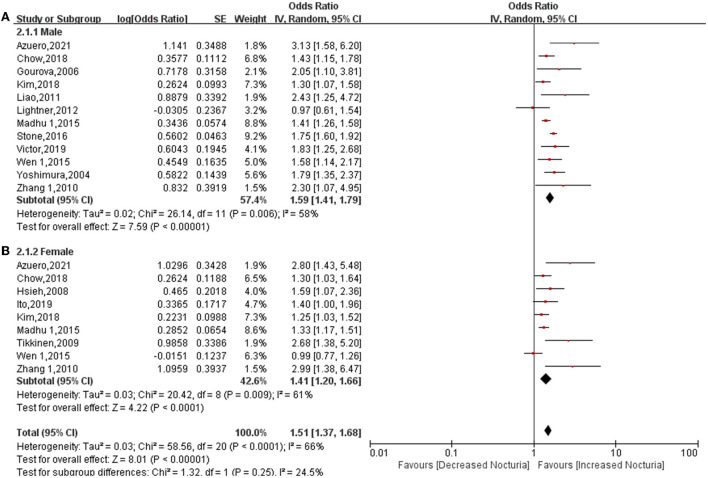
For subgroup classification according to gender, 12 studies provided data relating to men (6, 8, 11, 16, 18, 20, 21, 27, 29, 31, 32, 34) and nine studies provided data relating to women (6, 8, 12, 14, 16, 21, 28, 31, 34). Pooled OR showed that diabetes increases the risk of nocturia for men (OR: 1.59; 95% CI: 1.41, 1.79; P < 0.00001) and women (OR: 1.41; 95% CI: 1.20, 1.66; P < 0.0001) (Figure 3). Heterogeneity among both men (P = 0.006, I2 = 58%) and women (P = 0.009, I2 = 61%) was found to be lower than heterogeneity for the overall cohort (P < 0.0001, I2 = 66%).
Figure 3.
Forest plot for the association between diabetes and nocturia stratified by gender. Men (A), Women (B).
Stratification by country
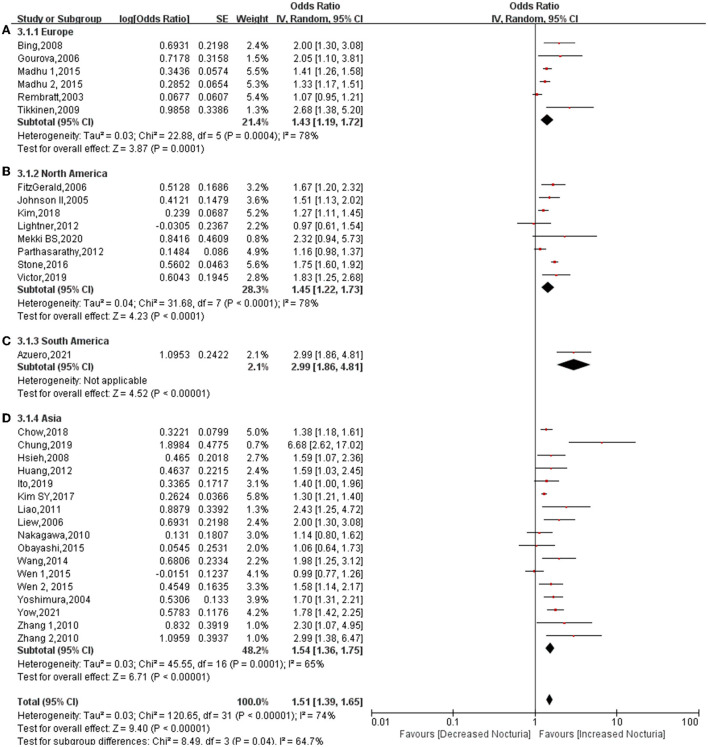
In total, 5 studies provided data relating to Europe (7, 11, 21, 26, 28), 8 studies provided data relating to North America (10, 15, 16, 20, 22, 25, 27, 29), 1 study provided data relating to South America (6), and 15 studies provided data relating to Asia (8, 9, 12–14, 17–19, 23, 24, 30–34). Regardless of the continent, diabetes increases the risk of diabetes. The pooled OR for the Asia subgroup was 1.54 (95% CI: 1.36, 1.75; P < 0.00001). The pooled OR for Asian participants was higher than for Europe subgroup (OR: 1.43; 95% CI: 1.19, 1.72; P = 0.0001) or North America (OR: 1.45; 95% CI: 1.22, 1.73; P < 0.0001) (Figure 4). A South American study showed that diabetes increases the risk of nocturia to a greatest extent (OR: 2.99; 95% CI: 1.86, 4.81; P < 0.00001). Heterogeneity among both Europe (P = 0.0004, I2 = 78%) and North America (P < 0.0001, I2 = 78%) was higher than the heterogeneity for the overall cohort (P < 0.00001, I2 = 74%). In contrast, the heterogeneity of the Asia participants (P = 0.0001, I2 = 65%) was lower than the overall cohort. High heterogeneity among subgroups (P = 0.04, I2 = 64.7%).
