Abstract
Objective
To assess adverse perinatal outcomes and caesarean section of low-risk women receiving elective induction of labour at 41 weeks or expectant management until 42 weeks according to their preferred and actual management strategy.
Design
Multicentre prospective cohort study alongside RCT.
Setting
90 midwifery practices and 12 hospitals in the Netherlands.
Population
3642 low-risk women with uncomplicated singleton late-term pregnancy.
Main outcome measures
Composite adverse outcome (perinatal death, Apgar score 5′ < 7, NICU admission, meconium aspiration syndrome), composite severe adverse perinatal outcome (all above with Apgar score 5′ < 4 instead of < 7) and caesarean section.
Results
From 2012–2016, 3642 women out of 6088 eligible women for the INDEX RCT, participated in the cohort study for observational data collection (induction of labour n = 372; expectant management n = 2174; unknown preference/management strategy n = 1096).
Adverse perinatal outcome occurred in 1.1 % (4/372) in the induction group versus 1.9 % (42/2174) in the expectant group (adjRR 0.56; 95 %CI: 0.17–1.79), with severe adverse perinatal outcome occurring in 0.3 % (1/372) versus 1.0 % (22/2174), respectively (adjRR 0.39; 95 % CI: 0.05–2.88). There were no stillbirths among all 3642 women; one neonatal death occurred in the unknown preference/management group. Caesarean section rates were 10.5 % (39/372) after induction and 8.9 % (193/2174) after expectant management (adjRR 1.32; 95 % CI: 0.95–1.84).
A higher incidence of adverse perinatal outcome was observed in nulliparous compared to multiparous women. Nulliparous 1.8 % (3/170) in the induction group versus 2.6 % (30/1134) in the expectant management group (adjRR 0.58; 95 % CI 0.14–2.41), multiparous 0.5 % (1/201) versus 1.1 % (11/1039) (adjRR 0.54; 95 % CI 0.07–24.19). One maternal death due to amniotic fluid embolism occurred after elective induction at 41 weeks + 6 days.
Conclusion
In this cohort study among low-risk women receiving the policy of their preference in late-term pregnancy, a non-significant difference was found between induction of labour at 41 weeks and expectant management until 42 weeks in absolute risks of composite adverse (1.1 % versus 1.9 %) and severe adverse (0.3 % versus 1.0 %) perinatal outcome. The risks in this cohort study were lower than in the trial setting. There were no stillbirths among all 3642 women. Caesarean section rates were comparable.
Keywords: Late-term pregnancy, Induction of labor, Expectant management, Perinatal outcome, Maternal outcome, Cesarean section
Highlights
-
•
No stillbirths in late-term pregnancy (IOL or EM) of 3642 women in INDEX-cohort.
-
•
Less adverse perinatal outcomes in INDEX-cohort than in trial with comparable risk difference.
-
•
More women approaching late-term pregnancy prefer EM, not elective induction.
1. Introduction
The risk of adverse perinatal outcomes gradually increases after 41 weeks [1]. The main concern is the risk of stillbirth, although the exact magnitude of risk reduction of perinatal mortality with a policy of induction of labour (induction) from 41 weeks onwards remains unclear [2], [3], [4]. Recent Individual Patient Data-Meta Analysis (IPD-MA) of trials comparing induction at 41 weeks with a policy of expectant management (expectant) until 42 weeks concluded that induction reduces the risk of adverse perinatal outcome for nulliparous women (induction 0.3 % versus expectant 1.6 %; RR 0.20; 95 % CI 0.07–0.60) but not for multiparous women (induction 0.6 % versus expectant 0.3 %; RR 1.59; 95 % CI 0.15–17.30). The risk of caesarean section was not increased for either nulliparous or multiparous women [5]. Though different guidelines and systematic reviews on this topic were published in recent years, the question who benefits most from elective induction at 41 weeks is not clear [2], [6], [7].
One of the included trials in the IPD-MA was the INDEX trial (N = 1801) in which a small, but significant difference of 1.4 % was found in the incidence of composite adverse perinatal outcomes in favour of induction (1.7 % versus 3.1 %; RR 0.54; 95 % CI 0.29–1.00; p-value=0.045), though the absolute risk of severe adverse perinatal outcome was low in both groups (induction 0.4 % versus expectant 1.3 %; RR 0,33; 95 % CI 0.11–1.03; p-value=0.06 [3]. A large proportion (69 %) of women invited for the INDEX trial did not want to be randomised, which is comparable to findings in similar trials (SWEPIS trial 78 %, ARRIVE trial 71 %, 35/39 trial 86 %) [4], [8], [9].
Since trials are designed to evaluate outcomes by comparing management strategies, whereas in an observational design outcomes of daily practice are evaluated [10]. This raises the question if characteristics and clinical outcomes of those women who participate in a trial differ from women who decline participation.
In this study we assessed the total group of women who were eligible for both management strategies but declined randomisation for the INDEX trial. Actual management strategy (induction or expectant) was mostly depended on woman’s preference and local protocol.
The aim of this prospective cohort study was to assess perinatal, maternal and labour outcomes of initially low-risk women according to their preferred and the actual management strategy after elective induction of labour at 41 weeks or expectant management until 42 weeks.
2. Materials and methods
2.1. Study design
We conducted this prospective cohort study alongside the INDEX trial. Our study was set in the Dutch Obstetric Consortium: a collaboration of obstetrical centres in the Netherlands in cooperation with the Midwifery Research Network of the Netherlands (MRNN). The study was conducted in 90 midwifery practices and 12 hospitals in the Netherlands in the period from May 2012 to March 2016.
2.2. Study population
In the INDEX trial — and therefore also in this cohort— eligible pregnant women at low risk visiting outpatient clinics or midwifery practices for the 40 weeks check-up, were counselled for participation in the INDEX trial. After informed consent was given between 40 weeks + 5 days to 41 weeks + 0 days they were randomly allocated to induction of labour at 41 weeks or to expectant management until 42 weeks. From 42 weeks onwards induction of labour was indicated according to the interprofessional Dutch Obstetrical Indication List [3], [11], [12], [13]. Women who did not want to be randomised were asked for their reason to decline. Reasons for decline were registered by the caregiver using a prespecified model with four options: “prefers elective induction”, “prefers expectant management”, “doesn’t want policy by randomisation” and “other”. Participants were enrolled in a midwifery practice by an independent midwife (primary care) or by a research nurse/midwife of the hospital (secondary care).
In- and exclusion criteria of both trial and cohort study were identical. Consenting women at low obstetrical risk, aged ≥ 18 years, with a singleton pregnancy and a fetus in stable cephalic position, and a gestational age of 40 weeks + 5 days to 41 weeks + 1 days confirmed by early ultrasound, were included in this cohort. Exclusion criteria at time of inclusion were obstetrical complications or conditions such as: hypertensive disorders (systolic 140 mmHg and/or diastolic 90 mmHg or more), proteinuria (=3 g/L), pre-existent maternal heart or kidney diseases, gestational diabetes, previous caesarean section, multiple pregnancy, intra-uterine growth retardation, non-reassuring fetal status (including absence of or decreased fetal movements, abnormal fetal heart rate), known fetal abnormalities which could influence perinatal outcome, abnormal karyotype, ruptured membranes at time of inclusion or other contra-indications for expectant management until 42 weeks + 0 days.
