Abstract
Objectives
Syndromic surveillance can be used to enhance notifiable disease case-based surveillance. We analyzed features of varicella reported in Georgia to evaluate case detection through syndromic surveillance and to compare varicella reported through syndromic surveillance with varicella reported from all other sources.
Methods
Syndromic surveillance was incorporated into case-based varicella surveillance by the Georgia Department of Public Health (GDPH) in May 2016. A cross-sectional study design evaluated syndromic and nonsyndromic varicella reported to GDPH from May 1, 2016, through December 31, 2019. Varicella was reported by nonsyndromic sources including health care providers, schools, and laboratories. We identified syndromic varicella cases from urgent care and emergency department visit data with discharge diagnoses containing the terms “varicella” or “chickenpox.”
Results
Syndromic notifications accounted for 589 of 2665 (22.1%) suspected varicella reports investigated by GDPH. The positive predictive value was 33.1% for syndromic notifications and 31.3% for nonsyndromic notifications. Mean days from rash onset to GDPH notification was 3.2 days fewer (P < .001) among patients identified through syndromic notification than among patients identified through nonsyndromic notification. The odds of varicella identified by syndromic notification being outbreak-associated were 0.18 (95% CI, 0.09-0.36) times those of varicella identified through nonsyndromic notification.
Practice Implications
Syndromic notifications were an effective, timely means for varicella case detection. Syndromic patients were significantly less likely than nonsyndromic patients to be outbreak-associated, possibly because of early detection. Syndromic surveillance enhanced case-based reporting for varicella in Georgia and was a useful tool to improve notifiable disease surveillance.
Keywords: vaccine-preventable diseases, varicella, syndromic surveillance, infectious disease surveillance, epidemiology
Varicella (chickenpox) is a highly infectious rash illness caused by the varicella-zoster virus. 1 Before introduction of the vaccine in 1995, varicella was an endemic childhood disease in the United States, 1 -4 with approximately 4 million cases and 100-150 deaths occurring annually. 1,2 Since the introduction of routine vaccination, case and outbreak incidence and varicella-related outpatient visits and hospitalizations have decreased dramatically. 1 -6 Disease reduction facilitated the implementation of case-based surveillance in many states. 7 -9 Case-based surveillance refers to capturing data on individual cases of varicella, allowing public health entities to monitor disease trends and the effect of routine vaccination. 7,10 Routine surveillance also allows public health to provide recommendations and implement disease control measures to prevent transmission. 10
Varicella was made nationally notifiable in 2003 and notifiable in Georgia in July 2011. 7 Since then, the Georgia Department of Public Health (GDPH) has conducted case-based varicella surveillance. All suspected cases of varicella in Georgia, including breakthrough varicella, should be reported to GDPH within 7 days. Breakthrough varicella refers to varicella occurring in people who received the varicella vaccine >42 days before illness onset. 1 Health care provider diagnosis and/or laboratory confirmation is not required to report varicella in Georgia. Varicella is reported to GDPH by health care providers, laboratories, and health departments. Additional reporters include facilities where cases and outbreaks commonly occur, including day care facilities, schools, and prisons. 11 -14 GDPH routinely follows up with patients’ households 21 days (the incubation period for varicella-zoster virus) 1,10 after rash onset to capture data on any secondary transmission. In May 2016, GDPH began using syndromic surveillance data for routine varicella reporting.
Syndromic surveillance describes automated querying of data sources, such as emergency department (ED) discharge data (eg, patient chief concern, discharge diagnosis) for indicators of emerging threats and unusual disease patterns. 15,16 A priority of syndromic surveillance systems is early detection of disease outbreaks or health events. 15,17 These systems have also identified clusters of notifiable conditions (including varicella), triggering a public health response. 18 -20 In addition, specialized syndromic surveillance queries are used to enhance routine notifiable disease surveillance. 16 -19 The National Syndromic Surveillance Program publishes reports from state health departments that have used specialized queries to detect individual cases of notifiable conditions, such as arboviral diseases and mumps, that might not have been reported otherwise. 21 Some research suggests that syndromic surveillance can enhance case-based varicella surveillance by increasing case ascertainment. 17 To describe the usefulness of hospital discharge data for routine notifiable disease surveillance, we analyzed varicella reported in Georgia since the implementation of syndromic surveillance as a routine reporting source in May 2016.
We analyzed features of varicella reported in Georgia from May 1, 2016, through December 31, 2019 (the study period) to evaluate case detection through syndromic surveillance and to compare varicella reported through syndromic surveillance with varicella reported from all other sources.
