Summary
Background
Breastfeeding has been associated with a reduced maternal long-term risk of chronic diseases, but its association with mortality is poorly known.
Methods
We included 166,708 female United States (US) nurses from the Nurses’ Health Study (1986-2016) and the Nurses’ Health Study II (1989-2019) who experienced at least one pregnancy lasting at least six months across their reproductive lifespan. Hazard ratios and 95% confidence intervals (CI) for mortality according to lifetime breastfeeding duration were estimated with time-dependent Cox proportional hazards regression models.
Findings
During 4,705,160 person-years of follow-up, 36,634 deaths were documented in both cohorts, including 9880 from cancer and 7709 from cardiovascular disease (CVD). Lifetime total breastfeeding duration was associated with a lower subsequent risk of all-cause mortality in a non-linear manner (p-value for non-linearity=0.0007). The pooled multivariable-adjusted hazard ratios of all-cause mortality were 0.95 (95% CI: 0.92 to 0.98), 0.94 (95% CI: 0.91 to 0.98), 0.93 (95% CI: 0.90 to 0.97), and 0.93 (95% CI: 0.89 to 0.97), respectively, for women reporting lifetime total breastfeeding duration of 4–6, 7–11, 12–23, and ≥24 months, compared to women who breastfed for ≤3 months over their reproductive lifespan. Cause-specific analysis showed a similar pattern of non-linear inverse associations between lifetime total breastfeeding duration and CVD and cancer mortality (both p-values for non-linearity <0.01). There was no evidence of interactions between breastfeeding duration and pre-pregnancy lifestyle factors on mortality risk.
Interpretation
Parous women with longer lifetime breastfeeding duration had a modestly lower risk of mortality.
Funding
The National Institutes of Health grants.
Keywords: Breastfeeding duration, Mortality, Cohort study, Women's health
Research in context.
Evidence before this study
Emerging evidence shows that breastfeeding is associated with a reduced maternal long-term risk of hypertension, endometriosis, diabetes, cardiovascular disease, metabolic syndrome, and certain types of cancer (e.g., ovarian and breast cancer), probably by increasing energy expenditure and providing a positive impact on lipid metabolism, glucose homeostasis, and insulin secretion. We searched PubMed and Web of Science from inception until March 22, 2022, using search terms (“breastfeeding” OR “lactation”) AND (“mortality” OR “death”) AND (“adulthood”). However, the cumulative role of lifetime breastfeeding on maternal mortality, a major public health focus, is poorly known.
Added value of this study
Among 166,708 parous women from two large prospective cohorts, we found non-linear associations between lifetime breastfeeding duration and the long-term risk of total, cancer, and cardiovascular disease mortality during adulthood. These non-linear associations were further confirmed in the cubic spline, where women who breastfed for a total of 18-24 months experienced the lowest risk. There was no evidence of interactions between breastfeeding duration and pre-pregnancy smoking status, dietary quality, alcohol consumption, physical activity, and overweight or obesity on mortality risk.
Implications of all the available evidence
Our results strengthen and refine the evidence of lifelong benefits of breastfeeding for mothers and highlight the importance for policymakers and social communities to protect, promote, and support breastfeeding and for healthcare professionals to inform its advantages to mothers and infants among the high-risk population with low breastfeeding initiation.
Alt-text: Unlabelled box
Introduction
The World Health Organization (WHO) has suggested exclusive breastfeeding for the first 6 months of infants’ life, followed by continued breastfeeding as solid foods are introduced for up to 2 years of age or beyond.1 In the United States (US), similar guidelines have also been recommended by major health organizations, such as the American Academy of Pediatrics and the Centres for Disease Control and Prevention.2 Although these initiatives have been established for more than two decades, the prevalence of exclusive breastfeeding is only about one-third among children younger than 6 months in low- and middle-income countries.3 In high-income countries, the estimated proportion of breastfeeding at 12 months is lower than 20%.3
Breastfeeding offers unrivalled benefits for all children by improving their survival, growth, and lifelong health.3 Meanwhile, emerging evidence shows that breastfeeding is associated with a reduced maternal long-term risk of hypertension,4 endometriosis,5 diabetes,4 cardiovascular diseases (CVD),6 metabolic syndromes,7 and certain types of cancer (e.g., ovarian and breast cancer),8, 9, 10 probably by increasing energy expenditure and providing a positive impact on lipid metabolism, glucose homeostasis, and insulin secretion.11 However, the cumulative role of a woman's lifetime breastfeeding on subsequent risk of mortality, a major public health focus, is poorly known. To fill the data gap, we evaluated the association of lifetime breastfeeding duration with the risk of all-cause and cause-specific mortality among women from two large ongoing prospective cohorts, the Nurses’ Health Study (NHS) and the Nurses’ Health Study II (NHS II), with continuous follow-up spanning three decades. Because lifestyle factors such as cigarette smoking, nutrition, physical activity, and overweight or obesity are important determinants of mortality and can be influenced by breastfeeding practices,12,13 we also investigated the potential interaction between breastfeeding duration and these lifestyle factors across women's reproductive lifespan on mortality risk.
Methods
Study population
The NHS was initiated in 1976 by recruiting 121,700 female US nurses aged 30–55 years at entry. The NHS II began in 1989 by recruiting 116,429 female US nurses aged 25–42 years. In both cohorts, data on demographic characteristics, reproductive and lifestyle factors, and medical history were biennially collected via postal or electronic questionnaires, with a follow-up rate of >90% for each survey cycle. Study procedures have been approved by the institutional review boards of the Brigham and Women's Hospital and Harvard T.H. Chan School of Public Health (Protocol number: 2009-P-002375), and those of participating registries as required. Returning completed questionnaires indicated informed consent. This study was conducted and reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement.
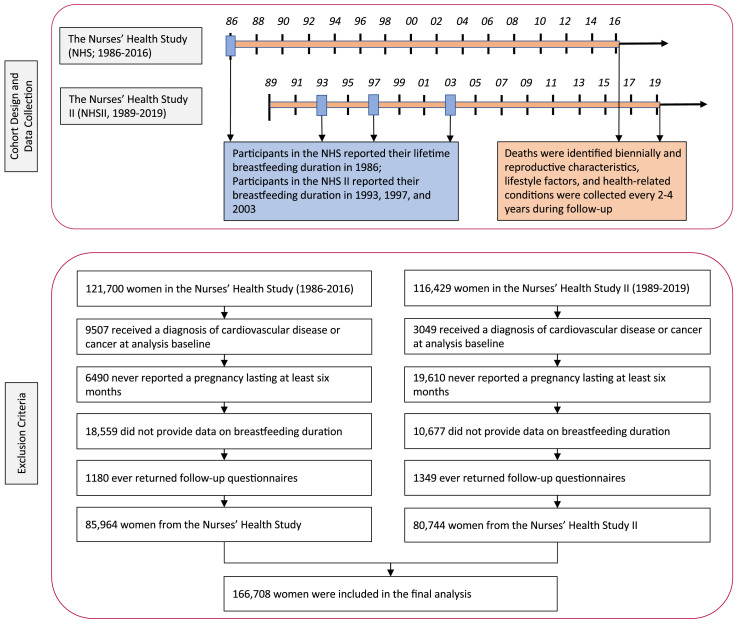
We excluded women who reported cancer or CVD at the analysis baseline, had missing data on breastfeeding duration, or never returned follow-up questionnaires (Figure 1). Our current analysis was finally limited to 166,708 parous women experiencing at least one pregnancy that lasted at least six months (85,964 in the NHS and 80,744 in the NHS II). The baseline demographic characteristics and prepregnancy lifestyle factors were similar between included women and those excluded due to missing data on breastfeeding duration (Appendix Table 1).
