Abstract
Background:
The use of advanced magnetic resonance imaging (MRI) techniques in MS research has led to new insights in lesion evolution and disease outcomes. It has not yet been determined if, or how, pre-lesional abnormalities in normal-appearing white matter (NAWM) relate to the long-term evolution of new lesions.
Objective:
To investigate the relationship between abnormalities in MRI measures of axonal and myelin volume fractions (AVF and MVF) in NAWM preceding development of black-hole (BH) and non-BH lesions in people with MS.
Methods:
We obtained magnetization transfer and diffusion MRI at 6-month intervals in patients with MS to estimate MVF and AVF during lesion evolution. Lesions were classified as either BH or non-BH on the final imaging visit using T1 maps.
Results:
Longitudinal data from 97 new T2 lesions from 9 participants were analyzed; 25 lesions in 8 participants were classified as BH 6–12 months after initial appearance. Pre-lesion MVF, AVF, and MVF/AVF were significantly lower, and T1 was significantly higher, in the lesions that later became BHs (p < 0.001) compared to those that did not. No significant pre-lesion abnormalities were found in non-BH lesions (p > 0.05).
Conclusion:
The present work demonstrated that pre-lesion abnormalities are associated with worse long-term lesion-level outcome.
Keywords: MRI, multiple sclerosis, demyelination, quantitative MRI, biomarkers, T2 lesions
Background
The use of advanced magnetic resonance imaging (MRI) techniques, new imaging biomarkers, and high- and ultra-high field imaging in multiple sclerosis (MS) research has led to new insights in lesion evolution and disease outcomes. During the acute phase of lesion development, T2-weighted (-w) hyperintensity and gadolinium (Gd)-based contrast agent enhancement on T1-w MRI are both indicative of inflammation, with T2-w being sensitive to a variety of pathological processes leading to an increase in local water content, and Gd T1-w enhancement demonstrating blood–brain barrier compromise. Evolution from an acute enhancing lesion to a chronic T1-hypointense “black-hole” (BH) has long been used as evidence of irreversible tissue damage, the persistent T1-w hypointensity indicating loss of myelin and axons.1,2 Early in disease, some lesions are capable of repair and remyelination, and do not become BH lesions. The likelihood of lasting damage resulting in a BH has been shown to correlate with duration of Gd T1-w enhancement and volume of enhancing lesions,1,3 and recent work also suggests Gd T1-w enhancement pattern (i.e. ring-like vs nodular) and presence of a phase rim on susceptibility-weighted MRI are associated with long-term tissue damage in developing MS lesions.4–6
While T1-w and T2-w imaging are clinical standard of care and provide good sensitivity to disease activity, other MRI techniques are better suited to investigating specific pathological features such as demyelination or axonal loss. The magnetization transfer ratio (MTR) is a simple, semi-quantitative measure that is fast, commonly available, and sensitive to myelin content in vivo.7,8 Focal MTR decreases have been observed in normal-appearing white matter (NAWM) preceding appearance of Gd-enhancing or T2-w lesions in relapsing-remitting9,10 and progressive forms of MS,10–12 suggesting pre-lesion abnormalities may influence lesion formation. Diffusion-weighted MRI techniques offer insight into additional tissue properties that may reflect axonal integrity, and also are sensitive to pre-lesion abnormalities in MS NAWM.13–16 Combining myelin-sensitive metrics and diffusion MRI may give a more complete picture of the pathology.17
Understanding eventual lesion outcome is important for development of neuroprotective therapeutic strategies. NAWM is abnormal on MRI prior to lesion development, but it has not yet been determined if, or how, pre-lesional abnormalities relate to the long-term evolution of new lesions. To address this question, we obtained magnetization transfer (MT) and diffusion MRI at 6-month intervals in patients with MS to assess longitudinal changes during lesion evolution. Magnetization transfer saturation (MTSat) is a refined version of MTR that has a strong linear correlation with myelin content and allows estimation of myelin volume fraction (MVF).18,19 Neurite orientation dispersion and density imaging (NODDI) is a biophysical model of diffusion MRI that attempts to disentangle microstructural features obscured by traditional diffusion measures.20 Combined with estimates of MVF, NODDI can provide estimates of axonal volume fraction (AVF) that are less contaminated by lack of fiber direction coherence compared to traditional diffusion tensor imaging (DTI) parameters. We thus investigated the relationship between AVF and MVF abnormalities in NAWM preceding development of BH and non-BH lesions in people with MS.
Methods
Participants
Participants diagnosed with MS according to the 2010 McDonald Criteria21 were recruited from the MS clinic at the Montreal Neurological Institute-Hospital. Exclusion criteria included contraindications to MRI and/or Gd-based contrast agents. All subjects provided written informed consent in compliance with the local Research Ethics Board requirements.
MRI acquisition
MRI data were collected at 6-month intervals (maximum four visits per subject) on a Siemens Prisma-Fit 3T instrument (Erlangen, Germany) equipped with a 64-channel phased-array head coil. A standardized MS protocol was acquired for T2-w, T1-w, new T2-w, and Gd-enhancing lesion segmentation: dual spin-echo PD/T2-w (repetition time (TR)/TE1 (echo time), TE2: 3000 ms/11, 99 ms; 1 mm × 1 mm × 2 mm voxel size; 72 axial slices); three-dimensional (3D) T2-w fluid-attenuated inversion recovery (FLAIR; TR/TE/inversion time (TI): 6000 ms/356 ms/2200 ms; 1 mm isotropic voxels; 176 sagittal slices); T1-w 3D gradient recalled echo (GRE) acquired before and after 0.1 mmol/kg Gd contrast (Gadovist, Bayer AG, Leverkusen, Germany) bolus injection (TR/TE/FA (flip angle): 28 ms/4.92 ms/25°; 1 mm isotropic voxels; 192 axial slices). Pre-contrast 3D MP2RAGE (TR/TE/FA1, FA2,/TI1, TI2: 5000 ms/2.76 ms/4°, 5°/940 ms, 2830 ms; 1 mm isotropic voxels; 208 sagittal slices) provided quantitative T1 maps for BH lesion classification.