The induction of labour group consisted of women who preferred induction at 41 weeks + 0/1 days at enrolment. In the days after enrolment but before the planned induction (≤ 41 weeks + 0/1 days), women could have a spontaneous onset of labour or labour induction; electively or medically indicated for a fetal or maternal condition that emerged.
The expectant management group consisted of women who preferred expectant management. In the days after enrolment, women could have had a spontaneous onset of labour or labour was induced on fetal or maternal indication between 41 and 42 weeks or because of postterm pregnancy ≥ 42 weeks + 0 days. Data were analysed according to assignment to these two groups.
The third group (unknown preference/management strategy) consisted of women without an expressed preference for either induction or expectant management, a preference for induction but an onset of labour after 41 weeks + 1 days or a preference for expectant management with elective induction of labour at 41 weeks + 2 days to 41 weeks + 6 days (Fig. 1). Because women’s preference and actual management were not consistent or unclear, it was not possible to assign them to the induction or expectant group, therefore this group is not included in the analysis. However, the group was described separately. (Supplementary tables 1 and 2).
Fig. 1.
Flowchart.
2.3. Data collection
Data were collected from caregivers in a Case Report Form (CRF) which was sent to the coordinating centre. Upon receipt of the CRF all data were checked by the first authors (AB, JKJK) or a research midwife connected to the research team (LS). Missing, illogical data and outliers were verified from the corresponding participating centres. After ascertainment, data were entered in an Oracle Clinical electronic database by trained midwifery or medical students. Data from secondary care were directly entered in the database by research nurses and midwives of the participating hospitals using the electronic CRF. Severe adverse perinatal and maternal outcomes (perinatal and maternal mortality, neonatal intensive care unit (NICU) and Intensive Care Unit (ICU) admission and postpartum haemorrhage ≥ 2000 mL) were verified against the records of the midwifery practice or hospital. The study was performed without external funding.
2.4. Outcomes of interest
2.4.1. Primary outcome
Our primary outcome was identical to the primary outcome of the INDEX trial and consisted of a composite of adverse perinatal outcomes [3]. This composite outcome was defined as having one or more of the following outcomes: perinatal mortality, 5 min Apgar score < 7, meconium aspiration syndrome (MAS) [14], plexus brachialis injury (with and without association with shoulder dystocia), or admission to a NICU. All individual components of the composite adverse perinatal outcome were also reported separately. In concordance with the INDEX-trial, we also defined a composite of severe adverse perinatal outcomes with similar components with the exception that the 5 min Apgar score should was replaced by < 4 instead of < 7 because of the ACOG and AAP statements that the inappropriate use of the Apgar score as outcome measure in studies has led to an erroneous definition of asphyxia. An Apgar score of 0–3 at 5 min or more can be considered as a nonspecific sign of illness, which may be one of the first indications of encephalopathy [15].
2.4.2. Secondary outcomes
Secondary outcomes consisted of maternal, labour and other perinatal outcomes. The composite adverse maternal outcome consisted of postpartum haemorrhage (≥ 1000 mL), manual removal of placenta, obstetrical anal sphincter injuries (OASIS), maternal admission to an intensive care unit (ICU) and maternal death. All individual components of the composite adverse maternal outcome were also reported separately. Labour outcomes included gestational age at onset of labour, onset of labour, reason for induction and mode of delivery. Secondary perinatal outcomes included neonatal admission to the medium care, congenital abnormality, hypoglycaemia, neonatal infection, neonatal sex, birthweight and small and large for gestational age (SGA < 2.3rd percentile and LGA > 97.7th percentile) [16]. As risks could differ between nulliparous and multiparous women, subgroup analysis according to parity was performed for adverse perinatal and maternal outcome and for caesarean section.
2.5. Statistical analysis
A target sample size was not calculated, since recruitment for this cohort took place alongside the INDEX trial, for which a sample calculation had been made. Relative Risks (RR) with 95 % confidence interval (CI) were calculated for composite adverse (severe) perinatal outcomes, composite adverse maternal outcomes, and caesarean section rates. With a generalised linear regression model (log-binomial) we estimated Relative Risks adjusted (adjRR) for ethnicity, SES, parity and BMI (Table 1)[5], [17], [18], [19], [20], [21]. BMI was dichotomised in < 25 and ≥ 25. Birth centiles were based on national reference data for the Netherlands on birthweight and gestational age by week and day [16].
Table 1.
Baseline characteristics.
| Preference for induction of labour ≤ 411/7 (n = 372) |
Preference for expectant management (n = 2174) |
p-value | |
|---|---|---|---|
| Maternal age (years, mean, SD) | 31.0 (4.8) | 31.4 (4.5) | 0.13* |
| Ethnicity | 0.71 | ||
| White | 302 (81.2 %) | 1893 (87.1 %) | |
| Other | 34 (9.1 %) | 234 (10.8 %) | |
| Missing | 36 (9.7 %) | 47 (2.2 %) | |
| Social economic status | < 0.001 | ||
| Low | 113 (30.4 %) | 480 (22.1 %) | |
| Medium | 173 (46.5 %) | 1052 (48.4 %) | |
| High | 82 (22.0 %) | 633 (29.1 %) | |
| Missing | 4 (1.1 %) | 9 (0.4 %) | |
| Parity | 0.025 | ||
| Nulliparous | 170 (45.7 %) | 1134 (52.2 %) | |
| Multiparous | 201 (54.2 %) | 1039 (47.8 %) | |
| Missing | 1 (0.3 %) | 1 (0.0 %) | |
| Body Mass Index (BMI) at start of pregnancy | |||
| BMI (median, IQR) | 23.4 (21.2–26.4) | 23.0 (21.0–25.7) | 0.07† |
| < 18.5 | 6 (1.6 %) | 64 (2.9 %) | 0.08† |
| 18.5 - < 25 | 220 (59.1 %) | 1421 (65.4 %) | |
| 25 - < 30 | 86 (23.1 %) | 487 (22.4 %) | |
| ≥ 30 | 34 (9.1 %) | 173 (8.0 %) | |
| Missing | 26 (7.0 %) | 29 (1.3 %) |
p-values are chi-squared tests, unless indicated otherwise. * t-test † Mann-Whitney U test. SD: standard deviation IQR: interquartile range.
2.5.1. Subgroup analysis
Because previous studies showed different outcomes according to parity [5], we performed a subgroup analysis according to parity. Analyses were performed using IBM SPSS Statistics for Windows (Version 26.0. Armonk, NY: IBM Corp. Released 2019).
2.6. Ethical approval
Both trial and cohort study were approved by the local ethics committee of the Academic Medical Centre, Amsterdam (No NL38455.018.11). The INDEX trial and cohort study were conducted under an otherwise equal protocol [11].
3. Results
Between May 2012 and March 2016, 4223 women who declined randomisation for the INDEX trial were prospectively recruited for this cohort study in 90 midwifery practices and 12 hospitals throughout The Netherlands. A total of 449 women (10.7 %) were lost to follow-up (Fig. 1). A group of 132 women had to be excluded because they did not meet the inclusion criteria, or labour onset had started before a gestational age of 40 weeks + 5 days. We assessed outcomes of 3642 women according to maternal preference and actual management strategy of whom 372 women in the induction group and 2174 in the expectant group (Table 1, Table 2, Table 3). From 1096 women preference and actual management were not consistent or unclear (Fig. 1).
