Abstract
Objective
Approximately 2.4 million people in the United States are living with hepatitis C virus (HCV) infection. The objective of our study was to describe demographic and socioeconomic characteristics, liver disease–related risk factors, and modifiable health behaviors associated with self-reported testing for HCV infection among adults.
Methods
Using data on adult respondents aged ≥18 from the 2013-2017 National Health Interview Survey, we summarized descriptive data on sociodemographic characteristics and liver disease–related risk factors and stratified data by educational attainment. We used weighted logistic regression to examine predictors of HCV testing.
Results
During the study period, 11.7% (95% CI, 11.5%-12.0%) of adults reported ever being tested for HCV infection. Testing was higher in 2017 than in 2013 (adjusted odds ratio [aOR] = 1.27; 95% CI, 1.18-1.36). Adults with ≥some college were significantly more likely to report being tested (aOR = 1.60; 95% CI, 1.52-1.69) than adults with ≤high school education. Among adults with ≤high school education (but not adults with ≥some college), those who did not have health insurance were less likely than those with private health insurance (aOR = 0.78; 95% CI, 0.68-0.89) to get tested, and non–US-born adults were less likely than US-born adults to get tested (aOR = 0.77; 95% CI, 0.68-0.87).
Conclusions
Rates of self-reported HCV testing increased from 2013 to 2017, but testing rates remained low. Demographic characteristics, health behaviors, and liver disease–related risk factors may affect HCV testing rates among adults. HCV testing must increase to achieve hepatitis C elimination targets.
Keywords: hepatitis C, hepatitis C virus (HCV) infection, viral hepatitis, health care use, baby boomers
Hepatitis C adversely affects adults in the United States, causing substantial mortality despite availability of curative treatments. 1 -4 Approximately 2.4 million people (1.0% of the US adult population) are living with chronic hepatitis C virus (HCV) infection. 1,5 HCV is transmitted by exposure to infectious blood or bodily fluids, 1 and chronic infection with HCV can lead to death and serious life-threatening liver disease. 6 -12 Injection drug use is the largest risk factor for acquiring HCV infection in the United States. 1 In the United States in 2018, 15 713 people died of HCV-related illnesses. 1 From 2017 to 2018, the number of new cases of HCV infection in the United States rose by 13.0%; adults aged 20-39 had the highest rate of new HCV infection, coincident with increases in injection drug use. 1,13 Thus, identifying people living with HCV infection is critical to eliminating it as a public health threat in the United States. 14 -16 Among adults, HCV testing has evolved from a risk-based strategy to a universal testing strategy.
Since 1998, the Centers for Disease Control and Prevention (CDC) has recommended HCV testing for people at increased risk for HCV infection. 17,18 In 1999, HCV testing was also recommended for people infected with HIV. 19 In 2012, CDC and the US Preventive Services Task Force (USPSTF) expanded HCV testing recommendations to include onetime testing for people born during 1945-1965. 20,21 In 2020, CDC recommended universal onetime HCV testing for adults aged ≥18 and pregnant people. 22 Despite these recommendations, national testing rates among people born during 1945-1965, who account for most people living with chronic HCV infection, are low. 23 Only half of people in the United States with HCV infection have been diagnosed and are aware of their HCV infection. 24 An opportunity exists to treat and cure HCV infection—if testing is expanded—so that more people know their status and access to care is made more widely available.
An analysis of factors that affect whether people receive an HCV test can inform public health strategies to eliminate hepatitis C in this country. Health insurance, educational attainment, poverty status, social support (eg, marital status), country of origin, and other environmental factors may play a role in HCV testing and awareness among adults. 23 -33 Because testing and treatment are cost-effective, can extend quality of life, and reduce transmission to others, it is imperative to examine the facilitators and barriers to testing. 34,35 Only a few studies have used the National Health Interview Survey (NHIS) to assess the social, economic, and other factors associated with HCV testing by education status among adults aged ≥18. 26 -29 The objective of our study was to describe the demographic and socioeconomic characteristics, liver disease–related risk factors, and modifiable health behaviors associated with self-reported HCV testing among adults, stratified by educational attainment.
Methods
We obtained data for 2013-2017 from the NHIS, an annual survey conducted by the National Center for Health Statistics at CDC. The survey is a nationally representative, cross-sectional household interview of civilian, noninstitutionalized people in the United States. 36 Conducted through computer-assisted personal interviewing, NHIS uses geographically clustered sampling techniques to select each household, yielding approximately 27 000 sampled adults per year. For this study, we used data obtained from the Sample Adult Interview, the Imputed Adult Income File, and the Person File. Methodologic details, protocols, and research ethics review board approval are described elsewhere. 36 We restricted all analyses to adults aged ≥18.
HCV Testing
The primary outcome variable was HCV testing, defined by using the question, “Ever had a blood test for hepatitis C?” The response options were yes, no, refused, not ascertained, or don’t know. We created a single yes/no variable, where no included both no and don’t know. We excluded refusals and responses that were not ascertained. We analyzed data for 2013-2017, when this question was asked.
Independent Variables
We included 5 demographic factors in our analyses: age (year of birth); sex (male or female); race/ethnicity (non-Hispanic White or “other,” which included Hispanic, non-Hispanic Black, American Indian/Alaska Native, Asian, and multiple races); marital status (currently married, single/never married, and separated/widowed/divorced); and place of birth (United States, not United States). We classified respondents into 3 birth cohorts: those born before 1945, those born during 1945-1965, and those born after 1965. Respondents born during 1945-1965 were the only birth cohort that was recommended to be universally tested for HCV infection during our study period. For the purposes of our study, this age group will be referred to as the birth cohort.
We assessed the association of HCV testing with 3 socioeconomic factors: education, federal poverty level (FPL), and health insurance type. We measured education by highest degree attained. The NHIS question was, “What is the highest level of school completed or the highest degree received?” We categorized these data into high level of education (≥some college) and low level of education (≤high school graduate). To measure income, we estimated the poverty–income ratio, which is the ratio of annual family income to the appropriate FPL. We classified respondents into 2 groups: poverty–income ratio ≤100% FPL or poverty–income ratio >100% FPL. We also categorized respondents into 5 mutually exclusive health insurance types: private health insurance; Medicaid, Medicare, or both; military insurance; other; and uninsured.
