Abstract
Epidemiological and mechanistic studies suggest that some US Food and Drug Administration (FDA)-approved drugs can reduce the incidence of cancer and inhibit tumor growth. Therefore, investigating FDA-approved drugs for cancer chemoprevention is a promising strategy. In this study, we screened FDA-approved drugs and found that azelnidipine, a Ca channel blocker widely used in the treatment of hypertension, inhibits the growth of esophageal squamous cell carcinoma (ESCC) in vitro and in vivo. We identified that MEK1/2 were direct targets of azelnidipine through pull-down assay and cellular thermal shift assay. Azelnidipine could suppress kinase activity of MEK1/2 through in vitro kinase assay. Hypophosphorylation of ERK1/2 decreased the levels of Cyclin D1/CDK6 in ESCC cells after azelnidipine treatment. More importantly, azelnidipine, like trametinib, inhibited the growth of ESCC in vivo. In conclusion, azelnidipine, a novel dual MEK1/2 inhibitor, exerted antitumor effects against ESCC cell lines and patient-derived xenograft in ESCC.
Keywords: esophageal squamous cell carcinoma, azelnidipine, proliferation, cell cycle, MEK1/2 inhibitor, MEK1/2-ERK1/2 signaling pathway
Graphical abstract

Azelnidipine, an FDA-approved drug, is a novel dual MEK1/2 inhibitor and suppresses ESCC growth by inhibiting the phosphorylation of ERK1/2. Azelnidipine also arrestes cells at G1 phase by reducing the levels of Cyclin D1 and CDK6. Azelnidipine may be a potent chemoprevention candidate for ESCC with a high level of MEK1/2.
Introduction
Esophageal squamous cell carcinoma (ESCC) is the seventh most common cancer worldwide1,2; it is also highly invasive and metastatic.3, 4, 5 Due to the lack of early diagnosis technology and nonspecific symptoms, more than 50% of patients with ESCC have been diagnosed at advanced stages.6 Early ESCC is often treated with surgery, while standard fluoropyrimidine-plus-platinum-based chemotherapy for advanced ESCC is the main means and has poor outcomes, with the median survival time being <1 year.7, 8, 9 Sintilimab and nivolumab are currently used to treat advanced ESCC with a median overall survival time of 15 months in combination with chemotherapy.7,10 This treatment still has a high rate of recurrence.11 Therefore, it is urgent to find a more effective cancer chemopreventive agent that can be widely used in ESCC patients for recurrence prevention.
Seven different mitogen-activated protein kinase (MEK) proteins are involved in sequence phosphorylation and transduction upstream. MEK1/2 phosphorylate serine/threonine and tyrosine residues of extracellular-regulated protein kinase 1/2 (ERK1/2), which is the only substrate of MEK1/2.12,13 The hyperactivation of the MEK-ERK pathway is an important and unresolved potential therapeutic target in many malignant tumors, such as melanoma, lung cancer, and colon cancer.14, 15, 16 Moreover, hypophosphorylation of ERK1/2 can decrease the level of cyclin-dependent kinase 6 (CDK6) and its activator Cyclin D1.17,18 Suppression of Cyclin D1/CDK6 can induces cell-cycle arrest at the G1/S phase.19 Inhibiting MEK1/2 is an effective way to inhibit the activity of the whole cascade.13,20 Activation of the MEK-ERK pathway can promote the growth of ESCC.21,22 Purpurogallin, a natural compound, could inhibit ESCC by targeting MEK1/2.4 However, the clinical trials for natural compounds are lacking. Therefore, MEK1/MEK2-based targeted therapies would have broad therapeutic potential for ESCC treatment and chemoprevention.
It became a promising strategy to retarget existing drugs for new indications, because they have safe pharmacokinetic data and can be developed with significant time and cost savings.23,24 There have been other studies showing that some Food and Drug Administration (FDA)-approved drugs are promising for cancer chemoprevention, such as nonsteroidal antiinflammatory drugs (NSAIDs) and metformin.25 Our research group recently also found FDA-approved drugs, such as Nuplazid,26 and tegaserod maleate27 have ESCC inhibitory effects.
In this study, we found azelnidipine, a Ca channel blocker widely used in the treatment of hypertension,28 inhibited the proliferation of ESCC cells in vitro and in vivo by targeting MEK1/2 and regulated downstream pathways. Also, patients with advanced ESCC may have hypertension as a side effect after chemotherapy.29 Therefore, azelnidipine may be a candidate for ESCC chemoprevention.
Results
Azelnidipine inhibits ESCC cell proliferation in vitro
To select effective drugs to inhibit ESCC proliferation, we screened an FDA-approved drug library. We discovered that azelnidipine inhibited the proliferation of ESCC cells. The chemical structure of azelnidipine was shown in Figure 1A. The antitumor effects of azelnidipine were assessed in ESCC cell lines. The half-maximal inhibitory concentration (IC50) of KYSE150 was 10.972 μM at 24 h and 9.411 μM at 48 h, the IC50 of KYSE450 was 13.822 μM at 24 h and 10.135 μM at 48 h, and the IC50 of Shantou human embryonic esophageal (SHEE), an immortalized esophageal epithelial cell, was 23.475 μM at 24 h and 19.236 μM at 48 h (Figure 1B). To investigate the effects of azelnidipine on ESCC cell growth, we measured the proliferation of ESCC and SHEE cells treated with different concentrations of azelnidipine (0, 1, 2.5, 5, and 10 μM) by cell proliferation assay. The inhibitory ratio of KYSE150 at 10 μM for 96 h was 66.43%, and the inhibitory ratio of KYSE450 was 36.05%. Azelnidipine inhibited the proliferation of ESCC cells in a concentration- and time-dependent manner and did not significantly inhibit the proliferation of SHEE (Figure 1C). Colony-formation assay and anchorage-independent cell growth assay were used to assess the effects of azelnidipine on cell colony number (Figures 1D, 1E, S1A, and S1B). The colony-formation inhibitory ratio of KYSE150 and KYSE450 was 85.23% and 84.43% at 10 μM by colony-formation assay (Figures 1D and S1A). Consistent with the result of colony-formation assay, azelnidipine also inhibited the colony-formation of ESCC cells in a concentration-dependent manner (Figures 1E and S1B). Together, these data suggest that azelnidipine can significantly inhibit ESCC cell proliferation in vitro.
Figure 1.
