Abstract
For the surveillance of pig herds infected with porcine pleuropneumonia, an enzyme-linked immunosorbent assay (ELISA) using the recombinant Actinobacillus pleuropneumoniae ApxII protein as species- but not serotype-specific antigen was developed. Using this ELISA, 243 of 400 animals from 22 A. pleuropneumoniae-infected herds were classified as seropositive.
Actinobacillus pleuropneumoniae is the etiologic agent of porcine pleuropneumonia, which has become a major problem in the swine industry, causing severe economic losses worldwide (2). The clinical course of disease can range from peracute to chronic, with infected pigs typically showing a hemorrhagic, necrotizing pneumonia often associated with fibrinous pleuritis (17). Pigs surviving the infection develop a serotype-specific protective immunity but may become subclinical carriers of the pathogen. These carriers are the most frequent source of infection in previously uninfected herds (10, 17).
To date, two biotypes and 14 serotypes of A. pleuropneumoniae have been recognized, and convalescent sera frequently show a serotype-specific reaction (7, 12). To control the disease, a variety of serological tests is being employed, with the complement fixation test (CFT) still being used as a reference test (9). In addition, several enzyme-linked immunosorbent assays (ELISAs) have been developed (6, 13–15, 18). All these tests, however, are serotype specific. This indicates the need for a test detecting antibodies directed against A. pleuropneumoniae irrespective of the serotype in routine diagnostics and for epidemiological surveys.
We have assessed the suitability of the recombinant A. pleuropneumoniae ApxII protein for this purpose. The ApxII protein is highly immunogenic and is present in all A. pleuropneumoniae serotypes except serotype 10 (3, 4, 8). The recombinant ApxII protein was prepared in aggregated form from Escherichia coli HB101 transformed with the plasmid pCY76/503 as previously described (5, 16). Protein aggregates were resuspended in H2O and dissolved by the addition of an equal volume of 7 M guanidine hydrochloride. To remove contaminating peptides, the solution was filtered through a Centricon 30 system (Amicon, Beverly, Mass.). The retentate was washed twice by repeating the filtration and will be referred to as ELISA antigen.
The positive control serum was a pool of sera from animals experimentally infected with A. pleuropneumoniae serotypes 2, 3, 5, 7, and 9. The negative control serum was obtained from one animal from an A. pleuropneumoniae-free herd. Fifty sera which, in a serotype-specific CFT, reacted solely with A. pleuropneumoniae serotype 2, 3, 5, 7, or 9 originated from a bank of convalescent sera.
Clinical samples were taken from 616 grower pigs and gilts; 216 of these sera originated from 13 A. pleuropneumoniae-free herds (as indicated by a complete lack of both clinical symptoms and detectable antibodies against serotypes 2, 3, 5, 7, and 9 by CFT) and 400 sera from 22 A. pleuropneumoniae-infected herds (as indicated by clinical symptoms in the herd and the presence of antibodies detectable by CFT in at least 40% of blood samples taken at random). CFT was performed as described by Lombin et al. (9); sera with less than 50% hemolysis at a dilution of 1:10 were judged positive.
For the ELISA, all reagents were applied in 100-μl volumes. Plates (Polysorb; Nunc, Wiesbaden, Germany) were coated (1 h at ambient temperature) using the ELISA antigen diluted in a carbonate-bicarbonate buffer (100 mM NaHCO3, 46 mM Na2CO3 [pH 9.6]). The optimal coating concentration as determined by checkerboard titration was 1.2 μg/ml. Plates were washed three times with PBST (phosphate-buffered saline with Tween 20; 150 mM NaCl, 1.5 mM KH2PO4, 9 mM Na2HPO4 · 12H2O, 2.5 mM KCl [pH 7.2], 0.05% Tween 20) by using a plate washer (Dynatech, Denkendorf, Germany) and stored at −20°C for up to 3 months. All subsequent incubations were performed for 15 min at 37°C with rotation at 800 rpm, and after each incubation step, plates were washed three times. Control sera and samples were titrated in PBST in a serial twofold dilution from 1:100 to 1:6,400. The conjugates (biotinylated antiporcine immunoglobulin G and streptavidin peroxidase) (both from Dianova, Hamburg, Germany) were added sequentially, and the ELISA was developed with ABTS [2,2′-azinobis(3-ethyl)benzothiazolinesulfonic acid] (Boehringer Mannheim, Mannheim, Germany); color development was measured in a spectrophotometer (MR7000; Dynatech) at 410 to 490 nm. Absorbances were analyzed by the reference standard method (1) and subsequent log-log transformation of data. The activity of the positive control serum was arbitrarily set to 100 ELISA units (EU).
