Abstract
Peripheral arterial disease (PAD) is common in elderly patients. Lower-extremity CT angiography (LE-CTA) can be useful for detecting PAD and planning its treatment. PAD can also be accurately evaluated on reconstructed monoenergetic images (MEIs) from low kiloelectron volt (keV) to high keV images using dual-energy CT. Low keV images generally provide higher contrast than high keV images but also feature more severe image noise. The noise-reduced virtual MEI reconstruction algorithm, called the Mono+ technique, was recently introduced to overcome such image noise. Therefore, this pictorial review aimed to present the imaging findings of PAD on LE-CTA and compare low and high keV images with those subjected to the Mono+ technique. We found that, in many cases, the overall and segmental image qualities were better and metal artifacts and venous contamination were decreased in the high keV images.
Keywords: Computed Tomography, X-Ray; Peripheral Arterial Disease; Lower Extremity
Abstract
말초동맥질환은 고령의 환자들에게서 흔하게 발생하며, 하지 동맥 단층촬영 혈관조영술은 말초동맥질환을 발견하고 치료 계획을 세우는데 유용하다. 특히, 이중에너지 단층 촬영을 통해 낮은 kiloelectron volt (이하 KeV) 영상부터 높은 KeV 영상까지 단일 에너지 영상을 재구성하면, 말초동맥질환을 정확하게 평가하는데 도움이 된다. 일반적으로 낮은 KeV 영상은 높은 대조도를 제공해 주지만, 낮은 KeV 영상은 높은 KeV 영상보다 더 심한 잡음을 제공한다는 단점도 있다. 최근에 낮은 KeV 영상에서 잡음을 극복하기 위해 Mono+ 기술이 도입되었다. 따라서, 본 임상 화보에서는 Mono+ 기법으로 시행한 하지동맥 단층촬영 혈관조영술에서의 말초동맥질환의 영상 소견을 보여주며 낮은 KeV 영상과 높은 KeV 영상의 특성이 어떻게 다른지 비교하여 보여주고자 한다. 많은 사례에서, 전체적인 영상의 질과 말초동맥질환을 평가하고자 하는 구간에서의 영상의 질은 모두 높은 KeV에서 더 좋았고, 금속 인공물과 정맥 오염은 높은 KeV 영상에서 감소했다.
INTRODUCTION
Peripheral arterial disease (PAD) is common in elderly patients and is one of the main causes of atherosclerotic cardiovascular morbidity (1). Lower extremity CT angiography (LE-CTA) can be useful for detecting PAD, evaluating the severity of atherosclerosis, and planning treatment (2,3). However, there are some limitations while evaluating PAD by LE-CTA, because most PAD patients are elderly with calcification in vessels or prostheses, which interfere with correct PAD evaluation.
Monoenergetic images (MEIs) from dual-energy CT can optimize the kiloelectron volt (keV) level to evaluate the objective and subjective image quality of vessels (4). Although low keV (40–50 keV) provides higher contrast than high keV (70–90 keV), its images have more severe noise than those obtained at high keV. Recently, a noise-reduced virtual MEI reconstruction algorithm (synonym Mono+) was developed to mitigate image noise at low energy levels (5). This algorithm can reduce the image noise at low keV and improve the image contrast at high keV.
Till now, Mono+ has not been used to optimize the keV level in LE-CTA to evaluate PAD. In this pictorial essay, we review various MEIs obtained from LE-CTA at different keV levels to evaluate PAD efficiently.
DECT
Dual energy CT (DECT) imaging permits a variety of image reconstructions that aid in pathological depiction and characterization (6). Recently, second or third-generation dual-source CT (SOMATOM FLASH or SOMATOM Force, Siemens Healthcare, Forchheim, Germany) has been developed that uses two tubes (tube A, low kVp; tube B, high kVp). A blend of low and high kVp images (A, B tube) provides an image quality similar to an approximately 120 kV image (e.g., 80/140, 80/150 using Tin filter, 80/140 using Tin filter kVp) (Fig. 1). A specific mixing ratio (e.g., A:B = 6:4) combined with the low and high kVp data is important to provide a blended image with a decrease in image noise. The reconstruction of virtual MEIs from low to high-keV images helps to accurately evaluate PAD (7). As low keV images enable increasing proportions of photons to undergo photoelectric absorptive interactions around the K-edge of iodine (33 keV), CT arteriography may reveal a high-contrast vessel image (6).
