Summary
Background
Summarized data of cardiovascular outcomes trials (CVOTs) of sodium glucose cotransporter 2 inhibitors (SGLT2i) and glucagon-like peptide 1 receptor agonists (GLP-1 RAs) have shown a reduction in major adverse cardiovascular event (MACE), whether these benefits are extended in certain risk groups (elderly or obese patients or those with a longer duration of diabetes) or certain minorities (Black participants) are not clearly established. We aimed to provide overall hazard ratios (HRs) estimates for MACE of SGLT2i and GLP-1 RAs stratified by age (< 65 years vs. ≥ 65 years and < 75 years vs. ≥ 75 years), sex (male vs. female), race (Black vs. White, Black vs. Asian, and White vs. Asian), body mass index (BMI: < 30 kg/m2 vs. ≥ 30 kg/m2), and duration of diabetes (< 10 years vs. ≥ 10 years).
Methods
We performed a MEDLINE database search from inception up to July 31, 2022 to identify all placebo-controlled phase 3 CVOTs that evaluated the efficacy of SGLT2i and GLP-1 RAs on vascular events at least 1-year after randomisation in participants with type 2 diabetes, and we selected those reporting hazard ratios (HRs) for the specific risk groups for MACE. Differences on MACE in risk groups were examined using a random-effect meta-analysis. The study protocol was registered on PROSPERO (CRD42022347901).
Findings
A total of 11 studies fulfilled the prespecified criteria, comprising 96,580 patients with T2D were included. Of these patients, 61,975 (64.2%) were male, 34,605 (35.8%) were female, and race groups included 74,982 (77.6%) White, 7760 (8.0%) Asian, and 4023 (4.2%) Black. In two SGLT2i trials, the HR (95% CI) for long-term diabetes duration more than10 years versus short duration was 0.84 (0.77–0.93) vs. 1.02 (0.89–1.16), respectively (Pinteraction = 0.03). In four SGLT2i trials, the MACE benefit was similar by sex (Pinteraction = 0.13), age (Pinteraction = 0.36), BMI (Pinteraction = 0.69), and race groups (Pinteraction = 0.86 between Black and White, Pinteraction = 0.98 between Black and Asian, and Pinteraction = 0.69 between White and Asian). For GLP-1 RAs, the MACE benefit from the seven trials tended to be greater for Asian (0.71, [0.58–0.87]) than for White (0.87, [0.81–0.94]), (Pinteraction = 0.07). In two GLP-1 RAs trials, the MACE outcome was reduced by 22% (0.78, 0.63–0.95) in elderly patients (≥ 75 years) while no difference was observed in those < 75 years (0.87; 0.75–1.01), (Pinteraction = 0.37). In the remaining risk groups, the MACE benefit was similar by sex (Pinteraction = 0.37), age < 65 years (Pinteraction = 0.80), duration of diabetes (Pinteraction = 0.70), and race (Pinteraction = 0.57 between Black and White, and Pinteraction = 0.15 between Black and Asian), BMI (Pinteraction = 0.78). Risk of bias was lower, and overall heterogeneity was high for sex with SGLT2i, and moderate to low for the remaining comparisons, with a I2 values ranging from 0% to 54%.
Interpretation
In patients with type 2 diabetes at highest risk of cardiovascular disease or established cardiovascular disease, a greater benefit on MACE was found for elderly patients and for Asian individuals compared with White individuals with GLP-1 RAs, and those with a long duration of diabetes with SGLT2i. These findings could help in providing guidance for treatment prescription and facilitate selection and stratification of patients for future CVOTs. Furthermore, pooled individual patient-level data are urgently needed to support our conclusions, and to derive definitive evidence.
Funding
None.
Keywords: Meta-analysis, Risk group differences, MACE, CVOTs
Research in context.
Evidence before this study
We searched PubMed using the search terms “glucagon-like peptide 1 receptor agonists (GLP-1 RAs)”, “sodium glucose cotransporter 2 inhibitors (SGLT2i)”, “randomized controlled trials”, “death”, “stroke”, “myocardial infarction”, and “death form cardiovascular causes” for reports published before July 31, 2022. Only placebo-controlled peer-reviewed, English-language reports were considered. We identified two meta-analyses that reported data on major adverse cardiovascular event (MACE) benefit in GLP-1 RAs and SGLT2i trials stratified by sex (Singh AK, 2020) and race (Lee MMY, 2021). Each of these meta-analyses investigated the effect of glucose-lowering therapy on vascular events only in one risk group: sex or race. These studies were very heterogeneous in terms of design, population, follow-up, and methods. No study meta-analysed treatment effects on MACE outcome stratified by age, body mass index (BMI), and duration of diabetes in patient with type 2 diabetes at highest risk of cardiovascular disease or established cardiovascular disease. In addition, the availability of data from the AMPLITUD-O trial for the sex and race subgroups will allow an update of the two previous meta-analyses.
Added value of this study
To the best of our knowledge, this systematic review is the first that investigates the MACE benefit of GLP-1 RAs and SGLT2i drugs stratified by age, BMI, and duration of diabetes in 11 large-scale trials of 96,580 patients with type 2 diabetes at highest risk of cardiovascular disease or established cardiovascular disease. We found that, patients in whom the duration of diabetes was over 10 years had a significantly greater benefit on MACE outcome with SGLT2i than those with those with a shorter duration of diabetes. Furthermore, in a subgroup of 2390 patients older than 75 years from pooled data from two GLP-1 RAs trials (HARMONY and EXSCEL), the MACE outcome was reduced by 22% (0.78, 0.63–0.95). The addition of SGLT2i or GLP-1 RAs to standard care, yielded similar benefits on MACE outcome according to age (< 75 years), sex, BMI, and other race groups.
Implications of all the available evidence
The available data extend the knowledge of the benefit of SGLT2i and GLP-1 RAs on major cardiovascular events in patients with type 2 diabetes according to the specific risk group. These findings suggest greater reduction in MACE outcome for patients with a long-term duration of diabetes with SGLT2i, and in those older than 75 years and in the Asian population with GLP-1 RAs. In contrast, the MACE benefit of SGLT2i and GLP-1 RAs was similar for age, sex, BMI, and other race groups. These findings could help in providing guidance for treatment prescription and facilitate selection and stratification of patients for future cardiovascular outcomes trials (CVOTs).
Alt-text: Unlabelled box
Introduction
The cardiovascular (CV) benefits of sodium glucose cotransporter 2 inhibitors (SGLT2i) and glucagon-like peptide 1 receptor agonists (GLP-1 RAs) for patients with type 2 diabetes (T2D) are well established,1,2 with possible extension of these benefits among some risk groups, particularly for the Asian participants in comparison to White participants.3 Meta-analyses examining sex differences have found a significant reduction in major adverse CV event (MACE) for men but not in women with SGLT2i, while for GLP-1 RAs, the pooled data demonstrates a significant reduction in MACE for both sexes. However, this study did not directly compare men to women.4 Regarding race difference, results from a recent meta-analysis of ten cardiovascular outcomes trials (CVOTs) indicated a significant reduction in MACE for Asian participants with GLP-1 RAs but not with SGLT2i.3 Furthermore, although black patients are more affected by cardiovascular disease (coronary heart disease and type 2 diabetes) and advanced kidney disease, and have more severe cardiovascular outcomes,5,6 they are unfortunately under-represented in clinical trials. In addition, as obesity has a critical role in the development and progression of T2D, the incidence of T2D increase with the rising prevalence of obesity and age.7 It remains unclear whether the CV benefits of SGLT2i or GLP-1 RAs are maintained in Black, elderly, and obese patients living with diabetes. Summarized data of CVOTs trials will help support guideline recommendations on the use of these new antihyperglycemic medications in the management of these risk groups. In addition, the availability of new data on the results of a GLP-1 RA trial (AMPLITUDE-O) which included T2D with an elevated HbA1c and established CV disease,8 gives the opportunity to update these previous meta-analyses.3,4 In the present study, we investigate whether there is a difference on MACE benefit of SGLT2i or GLP-1 RAs drugs stratified by age, sex, race, body mass index (BMI), and duration of diabetes. Given the increasing prevalence of diabetes worldwide, such data assume added importance.