The diffusion protocol consisted of one signal average at b-values of 300 s/mm2 (10 directions), 700 s/mm2 (30 directions) and 2500 s/mm2 (64 directions) and nine b = 0 s/mm2 images, acquired using a single-shot, spin-echo echo-planar imaging (EPI) sequence20 with monopolar gradients (TR/TE: 3000 ms/65 ms; 2 mm isotropic voxels; GRAPPA factor 2 and multiband22 acceleration factor of 3). Magnetic susceptibility–induced distortions were corrected using a blip-up, blip-down phase-encode strategy.23
The MTSat protocol was based on the work of Helms et al.,18,24 using a set of non-selective 3D FLASH (fast low angle shot) acquisitions to obtain MT-weighted (TR/TE/FA: 36 ms/4.92 ms/5°; MT preparation pulse: FA 500°, offset frequency +1200 Hz, pulse width 9.984 ms; 1 mm isotropic voxels; 192 axial slices), PD-w (FA 5°; no MT pulse) and T1-w (TR/FA: 28 ms/25°; no MT pulse) images. A pair of low spatial resolution–segmented EPI acquisitions (TR/TE: 4010 ms/46 ms; FA 1/FA 2: 60°/120°; 2 mm × 2 mm × 4 mm voxel size; 35 axial slices) were acquired to calculate B1+.
Image processing
Most image processing was done using locally developed tools; all images from all timepoints were registered into a patient-specific common reference space for analysis, as previously described.25 T2-w lesions were segmented on baseline data using a locally developed, automated, multispectral Bayesian technique26 using the PD-w, T2-w, FLAIR and T1-w images. Resulting lesion labels were reviewed and corrected as necessary by a trained reader using interactive software, as previously described.27 New T2-w lesions arising from previously NAWM were similarly identified on follow-up data and then manually corrected by an expert reviewer. New T2-w lesions were further manually classified as either (1) de novo new T2-w, arising entirely from NAWM with no adjacent voxels labeled as previously existing T2-w lesions or (2) enlarging/expanding new T2, which are areas of new T2-w hyperintensity adjoining T2-w lesions identified on the previous timepoint. Chronic T1-w BH regions within T2-w lesions were segmented automatically on quantitative T1 map reconstructions from the pre-contrast MP2RAGE acquisition at the final imaging timepoint to avoid transient T1 changes associated with acute inflammation during lesion formation. T2-w lesions containing clusters of at least eight spatially connected voxels with T1 > 1600 ms at the final timepoint were classified as BH-fate lesions. Gd-enhancing lesions were segmented on post-contrast T1-w images with reference to the registered pre-contrast T1-w images.
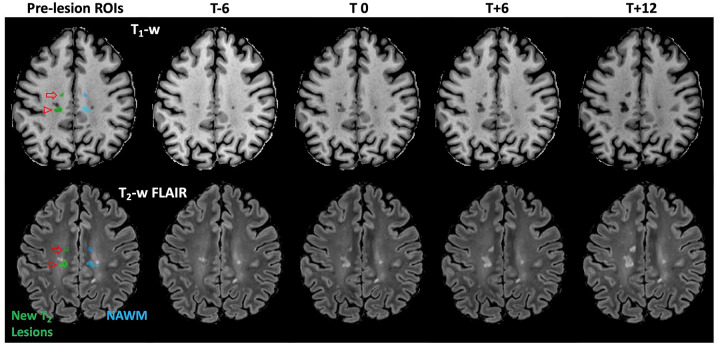
Myelin and axonal density vary spatially throughout the brain, so spatially matched contralateral NAWM regions of interest (ROIs) in homologous areas of the opposite hemisphere were used for pairwise analyses.9,10 Contralateral NAWM ROIs were prepared by first creating cumulative non-WM masks for each participant by summing tissue classification masks from all timepoints for gray matter, T2-w lesions, and cerebrospinal fluid, then dilating 1 mm to reduce partial volume contamination. Lesion masks were mirrored across the midline and non-WM masks were applied to ensure each ROI resided entirely within the NAWM at every imaging timepoint. ROIs were reviewed and manually corrected to ensure contralateral NAWM ROI volumes were matched to the corresponding lesion while allowing for anatomical variation between hemispheres. Representative ROI pairs are illustrated in Figure 1.
Figure 1.
Representative lesion masks. New T2-w lesion masks (green) and spatially matched contralateral NAWM masks (blue) are overlaid on the pre-lesion (6 months prior to lesion appearance (T-6)) T1-w anatomical (top) and T2-w FLAIR (bottom) images. The anterior lesion (open arrow) is a discrete de novo lesion arising entirely from NAWM with no voxels spatially connected to existing lesions. The second lesion (open arrowhead) is an expanding/enlarging lesion, and the spatially matched contralateral NAWM ROI was manually adjusted to avoid an existing T2 lesion while maintaining total ROI volume.