Fig. 1. Example of dual-energy CT protocol for lower-extremity CT angiography using SOMAOM FLASH or SOMATOM Force, Siemens Healthineers.
PERIPHERAL ARTERIAL DISEASE EVALUATION USING LE-CTA
LE-CTA may be useful for detecting PAD, evaluating anatomic variations and limb ischemia, and assessing stent placement (8). Current guidelines recommend LE-CTA examination to assess PAD, severity of arterial stenosis, and treatment planning (1,3,9). LE-CTA helps to determine whether endovascular therapy or surgery is appropriate because it can specify the exact location and extent of atherosclerosis.
For accurate analysis of LE-CTA images, it is necessary to know the characteristics of each KeV image, especially when the radiologist reconstructs CT images from low-KeV to high-KeV using dual-energy CT. Lower keV images on dual-energy CTA are able to improve the contrast-to-noise ratio. However, high density structures such as calcification or metal objects in low kVp or keV images may appear to be larger than the actual size, which are called “blooming artifacts.” Hence, due care is required while interpreting LE-CTA. It is useful to study the technical aspects of LE-CTA to accurately interpret PAD.
MEI
As mentioned above, virtual MEIs, from low to high-keV images, can be reconstructed using DECT. At low keV, high-density materials, such as iodine, become very bright and increase the contrast with the surrounding soft tissue. However, the noise is also high in these low keV images, as it may affect artifacts from high-density materials (10).
Recently, the Mono+ technique has been introduced to overcome image noise at low keV. In this technique, a combination of low-keV image and image noise from optimal keV (typically obtained at 70 keV) are computed. This approach takes advantage of the improved iodine, contrast-to-noise ratio (CNR) of virtual MEIs, in particular at low energy levels, while maintaining spatial resolution and fine noise texture. To avoid noise increase at lower calculated energies, which is a known drawback of virtual MEIs at low keV, a regional spatial frequency-based combination of the high contrast at lower energies and the superior noise properties at higher energies can be performed to optimize CNR (e.g., image noise from 70 keV) (Fig. 2) (5). Using this technique, it is possible to obtain improved contrast with low noise.
Fig. 2. Monoenergetic image plus technique.
Therefore, we expect low-keV images using the Mono+ technique, which are higher quality images than low-keV images without denoising. Although theoretically, low keV images using the Mono+ technique have better image quality than those using high keV images, high keV images may be of superior image quality to evaluate PAD caused due to calcifications and metal artifacts.
OVERALL IMAGE QUALITY OF MEI
LE-CTA is used to evaluate PAD. However, apart from the evaluation of vessels, it is also important to evaluate other organs, such as the liver, kidney, bowel, muscle, lymph nodes, and bones, to assess the overall condition of the patient. Thus, we review the overall CT image quality for various KeV images. In some patients, we obtained Mono+ images at 40, 50, 60, 70, and 80 keV, and we have reviewed the overall image quality of CT images at each KeV. In most of the images, for higher keV, the overall image quality is better with lower image noise. Lower keV reveals lower image quality, and is especially affected by beam hardening (Fig. 3) or other metal artifacts. For example, if the patient cannot raise their arm during CT examination, it causes streak artifacts, especially at lower keV, which affects the overall image quality (Fig. 4). This artifact can be considerably reduced by optimizing the window length/window width for each image (11,12).
Fig. 3. Lower-extremity CT angiography images using the MEI plus technique of a 75-year-old male with peripheral arterial disease (body mass index, 22.9 kg/m2; CT volume dose index, 8.45 mGy; and dose-length product, 1157 mGy·cm).
A-F. The MEIs (window level, 45 HU; window width, 450 HU) include (A) 40 keV, (B) 50 keV, (C) 60 keV, (D) 70 keV, and (E) 80 keV. An occlusion was noted in the left tibioperoneal trunk, proximal posterior tibial artery, and bilateral anterior tibial arteries (not shown). The overall image quality is adequate and good with beam hardening artifacts (white arrows) on the 40 and 50 keV MEIs, respectively (A, B). The beam hardening artifacts close to the right profunda femoris artery (black arrows) can affect the vessel evaluation by radiologists. However, the overall image quality is excellent with little beam hardening in the 60–80 keV MEIs (C-E). Poly-energetic (80/140 kVp with a tin filter) image appears similar to the 120 kVp image and the high keV images (F).