Methods
Search strategy and selection criteria
In this systematic review and meta-analysis, we searched MEDLINE (via PubMed) from database inception up to July 31, 2022 using the following search terms: “GLP-1 RAs”, “SGLT2 inhibitors”, “randomized placebo-controlled trials”, “death”, “stroke”, “myocardial infarction”, and “death form cardiovascular causes”. Published randomized placebo-controlled trials (RCTs) which evaluated the efficacy of the two newest classes of glucose-lowering drugs, the SGLT2i and GLP-1 RAs on cardiovascular outcomes at least 1-year after randomization in patients with type 2 diabetes were included. Observational studies (cohort, case-control), cases series or cases reports were excluded. This pooled analysis was aligned with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA).9 The study protocol was registered on PROSPERO (CRD42022347901).
Data analysis
Data extraction and trial bias assessment were done independently by two reviewers (AD and MCB). When there was disagreement between two reviewers, we addressed them in a panel discussion by referencing the original trials. For each CVOTs trials that met eligible criteria, hazard ratios (HRs) and their 95% confidence interval (CI) for MACE in the following specific risk groups: age (< 65 years vs. ≥ 65 years and < 75 years vs. ≥ 75 years), sex (male vs. female), race (Black vs. White, Black vs. Asian, and White vs. Asian), BMI (< 30 kg/m2 vs. ≥ 30 kg/m2), and duration of diabetes (< 10 years vs. ≥ 10 years) was extracted. The risk of bias was assessed using the Cochrane Collaboration's Risk of Bias Tool (Rob2) for RCTs.10 In Rob2, the following characteristics were considered: randomization sequence generation and allocation concealment (selection bias), blinding of participant and staff (performance bias), the outcome blinding (detection bias), incomplete outcome data (attrition bias), and selective reporting (reporting bias).
The primary outcome was the three-points MACE (MACE-3), a composite of death from cardiovascular cause, fatal or non-fatal stroke, and fatal or non-fatal myocardial infarction. The providing effect size estimate as HRs and their 95% CI form each trial were pooled based on intention-to-treat analysis.
Inverse-variance weighted random-effect meta-analysis with the restricted maximum likelihood estimation (RMLE), with tests for groups difference were used to compared CV benefits of SGLT2i or GLP-1 RAs drugs stratified by age, sex, race, BMI, and duration of diabetes. Heterogeneity across trials were investigated using the I² statistic and the between-study variance τ2. Heterogeneity was considered low, moderate, and high if the I2 was ≤ 25%, between >25% and <75%, and ≥ 75% respectively. All analyses were performed using the ‘meta’ R package, and P<0.05 was considered statistically significant, and for subgroup interactions of age and race the Bonferroni correction (P<0.02) was used to compensate for the effects of multiple testing.
Role of the funding source
There was no funding source for this study.
Results
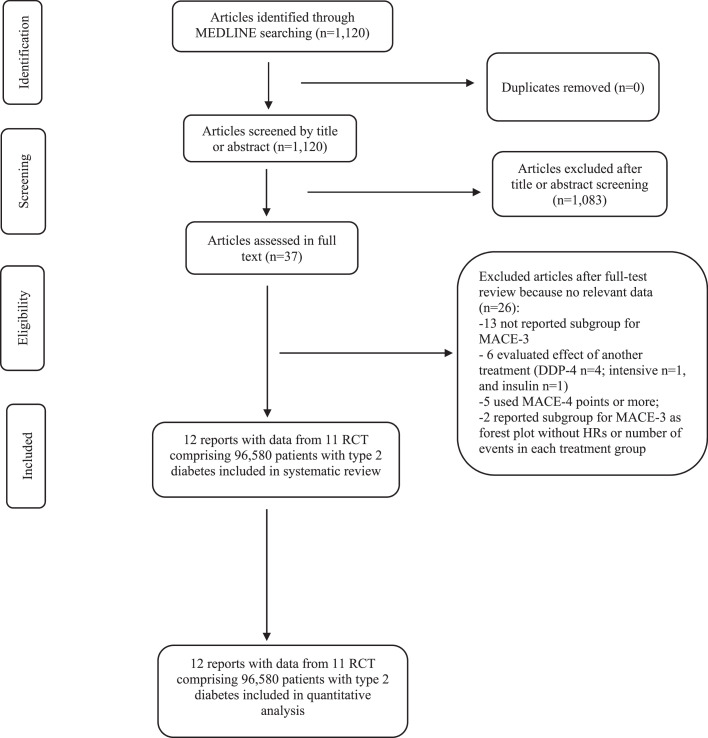
Of 1120 trials retrieved from MEDLINE, we excluded 1083 trials that did not meet the inclusion criteria. After assessing the 37 full-text trials, we excluded 13 trials that did not reported subgroups for major cardiovascular events, six that assessed the effects of other treatment on cardiovascular outcomes, five that used an extended list of major adverse cardiovascular event (more than three points), and two that reported subgroups for MACE-3 as a forest plot without HRs or number of events in each treatment group. Therefore, a total of 11 CVOTs trials (four evaluating SGLT2i versus placebo and seven evaluating GLP-1 RAs versus placebo) 8,11, 12, 13, 14, 15, 16, 17, 18, 19, 20 fulfilled the prespecified criteria (Figure 1), with low risk of bias (appendix, Figure S1), and comprising a total of 96,580 patients with type 2 diabetes were included. Of the 96,580 patients, 61,975 (64.2%) were male, 34,605 (35.8%) were female, and race groups included 74,982 (77.6%) White, 7760 (8.0%) Asian, and 4023 (4.2%) Black. The median length of follow-up ranged from 1.3 years in PIONEER 6 trial to 5.40 years in REWIND trial. At baseline, the mean age ranged from 62 years in EXSCEL to 66.2 years in REWIND, and glycated haemoglobin ranged from 7.2% in REWIND to 8.9% in AMPLITUDE-O (Table 1). A total of 44,758 (46%) participants received insulin at baseline, with a similar proportion of insulin-treated patients in both classes of new hypoglycaemic agents (46% for SGLT2 inhibitors vs 47% for GLP1-RAs; appendix, Table S1).
Figure 1.
PRISMA flowchart of studies selected for meta-analysis of glucose-lowering drugs. RCT: randomized controlled trial.
Table 1.