MTSat maps were computed with B1+-correction for non-uniform radio-frequency (RF) transmit field effects as previously described18,28–30 using pipelines developed locally using the Montreal Neurological Institute (MINC) toolkit (http://bic-mni.github.io/). MVF estimates were obtained from MTSat using a calibration factor obtained from a combined MRI/histology data set with the assumption of a linear relationship and zero-intercept.31 NODDI processing was performed using the freely available “AMICO” implementation of the NODDI model32 to obtain estimates of AVF
| (1) |
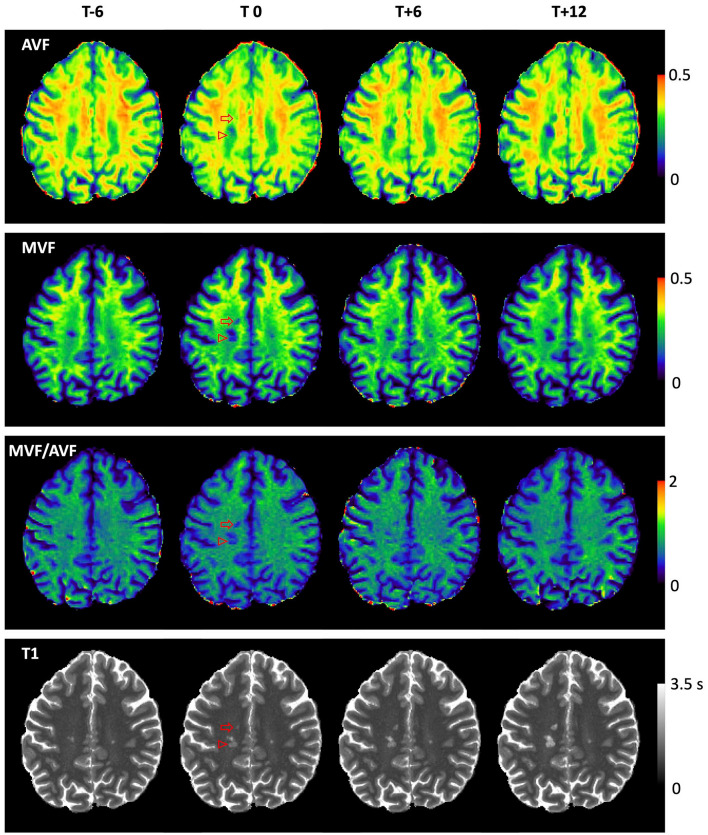
where fiso and fin are the isotropic and restricted (intra-neurite) signal fractions, respectively, also obtained from the NODDI model. While MVF provides an informative estimate of myelin content within a voxel, interpretation of changes in MVF will be different in a lesion with minor axonal loss versus a lesion with more profound axonal loss. For example, limited recovery of MVF may reflect poor remyelination if axons are spared but could indicate relatively good remyelination in the context of substantial axonal loss, where overall (stable) change in MVF (i.e. MVFdrop = MVFpost − MVFpre) is determined to some extent by the number of remaining axons available to remyelinate. To investigate relative remyelination within lesions while controlling for the number of axons within a voxel, we use the ratio MVF/AVF as a normalized MVF metric.31 Parametric maps demonstrating lesion evolution are shown in Figure 2.
Figure 2.
Parametric maps showing lesion evolution. Parametric maps demonstrating lesion evolution in the de novo (open arrow) and expanding/enlarging (open arrowhead) lesions from Figure 1. AVF change is not visually appreciable in this slice at T0 in the de novo lesion, where axonal loss follows acute inflammation and demyelination. AVF, MVF, and T1 worsen progressively over time in both lesions, ultimately resulting in black-hole fate at T+12.
Statistical analysis
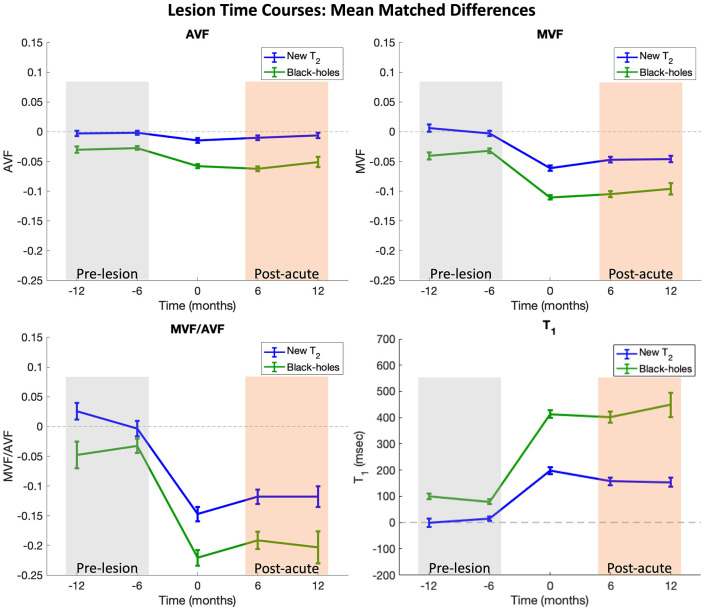
Pre-lesion and post-acute values were obtained for each ROI by averaging all available timepoints before, or after, lesion appearance, corresponding to the gray- and orange-shaded areas in Figure 3, respectively. Longitudinal changes within ROIs were calculated by subtracting post-acute ROI averages from pre-lesion ROI averages. One-sided paired t-tests were used to compare pre-lesion and post-acute values within ROIs. BH and non-BH lesion metrics, including ROI volumes, were compared with mixed-effect models with primary outcome (e.g. MVFdrop) as fixed effect and subject as random effect. Linear mixed-effects models were also used to compare BH and non-BH lesion to contralateral NAWM with ROI tissue type (i.e. NAWM, BH, non-BH) as fixed effect and lesion ID (i.e. a lesion and its corresponding contralateral NAWM ROI would have a common ID such as subject-X_lesion-Y) as a random effect; significance was set at the 0.05 level for all comparisons. Associations between lesion type (discrete/de novo or enlarging/expanding) and lesion fate were evaluated with a chi-square test.