Figure 4.
Forest plot for the association between diabetes and nocturia stratified by country. Europe (A), North America (B), South America (C), Asia (D).
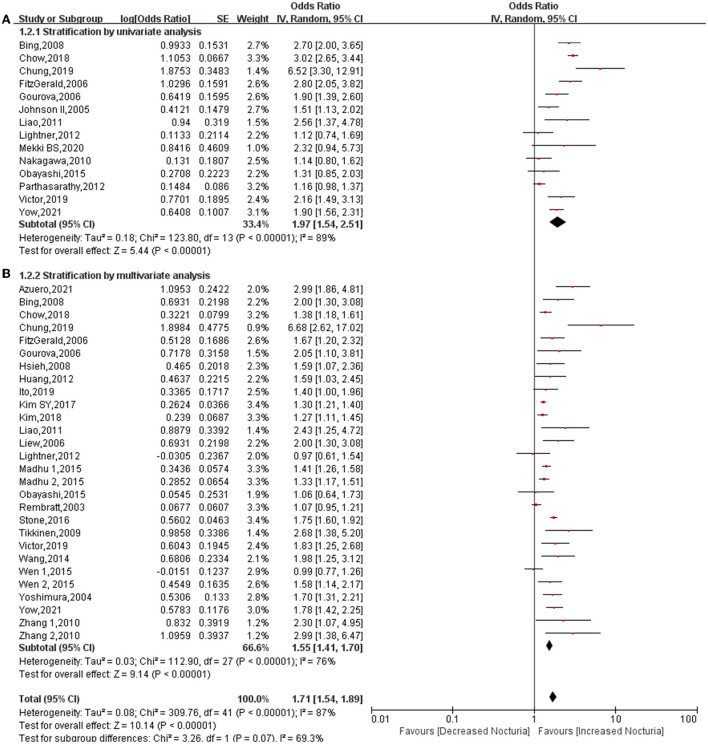
Stratification by univariate and multivariate analysis
A total of 14 studies provided data relating to univariate analysis (7–11, 15, 18, 20, 22–25, 29, 33) and 25 studies provided data relating to multivariate analysis (6–14, 16–21, 24, 26–34). The pooled results proved that diabetes significantly increases the risk of nocturia in univariate analysis (OR: 1.97; 95% CI: 1.54, 2.51; P < 0.00001). The pooled OR for univariate was found to be higher than the overall results (OR: 1.71; 95% CI: 1.54, 1.89; P < 0.00001), while the pooled OR for multivariate analysis (OR: 1.55; 95% CI: 1.41, 1.70; P < 0.00001) was lower than the overall results (Figure 5). Heterogeneity among univariate (P < 0.00001, I2 = 89%), multivariate (P < 0.00001, I2 = 76%), and overall analysis (P < 0.00001, I2 = 87%) was found to be higher. The heterogeneity among subgroups was high (P = 0.07, I2 =69.3%). This indicates that multivariate analysis can weaken the interference other factors have on the results.
Figure 5.
Forest plot for the association between diabetes and nocturia stratified by univariate analysis (A) and multivariate analysis (B).
Sensitivity analysis and publication bias
We constructed a funnel plot to detect publication bias for diabetes and nocturia frequency. There is no publication bias for all studies (Supplementary Figure 1). When performed sensitivity analysis by removing individual studies, no sources of heterogeneity were found.