Materials and Methods
Case-Based Varicella Reporting in Georgia
Reporters, including health care providers, laboratories, and childcare providers, report varicella via telephone, fax, the State Electronic Notifiable Disease Surveillance System (SendSS), electronic laboratory reporting, and syndromic surveillance notifications. SendSS is a customized, in-house application developed by GDPH that facilitates secure electronic reporting, data collection, and data storage for notifiable diseases in Georgia.
Notifiable disease reporting is required by law in Georgia; health care providers are not required to obtain patient consent to report a notifiable disease. Case investigators obtain verbal consent before patient interviews. State and district epidemiologists collect patient demographic, clinical, and epidemiologic information at the time of report via medical record reviews and patient and physician interviews. Patient demographic information includes residence, age, race, and ethnicity. Clinical information includes rash characteristics, other symptoms, and information on medical interventions (eg, diagnostic testing, hospitalizations, antiviral medications prescribed). Epidemiologic information, used to inform GDPH recommendations and response, includes indicators of disease transmission risk, such as attendance/employment at schools or day care facilities, employment in a health care setting, incarceration, and pregnancy. Case investigators routinely provide household-level prevention recommendations to patients with varicella and contact patients 21 days after rash onset to identify any transmission among contacts. We extracted varicella data from SendSS as a comma-separated values file. This research involved use of data collected through routine public health surveillance; as such, we determined that GDPH institutional review board approval was not required.
Syndromic Surveillance and Case-Based Varicella Reporting
Georgia’s syndromic surveillance system received visit data during the study period from 135 hospital EDs and urgent care facilities. Visit data included chief concern, discharge diagnosis, patient age, patient zip code, and patient identification or medical record number. Because of the high volume of varicella or chickenpox in chief concern fields and the subjective nature of this field, we evaluated discharge diagnosis, rather than chief concern, to detect varicella. Georgia’s syndromic surveillance epidemiologist and program coordinator (R.B.) queried data using Base SAS version 9.2 (SAS Institute, Inc) for visits with a discharge diagnosis field containing the terms varicella, chickenpox, or chicken pox. R.B. then sent data on suspected varicella cases via email as syndromic surveillance notifications (syndromic notifications) to state and district epidemiologists, who then followed up by calling the reporting facility or accessing the electronic medical record.
Syndromic Notifications Versus Nonsyndromic Notifications
We used a cross-sectional study design to describe patients with syndromic and nonsyndromic varicella reported during the study period. The study team matched syndromic notifications by medical record number to true-positive varicella reports with rash onset during the same period. True-positive varicella reports are reports verified as meeting the clinical, laboratory, and epidemiological criteria for national reporting, as defined by the Council for State and Territorial Epidemiologists. 1 We calculated positive predictive values (PPVs) of reporting sources by dividing the number of true-positive reports by the total number of reports. We compared features of patients with varicella reported through syndromic surveillance (syndromic patients) with features of patients with varicella reported from other sources (nonsyndromic patients). We extracted data on case features from case report forms in SendSS. Case information also indicated whether the infection was sporadic or epidemiologically linked to another known infection or outbreak. Case patients are epidemiologically linked if they have a known common exposure and exposure occurs within 1 incubation period (21 days) of one another. In Georgia, a varicella outbreak is defined as ≥3 epidemiologically linked case patients in the same setting, with transmission outside a household. We compared epidemiologic information between syndromic and nonsyndromic varicella to ascertain whether syndromic surveillance affected transmission and outbreak detection. For all comparisons, we used syndromic patients as the reference group, and we excluded missing or unknown observations.
We compared categorical features of syndromic and nonsyndromic varicella by using the χ2 test of association at a 5% significance level and compared continuous features by using a 2-sample t test at a 5% significance level. We analyzed varicella data by reporting source in Microsoft Excel (Microsoft Corp) and SAS Enterprise Guide version 7.1 (SAS Institute, Inc).
