Abstract
Aggressive behavior is common across childhood-onset psychiatric disorders and is associated with impairments in social cognition and communication. The present study examined whether amygdala connectivity and reactivity during face emotion processing in children with maladaptive aggression are moderated by social impairment. This cross-sectional study included a well-characterized transdiagnostic sample of 101 children of age 8–16 years old with clinically significant levels of aggressive behavior and 32 typically developing children without aggressive behavior. Children completed a face emotion perception task of fearful and calm faces during functional magnetic resonance imaging. Aggressive behavior and social functioning were measured by standardized parent ratings. Relative to controls, children with aggressive behavior showed reduced connectivity between the amygdala and the dorsolateral prefrontal cortex (PFC) during implicit emotion processing. In children with aggressive behavior, the association between reduced amygdala–ventrolateral PFC connectivity and greater severity of aggression was moderated by greater social impairment. Amygdala reactivity to fearful faces was also associated with severity of aggressive behavior for children without social deficits but not for children with social deficits. Social impairments entail difficulties in interpreting social cues and enacting socially appropriate responses to frustration or provocation, which increase the propensity for an aggressive response via diminished connectivity between the amygdala and the ventral PFC.
Keywords: amygdala functional connectivity, dorsolateral prefrontal cortex, maladaptive aggression, social impairment, ventral prefrontal cortex
Introduction
Maladaptive aggression is common across childhood-onset psychiatric disorders (Connor et al. 2019) and constitutes one of the main reasons for referral to mental health services (Costello et al. 2014). The causal pathways to childhood aggression involve multiple interacting biological, environmental, and psychosocial risk factors (Tremblay et al. 2018). One influential theory, the social information-processing model, identifies deficits in the detection and interpretation of social cues, as well as in the generation and enactment of socially appropriate responses, as key factors that can lead to maladaptive aggression (Crick and Dodge 1994). For example, children may be more likely to respond aggressively if perceived to be intentionally provoked or mistreated (Ray et al. 2008). This distortion in processing social information—referred to as hostile attribution bias—often leads to increased anger arousal and aggressive behavior (Verhoef et al. 2019). The construct of “aggression” refers to a broad category of behaviors, which often results in harm to self or others (Tremblay et al. 2018). In children with psychiatric disorders, affective, impulsive aggression—also referred to as reactive aggression—represents one of the most clinically challenging behaviors (Vitiello and Stoff 1997). Aggression is also linked to reduced ability to generate and implement socially appropriate solutions to provocation (Orue et al. 2019), as well as to overall impairments in social functioning (Burke et al. 2014). Children with aggressive behavior also show reduced ability to infer another person’s thoughts, intentions, and feelings (Happé and Frith 1996; Oliver et al. 2011; Mandy et al. 2013; Holl et al. 2018; Heleniak and McLaughlin 2020). These social cognitive processes, such as reappraisal of frustrating events and generation of nonaggressive responses to provocations, are also the targets of cognitive–behavioral interventions for childhood aggression (Sukhodolsky et al. 2004; Sukhodolsky, Smith, et al. 2016).
Neuroimaging studies of child aggression have identified deficits in emotion processing and reinforcement-based decision making (Alegria et al. 2016). There is also considerable literature on the neural mechanisms of impaired recognition of distress in others (i.e. affective empathy) in individuals with conduct problems and callous-unemotional (CU) traits (Lockwood et al. 2013; Sethi et al. 2018). However, the effects of social impairments other than the lack of affective empathy on the neural mechanisms of aggression have not been well studied. Given the increasing body of research on the neural circuitry of social dysfunction across psychiatric disorders, understanding brain mechanisms of social cognition in children with aggression can offer new insights into the pathophysiology of this common and impairing behavioral problem. Here, we examine if brain responses to emotional faces measured via functional magnetic resonance imaging (fMRI) and aggression severity are moderated by the degree of social impairment in a transdiagnostic sample of children with aggressive behavior.
Neural Correlates of Implicit Emotion Processing Deficits in Childhood Aggression
Implicit emotional face processing tasks are commonly used to study frontolimbic and frontoparietal networks implicated in both threat processing and social perception (Fusar-Poli et al. 2009; Del Casale et al. 2017). Children with disruptive behavior are reported to show over-reactivity in regions involved in emotion generation including the amygdala and insula (Herpertz et al. 2008; Viding et al. 2012), underactivity in prefrontal regions involved in the cognitive control of emotion including the ventromedial, ventrolateral, and dorsolateral prefrontal cortices (vmPFC, vlPFC, and dlPFC, respectively) (Marsh et al. 2008; Aghajani et al. 2017), and reduced connectivity between the amygdala and prefrontal regions (Marsh et al. 2008; Aghajani et al. 2017; Stoddard et al. 2017; Kryza-Lacombe et al. 2019). Disruptions in frontolimbic circuitry during face perception tasks are also well documented in children with autism spectrum disorder (ASD) (Pelphrey et al. 2007; Vandewouw et al. 2020). Our prior work demonstrated that children with ASD and co-occurring aggression showed reduced amygdala–vlPFC connectivity compared with children with ASD-without-aggression; in addition, weaker amygdala–vlPFC connectivity was associated with greater severity of disruptive behaviors after accounting for the presence of CU traits (Ibrahim et al. 2019). Given the central role of the vlPFC as a hub in social cognitive (Dal Monte et al. 2014) and emotion regulation (Silvers et al. 2016) processes, it is possible that social impairment could moderate the association between perturbed amygdala–vlPFC connectivity and maladaptive aggressive responses to threat and emotionally salient stimuli. Thus, in this study, we hypothesized that amygdala–vlPFC connectivity in children with aggression will be modulated by the severity of social deficits. To examine the moderating role of social impairment on frontoamygdala connectivity and aggression in youths—which was motivated by prior imaging work of aggressive behavior in ASD (Ibrahim et al. 2019)—we leveraged a well-established continuous measure of social functioning, the Social Responsiveness Scale—Second Edition (SRS-2) (Constantino 2005). We reasoned that the SRS-2 Social Communication and Interaction subscale—which measures social deficits in the domains of social awareness, motivation, communication, and cognition—would approximate social cognitive deficits implicated in childhood aggression. While the SRS was initially developed to measure core ASD social deficits, it has been shown to have transdiagnostic applications in youths and in the general population for assessing social cognitive impairments behaviorally (Cholemkery et al. 2014) and on a neural level in fMRI studies (Baribeau et al. 2019; Lake et al. 2019; Ibrahim, Noble, et al. 2021).
Evaluating a Neurobiological Mechanism of Aggression Modulated by Social Impairment
Examining the moderating effects of social impairments on the neural circuitry of aggressive behavior is important for at least three reasons. First, psychological deficits in social information processing, such as the ability to recognize and interpret social cues, are correlated with aggressive behavior in children (Capage and Watson 2001; Pardini and Frick 2013). Second, the neurocognitive mechanisms of aberrant emotion processing, particularly fearful faces, are implicated in both aggressive behavior and social impairments (Dawel et al. 2012; Pardini and Frick 2013; Moul et al. 2018). Third, there is increased recognition that social cognitive deficits may be shared across psychiatric and developmental disorders (Happé and Frith 2014; Cotter et al. 2018). To this end, the SRS (Constantino 2005) has been used to capture dimensional social constructs in child psychiatric disorders (Uljarević et al. 2019). The SRS has excellent dimensionality in the general population (Constantino and Todd 2003; Wigham et al. 2012) and was recommended by the NIMH workgroup for probing the Research Domain Criteria (RDoC) framework of social processes (NIMH 2016). Of relevance to this study, the SRS was shown to be correlated with cognitive empathy but not with affective empathy in children (Georgiou et al. 2019), and cognitive empathy is rarely tested in neuroimaging studies of aggressive behavior (Sebastian et al. 2012). In this study, the SRS was used in a dimensional analysis of the neural correlates of aggressive behavior to identify relative contributions of social cognitive deficits to the brain mechanisms of aggression.
Our primary aim was to investigate amygdala–prefrontal connectivity in a transdiagnostic sample of children with clinically significant levels of aggressive behavior. Based on the previous research (Marsh et al. 2008; Aghajani et al. 2017), we hypothesized that children with aggressive behavior would show reduced amygdala–prefrontal connectivity during implicit emotion processing compared with unaffected, healthy controls. Our second aim was to test if the association between the amygdala–prefrontal connectivity and the severity of aggression measured by the Child Behavior Checklist (CBCL) Aggressive Behavior Scale (Achenbach and Rescorla 2001) is moderated by severity of social impairment measured by the SRS-2 Social Communication and Interaction (SCI) subscale (Constantino 2005), as continuous measures of aggressive behavior and social functioning, respectively. These dimensional analyses were conducted in the sample of children with aggression (n = 101). In addition, the covariance between social deficits and CU traits was controlled by including the Inventory of Callous-Unemotional (ICU) traits (Frick 2003) score as a covariate. Given the influence of age on amygdala functional connectivity development (Gee et al. 2013; Gabard-Durnam et al. 2014; Gabard-Durnam et al. 2018), we also tested age-related differences between aggressive behavior and healthy control groups. Thus, even though exploratory in nature, we expected younger children versus adolescents with aggression to show distinct patterns of attenuation in amygdala–PFC connectivity relative to healthy controls. The third aim was to investigate amygdala reactivity to fearful faces as a potential transdiagnostic neuromarker of aggressive behavior. Amygdala reactivity to fearful faces has been shown to be increased in children with conduct problems without CU traits and decreased in children with conduct problems and high levels of CU traits (Sebastian et al. 2012; Lozier et al. 2014). Here, we tested if the association of amygdala reactivity to fearful expressions with aggressive behavior is moderated by the severity of social impairment controlling for CU traits. We predicted that amygdala reactivity to fearful faces would be attenuated in socially impaired children with aggression. We also conducted follow-up sensitivity analyses in which we systematically assessed the impact of covariates related to CU traits, co-occurring anxiety, and psychotropic medication use in both amygdala connectivity and region-of-interest (ROI) analyses.