Discussion
This is believed to be the first meta-analysis that explores the relationship between diabetes and nocturia. The conclusions reached following this systematic review have significant guiding value for clinical practice. First, of the 197,809 subjects that were analyzed, diabetes increased the risk of nocturia by approximately 49%, the probability increasing to 1.74-fold for subjects ≥ 1 void nocturia. In addition, in subgroup classification based on gender, diabetes increased the risk of nocturia among males (OR: 1.59; 95% CI: 1.41, 1.79; P < 0.00001) and females (OR: 1.41; 95% CI: 1.20, 1.66; P < 0.0001). The association between diabetes and nocturia was found to be stronger in male subjects than in female subjects. In addition, the pooled OR for Asia (OR: 1.54) was found to be higher than Europe (OR: 1.43) and North America (OR: 1.45). There is a greater likelihood of diabetes being related to nocturia in Asians than in Europeans and Americans. Furthermore, diabetes significantly increased the risk of nocturia in univariate analysis (OR: 1.97), but OR dropped to 1.55 in the multivariate analysis. This demonstrates that many factors interfere with the effect of diabetes on nocturia, and these factors will be discussed later.
Most studies have found that after making adjustments for other factors, diabetes is an independent risk factor for nocturia (6, 18, 32). However, relatively few studies have reported that diabetes and nocturia are two independent diseases (20, 24). Diabetes is a common cause of nocturia for several reasons. Osmotic diuresis secondary to hyperglycemia can significantly increase the output of urine during the night (35). In addition, diabetes-induced cerebrovascular disease or peripheral nerve stimulation resulting in bladder sensory dysfunction or detrusor overactivity may be a cause of overactive bladder (36). A survey that was conducted in Japan found that 25% of patients with diabetes also had bladder detrusor hyperreflexia (37).
Although a strong correlation exists between nocturia and age, the link between diabetes and nocturia appears to have no connection with age, potentially due to the fact that the prevalence of diabetes increases with age. Many studies have found that following adjustments for the effects of age, diabetes has a significant association with nocturia (18, 32). The results of this study are in accordance with previous studies that found diabetes to increase the risk of nocturia even following adjustments made for age, gender, and other factors in multivariate analysis (OR: 1.55).
The more robust relationship between diabetes and nocturia remains controversial in men in comparison to women. In the subgroup classification based on gender in this article, the association between diabetes and nocturia was found to be slightly stronger among men (OR: 1.59) than women (OR: 1.41). However, a meta-analysis indicated that the correlation between hypertension and nocturia is stronger in women (OR: 1.45) than in men (OR: 1.28) (38). This demonstrates that the influence of gender on nocturia is interfered with by accompanying diseases. In addition, the influence gender has on nocturia and the influence diabetes has on bladder function are also interfered with by several confounding factors. In a study that was conducted by Tikinen et al. (39) women younger than 50 years were found to have a higher incidence of nocturia than men of the same age, but the increase rate of nocturia in men was observed as being twice as fast as that of older women. Bing et al. noted that although there is a similar prevalence of nocturia in men and women, women have a higher tolerance to nocturia than men (7). A study found bladder dysfunction caused by diabetes to account for 59.26% of women and 74.07% of men (40). This proves that the degree of association between diabetes and nocturia differs between genders.
Many studies have reported the incidence of nocturia to vary between people of different races. Limited by several races included in one study, subgroup analysis could only be performed by continent. Only one study in South America has investigated the relationship between diabetes and nocturia, so the level of evidence for the results is incredibly low. A more robust association was found between diabetes and nocturia in Asia (OR: 1.54) than in Europe (OR: 1.43) or North America (OR: 1.45). The strong association between diabetes and nocturia in Asia may prove to be particularly useful, particularly considering the fact that Asians are more prone to organ damage resulting from diabetes. The inclusion of several races in individual studies has resulted in particularly high heterogeneity within the group. Therefore, the correlation between diabetes and nocturia in different continents warrants further study.