Figure 1.
Flow diagram for study design, data collection, and exclusion criteria.
Assessment of breastfeeding duration
Participants in the NHS reported their lifetime breastfeeding duration as “cannot remember”, “did not breastfeed”, “<1”, “1–3”, “4–6”, “7–11”, “12–17”, “18–23”, “24–35”, “36–47”, and “≥48 months” in 1986, when most of them had completed their reproductive lifespan. Participants in the NHS II reported their breastfeeding duration in 3 follow-up questionnaires. In 1993, women initially reported their cumulative breastfeeding duration as “cannot remember”, “did not breastfeed”, “<1”, “1–3”, “4–6”, “7–11”, “12–17”, “18–23”, “24–35”, “36–47”, and “≥48 months”. In 1997, a more detailed questionnaire was used to assess the breastfeeding duration of women reporting breastfeeding for each of their first 4 children. Participants who breastfed more than 4 children reported a total of additional months they breastfed for all subsequent children. Women reported additional births after 1997 by completing a similar breastfeeding questionnaire in 2003 when most women had completed their reproductive lifespan.5 To allow comparison with the data from NHS, we calculated the cumulative breastfeeding duration after each birth that the participants reported any breastfeeding for each survey cycle. In 1997 and 2003, women who had breastfed for at least 1 month were also asked “At what month did you start giving formula or purchased milk at least once daily?” and “At what month did you start giving solid food at least once daily?”, which could be reported as “0–2”, “3”, “4–5”, “6–7”, “8–11”, and “≥12 months”. We defined cumulative exclusive breastfeeding duration as the period of time during which women fed infants breast milk without any other liquid or solid food.5 Existing studies have consistently shown that both self-reported breastfeeding initiation and duration are highly reliable.14 Among 146 women whose daughters participated in the NHS, the correlation coefficient of their breastfeeding duration recalled on two occasions 2 years apart was 0.89, indicating high reproducibility.15
Ascertainment of deaths
Deaths in the NHS and NHS II were identified from state vital statistics records and the National Death Index or by reports from next of kin or the postal authorities, which were able to correctly identify >97% of deaths from clinical records.16 Physicians used the Eighth and Ninth Revisions of the International Classification (ICD-8 and ICD-9) to identify the underlying cause of death due to CVD and cancer by reviewing autopsy reports, death certificates, and medical records (Appendix Table 2).17
Assessment of covariates
In both cohorts, data on race/ethnicity, birthplace, and height were collected at recruitment or during follow-up. Parity (number of pregnancies lasting no less than 6 months), age, weight, age at menopause, postmenopausal hormone use, smoking habit, and history of major chronic conditions were updated biennially since recruitment. We calculated body mass index (BMI) by dividing weight in kilograms by the square of height in meters. Physical activity was assessed every 2–4 years using a validated questionnaire collecting the average time spent per week on walking or hiking, jogging, running, bicycling, swimming, weightlifting, working outdoors, playing squash, racquetball or tennis, engaging in lower-intensity exercise, and performing callisthenics and other aerobic exercises. We estimated the weekly time of doing moderate-to-vigorous activities that required at least 3 metabolic equivalent units per hour.18 Dietary intake, including alcohol consumption, was collected every 2–4 years using a validated semiquantitative food frequency questionnaire. We applied the Alternate Healthy Eating Index (AHEI) score to assess the overall quality of the diet.17 These self-reported lifestyle factors have been validated to be highly reliable in both cohorts.19
Statistical analysis
Descriptive analyses were conducted for baseline characteristics according to breastfeeding duration by standardizing to participants’ age distribution. We applied time-dependent Cox proportional hazards regression models to estimate the hazard ratios (HRs) and 95% confidence intervals (CI) for the associations between lifetime total breastfeeding duration and risk of all-cause and cause-specific mortality in both cohorts, jointly stratified by age in years at the start of follow-up and calendar years of the current questionnaire cycle that were equivalent due to the way we structured the data and formulated the Cox models.20 Because of the similarity of study design and population, we also pooled the hazard ratios in both cohorts using an inverse variance weighted meta-analysis with the fixed effects model to maximise statistical power.19 Follow-up times started from the return date of follow-up questionnaires when breastfeeding duration was initially reported until the onset of death or the end of follow-up (June 2016 in NHS and June 2019 in NHS II), whichever occurred first. Our main analysis categorised total breastfeeding duration as ≤3 (reference), 4–6, 7–11, 12–23, and ≥24 months. Tests for linear trends were evaluated using the Wald test on the continuous breastfeeding duration representing the median values of each category. The potential non-linear relationship between continuous breastfeeding duration and mortality risk was examined using restricted cubic splines with 3 knots since they provided the highest power to detect non-linearity.21
Multivariable Cox models were initially adjusted for race/ethnicity (non-Hispanic White or other), age at first birth (<20, 20–24, 25–29, ≥30 years), infertility history (yes or no), woman's own birth weight (<5.5, 5.5–6.9, 7.0–8.4, 8.5–9.9, ≥10 lbs), oral contraceptive use during puberty (yes or no), woman's parents worked as professionals, managers, or executives during infancy (yes or no), woman's parents owned home during infancy (yes or no), maternal history of cancer (yes or no), and parental history of CVD before age 60 years (yes or no). In a secondary model, we additionally adjusted for time-varying pre-pregnancy smoking status (never smoker, former smoker: 1–14, ≥15 cigarettes/d, and current smoker: 1–14, 15–24, ≥25 cigarettes/d), exercise at moderate-to-high intensity (0, 0.01–1.0, 1.1–3.4, 3.5–5.9, or ≥6.0 h/week), alternate healthy eating index (quintiles), alcohol drinking (0, 0.1–4.9, 5.0–14.9, 15.0–19.9, 20.0–29.9, or ≥30 g/d), and body mass index (<21, 21–24.9, 25–29.9, 30–31.9, or ≥32 kg/m2). Time-varying covariates with missing values at a given follow-up cycle were replaced with the information from the most recent cycle (mostly <5%); otherwise, we used the missing covariate indicator method to deal with missing values, which has been demonstrated to induce minimal bias in epidemiologic studies.22
Stratified analyses were performed to assess the effect modification by time-varying parity (1 or ≥2) and pre-pregnancy BMI, smoking status, dietary quality, alcohol consumption, and physical across women's reproductive lifespan. Multiplicative interaction was estimated using multiplicative interaction terms between dichotomous breastfeeding duration (≤3 versus >3 months) and these stratified variables based on the Wald test. Several sensitivity analyses were also conducted. First, to assess the potential confounding by parity, we reanalysed the association of lifetime total breastfeeding duration with risk of all-cause mortality by a) additionally adjusting for time-varying parity; and b) using the average lifetime breastfeeding duration per parity by categorising women as <1 (reference), 1–3, 4–6, and ≥7 months.5 Second, we additionally adjusted for the tier of birth (North, Middle, South, outside of US) to assess the influence of birthplace. Third, we remodelled the Cox proportional hazards regression models by stratifying the analyses jointly by calendar years and participants’ own age in months or broader age groups (<35, 35–39, 40–44, 45–49, 50–54, 55–59, and ≥60 years). Finally, we assessed the influence of pregnancy complications (i.e., gestational diabetes and hypertensive disorders) and exclusive breastfeeding duration among NHS II women who had completed data. All data were analysed using SAS 9.4 for UNIX (SAS Institute Inc).