HU = Hounsfield units, MEI = monoenergetic image
Fig. 4. Lower-extremity CT angiography images using the MEI plus technique of a 57-year-old male with peripheral arterial disease (body mass index, 25.4 kg/m2; CT volume dose index, 8.66 mGy; dose-length product, 1266 mGy·cm).
A-F. The MEIs shown include (A) 40 keV, (B) 50 keV, (C) 60 keV, (D) 70 keV, and (E) 80 keV. An occlusion was noted in the right internal iliac artery (not shown). The overall image quality is poor with severe streak artifacts (arrows) on the 40 keV MEI (A). The overall image quality is superior on the 70–80 keV (D, E) MEIs to the low keV MEIs (A-C). Poly-energetic (80/140 kVp with a tin filter) image appears similar to the 120 kVp image and the high keV images (F).
MEI = monoenergetic image
VENOUS CONTAMINATION IN MEIS
Venous contamination is defined as excessive venous enhancement at the arterial phase that interferes with the evaluation of arterial disease (13). Patients with a fast flow are more likely to have venous enhancement. Patients with inflammatory processes, such as cellulitis, are more likely to have venous contamination (13,14).
We evaluated venous contamination by dividing it into three cases where it was 1) not visible, 2) visible but did not affect diagnostic interpretation, or 3) visible and compromised diagnostic interpretation. CT images at lower keV showed an increase in venous contamination due to an increase in contrast, compared with those at higher keV (Figs. 5, 6, 7). Although venous contamination may be reduced by adjusting the window width and level of CT images in actual practice, it is generally greater in low-keV images.
Fig. 5. Lower-extremity CT angiography image using the MEI plus technique of a 63-year-old male with diabetes mellitus macroangiopathy and a below-the-left-knee amputation (body mass index, 21.2 kg/m2; CT volume dose index, 7.74 mGy; dose-length product, 978 mGy·cm).
A-F. The MEIs (window level, 45 HU; window width, 450 HU) shown include (A) 40 keV, (B) 50 keV, (C) 60 keV, (D) 70 keV, and (E) 80 keV. Venous contamination is visible (arrows) and compromised diagnostic interpretation at just above the knee level of the left leg on the 40–50 keV images (A, B). Although venous contamination is visible in the 60 keV image, it did not affect the diagnostic interpretation (D). Minimal venous contamination is visible in the 70–80 keV images (D, E). Poly-energetic (80/140 kVp with a tin filter) image shows similar to the 120 kVp image and the high keV images (F).
HU = Hounsfield units, MEI = monoenergetic image
Fig. 6. Lower-extremity CT angiography images using the MEI plus technique of an 86-year-old male with diffuse steno-occlusive disease in the bilateral BTK arteries (body mass index, 15.1 kg/m2; CT volume dose index, 7.09 mGy; dose-length product, 911 mGy·cm).
A-E. The MEIs (window level, 45 HU; window width, 450 HU) shown include (A) 40 keV, (B) 50 keV, (C) 60 keV, (D) 70 keV, and (E) 80 keV. Venous contamination is visible (arrows) that compromises the diagnostic interpretation at the BTK arteries of the left leg in the 40–50 keV images (A, B). Although venous contamination is visible on the 60–80 keV images, it did not affect diagnostic interpretation (C-E).
BTK = below-the-knee, HU = Hounsfield units, MEI = monoenergetic image
Fig. 7. Lower-extremity CT angiography images using the MEI plus technique of a 37-year-old male with left diabetes mellitus foot (body mass index, 26.5 kg/m2; CT volume dose index, 8.57 mGy; dose-length product, 1236 mGy·cm).
A-E. MEIs (window level, 45 HU; window width, 450 HU) shown include (A) 40 keV, (B) 50 keV, (C) 60 keV, (D) 70 keV, and (E) 80 keV. Venous contamination is visible (arrows) and compromised diagnostic interpretation in the left lower leg arteries in the 40–50 keV images (A, B). Although venous contamination is visible in the 60–80 keV images, it did not affect the diagnostic interpretation (C-E).