The strengths of this review include a contemporary search of studies published in English, duplicate assessment of inclusion criteria, and the quality of evidence and extracted data. This is believed to be the first meta-analysis that explores the relationship between diabetes and nocturia. Through an overall evaluation and subgroup analysis, the results of this study provide evidence of an association between diabetes and nocturia. However, there were inevitably some limitations with the meta-analysis in this study. First, many subjects were diagnosed with diabetes based on their medical histories rather than the current status of hyperglycemia. Second, the nocturia data that was obtained through questionnaires was found to be too subjective. Third, pertinent diseases such as hypertension, obesity, and other diseases that may strengthen the association between diabetes and nocturia were not examined. Fourth, the subgroup analysis by country was unable to provide an analysis based on race. At last, the significant difference in the number of cases that were included in the study may lead to biased results.
Conclusions
Diabetes has an association with a 1.49-fold higher risk of nocturia. This association is more robust for Asian and male subjects or those at a lower nocturia cut-off.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
ZF wrote the manuscript. FW collected and analyzed the data. XD helped the review and revised the manuscript. TZ helped to design the study and revised the article. All authors have read and approved the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are grateful to the authors and patients of the included studies.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.924488/full#supplementary-material
References
- 1.Hashim H, Blanker MH. International Continence Society (ICS) report on the terminology for nocturia and nocturnal lower urinary tract function. Neurourol Urodyn. (2019) 38:499–508. 10.1002/nau.23917 [DOI] [PubMed] [Google Scholar]
- 2.Bosch JL, Weiss JP. The prevalence and causes of nocturia. J Urol. (2013) 189:S86–92. 10.1016/j.juro.2012.11.033 [DOI] [PubMed] [Google Scholar]
- 3.Pesonen JS, Vernooij RWM, Cartwright R, Aoki Y, Agarwal A, Mangera A, et al. The Impact of nocturia on falls and fractures: a systematic review and meta-analysis. J Urol. (2020) 203:674–83. 10.1097/JU.0000000000000459 [DOI] [PubMed] [Google Scholar]
- 4.Pesonen JS, Cartwright R, Vernooij RWM, Aoki Y, Agarwal A, Mangera A, et al. The impact of nocturia on mortality: a systematic review and meta-analysis. J Urol. (2020) 203:486–95. 10.1097/JU.0000000000000463 [DOI] [PubMed] [Google Scholar]
- 5.Wells G, Shea B, 0'Connell D, Peterson J, Welch V, Losos M, et al. The Newcasstle-Ottawa Scale (NOS) for Assessing the Quality of Non-Randomised Studies in Meta-Analysis. Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed July 4, 2021).
- 6.Azuero J, Santander J, Trujillo CG, Caicedo JI, Zuluaga L, Becerra AM, et al. Potential associations of adult nocturia. Results from a national prevalence study. Neurourol Urodyn. (2021) 40:819–28. 10.1002/nau.24624 [DOI] [PubMed] [Google Scholar]
- 7.Bing MH, Moller LA, Jennum P, Mortensen S, Lose G. Nocturia and associated morbidity in a Danish population of men and women aged 60–80 years. BJU Int. (2008) 102:808–14. 10.1111/j.1464-410X.2008.07813.x [DOI] [PubMed] [Google Scholar]
- 8.Chow PM, Liu SP, Chuang YC, Lee KS, Yoo TK, Liao L, et al. The prevalence and risk factors of nocturia in China, South Korea, and Taiwan: results from a cross-sectional, population-based study. World J Urol. (2018) 36:1853–62. 10.1007/s00345-018-2329-0 [DOI] [PubMed] [Google Scholar]