Materials and Methods
Subjects
The sample included 101 children of age 8–16 years old with clinically significant aggressive behavior (28 females) and 32 healthy controls (13 females) without aggressive behavior. Table 1 shows demographic and clinical characteristics of participants. Detailed inclusion and exclusion criteria are provided in the Supplementary Material. Children with aggressive behavior participated in a treatment study of behavior therapy for irritability and aggression (Sukhodolsky, Wyk, et al. 2016), and this paper reports fMRI and clinical characterization data that were collected prior to initiating the treatment. One of the inclusion criteria for the treatment study was a T-score of 65 or greater on the CBCL Aggressive Behavior Scale (Achenbach and Rescorla 2001). In addition to high levels of aggression on the dimensional measure, all children met criteria for Oppositional Defiant Disorder, Conduct Disorder, or Disruptive Mood Dysregulation Disorder. Children were allowed to have co-occurring psychiatric disorders such as attention-deficit/hyperactivity disorder (ADHD), ASD, and anxiety if the presence of co-occurring disorders did not require immediate treatment. There were no significant differences in aggression severity between girls and boys in the aggressive behavior group (t99 = 0.81, P = 0.43) or in the total sample (t131 = 1.31, P = 0.19). There were also no significant correlations between CBCL aggression severity and gender in the aggressive behavior group (r = −0.08, P = 0.42) or in the total sample (r = −0.13, P = 0.12): See the Supplementary Results and Supplementary Table S10 for additional correlations between study variables. Each participant’s parent provided informed consent according to the institutional review board at the Yale University School of Medicine. Each child provided verbal and written assent. This study was reviewed and approved by the local ethical committee (institutional review board at the Yale University School of Medicine), and it was conducted in accordance with the declaration of Helsinki.
Table 1.
Participant demographics and clinical characteristics
| Variable | Children with aggressive behavior, n = 101 | Typically developing healthy controls, n = 32 | Total sample, N = 133 | P valuea |
|---|---|---|---|---|
| Age, years (SD) | 11.8 (2.2) | 12.7 (1.9) | 11.9 (2.1) | 0.03b |
| Male, % | 72.3 | 59.4 | 69.2 | 0.19 |
| Mean IQc (SD) | 107 (13.9) | 112 (12.3) | 107.7 (13.7) | 0.07 |
| Race, % | 0.37 | |||
| White | 77.2 | 65.6 | 74.4 | |
| Black | 11.9 | 21.9 | 14.3 | |
| American Indian/Alaska native | 1.5 | 0 | 1.5 | |
| Asian/Pacific Islander | 2.0 | 0 | 1.5 | |
| Other/more than one race | 6.9 | 12.5 | 8.3 | |
| Ethnicity | 0.48 | |||
| Hispanic | 15.8 | 12.5 | 15.0 | |
| Non-Hispanic | 84.2 | 87.5 | 85.0 | |
| Mean CBCL aggressive behavior T-score (SD) | 75.2 (7.5) | 50.9 (2.5) | 69.4 (12.3) | <0.001d |
| Mean ICU score (SD) | 33.2 (9.6) | 14.8 (6.4) | 28.7 (11.9) | <0.001d |
| Mean SRS-2 SCI T-score | 65.4 (11.3) | 45.4 (8.6) | 60.7 (13.7) | <0.001d |
| DSM-5 diagnosese (n, %) | ||||
| Oppositional defiant disorder | 81 (80.2) | |||
| Conduct disorder | 12 (11.9) | |||
| DBD-NOS | 3 (3) | |||
| DMDD | 16 (15.8) | |||
| ASD | 18 (17.8) | |||
| ADHD | 77 (76.2) | |||
| Anxiety disorder | 26 (25.7) | |||
| Depressive disorder | 4 (4) | |||
| Medication (n, %) | 47 (46.5) | |||
| Type of medication (n, %) | ||||
| Stimulants | 32 (31.7) | |||
| Nonstimulants | 20 (19.8) | |||
| Antidepressant | 13 (12.9) | |||
| Neuroleptics | 13 (12.9) | |||
| Mood stabilizers | 4 (4) | |||
| Benzodiazepines | 2 (2) | |||
Notes: ADHD, attention-deficit/hyperactivity disorder; CBCL, Child Behavior Checklist; DMDD, disruptive mood dysregulation disorder; HC, healthy controls; ICU, Inventory of Callous-Unemotional Traits; SRS-2 SCI, Social Responsiveness Scale-Second Edition (SRS-2) Social Communication and Interaction subscale score (SCI).
aSignificant group differences at P < 0.05 using Chi-square test for categorical variables or independent samples T-test.
bHC > Aggressive Behavior group.
cFull-scale IQ measured by the Wechsler Abbreviated Scale of Intelligence (Wechsler 1997).
dAggressive Behavior group > HC.
eFollowing DSM-5, oppositional defiant disorder diagnosis was not assigned to children who met criteria for DMDD.
Clinical Assessment
All children received a comprehensive diagnostic evaluation that included the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL) (Kaufman et al. 2016), a structured interview with excellent reliability that was conducted with the parent and child by an expert clinician to establish Diagnostic and Statistical Manual of Mental Disorders (DSM-5) diagnoses of Disruptive Behavior Disorders as well as co-occurring psychopathology. ASD diagnosis was confirmed with the Autism Diagnostic Interview—Revised (Le Couteur et al. 2003) and Autism Diagnostic Observation Schedule—Second Edition (Lord et al. 2012). Full-scale IQ was evaluated with the Wechsler Abbreviated Scale of Intelligence (Wechsler 1997). Parents completed demographics and medical history forms.
Parents also completed the CBCL, a well-established measure of child psychopathology (Achenbach and Rescorla 2001). The CBCL Aggressive Behavior Scale includes 16 items reflecting inappropriate anger outbursts, as well as verbal and physical aggression. A T-score of 65 represents a cutoff for a clinically significant level of aggression. The parent-rated SRS-2 Social Communication and Interaction (SCI) subscale score (53 items are included in the SRS-2 SCI subscale) (Constantino 2005) was used as a dimensional measure of severity of social impairments. The SCI represents four dimensions of social behaviors including social awareness, motivation, communication, and cognition. Higher scores on the SRS-2 SCI indicate greater social impairment. We reasoned that the SCI subscale would approximate deficits in social cognition related to maladaptive aggression. The SRS was initially developed to measure social impairment in ASD but was shown to capture social cognitive impairments in the general population, as well as in children with disruptive behavior disorders (Cholemkery et al. 2014). Parents also completed the ICU (Frick 2003) and the total score was used as a dimensional measure of CU traits.
Dimensional measures of aggression and social impairments were selected to allow comparison with prior fMRI studies. The CBCL aggression-related scales have been extensively used in prior neuroimaging studies with samples of children with disruptive behavior (Lozier et al. 2014; Cardinale et al. 2018; Ibrahim et al. 2019; Ibrahim, Kalvin, et al. 2021). The SRS-2 SCI subscale has also been used in neuroimaging research of children with and without ASD (Baribeau et al. 2019; Lake et al. 2019), and it is designated as a measure of social cognitive impairment in the RDoC social processes domain (Abram et al. 2015; Ibrahim and Sukhodolsky 2018; Clarkson et al. 2020), underscoring its utility in transdiagnostic samples.
Experimental Paradigm
Children completed a block-design fMRI task where they viewed emotionally expressive faces from the NimStim Face Stimulus Set (Tottenham et al. 2009) that depicted fearful and calm expressions with an equal number of male and female faces (Supplementary Fig. 1). The task used a pseudorandomized block design with 12 blocks that each contain two randomly selected faces per block exhibiting the same expression: 6 calm emotion and 6 fearful emotion blocks. The face-expression pair of images were randomly selected throughout the blocks and no individual face-expression image is displayed more than once throughout the paradigm. Each block was 12 s in length and consisted of two faces displayed for 5.5 s each that were separated by a 1-s intertrial fixation cross (i.e., two faces displayed in succession per block with only one face on the screen at a time). Blocks were separated by a jittered interblock interval between 8 and 12 s to optimize statistical efficiency. The interblock intervals were pseudorandomized such that the mean of all interblock intervals was 10 s. The first block was preceded by a 10-s initial fixation cross and the final block was succeeded by an identical 10-s fixation cross. The total duration of the paradigm was 284 s. To ensure attention to the stimuli, participants were instructed to perform an orthogonal gender identification task using a button press in their left or right hand to indicate male or female, respectively. We examined connectivity across both emotions (fearful and calm) to understand deficits in implicit emotion face processing associated with aggressive behavior and to ensure sufficient continuous voxel time course for psychophysiological interaction (PPI) analyses. For amygdala ROI analyses, to understand differences in amygdala reactivity to threat or fearful faces in children with aggression, fearful versus calm faces was the contrast of interest because the presentation of fearful faces is shown to strongly activate the amygdala (Morris et al. 1996; Fusar-Poli et al. 2009). Additional task details are also reported elsewhere (Sukhodolsky, Wyk, et al. 2016; Ibrahim et al. 2019; Ibrahim, Noble, et al. 2021): Behavioral performance results are reported in Supplementary Table 2. A mock scanner was used to acclimate participants to the scanning environment prior to the fMRI session (see Supplementary Material).