We also assessed associations between testing and 2 modifiable health behaviors that are associated with liver disease: smoking status (current smoker, former smoker, never smoker) and alcohol drinking status (lifetime abstainer, former drinker, current nonexcessive drinker, current excessive drinker). Smoking status was determined by 2 questions: “Have you smoked at least 100 cigarettes in your entire life?” and “Do you now smoke cigarettes every day, some days, or not at all?” For alcohol drinking status, a lifetime abstainer was defined as not having >12 alcoholic drinks in a lifetime. Former drinkers were defined as those who indicated they had >12 alcoholic drinks in their lifetime but had none in the past year. Current nonexcessive drinkers were defined as those who had >12 alcoholic drinks in their lifetime, and for male drinkers, had on average <15 drinks per week in the past year; for female drinkers, on average had <8 alcoholic drinks per week in the past year. Current excessive drinkers were defined as those who drink, on average, more than current nonexcessive drinkers in the past year.
We also examined body mass index (BMI), another known risk factor for liver-related disease. NHIS uses 2 questions to ascertain BMI (“How tall are you without shoes?” and “How much do you weigh without shoes?”) and calculates BMI by dividing weight in kilograms by height in meters squared (kg/m2). We categorized BMI data from the NHIS dataset into 2 categories: normal/underweight (<25.0 kg/m2) and overweight/obese (≥25.0 kg/m2).
Statistical Analysis
The NHIS uses a multistage probability design to obtain a sample; this design involves stratification, clustering, and oversampling. Accounting for the complex sampling design, we used weighted logistic regression to identify potential factors associated with HCV testing prevalence. We selected predictors a priori that we hypothesized might be associated with HCV testing prevalence. These predictors included demographic and socioeconomic characteristics, liver disease–related risk factors, and modifiable health behaviors. We did not set any criteria for removing variables from the models, given that we selected variables on the basis of plausible associations. After the initial analyses, we performed post hoc analyses for educational attainment for 2 reasons: (1) this factor was strongly associated with testing and (2) the factor is critical to designing messaging for the promotion of testing among groups at high risk of having HCV infection. We stratified our analyses by educational attainment (≥some college vs ≤high school graduate) to assess associations within each level. We calculated adjusted odds ratios (aORs), 95% CIs, and P values; we considered P < .05 to be significant. We used the R computing language version 3.35-1 (R Foundation for Statistical Computing) for all analyses.
Results
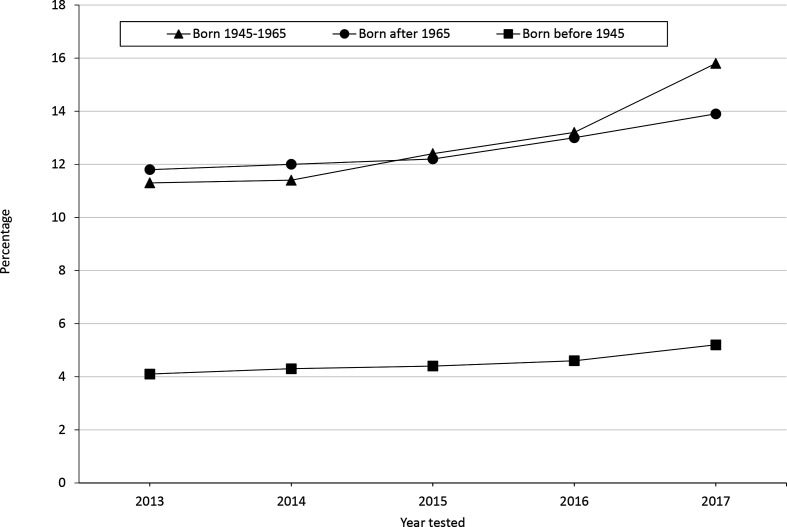
Based on weighted averages, 11.7% (95% CI, 11.5%-12.0%) of respondents reported receiving an HCV test (Table 1). HCV testing rates increased from 10.6% (95% CI, 10.1%-11.1%) in 2013 to 13.6% (95% CI, 13.0%-14.2%) in 2017 overall and among the birth cohort from 11.3% (95% CI, 10.6%-12.1%) to 15.8% (95% CI, 14.8%-16.7%) (Figure). Among respondents with ≥some college, 13.4% (95% CI, 13.1%-13.7%) reported testing; among respondents with ≤high school education, 9.1% (95% CI, 8.7%-9.5%) reported testing.
Table 1.
Weighted descriptive statistics for self-reported HCV testing among adults aged ≥18, United States, 2013-2017 a
| Characteristic | Weighted estimates | |
|---|---|---|
| Sample size, no. | % (95% CI) | |
| Received an HCV test | 27 933 983 | 11.7 (11.5-12.0) |
| Survey year | ||
| 2013 | 46 680 748 | 10.6 (10.1-11.1) |
| 2014 | 47 093 859 | 10.9 (10.3-11.4) |
| 2015 | 47 514 085 | 11.4 (10.8-11.9) |
| 2016 | 48 236 246 | 12.1 (11.6-12.7) |
| 2017 | 48 617 364 | 13.6 (13.0-14.2) |
| Birth year | ||
| Born after 1965 | 130 983 282 | 12.6 (12.3-13.0) |
| Born 1945-1965 | 79 490 134 | 12.8 (12.4-13.2) |
| Born before 1945 | 27 668 885 | 4.5 (4.1-4.8) |
| Sex | ||
| Female | 123 355 575 | 11.2 (10.9-11.5) |
| Male | 114 786 726 | 12.3 (11.9-12.6) |
| Race/ethnicity | ||
| Non-Hispanic White | 156 329 610 | 12.2 (11.9-12.5) |
| Other b | 79 531 402 | 10.6 (10.2-11.0) |
| Marital status | ||
| Married | 126 250 466 | 11.4 (11.1-11.7) |
| Single | 64 659 406 | 11.2 (10.8-11.6) |
| Separated/widowed/divorced | 46 800 887 | 13.3 (12.8-13.8) |
| Education | ||
| ≤High school graduate | 89 912 380 | 9.1 (8.7-9.5) |
| ≥Some college | 147 163 014 | 13.4 (13.1-13.7) |
| Federal poverty level | ||
| ≤100% poverty–income ratio | 30 820 340 | 12.3 (11.7-12.9) |
| >100% poverty–income ratio | 207 321 961 | 11.7 (11.4-11.9) |
| Health insurance | ||
| Private | 152 663 562 | 11.5 (11.2-11.8) |
| Medicaid/Medicare/both | 48 620 540 | 12.1 (11.6-12.6) |
| Military | 4 683 655 | 23.9 (22.1-25.8) |
| Other | 2 594 544 | 15.2 (12.9-17.4) |
| None | 28 320 288 | 10.1 (9.5-10.6) |
| Place of birth | ||
| United States | 196 294 899 | 12.3 (12.0-12.5) |
| Not United States | 41 661 068 | 9.3 (8.8-9.8) |
| Smoking status c | ||
| Never | 147 556 666 | 9.8 (9.6-10.1) |
| Former | 52 638 352 | 13.2 (12.8-13.7) |
| Current | 37 643 453 | 17.1 (16.5-17.8) |
| Alcohol consumption d | ||
| Abstain | 48 052 912 | 6.9 (6.5-7.3) |
| Former drinker | 32 676 126 | 14.2 (13.5-14.8) |
| Current drinker | 142 433 183 | 12.6 (12.3-12.9) |
| Current excessive drinker | 12 284 844 | 14.0 (13.0-15.0) |
| Body mass index | ||
| Normal/underweight (<25.0 kg/m2) | 82 855 506 | 10.8 (10.4-11.2) |
| Overweight/obese (≥25.0 kg/m2) | 148 0 792 | 12.4 (12.1-12.7) |
Abbreviation: HCV, hepatitis C virus.