- 9.Chung JH, Moon HS, Park SY, Kim KR, Cho SH, Kim YT. Effect of nocturnal hypoxia on nocturia in patients with obstructive sleep apnea. Int Neurourol J. (2019) 23:161–68. 10.5213/inj.1938026.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fitzgerald MP, Litman HJ, Link CL, McKinlay JB. The association of nocturia with cardiac disease, diabetes, body mass index, age and diuretic use: results from the BACH survey. J Urol. (2007) 177:1385–9. 10.1016/j.juro.2006.11.057 [DOI] [PubMed] [Google Scholar]
- 11.Gourova LW, van de Beek C, Spigt MG, Nieman FH, van Kerrebroeck PE. Predictive factors for nocturia in elderly men: a cross-sectional study in 21 general practices. BJU Int. (2006) 97:528–32. 10.1111/j.1464-410X.2006.06029.x [DOI] [PubMed] [Google Scholar]
- 12.Hsieh CH, Kuo TC, Hsu CS, Chang ST, Lee MC. Nocturia among women aged 60 or older in Taiwan. Aust N Z J Obstet Gynaecol. (2008) 48:312–6. 10.1111/j.1479-828X.2008.00870.x [DOI] [PubMed] [Google Scholar]
- 13.Huang MH, Chiu AF, Wang CC, Kuo HC. Prevalence and risk factors for nocturia in middle-aged and elderly people from public health centers in Taiwan. Int Braz J Urol. (2012) 38:818–24. 10.1590/1677-553820133806818 [DOI] [PubMed] [Google Scholar]
- 14.Ito H, Aoki Y, Oe H, Taga M, Tsuchiyama K, Yokoyama O. Low and high body mass index values are associated with female nocturia. Neurourol Urodyn. (2019) 38:2250–54. 10.1002/nau.24126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson T M., 2nd, Sattin R W, Parmelee P, Fultz N H, Ouslander J G. Evaluating potentially modifiable risk factors for prevalent and incident nocturia in older adults. J Am Geriatr Soc. (2005) 53:1011–6. 10.1111/j.1532-5415.2005.53321.x [DOI] [PubMed] [Google Scholar]
- 16.Kim JS, Chung HS, Yu JM, Cho ST, Moon S. Analyzing the factors associated with nocturia in older people in the United States. Ann Geriatr Med Res. (2018) 22:184–88. 10.4235/agmr.18.0044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim SY, Bang W, Kim MS, Park B, Kim JH, Choi HG. Analysis of the prevalence and factors associated with nocturia in adult Korean Men. Sci Rep. (2017) 7:41714. 10.1038/srep41714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liao CH, Chiang HS, Yu HJ. Serum testosterone levels significantly correlate with nocturia in men aged 40-79 years. Urology. (2011) 78:631–5. 10.1016/j.urology.2011.05.033 [DOI] [PubMed] [Google Scholar]
- 19.Liew LC, Tiong HY, Wong ML, Png DC, Tan JK. A population study of nocturia in Singapore. BJU Int. (2006) 97:109–12. 10.1111/j.1464-410X.2006.05867.x [DOI] [PubMed] [Google Scholar]
- 20.Lightner DJ, Krambeck AE, Jacobson DJ, McGree ME, Jacobsen SJ, Lieber MM, et al. Nocturia is associated with an increased risk of coronary heart disease and death. BJU Int. (2012) 110:848–53. 10.1111/j.1464-410X.2011.10806.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madhu C, Coyne K, Hashim H, Chapple C, Milsom I, Kopp Z. Nocturia: risk factors and associated comorbidities; findings from the EpiLUTS study. Int J Clin Pract. (2015) 69:1508–16. 10.1111/ijcp.12727 [DOI] [PubMed] [Google Scholar]
- 22.Mekki P, Monaghan TF. Nocturia and electrocardiographic abnormalities among patients at an inner-city cardiology clinic. Neurourol Urodyn. (2021) 40:509–14. 10.1002/nau.24590 [DOI] [PubMed] [Google Scholar]
- 23.Nakagawa H, Niu K, Hozawa A, Ikeda Y, Kaiho Y, Ohmori-Matsuda K, et al. Impact of nocturia on bone fracture and mortality in older individuals: a Japanese longitudinal cohort study. J Urol. (2010) 184:1413–8. 10.1016/j.juro.2010.05.093 [DOI] [PubMed] [Google Scholar]
- 24.Obayashi K, Saeki K, Kurumatani N. Relationship between asymmetric dimethylarginine and nocturia in the general elderly population: The HEIJO-KYO cohort. Neurourol Urodyn. (2015) 34:769–73. 10.1002/nau.22647 [DOI] [PubMed] [Google Scholar]