HU = Hounsfield units, MEI = monoenergetic image
PAD THROUGH MEI
Low keV images with high contrast can lead to misinterpretation or overestimation of PAD calcification (Figs. 8, 9, 10, 11). A prior study reported that low-keV images produced larger arterial plaque volumes than conventional 90/Sn150-kVp images (7,15). Although blooming artifacts due to small high-contrast structures can be reduced by optimizing the window length/window width (Fig. 10) (11), it may not be appropriate to perform PAD evaluation on low-keV MEIs.
Fig. 8. Lower-extremity CT angiography images using the MEI plus technique in a 79-year-old male (body mass index, 17.3 kg/m2; CT volume dose index, 7.20 mGy; dose-length product, 947 mGy·cm) with peripheral arterial disease. Severe stenosis was noted at the bilateral superficial femoral arteries (arrowheads).
A-E. MEIs (window level, 500 HU; window width, 2000 HU) shown include (A) 40 keV, (B) 50 keV, (C) 60 keV, (D) 70 keV, and (E) 80 keV. At 40 keV, the segmented image provides acceptable information but the image quality is unsatisfactory due to vessel calcification with mild blooming artifacts (arrowheads). At 50–60 keV, the segmented images satisfactorily provide information with adequate image quality. However, at 70–80 keV, the segmented images provide optimal information with excellent image quality and no blooming artifacts. The image quality of peripheral arterial disease with calcification on the higher keV images is superior to that on the lower keV images.
HU = Hounsfield unit, MEI = monoenergetic image
Fig. 9. Lower-extremity CT angiography images using the MEI plus technique of an 82-year-old female (body mass index, 40.7 kg/m2; CT volume dose index, 8.88 mGy; dose-length product, 1104 mGy·cm) with peripheral arterial disease. Severe stenosis is noted at the right superficial femoral artery (arrowheads).
A-E. MEIs (window level, 150 HU; window width, 600 HU) shown include (A) 40 keV, (B) 50 keV, (C) 60 keV, (D) 70 keV, and (E) 80 keV. At 40–50 keV, the segmented images provide acceptable information but the image quality is unsatisfactory due to vessel calcification with blooming artifacts (arrowheads) At 60 keV, the segmented images satisfactorily provide information with adequate image quality. However, at 70–80 keV, the segmented images provide optimal information with excellent image quality.
F-J. The MEIs with modification in the window setting (window level, 500 HU; window width, 2000 HU) shown include (F) 40 keV, (G) 50 keV, (H) 60 keV, (I) 70 keV, and (J) 80 keV. At 40–80 keV, the segmented images provide acceptable to optimal information of the evaluation of vessel calcification without blooming artifacts.
HU = Hounsfield units, MEI = monoenergetic image
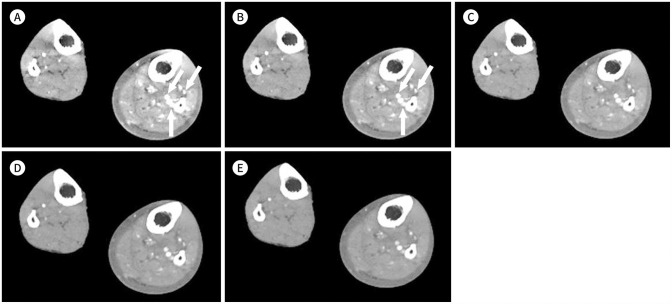
Fig. 10. Lower-extremity CT angiography images using the MEI plus technique in a 90-year-old female (body mass index, 21.9 kg/m2; CT volume dose index, 7.1 mGy; dose-length product, 936 mGy·cm) with peripheral arterial disease at the bilateral superficial femoral arteries, popliteal arteries, and below-the-knee arteries. Moderate stenosis is noted in the left popliteal artery (arrowhead).
A-E. MEIs (WL: 500 HU, WW: 2000 HU) shown include (A) 40 keV, (B) 50 keV, (C) 60 keV, (D) 70 keV, and (E) 80 keV. At 40 keV (arrowhead), the segmented image of the left popliteal artery provides unclear information with inadequate image quality due to severe blooming artifacts. However, at 50–80 keV, the segmented images provide optimal information with adequate to excellent image quality and a decrease in blooming artifacts compared with the 40 keV image.