Role of the funding source
All funding sources had no role in the conduct of the study; collection and analysis of the data; preparation and review of the manuscript; and the decision to submit the manuscript for publication. Y-XW and MA had full access to the data in the study and all authors had final responsibility for the decision to submit for publication.
Results
The baseline characteristics of 166,708 parous women in NHS and NHS II according to breastfeeding duration are shown in Table 1. Together, there were 72,589 (43.5%), 19,290 (11.6%), 21,565 (12.9%), 29,724 (17.8%), and 23,540 (14.1%) women who breastfed for a cumulative total of ≤3, 4–6, 7–11, 12–23, and ≥24 months, respectively. In both cohorts, women who cumulatively breastfed for less than or equal to 3 months had the lowest mean parity and the highest prevalence of smoking.
Table 1.
Age-standardised baseline characteristics of study participants from the Nurses’ Health Study (NHS) and the Nurses’ Health Study II (NHS II) according to lifetime total breastfeeding duration.
| Characteristicsa,b | Lifetime total breastfeeding duration (months) |
||||
|---|---|---|---|---|---|
| ≤3 | 4–6 | 7–11 | 12–23 | ≥24 | |
| The Nurses’ Health Study (1986) | |||||
| Number of women | 51,168 | 10,279 | 8333 | 10,419 | 5765 |
| Age (year), mean (SD) | 52.8 (7.0) | 52.2 (7.2) | 51.3 (7.2) | 51.0 (7.2) | 50.6 (7.2) |
| Non-Hispanic White, % | 49,936 (97.6) | 9988 (97.2) | 8118 (97.4) | 10,146 (97.3) | 5646 (97.8) |
| Woman's own birth weight less than 2.5kg, % | 3850 (7.6) | 701 (6.8) | 618 (7.3) | 740 (7.0) | 397 (6.8) |
| Oral contraceptive use during puberty, % | 3178 (6.1) | 725 (7.1) | 561 (6.9) | 620 (6.2) | 274 (5.1) |
| Parity, mean (SD) | 3.0 (1.4) | 3.1 (1.4) | 3.2 (1.4) | 3.5 (1.4) | 4.5 (1.9) |
| Age at first birth (year), mean (SD) | 25.1 (3.7) | 25.1 (3.8) | 25.2 (3.5) | 25.2 (3.5) | 25.2 (3.8) |
| Infertility history, % | 5162 (10.2) | 1098 (10.7) | 872 (10.4) | 958 (8.9) | 509 (8.1) |
| Pre-pregnancy cigarette smoking, % | 12,470 (24.5) | 2297 (22.3) | 1643 (19.7) | 1803 (17.4) | 831 (14.3) |
| Pre-pregnancy BMI (kg/m2), mean (SD) | 25.4 (4.9) | 25.2 (4.7) | 25.0 (4.5) | 25.2 (4.7) | 25.6 (4.8) |
| Pre-pregnancy diet quality (alternate healthy eating index), mean (SD) | 50.0 (12.1) | 50.0 (12.8) | 50.9 (12.5) | 50.7 (12.6) | 50.1 (12.8) |
| Pre-pregnancy alcohol intake (g/d), mean (SD) | 6.1 (10.6) | 6.5 (10.7) | 6.4 (10.9) | 6.1 (10.4) | 5.2 (9.6) |
| Pre-pregnancy moderate to vigorous intensity exercise (h/wk), mean (SD) | 2.0 (3.1) | 2.3 (3.5) | 2.3 (3.5) | 2.4 (3.5) | 2.4 (3.3) |
| Maternal history of cancer, % | 12,850 (25.1) | 2497 (24.3) | 2157 (25.8) | 2600 (24.8) | 1457 (24.9) |
| Parental history of CVD before age 60 years, % | 10,929 (21.4) | 2097 (20.4) | 1711 (20.4) | 2099 (20.1) | 1121 (19.2) |
| Woman's parents worked as professionals, managers, or executives during infancy, % | 14,633 (28.8) | 3236 (31.5) | 2725 (32.4) | 3549 (33.4) | 2030 (34.3) |
| Woman's parents owned home during infancy, % | 15,565 (30.5) | 3151 (30.6) | 2680 (31.9) | 3385 (32.2) | 1890 (32.6) |
| The Nurses’ Health Study II (1989) | |||||
| Number of women | 21,421 | 9011 | 13,232 | 19,305 | 17,775 |
| Age (year), mean (SD) | 34.7 (5.0) | 34.2 (4.7) | 34.2 (4.7) | 34.0 (4.6) | 34.0 (4.5) |
| Non-Hispanic White, % | 19,823 (92.5) | 8337 (92.5) | 12,377 (93.5) | 18,224 (94.4) | 17,031 (95.8) |
| Woman's own birth weight less than 2.5kg, % | 1499 (7.0) | 588 (6.6) | 849 (6.4) | 1203 (6.2) | 1042 (5.8) |
| Oral contraceptive use during puberty, % | 4592 (21.4) | 2151 (23.7) | 3085 (23.3) | 4293 (22.2) | 3630 (20.5) |
| Parity, mean (SD) | 1.5 (1.0) | 1.6 (1.0) | 1.6 (1.0) | 1.9 (1.0) | 2.3 (1.2) |
| Age at first birth (year), mean (SD) | 24.5 (4.2) | 25.7 (4.3) | 26.0 (4.2) | 26.0 (3.8) | 25.9 (3.5) |
| Infertility history, % | 5556 (26.0) | 2422 (26.9) | 3565 (27.0) | 4679 (24.2) | 3824 (21.4) |
| Pre-pregnancy cigarette smoking, % | 2050 (9.6) | 808 (8.9) | 964 (7.3) | 1102 (5.7) | 698 (3.9) |
| Pre-pregnancy BMI (kg/m2), mean (SD) | 24.5 (5.3) | 24.1 (4.9) | 23.9 (4.7) | 23.5 (4.4) | 23.2 (4.1) |
| Pre-pregnancy diet quality (alternate healthy eating index), mean (SD) | 46.2 (10.5) | 46.8 (10.4) | 47.8 (10.5) | 48.0 (10.5) | 48.1 (10.7) |
| Pre-pregnancy alcohol intake (g/d), mean (SD) | 2.9 (5.7) | 3.2 (6.2) | 3.3 (6.2) | 3.0 (5.6) | 2.6 (5.2) |
| Pre-pregnancy moderate to vigorous intensity exercise (h/wk), mean (SD) | 2.9 (5.1) | 2.7 (4.7) | 2.8 (4.7) | 2.8 (4.6) | 2.8 (4.6) |
| Maternal history of cancer, % | 5305 (24.8) | 2170 (24.3) | 3332 (25.2) | 4849 (25.1) | 4453 (24.9) |
| Parental history of CVD before age 60 years, % | 4815 (22.5) | 1876 (20.9) | 2751 (20.8) | 3720 (19.3) | 3272 (18.3) |
| Woman's parents worked as professionals, managers, or executives during infancy, % | 4426 (20.7) | 2180 (24.2) | 3616 (27.3) | 5689 (29.4) | 5722 (32.2) |
| Woman's parents owned home during infancy, % | 8504 (39.8) | 3660 (40.8) | 5681 (42.9) | 8195 (42.4) | 7878 (44.3) |
Values are means (SD) or N (percentages); means (SD) and percentages of all variables except for age are age-standardised.