Results
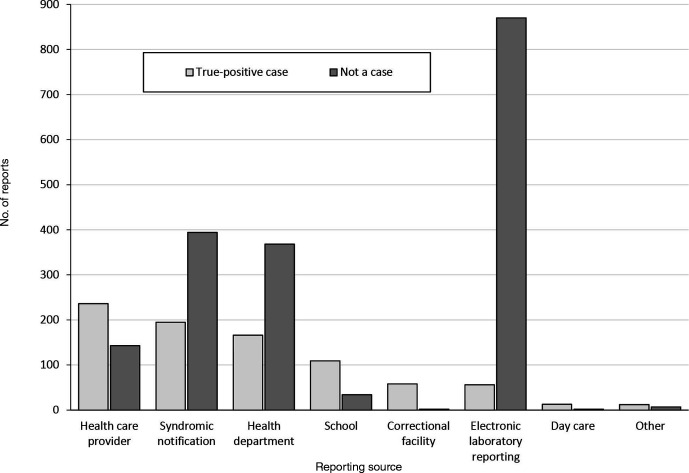
During the study period, GDPH received 2665 reports of suspected varicella diagnoses; 845 (31.7%) were classified as true-positive varicella after investigation, thus yielding an overall PPV of the GDPH varicella surveillance system of 31.7%. Of the 8 types of reporting sources, health care providers (236 cases), syndromic notifications (195 cases), and health departments (166 cases) identified the most true-positive cases (Figure 1). Syndromic notifications accounted for 589 of 2665 (22.1%) suspected varicella reports investigated by GDPH. The PPV of syndromic notifications was 33.1% (195 true positives/589 total reports), compared with a PPV of 31.3% (650 true positives/2076 total reports) for nonsyndromic notifications. In addition, syndromic notifications detected 3 household clusters and led to early detection of 2 outbreaks, one in a school and one in a community-based setting (Figure 2). Of 589 syndromic notifications, 394 (66.9%) did not detect varicella. Of these 394 notifications, 319 (81.0%) had insufficient information for ascertaining varicella and 62 (15.7%) did not meet the varicella case definition.
Figure 1.
Evaluation of varicella reporting sources in Georgia, May 1, 2016, through December 31, 2019. Electronic laboratory reporting was implemented for varicella in January 2016. Suspected varicella reports designated as “not a case” might include reports of patients with addresses outside Georgia. The health department category includes state or local health departments; these reports might include cases reported by external partners through faxed laboratory reports or telephone calls, in addition to cases diagnosed at health departments. The correctional facility category includes federal prisons, federal detention centers, US Immigration and Customs Enforcement processing centers, and state and county jails.
Figure 2.
Varicella case detection through syndromic surveillance notifications, Georgia, May 1, 2016, through December 31, 2019. Epidemiologically linked cases are cases in which patients with a known exposure occur within 1 incubation period (21 days) of one another. A household cluster is defined as ≥3 epidemiologically linked patients with varicella in the same household (not including residential settings, such as prisons and dormitories). An outbreak is defined as ≥3 epidemiologically linked patients with varicella in the same setting, with transmission outside a household. Abbreviation: GDPH, Georgia Department of Public Health.
Varicella Reporting
Syndromic varicella accounted for 195 of 845 (23.1%) true-positive varicella reports. The mean number of days from rash onset to GDPH notification was significantly fewer (P < .001) for syndromic varicella (4.7 days) than for nonsyndromic varicella (7.9 days; Table).
Table.
Comparison of syndromic and nonsyndromic patients with true-positive cases of varicella (N = 845) in Georgia, May 1, 2016, through December 31, 2019 a
| Variable | Syndromic case patients, b no. (%) (n = 195) | Nonsyndromic case patients, no. (%) (n = 650) | Odds ratio (95% CI) c | P value d |
|---|---|---|---|---|
| Epidemiologic information | ||||
| Epidemiologically linked e | 38 (19.6) | 238 (36.8) | 0.42 (0.28-0.62) | <.001 |
| Outbreak related f | 10 (5.1) | 147 (22.8) | 0.18 (0.09-0.36) | <.001 |
| Attended/employed in day care | 19 (11.1) | 75 (12.3) | 0.89 (0.52-1.52) | .67 |
| Attended/employed in school | 46 (25.0) | 213 (34.9) | 0.62 (0.43-0.90) | .01 |
| Days from rash onset to GDPH notification, mean (range) | 4.7 (0-25) | 7.9 (0-80) | 3.20 (1.79-4.62) c | <.001 |
| Patient demographic characteristics | ||||
| Male | 95 (49.0) | 358 (55.2) | 0.78 (0.56-1.07) | .12 |
| Hispanic/Latino ethnicity | 53 (29.6) | 133 (21.9) | 1.50 (1.03-2.18) | .03 |
| Black/African American race | 49 (26.5) | 103 (16.8) | 1.78 (1.21-2.63) | .003 |
| White race | 109 (58.9) | 412 (67.2) | 0.70 (0.50-0.98) | .04 |
| Asian race | 10 (5.4) | 51 (8.3) | 0.63 (0.31-1.27) | .19 |
| Other race g | 13 (7.0) | 35 (5.7) | 1.25 (0.65-2.41) | .51 |