Table 2.
Perinatal, maternal and labour outcomes.
| Preference for induction of labour ≤ 411/7 (n = 372) |
Preference for expectant management (n = 2174) |
Unadjusted RR (95 % CI) |
Adjusted RR (95 % CI)‡‡ |
|
|---|---|---|---|---|
| Composite adverse perinatal outcome* | 4 (1.1 %) | 42 (1.9 %) | 0.56 (0.20–1.54) | 0.56 (0.17–1.79) |
| Severe adverse perinatal outcome† (with Apgar score 5′< 4 instead of < 7) |
1 (0.3 %) | 22 (1.0 %) | 0.27 (0.04–1.97) | 0.39 (0.05–2.88) |
| Stillbirth | 0 | 0 | N/A | N/A |
| Neonatal death, post-partum†† | 0 | 0 | N/A | N/A |
| Apgar score 5 min post-partum | ||||
| < 7 | 3 (0.8 %) | 35 (1.6 %) | 0.50 (0.16–1.62) | 0.43 (0.10–1.78) |
| < 4 | 0 | 7 (0.3 %) | N/A | N/A |
| Admission neonate to | ||||
| Intensive Care (NICU) | 1 (0.3 %) | 18 (0.8 %) | 0.33 (0.04–2.43) | 0.51 (0.07–3.85) |
| Medium Care | 25 (6.7 %) | 143 (6.6 %) | 1.02 (0.68–1.54) | 0.97 (0.62–1.52) |
| Meconium aspiration syndrome | 0 | 6 (0.3 %) | N/A | N/A |
| Plexus brachialis injury | 0 | 0 | N/A | N/A |
| Congenital abnormality | 1 (0.3 %) | 9 (0.4 %) | 0.65 (0.08–5.11) | 0.72 (0.09–5.76) |
| Hypoglycaemia | 3 (0.8 %) | 13 (0.6 %) | 1.35 (0.39–4.71) | 1.67 (0.48–5.83) |
| Neonatal infection / sepsis | 6 (1.6 %) | 76 (3.5 %) | 0.46 (0.20–1.05) | 0.39 (0.14–1.05) |
| Neonatal sex, female | 180 (48.5 %) | 1078 (49.7 %) | 0.98 (0.87–1.09) | 0.96 (0.85–1.09) |
| Birthweight (g), mean (SD) | 3774 (418) | 3742 (424) | – | – |
| Small of gestational age (< 2.3rd percentile) | 6 (1.6 %) | 33 (1.5 %) | 1.06 (0.45–2.52) | 1.24 (0.52–2.92) |
| Large for gestational age (> 97.7th percentile) | 13 (3.5 %) | 43 (2.0 %) | 1.77 (0.96–3.25) | 1.69 (0.85–3.35) |
| Gestational age onset of labour (weeks+days), median (IQR) |
410/7 (406/7-411/7) | 412/7 (410/7-414/7) | – | – |
| Onset of labour | ||||
| Spontaneous | 154 (41.4 %) | 1879 (86.4 %) | 0.48 (0.42–0.54) | 0.25 (0.22–0.29) |
| Induction | 217 (58.3 %) | 292 (13.4 %) | 4.34 (3.79–4.98) | 4.02 (3.47–4.66) |
| Fetal condition | 19/217 (8.8 %) | 83/292 (28.4 %) | – | – |
| Maternal condition | 11/217 (5.1 %) | 27/292 (9.2 %) | – | – |
| > 24 h ruptured membranes | 0 | 5/292 (1.7 %) | – | – |
| Postterm pregnancy (≥ 420/7 weeks) | 0 | 177/292 (60.6 %) | – | – |
| Maternal preference | 187/217 (86.2 %) | 0 | N/A | N/A |
| Planned caesarean section | 1 (0.3 %) | 3 (0.1 %) | 1.95 (0.20–18.68) | 1.90 (0.20–18.52) |
| Mode of delivery | ||||
| Spontaneous vaginal delivery | 292 (78.5 %) | 1708 (78.6 %) | 1.00 (0.94–1.06) | 0.88 (0.73–1.07) |
| Operative vaginal delivery | 41 (11.0 %) | 273 (12.6 %) | 0.88 (0.64–1.20) | 0.99 (0.73–1.35) |
| Caesarean section (planned and secondary) | 39 (10.5 %) | 193 (8.9 %) | 1.18 (0.85–1.64) | 1.32 (0.95–1.84) |
| Composite adverse maternal outcome‡ | 43 (11.6 %) | 248 (11.4 %) | 1.01 (0.75–1.37) | 0.99 (0.71–1.39) |
| Maternal death†† | 0 | 0 | N/A | N/A |
| Postpartum blood loss (mL), median (IQR)§ | 300 (200–500) | 300 (200–500) | – | – |
| Haemorrhage postpartum ≥ 1000 mL | 30 (8.1 %) | 183 (8.4 %) | 0.96 (0.66–1.39) | 0.83 (0.54–1.27) |
| Haemorrhage postpartum ≥ 2000 mL | 7 (1.9 %) | 50 (2.3 %) | 0.82 (0.37–1.79) | 0.72 (0.29–1.79) |
| Manual removal of placenta¶ (vaginal birth only) |
9/333 (2.7 %) | 69/1981 (3.5 %) | 0.78 (0.40–1.55) | 0.77 (0.36–1.66) |
| Obstetrical anal sphincter injuries (OASIS)¶ | 11/333 (3.3 %) | 73/1981 (3.7 %) | 0.90 (0.48–1.67) | 1.16 (0.62–2.18) |
| Maternal admission to Intensive Care Unit (highest level of care) |
0 | 2 (0.1 %) | N/A | N/A |
*Defined as perinatal death and/or 5 min Apgar score < 7 and/or NICU admission and/or meconium aspiration syndrome and/or plexus brachialis injury.
†Defined as Composite adverse perinatal outcome, tough where 5 min. Apgar score < 4 instead of < 7.
‡Defined as haemorrhage postpartum ≥ 1000 mL, and/or manual removal of placenta, and/or 3rd or 4th degree tears (OASIS), and/or intensive care admission, and/or maternal death.
§Of postpartum blood loss 65 cases are missing.
¶Manual removal of placenta and OASIS of vaginal deliveries only.
††One neonatal death and one maternal death occurred in the undefined preference or management strategy group.
‡‡Adjusted for ethnicity, SES, parity and BMI. BMI dichotomised as obese (BMI ≥ 25 or < 25).
SD: standard deviation; IQR: interquartile range
Table 3.