Baseline characteristics and use of glucose-lowering drugs across trials.
| Authors (year of publication)Trials (participant; inclusion period; sites) | Key inclusion criteria | Drugs (dose, administration route) | aAge, years | Male | Female | aBMI, kg/m2 | aDiabetes duration, years | HbA1C, % | White | Black | Asian | Follow-up, years (median, IQR) | Event rates in placebo group | Cardiovascular composite outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gerstein et al. (2021)8 AMPLITUDE-O (n=4076; May 11, 2018 to April 25, 2019; 344 sites in 28 countries) |
age ≥ 18 years with prior CVD or age ≥ 50 years (if male) or age ≥ 55 years (if female) with ≥ 1 CV risk factor and eGFR 25.0 to 59.9 ml/min/1.73m2, and HbA1C ≥ 7% | Efpeglenatide, 4 mg/week or 6 mg/week, subcutaneous | 64.5 (8.2) | 2732 (67%) |
1344 (33%) |
32.7 (6.2) | 15.4 (8.8) | 8.9 (1.5) | 3534 (87%) |
143 (4%) | 267(7%) | 1.81 (1.69 – 1.98 |
5.3 per 100 person-years | MACE-3 (non-fatal stroke, non-fatal myocardial infarction, and death from CV causes or undetermined causes) |
| Cannon et al. (2020)11 VERTIS CV (n=8246 with 4023 patients from cohort 1: December 2013 to July 2015; and 4223 patients from cohort 2: June 2016 to April 2017; 567 sites in 34 countries) |
age ≥ 40 years with established ASCVD, and HbA1C 7.0 – 10.5% | Ertugliflozin, 5 mg/day or 15 mg/day, oral | 64.4 (8.1) | 5769 (70%) |
2477 (30%) |
32.0 (5.5) | 13.0 (8.4) | 8.2 (1.0) | 7240 (88%) |
235 (3%) | 497 (6%) | 3.5 (4.3 in cohort 1 and 2.7 in cohort 2 |
4.0 per 100 person-years | MACE-3 (non-fatal stroke, non-fatal myocardial infarction, and death from CV causes) |
| Zinman et al. (2015)12 EMPA-REG OUTCOME (n=7020; September 2010 to April 2013, 590 sites in 42 countries) |
age ≥ 18 years with BMI ≤ 45 kg/m2 and eGFR ≥ 30 ml/min/1.73m2 with no antidiabetic's drugs within 12 weeks and HbA1C ≥7.0 to ≤ 9.0 % or stable antidiabetics drugs within 12 weeks and HbA1C ≥7.0 to ≤ 10.0% | Empagliflozin, 10 mg/day or 25 mg/day, oral | 63.1 (8.6) | 5016 (71%) |
2004 (29%) | 30.6 (5.3) | NR | 8.1 (0.9) | 5081 (72%) |
339 (5%) | 1517 (22%) | 3.1 (NR) | 43.9 per 1000 person-years | MACE-3 (non-fatal stroke, non-fatal myocardial infarction, and death from CV causes) |
| Neal et al. (2017)13 CANVAS Program (n=10,142) including CANVAS (n=4330) and CANVAS-R (n=5812); 667 sites in 30 countries) |
age ≥ 30 years with history of symptomatic ASCVD or age ≥ 50 years ≥ 2 CV risk factor, and HbA1C ≥7 to ≤ 10.5% | Canagliflozin 100 mg/day or 300 mg/day, oral | 63.3 (8.3) | 6509 (64%) |
3633 (36%) | 32.0 (5.9) | 13.5 (7.8) | 8.2 (0.9) | 7944 (78%) |
336 (3%) | 1284 (13%) | 2.4 (NR) | 31.5 per 1000 person-years | MACE-3 (non-fatal stroke, non-fatal myocardial infarction, and death from CV causes) |
| Wiviott et al. (2018)14 DECLARE-TIMI 58 (n=17,160; 882 sites in 33 countries) |
age ≥ 40 years with eGFR ≥60 ml/min/1.73m2 and with ≥ 1 ASCVD risk factor or established ASCVD, and HbA1C 6.5– 12% | Dapagliflozin 10 mg/day, oral | 63.9 (6.8) | 10738 (63%) |
6422 (37%) |
32.1 (6.0) | 11.0 (6.0– 16.0) |
8.3 (1.2) | 13653 (80%) |
NR | NR | 4.2 (3.9–4.4) |
5.8 per 1000 person-years | MACE-3 (ischemic stroke, myocardial infarction, and death from CV causes) |
| Marso et al. (2016)15 LEADER (n=9340; September 2010 to April 2012; 410 sites in 32 countries) |
age ≥ 50 years with CVD/HF/CKD or age ≥ 60 years with ≥ 1 CV risk factor, and HbA1C ≥ 7% | Liraglutide, 1.8 mg/day, subcutaneous | 64 (7) | 6003 (64%) |
3337 (36%) | 32.5 (6.3) | 12.8 (8.0) | 8.7 (1.6) | 7238 (77%) |
777 (8%) | 936 (10%) | 3.8 (NR) | 3.9 per 100 person-years | MACE-3 (non-fatal stroke, non-fatal myocardial infarction, and death from CV causes) |
| Marso et al. (2016)16 SUSTAIN-6 (n=3297; February 2013 to December 2013; 230 sites in 20 countries) |
age ≥ 50 years with CVD/HF/CKD ≥ stage 3 or age ≥ 60 years with ≥ 1 CV risk factor, and HbA1C ≥ 7% | Semaglutide, 0.5 mg/week or 1.0 mg/week, subcutaneous | 64.6 (7.4) | 2002 (61%) |
1295 (39%) | 32.8 (6.2) | 13.9 (8.1) | 8.7 (1.5) | 2736 (83%) |
221 (7%) | 273 (8%) | 2.1 (NR) | 4.4 per 100 person-years | MACE-3 (non-fatal stroke, non-fatal myocardial infarction including silent, and death from CV causes) |
| Holman et al. (2017)17 EXSCEL (n=14,752; June 18, 2010 to September 16, 2015; 687 sites in 35 countries) |
age ≥ 18 years with primary prevention of CVD (30% of sample) or established CVD (70% of sample), and HbA1C 6.5 - 10% | Exenatide, 2 mg/week, subcutaneous | 62 (9) | 9149 (62%) |
5603 (38%) |
32.7 (6.4) | 12 (7– 18) | 8.0 (7.3 – 8.9) | 11175 (76%) |
878 (6%) | 1452 (10%) | 3.2 (2.2 – 4.4) |
4.0 per 100 person-years | MACE-3 (non-fatal stroke, non-fatal myocardial infarction, and death from CV causes) |
| Hernandez et al. (2018)18 HARMONY OUTCOMES (n=9463; July 1, 2015 to November 24, 2016; 610 sites in 28 countries) |
age ≥ 18 years with established CVD and HbA1C > 7.0% | Albiglutide, 30–50 mg/week, subcutaneous | 64.1 (8.7) | 6569 (69%) |
2894 (31%) | 32.3 (5.9) | 14.1 (8.7) | 8.7 (1.5) | 6583 (70%) |
225 (2%) | 470 (5%) | 1.6 (1.3 – 2.0) |
5.9 per 100 person-years | MACE-3 (non-fatal stroke, non-fatal myocardial infarction, and death from CV causes) |
| Gerstein et al. (2019)19 REWIND (n=9901; Aug 18, 2011 to Aug 14, 2014; 371 sites in 24 countries), |
age ≥50/55/60 years with CVD or high-risk CV; ≤ 2 antidiabetics drugs ± insulin, BMI ≥ 23 kg/m2, and HbA1C ≤ 9% | Dulaglutide, 1.5 mg/week, subcutaneous | 66.2 (6.5) | 5312 (54%) |
4589 (46%) | 32.3 (5.7) | 10.5 (7.2) | 7.3 (1.1) | 7498 (76%) |
677 (7%) | 434 (4%) | 5.4 (5.1 – 5.9) |
2.7 per 100 person-years | MACE-3 (non-fatal stroke, non-fatal myocardial infarction, and death from CV causes or unknown causes) |
| Husain et al (2020)20 PIONEER 6 (n=3183; January to Aug, 2017; 214 sites in 21 countries) |
age ≥ 50 years with CVD or CKD; or ≥ 60 years with ACSCVD only | Semaglutide, 14 mg once-daily oral | 66 (7) | 2176 (68%) |
1007 (32%) | 32.3 (6.5) | 14.9 (8.5) | 8.2 (1.6) | 2300 (72%) |
192 (6%) | 630 (20%) | 1.3 (0.33–1.67) |
3.7 per 100 person-years | MACE-3 (CV death, non-fatal myocardial infarction, non-fatal stroke) |
Data are mean (standard deviation); ACSCVD: atherosclerosis cardiovascular disease; MACE: major adverse cardiovascular event; CKD: chronic kidney disease; HbA1C: haemoglobin glycated (A1c); NR: not reported; CV: cardiovascular; CVD: cardiovascular disease; HF: hospitalization for heart failure; IQR: interquartile range; BMI: body mass index; REWIND: Researching Cardiovascular Event with a Weekly Incretin in Diabetes; LEADER: Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results; SUSTAIN-6: Trial to Evaluate Cardiovascular and Other Long-term Outcomes with Semaglutide in Subjects with Type 2 Diabetes; EXSCEL: Exenatide Study of Cardiovascular Event Lowering; EMPLITUDE-O: Effect of Efpeglenatide on Cardiovascular Outcomes in Type 2 Diabetes Patients at High Cardiovascular Risk; DECLARE-TIMI 58: Dapagliflozin Effect on Cardiovascular Events-Thrombolysis in Myocardial Infarction 58; VERTIS CV: Evaluation of Ertugliflozin Efficacy and Safety Cardiovascular Outcomes Trial; CANVAS: Canagliflozin Cardiovascular Assessment Study; EMPA-REG OUTCOME: Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients; PIONEER 6: Peptide Innovation for Early Diabetes Treatment; HARMONY OUTCOMES.