In the NHS, a total of 114 (0.1%), 8419 (9.8%), and 4468 (5.2%) women had missing data on baseline BMI, diet (including alcohol intake), and physical activity, respectively; in the NHS II, a total of 4860 (6.0%), 14,453 (17.9%), 6087 (7.5%), and 996 (1.2%) women had missing data on baseline BMI, diet (including alcohol intake), physical activity, and age at first birth, respectively.
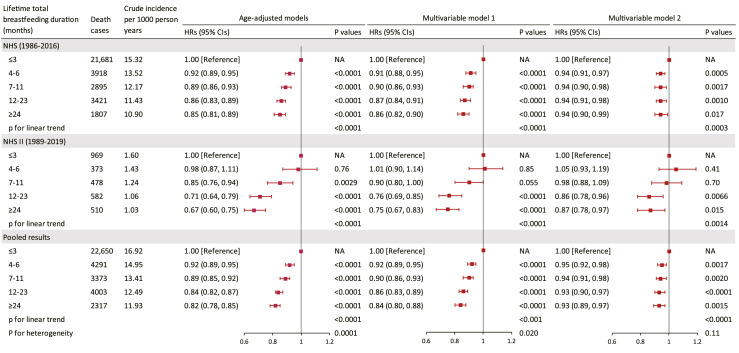
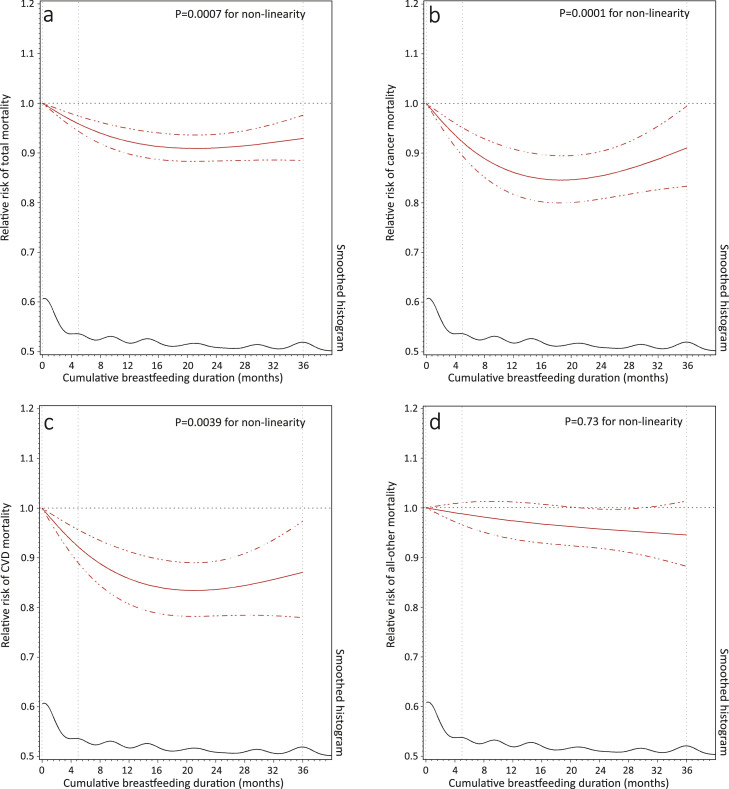
During 4,705,160 person-years of follow-up, 36,634 deaths were documented in the NHS and NHS II, including 9880 from cancer and 7709 from CVD. The pooled crude mortality rate per 1000 person-years of follow-up for women reporting lifetime total breastfeeding duration of ≤3, 4–6, 7–11, 12–23, and ≥24 months were 16.92, 14.95, 13.41, 12.49, and 11.93, respectively. In the age-adjusted model, a longer lifetime total breastfeeding duration was associated with a lower risk of all-cause mortality in both cohorts (Figure 2). The associations were similar after adjusting for ethnicity, birth weight, age at first birth, infertility history, oral contraceptive use during puberty, parents’ profession and homeownership, maternal history of cancer, and parental history of CVD (model 1) but were attenuated with additional adjustment for pre-pregnancy lifestyle factors (model 2). In the fully adjusted models, the pooled HRs for all-cause mortality during follow-up were 0.95 (95% CI: 0.92 to 0.98), 0.94 (95% CI: 0.91 to 0.98), 0.93 (95% CI: 0.90 to 0.97), and 0.93 (95% CI: 0.89 to 0.97), respectively, for women who breastfed for 4–6, 7–11, 12–23, and ≥24 months, compared to women breastfeeding for less than 4 months over their reproductive lifespan (Figure 2). When cause-specific mortality was explored, a similar pattern of non-linear inverse associations was observed between lifetime total breastfeeding duration and CVD and cancer mortality (Table 2). These non-linear inverse associations were further confirmed in the cubic spline model with adjustment for the same set of covariates (all p-values for non-linearity <0.01), where women breastfeeding for a total of 16–24 months showed the lowest risk (Figure 3).
Figure 2.
Hazard ratio (HR) (95% confidence interval (CI)) of all-cause mortality according to lifetime total breastfeeding duration among 166,708 women from the NHS (1986–2016) and NHS II (1989–2019). In age-adjusted Cox proportional hazard regression models, the analyses were stratified jointly by participants’ own age in years at the start of follow-up and calendar years of the current questionnaire cycle. Multivariable Cox model was further adjusted for race/ethnicity (non-Hispanic White; yes or no), age at first birth (<20, 20–24, 25–29, ≥30 years), woman's own birth weight (<5.5, 5.5–6.9, 7.0–8.4, 8.5–9.9, ≥10 lbs), oral contraceptive use during puberty (yes or no), infertility history (yes or no), woman's parents worked as professionals, managers, or executives during infancy (yes or no), woman's parents owned home during infancy (yes or no), maternal history of cancer (yes or no), and parental history of CVD before age 60 years (yes or no). Multivariable Cox model 2 was further adjusted for time-varying pre-pregnancy smoking status (never smoker, former smoker: 1–14, ≥15 cigarettes/d, and current smoker: 1–14, 15–24, ≥25 cigarettes/d), alcohol drinking (0, 0.1–4.9, 5.0–14.9, 15.0–19.9, 20.0–29.9, or ≥30 g/d), exercise at moderate-to-high intensity (0, 0.01–1.0, 1.1–3.4, 3.5–5.9, or ≥6.0 h/week), and alternate healthy eating index (five categories), and body mass index (<21, 21–24.9, 25–29.9, 30–31.9, or ≥32 kg/m2). Tests for linear trends were evaluated using the Wald test on the continuous breastfeeding duration representing the median values of each category. Tests for heterogeneity were conducted using an inverse variance weighted meta-analysis with the random-effects model. NA: not applicable.
Table 2.