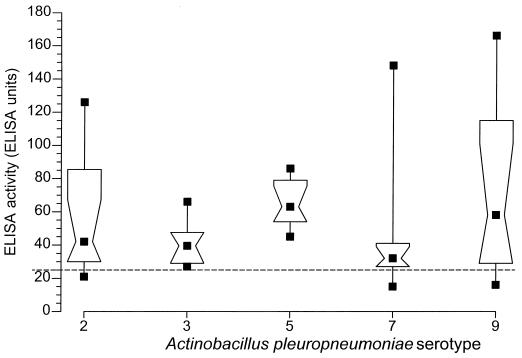
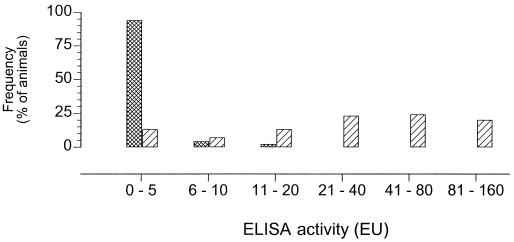
The ELISA was determined to have a well-to-well variation of 8% and an interassay variation of 26%; the arithmetic mean of the ELISA activities of 216 sera from animals from A. pleuropneumoniae-negative herds was 3.2 EU with a standard deviation of 6.2 EU; the cutoff value was set to 25 EU. The ELISA reaction was positive (>25 EU) with 46 of the 50 sera monospecific for A. pleuropneumoniae serotype 2, 3, 5, 7, or 9 in the CFT (Fig. 1). The feasibility of the cutoff value and the discriminatory efficacy of the ELISA were confirmed by testing 616 field sera (Fig. 2). None of the 216 animals from A. pleuropneumoniae-free herds had an ELISA activity of more than 25 EU, whereas 243 of 400 animals from infected herds had ELISA activities of >25 EU (Table 1).
FIG. 1.
ELISA reactivity patterns of sera from five groups of 10 animals each monospecific for A. pleuropneumoniae serotype 2, 3, 5, 7, or 9 (in CFT). The calculation of notch boxes was performed according to the method of McGill et al. (11). The dashed line represents the cutoff set to 25 EU.
FIG. 2.
Distribution of ELISA activities of 22 A. pleuropneumoniae-infected herds (400 animals) (cross-hatched bars) and 13 A. pleuropneumoniae-free herds (216 animals) (striped bars).
TABLE 1.
Comparison of ELISA and CFT results from 22 A. pleuropneumoniae-infected herds and A. pleuropneumoniae-free herds
| Herd type (no. of animals) | CFT result (dilution) | No. of animals with
an ELISA result of:
|
|
|---|---|---|---|
| Positive (>25 EU) | Negative (≤25 EU) | ||
| A. pleuropneumoniae positive (400) | Positive (≥1:10) | 138 | 32 |
| Negative (<1:10) | 105 | 125 | |
| A. pleuropneumoniae negative (216) | Positive (≥1:10) | 0 | 0 |
| Negative (<1:10) | 0 | 216 | |
ELISA and CFT results from animals of both infected and noninfected herds were compared by Spearman’s correlation and the McNemar test (using SAS software). Spearman’s correlation coefficient was found to be 0.709 as calculated for all 616 sera, indicating a strong correlation between both tests. In the McNemar test, a χ2 value of 27.35 indicated a significantly higher number of animals to be regarded as positive in the ELISA than in the CFT. ELISA and CFT results from animals of infected herds were further compared as indicated in Table 1. Using the ELISA, 243 of 400 animals were classified as seropositive, whereas the CFT detected only 170 seropositive animals. These results indicated the higher sensitivity of the ELISA as compared to that of the CFT.
In addition, the correlation of both tests allows an ELISA-based selection of positive sera for CFT analysis with A. pleuropneumoniae serotype-specific antigens. This approach might be financially favorable for epidemiologic monitoring requiring the identification of the infecting A. pleuropneumoniae serotype because it reduces the number of sera to be tested by the labor-intensive CFT using all possible different serotypes.
The results show that the ApxII-protein-based ELISA is a sensitive, inexpensive, and highly discriminatory method for clinical veterinary laboratories to continuously monitor the status of A. pleuropneumoniae-free herds.