Imaging Acquisition/Preprocessing
Functional MRI data were collected using a Siemens MAGNETOM Tim Trio 3 Tesla scanner with an upgrade for echoplanar images (EPI). A T1-weighted high-resolution anatomical scan was obtained for each participant for co-registration purposes: repetition time (TR) = 2530 ms; echo time (TE) = 3.31 ms; 1-mm isotropic voxels; 176 slices; flip angle = 7°; matrix size = 2562; field of view (FOV) = 256 mm. For each participant, 137 interleaved, oblique whole-brain functional volumes were collected in the axial plane parallel to the anterior-posterior commissure (AC-PC) line using an EPI gradient echo sequence: TR = 2000 ms; TE = 25 ms; voxel size = 3 × 3 × 4 mm, 34 slices; flip angle = 60°; matrix size = 642; FOV = 2202 mm.
Preprocessing and functional imaging statistical analyses were conducted using the fMRI of the Brain (FMRIB) Software Library (FSL Version 4.1.6; FMRIB, Oxford, United Kingdom) (Smith et al. 2004; Woolrich et al. 2009). Functional data were temporally realigned to correct for interleaved slice acquisition. Motion was corrected using FSL MCFLIRT linear realignment tool (Jenkinson et al. 2002). Eight children with aggressive behavior and one typically developing child were excluded from the final analysis owing to excessive motion (i.e., >3 mm/degrees of motion relative to the first undiscarded volume) and computer error during the scan, respectively. Data were spatially smoothed with a 5-mm full-width at half-maximum isotropic Gaussian kernel with a nonlinear high-pass filter (60-s cutoff). Individual participant analyses were conducted using FSL FMRI Expert Analysis Tool (FEAT). Functional images were registered to coplanar images, which were then registered to the high-resolution T1-weighted images and normalized to the Montreal Neurological Institute 152 template.
Data were corrected for structured noise associated with motion artifacts using the AROMA package (Pruim et al. 2015) (https://github.com/rhr-pruim/ICA-AROMA). AROMA is an independent component analysis (ICA)-based method that automatically classifies and removes components identified as noise. ICA-AROMA is a robust approach for denoising and removing motion artifact in pediatric fMRI data (Ciric et al. 2017; Gabard-Durnam et al. 2018) and preserving temporal degrees of freedom while eliminating distance-dependent artifact without inducing false anticorrelations (Pruim et al. 2015; Ciric et al. 2017). Noise components were detected and removed prior to any temporal filtering, so the resulting cleaned data were temporally high-pass filtered. At the single-subject level, we also regressed the white-matter time series, which was included as a confound variable in first-level FEAT analyses. No between-group differences were observed in mean head motion (Supplementary Table 1). Additional detail regarding data acquisition and preprocessing is also provided in the Supplementary Methods.
Psychophysiological Interaction Analysis
Categorical Analyses
We first examined differences in amygdala–prefrontal connectivity between the aggressive behavior and the HC groups using a PPI analysis (Friston et al. 1997). Whole-brain PPI tests were conducted across fearful and calm faces. Amygdala ROIs were anatomically defined using the Harvard-Oxford atlas, with a threshold set at ≥25% probability. A general linear model was created that included the following regressors: psychological (task), physiological (amygdala ROI time series), PPI, and nuisance (six motion parameters). We did not have a priori hemispheric hypotheses and therefore analyses were conducted for both the right and the left amygdala. Group-level analyses applied a cluster-forming threshold of Z > 3.1 (corresponding to P < 0.001) and a whole-brain correction at P < 0.05 family-wise error rate-corrected using random field theory. Additional detail of the PPI analysis is provided in the Supplementary Material. Age and IQ were included as covariates in all models. We also investigated age-related differences between the groups (group × age interaction).
Dimensional Analyses
Next, we conducted dimensional PPI analyses in the aggressive behavior group (n = 101) to assess whether the association between amygdala connectivity and aggression severity is moderated by severity of social impairments. Severity of aggressive behavior (using the CBCL Aggressive Behavior score) and social impairments (using the SRS-2 SCI score) were modeled as continuous variables to maximize statistical power, with the interaction of CBCL Aggressive Behavior total score × SRS-2 SCI total score. Whole-brain PPI tests were conducted across all emotions and the regression models included IQ and age as covariates. In addition, consistent with prior dimensional fMRI work in children with conduct problems (Lozier et al. 2014; Ibrahim et al. 2019; Ibrahim, Kalvin, et al. 2021), level of CU traits was included as a covariate to identify the unique contribution of social impairment to predicting amygdala connectivity in children with aggression. To visualize interactions, beta coefficient values averaged from each cluster were extracted for each subject using FSL Featquery and compared across groups in post hoc analyses for ventral PFC regions that emerged as significant and were plotted based on a median split in SRS-2 SCI scores. That is, for hypothesis testing, social impairment was treated as a continuous measure in dimensional PPI analyses. However, when significant associations with amygdala connectivity and aggression were observed, we used a post hoc comparison median split of SRS-2 SCI to illustrate social impairment moderation of amygdala–prefrontal connectivity differences among children with aggression (in the aggression group: n = 49 for low social impairment and n = 52 for high social impairment). We also investigated interactions with age, social impairment severity, and aggression severity (SRS-2 SCI × CBCL Aggressive Behavior × age interactions).
Amygdala ROI Analyses
To test amygdala reactivity to threat or fearful faces as a potential neurobiological marker of aggressive behavior, as well as moderation by social impairments, we conducted a ROI analysis of amygdala reactivity. Featquery was used to extract beta coefficients of the mean parameter estimate values for the contrast fearful versus calm using bilateral amygdala anatomical ROI masks (described above). The regression models included severity of aggression (using the CBCL Aggressive Behavior score) and social impairments (using the SRS-2 SCI score) modeled as continuous variables, the interaction of CBCL Aggressive Behavior × SRS-2 SCI, and amygdala reactivity to fearful faces as the dependent variable. Regression models included ICU scores, age, and IQ as covariates. Results are plotted according to a median split in SRS-2 SCI scores for visualization.
Exploratory Analysis of Whole-Brain Task Activation
For completeness, we conducted an exploratory whole-brain analysis to test between group differences in task activation during implicit emotion processing for regions not included in our a priori hypothesis. IQ and age were included as covariates. We also tested for age-related differences between the groups (group × age interaction). The identical statistical thresholds were used as mentioned above for the PPI analysis. We provide these findings in detail in the Supplementary Methods and Supplementary Results (Supplementary Table 7 and Supplementary Fig. 3) for the interested reader.
Follow-up Analyses
Potential Covariates
Additional analyses were conducted to test the robustness of significant effects. First, sensitivity analyses were conducted that accounted for CU traits using the ICU total score as well as co-occurring anxiety disorder diagnosis (0 = no, 1 = yes; based on the K-SADS diagnostic interview) and psychotropic medication use (0 = no, 1 = yes) included as covariates in the models. Sensitivity analyses were conducted by repeating the PPI models that emerged as significant for categorical analyses and dimensional analyses but with CU traits (ICU total score), anxiety disorder diagnosis, and psychotropic medication use included as covariates in the models. To account for co-occurring anxiety, we included anxiety diagnosis as a dichotomous variable. Participants with co-occurring disruptive behavior disorder and anxiety disorder (n = 26) met DSM-5 criteria based on a diagnostic interview using the K-SADS for a current comorbid diagnosis of generalized anxiety disorder, social anxiety disorder, separation anxiety disorder, or specific phobia. Anxiety disorder based on DSM-5 diagnosis as a dichotomous variable was used to account for the presence of comorbid anxiety in our transdiagnostic sample (Bubier and Drabick 2009; Dugré et al. 2020; Isdahl-Troye et al. 2021).
Psychotropic Medication Use
Given that psychotropic medications may influence functional brain connectivity (Linke et al. 2017), we examined the effects of medication status in a separate post hoc analysis (beyond using medication status as a covariate) on main connectivity findings. Specifically, we tested whether the main findings retained significance after excluding participants taking psychotropic medications and whether there were connectivity differences between medicated and non-medicated children in the aggression group.
Construct Specificity
Next, to test the construct specificity of prefrontal regions emerging as significant in PPI and ROI analyses in predicting aggression severity, we examined whether amygdala connectivity and reactivity were associated with social impairment and internalizing problems using a standardized continuous measure (CBCL Internalizing Problems × SRS-2 SCI interaction). Post hoc tests were also conducted to assess the robustness of findings using gender as a covariate (males = 0, females = 1) and in a gender-matched sample.
Results
Amygdala Functional Connectivity: Categorical Analyses
First, we examined if there were differential patterns of amygdala functional connectivity between children with aggressive behavior compared with healthy controls. Children with aggressive behavior showed reduced connectivity between the right amygdala and right dlPFC (center of mass: 26.4, 28.1, and 42.4) relative to healthy controls during implicit emotion processing (Fig. 1A). Children with aggressive behavior also showed hyperconnectivity between the right amygdala and two clusters in the left visual association cortex (center of mass: −41.7, −69.9, 9.1 and −19.3, −46.3, −1) compared with healthy controls (Fig. 1B). Peak coordinates are reported in Supplementary Table 3. Sensitivity analyses were conducted by repeating the main analysis and including potential covariates, which revealed a highly similar pattern of results after accounting for CU traits (using the ICU total score), as well as for anxiety disorder diagnosis and psychotropic medication use (as dichotomously coded variables) (all Ps < 0.03) (see Supplementary Results: Sensitivity Analyses). No significant group differences were found for left amygdala connectivity. There were also no significant interactions observed between group and age for either left or right amygdala connectivity.