Pooled hazard ratio (HR) (95% confidence interval (CI)) of cause-specific mortality according to lifetime total breastfeeding duration among 166,708 women from the NHS (1986–2016) and NHS II (1989–2019).
| Cause-specific mortality | Death cases | Crude incidence per 1000 person-years | HRs (95% CIs) |
||
|---|---|---|---|---|---|
| Age-adjusted modelsb | Multivariable model 1c | Multivariable model 2d | |||
| Cancer | |||||
| ≤3 | 5936 | 449 | 1.00 [Reference] | 1.00 [Reference] | 1.00 [Reference] |
| 4–11 | 2073 | 755 | 0.90 (0.86, 0.95) | 0.90 (0.86, 0.95) | 0.94 (0.90, 0.99) |
| 12–23 | 1146 | 344 | 0.85 (0.80, 0.91) | 0.87 (0.81, 0.93) | 0.95 (0.89, 1.01) |
| ≥24 | 725 | 345 | 0.87 (0.80, 0.94) | 0.89 (0.82, 0.96) | 0.99 (0.92, 1.08) |
| p for linear trenda | <0.0001 | <0.0001 | 0.40 | ||
| CVD | |||||
| ≤3 | 4972 | 353 | 1.00 [Reference] | 1.00 [Reference] | 1.00 [Reference] |
| 4–11 | 1515 | 560 | 0.87 (0.82, 0.93) | 0.88 (0.83, 0.93) | 0.91 (0.86, 0.96) |
| 12–23 | 787 | 255 | 0.87 (0.81, 0.94) | 0.89 (0.82, 0.96) | 0.94 (0.87, 1.02) |
| ≥24 | 435 | 249 | 0.87 (0.79, 0.96) | 0.89 (0.81, 0.98) | 0.96 (0.87, 1.06) |
| p for linear trend a | <0.0001 | 0.00020 | 0.079 | ||
| All other causes | |||||
| ≤3 | 11,742 | 877 | 1.00 [Reference] | 1.00 [Reference] | 1.00 [Reference] |
| 4–11 | 4086 | 1502 | 0.92 (0.89, 0.96) | 0.93 (0.89, 0.96) | 0.96 (0.93, 1.00) |
| 12–23 | 2070 | 641 | 0.83 (0.79, 0.87) | 0.85 (0.81, 0.89) | 0.92 (0.88, 0.96) |
| ≥24 | 1157 | 591 | 0.77 (0.72, 0.82) | 0.79 (0.74, 0.84) | 0.89 (0.83, 0.94) |
| p for linear trenda | <0.0001 | <0.0001 | <0.0001 | ||
Tests for linear trends were evaluated using the Wald test on the continuous breastfeeding duration representing the median values of each category.
Cox proportional hazard regression models were stratified jointly by participants’ own age in years at the start of follow-up and calendar years of the current questionnaire cycle.
Multivariable Cox model 1 was further adjusted for race/ethnicity (non-Hispanic White; yes or no), age at first birth (<20, 20–24, 25–29, ≥30 years), woman's own birth weight (<5.5, 5.5–6.9, 7.0–8.4, 8.5–9.9, ≥10 lbs), oral contraceptive use during puberty (yes or no), infertility history (yes or no), woman's parents worked as professionals, managers, or executives during infancy (yes or no), woman's parents owned home during infancy (yes or no), maternal history of cancer (yes or no), and parental history of CVD before age 60 years (yes or no).
Multivariable Cox model 2 was further adjusted for time-varying pre-pregnancy smoking status (never smoker, former smoker: 1–14, ≥15 cigarettes/d, and current smoker: 1–14, 15–24, ≥25 cigarettes/d), alcohol drinking (0, 0.1–4.9, 5.0–14.9, 15.0–19.9, 20.0–29.9, or ≥30 g/d), exercise at moderate-to-high intensity (0, 0.01–1.0, 1.0–3.5, 3.5–6.0, or ≥6.0 h/week), and alternate healthy eating index (five categories), and body mass index (<21, 21–24.9, 25–29.9, 30–31.9, or ≥32 kg/m2).
Figure 3.
Pooled dose-response associations between lifetime total breastfeeding duration and risks of all-cause (a) and cause-specific mortality (b-d) among 166,708 women from the NHS (1986–2016) and NHS II (1989–2019). Cubic spline models were adjusted for participants’ own age in years (continuous), race/ethnicity (non-Hispanic White; yes or no), age at first birth (<20, 20–24, 25–29, ≥30 years), woman's own birth weight (<5.5, 5.5–6.9, 7.0- 8.4, 8.5–9.9, ≥10 lbs), oral contraceptive use during puberty (yes or no), infertility history (yes or no), woman's parents worked as professionals, managers, or executives during infancy (yes or no), woman's parents owned home during infancy (yes or no), maternal history of cancer (yes or no), parental history of CVD before age 60 years (yes or no), and time-varying pre-pregnancy smoking status (never smoker, former smoker: 1–14, ≥15 cigarettes/d, and current smoker: 1–14, 15–24, ≥25 cigarettes/d), alcohol drinking (0, 0.1–4.9, 5.0–14.9, 15.0–19.9, 20.0–29.9, or ≥30 g/d), exercise at moderate-to-high intensity (0, 0.01–1.0, 1.1–3.4, 3.5–5.9, or ≥6.0 h/week), and alternate healthy eating index (five categories), and body mass index (<21, 21–24.9, 25–29.9, 30–31.9, or ≥32 kg/m2).
Cause-specific analyses were also disaggregated for diagnostic categories with at least 30 deaths attributed in both cohorts (Appendix Table 3). Compared to women who breastfed for ≤3 months over their reproductive lifespan, women who cumulatively breastfed for >3 months showed a lower risk of mortality due to malignant neoplasm of the respiratory system (pooled HR = 0.91; 95% CI: 0.84 to 1.00) and non-malignant diseases of the gastrointestinal system (pooled HR = 0.81; 95% CI: 0.70 to 0.93) and endocrine, nutritional, and metabolic diseases or immunity disorder (pooled HR = 0.70; 95% CI: 0.56 to 0.86), but a slightly higher risk of mortality due to suicide (pooled HR = 1.43; 95% CI: 1.00 to 2.04).
There was no evidence of interaction between lifetime total breastfeeding duration and parity, diet quality, alcohol consumption, and physical activity on the risk of all-cause mortality (Table 3). The pooled HRs for all-cause mortality in both cohorts were substantially unchanged when we used the average duration of total breastfeeding per parity, when we additionally adjusted for the Tier of birth or time-varying parity, and when we stratified the analyses jointly by calendar years and participants’ own age in months or broader age groups (Appendix Table 4–7). Among NHS II women, the non-linear inverse associations between breastfeeding duration and all-cause mortality persisted when we additionally adjusted for the history of gestational diabetes and hypertensive disorders (Appendix Table 8) and when we used exclusive breastfeeding duration (Appendix Table 9).
Table 3.