Azelnidipine (Az) inhibits ESCC cell proliferation in vitro
(A) Chemical structure of Az. (B) The KYSE150, KYSE450, and SHEE cells were treated to Az at 0, 5, 10, 15, and 20 μM for 24 and 48 h. The IC50 value of Az on ESCC cells and SHEE. (C) ESCC and SHEE cells were treated with Az for 24, 48, 72, and 96 h. The optical density(OD) value was evaluated by 3-(4,5)-dimethylthiahiazo (-z-y1)-3,5-di- phenytetrazoliumromide (MTT) assay and normalized to that of the control. (D) ESCC cells were treated with various concentrations of Az (0, 1, 2.5, 5, and 10 μM) for 10 days, followed by crystal violet staining to monitor colony formation. (E) ESCC cells were treated with various concentrations of Az (0, 1, 2.5, 5, and 10 μM) and measured at 9 days. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Azelnidipine can bind to MEK1 and MEK2
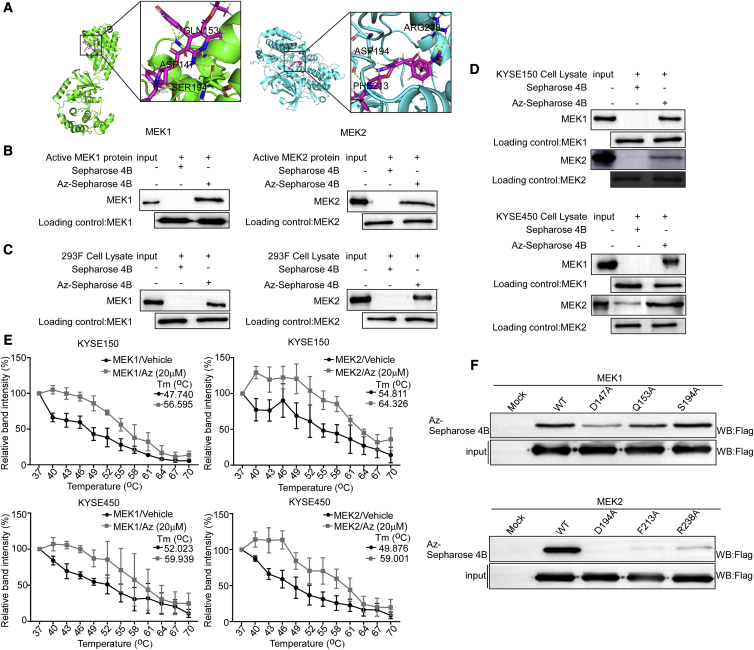
In order to explore the antitumor mechanism of azelnidipine on ESCC, we used the molecular modeling to evaluate the target of azelnidipine. We found MEK1/2 were potential targets of azelnidipine. The results declared that azelnidipine may bind with MEK1 at the binding sites Asp147, Ser194, and Gln153, while it may bind with MEK2 at the binding sites Asp194, Phe213, and Arg238 (Figure 2A). Then pull-down assays were used to prove the binding of azelnidipine with MEK1 and MEK2. First, we certified azelnidipine can bind with recombined MEK1 and MEK2 (Figure 2B). Next, we overexpressed MEK1 and MEK2 in HEK293F cells and found that azelnidipine could bind with MEK1 and MEK2 protein of HEK293F (Figure 2C). Furthermore, azelnidipine could bind with endogenous MEK1 and MEK2 by pull-down assay (Figure 2D). The results indicated that azelnidipine could bind with MEK1 and MEK2. The cellular thermal shift assay (CETSA) directly demonstrated that MEK1 and MEK2 protein were rapidly denatured and precipitated by high temperature, and the ESCC cells treated with azelnidipine were more stable (Figures 2E and S2A). The results of CETSA could further explore the mechanism of azelnidipine binding with MEK1/2 in intact cells. To further verify the sites of azelnidipine binding with MEK1/2, we constructed the mutant plasmid of MEK1/2 and expressed them in HEK293F cells. The results illustrated that the binding affinity between mutant MEK1 (D147A and Q153A), MEK2 (D194A, F213A, and R238A) protein, and azelnidipine was reduced by pull-down assay, suggesting that these sites were vital for binding (Figure 2F). Together, azelnidipine directly binds to MEK1 and MEK2.
Figure 2.
Azelnidipine can bind to MEK1 and MEK2
(A) Computational docking model between Az and MEK1 and MEK2. (B–D) Az directly binds to MEK1 and MEK2. The recombinant proteins (B) or cell lysates of HEK293F (C), KYSE150, and KYSE450 cells (D) were incubated with Az-conjugated Sepharose 4B beads or with Sepharose 4B beads alone. The results were analyzed by Western blot. (E) The binding capacity of Az to MEK1 and MEK2 in ESCC intact cells. The ESCC cells were treated with Az or DMSO for 24 h and treated with different temperatures. The protein bindings were visualized by western blot. (F) Cells ectopically expressing MEK1 (wild type [WT], mutant D147A, Q153A, or S194A) or MEK2 (WT, mutant D194A, F213A, or R238A) were incubated with Az-conjugated Sepharose 4B beads or with Sepharose 4B beads alone. The results were analyzed by Western blot.
Azelnidipine inhibits the MEK-ERK signaling pathway
To evaluate whether azelnidipine can regulate the kinase activity of MEK1 and MEK2, we used the in vitro kinase assay. The recombined active MEK1 protein and recombined MEK2 protein were treated with azelnidipine, and the phosphorylation of ERK1 and ERK2 was suppressed in a concentration-dependent manner (Figures 3A and 3B). These data manifested that azelnidipine could inhibit the kinase activity of MEK1/2. Next, we evaluated the protein levels of p-MEK1 S298, p-MEK2 T394, MEK1, and MEK2 in SHEE and ESCC cell lines (Figure S3A). The results stated that these protein levels in KYSE150 and KYSE450 were higher than in the SHEE. Then we evaluated the effect of azelnidipine on ESCC using KYSE150 and KYSE450 cell lines. We also determined whether azelnidipine affected the MEK1/2 downstream pathway. After ESCC cells were treated with azelnidipine for 24 h, azelnidipine gradually induced hypophosphorylation of ERK1/2 in a concentration-dependent manner by Western blot (Figure 3C). Further, we detected the level of p-ERK1/2 T202/Y204 and total ERK1/2 by immunofluorescence assay. The fluorescence intensity of p-ERK1/2 T202/Y204 gradually decreased with the increase of drug concentration (Figures 3D and S3B); however, the fluorescence intensity of ERK1/2 did not change with the concentration of the drug increased (Figures 3E and S3B). These results demonstrated azelnidipine could induce the inhibition of ERK1/2 phosphorylation by Western blot and immunofluorescence assay. Next, we detected the effect of azelnidipine on cell cycle in ESCC cells by flow cytometry. The results manifested that azelnidipine induced the G1/S cell-cycle arrest on ESCC cells (Figures 3F and S3C). Cyclin D1 interacts with CDK6 and is critical for G1/S transition during the cyclin process30; then the levels of Cyclin D1 and CDK6 were evaluated by Western blot. The results declared that azelnidipine could reduce the levels of Cyclin D1/CDK6 in a concentration-dependent manner (Figure 3G). These results suggest that azelnidipine can inhibit the kinase activity of MEK1/2 and down-regulate the MEK-ERK pathway, then arrest in G1 phase on ESCC cells.
Figure 3.