Adverse perinatal, maternal and caesarean section outcomes stratified by parity.
| Preference for induction of labour ≤ 411/7 * (n = 371) |
Preference for expectant management† (n = 2173) |
Unadjusted RR (95 % CI) |
Adjusted RR (95 % CI)‡ |
|||||
|---|---|---|---|---|---|---|---|---|
| Nulliparous (n = 170) |
Multiparous (n = 201) |
Nulliparous (n = 1134) |
Multiparous (n = 1039) |
Nulliparous | Multiparous | Nulliparous | Multiparous | |
| Composite adverse perinatal outcome | 3 (1.8 %) |
1 (0.5 %) |
30 (2.6 %) |
11 (1.1 %) |
0.67 (0.21–2.16) |
0.47 (0.06–3.62) |
0.58 (0.14–2.41) |
0.54 (0.07–4.19) |
| Severe adverse perinatal outcome (with 5 min. Apgar score < 4)§ |
0 | 1 (0.5 %) |
13 (1.0 %) |
8 (0.8 %) |
N/A | 0.65 (0.08–5.14) |
N/A | 0.77 (0.10–6.15) |
| Composite adverse maternal outcome | 23 (13.5 %) |
20 (10.0 %) |
163 (14.4 %) |
85 (8.2 %) |
0.94 (0.63–1.41) |
1.22 (0.77–1.93) |
0.85 (0.54–1.34) |
1.23 (0.75–2.03) |
| (Secondary) caesarean section | 36 (21.1 %) |
2 (1.0 %) |
171 (15.1 %) |
22 (2.1 %) |
1.18 (0.85–1.64) |
0.47 (0.11–1.98) |
1.41 (1.01–1.97) |
0.57 (0.14–2.44) |
*One woman in the IOL group had an unknown parity; † One women in the EM group had an unknown parity; ‡ Adjusted for ethnicity, SES and BMI. BMI dichotomised by BMI < 25 and ≥ 25.
§Defined as Composite adverse perinatal outcome, tough where 5 min. Apgar score < 4 instead of < 7.
Four women received a planned caesarean section (Fig. 1), the reasons being, respectively, maternal wish at 42 weeks + 0 days, fetal malpresentation at 41 weeks + 4 days, vasa praevia at 41 weeks + 3 days and non-reassuring fetal status all at routine consultation.
We observed that almost all centres (> 97 %) used a standard policy of additional fetal monitoring in secondary care for women with a late-term pregnancy.
3.1. Characteristics of study participants
In this cohort the proportion of women who preferred and received induction was smaller (n = 372) than those who preferred and received expectant management (n = 2174). We observed significant differences in social economic status (SES) and parity between the induction and expectant preference groups. There were no significant differences in maternal age, ethnicity and Body Mass Index (BMI). More women in the expectant group had a high SES (induction 22.0 %; expectant 29.1 %; p-value < 0.001) and were more often nulliparous (induction 45.7 %; expectant 52.2 %; p-value < 0.001).
More women in the induction group had a low SES (induction 30.4 %; expectant 22.1 %; p-value < 0.001) (Table 1).
3.2. Outcomes
In the induction group, 58.3 % (217/372) of the women had induction of labour and 41.4 % (154/372) had a spontaneous onset of labour. Of all women with induction, 86.2 % (187/217) had elective induction and 13.8 % (30/217) had medically indicated induction (Table 2).
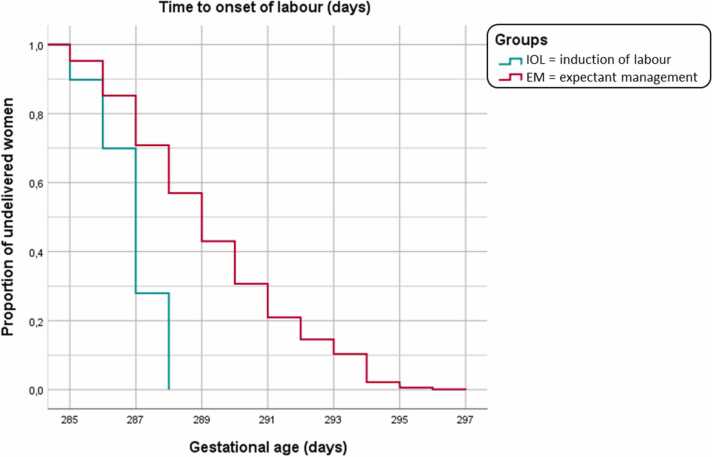
In the expectant group, 13.4 % (292/2174) had induction of labour and 86.4 % (1879/2174) started labour spontaneously. Of women with induction (n = 292), 39.4 % (115/292) were diagnosed to have a medical indication for induction between 40 weeks + 5 days and 41 weeks + 6 days and 60.6 % (177/292) had induction for postterm pregnancy (≥ 42 weeks + 0 days) (Table 2). Fig. 2 visualises the time to onset of labour for the two groups (IOL and EM) in a Kaplan Meier curve.
Fig. 2.
Time to onset of labour.
3.2.1. Perinatal outcomes
The composite adverse perinatal outcome was observed in 1.1 % (4/372) in the induction group versus 1.9 % (42/2174) in the expectant group (adjRR 0.56; 95 % CI 0.17–1.79). Severe adverse perinatal outcome occurred in 0.3 % (1/372) in the induction group versus 1.0 % (22/2174) in the expectant group (adjRR 0.39; 95 % CI 0.05–2.88).
There were no stillbirths in this study, while one neonatal death at day of birth occurred in the group with unknown preference/management strategy. This multiparous woman was referred for routine antenatal consultation (including CTG) in secondary care at 41 weeks + 2 days, the fetal condition was reassuring. After spontaneous onset of labour at 41 weeks + 2 days, she was referred to secondary care because off sudden excessive vaginal blood loss after spontaneous rupture of the membranes followed by fetal bradycardia (60 bpm). Emergency caesarean section was performed. The newborn was diagnosed with severe asphyxia and died the same day at the NICU. The parents refused post-mortem examination.
Six cases of MAS occurred, all in the expectant group, respectively once at 40 weeks + 6 days, twice at 41 weeks + 3 days, twice at 41 weeks + 4 days and once at 42 weeks + 0 days. In the total cohort no plexus brachialis injury occurred (Table 2).
3.2.2. Maternal and labour outcomes
The composite adverse maternal outcome was observed in 11.6 % (43/372) in the induction group versus 11.4 % (248/2174) in the expectant group (adjRR 0.99; 95 % CI 0.71–1.39). Caesarean section was performed in 10.5 % (39/372) in the induction group versus 8.9 % (193/2174) in the expectant group (adjRR 1.32; 95 % CI 0.95–1.84) (Table 2).
One maternal death occurred in our study. This concerned a multiparous woman with preference for expectant management and elective induction at 41 weeks + 6 days. An operative vaginal delivery was performed because of fetal distress, followed by a postpartum haemorrhage of 1600 mL. She was admitted to the ICU and died the day after she gave birth. Post-mortem examination concluded that amniotic fluid embolism was the cause for this maternal death.