Briefly, 4800 (5.0%) patients experienced a MACE-3 outcome; compared with placebo, adding GLP-1 RAs or SGLT2i to standard care was associated with a 12% reduction in MACE-3 (HR=0.88, 95% CI: 0.85–0.92) without heterogeneity (I2=0%, p=0.53), with a similar benefit for GLP-1 RAs (0.87, 0.83–0.91) and SGLT2i (0.91, 0.85–0.97), (P interaction = 0.25) (appendix, Figure S2).
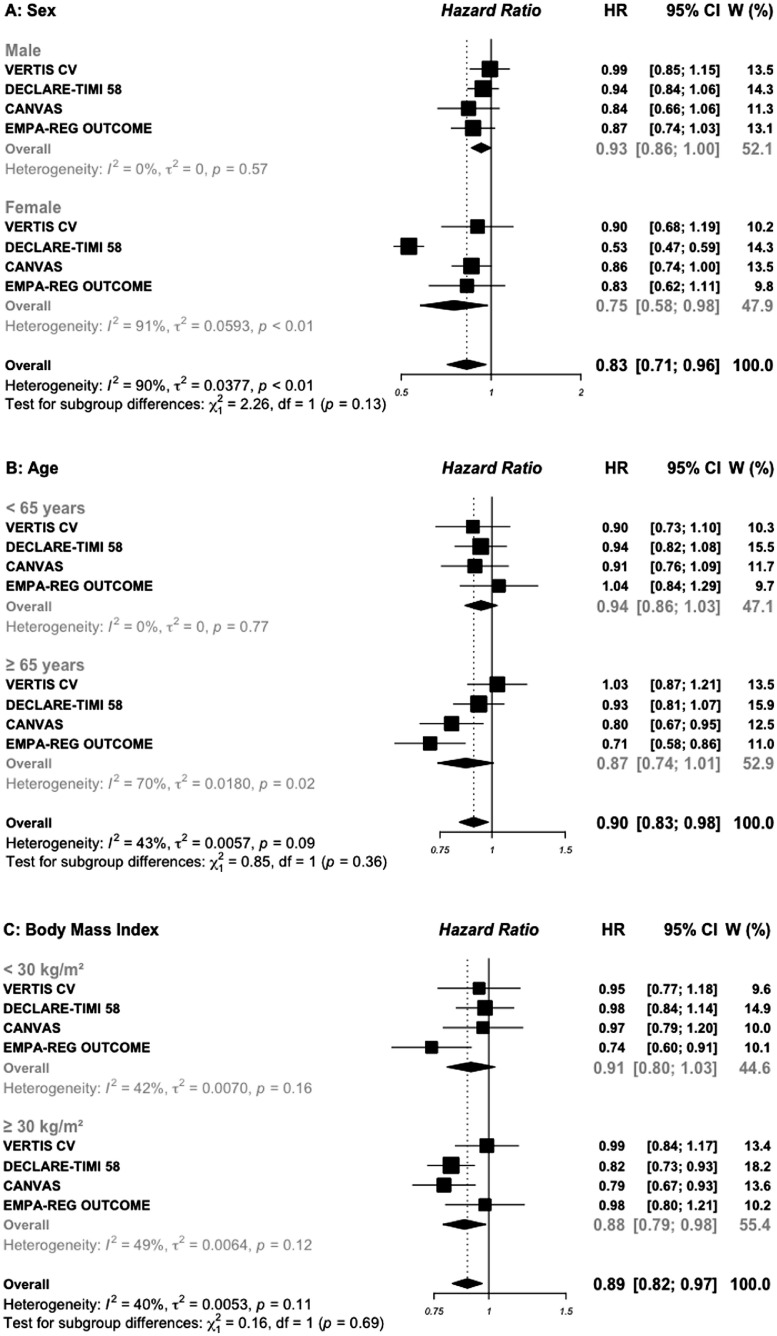
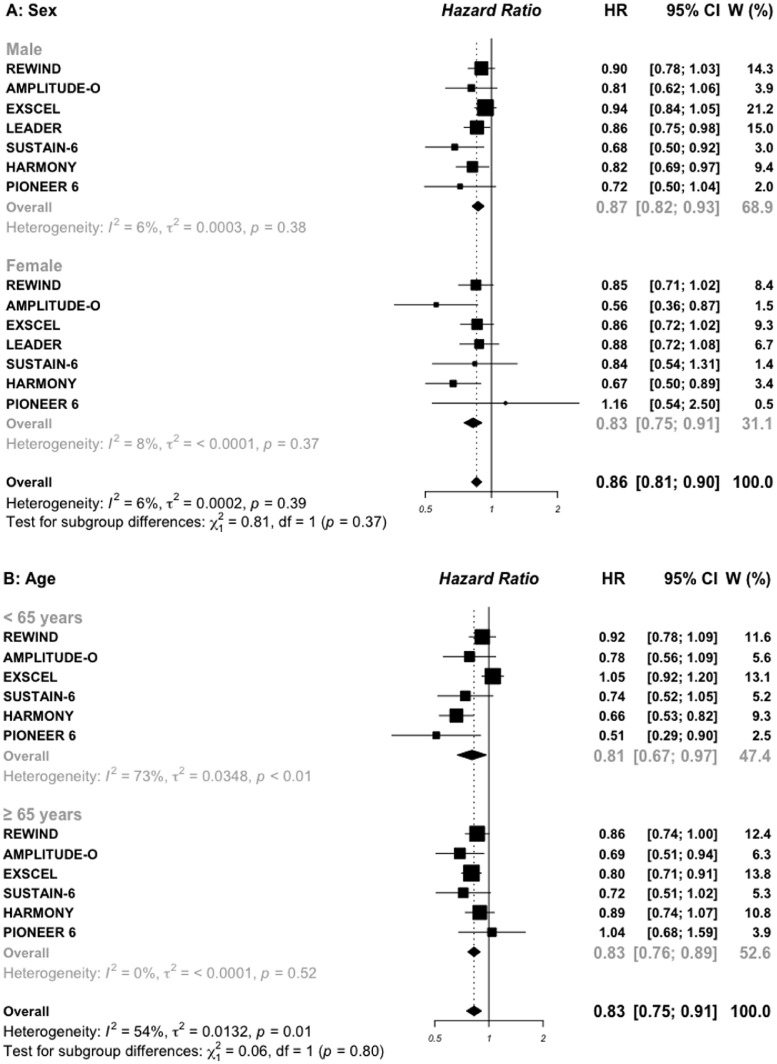
Subgroup analysis by sex was reported in all included trials (Figures 2A and 3A). In the four trials of SGLT2i, the HR (95%) for MACE outcome in 28,032 men versus in 14,536 women included was 0.93 (0.86–1.00) vs. 0.75 (0.58–0.98), respectively (P interaction =0.13). In the seven GLP-1 RAs trials, including 33,943 males and 20,069 females, the corresponding HRs were 0.87 (0.82–0.93) vs. 0.83 (0.75–0.91), respectively (P interaction = 0.39).