Azelnidipine inhibits the MEK-ERK signaling pathway
(A and B) In vitro kinase assay of active MEK1 and MEK2 and inactive ERK1 and ERK2. The active MEK1 (A) and MEK2 (B), inactive ERK1 and ERK2, and ATP mixture were treated with Az or DMSO at 30°C for 30 min, and p-ERK1/2 T202/Y204 were visualized by Western blot. (C) The levels of MEK1/2-ERK1/2 signaling pathway in ESCC cells after Az (0, 1, 2.5, 5, and 10 μM) treatment. (D and E) Immunofluorescence staining of p-ERK1/2 T202/Y204 (D) and ERK1/2 (E) in ESCC cells after Az treatment. (F) Cell cycle was analyzed by PI staining, and the number of cells in each phase was analyzed by Modfit (n = 3). (G) The levels of Cyclin D1 and CDK6 in ESCC cells after Az (0, 1, 2.5, 5, and 10 μM) treatment. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
MEK1 and MEK2 knockdown inhibits ESCC cell proliferation and reduces the sensitivity of ESCC cells to azelnidipine
To further evaluate the role of MEK1 and MEK2 kinases in ESCC cells, we constructed MEK1 or MEK2 knockdown cell lines. We verified the effect of knockdown by Western blot. The results illustrated that MEK1 or MEK2 knockdown cells had lower levels of MEK1 and MEK2 in ESCC cells, and the protein levels of p-ERK1/2 T202/Y204 decreased in shMEK1 or shMEK2 ESCC cells; however, the protein levels of ERK1/2 did not change (Figures 4A and 4B). Then we investigated whether knockdown of MEK1 or MEK2 would inhibit growth of ESCC cells. The results of the proliferation assay indicated that cell proliferation of the MEK1 or MEK2 knockdown cells was significantly reduced compared with the Mock cells (Figures 4C and 4D). We determined whether the growth of MEK1 or MEK2 knockdown cells would inhibit the colony-formation ability in ESCC by colony-formation assay. Consistent with the results of cell proliferation assay, the colony-formation ability of the MEK1 or MEK2 knockdown cells was significantly inhibited (Figures 4E, 4F, S4A, and S4B). Together, these data suggest that MEK1 and MEK2 kinases have a vital role in tumor growth of ESCC cells.
Figure 4.
MEK1 and MEK2 knockdown inhibits ESCC cell proliferation and reduces the sensitivity of ESCC cells to Azelnidipine
(A and B) The p-ERK1/2 protein expression in MEK1 (A) and MEK2 (B) knockdown KYSE 150 and KYSE 450 cells by Western blot. (C and D) The cell viability in MEK1 (C) or MEK2 (D) knockdown cells was evaluated by MTT assay and normalized to that of the control. (E and F) The colony-formation ability in MEK1 (E) or MEK2 (F) knockdown cells was normalized to that of the control. (G and H) MEK1 (G) or MEK2 (H) knockdown cells were treated with Az (0, 1, 2.5, 5, and 10 μM) for 96 h. The cell viability was evaluated by MTT assay and normalized to that of the Mock cells. (I and J) MEK1 (I) or MEK2 (J) knockdown cells were plated into six-well plates and treated with various concentrations of Az (0, 1, 2.5, 5, and 10 μM) for 10 days, followed by crystal violet staining to monitor colony formation. (K and L) EGF stimulator was treated for 30 min with MEK1 (K) or MEK2 (L) knockdown cells; then they were treated with various concentrations of Az (0 and 10 μM). The cell viability was evaluated by MTT assay and normalized to the Mock cells. (M and N) qPCR analysis was used to detect the expression levels of E2F1, Cyclin D1, and CDK6 of Az’s effect on MEK1 (M) or MEK2 (N) knockdown KYSE150 cells by EGF stimulator. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
To verify whether azelnidipine inhibits ESCC proliferation through MEK1 and MEK2, we treated MEK1 or MEK2 knockdown cells and their control cells with azelnidipine. As indicated by cell proliferation assay, the sensibility of the MEK1 or MEK2 knockdown cells to azelnidipine was significantly less than the Mock cells (Figures 4G and 4H). We further attested whether MEK1 or MEK2 knockdown cells reduced sensitivity to azelnidipine by the colony-formation assay. Consistent with the results of the cell proliferation assay, the inhibitory effect of azelnidipine on the proliferation of MEK1 or MEK2 cells was decreased compared with the Mock cells (Figures 4I, 4J, S4C, and S4D). Epidermal growth factor (EGF) can activate the mitogen-activated protein kinase (MAPK) signaling pathways and increase the p-MEK1/2 levels.31,32 We used EGF to stimulate MEK1 or MEK2 knockdown cells, then treated these cells with azelnidipine. The results revealed that MEK1 or MEK2 knockdown cells increased their sensitivity to azelnidipine after EGF treatment. Azelnidipine could significantly inhibit the proliferation of MEK1 or MEK2 knockdown cells after EGF stimulator (Figures 4K and 4L). MEK1/2 phosphorylates ERK1/2, and phosphorylated ERK1/2 regulates the expression of downstream transcription factors such as E2F1 and increases the expression of Cyclin D1 and CDK6.33 Then we assessed azelnidipine’s effect on the mRNA levels of E2F1, Cyclin D1, and CDK6 before and after MEK/ERK signaling activation by EGF in MEK1 and MEK2 knockdown cells by qPCR. In MEK1 and MEK2 knockdown cells, azelnidipine did not alter the mRNA levels of E2F1, Cyclin D1, and CDK6. After EGF stimulation on MEK1 or MEK2 knockdown cells, azelnidipine significantly decreased the mRNA levels of E2F1, Cyclin D1, and CDK6 (Figures 4M, 4N, S4E, and S4F). These results indicated that azelnidipine inhibits ESCC cells depending on MEK1/2 kinase.
Azelnidipine inhibits PDX tumor growth in vivo
To investigate the effect of azelnidipine on esophageal cancer (EC) in vivo, we established EC patient-derived xenograft (PDX) models in severe combined immunodeficiency (SCID) mice. Azelnidipine inhibited the growth of EC models EG20 and LEG34 following 42 and 18 days with a daily 2 or 20 mg/kg schedule via oral gavage, and the tumor sizes treated with azelnidipine were smaller compared with the vehicle group (Figure 5A). In order to digitally understand the suppression of EC PDX tumor growth, we weighed the tumors. The tumor weights of the azelnidipine-treated group were significantly reduced compared with the vehicle group (EG20: 66.45% at 2 mg/kg group and LEG34: 49.60% at 2 mg/kg group; Figure 5B), and the results indicated azelnidipine had a significant inhibition on EC PDX tumor growth. The results of tumor volume declared that azelnidipine inhibited the growth of EC (Figure 5C). Next, we explored the effect of azelnidipine on Ki67 and p-ERK1/2 T202/Y204 levels by immunohistochemistry (IHC) staining. The results showed that azelnidipine could significantly inhibit the levels of Ki67 and p-ERK1/2 T202/Y204 in tumor tissues compared with the vehicle group (Figures 5D and S5). These results prove that azelnidipine inhibits tumor growth by the MEK-ERK pathway.