Maternal admission to an ICU occurred six times in total, zero in the induction group, twice in the expectant group and four in the ‘unknown preference/management strategy’ group. Most of these women were admitted at the ICU after severe postpartum haemorrhage. Beside the woman who died, who was described above, there were five more cases. The second was a nulliparous woman with SOL and spontaneous delivery at 40 weeks + 6 days, followed by manual removal of the placenta and a total blood loss 5700 mL. She was treated with uterotonics and received packed cells. The third woman with SOL and spontaneous delivery at 41 weeks + 3 days had a postpartum haemorrhage of 3750 mL due to uterine atony. The fourth woman had induction at 41 weeks + 4 days on maternal indication, an emergency caesarean section was performed after failed operative vaginal delivery (for fetal distress), she had total blood loss of 2200 mL due to suspected disseminated intravascular coagulation after amniotic fluid embolism. The fifth woman had elective induction at 41 weeks + 6 days resulting in caesarean section due to failure to progress in the first stage. Three days postpartum, she was diagnosed with a bowel perforation combined with a pneumonia and sepsis. The sixth woman had SOL at 41 weeks + 6 days, after spontaneous vaginal delivery, the placenta was manually removed with a total blood loss of 4500 mL.
3.3. Subgroup analysis
In a subgroup analysis according to parity, we observed higher incidences of the main adverse outcomes in nulliparous women compared to multiparous women.
In nulliparous women the risk of adverse perinatal outcome was 1.8 % (3/170) in the induction group versus 2.6 % (30/1134) in the expectant group (adjRR 0.58; 95 % CI 0.14–2.41). Caesarean section was performed in 21.1 % (36/170) in the induction group versus 15.1 % (171/1134) in the expectant group (adjRR 1.41; 95 % CI 1.01–1.97). For multiparous women the risk of adverse perinatal outcome was 0.5 % (1/201) in the induction group versus 1.1 % (11/1039) in the expectant group (adjRR 0.54; 95 % CI 0.07–24.19). Caesarean section was performed in 1.0 % (2/201) in the induction group versus 2.1 % (22/1039) in the expectant group (adjRR 0.57; 95 % CI 0.14–2.44). (Table 3).
4. Discussion
4.1. Main findings
In this INDEX cohort study among low-risk women with late-term pregnancies, there was a non- significant difference in the absolute risk of adverse (induction 1.1 %; expectant 1.9 %) and severe adverse (induction 0.3 %; expectant 1.0 %) perinatal outcome between induction of labour at 41 weeks and expectant management until 42 weeks. The risks in both groups were lower than in the INDEX trial. Adverse maternal outcomes were comparable between the groups. The caesarean section rate in nulliparous women was lower in the expectant group. No stillbirths were observed. One neonatal death occurred after spontaneous onset of labour at 41 weeks + 2 days in the ‘unknown preference/management strategy’ group.
4.2. Strengths and limitations
This prospective cohort study alongside a randomised controlled trial collected outcomes from women with a preference and actual management of elective induction at 41 weeks + 0/1 days and from women with a preference and actual management of expectant management until 42 weeks. A strength in this study is the large number of women (n = 2174) preferring expectant management which allows risk estimation of rare events, although the smaller size of the induction group limited precision in estimating differences between groups. The INDEX trial and IPD-MA on the same subject concluded that the risk of these rare events —adverse perinatal outcomes— is higher with a policy of expectant management [3], [5]. The number of women in our expectant group was comparable with the expectant group of the IPD-MA (n = 2280).
A known limitation of cohort studies is loss to follow-up with a rule of thumb that < 5 % can be considered as little bias and > 20 % is a serious threat to validity [22]. In our cohort study, we had a loss to follow-up of 10.7 %. The loss to follow-up was mainly attributable to withdrawal of initially participating centres. Because centres provided data on a voluntary basis and did not receive financial compensation, a few centres stopped providing data.
Another limitation is that, due to analysing according to both preference and actual management strategy groups, we could not include all women with available data in the study. However, this strict classification (expectant and induction groups according to women’s preferred and followed management strategy) reflects ‘real world’ outcomes [23], [24]. The large group of women preferring expectant management in this cohort study made a considered choice for expectant management as opposed to the expectant group of women in the trial, which consisted largely of women with a preference for induction who participated in the study with the hope to be allocated to the induction group. Women who preferred expectant management were less anxious and reported less problems regarding quality of life than women preferring induction as was showed in a recently published study on women’s management preference among both INDEX trial and cohort participants. This may indicate a better health status in women preferring expectant management, which may be a contributing factor to the lower incidence of adverse outcomes in the expectant group in the cohort compared to the trial. The large expectant group of this cohort shows the ‘real world’ incidence of (severe) adverse outcomes, reflecting daily practice [25].
Although we were able to adjust for SES, BMI, ethnicity and parity in this non-randomised comparison, including this limited set of variables is unlikely to remove all confounding, so any interpretation of differences between groups should be handled with caution.
By performing a sub-analysis according to parity, the group of induction is further reduced and the power of the study to provide robust statements is limited. The point estimates will mainly give an indication of the risks within the compared groups.
4.3. Interpretation
A recently published meta-analysis on all relevant trials concluded that elective induction at 41 weeks reduces adverse perinatal outcome without an increase in caesarean section or adverse maternal outcomes compared to expectant management until 42 weeks, subgroup analysis showed that this concerned nulliparous women only [5]. In this cohort study among low-risk women with late-term pregnancies, the absolute risk of adverse perinatal was not significant different between induction at 41 weeks and expectant management until 42 weeks, with lowest risk for induction. Six cases of MAS occurred in the expectant group (0.3 %; 6/2174) versus zero cases in the induction group (0/372). This result is in line with the trial (induction 0 %, expectant 0.2 %). In four (4/6) children with MAS, the mother had a non-Caucasian ethnicity and in five (5/6) the mother was nulliparous. It was shown in previous studies that non-Western ethnicity (in particular women from Indian and African origin) is associated with a higher risk of perinatal morbidity and mortality in pregnancies exceeding 40 weeks [17]. Nulliparity as such is also associated with a higher risk of adverse perinatal outcomes [5], [26]. Further, we noticed that six women of the whole cohort (6/3642) were admitted to the ICU. Of whom 2 (2/2174) in the expectant group, zero in the induction group (0/372) and four in the ‘unknown preference/management strategy’ group (4/1096), mainly because of severe postpartum haemorrhage and coagulation abnormalities. Four of these women were nulliparous, two multiparous.
The absolute risk of adverse perinatal outcome in this cohort study was lower than in the trial. The absolute risk in the induction groups from the cohort and to the trial was 1.1 % versus 1.7 %. This also applied to the expectant groups from cohort and trial: 1.9 % and 3.1 % respectively. The risk difference between induction and expectant management for composite severe adverse perinatal outcome was comparable with that in the trial, 0.7 % in the cohort versus 0.9 % in the trial [3].
Women in the expectant groups of trial and cohort study differed in the way the management strategy was decided on. Women in the trial were allocated to expectant management regardless of their preference, whereas women in the cohort had expressed a preference for expectant management [25]. The lower composite adverse perinatal outcome in the expectant group of the cohort could be related to self-selection of these women. A recently published study on women’s preference for either induction at 41 weeks or expectant management among a subset of women of our cohort and the INDEX trial showed that women who declined policy by randomisation had lower levels of anxiety compared to women participating in the trial [25].