| Multiracial, unspecified | 3 (1.6) | 11 (1.8) | 0.90 (0.25-3.27) | .88 |
| Age, mean (range), y | 7.8 (0.1-49.0) | 14.2 (0.1-85.1) | 6.37 (4.03-8.72) c | <.001 |
| Clinical information | ||||
| Breakthrough case h | 70 (39.6) | 235 (40.9) | 0.94 (0.67-1.33) | .74 |
| Laboratory test done | 32 (16.5) | 208 (32.2) | 0.42 (0.28-0.63) | <.001 |
| ≥50 Lesions | 90 (54.2) | 332 (54.6) | 0.98 (0.70-1.39) | .93 |
| Reported fever | 106 (57.0) | 262 (43.4) | 1.73 (1.24-2.41) | .001 |
| Hospitalized | 7 (3.6) | 31 (4.8) | 0.74 (0.32-1.70) | .47 |
| Complications | 12 (6.3) | 34 (5.3) | 1.19 (0.60-2.34) | .62 |
| Comorbidities | 20 (11.1) | 64 (10.5) | 1.06 (0.63-1.81) | .83 |
| Immunocompromised | 3 (1.6) | 27 (4.3) | 0.36 (0.11-1.21) | .09 |
| Received antiviral treatment | 40 (21.0) | 189 (30.3) | 0.61 (0.42-0.90) | .01 |
Abbreviation: GDPH, Georgia Department of Public Health.
aData source: Georgia State Electronic Notifiable Disease Surveillance System (SendSS), a customized, in-house application developed by GDPH. Syndromic case patients were defined as those whose case of varicella was reported through syndromic surveillance; nonsyndromic case patients were defined as those whose case of varicella was reported from all other sources. Missing or unknown observations were excluded from analysis.
bSyndromic case is reference group.
cFor categories “days from rash onset to GDPH notification” and “age,” values are mean difference (95% CI).
d P values determined by χ2 test of association, except for categories “days from rash onset to GDPH notification” and “age,” where the pooled P value for the 2-sample t test is indicated. Significance set at P ≤ .05.
ePatients with a known exposure occurring within 1 incubation period (21 days) of one another.
fAt least 3 epidemiologically linked case patients with varicella in the same setting, with transmission outside a household.
gIncludes American Indian/Alaska Native and other race, unspecified.
hReceived varicella vaccine >42 days before illness onset.
Patient Demographic Characteristics
The mean age of syndromic patients (7.8 years) was significantly lower than the mean age of nonsyndromic patients (14.2 years; P < .001; Table). The odds of a syndromic patient being Hispanic or Latino were 1.50 (95% CI, 1.03-2.18) times those of a nonsyndromic patient. Syndromic patients, compared with nonsyndromic patients, were also significantly more likely to be Black/African American and significantly less likely to be White, not controlling for ethnicity. The odds of a syndromic patient being Black were 1.78 (95% CI, 1.21-2.63) times the odds of a nonsyndromic patient. The odds of a syndromic patient being White were 0.70 (95% CI, 0.50-0.98) times the odds of a nonsyndromic patient.
Epidemiologic Information
Syndromic varicella was more likely than nonsyndromic varicella to be sporadic (Table). The odds of a syndromic patient being epidemiologically linked to another known patient were 0.42 (95% CI, 0.28-0.62) times the odds of a nonsyndromic patient. In addition, the odds of a syndromic patient being associated with an outbreak were 0.18 (95% CI, 0.09-0.36) times the odds of a nonsyndromic patient.
The odds of syndromic patients being enrolled or employed in school were 0.62 (95% CI, 0.43-0.90) times the odds of nonsyndromic patients. We found no significant difference between the proportion of syndromic patients who attended or were employed in a day care facility as compared with nonsyndromic patients.
Clinical Features
The proportion of patients with breakthrough varicella was not significantly different among syndromic and nonsyndromic patients (Table). The odds of a syndromic patient reporting fever were 1.73 (95% CI, 1.24-2.41) times the odds of a nonsyndromic patient. We found no significant differences among other markers of disease severity. The odds of a syndromic patient receiving diagnostic testing were 0.42 (95% CI, 0.28-0.63) times the odds of a nonsyndromic patient, and the odds of a syndromic patient receiving antiviral treatment were 0.61 (95% CI, 0.42-0.9) times the odds of a nonsyndromic patient.