- 25.Parthasarathy S, Fitzgerald M, Goodwin JL, Unruh M, Guerra S, Quan S F. Nocturia, sleep-disordered breathing, and cardiovascular morbidity in a community-based cohort. PLoS ONE. (2012) 7:e30969. 10.1371/journal.pone.0030969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rembratt A, Norgaard J P, Andersson KE. Nocturia and associated morbidity in a community-dwelling elderly population. BJU Int. (2003) 92:726–30. 10.1046/j.1464-410X.2003.04467.x [DOI] [PubMed] [Google Scholar]
- 27.Stone BV, Shoag J, Halpern JA, Mittal S, Lewicki P, Golombos DM, et al. Prostate size, nocturia and the digital rectal examination: a cohort study of 30 500 men. BJU Int. (2017) 119:298–304. 10.1111/bju.13613 [DOI] [PubMed] [Google Scholar]
- 28.Tikkinen KA, Auvinen A, Johnson TM., 2nd, Weiss JP, Keränen T, Tiitinen A, et al. A systematic evaluation of factors associated with nocturia–the population-based FINNO study. Am J Epidemiol. (2009) 170:361–8. 10.1093/aje/kwp133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Victor RG, Li N, Blyler CA, Mason OR, Chang LC, Moy NPB, et al. Nocturia as an unrecognized symptom of uncontrolled hypertension in black men aged 35 to 49 years. J Am Heart Assoc. (2019) 8:e010794. 10.1161/JAHA.118.010794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Hu H, Xu K, Zhang X, Wang X, Na Y, et al. Prevalence, risk factors, and symptom bother of nocturia: a population-based survey in China. World J Urol. (2015) 33:677–83. 10.1007/s00345-014-1411-5 [DOI] [PubMed] [Google Scholar]
- 31.Wen L, Wen YB, Wang ZM, Wen JG, Li ZZ, Shang XP, et al. Risk factors of nocturia (two or more voids per night) in Chinese people older than 40 years. Neurourol Urodyn. (2015) 34:566–70. 10.1002/nau.22623 [DOI] [PubMed] [Google Scholar]
- 32.Yoshimura K, Terada N, Matsui Y, Terai A, Kinukawa N, Arai Y. Prevalence of and risk factors for nocturia: analysis of a health screening program. Int J Urol. (2004) 11:282–7. 10.1111/j.1442-2042.2004.00791.x [DOI] [PubMed] [Google Scholar]
- 33.Yow HY, Tiong JJL, Mai CW, van der Werf E, Zainuddin ZM, Toh CC, et al. Prevalence of nocturia among community-dwelling adults: a population-based study in Malaysia. BMC Urol. (2021) 21:95. 10.1186/s12894-021-00860-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang X, Zhang J, Chen J, Zhang C, Li Q, Xu T, et al. Prevalence and risk factors of nocturia and nocturia-related quality of life in the Chinese population. Urol Int. (2011) 86:173–8. 10.1159/000321895 [DOI] [PubMed] [Google Scholar]
- 35.Weiss JP, Weinberg AC, Blaivas JG. New aspects of the classification of nocturia. Curr Urol Rep. (2008) 9:362–7. 10.1007/s11934-008-0063-7 [DOI] [PubMed] [Google Scholar]
- 36.Barry MJ, Fowler FJ, O'Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, et al. The American urological association symptom index for benign prostatic hyperplasia. The measurement committee of the American urological association. J Urol. (1992) 148:1549–57. 10.1016/S0022-5347(17)36966-5 [DOI] [PubMed] [Google Scholar]
- 37.Ueda T, Yoshimura N, Yoshida O. Diabetic cystopathy: relationship to autonomic neuropathy detected by sympathetic skin response. J Urol. (1997) 157:580–4. 10.1016/S0022-5347(01)65209-1 [DOI] [PubMed] [Google Scholar]
- 38.Rahman SN, Cao DJ, Monaghan TF, Flores VX, Vaysblat M, Moy MW, et al. Phenotyping the association between nocturia and hypertension: a systematic review and meta-analysis. J Urol. (2021) 205:1577–83. 10.1097/JU.0000000000001433 [DOI] [PubMed] [Google Scholar]
- 39.Tikkinen KA, Tammela TL, Huhtala H, Auvinen A. Is nocturia equally common among men and women? A population based study in Finland. J Urol. (2006) 175:596–600. 10.1016/S0022-5347(05)00245-4 [DOI] [PubMed] [Google Scholar]
- 40.Kebapci N, Yenilmez A, Efe B, Entok E, Demirustu C. Bladder dysfunction in type 2 diabetic patients. Neurourol Urodyn. (2007) 26:814–9. 10.1002/nau.20422 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.