aData source: Centers for Disease Control and Prevention. 36
bIncludes Hispanic, non-Hispanic Black, American Indian/Alaska Native, Asian, and multiple races.
cSmoking status determined by 2 questions: “Have you smoked at least 100 cigarettes in your entire life?” and “Do you now smoke cigarettes every day, some days, or not at all?” Never smokers were defined as those who said no to both questions. Former smokers were defined as those who said yes to the first question and no to the second question. Current smokers were defined as those who said yes to both questions.
dAlcohol status defined in the following manner: lifetime abstainer was defined as not having >12 alcoholic drinks in a lifetime. Former drinkers were defined as those who indicated they had >12 alcoholic drinks in their lifetime but had none in the past year. Current nonexcessive drinkers were defined as those who had >12 alcoholic drinks in their lifetime and, for male drinkers, had on average <15 drinks per week in the past year; for female drinkers, on average had <8 alcoholic drinks per week in the past year. Current excessive drinkers were defined as those who drink, on average, more than current nonexcessive drinkers in the past year.
Figure.
Percentage of adults aged ≥18 who self-reported being tested for hepatitis C virus infection, by birth cohort and year, United States, 2013-2017. Data source: Centers for Disease Control and Prevention. 36
In the multivariate analysis (Table 2), compared with the birth cohort, respondents born before 1945 were less likely to receive an HCV test (aOR = 0.32; 95% CI, 0.29-0.35) and respondents born after 1965 were more likely to be tested (aOR = 1.19; 95% CI, 1.13-1.25). By educational status, respondents with ≥some college were more likely than respondents with ≤high school education to be tested (aOR = 1.60; 95% CI, 1.52-1.69).
Table 2.
Weighted adjusted odds ratios for self-reported HCV testing among adults aged ≥18, United States, 2013-2017 a
| Characteristic | Weighted estimates | |
|---|---|---|
| Adjusted odds ratio (95% CI) | P value b | |
| Survey year | ||
| 2013 | 1 [Reference] | |
| 2014 | 1.03 (0.95-1.11) | .46 |
| 2015 | 1.06 (0.98-1.14) | .15 |
| 2016 | 1.10 (1.03-1.19) | .007 c |
| 2017 | 1.27 (1.18-1.36) | <.001 |
| Birth year | ||
| Born 1945-1965 | 1 [Reference] | |
| Born after 1965 | 1.19 (1.13-1.25) | <.001 |
| Born before 1945 | 0.32 (0.29-0.35) | <.001 |
| Sex | ||
| Female | 1 [Reference] | |
| Male | 1.06 (1.01-1.11) | .01 c |
| Race/ethnicity | ||
| Non-Hispanic White | 1 [Reference] | |
| Other d | 0.98 (0.93-1.03) | .47 |
| Marital status | ||
| Married | 1 [Reference] | |
| Single | 0.91 (0.86-0.96) | <.001 c |
| Separated/widowed/divorced | 1.26 (1.20-1.33) | <.001 |
| Education | ||
| ≤High school graduate | 1 [Reference] | |
| ≥Some college | 1.60 (1.52-1.69) | <.001 |
| Federal poverty level | ||
| ≤100% poverty–income ratio | 1 [Reference] | |
| >100% poverty–income ratio | 0.92 (0.86-0.98) | .009 c |
| Health insurance | ||
| Private | 1 [Reference] | |
| Medicaid/Medicare/both | 1.30 (1.23-1.38) | <.001 |
| Military | 2.11 (1.90-2.34) | <.001 |
| Other | 1.43 (1.19-1.72) | <.001 |
| None | 0.87 (0.80-0.94) | <.001 |
| Place of birth | ||
| United States | 1 [Reference] | |
| Not United States | 0.89 (0.83-0.96) | .002 c |
| Smoking status e | ||
| Never | 1 [Reference] | |
| Former | 1.37 (1.30-1.44) | <.001 |
| Current | 1.75 (1.65-1.86) | <.001 |
| Alcohol consumption f | ||
| Lifetime abstainer | 1 [Reference] | |
| Former drinker | 1.83 (1.68-1.99) | <.001 |
| Current drinker | 1.45 (1.35-1.55) | <.001 |
| Current excessive drinker | 1.45 (1.29-1.63) | <.001 |
| Body mass index | ||
| Normal/underweight (<25.0 kg/m2) | 1 [Reference] | |
| Overweight/obese (≥25.0 kg/m2) | 1.13 (1.07-1.19) | <.001 |
Abbreviation: HCV, hepatitis C virus.
aData source: Centers for Disease Control and Prevention. 36
b P < .05 was considered significant.
cEstimates considered unstable because the relative SE was ≥30% of the estimate.
dIncludes Hispanic, non-Hispanic Black, American Indian/Alaska Native, Asian, and multiple races.
eSmoking status determined by 2 questions: “Have you smoked at least 100 cigarettes in your entire life?” and “Do you now smoke cigarettes every day, some days, or not at all?” Never smokers were defined as those who said no to both questions. Former smokers were defined as those who said yes to the first question and no to the second question. Current smokers were defined as those who said yes to both questions.
fAlcohol status defined in the following manner: lifetime abstainer was defined as not having >12 alcoholic drinks in a lifetime. Former drinkers were defined as those who indicated they had >12 alcoholic drinks in their lifetime but had none in the past year. Current nonexcessive drinkers were defined as those who had >12 alcoholic drinks in their lifetime and, for male drinkers, had on average <15 drinks per week in the past year; for female drinkers, on average had <8 alcoholic drinks per week in the past year. Current excessive drinkers were defined as those who drink, on average, more than current nonexcessive drinkers in the past year.