F-J. The MEIs shown include (F) 40 keV, (G) 50 keV, (H) 60 keV, (I) 70 keV, and (J) 80 keV with changes in window setting. In each keV image, the WL and WW were adjusted to optimize image quality (A) WL: 1040 HU, WW: 4200 HU, (B) WL: 800 HU, WW: 3400 HU, (C) WL: 500 HU, WW: 2100 HU, (D) WL: 600 HU, WW: 2200 HU, (E) WL: 530 HU, WW: 2000 HU). With the WW and WL adjustments, the image quality improved at all keV, even 40 keV, with a decrease in blooming artifacts, showing excellent image quality.
HU = Hounsfield units, MEI = monoenergetic image, WL = window level, WW = window width
Fig. 11. Lower-extremity CT angiography images using the MEI plus technique of a 69-year-old female (body mass index, 23.4 kg/m2; CT volume dose index, 7.16 mGy; dose-length product, 830 mGy·cm) with peripheral arterial disease. Mild stenosis is noted at the left superficial femoral artery (arrowheads).
A-E. The MEIs (window level, 45 HU; window width, 450 HU) shown include (A) 40 keV, (B) 50 keV, (C) 60 keV, (D) 70 keV, and (E) 80 keV. At 40–50 keV, the segmented images provide acceptable information but unsatisfactory image quality due to vessel calcification with blooming artifacts (arrowheads). At 60–80 keV, the segmented images provide satisfactory information with adequate image quality such that the vessel calcification is differentiated from the vessel enhancement.
F-J. The MEIs with modified window settings (window level, 600 HU; window width, 2000 HU) shown include (F) 40 keV, (G) 50 keV, (H) 60 keV, (I) 70 keV, and (J) 80 keV. Thus, at 40–80 keV, the segmented images provide acceptable to optimal information of the evaluation of vessel calcification without blooming artifacts.
HU = Hounsfield units, MEI = monoenergetic image
METAL ARTIFACTS IN MEI
CT image quality in high-keV MEIs is superior for metal artifacts compared to low-keV MEIs (4,7,16,17,18). Metal artifacts decrease in the CT images at high keV than those at low keV (Figs. 12, 13, 14). CT images at low keV yield higher objective image noise of metal artifacts than those at high keV.
Fig. 12. Lower-extremity CT angiography images using the MEI plus technique in a 60-year-old female (body mass index, 24.2 kg/m2; CT volume dose index, 7.78 mGy; dose-length product, 990 mGy·cm) with peripheral arterial disease.
A-E. MEIs shown include (A) 40 keV, (B) 50 keV, (C) 60 keV, (D) 70 keV, and (E) 80 keV. Metal artifacts caused by internal fixation material on the left femur affected the left superficial femoral artery evaluation. At 40–50 keV, the image quality is poor and non-diagnostic due to strong streak artifacts (arrows). In the 60–80 keV images, the image quality is poor due to severe artifacts, but the effect of the artifacts gradually decreases in the images from 40 to 80 keV.
F-J. The MEIs shown include (F) 40 keV, (G) 50 keV, (H) 60 keV, (I) 70 keV, and (J) 80 keV with optimized window settings (window level, 1000 HU; window width, 3000 HU) due to a decrease in metal artifacts. Despite the window setting changes, the low keV images (F, G) show remnant metal artifacts compared with the high keV images (H-J).
HU = Hounsfield units, MEI = monoenergetic image
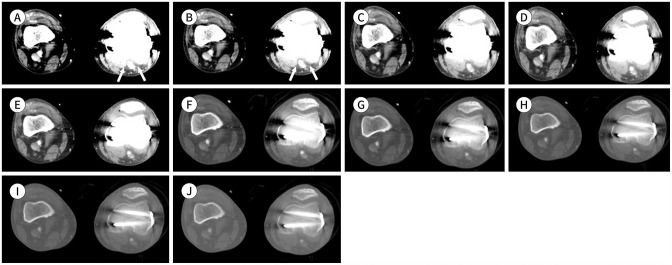
Fig. 13. Lower-extremity CT angiography images using the MEI plus technique of a 61-year-old male (body mass index, 18.5 kg/m2; CT volume dose index, 7.54 mGy; dose-length product, 939 mGy·cm) with peripheral arterial disease.
A-E. MEIs shown include (A) 40 keV, (B) 50 keV, (C) 60 keV, (D) 70 keV, and (E) 80 keV. Metal artifacts caused by the internal fixation material on the left femur and tibia affect the left popliteal artery evaluation. In the 40–50 keV images, the image quality is poor due to severe artifacts (arrows). In the 60–80 keV images, the image quality is adequate with slight metal artifacts. The metal artifacts gradually decrease in the images from 40 to 80 keV.