Discussion
Syndromic notifications detected the second highest number of varicella cases compared with other reporting sources and more cases than common reporting sources, such as schools. 7,9 Syndromic notifications also detected 2 outbreaks and were an effective component of routine varicella surveillance. One outbreak was a large, ongoing outbreak in a private school, which might not have been reported otherwise. Household cluster detection through syndromic surveillance also allowed public health to rapidly provide prevention recommendations, such as keeping patients out of school and day care until no longer infectious. Even when syndromic notifications did not lead to early detection of an outbreak, follow-up allowed public health to identify patients associated with known outbreaks and capture additional information. For example, 1 notification alerted public health to the hospitalization of a previously reported outbreak-associated case. Collecting complete case information for hospitalized cases is a varicella surveillance priority. 10
The time from rash onset to GDPH notification was significantly shorter among syndromic varicella reports than among nonsyndromic reports, indicating that using syndromic notifications can allow for earlier case investigations. This indication accords with a primary aim of syndromic surveillance systems: to increase early disease and outbreak detection. 22,23 Syndromic patients were less likely than nonsyndromic patients to be epidemiologically linked or outbreak associated, but whether this factor affected report timeliness is unclear. Early public health intervention can prevent transmission, particularly among groups at high risk of infection, sometimes by coordinating administration of postexposure prophylaxis (PEP) among vulnerable contacts (eg, people without varicella vaccination or history of varicella, immunocompromised people, pregnant people) shortly after exposure. 1,10 Infants aged <12 months are at risk for varicella because they are too young to receive the varicella vaccine. 1 Timeliness of reporting is critical for coordinating PEP administration. 4,10 To prevent or modify varicella, PEP must be administered within 5 days of exposure for patients who can receive live vaccines and within 10 days of exposure for patients with contraindications to live vaccines. 4 During the study period, a varicella syndromic notification alerted the case investigator early enough to arrange for a patient’s newborn contact to receive PEP. However, syndromic patients were also less likely than nonsyndromic patients to be enrolled or employed in a school, and schools are among the most common settings for US varicella outbreaks. 9,10,12 These findings indicate that the patient setting may also affect the likelihood of a case patient to transmit varicella.
Comparison between syndromic and nonsyndromic varicella also identified the education needs of health care providers. Syndromic patients were significantly less likely than nonsyndromic patients to have received diagnostic testing, indicating that some EDs and urgent care facilities diagnose varicella according to clinical presentation. Testing is encouraged among people who have been vaccinated against varicella, because symptoms of breakthrough varicella are often milder than symptoms of nonbreakthrough varicella, and the rash might appear atypically. 10 The similar proportion of breakthrough varicella among syndromic and nonsyndromic patients could indicate a need for setting-specific outreach to health care providers. Few published data exist on the prevalence of varicella diagnostic testing in outpatient settings. One survey among pediatric health care providers in Philadelphia showed that 13% of respondents would order testing to confirm varicella for a patient with a varicella-like rash, and more than half would order testing if the patient had been exposed. 24 Because varicella vaccination is part of routine childhood vaccinations, 4 it is important for public health to continue educating health care providers about diagnostic testing recommendations for varicella. 10 In addition, laboratory confirmation should be considered when assessing the administration of antiviral treatment. 1 Differences in testing between syndromic and nonsyndromic patients might also inform our finding that syndromic patients were significantly less likely than nonsyndromic patients to be prescribed antiviral treatment.