Among demographic factors, in unadjusted analyses, all covariates except for place of birth were significantly associated with rates of HCV testing. After adjustment, respondents with Medicaid, Medicare, or both; military insurance; and other health insurance were more likely than respondents with private health insurance to be tested. Respondents with military health insurance were substantially more likely than respondents with private health insurance to be tested (aOR = 2.11; 95% CI, 1.90-2.34). Similarly, respondents enrolled in Medicaid, Medicare, or both had higher rates of HCV testing than respondents with private health insurance (aOR = 1.30; 95% CI, 1.23-1.38). Uninsured respondents were less likely than respondents with private health insurance to be tested (aOR = 0.87; 95% CI, 0.80-0.94).
Respondents who reported smoking and excessive drinking were more likely than respondents who did not engage in these behaviors to receive an HCV test. Former smokers (aOR = 1.37; 95% CI, 1.30-1.44) and current smokers (aOR = 1.75; 95% CI, 1.65-1.86) were more likely than never smokers to be tested. Former drinkers (aOR = 1.83; 95% CI, 1.68-1.99), current drinkers (aOR = 1.45; 95% CI, 1.35-1.55), and current excessive drinkers (aOR = 1.45; 95% CI, 1.29-1.63) were more likely than lifetime abstainers to be tested. Finally, respondents who were overweight or obese were more likely than respondents who were normal weight or underweight to be tested (aOR = 1.13; 95% CI, 1.07-1.19).
We found differences in unadjusted prevalence estimates by survey year for both levels of educational attainment (Table 3). Respondents born before 1945 were significantly less likely than the birth cohort to be tested, but we found no differences between the birth cohort and respondents born after 1965 for either level of educational attainment. Among those with ≤high school education, respondents who were not non-Hispanic White were less likely than non-Hispanic White respondents to get tested. Among respondents who did not have health insurance, 7.5% (95% CI, 6.9%-8.1%) of respondents with ≤high school education reported being tested, compared with 13.9% (95% CI, 12.9%-14.9%) of respondents with ≥some college. In addition, among non–US-born respondents, the percentage of respondents who reported being tested was lower in the group with ≤high school education (6.1%; 95% CI, 5.5%-6.7%) than in the group with ≥some college (12.4%; 95% CI, 11.6%-13.1%).
Table 3.
Descriptive statistics for self-reported HCV testing stratified by education among adults aged ≥18, United States, 2013-2017 a
| Characteristic | Weighted estimates | |||
|---|---|---|---|---|
| ≤High school graduate | ≥Some college | |||
| Sample size, no. | % (95% CI) | Sample size, no. | % (95% CI) | |
| Received an HCV test | 8 186 633 | 9.1 (8.7-9.5) | 19 681 742 | 13.4 (13.1-13.7) |
| Survey year | ||||
| 2013 | 18 490 363 | 8.2 (7.6-8.9) | 27 981 878 | 12.2 (11.6-12.9) |
| 2014 | 18 406 349 | 8.1 (7.3-8.9) | 28 446 963 | 12.7 (12.0-13.3) |
| 2015 | 17 652 050 | 9.1 (8.3-9.8) | 29 616 983 | 12.8 (12.1-13.4) |
| 2016 | 17 908 193 | 9.7 (9.0-10.4) | 30 142 707 | 13.6 (13.0-14.3) |
| 2017 | 17 455 425 | 10.5 (9.7-11.3) | 30 974 482 | 15.4 (14.7-16.1) |
| Birth year | ||||
| Born after 1965 | 46 099 059 | 10.1 (9.5-10.7) | 84 427 474 | 14.0 (13.6-14.4) |
| Born 1945-1965 | 29 683 895 | 10.1 (9.6-10.7) | 49 430 968 | 14.5 (14.0-15.0) |
| Born before 1945 | 14 129 426 | 3.8 (3.3-4.2) | 13 304 572 | 5.3 (4.8-5.8) |
| Sex | ||||
| Female | 45 057 509 | 8.7 (8.3-9.2) | 77 771 924 | 12.7 (12.3-13.1) |
| Male | 44 854 871 | 9.5 (9.0-10.0) | 69 391 090 | 14.2 (13.7-14.6) |
| Race/ethnicity | ||||
| Non-Hispanic White | 51 258 802 | 9.9 (9.4-10.5) | 104 579 612 | 13.4 (13.0-13.7) |
| Other b | 37 596 925 | 7.9 (7.4-8.4) | 41 367 925 | 13.2 (12.6-13.8) |
| Marital status | ||||
| Married | 42 930 726 | 8.5 (8.1-8.9) | 82 820 904 | 13.0 (12.6-13.4) |
| Single | 25 616 331 | 9.2 (8.5-9.9) | 38 794 842 | 12.5 (12.0-13.0) |
| Separated/widowed/divorced | 21 218 489 | 10.2 (9.6-10.8) | 25 291 458 | 16.0 (15.4-16.6) |
| Federal poverty level | ||||
| ≤100% poverty–income ratio | 18 536 509 | 10.7 (9.9-11.4) | 12 017 074 | 14.9 (13.9-15.9) |
| >100% poverty–income ratio | 71 375 871 | 8.7 (8.3-9.1) | 135 145 940 | 13.2 (12.9-13.6) |
| Health insurance | ||||