Figure 2.
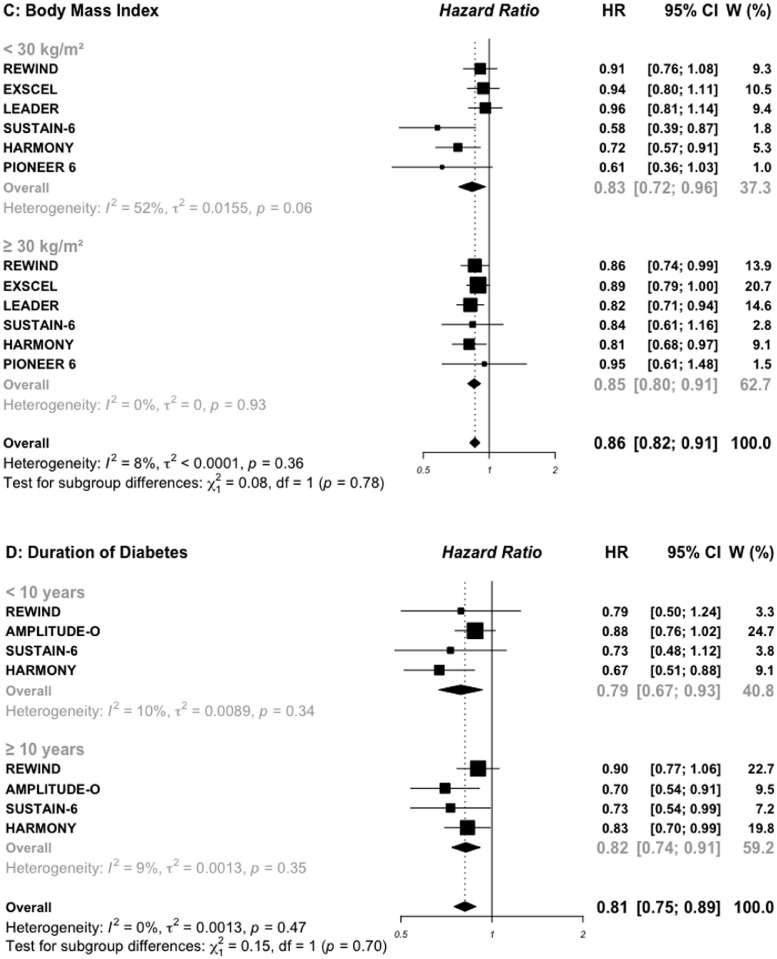
Risk for MACE outcome by sex (A), age (B), BMI (C), and duration of diabetes (D) reported in SGLT2 inhibitors cardiovascular outcomes trials.
MACE: major adverse cardiovascular events; SGLT2: sodium glucose cotransporter 2 inhibitors.
Figure 3.
Risk for MACE outcome by sex (A), age (B), BMI (C), and duration of diabetes (D) reported in GLP-1 RAs cardiovascular outcomes trials.
MACE: major adverse cardiovascular events; GLP-1 RAs: glucagon-like peptide 1 receptor agonists.
Pooled data for age subgroups with a cut-off of 65 years were reported in all included trials, except the LEADER trial which used the cut-off of 60 years, and was excluded for this subgroup analysis (Figures 2B and 3B). In the four SGLT2i trials, the HR for MACE outcome was 0.94 (0.86–1.03) in younger patients vs. 0.86 (0.74–1.01) in older patients, respectively (P interaction =0.36). In the six GLP-1 RAs trials, the corresponding HRs were 0.81 (0.67–0.97) vs. 0.83 (0.76–0.89), respectively (P interaction = 0.80). In addition, pooled data of two GLP-1 RAs trials indicated that, the addition of once-weekly exenatide (EXSCEL trial) or 30–50 mg of albiglutide (HARMONY trial) to standard care in 2390 patients older than 75 years was associated with a significant 22% reduction in MACE (0.78, 0.63–0.95) while no difference was observed in 21, 825 patients aged less than 75 years (0.87, 0.75–1.01), (P interaction = 0.37; appendix Figure S3).
AMPLITUDE-O trial reported subgroup analysis by obesity status using a cut-off of 31.9 kg/m2, then was excluded for BMI subgroups analysis (Figures 2C and 3C). In patients treated with SGLT2i, the HR for MACE outcome was 0.91 (0.80–1.03) in those with baseline BMI < 30 kg/m2 vs. 0.88 (0.79–0.99) in those with BMI ≥ 30 kg/m2, respectively (P interaction = 0.69). The corresponding HRs for MACE outcome in those treated with GLP-1 RAs were 0.83 (0.72–0.96) vs. 0.85 (0.80–0.91), respectively (P interaction = 0.78).
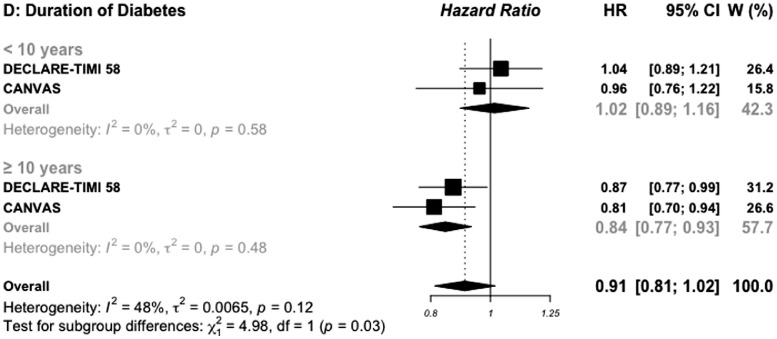
For duration of diabetes, pooled data were available in two SGLT2i and four GLP-1 RAs trials (Figures 2D and 3D). Patients in whom the duration of diabetes exceeded 10 years and treated with SGLT2i, had a great benefit for MACE outcome (0.84, 0.77–0.93) compared with those with a shorter duration of diabetes (1.02, 0.89–1.16), with significant interaction test (P interaction= 0.03). For patients treated with GLP-1 RAs, the HR for MACE outcome was similar (P interaction = 0.70) between those with a long (0.82, 0.74–0.91) versus short duration of diabetes (0.79, 0.67–0.93).