Figure 3.
Time course plots. Time course plots represent mean differences between lesion ROIs and matched contralateral NAWM ROIs. Contralateral ROIs were masked to remove non-WM tissue (i.e. cortex, lesion, cerebrospinal fluid) and were subsequently manually adjusted to maintain consistent ROI volume. Time courses were shifted in time such that Time 0 corresponds to the first time each lesion was observed on MRI. Lesions containing clusters of at least 8 connected voxels with T1 > 1600 ms at the last imaging timepoint were classified as black-hole lesions (plotted in green). Bars represent the standard errors. Pre-lesion and post-acute values are calculated for each lesion by averaging all available timepoints in the gray- and orange-shaded boxes, respectively.
Results
A total of 40 people with MS were recruited for this study: 29 with relapsing-remitting multiple sclerosis (RRMS), 10 with secondary-progressive multiple sclerosis (SPMS), and 1 with primary-progressive multiple sclerosis (PPMS). One participant classified as having RRMS was subsequently diagnosed with myelin oligodendrocyte glycoprotein antibody disease (MOGAD), and was removed from analysis. Ten participants withdrew (four after baseline visit, one after completing two visits, six after completing three visits); seventeen participants were lost to follow-up due to the COVID-19 pandemic (nine after baseline, three after completing two visits, five after completing three visits). Longitudinal data were available from twenty-five participants (thirteen with four completed visits, nine with three completed visits, three with two completed visits); demographics for these 25 participants are presented in Table 1. New T2 lesions (n = 118) were identified in 13 participants (10 RRMS, 3 SPMS). New T2 lesions were found only at the final timepoint in one participant, and three other participants with new T2 lesions were lost to follow-up due to the COVID-19 pandemic. Summary demographics comparing the participants with and without observed new lesions are given in Table 2. Briefly, the group with new lesions was slightly, but not significantly (unpaired t-test p > 0.05), younger with shorter disease duration, lower baseline Expanded Disability Status Scale (EDSS), and more EDSS increase during observation period, and had significantly higher annualized relapse rate (p = 0.03) compared to subjects with no new lesions. Finally, pre-lesion and post-acute data were available for 97 lesions from 9 participants (7 RRMS, 2 SPMS). Six of the nine subjects included in the longitudinal analysis were on DMT (three Teriflunomide, two Copaxone, one Gilenya). One SPMS subject (on Copaxone) did not have new BH on study. Twenty-five lesions in eight participants met our criteria for T1 BHs 6–12 months after lesion appearance. Eight lesions were Gd-enhancing at first observation, five of which had BH fate. Lesions with BH fate had significantly larger volume at first appearance than non-BH lesions (mean volume (±standard deviation): 257.8 (±243.9) vs 66.4 (±74.3) mm3; p < 0.001) but were not significantly more likely to arise from either enlarging/expanding or discrete/de novo lesions (chi-square p = 0.09).
Table 1.
Baseline demographic characteristics.
| Subtype | M/F | Age (years)a | Disease durationa | EDSSb | On DMT |
|---|---|---|---|---|---|
| RRMS | 5/13 | 47.8 (±10.7) | 16.3 (±8.5) | 2 | 14c |
| SPMS | 1/5 | 57.5 (±7.3) | 22.5 (±7.8) | 4.5 | 3d |
| PPMS | 0/1 | 66 (±0) | 38 (±0) | 6.5 | 0 |
RRMS: relapsing-remitting multiple sclerosis; SPMS: secondary-progressive multiple sclerosis; PPMS: primary-progressive multiple sclerosis; EDSS: Expanded Disability Status Scale.
Mean value (±standard deviation).
Median.
Alemtuzumab: n = 1, Avonex: n = 2, Copaxone: n = 5, Gilenya: n = 1, Teriflunomide: n = 5.
Copaxone: n = 1, Ocrelizumab: n = 1, Teriflunomide: n = 1.
Table 2.
Baseline demographics comparing participants with and without new T2 lesions.
| M/F | Age (years) | Baseline EDSS | EDSS change | Disease duration (years) | Number of MRI visits | Number of relapses | ARR | Participants with relapses | |
|---|---|---|---|---|---|---|---|---|---|
| Participants with new lesions | 5/8 | 47.62 (±12.5) | 2.5 (±1.93) | 0.5 (±0.98) | 15.69 (±8.14) | 3.31 (±0.63) | 0.46 (±0.66) | 0.3 (±0.52) | 5 |
| Participants without new lesions | 1/11 | 54.33 (±8.09) | 3.25 (±1.73) | 0.0 (±0.39) | 21.83 (±9.81) | 3.33 (±0.78) | 0.08 (±0.29) | 0.06 (±0.19) | 1 |
| p-value | 0.06 | 0.31 | 0.07 | 0.05 | 0.46 | 0.04 | 0.03 |
EDSS: Expanded Disability Status Scale; MRI: magnetic resonance imaging; ARR: annualized relapse rate.
Values reported as mean value (±standard deviation); EDSS and EDSS change are reported as median (±standard deviation).
p-values determined by unpaired one-tailed t-tests.