F-J. MEIs shown include (F) 40 keV, (G) 50 keV, (H) 60 keV, (I) 70 keV, and (J) 80 keV with optimized window settings (window level, 700 HU; window width, 3000 HU) due to the decrease in metal artifacts. With the window level and width adjustments, the vessel image quality is excellent without metal artifacts.
HU = Hounsfield units, MEI = monoenergetic image
Fig. 14. Lower-extremity CT angiography images using the MEI plus technique of a 79-year-old female (body mass index, 24.0 kg/m2; CT volume dose index, 7.28 mGy; dose-length product, 803 mGy·cm) with peripheral arterial disease.
A-E. MEIs shown include (A) 40 keV, (B) 50 keV, (C) 60 keV, (D) 70 keV, and (E) 80 keV. Metal artifacts caused by internal fixation material on the left femur affect the left superficial femoral artery evaluation. In the 40–50 keV images, the image quality is poor due to severe artifacts (arrows). In the 60–80 keV images, the image quality is adequate with slight metal artifacts. The metal artifacts gradually decrease in the images from 40 to 80 keV.
F-J. MEIs shown include (F) 40 keV, (G) 50 keV, (H) 60 keV, (I) 70 keV, and (J) 80 keV with optimized window settings (window level, 200 HU; window width, 2000 HU) due to the decrease in metal artifacts. With the window level and width adjustments, the vessel image quality is excellent without metal artifacts.
HU = Hounsfield units, MEI = monoenergetic image
CONCLUSION
We reviewed Mono+ images obtained from LE-CTA at different keV levels to evaluate PAD. A brief review of DECT, MEIs, and various image features of PAD on different MEIs can improve radiologists’ interpretation and diagnostic accuracy of PAD on LE-CTA. The overall image quality, segmental image quality, and diagnostic value of metal artifacts are better at higher keV than those at lower keV. This is because at lower keV, there is more severe noise than at higher keV, while lower keV leads to higher contrast than higher keV. The issue of noise has not been overcome, even though the Mono+ technique has been applied in patients with PAD mainly caused due to calcification lesions and metal artifacts. The image quality at higher keV is more acceptable to detect venous contamination, because higher contrast at lower keV tends to overestimate small venous contamination.
Footnotes
- Conceptualization, P.S.H.
- data curation, H.J.H., P.S., L.K., K.J.H., P.S.Y.
- formal analysis, K.J.S.
- investigation, H.J.H., P.S., L.K.
- methodology, P.S.H.
- project administration, P.S.H.
- resources, P.S.Y.
- software, P.S.Y.
- supervision, P.S.H.
- validation, P.S.H.
- visualization, L.K., K.J.S.
- writing—original draft, K.J.S.
- writing—review & editing, P.S.Y., S.B., P.S.H.
Conflicts of Interest: Seong Yong Pak and Bernhard Schmidts are employees of Siemens Healthineers. All other authors declare no conflicts of interest.
Funding: None
References
- 1.Fowkes FG, Rudan D, Rudan I, Aboyans V, Denenberg JO, McDermott MM, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–1340. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 2.Aboyans V, Ricco JB, Bartelink MEL, Bjorck M, Brodmann M, Cohnert T, et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS) Rev Esp Cardiol (Engl Ed) 2018;71:111. doi: 10.1016/j.rec.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 3.Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res. 2015;116:1509–1526. doi: 10.1161/CIRCRESAHA.116.303849. [DOI] [PubMed] [Google Scholar]
- 4.Mocanu I, Van Wettere M, Absil J, Bruneau M, Lubicz B, Sadeghi N. Value of dual-energy CT angiography in patients with treated intracranial aneurysms. Neuroradiology. 2018;60:1287–1295. doi: 10.1007/s00234-018-2090-5. [DOI] [PubMed] [Google Scholar]
- 5.Grant KL, Flohr TG, Krauss B, Sedlmair M, Thomas C, Schmidt B. Assessment of an advanced image-based technique to calculate virtual monoenergetic computed tomographic images from a dual-energy examination to improve contrast-to-noise ratio in examinations using iodinated contrast media. Invest Radiol. 2014;49:586–592. doi: 10.1097/RLI.0000000000000060. [DOI] [PubMed] [Google Scholar]