Our study also helped describe populations that visit EDs or urgent care facilities in Georgia. Several factors, including access to a usual care provider, socioeconomic status, and underlying medical conditions, affect whether a patient is likely to seek care in an ED or urgent care setting for a nonemergent concern. 25,26 In our study, syndromic patients were significantly younger than nonsyndromic patients. Research on the use of pediatric health care shows that younger age groups use urgent care facilities and EDs at a higher rate than older age groups do. Two studies by Montalbano et al examined Medicaid claims for US pediatric patients from 2010-2012 and 2013; both studies found that children aged 6-12 years accounted for the largest proportion of overall visits to urgent care facilities. 27,28 However, Medicaid claims from 2010-2012 showed that patients aged 0-2 years accounted for a larger proportion of ED visits than older age groups did, 28 and 2013 Medicaid claims data showed that children aged 3-5 years were more likely than other age groups to have high levels of urgent care use. 27 In our study, syndromic patients were also significantly more likely than nonsyndromic patients to have reported fever, in accordance with other research on ED and urgent care use in pediatric populations. In both Medicaid studies by Montalbano et al, fever was among the most frequent discharge diagnoses for urgent care visits. 27,28
We found that syndromic patients were significantly more likely to have reported Hispanic or Latino ethnicity than non-Hispanic ethnicity. Not controlling for ethnicity, syndromic patients were also more likely than nonsyndromic patients to have their race reported as Black and less likely as White. Data on US ambulatory care use suggest that patients reporting Hispanic or Latino ethnicity might be more likely than patients reporting non-Hispanic or Latino ethnicity to seek nonemergent care in these settings. 26 However, the 2013 pediatric Medicaid claims study 27 found that patients were more likely to be reported as non-Hispanic White than Hispanic. The same study also reported non-Hispanic Black patients as less likely than non-Hispanic White patients to have high urgent care use. 27 In another study, Zhang et al 29 used 2005-2016 National Hospital Ambulatory Medical Survey data to describe disparities in pediatric care received at EDs and found a similar low percentage of patients (overall, 5.3%; range, 4.3%-6.0%) visiting for symptoms related to skin, nails, and hair across racial/ethnic groups. In 2021, GDPH initiated efforts to increase completeness of patient demographic information in its syndromic surveillance system. During our study period, this system did not collect data on patient ethnicity. Further analysis of all urgent care and ED visits in Georgia, perhaps using similar methodology to the Medicaid claims studies by Montalbano et al, would provide useful context for our findings on patient demographic characteristics.
Limitations
This study had several limitations. First, varicella surveillance in Georgia is passive; thus, varicella is likely underreported. In addition, the completeness of syndromic discharge data might vary by reporting facility. During our study period, 20% of facilities reporting to Georgia’s syndromic surveillance system did not report discharge diagnosis, so any varicella diagnosed at these sites might have been missed.
Second, the resources of local public health systems vary; some systems did not routinely provide data on patient race/ethnicity. To increase data completeness in our analysis, we ascertained patient demographic information, including age, race, and ethnicity, through case follow-up (percentage completeness for these variables ranged from 93% to 100%) rather than discharge data. This process limited the ability to compare patient demographic characteristics for syndromic varicella with all other ED and urgent care visits.
Third, data collected through patient interviews were based on self-report, leading to potential case misclassification. Recall bias among patients likely affected the accuracy of clinical measures of case severity (eg, rash severity). Patients might also have been unwilling to share information about their exposure history.
Lastly, varicella can be reported multiple times, by different mechanisms, such as telephone, fax, or email. Determining whether a syndromic notification was the first and/or only varicella report is necessary to fully describe the effect of syndromic surveillance on varicella detection.
Practice Implications
Syndromic surveillance identified varicella and captured reports rapidly compared with other reporting sources. Case-based varicella surveillance was enhanced through the novel use of GDPH’s existing syndromic surveillance infrastructure. Hospital discharge data collected through syndromic surveillance systems should be considered for routine detection of notifiable conditions. Because of the high volume of varicella or chickenpox in chief concern fields, our study evaluated the use of discharge diagnosis, rather than chief concern, for disease detection. Evaluating the use of chief concern to detect varicella would be helpful to further inform best surveillance practices. In addition, we queried discharge data by text terms, rather than International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes. Multiple ICD-10-CM codes (Box) are associated with the terms varicella, chickenpox, or chicken pox, 30 and during our study, we detected 3 codes: “Varicella without complication (B01.9),” “Varicella with complication NEC [necrotizing enterocolitis] (B01.89),” and “Contact with and (suspected) exposure to varicella (Z20.820).” Querying by text terms rather than ICD-10-CM codes might not be possible in other jurisdictions; the ability to do so depends on the structure of syndromic surveillance systems’ data feed.
Box. International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes potentially associated with varicella diagnoses a .
| Diagnosis text (ICD-10-CM code) |
| Varicella without complication (B01.9) Varicella-associated complications
Congenital varicella (P35.8) Contact with and (suspected) exposure to varicella (Z20.820) |
aData source: Centers for Disease Control and Prevention. 30
Our study informed the GDPH Vaccine Preventable Disease (VPD) Epidemiology Team’s surveillance for other diseases, including measles, mumps, and pertussis. The routine responsibilities for the VPD surveillance specialist position now incorporate follow-up on syndromic notifications for these diseases. In addition, we are collecting data to measure the timeliness and effectiveness of using syndromic notifications for those diseases.