A comparison of baseline characteristics of our cohort and the INDEX trial participants showed that women in the cohort were older and more often of white ethnicity. The women in the cohort's induction group were more likely to have low SES compared to the trial's induction group (respectively 30.4 % and 24,3 %). The women in the expectant group of the cohort were more likely to have high SES compared to the expectant group of the trial (respectively 29.1 % and 25.9 %). With regard to BMI, both induction groups (cohort and trial) are comparable. However, in the expectant group of the cohort, we see more often a healthy weight and therefore less often overweight (BMI 25 to < 30) and obesity (BMI ≥ 30) compared to the expectant group of the trial. Since we know that white ethnicity, higher SES and healthy maternal weight are associated with a lower rate of adverse perinatal outcomes, these factors might have contributed to the difference in outcomes between cohort and trial [17], [18], [19], [27], [28]. In the cohort we observed that women with a preference for expectant were more often of white ethnicity, had a higher SES and had more often a healthy weight compared to the induction preference group. In the trial almost 54 % was nulliparous versus 51 % in this cohort. By adjusting risk ratios for these potential confounders, we could estimate the effect with reduced bias due to confounding.
In our cohort there were no stillbirths, compared to 0.4 % (5/1379) in the expectant group of the SWEPIS trial and 0.2 % (2/901) in the expectant group of the INDEX trial. In the induction group of SWEPIS, no stillbirth occurred and 0.1 % (1/900) in the induction group of the INDEX trial. To rule out confounding by indication we additionally contacted all participating centres to confirm if no perinatal or maternal death in the 41–42 timeframe of inclusion from May 2012 to March 2016 was lacking. In addition, the Dutch national perinatal registry in this time period of the INDEX trial was examined to analyse how many perinatal deaths occurred in late-term pregnancy and whether the study showed an under- or overestimation of the incidences. The incidence of fetal mortality in the 41–42 weeks’ timeframe in the Netherlands was 0.09 % (36/39.884) and the neonatal mortality rate was 0.04 % (17/39.884). Based on these national figures, for the size of our cohort two stillbirths and one neonatal death could have been expected for the size of our cohort [29].
Though a positive effect of fetal monitoring (ultrasound and/or CTG surveillance) has not been established in trials so far, a difference in the monitoring of the fetal condition in late-term pregnancy was noticed for Sweden and The Netherlands. All stillbirths in the SWEPIS trial occurred in Swedish regions outside the Stockholm region (19 hospitals) where no regular fetal monitoring was performed between 41 and 42 weeks (6/822), whereas fetal monitoring in late-term pregnancy has a prominent place in maternity care in the Netherlands the last decade [10]. In the Stockholm region, which accounted for half of the participants of the SWEPIS study, ultrasound for biometry and amniotic fluid measurement was performed routinely at 41 weeks before inclusion. In the Stockholm region, only women with a reassuring ultrasound could enter the SWEPIS study, no fetal death occurred here (0/557). In our cohort population over 97 % of the midwifery practices and hospitals followed a local protocol on fetal monitoring in late-term pregnancy during antenatal consultation between 41 weeks + 0 days and 42 weeks + 0 days of gestation. This could have had an effect on the incidence of perinatal mortality in both INDEX cohort and INDEX trial. The higher incidence of inductions on medical indication between 41 and 42 weeks in the expectant group of the INDEX trial compared to those in the expectant group of the SWEPIS trial, could be a reflection of the difference between the monitoring policies in both settings. Furthermore, the SWEPIS trial (N = 2760) was stopped earlier than planned (N = 10,000) for safety reasons due to a significantly higher rate of perinatal mortality in the expectant group (induction 0/1381 versus expectant 6/1379; p-value=0.03). The early stopping of the trial after 28 % of the planned number of patients were recruited, could have led to overestimation of the effect as the authors concluded already.
In our cohort, we observed a significant difference in caesarean section rates between induction and expectant management in nulliparous women (21.1 % versus 15.1 %) (adjRR 1.41; 95 % CI 1.01–1.97; p-value < 0.05). This was not observed in the group of multiparous women (adjRR 0.57; 95 % CI 0.14–2.44). In randomised trials no differences in caesarean section rate were found [3], [4], [30]. In a recently published whole-population study of 474,652 births, the incidence of caesarean section was increased in nulliparous women after elective induction compared to spontaneous onset of labour (29.3 % versus 13.8 %; RR 3.02; 95 % CI 2.90–3.15) [31]. These incidences in de nulliparous group are in line with the results of our study. The low incidence of caesarean section for multiparous women in this cohort could be explained by the inclusion criterion ‘no caesarean section in history’, multiparous women in our cohort have previously given birth vaginally. This low incidence of caesarean section for multipara is in line with the results of the IPD-MA [5].
The risk estimates for caesarean section in our cohort study were different from the recently updated Cochrane systematic review on induction of labour at or beyond 37 weeks’ gestation [2]. The Cochrane review showed a RR 0.90 (95 % CI 0.83–0.97) in favour of induction. This could be due to the different timeframes of comparison of the included studies: randomised controlled trials were included with expectant management strategies that went far beyond 42 weeks, some even without an upper limit of gestational age [2], [32]. One large trial was included with incomparable treatment arms considering that prostaglandins in case of an unfavourable cervix was restricted to use only in the induction group, it was not available for women in the expectant group with indicated IOL. [33]. The Cochrane review compares trials that investigate intended policies, whereas cohort studies examines actual policy.
No other large prospective cohort studies have been conducted into induction at 41 weeks versus expectant management until 42 weeks in low-risk pregnant women. A systematic review and meta-analysis of 12 cohort studies — mostly retrospective (n = 10) — shows that there is a significant increase in the risk of stillbirth from 41 weeks without a corresponding decrease in the risk of neonatal mortality [1]. Observational studies comparing induction and expectant management for the entire term period or starting at 41 weeks, involved a policy of expectant management beyond a gestational age of 42 weeks in most studies. They showed higher rates of caesarean section after induction compared to expectant management. Because of the different timeframes, a proper comparison of management strategies in the late term period is not possible [34], [35], [36], [37].
With a policy of expectant management, there is always a risk of developing pathological conditions, such as hypertensive disorders. The moment elective induction is performed at 41 weeks, the probability of a medical reason — maternal or fetal — for induction of labour disappears. On the other hand, there is a high chance (60 %) that women will have spontaneous onset of labour between 41 weeks + 0 days and 41 weeks + 4 day s [3]. The group with unknown preference/management strategy (n = 1096) does not fit into the comparison induction versus expectant management. This group represent women who did not made a clear choice for one of the two options and therefore comparison of induction versus expectant management was not possible. Though this situation occurs more often in daily practice, it is unclear how to consider the results of this group. In order to provide insight in all available data we present the outcomes of the unknown preference/management group in the supplementary tables 1 and 2.
4.4. Validity and generalisability
In this cohort study we compared groups according to maternal preference and actual management strategy in order to obtain real-world evidence [38]. Daily practice is influenced by multiple factors, such as maternal preference, caregivers’ influence, local policy, and availability of care [10].
Most women who participated in the parallel trial had a preference for induction (74.6 %); only 9.7 % expressed a preference for expectant management [3]. This in contrast to women in this cohort study, of whom 17.4 % indicated to have a preference for induction and 71.5 % for expectant management [25]. The heterogeneity of preferences in both expectant groups (cohort and trial), may have contributed to the difference in composite adverse perinatal outcomes. Women with a preference for induction are more anxious and have a lower quality of life compared to women with a preference for expectant management [25]. The women in the large expectant group in this cohort study preferred and received the expectant management strategy. To what extend self-selection plays a role in the low incidence of adverse perinatal outcome in this group of women is not clear yet, though it seems plausible to consider this group as representative for low-risk women preferring expectant management in late-term pregnancy. To analyse both the treatment effect and the influence of patient preferences, a partially randomised preference trial design could be used in future research [39], [40].