Figure 1.
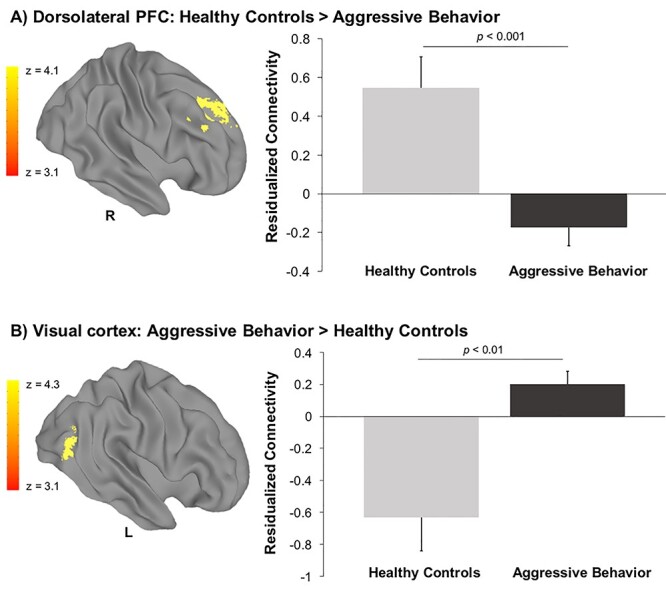
Reduced amygdala–prefrontal connectivity in children with aggressive behavior. Decreased levels of connectivity were observed between the right amygdala and the right dorsolateral prefrontal cortex (PFC) in children with aggressive behavior (n = 101) compared with healthy controls (n = 32) during implicit emotion processing (A). Hyperconnectivity between the right amygdala and the left visual association cortex was observed for children with aggressive behavior compared with healthy controls (B). Standard error is represented in bar graph error bars. The y-axis shows residualized connectivity strength with age and IQ partialled out.
Exploratory analyses were also conducted to examine connectivity differences in subgroups of children with aggression and low versus high social impairment that were formed based on a median split of a T-score of 65 on SRS-2 SCI subscale (Supplementary Material). We reasoned that this could inform future work in understanding categorical versus RDoC dimensional approaches in characterizing psychopathology, particularly childhood aggression (Parkes et al. 2020). Results of these analyses are provided in the Supplementary Results for the interested reader.
Amygdala Functional Connectivity: Dimensional Analyses
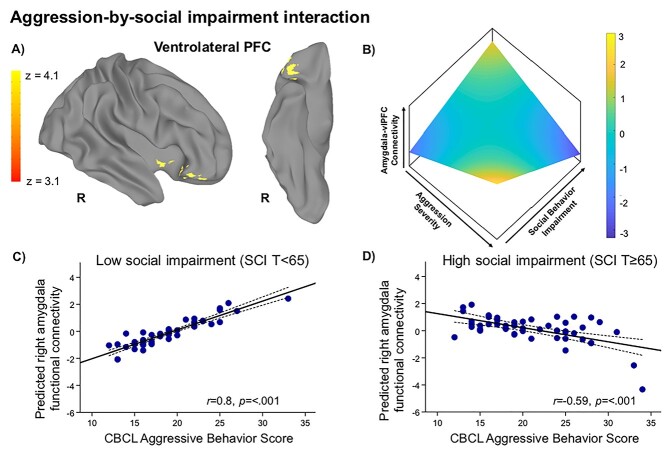
Next, we examined the relationship between amygdala connectivity, aggression severity, and social impairment modeled dimensionally. In the aggressive behavior group (n = 101), there was a CBCL Aggressive Behavior × SRS-2 SCI interaction for right amygdala connectivity with a cluster in the right vlPFC (center of mass: 43.1, 27.3, −11.6) during implicit emotion processing (Fig. 2). Specifically, children with higher severity of social impairment showed reductions in right amygdala–vlPFC connectivity with increasing severity of aggression, while children with low severity of social impairment showed the opposite pattern. Peak coordinates are reported in Supplementary Table 4. Sensitivity analyses conducted by repeating the main analysis and controlling for CU traits (ICU total score) as well as co-occurring anxiety disorder diagnosis and medication use (as dichotomous variables) did not alter these findings (P < 0.01) (see Supplementary Results: Sensitivity Analyses). No significant associations between social impairment and aggression severity were found for left amygdala connectivity. There were also no significant two-way or three-way interactions observed between social impairment severity, aggressive behavior severity, and age for either left or right amygdala connectivity. We also repeated this analysis in the total sample (N = 133), which revealed a similar pattern of amygdala–PFC connectivity, and report findings in Supplementary Table 9 and Supplementary Figure 5 for the interested reader.
Figure 2.
Associations between amygdala–prefrontal connectivity and severity of aggression is modulated by social impairment in youth. In dimensional analyses restricted to the aggressive behavior group (n = 101), increasing severity of aggressive behavior and social impairment were associated with reduced connectivity between the right amygdala and the right ventrolateral prefrontal cortex (vlPFC) during implicit emotion processing after accounting for the covariance with CU traits (A). The CBCL Aggressive Behavior Scale score was used as a continuous measure of severity of aggressive behavior and the Social Responsiveness Scale—Second Edition (SRS-2) Social Communication and Interaction (SCI) score was used as a continuous measure of severity of social impairments. A 3D representation is shown for the relationships driving this interaction between the two behavioral dimensions—aggression severity (x-axis) and social impairment severity (y-axis)—and the residuals of the dependent variable amygdala–vlPFC connectivity (z-axis), after adjusting for age, IQ, and CU traits (B). To visualize the interaction effects, mean extracted values for the significant vlPFC cluster are plotted separately by severity of social impairment using a median split T-score of 65 on the SRS-2 SCI subscale to form low social impairment (n = 49) (C) and high social impairment (n = 52) (D) subgroups. The y-axis represents predicted connectivity strength with the effects of age, IQ, and CU traits partialled out.
Amygdala Activation: ROI Analyses
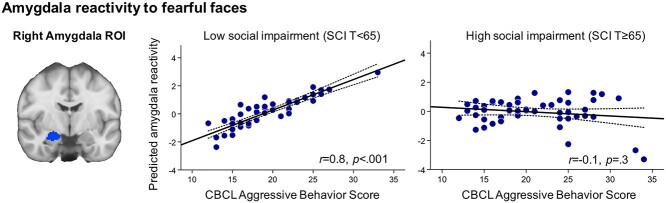
We then examined whether amygdala reactivity to threat or fear was modulated by social impairment in children with aggression. In the aggressive behavior group (n = 101), regression analyses revealed a significant CBCL Aggressive Behavior × SRS-2 SCI interaction for right amygdala reactivity to fearful versus calm faces after accounting for age, IQ, and CU traits (β = −1.1, t = −2.1, P = 0.04; Fig. 3). Specifically, in children with low severity of social impairment, right amygdala reactivity to fear was positively associated with aggression severity, but no significant association was found for children with high severity of social impairment. Sensitivity analyses in which the main analyses were repeated controlling for co-occurring anxiety disorder diagnosis and medication use (as dichotomous variables) did not alter these findings, and these variables did not make significant independent contributions to the variance in amygdala reactivity to fearful faces (P = 0.5 and P = 0.8 for anxiety and medication, respectively). There was no significant CBCL Aggressive Behavior × SRS-2 SCI interaction for left amygdala reactivity to fear (P = 0.13). We also repeated this analysis in the total sample (N = 133) and report findings in the Supplementary Results. Mean beta coefficients for the bilateral amygdala ROIs are also show in Supplementary Figure 6.
Figure 3.
Amygdala reactivity to fearful faces is associated with severity of aggressive behavior in youth. In the aggressive behavior group (n = 101), results of regression analyses revealed a significant CBCL Aggressive Behavior × SRS-2 SCI interaction for right amygdala reactivity to fearful versus calm faces. That is, right amygdala reactivity to fearful faces was associated with severity of aggressive behavior for children with aggression without social deficits (β = −1.1, t = −2.1, P = 0.04) but not for children with aggression with social deficits (P > 0.1) after controlling for age, IQ, and CU traits. The x-axis shows CBCL Aggressive Behavior Scale scores, which was used as a continuous measure of severity of aggression. The y-axis shows residualized amygdala reactivity with age, IQ, and CU traits partialled out. The left panel shows the right amygdala region-of-interest, which was structurally defined using the Harvard-Oxford atlas in FSL. For visualization, scatterplots display a median split using a T-score of 65 on the SRS-2 SCI subscale.
Follow-up Analyses
Psychotropic Medication Use
As a check on our connectivity findings, we found that after excluding subjects taking psychotropic medications, connectivity findings remained significant for both the right amygdala–dlPFC cluster (in analyses comparing the group of 54 children with aggressive behavior who were not taking medication to 32 healthy controls) and the right amygdala–vlPFC cluster (in dimensional analyses conducted in the group of 54 children with aggressive behavior who were not taking medication) (see Supplementary Fig. 4 and Supplementary Table 8).
Next, we tested for any amygdala connectivity differences between medicated and non-medicated subjects in the aggressive behavior group (47 taking psychotropic medications and 54 not taking psychotropic medications). There were no significant differences in connectivity observed between medicated and non-medicated subjects (n = 47 taking psychotropic medications; and n = 54 non-medicated) for either right or left amygdala connectivity (i.e. null maps). There were also no significant differences in task activation between medicated and non-medicated subjects (i.e. null maps) in the aggressive behavior group.