In 2020, the number of some notifiable disease reports, including varicella, decreased in Georgia, possibly because of the effect of the COVID-19 pandemic on transmission dynamics, health care use, and public health capacity. Syndromic surveillance can help maintain capacity for notifiable disease reporting when prolonged public health emergencies strain the public health system. Because the incidence of COVID-19 illness remains high, syndromic surveillance might be better suited to inform overall disease trends rather than used to enhance case-based surveillance of COVID-19. However, GDPH has used methodology described herein to help identify rare COVID-19–related syndromes, such as multisystem inflammatory syndrome in children. Syndromic surveillance might also enhance surveillance of breakthrough COVID-19 as it has for varicella and other vaccine-preventable diseases. Although the incidence of COVID-19 will likely decrease because of widespread vaccination, it will remain important to identify and rapidly respond to increases in transmission. Capturing data on breakthrough COVID-19 cases will also be important to inform national data on long-term vaccine effectiveness and describe disease trends. Syndromic surveillance should be considered as a vital tool in protecting populations from infectious diseases through routine public health surveillance.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Carolyn M. Adam, MPH https://orcid.org/0000-0001-7159-2515
References
- 1. Centers for Disease Control and Prevention . Varicella. In: Hamborsky JK., Kroger A., Wolfe C., eds. Epidemiology and Prevention of Vaccine-Preventable Diseases. 13th ed. Public Health Foundation; 2015:353-372. [Google Scholar]
- 2. Lopez A., Zhang J., Marin M. Epidemiology of varicella during the 2-dose varicella vaccination program—United States, 2005-2014. MMWR Morb Mortal Wkly Rep. 2016;65(34):902-905. 10.15585/mmwr.mm6534a4 [DOI] [PubMed] [Google Scholar]
- 3. Bialek SR., Perella D., Zhang J. et al. Impact of a routine two-dose varicella vaccination program on varicella epidemiology. Pediatrics. 2013;132(5):e1134-e1140. 10.1542/peds.2013-0863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marin M., Güris D., Chaves SS., Schmid S., Seward JF. Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2007;56(RR-4):1-40. [PubMed] [Google Scholar]
- 5. Leung J., Harpaz R. Impact of the maturing varicella vaccination program on varicella and related outcomes in the United States: 1994-2012. J Pediatric Infect Dis Soc. 2016;5(4):395-402. 10.1093/jpids/piv044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhou F., Harpaz R., Jumaan AO., Winston CA., Shefer A. Impact of varicella vaccination on health care utilization. JAMA. 2005;294(7):797-802. 10.1001/jama.294.7.797 [DOI] [PubMed] [Google Scholar]
- 7. Lopez AS., Lichtenstein M., Schmid SD., Bialek S. Assessment of varicella surveillance and outbreak control practices—United States, 2012. MMWR Morb Mortal Wkly Rep. 2014;63(36):785-788. [PMC free article] [PubMed] [Google Scholar]
- 8. Din E., Bialek SR., Lopez AS. Evolution of varicella surveillance—selected states, 2000-2010. MMWR Morb Mortal Wkly Rep. 2012;61(32):609-612. [PubMed] [Google Scholar]
- 9. Leung J., Rue A., Lopez A. et al. Varicella outbreak reporting, response, management, and national surveillance. J Infect Dis. 2008;197(suppl 2):S108-S113. 10.1086/522138 [DOI] [PubMed] [Google Scholar]
- 10. Lopez AL., Leung J., Schmid SD., Marin M. Chapter 17: varicella. In: Roush SW, Baldy LM, Kirkonnell MA, eds. Manual for the Surveillance of Vaccine-Preventable Diseases. National Center for Immunizations and Respiratory Diseases; 2018. Accessed July 13, 2021. https://www.cdc.gov/vaccines/pubs/surv-manual/chpt17-varicella.pdf
- 11. Leung J., Lopez AS., Blostein J. et al. Impact of the US two-dose varicella vaccination program on the epidemiology of varicella outbreaks: data from nine states, 2005-2012. Pediatr Infect Dis J. 2015;34(10):1105-1109. 10.1097/INF.0000000000000821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lopez AS., Leung J., Marin M. Varicella outbreak surveillance in the United States, 2015-2016. Open Forum Infect Dis. 2017;4(suppl 1):S461. 10.1093/ofid/ofx163.1176 [DOI] [Google Scholar]