| Private | 42 500 169 | 8.4 (7.9-8.9) | 109 781 042 | 12.7 (12.4-13.1) |
| Medicaid/Medicare/both | 27 443 443 | 10.5 (9.9-11.1) | 20 791 058 | 14.4 (13.7-15.2) |
| Military | 1 287 489 | 22.5 (18.4-26.5) | 3 386 354 | 24.5 (22.5-26.6) |
| Other | 1 321 140 | 10.3 (7.7-12.9) | 1 256 033 | 20.5 (16.8-24.2) |
| None | 16 771 364 | 7.5 (6.9-8.1) | 11 368 012 | 13.9 (12.9-14.9) |
| Place of birth | ||||
| United States | 70 152 993 | 10.0 (9.5-10.4) | 125 523 319 | 13.6 (13.2-13.9) |
| Not United States | 19 696 059 | 6.1 (5.5-6.7) | 21 540 936 | 12.4 (11.6-13.1) |
| Smoking status c | ||||
| Never | 49 450 698 | 6.3 (5.9-6.7) | 97 491 937 | 11.7 (11.3-12.0) |
| Former | 20 078 211 | 10.3 (9.6-10.9) | 32 323 025 | 15.1 (14.5-15.7) |
| Current | 20 229 016 | 14.9 (14.0-15.8) | 17 223 089 | 19.8 (18.9-20.7) |
| Alcohol consumption d | ||||
| Abstain | 24 777 362 | 5.4 (4.9-5.9) | 22 889 796 | 8.7 (8.1-9.3) |
| Former drinker | 16 290 412 | 11.6 (10.8-12.4) | 16 201 820 | 16.8 (15.9-17.7) |
| Current drinker | 43 306 797 | 10.0 (9.4-10.5) | 98 731 339 | 13.8 (13.5-14.2) |
| Current excessive drinker | 4 282 540 | 12.8 (11.2-14.5) | 7 971 077 | 14.6 (13.3-15.9) |
| Body mass index | ||||
| Normal/underweight (<25.0 kg/m2) | 28 313 497 | 8.6 (8.0-9.2) | 54 186 538 | 12.0 (11.5-12.5) |
| Overweight/obese (≥25.0 kg/m2) | 58 829 090 | 9.5 (9.0-9.9) | 89 212 973 | 14.4 (13.9-14.8) |
Abbreviation: HCV, hepatitis C virus.
aData source: Centers for Disease Control and Prevention. 36
bIncludes Hispanic, non-Hispanic Black, American Indian/Alaska Native, Asian, and multiple races.
cSmoking status determined by 2 questions: “Have you smoked at least 100 cigarettes in your entire life?” and “Do you now smoke cigarettes every day, some days, or not at all?” Never smokers were defined as those who said no to both questions. Former smokers were defined as those who said yes to the first question and no to the second question. Current smokers were defined as those who said yes to both questions.
dAlcohol status defined in the following manner: lifetime abstainer was defined as not having >12 alcoholic drinks in a lifetime. Former drinkers were defined as those who indicated they had >12 alcoholic drinks in their lifetime but had none in the past year. Current nonexcessive drinkers were defined as those who had >12 alcoholic drinks in their lifetime and, for male drinkers, had on average <15 drinks per week in the past year; for female drinkers, on average had <8 alcoholic drinks per week in the past year. Current excessive drinkers were defined as those who drink, on average, more than current nonexcessive drinkers in the past year.
After adjustment for analyses stratified by educational attainment (Table 4), among respondents with ≤high school education, we found significant differences by survey year, birth year, marital status, poverty status, health insurance type, place of birth, smoking status, drinking status, and BMI. Respondents with ≤high school education were more likely to report HCV testing in 2017 than in 2013 (aOR = 1.26; 95% CI, 1.10-1.43). Compared with the birth cohort, respondents born before 1945 were less likely to report testing (aOR = 0.34; 95% CI, 0.29-0.39) and respondents born after 1965 were more likely to report testing (aOR = 1.27; 95% CI, 1.15-1.41). Respondents who were separated, widowed, or divorced were more likely than married respondents to report testing (aOR = 1.20; 95% CI, 1.10-1.32). Respondents with a poverty–income ratio >100% FPL were less likely than respondents with a poverty–income ratio ≤100% FPL to get tested. Respondents with military health insurance were more likely than respondents with private health insurance to report testing (aOR = 2.65; 95% CI, 2.11-3.32). In addition, respondents enrolled in Medicaid, Medicare, or both were more likely than respondents with private health insurance to report testing (aOR = 1.35; 95% CI, 1.22-1.50). Uninsured respondents were less likely than respondents with private health insurance to be tested (aOR = 0.78; 95% CI, 0.68-0.89). Non–US-born respondents were less likely than US-born respondents to report testing (aOR = 0.77; 95% CI, 0.68-0.87). For smoking and alcohol use, effect sizes were similar to effect sizes for the overall sample. The difference by BMI status was not a stable estimate because the relative SE was >30%.
Table 4.