Construct Specificity
We then further assessed the construct specificity of the association between aggression severity, social impairment, and amygdala connectivity and reactivity. We tested whether regions emerging as significant in dimensional analyses (i.e. right vlPFC in Fig. 2; right amygdala in Fig. 3) predicted a CBCL Internalizing Problems × SRS-2 SCI interaction. For dimensional analyses in the aggression group (n = 101), even when social impairment moderated the association between right amygdala–vlPFC connectivity and aggression (Fig. 2), amygdala connectivity to this vlPFC region did not predict a CBCL Internalizing Problems × SRS-2 SCI interaction (i.e. null maps). For ROI analyses in the aggression group (Fig. 3), right amygdala reactivity did not predict a CBCL Internalizing Problems × SRS-2 SCI interaction (β = −0.56, t = −1.4, P = 0.14).
We also tested whether findings for amygdala connectivity and reactivity remained significant after accounting for gender as a dichotomous variable (0 = male, 1 = female). The pattern of findings did not change when including gender as a covariate for all analyses: amygdala–dlPFC cluster in categorical analyses (center of mass: 26.4, 28, 42.4, z = 4.2, P = 1.55E-08), amygdala–vlPFC cluster in dimensional analyses in the aggression group (center of mass: 44.9, 33.5, −12.8, z = 3.5, P = 0.004), or right amygdala reactivity in ROI analyses in the aggression group (β = −1.1, t = −2.0, P = 0.04).
As a more robust check on these main findings for potential effects of gender, analyses were then repeated in a gender-matched subgroup of children with aggression (n = 59). Gender-matched analyses resulted in a pattern of findings that were highly similar to those reported above for right amygdala–dlPFC connectivity (center of mass: 28.2, 26, 43) in categorical analyses as well as right amygdala–vlPFC connectivity (center of mass: 41.8, 21.6, −12.2) and right amygdala reactivity (β = −1.4, t = −2.3, P = 0.02) for the CBCL Aggressive Behavior × SRS-2 SCI interactions in the aggression group. Significant PPI clusters are reported in the Supplementary Results (Supplementary Table 5).
Follow-up Analyses for Sex Differences in Amygdala Connectivity
To facilitate comparisons with prior research examining sex differences in brain structure in children with aggressive behavior (Smaragdi et al. 2017; Ibrahim, Kalvin, et al. 2021), PPI analyses of amygdala connectivity were also conducted to test for the interaction between sex and group (healthy controls, aggressive behavior). A sex × group interaction was observed for connectivity between the left amygdala and the bilateral supramarginal gyrus (right hemisphere center of mass: 50.1, −47.4, 44.8, z = 4.7, P = 0.0007; left hemisphere center of mass: −53.2, −45.4, 46.5, z = 3.9, P = 0.002); that is, boys with aggression showed decreased amygdala–supramarginal gyrus connectivity, but girls with aggression showed the opposite pattern relative to respective control groups. We describe these findings in detail in the Supplementary Results, Supplementary Table 6, and Supplementary Figure 2 for the interested reader.
Discussion
This study examined interactions among aggression, social impairment, and amygdala–prefrontal connectivity in a transdiagnostic sample of children with aggressive behavior. Three key findings emerged. First, during implicit emotion processing, children with aggressive behavior showed reduced amygdala–PFC connectivity. Specifically, decreased amygdala–dlPFC connectivity was observed in children with aggressive behavior during implicit emotion processing compared with controls. Second, amygdala–prefrontal connectivity varied by both severity of aggression and social impairment in children with aggressive behavior. That is, during the processing of emotional faces, children with lower severity of social impairment showed a positive correlation between right amygdala–vlPFC connectivity and severity of aggression, while children with high severity of social impairments showed a negative correlation between right amygdala–vlPFC connectivity and severity of aggression. Third, the association between amygdala reactivity to fearful faces and severity of aggression was moderated by the severity of social impairment in children with aggressive behavior. Follow-up tests revealed that this association was present in children with aggressive behavior without social impairments, but not in children with both aggressive behavior and social impairment. These networks and regions (i.e., amygdala–dlPFC, amygdala–vlPFC, and right amygdala reactivity) also emerged as consistently robust in sensitivity analyses despite co-occurring internalizing symptoms, gender, and psychotropic medication use. Consistent with prior work, these findings demonstrate disruptions in amygdala reactivity and connectivity in youth with aggressive behavior (Lozier et al. 2014; Aghajani et al. 2017; Cardinale et al. 2018; Ibrahim et al. 2019). This study also demonstrates for the first time that the association of aggression with amygdala–prefrontal connectivity and amygdala reactivity is moderated by social cognitive deficits. These findings lend neurobiological support to the social information processing model of aggression—which posits that deficits in the detection and interpretation of social cues, as well as in the generation and enactment of socially appropriate responses can lead to maladaptive aggression (Crick and Dodge 1994)—in the context of aberrant frontolimbic coupling that is moderated by social deficits in children with aggression.
Consistent with prior research, children with aggressive behavior relative to unaffected controls evidenced reduced amygdala–dlPFC connectivity during the processing of emotional faces (Marsh et al. 2008; Ibrahim et al. 2019). Thus, reduced amygdala–prefrontal connectivity represents one of the few consistent and replicable findings in research on aggressive behavior using face emotion processing tasks. A recent study of pediatric irritability, a construct similar to maladaptive aggression, also reported reduced connectivity between the right amygdala and left dlPFC when processing fearful faces (Kryza-Lacombe et al. 2019). Despite the laterality differences observed in amygdala–dlPFC connectivity between the current study and Kryza-Lacombe et al. (2019) (left vs. right dlPFC, respectively), the connectivity findings were similar and in comparable middle/superior frontal cortex regions spanning the dlPFC. The dorsal and ventral PFC primarily connects to parietal and limbic regions, such as the amygdala, forming a frontoparietal and frontolimbic network that is tightly coupled with the cognitive control of emotion (Etkin et al. 2011). Therefore, projections between the amygdala and the dorsal and ventral PFC are critical in dampening the experience and expression of negatively valenced emotions (Milad and Quirk 2002; Silvers et al. 2016). In addition, amygdala–dlPFC connectivity represents a specific sub-network implicated in emotion regulation (e.g. reappraisal), which involves a conscious effort or the active monitoring of emotion, and thus self-awareness. Our findings show group differences in amygdala–dlPFC connectivity in a transdiagnostic sample of children with aggression relative to children without aggression. Exploratory analyses suggested that there were no differences in amygdala–dlPFC connectivity between subgroups of aggressive children with and without social deficits. Furthermore, recent meta-analytic work also implicates aberrant right dlPFC activation in aggression, particularly related to emotion/threat processing and social cognitive processes (Dugré et al. 2020). Thus, reduced amygdala–dlPFC connectivity may index a transdiagnostic vulnerability for maladaptive aggression regardless of the presence of social deficits.
This study is the first to show that severity of social impairment moderates the association between amygdala–prefrontal connectivity and aggressive behavior. Specifically, in children with aggression with social impairment, greater severity of aggression was associated with reduced amygdala–vlPFC connectivity. However, children with aggression without social impairment showed the opposite pattern (i.e. greater severity of aggression was associated with greater amygdala–vlPFC connectivity). In addition, social deficits indexed by the SRS-2 SCI moderated the association of amygdala–vlPFC connectivity with aggression severity even after controlling for CU traits. The vlPFC is involved in social perception and emotion processing, including higher-order social cognition referred to as theory of mind (Fusar-Poli et al. 2009; Dal Monte et al. 2014). Behavioral studies show that impairments in emotion recognition and theory of mind are associated with aggressive behavior in children (Capage and Watson 2001; Mandy et al. 2013). Reduced vlPFC and prefrontal activation during social perception tasks is also reported in youth with conduct problems relative to controls (Sebastian et al. 2012; Alegria et al. 2016). We speculate that in aggressive children without social impairment, heightened amygdala–vlPFC connectivity may subserve social processing distortions, such as hostile attribution bias or rumination on slights and snubs. In other words, there may be recruitment of social cognitive circuitry for the generation of hostile or maladaptive thoughts, which, in turn, can contribute to aggressive actions. In contrast, in children with aggressive behavior and social impairment, there was a significant negative association between amygdala–vlPFC connectivity and aggression severity. We argue that attenuated amygdala–vlPFC connectivity in this high social impairment subgroup may represent a deficit, or lack of social cognitive skills rather than a distortion of social processing. In other words, it is possible that children in this high social impairment subgroup may not be “registering” the social context when they react aggressively to frustration or provocation. In support of this, Dugré et al. (2020) reported disruptions in lateral PFC regions, including the right vlPFC, during social cognitive and cognitive control processes in individuals with aggression. When dimensional analyses were repeated in the total sample (N = 133) that combined children in the aggression group and HC children, we found a highly similar pattern in which social impairment moderated the association between aggressive behavior and amygdala–PFC connectivity (that included the dorsomedial PFC and orbitofrontal cortex/vlPFC regions). There were differences with laterality in which left and right amygdala–PFC connectivity emerged as significant in the total sample and aggression group, respectively. These laterality differences may be attributed to the distribution of aggressive behavior and social impairment severity scores when combining samples of healthy controls and children with high levels of aggressive behavior. In addition, amygdala lateralization remains poorly understood despite some evidence to suggest that the right amygdala may be implicated in emotionally arousing stimuli while the left amygdala may be implicated in emotion processing involving cognitive and perceptual processes (Wright et al. 2001; Gläscher and Adolphs 2003). Importantly, similar key regions of the PFC (i.e., dorsomedial PFC and orbitofrontal cortex/vlPFC) involved in emotion regulation and in conduct problems in youths (Alegria et al. 2016) as well as adults (Deming and Koenigs 2020; Dugré and Potvin 2021) emerged as significant in both models, which emphasizes the relationship between aggression severity and amygdala–PFC connectivity in heterogeneous as well as clinical samples. However, caution is warranted when interpreting these findings that were based on moderation analyses of social impairment. Future work is needed to examine mediation models as well as longitudinal studies to test a causal link between social information processing, disrupted frontolimbic connectivity, and increased aggression in youths.