- 6.Vlahos I, Chung R, Nair A, Morgan R. Dual-energy CT: vascular applications. AJR Am J Roentgenol. 2012;199:S87–S97. doi: 10.2214/AJR.12.9114. [DOI] [PubMed] [Google Scholar]
- 7.Symons R, Choi Y, Cork TE, Ahlman MA, Mallek M, Bluemke DA, et al. Optimized energy of spectral coronary CT angiography for coronary plaque detection and quantification. J Cardiovasc Comput Tomogr. 2018;12:108–114. doi: 10.1016/j.jcct.2018.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Horehledova B, Mihl C, Milanese G, Brans R, Eijsvoogel NG, Hendriks BMF, et al. CT angiography in the lower extremity peripheral artery disease feasibility of an ultra-low volume contrast media protocol. Cardiovasc Intervent Radiol. 2018;41:1751–1764. doi: 10.1007/s00270-018-1979-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aboyans V, Ricco JB, Bartelink MEL, Björck M, Brodmann M, Cohnert T, et al. 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS): document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries. Endorsed by: the European Stroke Organization (ESO) the task force for the diagnosis and treatment of peripheral arterial diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS) Eur Heart J. 2018;39:763–816. doi: 10.1093/eurheartj/ehx095. [DOI] [PubMed] [Google Scholar]
- 10.Yu L, Leng S, McCollough CH. Dual-energy CT-based monochromatic imaging. AJR Am J Roentgenol. 2012;199:S9–S15. doi: 10.2214/AJR.12.9121. [DOI] [PubMed] [Google Scholar]
- 11.Pollak AW, Norton PT, Kramer CM. Multimodality imaging of lower extremity peripheral arterial disease: current role and future directions. Circ Cardiovasc Imaging. 2012;5:797–807. doi: 10.1161/CIRCIMAGING.111.970814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee KH, Shim YS, Park SH, Park SH, Choi SJ, Pak SY, et al. Comparison of standard-dose and half-dose dual-source abdominopelvic CT scans for evaluation of acute abdominal pain. Acta Radiol. 2019;60:946–954. doi: 10.1177/0284185118809544. [DOI] [PubMed] [Google Scholar]
- 13.ÇildağMB, Ertuğrul MB, Köseoğlu ÖF, Armstrong DG. A factor increasing venous contamination on bolus chase three-dimensional magnetic resonance imaging: Charcot neuroarthropath. J Clin Imaging Sci. 2018;8:13. doi: 10.4103/jcis.JCIS_77_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Chen CZ, Chabra SG, Winchester PA, Khilnani NM, Watts R, et al. Bolus arterial-venous transit in the lower extremity and venous contamination in bolus chase three-dimensional magnetic resonance angiography. Invest Radiol. 2002;37:458–463. doi: 10.1097/00004424-200208000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Al-Baldawi Y, Große Hokamp N, Haneder S, Steinhauser S, Püsken M, Persigehl T, et al. Virtual mono-energetic images and iterative image reconstruction: abdominal vessel imaging in the era of spectral detector CT. Clin Radiol. 2020;75:641.e9–641.e18. doi: 10.1016/j.crad.2020.03.036. [DOI] [PubMed] [Google Scholar]
- 16.Magarelli N, De Santis V, Marziali G, Menghi A, Burrofato A, Pedone L, et al. Application and advantages of monoenergetic reconstruction images for the reduction of metallic artifacts using dual-energy CT in knee and hip prostheses. Radiol Med. 2018;123:593–600. doi: 10.1007/s11547-018-0881-8. [DOI] [PubMed] [Google Scholar]
- 17.Shinohara Y, Sakamoto M, Iwata N, Kishimoto J, Kuya K, Fujii S, et al. Usefulness of monochromatic imaging with metal artifact reduction software for computed tomography angiography after intracranial aneurysm coil embolization. Acta Radiol. 2014;55:1015–1023. doi: 10.1177/0284185113510492. [DOI] [PubMed] [Google Scholar]
- 18.Hwang JH, Kang JM, Park S, Park SH, Kim JH, Lee KH, et al. Advanced virtual monoenergetic imaging algorithm for lower extremity CT angiography: effects on image quality, artifacts, and peripheral arterial disease evaluation. Diagn Interv Radiol. 2022 doi: 10.5152/dir.2022.21551. [Epub] [DOI] [PMC free article] [PubMed] [Google Scholar]