Weighted adjusted odds ratios for self-reported HCV testing stratified by education among adults aged ≥18, United States, 2013-2017 a
| Characteristic | Weighted estimates | |||
|---|---|---|---|---|
| ≤High school graduate | ≥Some college | |||
| Adjusted odds ratio (95% CI) | P value b | Adjusted odds ratio (95% CI) | P value b | |
| Survey year | ||||
| 2013 | 1 [Reference] | 1 [Reference] | ||
| 2014 | 0.97 (0.85-1.12) | .71 | 1.06 (0.97-1.15) | .23 |
| 2015 | 1.09 (0.95-1.24) | .21 | 1.05 (0.96-1.14) | .30 |
| 2016 | 1.13 (1.00-1.29) | .06 | 1.10 (1.01-1.19) | .03 c |
| 2017 | 1.26 (1.10-1.43) | <.001 | 1.28 (1.17-1.39) | <.001 |
| Birth year | ||||
| Born 1945-1965 | 1 [Reference] | 1 [Reference] | ||
| Born after 1965 | 1.27 (1.15-1.41) | <.001 | 1.16 (1.09-1.23) | <.001 |
| Born before 1945 | 0.34 (0.29-0.39) | <.001 | 0.31 (0.28-0.35) | <.001 |
| Sex | ||||
| Female | 1 [Reference] | 1 [Reference] | ||
| Male | 0.97 (0.90-1.05) | .42 | 1.09 (1.04-1.15) | <.001 |
| Race/ethnicity | ||||
| Non-Hispanic White | 1 [Reference] | 1 [Reference] | ||
| Other d | 0.94 (0.85-1.03) | .19 | 1.01 (0.94-1.08) | .81 |
| Marital status | ||||
| Married | 1 [Reference] | 1 [Reference] | ||
| Single | 0.95 (0.85-1.05) | .30 | 0.90 (0.84-0.95) | <.001 |
| Separated/widowed/divorced | 1.20 (1.10-1.32) | <.001 | 1.29 (1.21-1.38) | <.001 |
| Federal poverty level | ||||
| ≤100% poverty–income ratio | 1 [Reference] | 1 [Reference] | ||
| >100% poverty–income ratio | 0.85 (0.78-0.94) | .002 | 0.97 (0.89-1.06) | .50 |
| Health insurance | ||||
| Private | 1 [Reference] | 1 [Reference] | ||
| Medicaid/Medicare/both | 1.35 (1.22-1.50) | <.001 | 1.25 (1.16-1.35) | <.001 |
| Military | 2.65 (2.11-3.32) | <.001 | 1.96 (1.74-2.21) | <.001 |
| Other | 1.12 (0.84-1.49) | .45 | 1.70 (1.34-2.14) | <.001 |
| None | 0.78 (0.68-0.89) | <.001 | 0.98 (0.89-1.08) | .72 |
| Place of birth | ||||
| United States | 1 [Reference] | 1 [Reference] | ||
| Not United States | 0.77 (0.68-0.87) | <.001 | 0.98 (0.90-1.07) | .68 |
| Smoking status e | ||||
| Never | 1 [Reference] | 1 [Reference] | ||
| Former | 1.56 (1.40-1.73) | <.001 | 1.30 (1.22-1.39) | <.001 |
| Current | 1.98 (1.77-2.20) | <.001 | 1.61 (1.50-1.72) | <.001 |
| Alcohol consumption f | ||||
| Abstainer | 1 [Reference] | 1 [Reference] | ||
| Former drinker | 1.85 (1.63-2.11) | <.001 | 1.82 (1.64-2.03) | <.001 |
| Current drinker | 1.49 (1.33-1.67) | <.001 | 1.43 (1.31-1.56) | <.001 |
| Current excessive drinker | 1.64 (1.36-1.99) | <.001 | 1.39 (1.21-1.59) | <.001 |
| Body mass index | ||||
| Normal/underweight (<25.0 kg/m2) | 1 [Reference] | 1 [Reference] | ||
| Overweight/obese (≥25.0 kg/m2) | 1.10 (1.01-1.20) | .033 c | 1.14 (1.07-1.22) | <.001 |
Abbreviation: HCV, hepatitis C virus.
aData source: Centers for Disease Control and Prevention. 36
b P < .05 was considered significant.
cEstimates considered unstable because the relative SE was ≥30% of the estimate.
dIncludes Hispanic, non-Hispanic Black, American Indian/Alaska Native, Asian, and multiple races.
eSmoking status determined by 2 questions: “Have you smoked at least 100 cigarettes in your entire life?” and “Do you now smoke cigarettes every day, some days, or not at all?” Never smokers were defined as those who said no to both questions. Former smokers were defined as those who said yes to the first question and no to the second question. Current smokers were defined as those who said yes to both questions.
fAlcohol status defined in the following manner: lifetime abstainer was defined as not having >12 alcoholic drinks in a lifetime. Former drinkers were defined as those who indicated they had >12 alcoholic drinks in their lifetime but had none in the past year. Current nonexcessive drinkers were defined as those who had >12 alcoholic drinks in their lifetime and, for male drinkers, had on average <15 drinks per week in the past year; for female drinkers, on average had <8 alcoholic drinks per week in the past year. Current excessive drinkers were defined as those who drink, on average, more than current nonexcessive drinkers in the past year.
Among respondents with ≥some college, we found several differences relative to the group with ≤high school education. Respondents who were single were less likely than respondents who were separated, widowed, or divorced to report HCV testing. We found no difference by poverty–income ratio. In addition, compared with respondents with private health insurance, respondents with “other” health insurance were more likely to report testing, whereas uninsured respondents were not less likely to get tested. Finally, the testing rates of non–US-born respondents did not differ from the testing rates of US-born respondents. Smoking status, drinking status, and BMI were all significant predictors in the expected direction.
Discussion
Our findings demonstrated significant increases in self-reported HCV testing rates from 2013 to 2017. However, overall self-reported testing rates were relatively low, approximately 12%. On a positive note, these self-reported testing rates increased more among the birth cohort, for whom universal testing is recommended, than among the other 2 groups. HCV testing rates among people born after 1965 were on average higher than the rates among the birth cohort, whereas rates among people born before 1945 were lower than among the birth cohort. The overall low rates of testing are consistent with findings of other studies showing late diagnosis and limited testing among people in the birth cohort and other age cohorts. 26 -29,37,38 Low testing rates and late diagnosis among the birth cohort may also be the result of lack of awareness about hepatitis C. 25 Thus, educational campaigns and strategies that promote HCV testing may be warranted.
We found large differences in testing rates by educational attainment. HCV testing also varied by race/ethnicity and place of birth, with lower rates of testing among non–US-born people than among US-born people. Similar results have been reported in other studies. 26 -29,37,38 We also found that health insurance played an important role in testing differences. Respondents with public health insurance were tested at higher rates than respondents with private health insurance, and respondents without health insurance were tested at lower rates than respondents with “other” health insurance. Future research should be conducted with the goal of developing interventions that increase HCV testing among groups that have low rates of HCV testing. When we stratified our analyses by education, we found that health insurance status was an important predictor of testing. Being uninsured was a significant predictor only for the group with ≤high school education. Together, our results suggest that education and other social determinants of health are affecting whether people are tested for HCV infection.
We found significant differences in HCV testing among modifiable health behaviors. Smoking and drinking excessive amounts of alcohol were associated with higher testing rates; this result might be expected, because people who have these behaviors may have more interactions with health care providers and, therefore, more opportunities to test, compared with people who do not smoke or drink excessively. Furthermore, obesity and excessive alcohol use can adversely affect the liver and result in laboratory findings that may prompt testing for viral hepatitis, including hepatitis C.