Amygdala ROI analyses of task activation revealed that right amygdala reactivity to threat or fearful faces was positively associated with aggression severity in children with aggressive behavior without social deficits, but not in children with aggressive behavior with social deficits after accounting for CU traits. Thus, the presence of social deficits measured by the SRS-2 SCI dampened amygdala reactivity to fearful faces. In categorical analyses, no significant between group differences were found for amygdala activation (Supplementary Fig. 4). These findings are consistent with prior studies of youth with conduct problems versus healthy controls (Lozier et al. 2014) and a related construct of irritability (Kryza-Lacombe et al. 2019). In addition, aberrant amygdala activation, particularly in the right hemisphere, has been implicated in conduct problems in children (Sebastian et al. 2012; Lozier et al. 2014; Ibrahim et al. 2019) and adults (Poeppl et al. 2019). Here, we also showed that this association between amygdala reactivity and aggression was only significant in aggressive children without social deficits, which could be important for identifying subgroups of children with aggression likely to respond to a particular treatment approach. Amygdala reactivity may also play a role in shaping connectivity between the amygdala and the PFC. For instance, individual differences in amygdala reactivity may influence the maturation of connectivity patterns or reciprocal functional connections between the amygdala and other regions important for cognitive control, potentially placing a child at greater or lesser risk for aggression psychopathology. Longitudinal studies of frontolimbic connectivity during the key periods of socioemotional development are needed to examine how changes in amygdala–PFC connectivity may affect developmental trajectories of child aggressive behavior.
Study Limitations
First, the cross-sectional design of this study was a fundamental limitation, although findings may guide future longitudinal research. Second, the amygdala has distinct subregions, including the basolateral, superficial, and centromedial regions, with structurally and functionally distinct subnuclei that have different patterns of connectivity with prefrontal networks (Aghajani et al. 2017). Investigation of amygdala subregional connectivity was not part of our initial hypotheses and beyond the scope of the current study. However, future work is needed to understand whether differential connectivity with amygdala subregions is associated with severity of aggression and social impairments. Third, this study consisted of predominately boys, and it will be important to investigate sex differences in the functional neural architecture of aggression. However, it is important to note that the ratio of males to females in the current study is similar to reported estimates of male to female ratios for children with disruptive behavior disorders (2–3:1) (Wittchen et al. 2011; Erskine et al. 2013; Demmer et al. 2017). This also illustrates a common challenge in recruiting and scanning a sufficiently sized sample of girls with clinically significant levels of aggression or disruptive behavior disorders, particularly to provide the necessary statistical power to examine sex differences in brain connectivity. Future studies that leverage large-scale data sets will also provide an ideal opportunity to clarify associations among sex, network connectivity, and aggressive behavior in youths. Fourth, because of the task acquisition length, examining connectivity for emotion contrasts (e.g., fearful vs. calm) would have resulted in an exceedingly short time course for robust connectivity analyses. Thus, it will be important for future studies of aggression in youths to acquire an fMRI acquisition length sufficient for examining network connectivity across and between specific emotion domains. Lastly, because the current study focused on aggression measured by the CBCL Aggressive Behavior subscale, which reflects mostly the reactive aggression construct and irritability/anger, the results of this study may not generalize to youths with more proactive forms of aggressive behavior or serious conduct problems. Given that some studies have suggested distinct neural correlates of reactive and proactive forms of aggression (Naaijen et al. 2020; Werhahn et al. 2020), future studies will be important to elucidate shared and unique patterns of network connectivity implicated in subtypes of aggression during implicit emotion processing. It will also be important to understand the difference in neural mechanisms of behavioral difficulties, such as anger outbursts and reactive aggression, in children seeking outpatient treatment for these behaviors versus neural mechanisms of severe conduct disorder or juvenile delinquency as seen in forensic samples.
Conclusion
Children with aggressive behavior showed reduced amygdala–dlPFC connectivity relative to unaffected controls. Social deficits moderated the association between aggression severity and amygdala–vlPFC connectivity during implicit emotion processing. In addition, increased amygdala reactivity to fearful faces was observed in children with aggression without social deficits, but not in children with aggression with social deficits. These results have implications for neurobiologically defined subgroups of children with aggressive behavior.
Supplementary Material
Contributor Information
Karim Ibrahim, Child Study Center, Yale University School of Medicine, New Haven, CT 06520, USA.
Carla Kalvin, Child Study Center, Yale University School of Medicine, New Haven, CT 06520, USA.
Simon Morand-Beaulieu, Child Study Center, Yale University School of Medicine, New Haven, CT 06520, USA.
George He, Department of Psychology, Yale University, New Haven, CT 06520, USA.
Kevin A Pelphrey, Department of Neurology, University of Virginia, Charlottesville, VA 22903, USA.
Gregory McCarthy, Department of Psychology, Yale University, New Haven, CT 06520, USA.
Denis G Sukhodolsky, Child Study Center, Yale University School of Medicine, New Haven, CT 06520, USA.
Data Availability
To promote data transparency, anonymized data will be available upon reasonable request. Data from the study reported in this paper has also been shared on the National Institute of Mental Health Data Archive (NDA; https://nda.nih.gov/).
Funding
National Institute of Mental Health (R01MH101514 to D.G.S. and K.A.P.); National Center for Advancing Translational Sciences (KL2 TR001862, TL1 TR001864 to K.I.); Translational Developmental Neuroscience Training Program (T32 MH18268 to K.I. and C.K.) directed by Dr Michael Crowley.
Notes
We thank Ms Sonia Rowley and Ms Julia Zhong at the Yale Child Study Center for their assistance with reviewing the final version of the manuscript, Ms Yasmeen Alawadhi for her assistance with formatting Supplementary Figure 5, Mr Jeffrey A. Eilbott and Mr Fangyong Li for their assistance in data analysis, Dr Megan Tudor for subject characterization assessments, and Ms Emilie Bertschinger, Ms Tess Gladstone and Ms Carolyn Marsh for study coordination. Conflict of Interest: Dr Sukhodolsky receives royalties from Guilford Press for a treatment manual on CBT for anger and aggression in children. Drs Ibrahim, Kalvin, Morand-Beaulieu, He, Pelphrey, and McCarthy report no competing interests.