Backward analysis of pre-lesion abnormalities
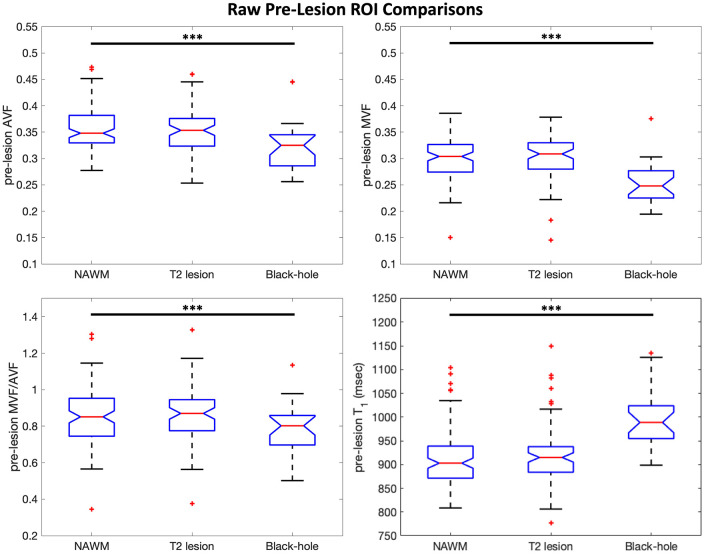
ROI time courses for mean matched differences (e.g. ≡ MVFlesion − MVFNAWM) are shown in Figure 3. Time courses were temporally shifted such that Time 0 corresponds to the first time each lesion was observed on MRI. In these plots, values close to 0 on the y-axis indicate no difference between lesion and contralateral NAWM; qualitatively, non-BH lesions were very similar to matched contralateral NAWM prior to T2 lesion appearance (gray shading) and areas where BH lesions formed were abnormal compared to contralateral NAWM before T2 lesion appearance. The largest deviations away from 0 are observed at lesion appearance where acute inflammation is likely captured in addition to frank tissue damage. Some recovery is then observed in the post-acute period (orange shading) for both BH and non-BH lesions. Boxplots in Figure 4 compare raw pre-lesion AVF, MVF, MVF/AVF, and T1 values for each ROI group. The pre-lesion MVF, AVF, and MVF/AVF were significantly lower, and T1 was significantly higher, in the ROIs that later became BHs (mixed-effects models, p < 0.001) compared to those that did not. No significant pre-lesion abnormalities were found in non-BH ROIs (p > 0.05).
Figure 4.
Pre-lesion boxplots. Boxplots comparing pre-lesion values (calculated for each ROI by averaging all available timepoints in the Figure 3 gray-shaded boxes) between lesion ROIs and contralateral NAWM ROIs matched in size and homologous location to each lesion ROI. Pre-lesion MVF, AVF, MVF/AVF, and T1 in black-hole lesions are significantly different from contralateral NAWM. Mixed-effects models were used to calculate p-values and significance was set at the 0.05 level. Outliers are indicated by red + symbols.
***p < 0.001.
Forward analysis of residual post-acute abnormalities relative to NAWM
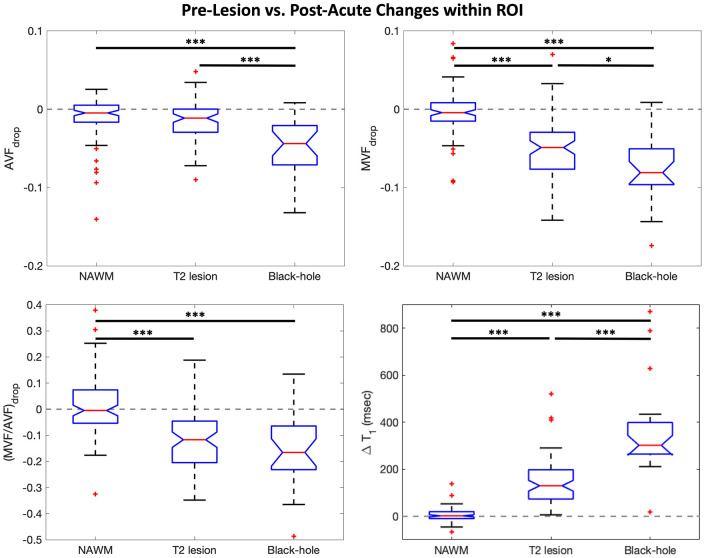
Post-acute lesions demonstrated significant abnormalities compared to contralateral NAWM, and BH lesions demonstrated greater residual tissue damage (i.e. greater initial acute injury and/or poorer recovery) compared to non-BH lesions. All markers were significantly decreased in post-acute BH compared to NAWM (AVF in non-BH, p = 0.03, all others p < 0.001). We estimated the magnitude of change in each parameter from a pre-lesion timepoint to a stable post-acute timepoint (>3 months after lesion appearance), as has been previously reported in normalized MTR.33 This approach provides a measure of unrepaired tissue injury associated with the new lesion, while avoiding the acute phase when inflammation and edema are most likely to affect MRI metrics, and is not sensitive to the precise timing of the MRI scans with respect to the time of lesion formation. Boxplots in Figure 5 show parameter changes between pre-lesion and post-acute measurements; hence, 0 represents no net change over time within each ROI. On average, NAWM showed no change between pre-lesion and post-acute measurements.
Figure 5.
Parameter change boxplots. Boxplots illustrating parameter change for each lesion type and NAWM (i.e. MVFdrop = post-acute MVF − pre-lesion MVF). On average, NAWM ROIs showed no significant change over time. Aside from AVF in non-BH lesions, all other signals showed longitudinal changes that were significantly greater than in contralateral NAWM ROIs matched in size and homologous location to each lesion ROI. Recovery in BH lesions was substantially attenuated compared to non-BH lesions: MVF and AVF were significantly decreased, and T1 was significantly increased (p < 0.001 for all comparisons). Interestingly, (MVF/AVF)drop was not different between BH and non-BH lesions. Mixed-effects models were used to calculate p-values and significance was set at the 0.05 level. Outliers are indicated by red + symbols.