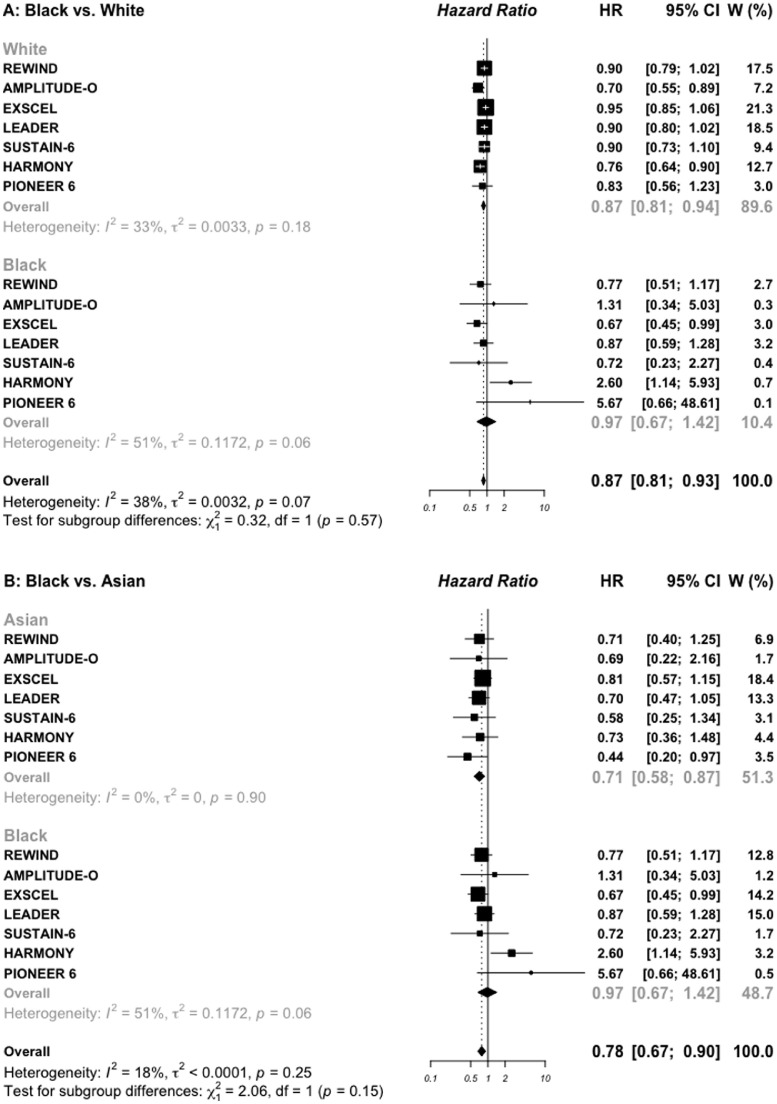
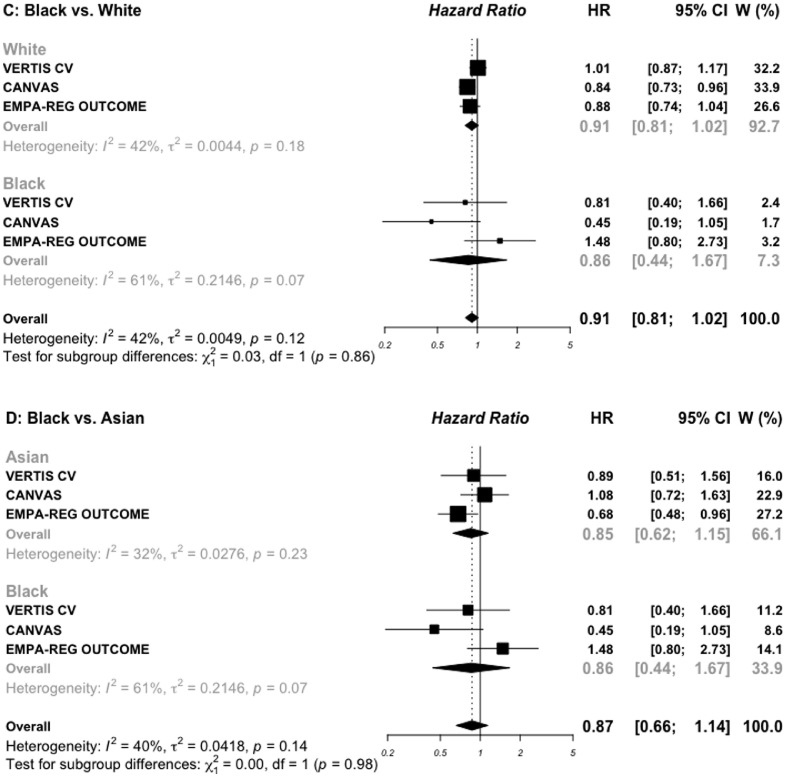
For race subgroups, three comparisons were made (White vs. Black, White vs. Asian, and Black vs. Asian). The DECLARE-TIMI-58 trial did not reported subgroups for Black and Asian individuals, and was then excluded for this subgroup analysis, leaving three trials with SGLT2i and seven trials with GLP-1 RAs. For patients treated with SGLT2i (20,265 White and 910 Black) (Figure 4C), the HR for MACE outcome was 0.91 (0.81–1.02) vs. 0.86 (0.44–1.67), respectively (P interaction = 0.86). The corresponding HRs for MACE outcome in 41,064 White and 3113 Black treated with GLP-1 RAs were 0.87 (0.81–0.94) vs. 0.97 (0.67–1.42), respectively (P interaction = 0.57) (Figure 4A). For the comparison between 20,265 White and 3298 Asian treated with SGLT2i (appendix, Figure S4), the HRs for MACE outcome were 0.90 (0.81–1.08) vs. 0.85 (0.62–1.15), respectively (P interaction = 0.69). The corresponding HRs for MACE outcome in 41,064 White and 4462 Asian treated with GLP-1 RAs were 0.87 (0.81–0.94) vs. 0.71 (0.58–0.87), respectively (P interaction = 0.07). For the comparison between Black and Asian treated with SGLT2i (Figure 4D), the HRs for MACE outcome were 0.85 (0.62–1.15) vs. 0.86 (0.44–1.67), respectively (P interaction = 0.98). The corresponding HRs for MACE outcome in those treated with GLP-1 RAs were 0.71 (0.58–0.87) vs. 0.97 (0.67–1.42), respectively (P interaction= 0.15) (Figure 4B).
Figure 4.
Risk for MACE outcome by race reported in GLP-1 RAs (A and B) and SGLT2 inhibitors (C and D) cardiovascular outcomes trials.
MACE: major adverse cardiovascular events; SGLT2: sodium glucose cotransporter 2 inhibitors; GLP-1 RAs: glucagon-like peptide 1 receptor agonists.