5. Conclusion
In this cohort study — alongside the INDEX-RCT — among low-risk women receiving the policy of their preference in late-term pregnancy, a non-significant difference was found between induction of labour at 41 weeks and expectant management until 42 weeks in absolute risks of composite adverse (1.1 % versus 1.9 %) and severe adverse (0.3 % versus 1.0 %) perinatal outcome. The risks were lower than in the trial setting.
There were no stillbirths among all 3642 women. Caesarean section rates were comparable, for nulliparous women the risk of caesarean section was increased after induction at 41 weeks.
Funding
None. The original RCT was funded by ZonMw: number NTR3431, Netherlands Trial Registy.
Contributions
AB and JKJK are joint first authors and contributed equally to the study. EdM and BWM initiated the INDEX study. EdM and JvdP supervised this study. AB and JKJK conducted the statistical analyses and take responsibility for the integrity of the data and accuracy of the data analyses, both wrote the first and subsequent drafts of the paper. PB and RD advised on statistical issues and interpretation of the results. JCK, JvD, RD, PB, AvK, JvdP, BWM and EdM provided feedback on all preliminary versions. All authors have approved the final version of this manuscript submitted for publication. AB, JKJK and EdM are guarantors. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Acknowledgements
We would like to thank all women who participated in this cohort, and all midwives, gynecologists residents and nurses of the participating centers. We would especially thank Loulou Schottelndreier (LS) and Julie van der Post for their contribution of the data management and Anita Ravelli for her comprehensive analysis of data from the Perinatal Registry of The Netherlands (Perined).
Disclosure of interests
BWM is supported by a NHMRC Investigator grant (GNT1176437).
BWM reports consultancy for ObsEva. BMW has received research funding from Ferring and Merck.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.eurox.2022.100165.
Contributor Information
Aafke Bruinsma, Email: a.bruinsma@amsterdamumc.nl.
Judit KJ Keulen, Email: judit.keulen@zuyd.nl.
Joep C Kortekaas, Email: jc.kortekaas@elkerliek.nl.
Jeroen van Dillen, Email: Jeroen.vanDillen1@radboudumc.nl.
Ruben G Duijnhoven, Email: r.g.duijnhoven@amsterdamumc.nl.
Patrick MM Bossuyt, Email: p.m.bossuyt@amsterdamumc.nl.
Anton H van Kaam, Email: a.h.vankaam@amsterdamumc.nl.
Joris AM van der Post, Email: j.a.vanderpost@amsterdamumc.nl.
Ben W Mol, Email: ben.mol@monash.edu.
Esteriek de Miranda, Email: e.demiranda@amsterdamumc.nl.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Muglu J., Rather H., Arroyo-Manzano D., Bhattacharya S., Balchin I., Khalil A., et al. Risks of stillbirth and neonatal death with advancing gestation at term: a systematic review and meta-analysis of cohort studies of 15 million pregnancies. PLoS Med. 2019;16(7) doi: 10.1371/journal.pmed.1002838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Middleton P., Shepherd E., Morris J., Crowther C.A., Gomersall J.C. Induction of labour at or beyond 37 weeks' gestation. Cochrane Database Syst Rev. 2020;7 doi: 10.1002/14651858.CD004945.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keulen J.K., Bruinsma A., Kortekaas J.C., van Dillen J., Bossuyt P.M., Oudijk M.A., et al. Induction of labour at 41 weeks versus expectant management until 42 weeks (INDEX): multicentre, randomised non-inferiority trial. BMJ. 2019;364:l344. doi: 10.1136/bmj.l344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wennerholm U.B., Saltvedt S., Wessberg A., Alkmark M., Bergh C., Wendel S.B., et al. Induction of labour at 41 weeks versus expectant management and induction of labour at 42 weeks (SWEdish Post-term Induction Study, SWEPIS): multicentre, open label, randomised, superiority trial. BMJ. 2019;367:l6131. doi: 10.1136/bmj.l6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alkmark M., Keulen J.K.J., Kortekaas J.C., Bergh C., van Dillen J., Duijnhoven R.G., et al. Induction of labour at 41 weeks or expectant management until 42 weeks: a systematic review and an individual participant data meta-analysis of randomised trials. PLoS Med. 2020;17(12) doi: 10.1371/journal.pmed.1003436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.ACOG Practice bulletin no. 146: management of late-term and postterm pregnancies. Obstet Gynecol. 2014;124(2 Pt 1):390–396. doi: 10.1097/01.AOG.0000452744.06088.48. [DOI] [PubMed] [Google Scholar]
- 7.WHO. WHO Recommendations. Induction of labour at or beyond term. [Available from: 〈https://appswhoint/iris/bitstream/handle/10665/277233/9789241550413-engpdf]〉. 2019. [PubMed]
- 8.Grobman W.A., Rice M.M., Reddy U.M., Tita A.T.N., Silver R.M., Mallett G., et al. Labor induction versus expectant management in low-risk nulliparous women. N Engl J Med. 2018;379(6):513–523. doi: 10.1056/NEJMoa1800566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walker K.F., Bugg G.J., Macpherson M., McCormick C., Grace N., Wildsmith C., et al. Randomized trial of labor induction in women 35 years of age or older. N Engl J Med. 2016;374(9):813–822. doi: 10.1056/NEJMoa1509117. [DOI] [PubMed] [Google Scholar]
- 10.Kortekaas J.C., Bruinsma A., Keulen J.K.J., Vandenbussche F., van Dillen J., de Miranda E. Management of late-term pregnancy in midwifery- and obstetrician-led care. BMC Pregnancy Childbirth. 2019;19(1):181. doi: 10.1186/s12884-019-2294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kortekaas J.C., Bruinsma A., Keulen J.K., van Dillen J., Oudijk M.A., Zwart J.J., et al. Effects of induction of labour versus expectant management in women with impending post-term pregnancies: the 41 week - 42 week dilemma. BMC Pregnancy Childbirth. 2014;14:350. doi: 10.1186/1471-2393-14-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Obstetric Vademecum, 2003. guideline from the 'Committee of Midwifery' and 'Health Care Insurance Board'. 2003.
- 13.Nederlandse Vereniging voor Obstetrie & Gynaecologie, 2007. Guideline Prolonged Pregnancy (Seroniteit). 2007.
- 14.ICD-10-CM Diagnosis Code P24.01 Meconium aspiration with respiratory symptoms [Available from: 〈https://www.icd10data.com/ICD10CM/Codes/P00-P96/P19-P29/P24-/P24.01]〉.
- 15.American Academy Of Pediatrics Committee On F, Newborn, American College Of O, gynecologists committee on obstetric P. the apgar score. Pediatrics. 2015;136(4):819–822. [Google Scholar]
- 16.Perined. P.R.N. geboortegewichtcurven. 2008.