*p < 0.05; ***p < 0.001.
Within-lesion changes as a measure of unrepaired tissue injury
While AVFdrop was qualitatively, but not significantly (p = 0.08), different from NAWM in non-BH lesions, both BH and non-BH lesions showed changes in MVF, MVF/AVF, and T1 that were significantly greater (p < 0.001) than in spatially matched contralateral NAWM. AVFdrop was significantly different from spatially matched contralateral NAWM only in BH lesions. Mixed-effects models excluding NAWM ROIs demonstrated additional significant differences between BH and non-BH lesions. Axon and myelin loss, estimated by AVFdrop and MVFdrop, respectively, were significantly greater in BH lesions compared to non-BH lesions (p < 0.001 for all comparisons). T1 increase was significantly greater in BH than non-BH lesions (p < 0.001). However, change in relative myelination, estimated as (MVF/AVF)drop, was not different between the two lesion types.
Discussion and conclusion
Longitudinal evaluation of MRI microstructural markers provides important insight into disease pathogenesis and lesion evolution in MS. Previous studies provided evidence of NAWM abnormalities preceding lesion formation, and we demonstrate here that the magnitude of pre-lesion abnormalities is associated with lesion outcomes. We show that MVF and AVF are uniquely decreased (compared to spatially matched contralateral NAWM) preceding appearance of new T2 lesions that eventually become BHs, suggesting subtle pathology beginning at least several months before T2 lesion appearance is associated with more severe tissue injury and loss.
The present work demonstrated that pre-lesion abnormalities are associated with worse long-term lesion-level outcome, extending prior observations of pre-lesion abnormalities.9–15 Large pre-lesion abnormalities observed in this study could reflect previous pathological insult that was (1) subclinical: did not create clinical event, was not captured on MRI, and resolved sufficiently to not meet T2 lesion criteria or (2) subacute: pathological event associated with low-grade, focal inflammation below detection threshold for what would be considered T2 lesion. In either case, such regions represent tissue affected by microstructural changes and/or inflammation34 that would be susceptible to greater damage after a second or new hit. Alternatively, it could reflect intrinsic characteristics of tissue that is more likely to develop more severe lesions.
The AVFdrop and MVFdrop results are intuitive and consistent with what is known about BH and non-BH lesions: BH lesions are associated with substantial axonal loss, which is reflected in significantly decreased AVF; non-BH lesions exhibit demyelination with relatively preserved axonal content, consistent with small and non-significant AVFdrop. Both lesion types show substantial demyelination which is reflected as significant MVFdrop. The magnitude of change is higher in MVF than in AVF for both BH and non-BH lesions, suggesting primary tissue damage is demyelination in both lesion types.
We found preliminary evidence of no difference in relative myelin content change (MVF/AVF)drop between BH and non-BH lesions. This finding suggests that, at the level of the axon, the extent of remyelination of surviving axons was similar between BH and non-BH lesions. Thus, because the extent of remyelination per axon is similar between the two lesion types, the larger drops in BH lesions are likely due to greater axonal loss during acute lesion formation.35 At the aggregate level, this results in lower AVF and MVF (since all the myelin associated with degenerated axons is also lost).
Limitations
This methodology used for MVF and AVF estimation from MRI data is at the development stage, and relies on several assumptions that have been detailed elsewhere.19,36 The importance and influence of assumptions such as equal intra- and extra-neurite T2 relaxation times37 and fixed diffusivities in the NODDI model are unclear and need further investigation in the context of MS pathology. For example, if the extra-axonal T2 is elevated as has been reported in age-related white matter lesions,38 the MVF/AVF differences between non-BH and BH lesions could be underestimated.
Due to participant withdrawal and restricted in-person research during the COVID-19 pandemic, some lesions only have ~6 months follow-up. However, previous work has demonstrated that MTR in Gd-enhancing lesions typically is fully resolved by 6 months;39 it is reasonable to assume all BH lesions were at least 6 months old at the final visit, and thus transient edema and other acute effects that may spuriously increase T1 should be resolved and not result in false-positive BH classification.
This study was not powered to evaluate associations between lesion outcomes and clinical outcomes. However, recently BH lesions have been used primarily to evaluate tissue damage and demonstrate protective effects of DMTs rather than to predict clinical course.40,41 Larger studies enrolling more subjects with longer follow-up periods will be necessary to investigate the association between pre-lesion abnormalities and conversion to chronic active/slowly expanding lesions, to assess associations with clinical outcomes such as recovery (or not) from relapses, and to establish whether pre-lesion abnormalities can be used to build a model predictive of lesion fate that can be used to determine outcomes in trials of neuroprotective/remyelination therapies.
Summary
We report two important novel observations regarding MS lesion evolution: (1) that more destructive BH lesions arise from tissue with more severe pre-lesional abnormalities and (2) that more destructive BH lesions may have an MVF/AVF that is the same as less destructive lesions but less than NAWM, suggesting that there may be room for improved remyelination independent of lesion severity.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by funds from Mitacs Elevate Fellowship, Conrad F. Harrington Fellowship, Canadian Institutes of Health Research, Myelin Repair Foundation, and Novartis, and GBP acknowledges support from NSERC (RGPIN-03880).