References
- Abram KM, Zwecker NA, Welty LJ, Hershfield JA, Dulcan MK, Teplin LA. 2015. Comorbidity and continuity of psychiatric disorders in youth after detention: a prospective longitudinal study. JAMA Psychiat. 72:84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achenbach TM, Rescorla LA. 2001. Manual for the ASEBA school—age forms & profiles. Burlington (VT): University of Vermont, Research Center for Children, Youth, and Families. [Google Scholar]
- Aghajani M, Klapwijk ET, van der Wee NJ, Veer IM, Rombouts SA, Boon AE, van Beelen P, Popma A, Vermeiren RR, Colins OF. 2017. Disorganized amygdala networks in conduct-disordered juvenile offenders with callous-unemotional traits. Biol Psychiatry. 82:283–293. [DOI] [PubMed] [Google Scholar]
- Alegria AA, Radua J, Rubia K. 2016. Meta-analysis of fMRI studies of disruptive behavior disorders. Am J Psychiatry. 173:1119–1130. [DOI] [PubMed] [Google Scholar]
- Baribeau DA, Dupuis A, Paton TA, Hammill C, Scherer SW, Schachar RJ, Arnold PD, Szatmari P, Nicolson R, Georgiades S. 2019. Structural neuroimaging correlates of social deficits are similar in autism spectrum disorder and attention-deficit/hyperactivity disorder: analysis from the POND network. Transl Psychiatry. 9:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubier JL, Drabick DA. 2009. Co-occurring anxiety and disruptive behavior disorders: the roles of anxious symptoms, reactive aggression, and shared risk processes. Clin Psychol Rev. 29:658–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JD, Rowe R, Boylan K. 2014. Functional outcomes of child and adolescent oppositional defiant disorder symptoms in young adult men. J Child Psychol Psychiatry. 55:264–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capage L, Watson AC. 2001. Individual differences in theory of mind, aggressive behavior, and social skills in young children. Early Educ Dev. 12:613–628. [Google Scholar]
- Cardinale EM, O'Connell K, Robertson EL, Meena LB, Breeden AL, Lozier LM, VanMeter JW, Marsh AA. 2018. Callous and uncaring traits are associated with reductions in amygdala volume among youths with varying levels of conduct problems. Psychol Med. 49:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cholemkery H, Kitzerow J, Rohrmann S, Freitag CM. 2014. Validity of the social responsiveness scale to differentiate between autism spectrum disorders and disruptive behaviour disorders. Eur Child Adolesc Psychiatry. 23:81–93. [DOI] [PubMed] [Google Scholar]
- Ciric R, Wolf DH, Power JD, Roalf DR, Baum GL, Ruparel K, Shinohara RT, Elliott MA, Eickhoff SB, Davatzikos C. 2017. Benchmarking of participant-level confound regression strategies for the control of motion artifact in studies of functional connectivity. NeuroImage. 154:174–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson T, Kang E, Capriola-Hall N, Lerner MD, Jarcho J, Prinstein MJ. 2020. Meta-analysis of the RDoC social processing domain across units of analysis in children and adolescents. J Clin Child Adolesc Psychol. 49:297–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor DF, Newcorn JH, Saylor KE, Amann BH, Scahill L, Robb AS, Jensen PS, Vitiello B, Findling RL, Buitelaar JK. 2019. Maladaptive aggression: with a focus on impulsive aggression in children and adolescents. J Child Adolesc Psychopharmacol. 29:576–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN. 2005. Social responsiveness scale (SRS). Los Angeles: Western Psychological Services. [Google Scholar]
- Constantino JN, Todd RD. 2003. Autistic traits in the general population: a twin study. Arch Gen Psychiatry. 60:524–530. [DOI] [PubMed] [Google Scholar]
- Costello EJ, He J-P, Sampson NA, Kessler RC, Merikangas KR. 2014. Services for adolescents with psychiatric disorders: 12-month data from the National Comorbidity Survey—Adolescent. Psychiatr Serv. 65:359–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter J, Granger K, Backx R, Hobbs M, Looi CY, Barnett JH. 2018. Social cognitive dysfunction as a clinical marker: a systematic review of meta-analyses across 30 clinical conditions. Neurosci Biobehav Rev. 84:92–99. [DOI] [PubMed] [Google Scholar]
- Crick NR, Dodge KA. 1994. A review and reformulation of social information-processing mechanisms in children's social adjustment. Psychol Bull. 115:74. [Google Scholar]
- Dal Monte O, Schintu S, Pardini M, Berti A, Wassermann EM, Grafman J, Krueger F. 2014. The left inferior frontal gyrus is crucial for reading the mind in the eyes: brain lesion evidence. Cortex. 58:9–17. [DOI] [PubMed] [Google Scholar]
- Dawel A, O’Kearney R, McKone E, Palermo R. 2012. Not just fear and sadness: meta-analytic evidence of pervasive emotion recognition deficits for facial and vocal expressions in psychopathy. Neurosci Biobehav Rev. 36:2288–2304. [DOI] [PubMed] [Google Scholar]
- Del Casale A, Kotzalidis GD, Rapinesi C, Janiri D, Aragona M, Puzella A, Spinazzola E, Maggiora M, Giuseppin G, Tamorri SM. 2017. Neural functional correlates of empathic face processing. Neurosci Lett. 655:68–75. [DOI] [PubMed] [Google Scholar]
- Deming P, Koenigs M. 2020. Functional neural correlates of psychopathy: a meta-analysis of MRI data. Transl Psychiatry. 10:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmer DH, Hooley M, Sheen J, McGillivray JA, Lum JA. 2017. Sex differences in the prevalence of oppositional defiant disorder during middle childhood: a meta-analysis. J Abnorm Child Psychol. 45:313–325. [DOI] [PubMed] [Google Scholar]
- Dugré JR, Potvin S. 2021. Impaired attentional and socio-affective networks in subjects with antisocial behaviors: a meta-analysis of resting-state functional connectivity studies. Psychol Med. 51: 1–11. [DOI] [PubMed] [Google Scholar]
- Dugré JR, Dumais A, Dellazizzo L, Potvin S. 2020. Developmental joint trajectories of anxiety-depressive trait and trait-aggression: implications for co-occurrence of internalizing and externalizing problems. Psychol Med. 50:1338–1347. [DOI] [PubMed] [Google Scholar]
- Erskine HE, Ferrari AJ, Nelson P, Polanczyk GV, Flaxman AD, Vos T, Whiteford HA, Scott JG. 2013. Research review: epidemiological modelling of attention-deficit/hyperactivity disorder and conduct disorder for the global burden of disease study 2010. J Child Psychol Psychiatry. 54:1263–1274. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. 2011. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 15:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick PJ. 2003. The inventory of callous-unemotional traits. New Orleans: University of New Orleans. [Google Scholar]
- Friston K, Buechel C, Fink G, Morris J, Rolls E, Dolan R. 1997. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage. 6:218–229. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Landi P, Allen P, Surguladze S, Benedetti F, Abbamonte M, Gasparotti R, Barale F. 2009. Functional atlas of emotional faces processing: a voxel-based meta-analysis of 105 functional magnetic resonance imaging studies. J Psychiatry Neurosci. 34:418–432. [PMC free article] [PubMed] [Google Scholar]
- Gabard-Durnam LJ, Flannery J, Goff B, Gee DG, Humphreys KL, Telzer E, Hare T, Tottenham N. 2014. The development of human amygdala functional connectivity at rest from 4 to 23 years: a cross-sectional study. NeuroImage. 95:193–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabard-Durnam L, O’Muircheartaigh J, Dirks H, Dean D III, Tottenham N, Deoni S. 2018. Human amygdala functional network development: a cross-sectional study from 3 months to 5 years of age. Dev Cogn Neurosci. 34:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, Hare TA, Bookheimer SY, Tottenham N. 2013. A developmental shift from positive to negative connectivity in human amygdala–prefrontal circuitry. J Neurosci. 33:4584–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiou G, Kimonis ER, Fanti KA. 2019. What do others feel? Cognitive empathy deficits explain the association between callous-unemotional traits and conduct problems among preschool children. Eur J Dev Psychol. 16:633–653. [Google Scholar]
- Gläscher J, Adolphs R. 2003. Processing of the arousal of subliminal and supraliminal emotional stimuli by the human amygdala. J Neurosci. 23:10274–10282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happé F, Frith U. 1996. Theory of mind and social impairment in children with conduct disorder. Br J Dev Psychol. 14:385–398. [Google Scholar]
- Happé F, Frith U. 2014. Annual research review: towards a developmental neuroscience of atypical social cognition. J Child Psychol Psychiatry. 55:553–577. [DOI] [PubMed] [Google Scholar]
- Heleniak C, McLaughlin KA. 2020. Social-cognitive mechanisms in the cycle of violence: cognitive and affective theory of mind, and externalizing psychopathology in children and adolescents. Dev Psychopathol. 32:735–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herpertz SC, Huebner T, Marx I, Vloet TD, Fink GR, Stoecker T, Jon Shah N, Konrad K, Herpertz-Dahlmann B. 2008. Emotional processing in male adolescents with childhood-onset conduct disorder. J Child Psychol Psychiatry. 49:781–791. [DOI] [PubMed] [Google Scholar]
- Holl AK, Kirsch F, Rohlf H, Krahé B, Elsner B. 2018. Longitudinal reciprocity between theory of mind and aggression in middle childhood. Int J Behav Dev. 42:257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim K, Sukhodolsky DG. 2018. RDoC and autism. In: Volkmar F, editor. Encyclopedia of autism spectrum disorders. New York: Springer. [Google Scholar]
- Ibrahim K, Eilbott J, Ventola P, He G, Pelphrey KA, McCarthy G, Sukhodolsky DG. 2019. Reduced amygdala–prefrontal functional connectivity in children with autism spectrum disorder and co-occurring disruptive behavior. Biol Psychiatry Cogn Neurosci Neuroimaging. 4:1031–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim K, Kalvin C, Li F, He G, Pelphrey KA, McCarthy G, Sukhodolsky DG. 2021. Sex differences in medial prefrontal and parietal cortex structure in children with disruptive behavior. Dev Cogn Neurosci. 47:100884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim K, Noble S, He G, Lacadie C, Crowley M, McCarthy G, Scheinost D, Sukhodolsky D. 2021. Large-scale functional brain networks of maladaptive childhood aggression identified by connectome-based predictive modeling. Mol Psychiatry. 10.1038/s41380-021-01317-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isdahl-Troye A, Villar P, Domínguez-Álvarez B, Romero E, Deater-Deckard K. 2021. The development of co-Occurrent anxiety and externalizing problems from early childhood: a latent transition analysis approach. Res Child Adolesc Psychopathol. 1–15. 10.1007/s10802-021-00865-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. 2002. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 17:825–841. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher, B., Axelson, D., Perepletchikova, F., Brent, D., & Ryan, N. 2016. Schedule for affective disorders and schizophrenia for school aged children: present and lifetime version for DSM-5 (K-SADS-PL). Pittsburgh: Western Psychiatric Institute and Clinic https://www.pediatricbipolar.pitt.edu/resources/instruments.