Figure 5.
Azelnidipine inhibits PDX tumor growth in vivo
(A) EG20 and LEG34 SCID mice received 2 and 20 mg/kg Az. Tumor images of different groups after sacrifice. (B and C) The tumor weights (B) and tumor volumes (C) of vehicle and Az groups were measured. (D) Immunohistochemistry was used to analyze the level of Ki67 and p-ERK1/2 T202/Y204 levels in tumor tissues from treated or untreated groups of mice. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Azelnidipine inhibits tumor growth compared with another MEK1/2 inhibitor, trametinib
Trametinib is a MEK1/2 inhibitor in clinical application.34 According to our study, azelnidipine is an inhibitor of MEK1/2. Then we compared the inhibitory effects between azelnidipine and trametinib in the PDX model. The tumor images of different groups were displayed in Figure 6A. Then we weighed the tumors. The antitumor rate of the 2 mg/kg group was 71.67%, the antitumor rate of the 20 mg/kg group was 73.79%, and the antitumor rate of the trametinib group was 77.15% (Figure 6B); the tumor weights of the azelnidipine-treated group were significantly reduced compared with the vehicle group. There was no statistical difference in antitumor rate among the trametinib- and azelnidipine-treated groups. We then measured the volumes of tumors in all four groups of mice. The tumor volumes of the 20 mg/kg group were similar to those of the trametinib group (Figure 6C). Subsequently, we detected the levels of p-ERK1/2 T202/Y204 and Ki67 in tumor tissues by IHC staining. The results showed that the levels of p-ERK1/2 T202/Y204 and Ki67 in tumor tissues were significantly lower than those of the vehicle group (Figures 6D and S6). Interestingly, we found the trametinib-treated mice had significantly reduced body weight compared with other groups on the 24th day (Figure 6E). In conclusion, azelnidipine has the same antitumor effect as trametinib.
Figure 6.
Azelnidipine inhibits tumor growth compared with another MEK1 inhibitor, trametinib
(A) Tumor images of LEG110 SCID mice received 2 and 20 mg/kg Az and 2 mg/kg trametinib. (B and C) The tumor volumes (B) and tumor weights (C) of vehicle, Az, and trametinib groups were measured. (D) Immunohistochemistry was used to analyze the protein levels of Ki67 and p-ERK1/2 T202/Y204 in tumor tissues from treated or untreated groups of mice. (E) Body weights of vehicle, Az, and trametinib groups were measured. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Discussion
Because FDA-approved drugs have established pharmacokinetics, stronger safety, and lower toxicity, drug repurposing can shorten the clinical trial cycles. FDA-approved drugs are a great asset for finding effective chemoprevention drugs for ESCC. Currently, trametinib, the second generation of MEK1/2 inhibitors, has been put into clinical application for many cancers,35 such as melanoma, breast cancer, and non-small cell lung cancer. Studies have shown that trametinib effectively inhibits the growth of ESCC and increases median survival,36 which provides powerful evidence for clinical application. However, trametinib in clinical has significant adverse reactions, such as rash, diarrhea, peripheral edema, and fatigue.37 Also, according to our research, trametinib significantly reduced body weight in mice. Therefore, the high-efficiency and non-toxic MEK1/2 inhibitors are needed.
Through screening of FDA-approved drug libraries, we found that azelnidipine had a significant inhibitory effect on ESCC cells. Also, azelnidipine inhibited ESCC cell proliferation in a concentration- and time-dependent manner in vitro (Figure 1). It has been reported that azelnidipine may be used in cancer immunotherapy for colorectal cancer cells,38 which significantly inhibited the growth of colorectal cancer by enhancing the quantity and activation of CD8+ T cells in the tumor tissues. However, the mechanism by which azelnidipine inhibits the proliferation of ESCC is not studied. This study provides a theoretical basis for clinical application of azelnidipine on ESCC.
Activation of the MEK-ERK signaling pathway occurs frequently in cancers.15,39 In the MEK-ERK pathway, MEK1/2 is a “gatekeeper,” which conducts signals from multiple upstream regulators to ERK1/2,13 and the high activity of MEK1/2 directly affects cell proliferation and drug resistance.16,40,41 We predicted by computational modeling that MEK1 and MEK2 may be the potential targets of azelnidipine and found that azelnidipine binds with MEK1 at Asp147, Ser194, and Gln153 and MEK2 at Asp194, Phe213, and Arg238. The full-length MEK1 or MEK2 kinase protein consists of 399 and 400 amino acids, respectively. Asp147, Ser194, and Gln153 are located in the kinase domain of MEK1 protein.42 Azelnidipine binds in the novel binding pocket of MEK1, which is separated from but adjacent to the MgATP site by Asp147 and Gln153. Similarly, Asp194, Phe213, and Arg238 were also in the kinase domain of MEK2 protein, and azelnidipine was located in the binding pockets through Asp194, Phe213, and Arg23 because the structure of MEK2 is highly homologous to MEK1 (Figure 2F). These data indicated that azelnidipine may be a novel noncompetitive inhibitor of MEK1/2. Our results also illustrated that MEK1/2 knockdown inhibits the proliferation of ESCC cells, which reduces the drug sensitivity to azelnidipine, and MEK1 and MEK2 might play an important role in ESCC pathogenesis and chemoprevention. Therefore, azelnidipine inhibits the growth of ESCC in vivo and in vitro by targeting MEK1/2. ERK1/2 acts as a cascade downstream of the MEK/ERK signaling pathway, which can decrease the levels of CDK6 and Cyclin D1.17,18 Our study demonstrated that azelnidipine reduces the levels of p-ERK1/2 T202/Y204, CDK6, and Cyclin D1 in ESCC cells, which arrested cells to G1 phase.
Esophagectomy is the first choice for advanced ESCC.43 The main postoperative complication of patients treated with esophagostomy was hypertension.44 It has also been reported that cancer patients are exposed to several types of kidney injury, such as obstructive and functional kidney injury, because acute kidney injury has a high mortality rate,12,45 and this can lead to hypertension. Also, hypertension is a basic disease of the elderly.46 The low dose (2 mg/kg) of azelnidipine was calculated according to the clinical dose.47 Then our studies showed that azelnidipine inhibited the growth of the PDX model of ESCC in vivo (Figure 5). Furthermore, the azelnidipine group had a similar inhibitory effect in PDX mice compared with the trametinib group in LEG110, another ESCC PDX model. Azelnidipine has no obvious toxic side effects in the clinical treatment of hypertension.48 Therefore, azelnidipine is a valuable FDA-approved drug targeting MEK1/2 in the chemoprevention of ESCC.
Our results suggest that azelnidipine can directly bind to MEK1/2, inhibit their kinase activity, and suppress the growth of ESCC in vivo and in vitro. This identified that appropriate application of azelnidipine may be a beneficial chemoprevention strategy for ESCC patients with high MEK1/2 protein levels.