ORCID iDs: Ian J Tagge  https://orcid.org/0000-0002-5260-7117
https://orcid.org/0000-0002-5260-7117
Dumitru Fetco  https://orcid.org/0000-0003-1335-8274
https://orcid.org/0000-0003-1335-8274
Robert A Brown  https://orcid.org/0000-0002-0632-2102
https://orcid.org/0000-0002-0632-2102
Paul S Giacomini  https://orcid.org/0000-0002-1346-3042
https://orcid.org/0000-0002-1346-3042
Douglas L Arnold  https://orcid.org/0000-0003-4266-0106
https://orcid.org/0000-0003-4266-0106
Contributor Information
Ian J Tagge, McConnell Brain Imaging Center, Montreal Neurological Institute & Hospital, Montreal, QC, Canada.
Ilana R Leppert, McConnell Brain Imaging Center, Montreal Neurological Institute & Hospital, Montreal, QC, Canada.
Dumitru Fetco, McConnell Brain Imaging Center, Montreal Neurological Institute & Hospital, Montreal, QC, Canada.
Jennifer SW Campbell, McConnell Brain Imaging Center, Montreal Neurological Institute & Hospital, Montreal, QC, Canada.
David A Rudko, McConnell Brain Imaging Center, Montreal Neurological Institute & Hospital, Montreal, QC, Canada.
Robert A Brown, McConnell Brain Imaging Center, Montreal Neurological Institute & Hospital, Montreal, QC, Canada.
Nikola Stikov, Electrical Engineering, Polytechnique Montreal, Montreal, QC, Canada.
G Bruce Pike, Departments of Radiology and Clinical Neurosciences, Hotchkiss Brain Institute, University of Calgary, Calgary, AB, Canada.
Paul S Giacomini, Neurology and Neurosurgery, Montreal Neurological Institute & Hospital, Montreal, QC, Canada.
Douglas L Arnold, McConnell Brain Imaging Center, Montreal Neurological Institute & Hospital, Montreal, QC, Canada.
Sridar Narayanan, McConnell Brain Imaging Center, Montreal Neurological Institute & Hospital, Montreal, QC, Canada.
References
- 1. Bagnato F, Jeffries N, Richert ND, et al. Evolution of T1 black holes in patients with multiple sclerosis imaged monthly for 4 years. Brain 2003; 126(Pt 8): 1782–1789. [DOI] [PubMed] [Google Scholar]
- 2. Sahraian MA, Radue EW, Haller S, et al. Black holes in multiple sclerosis: Definition, evolution, and clinical correlations. Acta Neurol Scand 2010; 122(1): 1–8. [DOI] [PubMed] [Google Scholar]
- 3. Cotton F, Weiner HL, Jolesz FA, et al. MRI contrast uptake in new lesions in relapsing-remitting MS followed at weekly intervals. Neurology 2003; 60: 640–646. [DOI] [PubMed] [Google Scholar]
- 4. Absinta M, Sati P, Schindler M, et al. Persistent 7-tesla phase rim predicts poor outcome in new multiple sclerosis patient lesions. J Clin Invest 2016; 126: 2597–2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van den Elskamp IJ, Lembcke J, Dattola V, et al. Persistent T1 hypointensity as an MRI marker for treatment efficacy in multiple sclerosis. Mult Scler 2008; 14(6): 764–769. [DOI] [PubMed] [Google Scholar]
- 6. McFarland HF, Barkhof F, Antel J, et al. The role of MRI as a surrogate outcome measure in multiple sclerosis. Mult Scler 2002; 8(1): 40–51. [DOI] [PubMed] [Google Scholar]
- 7. Tagge I, O’Connor A, Chaudhary P, et al. Spatio-temporal patterns of demyelination and remyelination in the cuprizone mouse model. PLoS ONE 2016; 11(4): e0152480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bagnato F, Gauthier SA, Laule C, et al. Imaging mechanisms of disease progression in multiple sclerosis: Beyond brain atrophy. J Neuroimaging 2020; 30(3): 251–266. [DOI] [PubMed] [Google Scholar]
- 9. Goodkin DE, Rooney WD, Sloan R, et al. A serial study of new MS lesions and the white matter from which they arise. Neurology 1998; 51(6): 1689–1697. [DOI] [PubMed] [Google Scholar]
- 10. Elliott C, Momayyezsiahkal P, Arnold DL, et al. Abnormalities in normal-appearing white matter from which multiple sclerosis lesions arise. Brain Commun 2021; 3(3): fcab176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Filippi M, Rocca MA, Martino G, et al. Magnetization transfer changes in the normal appearing white matter precede the appearance of enhancing lesions in patients with multiple sclerosis. Ann Neurol 1998; 43(6): 809–814. [DOI] [PubMed] [Google Scholar]
- 12. Pike GB, De Stefano N, Narayanan S, et al. Multiple sclerosis: Magnetization transfer MR imaging of white matter before lesion appearance on T2-weighted images. Radiology 2000; 215: 824–830. [DOI] [PubMed] [Google Scholar]
- 13. Rocca MA, Cercignani M, Iannucci G, et al. Weekly diffusion-weighted imaging of white matter in MS. Neurology 2000; 55: 882–884. [DOI] [PubMed] [Google Scholar]
- 14. Wuerfel J, Bellmann-Strobl J, Brunecker P, et al. Changes in cerebral perfusion precede plaque formation in multiple sclerosis: A longitudinal perfusion MRI study. Brain 2004; 127(Pt 1): 111–119. [DOI] [PubMed] [Google Scholar]
- 15. Werring DJ, Brassat D, Droogan AG, et al. The pathogenesis of lesions and normal-appearing white matter changes in multiple sclerosis. Brain 2000; 123(Pt 8): 1667–1676. [DOI] [PubMed] [Google Scholar]