Future research and additional resources need to be directed toward examining how public health strategies, interventions, and policies could increase testing rates for HCV infection in the United States. Furthermore, research should be conducted to assess barriers to HCV testing. This research could include evaluation of physicians’ knowledge and awareness of CDC’s and USPSTF’s recent expansion of HCV testing recommendation to all adults aged ≥18. 22,39,40 Along with research, opportunities exist to improve health care provider education about HCV infection and the care and treatment of patients with the disease. In a 2018 study, health care providers reported limited knowledge about whom to test and how to discuss testing with their patients. 41 Offering a more detailed curriculum on hepatitis C during medical or nursing school training, continuing education opportunities for hepatitis C and patient consultation techniques, and ongoing testing campaigns can help to promote HCV testing. To encourage people to get tested for HCV infection, CDC has conducted national promotional campaigns targeted toward all adults with messages about getting tested. Other sectors outside the health care sector, such as education, could consider the Health in All Policies approach, which promotes the inclusion of health policies designed to improve health across all communities and for all people. 42 Including health messages at schools and in the workplace can help to increase opportunities for encouraging testing for HCV infection and other important infectious diseases.
Limitations
Our study had several limitations. First, NHIS uses a cross-sectional study design; therefore, test–retest reliability and causal inferences cannot be made. 36 Second, NHIS excludes institutionalized populations and people not living in households (eg, incarcerated, homeless) who are more likely not to have health insurance and are at increased risk for HCV infection; thus, our results are not generalizable to the entire US population. 5,43 Lastly, we used self-reported data on testing, which could have resulted in recall or social desirability biases. Despite these limitations, our results provide some evidence that demographic characteristics, health behaviors, and liver disease–related factors may affect HCV testing rates.
Conclusion
Rates of self-reported HCV testing increased from 2013 to 2017, but testing rates remained low. Demographic characteristics, health behaviors, and liver disease–related factors may affect HCV testing rates among adults. HCV testing must increase to achieve hepatitis C elimination targets. 14 -16
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Hope King, PhD, MSPH https://orcid.org/0000-0002-3278-4968
References
- 1. Centers for Disease Control and Prevention . Viral hepatitis surveillance—United States, 2018. July 2020. Accessed January 15, 2020. https://www.cdc.gov/hepatitis/statistics/SurveillanceRpts.htm
- 2. McHutchison JG., Lawitz EJ., Shiffman ML. et al. Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N Engl J Med. 2009;361(6):580-593. 10.1056/NEJMoa0808010 [DOI] [PubMed] [Google Scholar]
- 3. Afdhal N., Zeuzem S., Kwo P. et al. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370(20):1889-1898. 10.1056/NEJMoa1402454 [DOI] [PubMed] [Google Scholar]
- 4. Ferenci P., Bernstein D., Lalezari J. et al. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370(21):1983-1992. 10.1056/NEJMoa1402338 [DOI] [PubMed] [Google Scholar]
- 5. Hofmeister MG., Rosenthal EM., Barker LK. et al. Estimating prevalence of hepatitis C virus infection in the United States, 2013-2016. Hepatology. 2019;69(3):1020-1031. 10.1002/hep.30297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thomas DL., Astemborski J., Rai RM. et al. The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA. 2000;284(4):450-456. 10.1001/jama.284.4.450 [DOI] [PubMed] [Google Scholar]
- 7. Westbrook RH., Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014;61(1 suppl):S58-S68. 10.1016/j.jhep.2014.07.012 [DOI] [PubMed] [Google Scholar]
- 8. Moorman AC., Rupp LB., Gordon SC. et al. Long-term liver disease, treatment, and mortality outcomes among 17,000 persons diagnosed with chronic hepatitis C virus infection: current chronic hepatitis cohort study status and review of findings. Infect Dis Clin North Am. 2018;32(2):253-268. 10.1016/j.idc.2018.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ly KN., Hughes EM., Jiles RB., Holmberg SD. Rising mortality associated with hepatitis C virus in the United States, 2003-2013. Clin Infect Dis. 2016;62(10):1287-1288. 10.1093/cid/ciw111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ly KN., Xing J., Klevens RM., Jiles RB., Holmberg SD. Causes of death and characteristics of decedents with viral hepatitis, United States, 2010. Clin Infect Dis. 2014;58(1):40-49. 10.1093/cid/cit642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moorman AC., Gordon SC., Rupp LB. et al. Baseline characteristics and mortality among people in care for chronic viral hepatitis: the Chronic Hepatitis Cohort Study. Clin Infect Dis. 2013;56(1):40-50. 10.1093/cid/cis815 [DOI] [PubMed] [Google Scholar]
- 12. Volk ML., Tocco R., Saini S., Lok ASF. Public health impact of antiviral therapy for hepatitis C in the United States. Hepatology. 2009;50(6):1750-1755. 10.1002/hep.23220 [DOI] [PubMed] [Google Scholar]
- 13. Zibbell JE., Asher AK., Patel RC. et al. Increases in acute hepatitis C virus infection related to a growing opioid epidemic and associated injection drug use, United States, 2004 to 2014. Am J Public Health. 2018;108(2):175-181. 10.2105/AJPH.2017.304132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Buckley GJ., Strom BL, eds. Eliminating the Public Health Problem of Hepatitis B and C in the United States: Phase One Report. National Academies Press; 2016. [PubMed] [Google Scholar]
- 15. Strom BL., Buckley GJ, eds. A National Strategy for the Elimination of Hepatitis B and C: Phase Two Report. National Academies Press; 2017. [PubMed] [Google Scholar]
- 16. US Department of Health and Human Services . Viral Hepatitis National Strategic Plan for the United States: A Roadmap to Elimination (2021-2025). 2020. Accessed January 15, 2021. https://www.hhs.gov/hepatitis/viral-hepatitis-national-strategic-plan/index.html