Materials and methods
Cell culture
KYSE150 and KYSE450 were bought from the Culture Collection of Chinese Academy of Sciences (Shanghai, China), and the SHEE cell line was obtained from Dr. Enmin Li.
Reagents and antibodies
Azelnidipine was purchased from Meilunbio (Dalian, China). Antibodies to detect MEK1 (#12671S), MEK2 (#9125S),p-ERK1/2 T202/Y204 (#94370S), and ERK1/2 (#9102S) were purchased from CST (Beverly, MA, USA)Antibodies to detect p-MEK1 S298 (ab96379), p-MEK2 T394 (ab30622), Cyclin D1 (ab124821), CDK6 (ab16667), and Ki67 (ab16667) were obtained from Abcam (Cambridge, MA, USA). Antibodies to measure FLAG (M185-3L) were acquired from HuaBio (Hangzhou, China).
Cytotoxicity assay and proliferation assay
Cells (KYSE150, KYSE450, and SHEE) were treated with various concentrations of azelnidipine (0, 5, 10, 15, and 20 μM) for 24 and 48 h. Cells (KYSE150, KYSE450, and SHEE) were treated with various concentrations of azelnidipine (0, 1, 2.5, 5, and 10 μM) for 0, 24, 48, 72, and 96 h. The OD values were determined by MTT assay. The plates were added by MTT (5 mg/mL, 10 μL/well) and were incubated at 37°C for 2 h. A total of 150 μL DMSO was added to each well, and it detected the OD value of each well at 570 nm. IC50 values were calculated in GraphPad Prism 7.0.
Colony-formation assay
Cells (KYSE150 and KYSE450) were treated with various concentrations of azelnidipine (0, 1, 2.5, 5, and 10 μM) in six-well plates. Then the cells colonies were dyed by crystal violet. The cell colonies were photographed and counted.
Anchorage-independent cell growth
KYSE150 and KYSE450 cells (8,000 cells/well) were seeded in 10% FBS and 0.3% agar in the top gel, which was treated with various concentrations of azelnidipine (0, 1, 2.5, 5, and 10 μM). After incubation for 7 days, the colonies were photographed by IN Cell Analyzer 6000.
Computational modeling of MEK1/2 with azelnidipine
To simulate the binding of azelnidipine to MEK1/2, we downloaded the crystal structures of MEK1 (PDB: 5EYM) and MEK2 (PDB: 1S9I) from the PDB database (https://www.rcsb.org/pdb). The structural formula for azelnidipine was available for download from the PubChem database (https://pubchem.ncbi.nlm.nih.gov/). The AutoDock 4.2.6 software was used for docking analysis. The optimal complex was visualized by PyMOL (PyMOLmolecular graphics system, version 2.3.4).
Western blot
KYSE150 and KYSE450 cells were treated with various concentrations of azelnidipine (0, 1, 2.5, 5, and 10 μM). The collected protein was determined from the concentration by the protein assay kit (BCA Protein Assay Kit; Beyotime; China). Then we used SDS-PAGE gels to separate the same amount of protein and used the polyvinylidene fluoride (PVDF) membrane to transfer the separated protein for 2 h at 90 V. After incubating with fat-free milk for 1 h, primary antibody for 4°C was hatched overnight. Finally, we incubated with corresponding secondary antibodies and exposed PVDF membrane.
Pull-down assay
Azelnidipine-conjugated Sepharose 4B beads (Sepharose 4B beads as control) were incubated with MEK1 and MEK2 protein or cell lysates (1,000 μg) in reaction buffer overnight at 4°C. Also, azelnidipine-conjugated Sepharose 4B beads and Sepharose 4B beads were washed three times in the washing buffer. The binding was assessed by Western blot. The composition of washing buffer was 2 mg/mL BSA, 50 mM Tris-HCl (pH 7.4), 200 mM NaCl, 5 mM EDTA, 1 mM dithiothreitol, and 0.01% Nonidet P-40 (NP-40).
CETSA
KYSE150 and KYSE450 cells (4.5 × 106) were treated with azelnidipine (0 and 20 μM). Then they were harvested and resuspended in PBS. The cells were divided into 12 tubes equally. The control group and azelnidipine treatment group were heated at 37°C, 40°C, 43°C, 46°C, 49°C, 52°C, 55°C, 58°C, 61°C, 64°C, 67°C, and 70°C for 3 min. Then the samples were quickly frozen twice in liquid nitrogen and centrifuged at 12,000 rpm for 20 min at 4°C. The proteins were transferred to new tubes and still on ice for 30 min. Finally, the samples were analyzed by Western blot.
In vitro kinase assay
The active MEK1 and MEK2 (100 ng) were blended by various concentrations of azelnidipine in kinase buffer II (Cat #K02–09; SignalChem, Canada). Then the inactive ERK1 and ERK2 proteins (200 ng), 100 μM adenosine triphosphate (ATP), and kinase buffer II were added and incubated for 30 min at 30°C. The reactions were terminated by adding loading buffer and heated for 5 min at 95°C, and MEK1/2 activity was detected by p-ERK1/2 T202/Y204 antibody.
Immunofluorescence assay
The slides were put in a 24-well plate, and then 2 × 104 KYSE150 or KYSE450 cells were seeded in each well. After 16–18 h, cells were treated with various concentrations of azelnidipine for 24 h. The medium was then aspirated from the plate, and the cells were fixed with 4% paraformaldehyde in PBS for 30 min. The cells were washed three times with PBS and incubated primary antibody (p-ERK1/2 T202/Y204 and ERK1/2 1:50) overnight at 4°C. Next, slides were incubated with the fluorescent secondary antibody (1:50) for 1.5–2 h in the dark. After several washes, slides were stained with DAPI (1:10,000) for 5 min at 37°C. The images were captured and analyzed by using IN Cell Analyzer 6000 software.
Cell-cycle assay
After 48 h of treatment with azelnidipine for ESCC, cell pellets were collected and fixed to 70% ethanol. A total of 10 mg/mL RNase A (R1030; Solarbio) was added and incubated at room temperature for 1 h. Fixed cells were added with 50 μg/mL propidium iodide (PI) and incubated in a dark room for 30 min. The stained cells were analyzed by flow cytometry, and the FlowJo software was used to process the data.
Knockdown of MEK1/2 by short hairpin RNA (shRNA)
We inserted shRNA for human MEK1 and MEK2 into the pLKO.1 vector. ESCC cells were transfected with shRNA, and the sequence of shRNA was shown in Table 1. Western blot was used to verify the transfection efficiency.
Table 1.