- 16. Naismith RT, Xu J, Tutlam NT, et al. Increased diffusivity in acute multiple sclerosis lesions predicts risk of black hole. Neurology 2010; 74: 1694–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yu F, Fan Q, Tian Q, et al. Imaging G-ratio in multiple sclerosis using high-gradient diffusion MRI and macromolecular tissue volume. Am J Neuroradiol 2019; 40(11): 1871–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Helms G, Dathe H, Kallenberg K, et al. High-resolution maps of magnetization transfer with inherent correction for RF inhomogeneity and T1 relaxation obtained from 3D FLASH MRI. Magn Reson Med 2008; 60(6): 1396–1407. [DOI] [PubMed] [Google Scholar]
- 19. Campbell JSW, Leppert IR, Narayanan S, et al. Promise and pitfalls of g-ratio estimation with MRI. NeuroImage 2018; 182: 80–96. [DOI] [PubMed] [Google Scholar]
- 20. Zhang H, Schneider T, Wheeler-Kingshott CA, et al. NODDI: Practical in vivo neurite orientation dispersion and density imaging of the human brain. NeuroImage 2012; 61: 1000–1016. [DOI] [PubMed] [Google Scholar]
- 21. Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011; 69(2): 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Setsompop K, Cohen-Adad J, Gagoski BA, et al. Improving diffusion MRI using simultaneous multi-slice echo planar imaging. NeuroImage 2012; 63: 569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Andersson JL, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: Application to diffusion tensor imaging. NeuroImage 2003; 20(2): 870–888. [DOI] [PubMed] [Google Scholar]
- 24. Helms G, Dathe H, Kallenberg K, et al. High-resolution maps of magnetization transfer with inherent correction for RF inhomogeneity and T1 relaxation obtained from 3D FLASH MRI [Erratum]. Magn Reson Med 2010; 64: 1856. [DOI] [PubMed] [Google Scholar]
- 25. Maranzano J, Dadar M, Rudko DA, et al. Comparison of multiple sclerosis cortical lesion types detected by multicontrast 3T and 7T MRI. Am J Neuroradiol 2019; 40(7): 1162–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Francis SJ. Automatic lesion identification in MRI of multiple sclerosis patients. Montreal, QC, Canada: McGill University, 2004. [Google Scholar]
- 27. Ghassemi R, Narayanan S, Banwell B, et al. Quantitative determination of regional lesion volume and distribution in children and adults with relapsing-remitting multiple sclerosis. PLoS ONE 2014; 9(2): e85741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Helms G, Dathe H, Dechent P. Modeling the influence of TR and excitation flip angle on the magnetization transfer ratio (MTR) in human brain obtained from 3D spoiled gradient echo MRI. Magn Reson Med 2010; 64(1): 177–185. [DOI] [PubMed] [Google Scholar]
- 29. Weiskopf N, Suckling J, Williams G, et al. Quantitative multi-parameter mapping of R1, PD*, MT, and R2* at 3T: A multi-center validation. Front Neurosci 2013; 7: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Helms G. Correction for residual effects of B1+ inhomogeniety on MT saturation in FLASH-based multi-parameter mapping of the brain. Proc ISMRM 2015; 34: 3360. [Google Scholar]
- 31. Stikov N, Campbell JS, Stroh T, et al. In vivo histology of the myelin g-ratio with magnetic resonance imaging. NeuroImage 2015; 118: 397–405. [DOI] [PubMed] [Google Scholar]
- 32. Daducci A, Canales-Rodríguez EJ, Zhang H, et al. Accelerated microstructure imaging via convex optimization (AMICO) from diffusion MRI data. NeuroImage 2015; 105: 32–44. [DOI] [PubMed] [Google Scholar]
- 33. Brown RA, Narayanan S, Arnold DL. Imaging of repeated episodes of demyelination and remyelination in multiple sclerosis. NeuroImage Clin 2014; 6: 20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Moccia M, van de Pavert S, Eshaghi A, et al. Pathologic correlates of the magnetization transfer ratio in multiple sclerosis. Neurology 2020; 95: e2965–e2976. [DOI] [PubMed] [Google Scholar]
- 35. Trapp BD, Peterson J, Ransohoff RM, et al. Axonal transection in the lesions of multiple sclerosis. N Engl J Med 1998; 338: 278–285. [DOI] [PubMed] [Google Scholar]
- 36. Mohammadi S, Callaghan MF. Towards in vivo g-ratio mapping using MRI: Unifying myelin and diffusion imaging. J Neurosci Methods 2021; 348: 108990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gong T, Tong Q, He H, et al. MTE-NODDI: Multi-TE NODDI for disentangling non-T2-weighted signal fractions from compartment-specific T2 relaxation times. NeuroImage 2020; 217: 116906. [DOI] [PubMed] [Google Scholar]
- 38. Lampinen B, Szczepankiewicz F, Mårtensson J, et al. Towards unconstrained compartment modeling in white matter using diffusion-relaxation MRI with tensor-valued diffusion encoding. Magn Reson Med 2020; 84(3): 1605–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Richert ND, Ostuni JL, Bash CN, et al. Interferon beta-1 b and intravenous methylprednisolone promote lesion recovery in multiple sclerosis. Mult Scler 2001; 7: 49–58. [DOI] [PubMed] [Google Scholar]
- 40. Oh J, Ontaneda D, Azevedo C, et al. Imaging outcome measures of neuroprotection and repair in MS. Neurology 2019; 92: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kolb H, Absinta M, Beck ES, et al. 7T MRI differentiates remyelinated from demyelinated multiple sclerosis lesions. Ann Neurol 2021; 90: 612–626. [DOI] [PMC free article] [PubMed] [Google Scholar]