Discussion
In this large meta-analysis of 96,580 patients with type 2 diabetes treated with SGLT2i or GLP-1 RAs in addition to standard of care, there was a similar reduction in MACE among patients regardless of differences in age, sex, race, and BMI. Patients with prolonged diabetes (≥ 10 years) who were treated with SGLT2 inhibitors had a significant reduction in MACE compared to those patients with a short duration of diabetes. This result derived from the pooled data of CANVAS and DECLARE-TIMI 58 trials, where for patients with long-term of duration of diabetes, the MACE outcome was reduced by 19% and 13% respectively. A larger sample-size (more than 10,000 in each trial) with a high proportion of patients with established atherosclerosis cardiovascular disease (ranging from 41% to 72%), and long-term duration of follow-up, from 2.4 years (24,340 person-years) in the CANVAS trial to 4.3 years (72,072 person-years) in the DECLARE-TIMI 58 trial could explain these benefits. Because the action of GLP-1 RAs involves stimulation of glucose-dependent pancreatic insulin secretion, one might have expected fewer insulin-treated patients to be included in GLP-1 RAs trials. However, nearly one in two patients received insulin at inclusion for both drugs classes. It is important that these patients are not excluded from the trials, as they often find themselves in therapeutic impasse due to the long duration and multicomplex nature of their diabetes. In addition, the complementary mechanism of action of GLP1-RAs and insulin therapy in the significative reduction in HbA1c, fasting and postprandial glucose, lowering risk of hypoglycaemia, prevention of weight gain, and concurrent reduction of insulin doses offers a unique advantage in the management of these patients.21 The value of these treatments’ insulin-treated patients is supported by data from a large routine clinical practice cohort that suggests that the addition of a GLP-1 RAs as an adjunct to insulin therapy in overweight patients with diabetes is associated with a significant 36% risk reduction in MACE outcome.22
For sex, the results of a recent meta-analysis from three SGLT2 inhibitors studies showed that, the reduction in MACE appears to be significant only in men with no benefit observed in women, while data for six GLP-1 RAs trials showed a similar reduction in MACE, irrespective of sex subgroup. However, this study provided only sex-pooled data, without testing for sex subgroup difference.4 The addition of VERTIS CV trial for SGLT2 inhibitors and AMPLITUDE-O trial for GLP-1 RAs increased the statistical power of this previous report, and shown that, although there was no difference compared to men, women had a greater reduction in major cardiovascular events, 25% for SGLT2 inhibitors and 17% for GLP-1 RAs. In addition, there is also a high heterogeneity (91%) between SGLT2 trials reporting data for female subgroup. However, a smaller number of trials (four) and participant contributed data to the female subgroup (34%) than to the men subgroup (66%), meaning that the analysis may not be able to detect subgroup difference. Several hypotheses could explain these gender differences. Firstly, results of real-world study found a differential response to short-acting exenatide. In this study, after a 1-year follow-up, weight loss was found to be significantly higher in women than in men, and inversely, the proportion of patients with a target HbA1c of < 7% was lower in women than in men.23 These response disparities have been shown for other CV drugs used in CVOTs as a standard of care such as aspirin or statins.24,25 Briefly, in primary prevention trials, the benefit of aspirin for the prevention of stroke was greater in women than in men, while, the benefit was lower for prevention of myocardial infarction in women than in men.24 The use of statins use for primary prevention in women is not definitively established.25 Secondly, despite the greater frequency and severity of type 2 diabetes in women compared to men, women are under-represented in CVOTs studies. One of the possible explanations is the difficulty of recruiting pre-menopausal women into clinical trials because of the potential pregnancy risks of most CV management drugs such as statins, renin-angiotensin system blockers, and some oral antidiabetics (although some scientific societies consider metformin to be risk-free). Thirdly, there is some evidence that therapeutic inertia is a possible explanation, and that compared with men, women receive inadequate dosing of statins and aspirin, and underwent lesser revascularization procedure for established coronary heart disease.26 Finally, compared with men, women would be less compliant to treatments and more likely to experience side effects of CV drugs.25
With respect to age, no significant reduction in MACE was observed in either the younger and older patients with SGLT2 drugs, while a significant reduction in MACE was observed with GLP-1 RAs, irrespective of the age groups. As for women, the elderly people are under-represented in CVOTs studies, mainly due to the presence of multiple co-morbidities and frailty. With the increasing risk of micro and macro-vascular complications with age, more data on the efficacy and safety of these new therapies in elderly and very elderly people are needed.
Results from a recent meta-analysis of ten CVOTs indicated that compared with White, the Asian participants may derive greater MACE benefit with GLP-1 RAs but not with SGLT2i.3 However, when data from 4076 participants among whom 2717 received 2 or 4 mg of efperglenatide during a median follow-up of 1·81 years from AMPLITUDE-O trial, are added, this benefit no longer appears to be significant. The small number of Asian patients (7%) included in this study and the very short follow-up time could possibly explain this difference, suggesting greater caution in the interpretation of these data whose conclusions may change as more results become available. Whatever the lowering-glucose drugs used, we did not find a benefit in MACE reduction for Black patients. Furthermore, despite their higher burden of cardiovascular and advanced kidney disease and higher experience of poor cardiovascular outcomes,5,6 we did not find a differential benefit compared with White or Asian participants with both GLP-1 RAs and SGLT2i. Disparities and barriers to accessing therapy with clinical benefit may contribute to worse cardiovascular outcomes in Black patients. Data from a large cohort study suggest the presence of inequities in access to SGLT2 inhibitors for Black, female, and those with lower household incomes.27 Therefore, many efforts are needed to further investigate barriers to access these new glucose-lowering drugs and implement strategies to include more Black patients in future clinical trials. Pharmaco-epidemiological studies are also needed to assess the safety and efficacy of GLP-1 RAs and SGLT2 drugs in these populations.
For body weight, the significant reduction of 12% in MACE with SGLT2 drugs was observed only in obese patients, while with GLP-1 RAs, we observed a significant reduction in MACE in both groups (17% vs 15%). Results from meta-analyses suggest a weight loss of 2.8 kg with GLP-1 RAs and from 2 to 3 kg with SGLT2i, with more important weight loss for patients with a baseline BMI > 40 kg/m2.28
The strengths of this study include the large specific risk subgroup considered compared with the previous meta-analyses. Nevertheless, we noted some limitations. First, there was significant heterogeneity between trials, particularly in the SGLT2i outcomes trials, with I2 ranging from 27% for race (Asian and White) to 90% for sex comparisons, while in the GLP-1 RAs outcomes trials, the significant heterogeneity was observed only for age subgroup comparison (I2 = 54%). Although, we were not able to examine this source of heterogeneity, difference in participant characteristics and background especially for the history of cardiovascular disease ranging from 40% in DECLARE-TIMI 58 trial to 99% in EMPA-REG OUTCOME trial may explain this heterogeneity. Second, some trials were excluded from the analyses because of different cut-offs used in the subgroup's analyses or because of lack of information, thus limiting the sample size. Efforts should be made to improve data reporting. In addition, other studies such as ELIXA were excluded because they reported a 4-point MACE. Although in this landmark trial which included over 6,000 patients with type 2 diabetes and acute coronary syndrome, the addition of lixisenatide to usual care did not significantly alter the rate of major CV events, they would still have contributed to the power of this meta-analysis. The availability of individual patient data would helped to overcome miss information and to standardized analysis, and to assess the impact of these treatments on certain risk groups such as smoking status or to assess treatment adherence among subgroups. Third, while the Asian participants, which accounts for nearly 60% of the world's population, is a very heterogeneous ethnic group including South Asians (Indian) and East/Southeast Asian (Chinese or Japanese), in clinical studies they are considered as a single entity. Efficacy data are needed for these more specific subgroups. Four, the exclusion of analyses from the LEADER trial for the age subgroup (60-years cut-off) and AMPLITUD-O trial for the BMI subgroup (31.9 kg/m2) could certainly induce a selection bias, but this seems limited because only one study was excluded for each comparison.