The oligonucleotide sequences of MEK1 and MEK2 short hairpin RNA (shRNA)
| Gene name | Primer sequences 5′–3′ |
|---|---|
| MEK1#1 | F: CCGGGTCCTACATGTCGCC AGAAAGCTCGAGCTTTCTGG CGACATGTAGGACTTTTTG |
| R: AATTCAAAAAGTCCTACATG TCGCCAGAAAGCTCGAGCT TTCTGGCGACATGTAGGAC |
|
| MEK1#2 | F: CCGGGCTTCTATGGTGCGT TCTACACTCGAGTGTAGAA CGCACCATAGAAGCTTTTTG |
| R: AATTCAAAAAGCTTCTATGG TGCGTTCTACACTCGAGTGT AGAACGCACCATAGAAGC |
|
| MEK2#1 | F: CCGGTTTGAACTCCTGGAC TATATTCTCGAGAATATAGT CCAGGAGTTCAAATTTTTG |
| R: AATTCAAAAATTTGAACTCC TGGACTATATTCTCGAGAAT ATAGTCCAGGAGTTCAAA |
|
| MEK2#2 | F: CCGGTTCCAGGAGTTTGTCA ATAAACTCGAGTTTATTGAC AAACTCCTGGAATTTTTG |
| R: AATTCAAAAATTCCAGGAG TTTGTCAATAAACTCGAGTT TATTGACAAACTCCTGGAA |
F, forward; R, reverse.
qPCR
Total RNA isolation was achieved by using TRIzol Reagent (15596018; Ambion) and the manufacturer’s instructions. RT-PCR was performed to generate cDNA in a total volume of 20 μL. qRT-PCR was accomplished with an SYBR Green kit (Q712-02; Vazyme) and a 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). Specific primer sequences are listed in Table 2.
Table 2.
The oligonucleotide sequences of qPCR primers
| Primer | Sequence (5′–3′) |
|---|---|
| CDK6-F | GGATAAAGTTCCAGAGCCTGGAG |
| CDK6-R | GCGATGCACTACTCGGTGTGAA |
| CyclinD1-F | TCTACACCGACAACTCCATCCG |
| CyclinD1-R | TCTGGCATTTTGGAGAGGAAGTG |
| E2F1-F | GGATCTGGAGACTGACCATCAG |
| E2F1-R | GGTTTCATAGCGTGACTTCTCCC |
PDX model
This study was performed by the Bioethics Committee of Zhengzhou University Institutional Animal Care and Use Committee. SCID/CB17 mice were purchased from the Beijing Vital River Laboratory Animal Technology (Beijing, China). First, the tumor tissues of 15–20 mm3 were implanted under the back of mice. When the tumor tissue grew to about 100 mm3, the mice were randomly divided into three groups as follows: (1) vehicle, (2) azelnidipine treatment (2 mg/kg), and (3) azelnidipine treatment (20 mg/kg). Tumor volumes were calculated by the following formula: tumor volume (mm3) = (length × width2)/2. Finally, the tumor tissues were removed when they grew to about 1,000 mm3.
IHC
The tumor tissues were embedded in paraffin and cut into 4-μm sections for IHC staining. After the slides were cutted, xylene and ethanol were used for dewaxing and dehydration. Next, the slides were processed for antigen retrieval. Then 3% H2O2 was dropped onto the slides for 8 min. The tissues were incubated with primary antibody Ki67 and p-ERK1/2 T202/Y204 at 4°C overnight. The slides were incubated with HRP-IgG secondary antibody for 20 min at 37°C. Finally, the slides were stained with diaminobenzidine (DAB) for 2 min and restained with hematoxylin. TissueFAXS (version 4.2; TissueGnostics) was used to scan the slides, and the Image-Pro Plus software program (Media Cybernetics, Rockville, MD, USA) was used to calculate positive cells.
Statistical analysis
Significant differences were assessed by using SPSS 21.0. Statistical analysis was performed by one-way ANOVA or non-parametric test. The quantitative results were expressed as the mean ± SD. p < 0.05 was considered statistically significant.
Data availability
The data that support the findings of this study are available from the corresponding author on reasonable request.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81872335), National Natural Science Youth Foundation (81902486), The Central Plains Science and Technology Innovation Leading Talents (224200510015), and the Science and Technology Project of Henan Province (212102310187).
Author contributions
K.L., Y.J., and X. Li designed the research. L.Z. and Y.Z. carried out the experiments, performed data analysis, and wrote the manuscript. A.L., X. Lu, M.Z.L., Q.Y., N.Y., and X.Z. participated in part of the experiments. All authors reviewed and approved the final version of the manuscript.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.omto.2022.09.007.
Contributor Information
Xin Li, Email: lixin0930@zzu.edu.cn.
Yanan Jiang, Email: yananjiang@zzu.edu.cn.
Kangdong Liu, Email: kdliu@zzu.edu.cn.
Supplemental information
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Tong M., Chan K.W., Bao J.Y.J., Wong K.Y., Chen J.N., Kwan P.S., Tang K.H., Fu L., Qin Y.R., Lok S., et al. Rab25 is a tumor suppressor gene with antiangiogenic and anti-invasive activities in esophageal squamous cell carcinoma. Cancer Res. 2012;72:6024–6035. doi: 10.1158/0008-5472.CAN-12-1269. [DOI] [PubMed] [Google Scholar]
- 3.Song W., Wang K., Yang X., Dai W., Fan Z. Long noncoding RNA BANCR mediates esophageal squamous cell carcinoma progression by regulating the IGF1R/Raf/MEK/ERK pathway via miR3383p. Int. J. Mol. Med. 2020;46:1377–1388. doi: 10.3892/ijmm.2020.4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie X., Zu X., Liu F., Wang T., Wang X., Chen H., Liu K., Wang P., Liu F., Zheng Y., et al. Purpurogallin is a novel mitogen-activated protein kinase kinase 1/2 inhibitor that suppresses esophageal squamous cell carcinoma growth in vitro and in vivo. Mol. Carcinog. 2019;58:1248–1259. doi: 10.1002/mc.23007. [DOI] [PubMed] [Google Scholar]
- 5.Yu J., Wang W., Yao W., Yang Z., Gao P., Liu M., Wang H., Chen S., Wang D., Wang W., Sun G. Gambogic acid affects ESCC progression through regulation of PI3K/AKT/mTOR signal pathway. J. Cancer. 2020;11:5568–5577. doi: 10.7150/jca.41115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wei L., Wang B., Hu L., Xu Y., Li Z., Shen Y., Huang H. MEX3A is upregulated in esophageal squamous cell carcinoma (ESCC) and promotes development and progression of ESCC through targeting CDK6. Aging (Albany NY) 2020;12:21091–21113. doi: 10.18632/aging.103196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doki Y., Ajani J.A., Kato K., Xu J., Wyrwicz L., Motoyama S., Ogata T., Kawakami H., Hsu C.H., Adenis A., et al. Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. N. Engl. J. Med. 2022;386:449–462. doi: 10.1056/NEJMoa2111380. [DOI] [PubMed] [Google Scholar]