Pooled hazard ratio (HR) (95% confidence interval (CI)) for the risk of all-cause mortality in relation to lifetime total breastfeeding duration, stratified by pre-pregnancy lifestyle factors and parity across the reproductive lifespan among 166,708 women from the NHS (1986-2016) and NHS II (1989-2019).a
| Stratified factors across the reproductive lifespan | Lifetime total breastfeeding duration |
|
|---|---|---|
| ≤3 months | >3 months | |
| AHEI diet quality score | ||
| Top 40% (n=13,821 deaths) | 1.00 [Reference] | 0.95 (0.92, 0.99) |
| Bottom 60% (n=22,813 deaths) | 1.00 [Reference] | 0.93 (0.91, 0.96) |
| P for multiplicative interactionb | 0.32 | |
| Smoking status | ||
| Never (n=14,074 deaths) | 1.00 [Reference] | 0.95 (0.91, 0.98) |
| Current/ever (n=22,560 deaths) | 1.00 [Reference] | 0.92 (0.90, 0.95) |
| P for multiplicative interactionb | 0.17 | |
| BMI | ||
| <25 kg/m2 (n=16,187 deaths) | 1.00 [Reference] | 0.97 (0.94, 1.00) |
| ≥25 kg/m2 (n=20,391 deaths) | 1.00 [Reference] | 0.92 (0.89, 0.94) |
| P for multiplicative interactionb | 0.18 | |
| Physical activity | ||
| ≥30 min/day (n=4387 deaths) | 1.00 [Reference] | 0.94 (0.88, 1.00) |
| <30 min/day (n=32,247 deaths) | 1.00 [Reference] | 0.93 (0.91, 0.95) |
| P for multiplicative interactionb | 0.99 | |
| Alcohol consumption | ||
| 5-15 g/day (n=6013 deaths) | 1.00 [Reference] | 0.94 (0.89, 0.99) |
| <5 or >15 g/day (n=30,621 deaths) | 1.00 [Reference] | 0.94 (0.92, 0.96) |
| P for multiplicative interactionb | 0.92 | |
| Parity | ||
| 1 (n=3395 deaths) | 1.00 [Reference] | 0.88 (0.81, 0.96) |
| ≥2 (n=33,239 deaths) | 1.00 [Reference] | 0.95 (0.93, 0.98) |
| P for multiplicative interactionb | 0.057 | |
Cox proportional hazards regression models were stratified jointly by participants’ own age in years at the start of follow-up and calendar years of the current questionnaire cycle, with adjustment for race/ethnicity (non-Hispanic White; yes or no), age at first birth (<20, 20–24, 25–29, ≥30 years), woman's own birth weight (<5.5, 5.5–6.9, 7.0–8.4, 8.5–9.9, ≥10 lbs), oral contraceptive use during puberty (yes or no), infertility history (yes or no), woman's parents worked as professionals, managers, or executives during infancy (yes or no), woman's parents owned home during infancy (yes or no), maternal history of cancer (yes or no), parental history of CVD before age 60 years (yes or no), and time-varying pre-pregnancy smoking status (never smoker, former smoker: 1–14, ≥15 cigarettes/d, and current smoker: 1–14, 15–24, ≥25 cigarettes/d), alcohol drinking (0, 0.1–4.9, 5.0–14.9, 15.0–19.9, 20.0–29.9, or ≥30 g/d), exercise at moderate-to-high intensity (0, 0.01–1.0, 1.1–3.4, 3.5–5.9, or ≥6.0 h/week), and alternate healthy eating index (five categories), and body mass index (<21, 21–24.9, 25–29.9, 30–31.9, or ≥32 kg/m2), excluding the stratified variables.
Multiplicative interaction was estimated using multiplicative interaction terms between dichotomous breastfeeding duration and these stratified variables based on the Wald test.
Discussion
In these two large cohorts involving 166,708 parous women, we found that a longer lifetime total breastfeeding duration was associated with a modestly lower risk of all-cause mortality later in life. This inverse association appeared to be independent of parity and persisted when we used the average duration of breastfeeding per child. When cause-specific mortality was explored, a similar pattern of non-linear inverse associations was observed for CVD and cancer mortality. These non-linear associations were further confirmed in cubic spline models, where women who breastfed for a total of 18–24 months experienced the lowest risk.
The observed inverse association between breastfeeding duration and mortality risk is biologically plausible given the pregnancy-associated physiological changes such as fat accumulation that are essential for the development of the fetus and anticipation of lactation. This energy storage is characterised by well-described increases in visceral fat, insulin resistance, insulin production, and circulating lipid levels,23 which could contribute to an elevated risk of CVD and cancer, and eventually to mortality. Animal studies have demonstrated that breastfeeding plays an important role in mobilizing stored fat,24 reestablishing glucose homeostasis,24,25 regulating insulin secretion,26 and promoting lipid metabolism after delivery.27 Population evidence from observational studies also suggests that breastfeeding is associated with improved glucose metabolism and pancreatic beta-cell function,28 more favourable lipid profiles,29 and reduced weight loss and risk of metabolic diseases.30 Meanwhile, several randomised controlled trials have demonstrated that greater intensity of lactation is associated with greater postpartum weight loss.31 Collectively, these findings suggest that breastfeeding plays a critical role in “resetting” maternal metabolism by increasing metabolic expenditure, which reduces the maternal risk of developing CVD and cancer and eventually the risk of mortality later in life.11
To date, very few studies have examined the association between breastfeeding duration and subsequent risk of mortality, although breastfeeding duration has been associated with a reduced risk of various maternal health outcomes, including hypertension,4 diabetes,4 CVD,6 metabolic syndromes,7 and certain types of cancer (e.g., ovarian and breast cancer).8, 9, 10,32 In support of our findings, Nguyen and colleagues reported that ever breastfeeding was associated with a lower risk of incident CVD hospitalization and mortality among 100,864 parous Australian women aged ≥45 years.33 Similarly, in a Norwegian prospective cohort study consisting of 21,889 parous women aged 30–85 years, Fagerhaug and colleagues reported a higher risk of CVD mortality among women younger than 65 years who never breastfed.34 A more recent study consisting of >320,000 European women aged 25–70 years at entry, also found a lower risk of all-cause and circulatory disease mortality among women who ever versus never breastfed.35,36 In contrast to our findings, they did not find any convincing association between breastfeeding duration and cancer mortality.35 Our results were also contrasted with the results from a Chinese cohort showing that breastfeeding duration was unrelated to CVD mortality risk among 267,400 female textile workers aged ≥30 years at enrollment.37 The differences between studies could partly be explained by the differences in population characteristics, study design, and sample size. For instance, most of these previous studies determined breastfeeding duration at a single time point by recalling at recruitment when a large portion of women hadn't completed their reproductive careers, which may have resulted in exposure misclassification. Besides, previous studies mostly classified participants into a few exposure categories (e.g., ever vs. never), which may have been insufficient to detect potential non-linear relationships. Finally, these previous studies lacked detailed information on various relevant confounders such as pre-pregnancy lifestyle factors that are important determinants of mortality and can be influenced by breastfeeding practices.12,13 In our present study, we did not observe any evidence of interaction between breastfeeding duration and cigarette smoking, nutrition, physical activity, and overweight or obesity on mortality risk. However, the associations between breastfeeding duration and total and cause-specific mortality were attenuated when we additionally adjusted for these pre-pregnancy lifestyle factors.
We found non-linear associations between lifetime breastfeeding duration and the risk of total, cancer, and CVD mortality. A recent meta-analysis consisting of 18 cohort studies reported a U-shaped association between parity and all-cause mortality where women with three to four live births had the lowest risk,38 suggesting that high parity may have offset the protective effect of breastfeeding. Interestingly, the inverse association between lifetime breastfeeding duration and mortality persisted when we restricted the analysis among women reporting single parity and when we used the average duration of breastfeeding per child. We also observed a slightly higher risk of suicide mortality among women who cumulatively breastfed for >3 months than those breastfeeding for ≤3 months. However, the possibility of a chance finding cannot be fully ruled out given the limited number of suicide deaths (n=143). More studies are needed to verify our findings and investigate the mechanisms underlying these observed associations.