- 17.Ravelli A.C., Schaaf J.M., Eskes M., Abu-Hanna A., de Miranda E., Mol B.W. Ethnic disparities in perinatal mortality at 40 and 41 weeks of gestation. J Perinat Med. 2013;41(4):381–388. doi: 10.1515/jpm-2012-0228. [DOI] [PubMed] [Google Scholar]
- 18.Kazemier B.M., Ravelli A.C., de Groot C.J., Mol B.W. Optimal timing of near-term delivery in different ethnicities: a national cohort study. BJOG. 2014;121(10):1274–1282. doi: 10.1111/1471-0528.12938. [DOI] [PubMed] [Google Scholar]
- 19.Blumenshine P., Egerter S., Barclay C.J., Cubbin C., Braveman P.A. Socioeconomic disparities in adverse birth outcomes a systematic review. Am J Prev Med. 2010;39(3):263–272. doi: 10.1016/j.amepre.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 20.Metcalfe A., Lail P., Ghali W.A., Sauve R.S. The association between neighbourhoods and adverse birth outcomes: a systematic review and meta-analysis of multi-level studies. Paediatr Perinat Epidemiol. 2011;25(3):236–245. doi: 10.1111/j.1365-3016.2011.01192.x. [DOI] [PubMed] [Google Scholar]
- 21.Sebastian Manzanares G., Angel Santalla H., Irene Vico Z., Lopez, Criado M.S. Alicia Pineda L, Jose Luis Gallo V. Abnormal maternal body mass index and obstetric and neonatal outcome. J Matern Fetal Neonatal Med. 2012;25(3):308–312. doi: 10.3109/14767058.2011.575905. [DOI] [PubMed] [Google Scholar]
- 22.Haynes R.B.S., David L., Richardson W.S., William R., Langley G., Ross E. Evidence-based medicine: how to practice & teach EBM. Churchill Livingstone; New-York: 1997. [Google Scholar]
- 23.Bartlett V.L., Dhruva S.S., Shah N.D., Ryan P., Ross J.S. Feasibility of using real-world data to replicate clinical trial evidence. JAMA Netw Open. 2019;2(10) doi: 10.1001/jamanetworkopen.2019.12869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.U.S. Food, Drug Administration, 2018. Framework for FDA’S Real-World Evidence Program. 2018.
- 25.Keulen J.K.J., Nieuwkerk P.T., Kortekaas J.C., van Dillen J., Mol B.W., van der Post J.A.M., et al., 2020. What women want and why. Women's preferences for induction of labour or expectant management in late-term pregnancy. Women Birth. 2020. [DOI] [PubMed]
- 26.Yim C., Wong L., Cabalag C., Wallace E.M., Davies-Tuck M. Post-term surveillance and birth outcomes in South Asian-born compared with Australian-born women. J Perinatol. 2017;37(2):139–143. doi: 10.1038/jp.2016.190. [DOI] [PubMed] [Google Scholar]
- 27.Kortekaas J.C., Kazemier B.M., Keulen J.K.J., Bruinsma A., Mol B.W., Vandenbussche F., et al. Risk of adverse pregnancy outcomes of late- and postterm pregnancies in advanced maternal age: a national cohort study. Acta Obstet Gynecol Scand. 2020;99(8):1022–1030. doi: 10.1111/aogs.13828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marchi J., Berg M., Dencker A., Olander E.K., Begley C. Risks associated with obesity in pregnancy, for the mother and baby: a systematic review of reviews. Obes Rev. 2015;16(8):621–638. doi: 10.1111/obr.12288. [DOI] [PubMed] [Google Scholar]
- 29.Perined, 2017. Perinatale Zorg in Nederland. (2012 through 2016). 2017.
- 30.Gelisen O., Caliskan E., Dilbaz S., Ozdas E., Dilbaz B., Ozdas E., et al. Induction of labor with three different techniques at 41 weeks of gestation or spontaneous follow-up until 42 weeks in women with definitely unfavorable cervical scores. Eur J Obstet Gynecol Reprod Biol. 2005;120(2):164–169. doi: 10.1016/j.ejogrb.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 31.Dahlen H.G., Thornton C., Downe S., de Jonge A., Seijmonsbergen-Schermers A., Tracy S., et al. Intrapartum interventions and outcomes for women and children following induction of labour at term in uncomplicated pregnancies: a 16-year population-based linked data study. BMJ Open. 2021;11(6) doi: 10.1136/bmjopen-2020-047040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keulen J.K.J., Bruinsma A., Kortekaas J.C., van Dillen J., van der Post J.A.M., de Miranda E. Timing induction of labour at 41 or 42 weeks? A closer look at time frames of comparison: a review. Midwifery. 2018;66:111–118. doi: 10.1016/j.midw.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 33.Hannah M.E., Hannah W.J., Hellmann J., Hewson S., Milner R., Willan A. Induction of labor as compared with serial antenatal monitoring in post-term pregnancy. A randomized controlled trial. The Canadian multicenter post-term pregnancy trial group. N Engl J Med. 1992;326(24):1587–1592. doi: 10.1056/NEJM199206113262402. [DOI] [PubMed] [Google Scholar]
- 34.Ehrenthal D.B., Jiang X., Strobino D.M. Labor induction and the risk of a cesarean delivery among nulliparous women at term. Obstet Gynecol. 2010;116(1):35–42. doi: 10.1097/AOG.0b013e3181e10c5c. [DOI] [PubMed] [Google Scholar]
- 35.Teo E.Y., Kumar S. Intrapartum intervention rates and perinatal outcomes following induction of labour after 41 + 0 weeks compared to expectant management. J Matern Fetal Neonatal Med. 2017;30(21):2517–2520. doi: 10.1080/14767058.2016.1255190. [DOI] [PubMed] [Google Scholar]
- 36.Rydahl E., Eriksen L., Juhl M. Effects of induction of labor prior to post-term in low-risk pregnancies: a systematic review. JBI Database Syst Rev Implement Rep. 2019;17(2):170–208. doi: 10.11124/JBISRIR-2017-003587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burgos J., Rodriguez L., Otero B., Cobos P., Osuna C., Centeno Mdel M., et al. Induction at 41 weeks increases the risk of caesarean section in a hospital with a low rate of caesarean sections. J Matern Fetal Neonatal Med. 2012;25(9):1716–1718. doi: 10.3109/14767058.2012.663018. [DOI] [PubMed] [Google Scholar]
- 38.de Lusignan S., Crawford L., Munro N. Creating and using real-world evidence to answer questions about clinical effectiveness. J Innov Health Inf. 2015;22(3):368–373. doi: 10.14236/jhi.v22i3.177. [DOI] [PubMed] [Google Scholar]
- 39.Walter S.D., Turner R., Macaskill P., McCaffery K.J., Irwig L. Beyond the treatment effect: evaluating the effects of patient preferences in randomised trials. Stat Methods Med Res. 2017;26(1):489–507. doi: 10.1177/0962280214550516. [DOI] [PubMed] [Google Scholar]
- 40.Walter S.D., Bian M. Relative efficiencies of alternative preference-based designs for randomised trials. Stat Methods Med Res. 2020;29(12):3783–3803. doi: 10.1177/0962280220941874. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material