- 8.Lee S.J., Kim S., Kim M., Lee J., Park Y.H., Im Y.H., Park S.H. Capecitabine in combination with either cisplatin or weekly paclitaxel as a first-line treatment for metastatic esophageal squamous cell carcinoma: a randomized phase II study. BMC Cancer. 2015;15:693. doi: 10.1186/s12885-015-1716-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moehler M., Maderer A., Thuss-Patience P.C., Brenner B., Meiler J., Ettrich T.J., Hofheinz R.D., Al-Batran S.E., Vogel A., Mueller L., et al. Cisplatin and 5-fluorouracil with or without epidermal growth factor receptor inhibition panitumumab for patients with non-resectable, advanced or metastatic oesophageal squamous cell cancer: a prospective, open-label, randomised phase III AIO/EORTC trial (POWER) Ann. Oncol. 2020;31:228–235. doi: 10.1016/j.annonc.2019.10.018. [DOI] [PubMed] [Google Scholar]
- 10.Xu J., Li Y., Fan Q., Shu Y., Yang L., Cui T., Gu K., Tao M., Wang X., Cui C., et al. Clinical and biomarker analyses of sintilimab versus chemotherapy as second-line therapy for advanced or metastatic esophageal squamous cell carcinoma: a randomized, open-label phase 2 study (ORIENT-2) Nat. Commun. 2022;13:857. doi: 10.1038/s41467-022-28408-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly R.J., Ajani J.A., Kuzdzal J., Zander T., Van Cutsem E., Piessen G., Mendez G., Feliciano J., Motoyama S., Lièvre A., et al. Adjuvant Nivolumab in resected esophageal or gastroesophageal junction cancer. N. Engl. J. Med. 2021;384:1191–1203. doi: 10.1056/NEJMoa2032125. [DOI] [PubMed] [Google Scholar]
- 12.Zennadi R. MEK1/2 as a therapeutic target in sickle cell disease. Int. J. Blood Res. Disord. 2019;6:38. doi: 10.23937/2469-5696/1410038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caunt C.J., Sale M.J., Smith P.D., Cook S.J. MEK1 and MEK2 inhibitors and cancer therapy: the long and winding road. Nat. Rev. Cancer. 2015;15:577–592. doi: 10.1038/nrc4000. [DOI] [PubMed] [Google Scholar]
- 14.Gilmartin A.G., Bleam M.R., Groy A., Moss K.G., Minthorn E.A., Kulkarni S.G., Rominger C.M., Erskine S., Fisher K.E., Yang J., et al. GSK1120212 (JTP-74057) is an inhibitor of MEK activity and activation with favorable pharmacokinetic properties for sustained in vivo pathway inhibition. Clin. Cancer Res. 2011;17:989–1000. doi: 10.1158/1078-0432.CCR-10-2200. [DOI] [PubMed] [Google Scholar]
- 15.Burotto M., Chiou V.L., Lee J.M., Kohn E.C. The MAPK pathway across different malignancies: a new perspective. Cancer. 2014;120:3446–3456. doi: 10.1002/cncr.28864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCubrey J.A., Steelman L.S., Chappell W.H., Abrams S.L., Wong E.W.T., Chang F., Lehmann B., Terrian D.M., Milella M., Tafuri A., et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim. Biophys. Acta. 2007;1773:1263–1284. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiu C.Y., Kuo K.K., Kuo T.L., Lee K.T., Cheng K.H. The activation of MEK/ERK signaling pathway by bone morphogenetic protein 4 to increase hepatocellular carcinoma cell proliferation and migration. Mol. Cancer Res. 2012;10:415–427. doi: 10.1158/1541-7786.MCR-11-0293. [DOI] [PubMed] [Google Scholar]
- 18.Liu L., Zhu Y., Xu Y., Reiter R.J. Prevention of ERK activation involves melatonin-induced G(1) and G(2)/M phase arrest in the human osteoblastic cell line hFOB 1.19. J. Pineal Res. 2012;53:60–66. doi: 10.1111/j.1600-079X.2011.00971.x. [DOI] [PubMed] [Google Scholar]
- 19.Dozier C., Mazzolini L., Cénac C., Froment C., Burlet-Schiltz O., Besson A., Manenti S. CyclinD-CDK4/6 complexes phosphorylate CDC25A and regulate its stability. Oncogene. 2017;36:3781–3788. doi: 10.1038/onc.2016.506. [DOI] [PubMed] [Google Scholar]
- 20.Chesnokov M.S., Khan I., Park Y., Ezell J., Mehta G., Yousif A., Hong L.J., Buckanovich R.J., Takahashi A., Chefetz I. The MEK1/2 pathway as a therapeutic target in high-grade serous ovarian carcinoma. Cancers (Basel) 2021;13:1369. doi: 10.3390/cancers13061369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen B., Liu S., Gan L., Wang J., Hu B., Xu H., Tong R., Yang H., Cristina I., Xue J., et al. FGFR1 signaling potentiates tumor growth and predicts poor prognosis in esophageal squamous cell carcinoma patients. Cancer Biol. Ther. 2018;19:76–86. doi: 10.1080/15384047.2017.1394541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maehara O., Suda G., Natsuizaka M., Ohnishi S., Komatsu Y., Sato F., Nakai M., Sho T., Morikawa K., Ogawa K., et al. Fibroblast growth factor-2-mediated FGFR/Erk signaling supports maintenance of cancer stem-like cells in esophageal squamous cell carcinoma. Carcinogenesis. 2017;38:1073–1083. doi: 10.1093/carcin/bgx095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ashburn T.T., Thor K.B. Drug repositioning: identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 2004;3:673–683. doi: 10.1038/nrd1468. [DOI] [PubMed] [Google Scholar]
- 24.Würth R., Thellung S., Bajetto A., Mazzanti M., Florio T., Barbieri F. Drug-repositioning opportunities for cancer therapy: novel molecular targets for known compounds. Drug Discov. Today. 2016;21:190–199. doi: 10.1016/j.drudis.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 25.Huang Z., Wang X., Zou Q., Zhuang Z., Xie Y., Cai D., Bai L., Tang G., Huang M., Luo Y., Yu H. High platelet-to-lymphocyte ratio predicts improved survival outcome for perioperative NSAID use in patients with rectal cancer. Int. J. Colorectal Dis. 2020;35:695–704. doi: 10.1007/s00384-020-03528-8. [DOI] [PubMed] [Google Scholar]
- 26.Wei Y., Wu W., Jiang Y., Zhou H., Yu Y., Zhao L., Wu X., Lu X., Yuan Q., Wang Z., et al. Nuplazid suppresses esophageal squamous cell carcinoma growth in vitro and in vivo by targeting PAK4. Br. J. Cancer. 2021;126:1037–1046. doi: 10.1038/s41416-021-01651-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu X., Wang Z., Jiang Y., Zhou H., Li A., Wei Y., Bao Z., Wang D., Zhao J., Chen X., et al. Tegaserod maleate inhibits esophageal squamous cell carcinoma proliferation by suppressing the peroxisome pathway. Front. Oncol. 2021;11:683241. doi: 10.3389/fonc.2021.683241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takihata M., Nakamura A., Kondo Y., Kawasaki S., Kimura M., Terauchi Y. Comparison of azelnidipine and trichlormethiazide in Japanese type 2 diabetic patients with hypertension: the COAT randomized controlled trial. PLoS One. 2015;10:e0125519. doi: 10.1371/journal.pone.0125519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng Z., Wei J., Wang F., Ying J., Deng Y., Gu K., Cheng Y., Yuan X., Xiao J., Tai Y., et al. Camrelizumab combined with chemotherapy followed by camrelizumab plus apatinib as first-line therapy for advanced gastric or gastroesophageal junction adenocarcinoma. Clin. Cancer Res. 2021;27:3069–3078. doi: 10.1158/1078-0432.CCR-20-4691. [DOI] [PubMed] [Google Scholar]