The strengths of our current study include its prospective design, extensive follow-up period, large population size, sufficient and valid death cases, high response rate, and repeated collection of various reproductive and lifestyle factors across most of the women's reproductive lifespan. Our study also has some limitations. First, we relied on self-reported breastfeeding duration to assign participants’ exposure, which may have resulted in exposure misclassification, although validation studies have consistently shown high validity of self-reported breastfeeding duration.14,15 In this case, however, such misclassification would be non-differential with respect to deaths because of our prospective study design, which would have biased risk estimations toward the null. Second, our participants were female nurses who had homogeneous race and ethnicity, educational attainment, and socioeconomic status, which may limit the generalizability of our findings, particularly for women in low-middle income countries. However, there is no evidence showing that the influence of breastfeeding on maternal health differs by race, ethnicity, or socioeconomic status. Third, our analyses used observational data, which cannot demonstrate any causal effects. However, as it is ethically problematic to randomise parous women to different categories of breastfeeding duration, a randomised controlled trial is infeasible and analyses of well-designed cohort studies such as ours are needed to advance our understanding of the lifelong benefits of breastfeeding for parous mothers.
Over the past two decades, the recommendations established by WHO and other health organizations have promoted the increasing trends in breastfeeding rates on a global scale.39 However, the prevalence of exclusive breastfeeding remains far below international targets of 50% by 2025 and 70% by 2030, particularly in low-income and middle-income countries.39 In the present study involving 166,708 parous women from two large prospective cohorts, we found that a longer lifetime breastfeeding duration was associated with a lower risk of mortality in later life in a non-linear manner, where women who breastfed for a total of 18–24 months experienced the lowest risk. Our results strengthen and refine the evidence of lifelong benefits of breastfeeding for parous mothers and highlight the importance for policymakers and social communities to protect, promote, and support breastfeeding and for healthcare professionals to inform its advantages to mothers and infants among the high-risk population with low breastfeeding initiation.
Contributors
Y-XW analysed and drafted the manuscript. Y-XW and JEC were involved in the study conception and design. MA verified the underlying data and conducted a technical review. JEM and JEC obtained funding for the study. Y-XW, MA, JWR-E, JEM, LW, SAM, and JEC participated in the interpretation of the results and critical revision of the manuscript. Y-XW, JEC, and MA had full access to all the data in the study. All authors accept the responsibility to submit for publication.
Data sharing statement
Data described in the manuscript, code book, and analytic code will not be made publicly available. Further information including the procedures for obtaining and accessing data from the Nurses’ Health Studies II is described at https://www.nurseshealthstudy.org/researchers (email: nhsaccess@channing.harvard.edu).
Declaration of interests
JEM reports financial support from the National Institutes of Health outside the submitted work. SAM reports personal fees from AbbVie and Roche outside the submitted work. J.E.C. reports grants from the National Institutes of Health, Centres for Disease Control and Prevention, and Food and Drug Administration, outside the submitted work.
Acknowledgements
The authors would like to acknowledge Ellen Hertzmark's technical assistance regarding data analyses and the contribution to this study from central cancer registries supported through the Centres for Disease Control and Prevention's National Program of Cancer Registries (NPCR) and/or the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) Program. Central registries may also be supported by state agencies, universities, and cancer centres. Participating central cancer registries include the following: Alabama, Alaska, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Hawaii, Idaho, Indiana, Iowa, Kentucky, Louisiana, Massachusetts, Maine, Maryland, Michigan, Mississippi, Montana, Nebraska, Nevada, New Hampshire, New Jersey, New Mexico, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Puerto Rico, Rhode Island, Seattle SEER Registry, South Carolina, Tennessee, Texas, Utah, Virginia, West Virginia, Wyoming. The study was funded by The National Institutes of Health grants U01-HL145386, U01-CA176726, R01-HL034594, R01-HL088521, UM-CA186107, P01-CA87969, R01-CA49449, R01-CA67262, U01-HL145386, U01-CA167552, R01-HL35464, and R24-ES028521–01.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101693.
Appendix. Supplementary materials
References
- 1.Organization WH . World Health Organization; 2003. Global strategy for infant and young child feeding. [Google Scholar]
- 2.Breastfeeding and the use of human milk. American Academy of Pediatrics Work Group on Breastfeeding. Pediatrics. 1997;100(6):1035–1039. doi: 10.1542/peds.100.6.1035. [DOI] [PubMed] [Google Scholar]
- 3.Victora CG, Bahl R, Barros AJ, et al. Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387(10017):475–490. doi: 10.1016/S0140-6736(15)01024-7. [DOI] [PubMed] [Google Scholar]
- 4.Rameez RM, Sadana D, Kaur S, et al. Association of maternal lactation with diabetes and hypertension: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(10) doi: 10.1001/jamanetworkopen.2019.13401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farland LV, Eliassen AH, Tamimi RM, Spiegelman D, Michels KB, Missmer SA. History of breast feeding and risk of incident endometriosis: prospective cohort study. BMJ. 2017;358:j3778. doi: 10.1136/bmj.j3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tschiderer L, Seekircher L, Kunutsor SK, Peters SAE, O'Keeffe LM, Willeit P. Breastfeeding is associated with a reduced maternal cardiovascular risk: systematic review and meta-analysis involving data from 8 studies and 1 192 700 parous women. J Am Heart Assoc. 2022;11(2) doi: 10.1161/JAHA.121.022746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torris C, Bjornnes AK. Duration of lactation and maternal risk of metabolic syndrome: a systematic review and meta-analysis. Nutrients. 2020;12(9):2718. doi: 10.3390/nu12092718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fortner RT, Sisti J, Chai B, et al. Parity, breastfeeding, and breast cancer risk by hormone receptor status and molecular phenotype: results from the Nurses' Health Studies. Breast Cancer Res. 2019;21(1):40. doi: 10.1186/s13058-019-1119-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danforth KN, Tworoger SS, Hecht JL, Rosner BA, Colditz GA, Hankinson SE. Breastfeeding and risk of ovarian cancer in two prospective cohorts. Cancer Causes Control. 2007;18(5):517–523. doi: 10.1007/s10552-007-0130-2. [DOI] [PubMed] [Google Scholar]