- Kryza-Lacombe M, Iturri N, Monk CS, Wiggins JL. 2019. Face emotion processing in pediatric irritability: neural mechanisms in a sample enriched for irritability with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry. 59:1380–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake EM, Finn ES, Noble SM, Vanderwal T, Shen X, Rosenberg MD, Spann MN, Chun MM, Scheinost D, Constable RT. 2019. The functional brain organization of an individual allows prediction of measures of social abilities transdiagnostically in autism and attention-deficit/hyperactivity disorder. Biol Psychiatry. 86:315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Couteur A, Lord C, Rutter M. 2003. The autism diagnostic interview-revised (ADI-R). Los Angeles (CA): Western Psychological Services. [Google Scholar]
- Linke AC, Olson L, Gao Y, Fishman I, Müller R-A. 2017. Psychotropic medication use in autism spectrum disorders may affect functional brain connectivity. Biol Psychiatry: Cogn Neurosci Neuroimaging. 2:518–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood PL, Sebastian CL, McCrory EJ, Hyde ZH, Gu X, De Brito SA, Viding E. 2013. Association of callous traits with reduced neural response to others’ pain in children with conduct problems. Curr Biol. 23:901–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S. 2012. Autism diagnostic observation schedule—second edition (ADOS-2). Los Angeles (CA): Western Psychological Services. [Google Scholar]
- Lozier LM, Cardinale EM, VanMeter JW, Marsh AA. 2014. Mediation of the relationship between callous-unemotional traits and proactive aggression by amygdala response to fear among children with conduct problems. JAMA Psychiat. 71:627–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandy W, Skuse D, Steer C, St Pourcain B, Oliver BR. 2013. Oppositionality and socioemotional competence: interacting risk factors in the development of childhood conduct disorder symptoms. J Am Acad Child Adolesc Psychiatry. 52:718–727. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Mitchell DG, Reid ME, Sims C, Kosson DS, Towbin KE, Leibenluft E, Pine DS, Blair R. 2008. Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behavior disorders. Am J Psychiatry. 165:712–720. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. 2002. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 420:70–74. [DOI] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, Dolan RJ. 1996. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 383:812. [DOI] [PubMed] [Google Scholar]
- Moul C, Hawes DJ, Dadds MR. 2018. Mapping the developmental pathways of child conduct problems through the neurobiology of empathy. Neurosci Biobehav Rev. 91:34–50. [DOI] [PubMed] [Google Scholar]
- Naaijen J, Mulder LM, Ilbegi S, de Bruijn S, Kleine-Deters R, Dietrich A, Hoekstra PJ, Marsman J-BC, Aggensteiner PM, Holz NE. 2020. Specific cortical and subcortical alterations for reactive and proactive aggression in children and adolescents with disruptive behavior. Neuroimage: Clin. 27:102344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIMH . 2016. National advisory mental health council workgroup on tasks and measures for research domain criteria. Bethesda, MD: Behavioral Assessment Methods for RDoC Constructs. [Google Scholar]
- Oliver BR, Barker ED, Mandy WP, Skuse DH, Maughan B. 2011. Social cognition and conduct problems: a developmental approach. J Am Acad Child Adolesc Psychiatry. 50:385–394. [DOI] [PubMed] [Google Scholar]
- Orue I, Calvete E, Fernández-González L. 2019. Early maladaptive schemas and social information processing in child-to-parent aggression. J Interpers Violence. 36:6931–6955. [DOI] [PubMed] [Google Scholar]
- Pardini D, Frick PJ. 2013. Multiple developmental pathways to conduct disorder: current conceptualizations and clinical implications. J Can Acad Child Adolesc Psychiatry. 22:20. [PMC free article] [PubMed] [Google Scholar]
- Parkes L, Satterthwaite TD, Bassett DS. 2020. Towards precise resting-state fMRI biomarkers in psychiatry: synthesizing developments in transdiagnostic research, dimensional models of psychopathology, and normative neurodevelopment. Curr Opin Neurobiol. 65:120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelphrey KA, Morris JP, McCarthy G, Labar KS. 2007. Perception of dynamic changes in facial affect and identity in autism. Soc Cogn Affect Neurosci. 2:140–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeppl TB, Donges MR, Mokros A, Rupprecht R, Fox PT, Laird AR, Bzdok D, Langguth B, Eickhoff SB. 2019. A view behind the mask of sanity: meta-analysis of aberrant brain activity in psychopaths. Mol Psychiatry. 24:463–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruim RH, Mennes M, van Rooij D, Llera A, Buitelaar JK, Beckmann CF. 2015. ICA-AROMA: a robust ICA-based strategy for removing motion artifacts from fMRI data. NeuroImage. 112:267–277. [DOI] [PubMed] [Google Scholar]
- Ray RD, Wilhelm FH, Gross JJ. 2008. All in the mind's eye? Anger rumination and reappraisal. J Pers Soc Psychol. 94:133–145. [DOI] [PubMed] [Google Scholar]
- Sebastian CL, McCrory EJ, Cecil CA, Lockwood PL, De Brito SA, Fontaine NM, Viding E. 2012. Neural responses to affective and cognitive theory of mind in children with conduct problems and varying levels of callous-unemotional traits. Arch Gen Psychiatry. 69:814–822. [DOI] [PubMed] [Google Scholar]
- Sethi A, O'Nions E, McCrory E, Bird G, Viding E. 2018. An fMRI investigation of empathic processing in boys with conduct problems and varying levels of callous-unemotional traits. NeuroImage Clin. 18:298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvers JA, Insel C, Powers A, Franz P, Helion C, Martin RE, Weber J, Mischel W, Casey B, Ochsner KN. 2016. vlPFC–vmPFC–amygdala interactions underlie age-related differences in cognitive regulation of emotion. Cereb Cortex. 27:3502–3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smaragdi A, Cornwell H, Toschi N, Riccelli R, Gonzalez-Madruga K, Wells A, Clanton R, Baker R, Rogers J, Martin-Key N. 2017. Sex differences in the relationship between conduct disorder and cortical structure in adolescents. J Am Acad Child Adolesc Psychiatry. 56:703–712. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE. 2004. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 23:S208–S219. [DOI] [PubMed] [Google Scholar]
- Stoddard J, Tseng W-L, Kim P, Chen G, Yi J, Donahue L, Brotman MA, Towbin KE, Pine DS, Leibenluft E. 2017. Association of irritability and anxiety with the neural mechanisms of implicit face emotion processing in youths with psychopathology. JAMA Psychiat. 74:95–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhodolsky DG, Kassinove H, Gorman BS. 2004. Cognitive-behavioral therapy for anger in children and adolescents: a meta-analysis. Aggress Violent Behav. 9:247–269. [Google Scholar]
- Sukhodolsky DG, Smith SD, McCauley SA, Ibrahim K, Piasecka JB. 2016. Behavioral interventions for anger, irritability, and aggression in children and adolescents. J Child Adolesc Psychopharmacol. 26:58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhodolsky DG, Wyk BV, Eilbott JA, McCauley SA, Ibrahim K, Crowley MJ, Pelphrey KA. 2016. Neural mechanisms of cognitive-behavioral therapy for aggression in children and adolescents: design of a randomized controlled trial within the national institute for mental health research domain criteria construct of frustrative non-reward. J Child Adolesc Psychopharmacol. 26:38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey BJ, Nelson C. 2009. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res. 168:242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay RE, Vitaro F, Côté SM. 2018. Developmental origins of chronic physical aggression: a bio-psycho-social model for the next generation of preventive interventions. Ann Rev Psychol., 69:383–407. [DOI] [PubMed] [Google Scholar]
- Uljarević M, Frazier TW, Phillips JM, Jo B, Littlefield S, Hardan AY. 2019. Mapping the research domain criteria social processes constructs to the social responsiveness scale. J Am Acad Child Adolesc Psychiatry. 59:1252–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandewouw MM, Choi EJ, Hammill C, Lerch J, Anagnostou E, Taylor MJ. 2020. Changing faces: dynamic emotional face processing in autism spectrum disorder across child and adulthood. Biol Psychiat Cogn Neurosci Neuroimaging. 6:825–836. [DOI] [PubMed] [Google Scholar]
- Verhoef REJ, Alsem SC, Verhulp EE, De Castro BO. 2019. Hostile intent attribution and aggressive behavior in children revisited: a meta-analysis. Child Dev. 90:e525–e547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viding E, Sebastian CL, Dadds MR, Lockwood PL, Cecil CA, De Brito SA, McCrory EJ. 2012. Amygdala response to preattentive masked fear in children with conduct problems: the role of callous-unemotional traits. Am J Psychiatry. 169:1109–1116. [DOI] [PubMed] [Google Scholar]
- Vitiello B, Stoff DM. 1997. Subtypes of aggression and their relevance to child psychiatry. J Am Acad Child Adolesc Psychiatry. 36:307–315. [DOI] [PubMed] [Google Scholar]
- Wechsler D. 1997. WAIS-III administration and scoring manual. San Antonio (TX): The Psychological Corporation. [Google Scholar]
- Werhahn JE, Mohl S, Willinger D, Smigielski L, Roth A, Hofstetter C, Stämpfli P, Naaijen J, Mulder LM, Glennon JC. 2020. Aggression subtypes relate to distinct resting state functional connectivity in children and adolescents with disruptive behavior. Eur Child Adolesc Psychiatry. 30:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigham S, McConachie H, Tandos J, Le Couteur AS, team GMSc. 2012. The reliability and validity of the social responsiveness scale in a UK general child population. Res Dev Disabil. 33:944–950. [DOI] [PubMed] [Google Scholar]
- Wittchen H-U, Jacobi F, Rehm J, Gustavsson A, Svensson M, Jönsson B, Olesen J, Allgulander C, Alonso J, Faravelli C. 2011. The size and burden of mental disorders and other disorders of the brain in Europe 2010. Eur Neuropsychopharmacol. 21:655–679. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, Beckmann C, Jenkinson M, Smith SM. 2009. Bayesian analysis of neuroimaging data in FSL. NeuroImage. 45:S173–S186. [DOI] [PubMed] [Google Scholar]
- Wright CI, Fischer H, Whalen PJ, McInerney SC, Shin LM, Rauch SL. 2001. Differential prefrontal cortex and amygdala habituation to repeatedly presented emotional stimuli. Neuroreport. 12:379–383. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
To promote data transparency, anonymized data will be available upon reasonable request. Data from the study reported in this paper has also been shared on the National Institute of Mental Health Data Archive (NDA; https://nda.nih.gov/).