- 30.Qi Y., Wang D., Huang W., Wang B., Huang D., Xiong F., Chen X., Chen Y. CyclinD1 inhibits dicer and crucial miRNA expression by chromatin modification to promote the progression of intrahepatic cholangiocarcinoma. J. Exp. Clin. Cancer Res. 2019;38:413. doi: 10.1186/s13046-019-1415-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang C., Jin Y., Marchetti M., Lewis M.R., Hammouda O.T., Edgar B.A. EGFR signaling activates intestinal stem cells by promoting mitochondrial biogenesis and beta-oxidation. Curr. Biol. 2022;32:3704–3719.e7. doi: 10.1016/j.cub.2022.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Teh J.L.F., Cheng P.F., Purwin T.J., Nikbakht N., Patel P., Chervoneva I., Ertel A., Fortina P.M., Kleiber I., HooKim K., et al. In vivo E2F reporting reveals efficacious schedules of MEK1/2-CDK4/6 targeting and mTOR-S6 resistance mechanisms. Cancer Discov. 2018;8:568–581. doi: 10.1158/2159-8290.CD-17-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shen Q., Uray I.P., Li Y., Krisko T.I., Strecker T.E., Kim H.T., Brown P.H. The AP-1 transcription factor regulates breast cancer cell growth via cyclins and E2F factors. Oncogene. 2008;27:366–377. doi: 10.1038/sj.onc.1210643. [DOI] [PubMed] [Google Scholar]
- 34.Lima B., Abreu M.H., Sousa S., Bartosch C., Pereira D. Impressive and durable clinical responses obtained with dabrafenib and trametinib in low-grade serous ovarian cancer harbouring a BRAF V600E mutation. Gynecol. Oncol. Rep. 2022;40:100942. doi: 10.1016/j.gore.2022.100942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watanabe M., Iizumi Y., Sukeno M., Iizuka-Ohashi M., Sowa Y., Sakai T. The pleiotropic regulation of cyclin D1 by newly identified sesaminol-binding protein ANT2. Oncogenesis. 2017;6:e311. doi: 10.1038/oncsis.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao M., Scott S., Evans K.W., Yuca E., Saridogan T., Zheng X., Wang H., Korkut A., Cruz Pico C.X., Demirhan M., et al. Combining neratinib with CDK4/6, mTOR, and MEK inhibitors in models of HER2-positive cancer. Clin. Cancer Res. 2021;27:1681–1694. doi: 10.1158/1078-0432.CCR-20-3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Srinivas N.R. Pharmacology of pimasertib, A selective MEK1/2 inhibitor. Eur. J. Drug Metab. Pharmacokinet. 2018;43:373–382. doi: 10.1007/s13318-018-0466-x. [DOI] [PubMed] [Google Scholar]
- 38.Zhou X., Jiao L., Qian Y., Dong Q., Sun Y., Zheng W.V., Zhao W., Zhai W., Qiu L., Wu Y., et al. Repositioning azelnidipine as a dual inhibitor targeting CD47/SIRPalpha and TIGIT/PVR pathways for cancer immuno-therapy. Biomolecules. 2021;11:706. doi: 10.3390/biom11050706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dhillon A.S., Hagan S., Rath O., Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–3290. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- 40.Zhang W., Liu H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002;12:9–18. doi: 10.1038/sj.cr.7290105. [DOI] [PubMed] [Google Scholar]
- 41.Liu S., Zha J., Lei M. Inhibiting ERK/Mnk/eIF4E broadly sensitizes ovarian cancer response to chemotherapy. Clin. Transl. Oncol. 2018;20:374–381. doi: 10.1007/s12094-017-1724-0. [DOI] [PubMed] [Google Scholar]
- 42.Ohren J.F., Chen H., Pavlovsky A., Whitehead C., Zhang E., Kuffa P., Yan C., McConnell P., Spessard C., Banotai C., et al. Structures of human MAP kinase kinase 1 (MEK1) and MEK2 describe novel noncompetitive kinase inhibition. Nat. Struct. Mol. Biol. 2004;11:1192–1197. doi: 10.1038/nsmb859. [DOI] [PubMed] [Google Scholar]
- 43.Ising M.S., Smith S.A., Trivedi J.R., Martin R.C., Phillips P., Van Berkel V., Fox M.P. Minimally invasive esophagectomy is associated with superior survival compared to open surgery. Am. Surg. 2022 doi: 10.1177/00031348221078962. [DOI] [PubMed] [Google Scholar]
- 44.Liu X.L., Wang R.C., Liu Y.Y., Chen H., Qi C., Hu L.W., Yi J., Wang W. Risk prediction nomogram for major morbidity related to primary resection for esophageal squamous cancer. Medicine (Baltimore) 2021;100:e26189. doi: 10.1097/MD.0000000000026189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aloy B., Janus N., Isnard-Bagnis C., Deray G., Launay-Vacher V. [Renal toxicity of anticancer drugs] Nephrol. Ther. 2021;17:553–563. doi: 10.1016/j.nephro.2021.09.001. [DOI] [PubMed] [Google Scholar]
- 46.Ye Z., Li X., Han Y., Wu Y., Fang Y. Association of long-term exposure to PM2.5 with hypertension and diabetes among the middle-aged and elderly people in Chinese mainland: a spatial study. BMC Public Health. 2022;22:569. doi: 10.1186/s12889-022-12984-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miyoshi T., Onoue G., Ito H. Effect of switching to azilsartan from fixed-dose combination of an angiotensin II receptor blocker and calcium channel blocker or a thiazide in patients with hypertension. J. Clin. Med. Res. 2019;11:202–207. doi: 10.14740/jocmr3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jadhav U., Mohanan P.P., Almeida A.F., Abraham G., Khan M.Y., Gaurav K., Mane A., Vikas S., Jain M., Meel B. Effectiveness and effect on renal parameters of amlodipine vs. Other dihydropyridine calcium channel blockers in patients with essential hypertension: retrospective observational study based on real-world evidence from electronic medical records. Cardiol. Ther. 2021;10:465–480. doi: 10.1007/s40119-021-00224-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on reasonable request.