REFERENCES
- 1.Butler J E, Heo Y, Adams P, Richardson H B. The enzyme-linked immunosorbent assay (ELISA): a measure of antibody concentration and affinity? Immunochemistry. 1978;15:131–146. doi: 10.1016/0161-5890(78)90053-6. [DOI] [PubMed] [Google Scholar]
- 2.Fenwick B W, Henry S. Porcine pleuropneumonia. J Am Vet Med Assoc. 1994;204:1334–1340. [PubMed] [Google Scholar]
- 3.Frey J. Virulence in Actinobacillus pleuropneumoniae and RTX toxins. Trends Microbiol. 1995;3:257–261. doi: 10.1016/s0966-842x(00)88939-8. [DOI] [PubMed] [Google Scholar]
- 4.Frey J, Bosse J T, Chang Y-F, Cullen J M, Fenwick B, Gerlach G-F, Gygi D, Haesenbrouck F, Inzana T J, Jansen R, Kamp E M, Segers J M, Smits M, Stenbaek E, Struck D K, Van den Bosch J F, Willson P J, Young R. Actinobacillus pleuropneumoniae RTX-toxins: uniform designation of haemolysins, cytolysins, pleurotoxin and their genes. J Gen Microbiol. 1993;139:1723–1728. doi: 10.1099/00221287-139-8-1723. [DOI] [PubMed] [Google Scholar]
- 5.Gerlach G-F, Anderson C, Potter A A, Klashinsky S, Willson P J. Cloning and expression of a transferrin-binding protein from Actinobacillus pleuropneumoniae. Infect Immun. 1992;60:892–898. doi: 10.1128/iai.60.3.892-898.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gottschalk M, Altman E, Charland N, De Lasalle F, Dubreuil J D. Evaluation of a saline boiled extract, capsular polysaccharides and long-chain lipopolysaccharides of Actinobacillus pleuropneumoniae serotype 1 as antigens for the serodiagnosis of swine pleuropneumonia. Vet Microbiol. 1994;42:91–104. doi: 10.1016/0378-1135(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 7.Inzana T J, Clar G F, Todd J. Detection of serotype-specific antibodies or capsular antigen of Actinobacillus pleuropneumoniae by a double-label radioimmunoassay. J Clin Microbiol. 1990;28:312–318. doi: 10.1128/jcm.28.2.312-318.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamp E M, Popma J K, Anakotta J, Smits M A. Identification of hemolytic and cytotoxic proteins of Actinobacillus pleuropneumoniae by use of monoclonal antibodies. Infect Immun. 1991;59:3079–3085. doi: 10.1128/iai.59.9.3079-3085.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lombin L H, Rosendal S, Mitchell W R. Evaluation of the complement fixation test for the diagnosis of pleuropneumonia in swine caused by Haemophilus pleuropneumoniae. Can J Comp Med. 1982;46:109–114. [PMC free article] [PubMed] [Google Scholar]
- 10.MacInnes J I, Rosendal S. Prevention and control of Actinobacillus (Haemophilus) pleuropneumoniae infection in swine: a review. Can Vet J. 1988;29:572–573. [PMC free article] [PubMed] [Google Scholar]
- 11.McGill R, Tukey J W, Larsen W A. Variation of box plots. Am Stat. 1978;32:12–16. [Google Scholar]
- 12.Nicolet J. Taxonomy and serological identification of Actinobacillus pleuropneumoniae. Can Vet J. 1988;29:578–579. [PMC free article] [PubMed] [Google Scholar]
- 13.Nicolet J, Paroz P H, Krawinkler M, Baumgarten A. An enzyme-linked immunosorbent assay, using an EDTA-extracted antigen for the serology of Haemophilus pleuropneumoniae. Am J Vet Res. 1981;42:2139–2142. [PubMed] [Google Scholar]
- 14.Nielsen R, Plambeck T, Foged N T. Blocking enzyme-linked immunosorbent assay for detection of antibodies to Actinobacillus pleuropneumoniae serotype 2. J Clin Microbiol. 1991;29:794–797. doi: 10.1128/jcm.29.4.794-797.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Radacovici S, Gottschalk M, Dubreuil J D. Lipopolysaccharides of Actinobacillus pleuropneumoniae (serotype 1): a readily obtainable antigen for ELISA serodiagnosis of pig pleuropneumonia. Vet Microbiol. 1994;39:219–230. doi: 10.1016/0378-1135(94)90159-7. [DOI] [PubMed] [Google Scholar]
- 16.Rossi-Campos A, Anderson C, Gerlach G-F, Klashinsky S, Potter A A, Willson P J. Immunization of pigs against Actinobacillus pleuropneumoniae with two recombinant protein preparations. Vaccine. 1992;10:512–518. doi: 10.1016/0264-410x(92)90349-o. [DOI] [PubMed] [Google Scholar]
- 17.Sebunya T N L, Saunders K R. Haemophilus pleuropneumoniae infection in swine: a review. J Am Vet Med Assoc. 1983;182:1331–1337. [PubMed] [Google Scholar]
- 18.Trottier Y-L, Wright P F, Lariviére S. Optimization and standardization of an enzyme-linked immunosorbent assay protocol for serodiagnosis of Actinobacillus pleuropneumoniae serotype 5. J Clin Microbiol. 1992;30:46–53. doi: 10.1128/jcm.30.1.46-53.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]