- 17. Alter MJ., Margolis HS., Bell BP. et al. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR Recomm Rep. 1998;47(RR-19):1-39. [PubMed] [Google Scholar]
- 18. US Public Health Service . Updated US Public Health Service guidelines for the management of occupational exposures to HBV, HCV, and HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep. 2001;50(RR-11):1-52. [PubMed] [Google Scholar]
- 19. Masur H., Holmes KK., Kaplan JE. Introduction to the 1999 USPHS/IDSA guidelines for the prevention of opportunistic infections in persons infected with human immunodeficiency virus. Clin Infect Dis. 2000;30(suppl 1):S1-S4. 10.1086/313847 [DOI] [PubMed] [Google Scholar]
- 20. Smith BD., Morgan RL., Beckett GA. et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945-1965. MMWR Recomm Rep. 2012;61(RR-4):1-32. [PubMed] [Google Scholar]
- 21. Summaries for patients: screening for hepatitis C virus infection in adults: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159(5):1-32. 10.7326/0003-4819-159-5-201309030-00677 [DOI] [PubMed] [Google Scholar]
- 22. Schillie S., Wester C., Osborne M., Wesolowski L., Ryerson AB. CDC recommendations for hepatitis C screening among adults—United States, 2020. MMWR Recomm Rep. 2020;69(2):1-17. 10.15585/mmwr.rr6902a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jemal A., Fedewa SA. Recent hepatitis C virus testing patterns among baby boomers. Am J Prev Med. 2017;53(1):e31-e33. 10.1016/j.amepre.2017.01.033 [DOI] [PubMed] [Google Scholar]
- 24. Kim H-S., Yang JD., El-Serag HB., Kanwal F. Awareness of chronic viral hepatitis in the United States: an update from the National Health and Nutrition Examination Survey. J Viral Hepat. 2019;26(5):596-602. 10.1111/jvh.13060 [DOI] [PubMed] [Google Scholar]
- 25. Denniston MM., Klevens RM., McQuillan GM., Jiles RB. Awareness of infection, knowledge of hepatitis C, and medical follow-up among individuals testing positive for hepatitis C: National Health and Nutrition Examination Survey 2001-2008. Hepatology. 2012;55(6):1652-1661. 10.1002/hep.25556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Patel EU., Mehta SH., Boon D., Quinn TC., Thomas DL., Tobian AAR. Limited coverage of hepatitis C virus testing in the United States, 2013-2017. Clin Infect Dis. 2019;68(8):1402-1405. 10.1093/cid/ciy803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kasting ML., Giuliano AR., Reich RR. et al. Hepatitis C virus screening trends: a 2016 update of the National Health Interview Survey. Cancer Epidemiol. 2019;60:112-120. 10.1016/j.canep.2019.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kasting ML., Giuliano AR., Reich RR. et al. Hepatitis C virus screening trends: serial cross-sectional analysis of the National Health Interview Survey population, 2013-2015. Cancer Epidemiol Biomarkers Prev. 2018;27(4):503-513. 10.1158/1055-9965.EPI-17-0855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nili M., Luo L., Feng X., Chang J., Tan X. Disparities in hepatitis C virus infection screening among baby boomers in the United States. Am J Infect Control. 2018;46(12):1341-1347. 10.1016/j.ajic.2018.06.008 [DOI] [PubMed] [Google Scholar]
- 30. Rehm J., Shield KD. Global burden of alcohol use disorders and alcohol liver disease. Biomedicines. 2019;7(4):99. 10.3390/biomedicines7040099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Polyzos SA., Kountouras J., Mantzoros CS. Obesity and nonalcoholic fatty liver disease: from pathophysiology to therapeutics. Metabolism. 2019;92:82-97. 10.1016/j.metabol.2018.11.014 [DOI] [PubMed] [Google Scholar]
- 32. Kim D., Kim WR. Nonobese fatty liver disease. Clin Gastroenterol Hepatol. 2017;15(4):474-485. 10.1016/j.cgh.2016.08.028 [DOI] [PubMed] [Google Scholar]
- 33. Abdel-Rahman O., Helbling D., Schöb O. et al. Cigarette smoking as a risk factor for the development of and mortality from hepatocellular carcinoma: an updated systematic review of 81 epidemiological studies. J Evid Based Med. 2017;10(4):245-254. 10.1111/jebm.12270 [DOI] [PubMed] [Google Scholar]
- 34. World Health Organization . Guidelines for the screening, care and treatment of persons with chronic hepatitis C infection. Updated April 2016. Accessed January 15, 2021. http://apps.who.int/iris/bitstream/10665/205035/1/9789241549615_eng.pdf?ua=1 [PubMed]
- 35. Rein DB., Wittenborn JS., Smith BD., Liffmann DK., Ward JW. The cost-effectiveness, health benefits, and financial costs of new antiviral treatments for hepatitis C virus. Clin Infect Dis. 2015;61(2):157-168. 10.1093/cid/civ220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Centers for Disease Control and Prevention . About the National Health Interview Survey. Accessed May 19, 2021. https://www.cdc.gov/nchs/nhis/about_nhis.htm
- 37. Spradling PR., Rupp L., Moorman AC. et al. Hepatitis B and C virus infection among 1.2 million persons with access to care: factors associated with testing and infection prevalence. Clin Infect Dis. 2012;55(8):1047-1055. 10.1093/cid/cis616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Isenhour CJ., Hariri SH., Hales CM., Vellozzi CJ. Hepatitis C antibody testing in a commercially insured population, 2005-2014. Am J Prev Med. 2017;52(5):625-631. 10.1016/j.amepre.2016.12.016 [DOI] [PubMed] [Google Scholar]
- 39. Joshi SN. Hepatitis C screening. Ochsner J. 2014;14(4):664-668. [PMC free article] [PubMed] [Google Scholar]
- 40. Falade-Nwulia O., McAdams-Mahmoud A., Irvin R. et al. Primary care providers knowledge, attitude and practices related to hepatitis C screening and treatment in the oral direct acting antiviral agents era. J Community Med Health Educ. 2016;6(5): 10.4172/2161-0711.1000481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shehata N., Austin T., Ha S., Timmerman K. Barriers to and facilitators of hepatitis C virus screening and testing: a scoping review. Can Commun Dis Rep. 2018;44(7-8):166-172. 10.14745/ccdr.v44i78a03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rudolph L., Caplan J., Ben-Moshe K., Dillon L. Health in All Policies: A Guide for State and Local Governments. American Public Health Association and Public Health Institute; 2013. [Google Scholar]
- 43. Edlin BR., Eckhardt BJ., Shu MA., Holmberg SD., Swan T. Toward a more accurate estimate of the prevalence of hepatitis C in the United States. Hepatology. 2015;62(5):1353-1363. 10.1002/hep.27978 [DOI] [PMC free article] [PubMed] [Google Scholar]