- 13. Murphy M., Berns AL., Bandyopadhyay U. et al. Varicella in the prison setting: a report of three outbreaks in Rhode Island and a review of the literature. Vaccine. 2018;36(37):5651-5656. 10.1016/j.vaccine.2018.07.031 [DOI] [PubMed] [Google Scholar]
- 14. Leung J., Lopez AS., Tootell E. et al. Challenges with controlling varicella in prison settings: experience of California, 2010 to 2011. J Correct Health Care. 2014;20(4):292-301. 10.1177/1078345814541535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Henning K. Overview of syndromic surveillance: what is syndromic surveillance? MMWR Morb Mortal Wkly Rep. 2004;53(suppl):5-11. [Google Scholar]
- 16. Paterson BJ., Durrheim DN. The remarkable adaptability of syndromic surveillance to meet public health needs. J Epidemiol Glob Health. 2013;3(1):41-47. 10.1016/j.jegh.2012.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. O’Connell EK., Zhang G., Leguen F., Llau A., Rico E. Innovative uses for syndromic surveillance. Emerg Infect Dis. 2010;16(4):669-671. 10.3201/eid1604.090688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yih WK., Deshpande S., Fuller C. et al. Evaluating real-time syndromic surveillance signals from ambulatory care data in four states. Public Health Rep. 2010;125(1):111-120. 10.1177/003335491012500115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eggers C., Hamilton J., Hopkins R. Utility of a syndromic surveillance system to identify disease outbreaks with reportable disease data. Online J Public Health Inform. 2014;6(1): 10.5210/ojphi.v6i1.5197 [DOI] [Google Scholar]
- 20. Borroto R., Pavlick J., Soetebier K., Williamson B., Pitcher P., Drenzek C. Detection of a salmonellosis outbreak using syndromic surveillance in Georgia. Online J Public Health Inform. 2019;11(1): 10.5210/ojphi.v11i1.9855 [DOI] [Google Scholar]
- 21. Centers for Disease Control and Prevention . National Syndromic Surveillance Program (NSSP). Syndromic surveillance in action: NSSP success stories. Accessed July 13, 2021. https://www.cdc.gov/nssp/success-stories.html
- 22. Buehler J., Hopkins R., Overhage JM., Sosin D., Tong V. Framework for evaluating public health surveillance systems for early detection of outbreaks. MMWR Morb Mortal Wkly Rep. 2004;53(RR-5):1-11. [PubMed] [Google Scholar]
- 23. Hopkins RS., Tong CC., Burkom HS. et al. A practitioner-driven research agenda for syndromic surveillance. Public Health Rep. 2017;132(1 suppl):116S-126S. 10.1177/0033354917709784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Daskalaki I., Viner KM., Perella D., Newbern EC., Johnson CC., Watson BM. Knowledge, attitudes, and practices for diagnosing breakthrough varicella in the outpatient setting. Public Health Rep. 2012;127(6):585-590. 10.1177/003335491212700608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Villani J., Mortensen K. Nonemergent emergency department use among patients with a usual source of care. J Am Board Fam Med. 2013;26(6):680-691. 10.3122/jabfm.2013.06.120327 [DOI] [PubMed] [Google Scholar]
- 26. Hong R., Baumann BM., Boudreaux ED. The emergency department for routine healthcare: race/ethnicity, socioeconomic status, and perceptual factors. J Emerg Med. 2007;32(2):149-158. 10.1016/j.jemermed.2006.05.042 [DOI] [PubMed] [Google Scholar]
- 27. Montalbano A., Rodean J., Canares T. et al. Urgent care utilization in the pediatric Medicaid population. J Pediatr. 2017;191:238-243. 10.1016/j.jpeds.2017.08.035 [DOI] [PubMed] [Google Scholar]
- 28. Montalbano A., Rodean J., Kangas J., Lee B., Hall M. Urgent care and emergency department visits in the pediatric Medicaid population. 2016;137(4): 10.1542/peds.2015-3100 [DOI] [PubMed] [Google Scholar]
- 29. Zhang X., Carabello M., Hill T., He K., Friese CR., Mahajan P. Racial and ethnic disparities in emergency department care and health outcomes among children in the United States. Front Pediatr. 2019;7:525. 10.3389/fped.2019.00525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. National Center for Health Statistics . Classification of Diseases, Functioning, and Disability. Accessed July 13, 2021. https://www.cdc.gov/nchs/icd/icd10cm.htm