- 10.Islami F, Liu Y, Jemal A, et al. Breastfeeding and breast cancer risk by receptor status–a systematic review and meta-analysis. Ann Oncol. 2015;26(12):2398–2407. doi: 10.1093/annonc/mdv379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stuebe AM, Rich-Edwards JW. The reset hypothesis: lactation and maternal metabolism. Am J Perinatol. 2009;26(1):81–88. doi: 10.1055/s-0028-1103034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goon DT, Ajayi AI, Adeniyi OV. Sociodemographic and lifestyle correlates of exclusive breastfeeding practices among mothers on antiretroviral therapy in the Eastern Cape, South Africa. Int Breastfeed J. 2021;16(1):18. doi: 10.1186/s13006-021-00366-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colpani V, Baena CP, Jaspers L, et al. Lifestyle factors, cardiovascular disease and all-cause mortality in middle-aged and elderly women: a systematic review and meta-analysis. Eur J Epidemiol. 2018;33(9):831–845. doi: 10.1007/s10654-018-0374-z. [DOI] [PubMed] [Google Scholar]
- 14.Li R, Scanlon KS, Serdula MK. The validity and reliability of maternal recall of breastfeeding practice. Nutr Rev. 2005;63(4):103–110. doi: 10.1111/j.1753-4887.2005.tb00128.x. [DOI] [PubMed] [Google Scholar]
- 15.Tomeo CA, Rich-Edwards JW, Michels KB, et al. Reproducibility and validity of maternal recall of pregnancy-related events. Epidemiology. 1999;10(6):774–777. [PubMed] [Google Scholar]
- 16.Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the national death index and equifax nationwide death search. Am J Epidemiol. 1994;140(11):1016–1019. doi: 10.1093/oxfordjournals.aje.a117191. [DOI] [PubMed] [Google Scholar]
- 17.Wang YX, Minguez-Alarcon L, Gaskins AJ, et al. Association of spontaneous abortion with all cause and cause specific premature mortality: prospective cohort study. BMJ. 2021;372:n530. doi: 10.1136/bmj.n530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shan Z, Li Y, Zong G, et al. Rotating night shift work and adherence to unhealthy lifestyle in predicting risk of type 2 diabetes: results from two large US cohorts of female nurses. BMJ. 2018;363:k4641. doi: 10.1136/bmj.k4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang YX, Li Y, Rich-Edwards JW, et al. Associations of birth weight and later life lifestyle factors with risk of cardiovascular disease in the USA: a prospective cohort study. EClinicalMedicine. 2022;51 doi: 10.1016/j.eclinm.2022.101570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang YX, Arvizu M, Rich-Edwards JW, et al. Hypertensive disorders of pregnancy and subsequent risk of premature mortality. J Am Coll Cardiol. 2021;77(10):1302–1312. doi: 10.1016/j.jacc.2021.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. 2010;29(9):1037–1057. doi: 10.1002/sim.3841. [DOI] [PubMed] [Google Scholar]
- 22.Song M, Zhou X, Pazaris M, Spiegelman D. The missing covariate indicator method is nearly valid almost always. arXiv preprint arXiv:2111.00138, 2021. https://arxiv.org/ftp/arxiv/papers/2111/2111.00138.pdf.
- 23.Einstein FH, Fishman S, Muzumdar RH, Yang XM, Atzmon G, Barzilai N. Accretion of visceral fat and hepatic insulin resistance in pregnant rats. Am J Physiol Endocrinol Metabol. 2008;294(2):E451–E455. doi: 10.1152/ajpendo.00570.2007. [DOI] [PubMed] [Google Scholar]
- 24.Zhong S, Almario R, Dubrinsky M, et al. Repeated pregnancy without lactation: effects on maternal glycemic control, pregnancy outcome, carcass composition, and fat distribution in rats. Metabolism. 1990;39(11):1127–1132. doi: 10.1016/0026-0495(90)90083-o. [DOI] [PubMed] [Google Scholar]
- 25.Jones RG, Ilic V, Williamson DH. Physiological significance of altered insulin metabolism in the conscious rat during lactation. Biochem J. 1984;220(2):455–460. doi: 10.1042/bj2200455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karnik SK, Chen H, McLean GW, et al. Menin controls growth of pancreatic beta-cells in pregnant mice and promotes gestational diabetes mellitus. Science. 2007;318(5851):806–809. doi: 10.1126/science.1146812. [DOI] [PubMed] [Google Scholar]
- 27.Smith JL, Lear SR, Forte TM, Ko W, Massimi M, Erickson SK. Effect of pregnancy and lactation on lipoprotein and cholesterol metabolism in the rat. J Lipid Res. 1998;39(11):2237–2249. [PubMed] [Google Scholar]
- 28.Ramos-Roman MA, Syed-Abdul MM, Adams-Huet B, Casey BM, Parks EJ. Lactation versus formula feeding: insulin, glucose, and fatty acid metabolism during the postpartum period. Diabetes. 2020;69(8):1624–1635. doi: 10.2337/db19-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin FP, Moco S, Montoliu I, et al. Impact of breast-feeding and high- and low-protein formula on the metabolism and growth of infants from overweight and obese mothers. Pediatr Res. 2014;75(4):535–543. doi: 10.1038/pr.2013.250. [DOI] [PubMed] [Google Scholar]
- 30.Gunderson EP, Lewis CE, Wei GS, Whitmer RA, Quesenberry CP, Sidney S. Lactation and changes in maternal metabolic risk factors. Obstet Gynecol. 2007;109(3):729–738. doi: 10.1097/01.AOG.0000252831.06695.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dewey KG, Cohen RJ, Brown KH, Rivera LL. Effects of exclusive breastfeeding for four versus six months on maternal nutritional status and infant motor development: results of two randomized trials in Honduras. J Nutr. 2001;131(2):262–267. doi: 10.1093/jn/131.2.262. [DOI] [PubMed] [Google Scholar]
- 32.Stuebe AM, Willett WC, Xue F, Michels KB. Lactation and incidence of premenopausal breast cancer: a longitudinal study. Arch Intern Med. 2009;169(15):1364–1371. doi: 10.1001/archinternmed.2009.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nguyen B, Gale J, Nassar N, Bauman A, Joshy G, Ding D. Breastfeeding and cardiovascular disease hospitalization and mortality in parous women: evidence from a large australian cohort study. J Am Heart Assoc. 2019;8(6) doi: 10.1161/JAHA.118.011056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Natland Fagerhaug T, Forsmo S, Jacobsen GW, Midthjell K, Andersen LF, Ivar Lund Nilsen T. A prospective population-based cohort study of lactation and cardiovascular disease mortality: the HUNT study. BMC Public Health. 2013;13:1070. doi: 10.1186/1471-2458-13-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Merritt MA, Riboli E, Murphy N, et al. Reproductive factors and risk of mortality in the European Prospective Investigation into Cancer and Nutrition; a cohort study. BMC Medicine. 2015;13:252. doi: 10.1186/s12916-015-0484-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vergnaud AC, Romaguera D, Peeters PH, et al. Adherence to the world cancer research fund/american institute for cancer research guidelines and risk of death in Europe: results from the European Prospective Investigation into Nutrition and Cancer cohort study1,4. Am J Clin Nutr. 2013;97(5):1107–1120. doi: 10.3945/ajcn.112.049569. [DOI] [PubMed] [Google Scholar]
- 37.Gallagher LG, Davis LB, Ray RM, et al. Reproductive history and mortality from cardiovascular disease among women textile workers in Shanghai, China. Int J Epidemiol. 2011;40(6):1510–1518. doi: 10.1093/ije/dyr134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zeng Y, Ni ZM, Liu SY, et al. Parity and all-cause mortality in women and men: a dose-response meta-analysis of cohort studies. Sci Rep. 2016;6:19351. doi: 10.1038/srep19351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neves PAR, Vaz JS, Maia FS, et al. Rates and time trends in the consumption of breastmilk, formula, and animal milk by children younger than 2 years from 2000 to 2019: analysis of 113 countries. Lancet Child Adolesc Health. 2021;5(9):619–630. doi: 10.1016/S2352-4642(21)00163-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.