To conclude, our data extend the knowledge of the SGLT2i and GLP-1 RAs benefits on major cardiovascular events in patients with type 2 diabetes according to specific risk subgroups. These findings suggest greater reduction in MACE outcome for patients with a long-term duration of diabetes with SGLT2i, and for elderly patients with GLP-1 RAs. In contrast, the MACE benefit of SGLT2i and GLP-1 RAs was similar across age, sex, BMI, and other race subgroups. Furthermore, these findings could help in providing guidance for treatment prescription, and facilitate selection and stratification of patients for future CVOTs.
Contributors
AD conceived the study design, analyzed the data, and drafted the first version of the manuscript, MCB supervision of data collection, and critical revision of the manuscript, and FG interpreted and substantially revised the manuscript. All authors have accessed to the full data, FG and MCB verified the full data, and all authors approved the final version of the manuscript.
Data sharing statement
AD, MCB, and FG had access to the data. Datasets of this meta-analysis is available upon reasonable requested to the corresponding authors (alhassane.diallo@chu-montpellier.fr).
Declaration of interests
AD, MCB, and FG declare no competing interests.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101697.
Appendix. Supplementary materials
References
- 1.Sattar N, Lee MMY, Kristensen SL, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 2021;9:653–662. doi: 10.1016/S2213-8587(21)00203-5. [DOI] [PubMed] [Google Scholar]
- 2.Yamada T, Wakabayashi M, Bhalla A, et al. Cardiovascular and renal outcomes with SGLT-2 inhibitors versus GLP-1 receptor agonists in patients with type 2 diabetes mellitus and chronic kidney disease: a systematic review and network meta-analysis. Cardiovasc Diabetol. 2021;20:14. doi: 10.1186/s12933-020-01197-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee MMY, Ghouri N, McGuire DK, Rutter MK, Sattar N. Meta-analyses of results from randomized outcome trials comparing cardiovascular effects of SGLT2is and GLP-1RAs in Asian versus white patients with and without type 2 diabetes. Diabetes Care. 2021;44:1236–1241. doi: 10.2337/dc20-3007. [DOI] [PubMed] [Google Scholar]
- 4.Singh AK, Singh R. Gender difference in cardiovascular outcomes with SGLT-2 inhibitors and GLP-1 receptor agonist in type 2 diabetes: a systematic review and meta-analysis of cardio-vascular outcome trials. Diabetes Metab Syndr Clin Res Rev. 2020;14:181–187. doi: 10.1016/j.dsx.2020.02.012. [DOI] [PubMed] [Google Scholar]
- 5.Durstenfeld MS, Ogedegbe O, Katz SD, Park H, Blecker S. Racial and ethnic differences in heart failure readmissions and mortality in a large municipal healthcare system. JACC Heart Fail. 2016;4:885–893. doi: 10.1016/j.jchf.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi AI, Rodriguez RA, Bacchetti P, Bertenthal D, Hernandez GT, O'Hare AM. White/black racial differences in risk of end-stage renal disease and death. Am J Med. 2009;122:672–678. doi: 10.1016/j.amjmed.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Narayan KMV, Boyle JP, Thompson TJ, Gregg EW, Williamson DF. Effect of BMI on lifetime risk for diabetes in the U.S. Diabetes Care. 2007;30:1562–1566. doi: 10.2337/dc06-2544. [DOI] [PubMed] [Google Scholar]
- 8.Gerstein HC, Sattar N, Rosenstock J, et al. Cardiovascular and renal outcomes with efpeglenatide in type 2 diabetes. N Engl J Med. 2021;385(10):896–907. doi: 10.1056/NEJMoa2108269. [DOI] [PubMed] [Google Scholar]
- 9.Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- 10.Higgins JPT, Altman DG, Gotzsche PC, et al. The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343 doi: 10.1136/bmj.d5928. d5928–d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cannon CP, Pratley R, Dagogo-Jack S, et al. Cardiovascular outcomes with ertugliflozin in type 2 diabetes. N Engl J Med. 2020;383:1425–1435. doi: 10.1056/NEJMoa2004967. [DOI] [PubMed] [Google Scholar]
- 12.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 13.Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 14.Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 15.Marso SP, Daniels GH, Brown-Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 17.Holman RR, Bethel MA, Mentz RJ, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377:1228–1239. doi: 10.1056/NEJMoa1612917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernandez AF, Green JB, Janmohamed S, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392:1519–1529. doi: 10.1016/S0140-6736(18)32261-X. [DOI] [PubMed] [Google Scholar]
- 19.Gerstein HC, Colhoun HM, Dagenais GR, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121–130. doi: 10.1016/S0140-6736(19)31149-3. [DOI] [PubMed] [Google Scholar]
- 20.Husain M, Birkenfeld AL, Donsmark M, et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381:841–851. doi: 10.1056/NEJMoa1901118. [DOI] [PubMed] [Google Scholar]
- 21.Ahrén B. Insulin plus incretin: a glucose-lowering strategy for type 2-diabetes. World J Diabetes. 2014;5:40. doi: 10.4239/wjd.v5.i1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anyanwagu U, Mamza J, Donnelly R, Idris I. Effect of adding GLP-1RA on mortality, cardiovascular events, and metabolic outcomes among insulin-treated patients with type 2 diabetes: a large retrospective UK cohort study. Am Heart J. 2018;196:18–27. doi: 10.1016/j.ahj.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Seghieri G, Anichini CS, Carlo Di, et al. Gender difference in response predictors after 1-year exenatide therapy twice daily in type 2 diabetic patients: a real world experience. Diabetes Metab Syndr Obes. 2013;6:123–129. doi: 10.2147/DMSO.S42729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berger JS, Roncaglioni MC, Avanzini F, Pangrazzi I, Tognoni G, Brown DL. Aspirin for the primary prevention of cardiovascular events in women and men: a sex-specific meta-analysis of randomized controlled trials. JAMA. 2006;295:306. doi: 10.1001/jama.295.3.306. [DOI] [PubMed] [Google Scholar]
- 25.Rosano GMC, Collins P. Gender differences in treatment of cardiovascular disease: a task force on gender of the ESC proposal on gender specific studies in cardiovascular pharmacology: editorial. Fundam Clin Pharmacol. 2010;24:662–663. doi: 10.1111/j.1472-8206.2010.00895_2.x. [DOI] [PubMed] [Google Scholar]
- 26.Vaccarino V, Rathore SS, Wenger NK, et al. Sex and racial differences in the management of acute myocardial infarction, 1994 through 2002. N Engl J Med. 18 août. 2005;353:671–682. doi: 10.1056/NEJMsa032214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eberly LA, Yang L, Eneanya ND, et al. Association of race/ethnicity, gender, and socioeconomic status with sodium-glucose cotransporter 2 inhibitor use among patients with diabetes in the US. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.6139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cai X, Ji L, Chen Y, et al. Comparisons of weight changes between sodium-glucose cotransporter 2 inhibitors treatment and glucagon-like peptide-1 analogs treatment in type 2 diabetes patients: a meta-analysis. J Diabetes Investig. 2017;8:510–517. doi: 10.1111/jdi.12625. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.